Abstract
Mutations in the isocitrate dehydrogenase (IDH) genes are frequently found in gliomas and in a fraction of acute myeloid leukemia patients. This results in the production of an oncometabolite, 2-hydroxyglutarate (2-HG). Glioma patients harboring IDH mutations have a longer survival than their wild-type counterparts. 2-HG has been detected noninvasively in gliomas with IDH mutations using magnetic resonance spectroscopy (MRS), suggesting its potential clinical relevance for identifying glioma subtypes with better prognosis. In this paper, the recent developments in the MRS detection of the 2-HG in gliomas are reviewed, including the therapeutic potentials and translational values.
Introduction
Somatic mutations in the genes isocitrate dehydrogenase 1 (IDH1) and IDH2 have been identified in a subset of gliomas, acute myeloid leukemia, and less frequently in other malignancies [1–4]. These mutations result in the substitution of the arginine 132 (in IDH1) and arginine 172 (in IDH2) codons by histidine, causing alterations in the normal enzymatic activities of IDH1-R132 and IDH2-R172 [1]. IDH mutations are associated with alterations in DNA methylation and impede the oxidative decarboxylation of isocitrate, resulting in overproduction and accumulation of the oncometabolite 2-hydroxyglutarate (2-HG) instead of α-ketoglutarate [1,5,6].
Being a direct and noninvasively detectable metabolic consequence of a genetic mutation in cancer, 2-HG detection in glioma serves as a unique biomarker for identifying IDH mutations. The potential diagnostic value of 2-HG as a biomarker of patient survival is further confirmed by better survival in patients with IDH mutations than wild type. IDH mutations have been reported in more than 70% of low-grade gliomas [World Health Organization (WHO) grades II and III] and secondary glioblastomas (Table 1), while the frequency of IDH mutations in primary glioblastoma is much lower (<10%) [3,7,8]. Secondary glioblastomas may often be incorrectly diagnosed as primary glioblastomas [9]. Hence, the detection of 2-HG could be a potential tool for in vivo distinction of secondary from primary glioblastomas [10]. The molecular pathogenesis of IDH1/2 mutations in the development of gliomas has yet to be identified. However, such identification may improve our understanding of the mechanisms of glioma development and may lead to the development of novel molecular classification and therapy. 2-HG being a magnetic resonance (MR)-visible indicator of IDH mutation offers unique possibilities in monitoring and tracking glioma patients.
Table 1.
Frequency of IDH Mutations in Various Glial Brain Tumors.
| WHO Grade | Glioma Type | n | IDH Mutation Status | References | ||
| IDH1 | IDH2 | Combined (%) | ||||
| II | A | 405 | 282 | 4 | 74 | [3,4,6,7,24,35] |
| OA | 196 | 150 | 1 | |||
| O | 363 | 268 | 8 | |||
| III | A | 398 | 257 | 4 | 76 | [3,4,6,7,24,35] |
| OA | 279 | 229 | 11 | |||
| O | 245 | 184 | 12 | |||
| IV | GBM secondary | 85 | 69 | 0 | 82 | [3,6,7,9] |
| IV | GBM primary | 673 | 32 | 0 | 5 | [3,6,7,9] |
Abbreviations: A, astrocytoma; OA, oligoastrocytoma; O, oligodendroglioma.
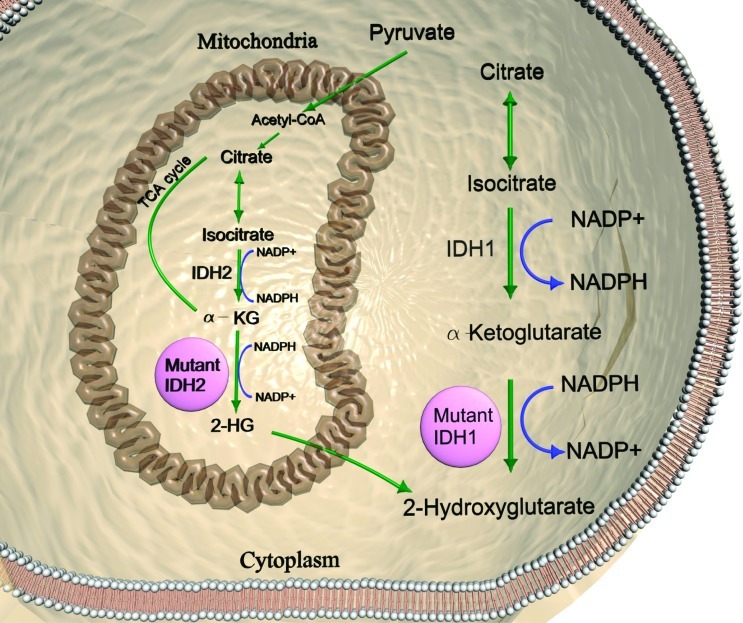
Both IDH1 and IDH2 are NADP+-dependent (oxidized form of nicotinamide adenine dinucleotide phosphate) enzymes catalyzing the conversion of isocitrate to α-ketoglutarate (Figure 1). In mutant tumors, the NADPH levels are decreased, causing a potential effect in cellular biosynthetic processes, such as lipogenesis [2]. By metabolic profiling, more than 200 metabolites have been analyzed in human oligodendroglioma cells engineered to express mutant IDH1/IDH2 [11]. The major biologic alterations identified were increased levels of free amino acids and lipid precursors and depletion of metabolites involved in the tricarboxylic acid cycle (also known as Krebs cycle): citrate, α-ketoglutarate, fumarate, cis-aconitate, and malate [11].
Figure 1.
Mutant IDH1/2 causes accumulation of the oncometabolite 2-HG. Metabolic pathway of 2-HG production follows the catalytic reduction of α-ketoglutarate by IDH1 (cytoplasmic) and IDH2 (mitochondrial) through the conversion of NADPH to NADP+.
MR spectroscopy (MRS) has been widely used as a quantitative analytical tool for monitoring metabolism in different types of malignancies [12,13]. Metabolic profiling helps in assessing pathophysiological processes associated with tumor biology and is a valuable monitoring tool for developing novel therapeutic anticancer agents [12]. More recently, promising results have been obtained using this technique for 2-HG detection in vivo [14,15]. Crucial to these approaches were the determination of whether the accumulation of 2-HG in IDH-mutated gliomas was within the range of MRS-detectable levels and in the precise assignment of 2-HG resonances overlapping with other metabolites using conventional one-dimensional (1D) MRS. This mini-review highlights the recent developments in the detection of the 2-HG metabolite in gliomas using MRS, including the therapeutic potentials of targeting IDH mutant cells. The potential translational values of MRS-based IDH detection and future directions are discussed.
MRS Principles
MRS can map metabolic profiles and dynamics in vivo or within tissue extracts or intact tissue samples in a laboratory setting. MRS detects MR signals from stimulated nuclear spins in a strong static magnetic field. When placed in an external strong magnetic field (B0), atomic nuclei with a magnetic dipole moment (such as 1H, 13C, or 31P) precess around B0 at specific frequencies. By applying an excitation radio-frequency (RF) pulse at resonance with this precession, nuclei can be brought to a higher energy state. Following the excitation, the nuclei recover to the equilibrium state and the absorbed energies are released, emitting RF signals. The RF receiver coils are tuned to detect only the RF signals originating from the excited nuclei. Different nuclei of the same type, e.g., 1H, experience slightly different magnetic fields with respect to their molecular environment and therefore precess at slightly different frequencies. The frequency of detected signals is described on a field-independent dimensionless scale called chemical shift (δ), which is expressed in parts per million (ppm; see [16]). Individual molecular properties will be characterized by a single resonance (e.g., N-acetylaspartic acid) or multiple resonances (e.g., lactate, a doublet resonance) in the MR spectra (Figure 2A). The signal intensity reflects the number of excited nuclei in the molecule. The MRS technique enables detection of a wide array of metabolites simultaneously. In this review, we refer to studies observing the nucleus of the hydrogen atom (proton, 1H), which is most commonly used in MRS due to its high intrinsic sensitivity.
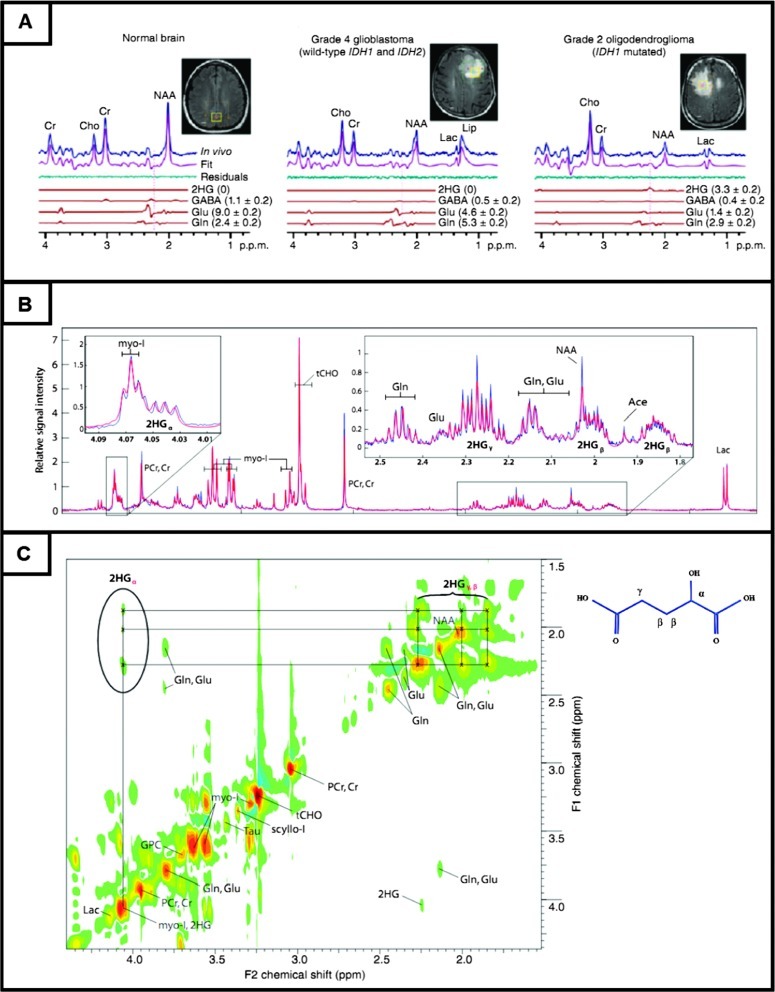
Figure 2.
2-HG detection in IDH mutant gliomas and its MRS signature in 1H MR spectra. (A) Representative in vivo 1H MR spectra from normal brain and gliomas. Single voxel (2 x 2 x 2 cm3)-localized PRESS spectra at 3 T together with spectral fits to the components of 2-HG, γ-aminobutyric acid, glutamate, and glutamine are shown. Vertical lines are drawn at 2.25 ppm to indicate the Hβ multiplet of 2-HG in the PRESS-edited spectra. (B) HRMAS spectra from surgical specimens ex vivo obtained from glioma sample of WHO grade III astrocytoma; blue and red traces represent the acquired and fitted spectra, respectively. (C) A 2D spectrum acquired from the same tissue sample representing the resonance cross-peaks about the F1/F2 diagonal. Three well-resolved proton resonances of 2-HG are located along a vertical column (cross-peaks of 2-HGα,2-HGβ, and 2-HGγ) at F2 = 4.05/F1 = 1.85 ppm, 4.05/2.01 ppm, and 4.05/2.28 ppm. Adapted with permission from [14,27].
Ex Vivo MRS of Biopsy Specimens
High-resolution magic angle spinning (HRMAS) is a relatively new solid-state MRS technique for metabolic profiling of intact tissue samples. In solid state, molecules experience significant motion restriction. The major reasons are molecular dipole-dipole interactions and chemical shift anisotropy, resulting in spectral broadening with an angular dependency. By rapidly spinning (typically 5000 Hz) the sample at an angle (θ = 54.7°) to the main magnetic field, the so-called “magic angle,” the line broadening is markedly reduced, and the tissue samples thus resemble a semiliquid phase [17]. The sample preparation is simple and straightforward. It typically includes weighing the samples (approximately 5–30 mg), thawing them on an ice bed, and cutting tissue samples either for direct loading into the MAS zirconium rotors (small holders with air turbine to spin the sample) or for loading into disposable inserts fitting the MAS rotors. A small amount of suitable buffer (<5 µl) is used for chemical shift referencing and field locking [18,19]. HRMAS is nondestructive, enabling subsequent biologic evaluations such as histopathology or gene expression profiling in the same tissue sample. This technique is well suited for the examination of unprocessed surgical specimens ex vivo in a laboratory setting and has the potential to be implemented in clinical workflows.
In Vivo MRS
When used in vivo, spatially localized MRS or MR spectroscopic imaging is combined with conventional MR imaging to allow the investigation of metabolic distribution within determined anatomic regions. The MR spectra obtained using clinical scanners suffer from poorer signal-to-noise ratio and spectral resolution compared to ex vivo MRS. However, recent advances in MR technology, including access to higher magnetic field strengths, have boosted the clinical potential of in vivo MRS. In vivo MRS detection of 2-HG in IDH-mutated glioma patients holds promise for reliable identification of this metabolite. Using a common spectroscopy sequence and field strength of 3 T [14], which is commonly used in the clinical setting, the 2-HG resonances have been identified without need for more specialized instrumentations. The data acquisition and post-processing methods used in these studies could be also implemented on standard hardware already in place in many MR imaging centers. Thus, the significance of 2-HG detection could be assessed with well-defined clinical settings and larger cohorts.
Detection of 2-HG by MRS
Detection of the 2-HG Metabolite Ex Vivo
HRMAS MRS provides a vast amount of biochemical information from intact tissue samples with minimal sample preparation [18,20]. Many studies have demonstrated the ability of this method to investigate and quantify malignancy-associated metabolites in diagnosis, prognosis, and treatment monitoring in cancer [21–25]. Using HRMAS MRS, the oncometabolite 2-HG has been detected in tissue specimens resected from glioma patients harboring IDH1/IDH2 mutations ex vivo [26–28]. The MR signal arising from the 2-HG metabolite exhibits complex features and overlaps with neighborhood resonances, such as myo-inositol, glutamine, glutamate, and γ-aminobutyric acid (Figure 2B). By using two-dimensional (2D) correlation spectroscopy (COSY) [29], the distinctive metabolite cross-peak pattern from the proton correlations in a molecule can be distinguished (Figure 2C).
In a study by Elkhaled et al. [27], there was a significant correlation between the presence of 2-HG as determined by MRS and IDH1 mutation status as determined by IDH1-R132H immunostaining and direct genetic sequencing of IDH1. The authors found a strong correlation between 2-HG levels and the presence of IDH mutation. They also correlated the 2-HG levels with histopathologic parameters, including cell density in tumor. Kalinina et al. [28] investigated whether the presence of 2-HG in glioma cohorts was reflected as a consequence of IDH1/IDH2 mutations across the subtypes and grades including nontumorous controls. Beyond the complexity, the 2D MR spectra clearly demonstrated the resolved proton resonances of 2-HG. High sensitivity, specificity, and accuracy (>95%) for the MRS-based identification of IDH1/IDH2 mutant gliomas with high levels of 2-HG were obtained.
In Vivo MRS of Glioma Patients
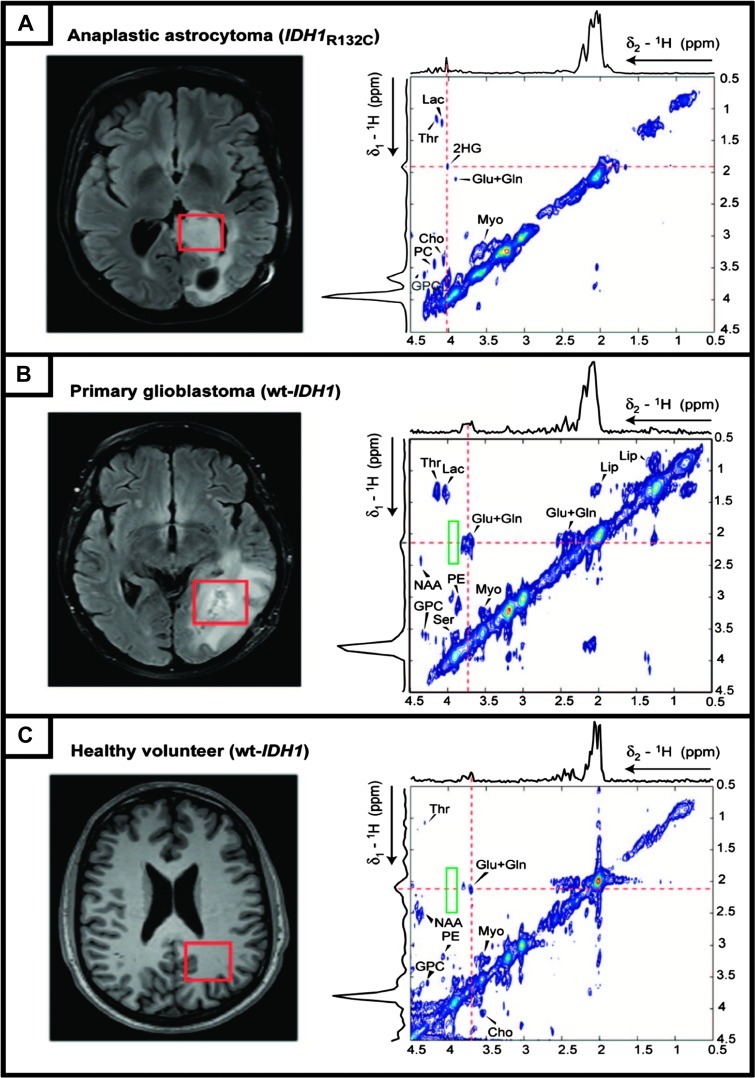
The feasibility of using 2-HG as a biomarker of IDH mutation in gliomas has been further established in vivo by using both 1D spectral editing and 2D MRS techniques [14,15]. Using an edited point resolved spectroscopy sequence (PRESS) [30], Choi et al. [14] identified the 2-HG resonances in 1H MR spectra of IDH-mutated gliomas at clinical field strength (3 T; Figure 2A). In an investigation of glioma patients harboring IDH1 mutations, primary glioblastoma multiforme (GBM) patients lacking IDH mutations, and healthy volunteers, Andronesi et al. [15] found a correlation between the 2-HG accumulation and IDH-mutated gliomas (Figure 3). Among 30 patients, the 2-HG resonances were detected only in the 1H MR spectra of IDH1-mutated gliomas, not in the GBM and healthy control cohorts, confirming the high specificity [23] of the method. The feasibility of detecting 2-HG using a localized 2D MRS sequence [31] using a 3-T clinical scanner was also proven [15]. The 2-HG metabolites were also assigned in the 1H MR spectra using localized 1D MRS.
Figure 3.
In vivo 2D MRS spectra of human brain subjects acquired at 3 T. Single voxels (red rectangles, 3 x 3 x 3 cm3) are placed on the basis of fluid attenuated inversion recovery images prescribing abnormalities in tumor patients (left column), and the corresponding metabolite cross-peak are depicted as contour maps (right column). (A) Astrocytoma patient with IDH1-R132C; the Hα-Hβ cross-peak of 2-HG located at 4.02/1.91 ppm (δ2/δ1). The 2D spectra acquired (voxel size of 3.5 x 3.5 x 3.5 cm3) from a primary glioblastoma patient with wt-IDH1 (B) and healthy volunteer with wt-IDH1 (C) do not contain any 2-HG cross-peak (outlined by the green rectangle). All 2D spectra were acquired using a developed 2D localized adiabatic selective refocusing-COSY sequence with a repetition time of 45 milliseconds, 64 increments in F1 direction, 8 averages per F1 transient, and a total acquisition time of 12.8 minutes. Adiabatic pulses improve the sequence performance by providing sharp and uniform excitation slices and a robust flip angle and by significantly decreasing the chemical shift displacement error (see [31]). Adapted with permission from [15].
Pope et al. [32] found the presence of 2-HG (with a sensitivity of 100%) in low-grade and recurrent GBM with IDH1 mutation. A conventional single-voxel MRS sequence was used in this study, showing the feasibility of detecting 2-HG resonances with current clinical uses. Analyzing the MR spectra obtained from two cohorts of patients, they found a significantly higher 2-HG level in mutant IDH1 genotypes than in wild type. The metabolite peaks were fitted using the LC Model software package [33] for estimating the metabolite level of 2-HG. When the glutamine and glutamate levels were compared ex vivo, no significant differences were found between wild-type and IDH1 mutant subgroups. Thus, the 2-HG peaks contributed predominantly to the increases in the detected MRS signals in vivo of glutamate + glutamine + 2-HG based on the LC Model measurements [32]. However, the in vivo assignment of 2-HG resonances is an important technical challenge because of the complex spin-coupling features. This could lead to false-positive 2-HG detection in wild-type cohorts [32]. The MRS sequences may still need further optimization and refinement to achieve reproducible sensitivity and specificity applicable in clinical settings. In addition, it is important to validate in vivo proof-of-principle data by implementing the protocols in a larger cohort.
One of the challenges in studying the underlying biology of IDH mutant gliomas in vivo could be the lack of reliable preclinical models that can also be used to develop novel therapeutic strategies targeting the IDH mutant cells. For this purpose, Luchman et al. [34] established an orthotopic anaplastic oligoastrocytoma xenograft model with endogenous IDH1 mutations.
Clinical Perspective
More than 70% of low-grade glioma patients (grade II or III) carry IDH1/IDH2 mutations. Most of the grade II and III gliomas progress over time to glioblastomas and are called secondary glioblastomas. The frequency of IDH mutations is high (>70%) among secondary glioblastomas compared to primary glioblastomas (<10%). (Table 1). Thus, 2-HG may potentially distinguish primary glioblastomas from secondary glioblastomas [10,35,36]. Moreover, IDH mutations provide overall survival prediction, irrespective of glioma grade. A better prognosis has been generally reported in glioma patients carrying an IDH mutation [37,38]. Furthermore, a reduction in cell proliferation, as a result of 2-HG accumulation in glioma cell lines, and prolonged survival in mice injected with IDH1-R132H mutant cells have been found [39]. Bralten et al. concluded that IDH1-R132H mutations in gliomas are associated with better prognosis and reduced aggressiveness, regarding both in vitro and in vivo results. Translating noninvasive detection of 2-HG by MRS to clinical applications may have a favorable impact on diagnosis, prognosis, treatment stratification, and management of glioma patients.
Summary and Conclusion
Using MRS, the oncometabolite 2-HG can be detected with high sensitivity (>90%) and specificity in gliomas harboring IDH mutations ex vivo and in vivo. The capability of this technique in tracking a mutation event suggests that 2-HG is a clinical valuable biomarker. Thus, it may potentially help to investigate how the IDH mutation accompanies gliomagenesis. Ex vivo analysis using HRMAS MRS provides a nondestructive and highly sensitive method in which tissue samples can be used for further histopathology and genomic analyses, aiding a better understanding of tumor biology. In vivo studies demonstrated the feasibility of detecting 2-HG noninvasively using current clinical scanners. Further validation in larger cohorts may improve the molecular characterization of IDH-mutant tumors. In conclusion, MRS is a useful and promising method for analyzing cancer-associated metabolic alteration, providing mechanistic insights into tumorigenesis, and presents a potential for therapeutic intervention.
Footnotes
No competing interests were declared.
References
- 1.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang C-M. Molecular targets of CNS tumors. 2011. [March 6, 2013]. Available at: http://www.intechopen.com/books/molecular-targets-of-cns-tumors.
- 3.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–474. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 5.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 11.Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci USA. 2011;108:3270–3275. doi: 10.1073/pnas.1019393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004;4:551–561. doi: 10.1038/nrc1390. [DOI] [PubMed] [Google Scholar]
- 13.McIntyre DJ, Madhu B, Lee SH, Griffiths JR. Magnetic resonance spectroscopy of cancer metabolism and response to therapy. Radiat Res. 2012;177:398–435. doi: 10.1667/rr2903.1. [DOI] [PubMed] [Google Scholar]
- 14.Choi C, Ganji SK, Deberardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andronesi OC, Kim GS, Gerstner E, Batchelor T, Tzika AA, Fantin VR, Vander Heiden MG, Sorensen AG. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4:116ra114. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuperman V. Magnetic Resonance Imaging: Physical Principles and Applications. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 17.Andrew ER, Bradbury A, Eades RG. Nuclear magnetic resonance spectra from a crystal rotated at high speed. Nature. 1958;182:1659. [Google Scholar]
- 18.Sitter B, Sonnewald U, Spraul M, Fjosne HE, Gribbestad IS. High-resolution magic angle spinning MRS of breast cancer tissue. NMR Biomed. 2002;15:327–337. doi: 10.1002/nbm.775. [DOI] [PubMed] [Google Scholar]
- 19.Bathen TF, Jensen LR, Sitter B, Fjosne HE, Halgunset J, Axelson DE, Gribbestad IS, Lundgren S. MR-determined metabolic phenotype of breast cancer in prediction of lymphatic spread, grade, and hormone status. Breast Cancer Res Treat. 2007;104:181–189. doi: 10.1007/s10549-006-9400-z. [DOI] [PubMed] [Google Scholar]
- 20.Cheng LL, Chang IW, Louis DN, Gonzalez RG. Correlation of high-resolution magic angle spinning proton magnetic resonance spectroscopy with histopathology of intact human brain tumor specimens. Cancer Res. 1998;58:1825–1832. [PubMed] [Google Scholar]
- 21.Cao MD, Sitter B, Bathen TF, Bofin A, Lonning PE, Lundgren S, Gribbestad IS. Predicting long-term survival and treatment response in breast cancer patients receiving neoadjuvant chemotherapy by MR metabolic profiling. NMR Biomed. 2012;25:369–378. doi: 10.1002/nbm.1762. [DOI] [PubMed] [Google Scholar]
- 22.Moestue S, Sitter B, Bathen TF, Tessem MB, Gribbestad IS. HR MAS MR spectroscopy in metabolic characterization of cancer. Curr Top Med Chem. 2011;11:2–26. doi: 10.2174/156802611793611869. [DOI] [PubMed] [Google Scholar]
- 23.Bathen TF, Sitter B, Sjobakk TE, Tessem MB, Gribbestad IS. Magnetic resonance metabolomics of intact tissue: a biotechnological tool in cancer diagnostics and treatment evaluation. Cancer Res. 2010;70:6692–6696. doi: 10.1158/0008-5472.CAN-10-0437. [DOI] [PubMed] [Google Scholar]
- 24.Giskeodegard GF, Lundgren S, Sitter B, Fjosne HE, Postma G, Buydens LM, Gribbestad IS, Bathen TF. Lactate and glycine—potential MR biomarkers of prognosis in estrogen receptor-positive breast cancers. NMR Biomed. 2012;25:1271–1279. doi: 10.1002/nbm.2798. [DOI] [PubMed] [Google Scholar]
- 25.Swanson MG, Keshari KR, Tabatabai ZL, Simko JP, Shinohara K, Carroll PR, Zektzer AS, Kurhanewicz J. Quantification of choline- and ethanolamine-containing metabolites in human prostate tissues using 1H HR-MAS total correlation spectroscopy. Magn Reson Med. 2008;60:33–40. doi: 10.1002/mrm.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yen KE, Bittinger MA, Su SM, Fantin VR. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene. 2010;29:6409–6417. doi: 10.1038/onc.2010.444. [DOI] [PubMed] [Google Scholar]
- 27.Elkhaled A, Jalbert LE, Phillips JJ, Yoshihara HA, Parvataneni R, Srinivasan R, Bourne G, Berger MS, Chang SM, Cha S, et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012;4:116ra115. doi: 10.1126/scitranslmed.3002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalinina J, Carroll A, Wang L, Yu Q, Mancheno DE, Wu S, Liu F, Ahn J, He M, Mao H, et al. Detection of “oncometabolite” 2-hydroxyglutarate by magnetic resonance analysis as a biomarker of IDH1/2 mutations in glioma. J Mol Med (Berl) 2012;90:1161–1171. doi: 10.1007/s00109-012-0888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ernst RR. Sterilization by means of ethylene oxide. Acta Pharm Suec. 1975;12:44–64. [PubMed] [Google Scholar]
- 30.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- 31.Andronesi OC, Ramadan S, Mountford CE, Sorensen AG. Low-power adiabatic sequences for in vivo localized two-dimensional chemical shift correlated MR spectroscopy. Magn Reson Med. 2010;64:1542–1556. doi: 10.1002/mrm.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope WB, Prins RM, Albert Thomas M, Nagarajan R, Yen KE, Bittinger MA, Salamon N, Chou AP, Yong WH, Soto H, et al. Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107:197–205. doi: 10.1007/s11060-011-0737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 34.Luchman HA, Stechishin OD, Dang NH, Blough MD, Chesnelong C, Kelly JJ, Nguyen SA, Chan JA, Weljie AM, Cairncross JG, et al. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol. 2012;14:184–191. doi: 10.1093/neuonc/nor207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capper D, Weissert S, Balss J, Habel A, Meyer J, Jager D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20:245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichimura K, Pearson DM, Kocialkowski S, Backlund LM, Chan R, Jones DT, Collins VP. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11:341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Bent MJ, Dubbink HJ, Marie Y, Brandes AA, Taphoorn MJ, Wesseling P, Frenay M, Tijssen CC, Lacombe D, Idbaih A, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16:1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 38.Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 39.Bralten LB, Kloosterhof NK, Balvers R, Sacchetti A, Lapre L, Lamfers M, Leenstra S, de Jonge H, Kros JM, Jansen EE, et al. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69:455–463. doi: 10.1002/ana.22390. [DOI] [PubMed] [Google Scholar]