Abstract
BACKGROUND: Signaling through stromal cell-derived factor-1α (SDF-1α), strongly secreted by bone marrow stromal cells and the CXC chemokine receptor 4 (CXCR4) exposed on tumor cells has pivotal roles in proliferation, metastasis, and tumor cell “dormancy.” Dormancy is associated with cytostatic drug resistance and is probably a property of tumor stem cells and minimal residual disease. Thus, hampering the SDF-1α/CXCR4 cross talk by a CXCR4 antagonist like Plerixafor (AMD3100) should overcome tumor cell dormancy bymobilization of tumor cells from “sanctuary” niches. Our aim was to elucidate the direct effects exerted by SDF-1α and Plerixafor on proliferation, chemosensitivity, and apoptosis of CXCR4-expressing tumor cells. METHODS: The ability of SDF-1α and Plerixafor to regulate intracellular signaling, proliferation, and invasion was investigated using two colon cancer cell lines (HT-29 and SW480) with either high endogenous or lentiviral expression of CXCR4 compared to their respective low CXCR4-expressing counterparts as a model system. Efficacy of Plerixafor on sensitivity of these cell lines against 5-fluorouracil, irinotecan, or oxaliplatin was determined in a cell viability assay as well as stroma-dependent cytotoxicity and apoptosis assays. RESULTS: SDF-1α increased proliferation, invasion, and ERK signaling of endogenously and lentivirally CXCR4-expressing cells. Exposure to Plerixafor reduced proliferation, invasion, and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling. Combination of chemotherapy with Plerixafor showed an additive effect on chemosensitivity and apoptosis in CXCR4-overexpressing cells. An SDF-1-secreting feeder layer provideda“protective niche” for CXCR4-overexpressing cells resulting in decreased chemosensitivity. CONCLUSION: CXCR4-antagonistic therapy mobilizes and additionally sensitizes tumor cells toward cytoreductive chemotherapy.
Introduction
Cytostatic drug resistance is a major obstacle for successful treatment of metastatic colorectal cancer (CRC) and is strongly associated with a poor outcome of this disease. Once metastases occur, prognosis significantly declines [1]; due to drug resistance, monotherapies with 5-fluorouracil (5-FU), irinotecan, or oxaliplatin in the meantime have been replaced by combination regimens, e.g., FOLFOX or FOLFIRI [2,3].
The development of drug resistance is supported by the tumor microenvironment where chemokine interactions appear to play pivotal roles for tumor progression, metastasis, and tumor cell dormancy. To date, 46 different human chemokines are described as ligands for at least 18 G protein-coupled receptors [4]. High CXC chemokine receptor 4 (CXCR4) expression was observed in primary tumors of CRC patients (stage IV) and correlated with reduced overall median survival due to liver metastasis [5,6]. For hematopoietic stem cells (HSCs), the importance of CXCR4 surface expression was demonstrated for their homing and engraftment in bone marrow stroma niches through binding to the CXCR4 ligand chemokine stromal cell-derived factor-1 (SDF-1; CXCL12) [7]. However, SDF-1 is also secreted by cells of lymph nodes, liver, and lungs and disperses through the blood flow, thereby sustaining a gradient for CXCR4-expressing metastatic tumor cells that have detached from the primary tumor bulk [8–10]. After settlement, metastatic cells are embedded in the stromal microenvironment, where sustained SDF-1 expression [11,12] either provides signals promoting tumor progression [13] or induces tumor cell dormancy through CXCR4 signaling [14–16]. Quiescent tumor cells might therefore be protected in such niches from chemotherapeutic cytotoxicity [17]. There is evidence that the level of CXCR4 expression of tumor cells displays a prognostic measure for disease progression and survival of CRC patients.
Removal of quiescent tumor cells from their niches into the blood stream is a promising approach to increase the susceptibility of these cells to chemotherapeutic drugs. In a previous study, combination therapy with CXCR4 antagonists was investigated to enhance the efficacy of conventional cytoreductive treatment [18]. The synthetic compound AMD3100 (Plerixafor, Mozobil) was originally developed for the treatment of CD4/CXCR4-mediated human immunodeficiency virus 1 infection. Unexpectedly, it was also seen to trigger a dose-dependent release of HSC into the peripheral blood of patients and volunteers [19,20] due to the interruption of the SDF-1/CXCR4 axis between HSC and the bone marrow microenvironment [21]. In vitro, Plerixafor inhibited migration, invasion, adhesion, or it prolonged survival of cells of various tumor entities [22,23].
These capacities in vitro as well as the high clinical efficacy and tolerability observed in numerous clinical studies for stem cell mobilization [24] prompted Plerixafor as a promising add-on to cancer chemotherapy, since restored cell cycle activity following mobilization might enhance the effect of chemotherapy.
In this study, we present both endogenous and lentiviral CXCR4-overexpressing models in colon cancer cell lines to elucidate the activity of SDF-1α and Plerixafor on proliferation, chemotherapy-induced sensitivity, and apoptosis. We show for the first time that expression of CXCR4 enhances chemosensitivity and that treatment with Plerixafor—besides its mobilization effect—might chemosensitize tumor cells.
Materials and Methods
Cell Lines
The human colon cancer cell lines SW480 and HT-29 were cultured in RPMI 1640 medium (Invitrogen, Karlsruhe, Germany) supplemented with 10% fetal calf serum (FCS; PAA, Pasching, Austria), penicillin (100 IU/ml; Invitrogen), and streptomycin (100 µg/ml; Invitrogen) and incubated in a 37°C humidified atmosphere containing 5% CO2. The human fibrosarcoma cell line HT1080 and the human embryonic kidney cell line 293T were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FCS under the same standard conditions. All cell lines were obtained from ATCC (Manassas, VA). Mouse FBMD-1 feeder cells were obtained from Dr D.A. Breems and cultured in particular medium [25] in a 33°C standard incubator.
Lentiviral Vector Construction, Virus Production, Transduction, and Selection
Construction of lentiviral vectors pHR′SIN-CXCR4-IRES-enhanced green fluorescent protein (EGFP) [23] and pHR′SINcPPT-SEW with EGFP as a transgene [26] has already been described. Lentiviral stocks were produced and titrated as previously explained [23]. SW480 cells were transduced with lentiviral supernatant of pHR′SIN-CXCR4-IRES-EGFP or pHR′SINcPPT-SEW in the presence of polybrene (8 µg/ml; Sigma-Aldrich, Deisenhofen, Germany) at a multiplicity of infection of 3. Cells were sorted using a FACSVantage SE cell sorter (BD Biosciences, Heidelberg, Germany) on the basis of CXCR4 or EGFP expression, either to reach completely transduced SW480 cell pools or to separate populations of endogenously high and low CXCR4-expressing HT-29 cells. The presence of endogenous CXCR4 expression in HT-29 cells has been determined before [23].
Fluorescence-activated Cell Sorting Analyses
The percentage of cells expressing CXCR4 was determined using a phycoerythrin (PE)-conjugated anti-human CXCR4 antibody (clone 12G5; BD Biosciences). EGFP expression was identified by fluorescence intensity. Briefly, cells were incubated with anti-CXCR4 for 30 minutes at 4°C, washed twice, and finally resuspended in 200 µl of staining medium [phosphate-buffered saline (PBS), 4% FCS]. For CXCR4 inhibition, cells were pretreated with various concentrations (1–100 µM) of Plerixafor (Sigma-Aldrich) for 1 hour at 4°C. Acquisition was carried out on a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed with the CellQuest software (BD Biosciences).
Western Blot Analyses
SW480 cells were incubated with 20 µM mitogen-activated protein/extracellular signal-regulated kinase 1/2 (MEK1/2) inhibitor UO126 (Cell Signaling Technology, Danvers, Massachusetts) or 100 µM Plerixafor before treatment with 100 ng/ml human recombinant SDF-1α (PeproTech GmbH, Hamburg, Germany) for 5 and 15 minutes. Preparation of protein lysates, electrophoretic separation, and blot analysis were previously explained [27]. The membranes were probed with primary antibodies against extracellular signal-regulated kinase 1/2 (ERK1/2), phospho-ERK1/2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) overnight at 4°C, incubated with the appropriate HRP-conjugated secondary antibody (α-rabbit and α-mouse; Cell Signaling Technology), and visualized by chemiluminescence (ECL; Amersham Biosciences, Freiburg, Germany).
Invasion Assay
Invasion of HT-29 cells untreated or pretreated with Plerixafor (100 µM) toward medium containing 100 ng/ml SDF-1α was examined using Matrigel-coated transwell systems (BD Biosciences and Corning, Amsterdam, The Netherlands) as described before [23].
Proliferation Assay
Cells (104) were pretreated with Plerixafor (100 µM) for 1 hour at 4°C, suspended in growth medium, and supplemented with either human recombinant SDF-1α (100 ng/ml) or Plerixafor in 96-well plates. Medium was replaced on the second day. Proliferation was measured after 24, 48, and 72 hours by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method as described by Maier et al. [28].
Cytotoxicity and Caspase Assay
Cells (104) were pretreated with Plerixafor (100 µM) for 1 hour at 4°C and suspended in growth medium in 96-well plates. On the next day, medium was replaced by medium containing the chemotherapeutic drug in increasing concentrations (50–1600 µg/ml 5-FU, 3.2–102.4 µg/ml oxaliplatin, 25–400 µg/ml irinotecan; Sigma-Aldrich) ± 1, 10, or 100 µM Plerixafor. Following 48-hour incubation at 37°C, cytotoxicity was analyzed by MTT assay (see above). Half-maximal inhibitory concentration (IC50) values were determined by the CalcuSyn Software (Biosoft, Cambridge, United Kingdom) as described by Chou and Talalay [29]. Apoptosis of all SW480- and HT-29-derived cell lines treated with IC50 of 5-FU obtained in the cytotoxicity assay was studied using Caspase-Glo 3/7 assay (Promega, Mannheim, Germany) following the manufacturer's instructions.
Stroma-dependent Cytotoxicity and Apoptosis Assay
A 96-well plate was coated with PBS/0.1% gelatin solution and a feeder layer of FBMD-1 cells was established, as described in previous work [30]. SW480 and HT-29 cells were incubated for 1 hour at 4°C in medium supplemented with Plerixafor (1, 10, or 100 µM). Subsequently, cell suspensions were added to the preestablished feeder layer and allowed to migrate for 24 hours at 37°C. Thereafter, medium supplemented with 1, 10, and 100 µM Plerixafor ± 5-FU in increasing concentrations (4–16 mg/ml) was added to the respective wells with PBS as negative control. After further 48 hours of incubation, all cells were harvested and analyzed by fluorescence-activated cell sorting (FACS). To differentiate between human colon cancer cells and murine FBMD-1 cells, the former cells were stained with anti-human MHC class I-related chain (MIC) A/B (1:10; eBioscience, Frankfurt, Germany) for 30 minutes at 4°C. In parallel, induction of apoptosis was analyzed by staining the cells with Annexin V (1:25 diluted in Binding Buffer; BD Pharmingen) for 15 minutes at 4°C. Dead cells were excluded using propidium iodide (PI, 1 mg/ml; Sigma-Aldrich).
Statistical Analysis
As indicated in the figure legends, the results are presented as means ± SD and usually represent three independent experiments. P values (calculated by the two-sided, paired Welch's t test) ≤ .05 were defined as statistically significant.
Results
Establishment of Endogenously and Lentivirally High CXCR4-Expressing Colon Cancer Cell Lines
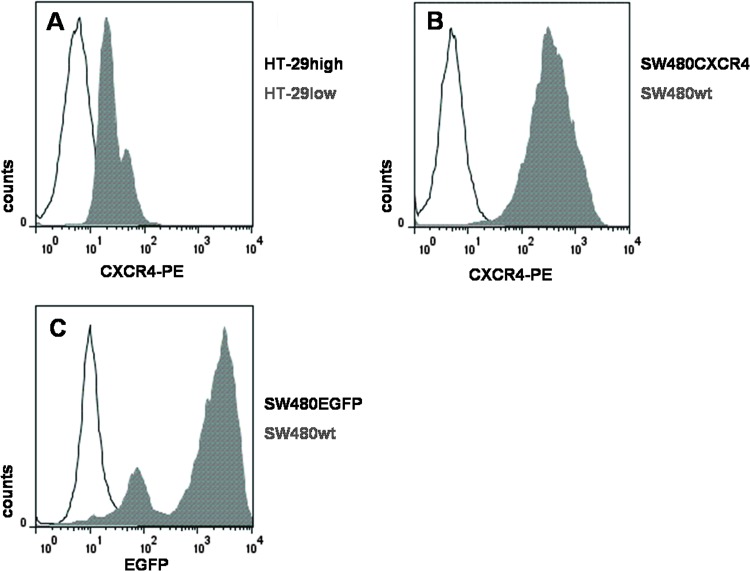
Endogenously CXCR4-expressing colon cancer cell lines. HT-29 cells (a human colon adenocarcinoma cell line) are endogenously expressing CXCR4. To separate the CXCR4 high- (HT-29high) from the low-expressing (HT-29low) population, we used flow cytometric sorting (Figure 1A) to analyze proliferation and chemosensitivity of different CXCR4-expressing cells.
Figure 1.
CXCR4 expression profiles of different colon cancer cell lines. (A) Endogenous expression of CXCR4 in HT-29 cells, sorted by FACS in two distinct populations HT-29high (displayed in gray) and HT-29low. SW480 cells transduced with (B) HR′SIN-CXCR4-IRES-EGFP (CXCR4; displayed in gray) and (C) HR′SINcPPT-SEW (EGFP; displayed in gray), both compared to wild-type SW480 cells. Amounts of CXCR4- and/or EGFP-expressing cells were determined by FACS analyses measuring direct staining with the PE-conjugated anti-human CXCR4 antibody and/or EGFP autofluorescence. Threshold lines were defined by signals of isotype control (IgG2-PE; BD Pharmingen).
Lentivirally high CXCR4-expressing colon cancer cell lines. In SW480 cells, overexpression of CXCR4 was achieved by lentiviral transduction with HR′SIN-CXCR4-IRES-EGFP [23], followed by a CXCR4-selective FACS analysis sorting for CXCR4-positive cells (SW480CXCR4; Figure 1B). As a control, SW480 cells were transduced with the vector HR′SINcPPT-SEW (SW480EGFP) [26] (Figure 1C).
Intracellular Signaling and Cell Invasion Regulated by SDF-1α and Plerixafor
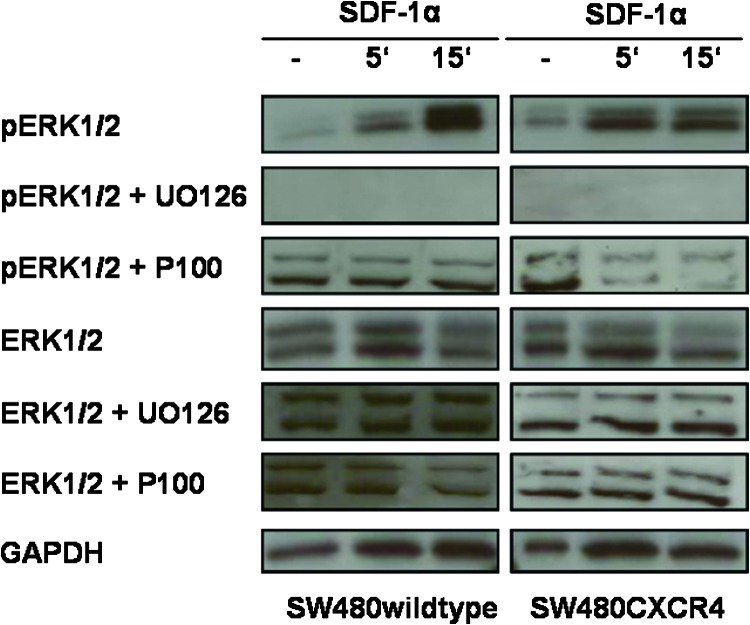
Activation of ERK1/2 through SDF-1α/CXCR4 mediates cell proliferation, migration, and invasion [31,32]. Thus, we assayed the functionality of lentivirally overexpressed CXCR4 by analyzing the phosphorylation status of ERK1/2 after exposure to SDF-1α or Plerixafor, its synthetic “competitor.” First, SDF-1α induced phosphorylation of ERK1/2 in CXCR4-expressing SW480 cells within minutes, while the amount of total ERK1/2 was constant in the cytoplasm. UO126, an inhibitor of MEK1/2 (the kinase directly upstream of ERK1/2), completely inhibited phosphorylation of ERK1/2 after exposure to SDF-1α. Plerixafor significantly reduced signaling through SDF-1α/CXCR4 in SW480CXCR4 cells only but not in SW480 wild-type cells (Figure 2).
Figure 2.
Activation of ERK1/2 signaling downstream of CXCR4 after exposure to Plerixafor (1 hour, 100 µM, P100) or UO126 (2 hours, 20 µM) and subsequent SDF-1α (5 or 15 minutes, 100 ng/ml) in SW480 cells. Western blots with lysates from different preparations gave similar results. Expression of GAPDH was used to demonstrate that equal amounts of protein extracts were loaded.
According to previous migration and SDF-1-binding experiments [30,33], we used a concentration of 100 µM Plerixafor that was shown to be sufficient for significant inhibition of binding of the specific antibody to endogenously expressed CXCR4 and to block the recognition of lentivirally expressed CXCR4 almost completely [23].
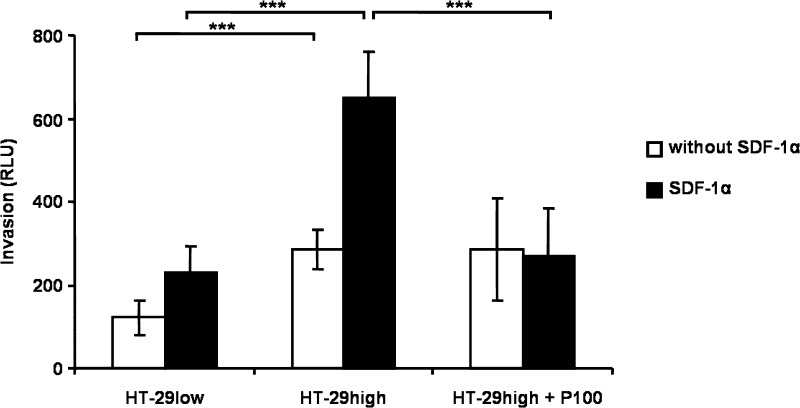
Previously, we showed that SDF-1α escalated invasion of CXCR4-expressing SW480 cells [23]. Treatment of HT-29high cells with SDF-1α resulted in 2.8-fold increase in invasive capacity compared to HT-29low cells (P < .001), while Plerixafor blocked SDF-1α-induced invasion (P < .001; Figure 3).
Figure 3.
Cell invasion of HT-29 cells. HT-29high and HT-29low cells were placed in the upper chamber of a transwell migration flask. During 24 hours, cells were allowed to invade through a membrane, previously coated with Matrigel, toward growth medium with or without chemoattracting SDF-1α (100 ng/ml) in the lower chamber. Pretreatment with 100 µM Plerixafor (P100) inhibited invasion of CXCR4-expressing cells significantly. Results denote mean ± SD of luminescence signal-derived relative light units of three independent measurements. See text for exact P values.
These results highlight that SDF-1α activated intracellular signaling and cell invasion in endogenously CXCR4-expressing HT-29 cells and in lentivirally CXCR4-expressing SW480 cells.
Cell Proliferation under SDF-1α or Plerixafor Exposure
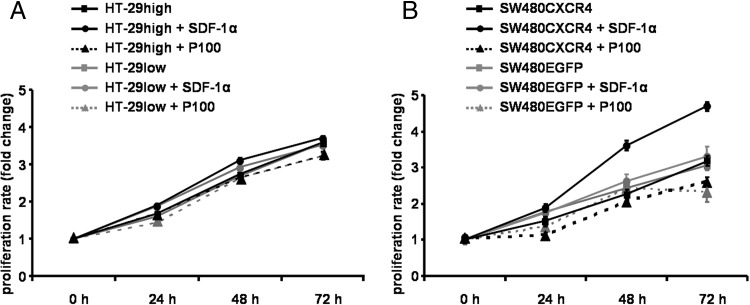
To investigate the effect of the CXCR4 ligand SDF-1α and Plerixafor on proliferation of the four established colon cancer cell lines (HT-29 high, HT-29low, SW480CXCR4, and SW480EGFP), growth curves were assessed under SDF-1α or Plerixafor exposure (Figure 4). In all cell lines, no correlation between CXCR4 expression and proliferation was discernible.
Figure 4.
Impact of SDF-1α or Plerixafor (P100) on proliferation of HT-29high and HT-29low (A) and SW480CXCR4 and SW480EGFP (B) cell lines. MTT measurements were performed twice in quadruplicate (mean ± SE).
In HT-29high cells, medium supplemented with SDF-1α slightly increased proliferation (compared to untreated HT-29high cells). In SW480CXCR4 cells, proliferation was distinctly increased by SDF-1α compared to untreated SW480CXCR4 cells (P = .021). In HT-29low and SW480EGFP cells, the proliferative effect of SDF-1α was marginal and not significant.
Thus, SDF-1α stimulated the proliferation of HT-29high and SW480CXCR4 cells compared to HT-29low and SW480EGFP cells (P < .026).
While Plerixafor did not affect proliferation of HT-29high and HT-29low cells (Figure 4A), it decreased significantly the proliferation rate of SW480CXCR4 cells (Figure 4B; P = .016). A comparable antiproliferative effect of Plerixafor was observed in SW480EGFP cells (Figure 4B).
CXCR4 Expression and Chemosensitivity: Combination Treatment with Cytostatic Drugs ± SDF-1α or Plerixafor
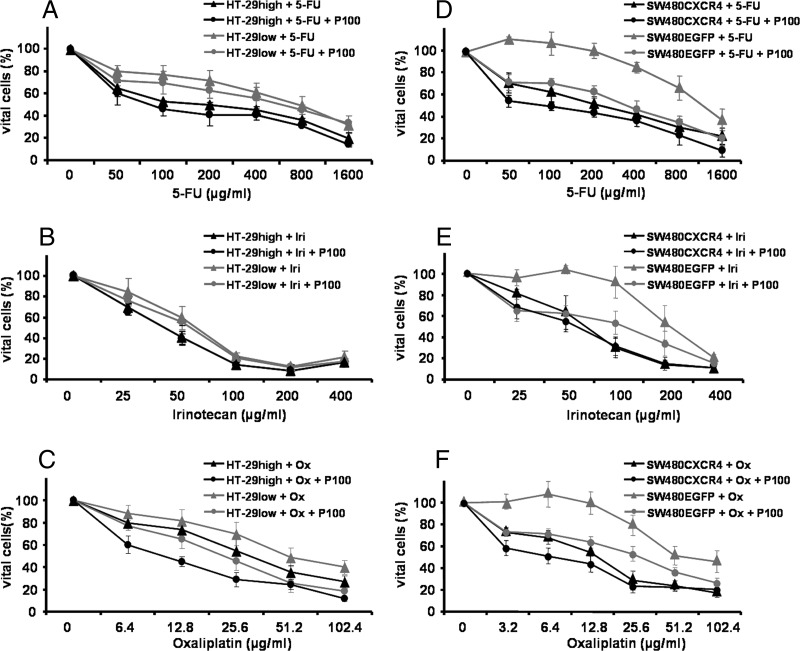
We investigated the ability of CXCR4 expression to modulate chemosensitivity to cytostatic drugs in combination with SDF-1α or Plerixafor in the MTT assay. In HT-29 (Figure 5, A–C) and SW480 cells (Figure 5, D–F), the cytotoxicity of increasing concentrations of 5-FU (Figure 5, A and D), irinotecan (Figure 5, B and E), and oxaliplatin (Figure 5, C and F) was analyzed, and IC50 values were calculated (Table 1).
Figure 5.
Combination treatment of HT-29high and HT-29low as well as SW480CXCR4 and SW480EGFP cells with cytostatic drugs and Plerixafor. Tumor cells were treated with increasing concentrations of 5-FU, irinotecan, or oxaliplatin ± 100 µM Plerixafor (P100; HT-29: A–C; SW480: D–F). Results are presented as mean ± SD of not less than three independent measurements. See text for exact P values.
Table 1.
IC50 Values (µg/ml) ± SD of Chemotherapy ± SDF-1α or ± Plerixafor in HT-29 and SW480 Cells.
| Chemotherapeutics | HT-29low | HT-29high | SW480EGFP | SW480CXCR4 | SW480CXCR4sh |
| 5-FU | 698 ± 195 | 201 ± 37 | 1304 ± 285 | 266 ± 47 | 842 ± 81 |
| 5-FU + SDF-1α | 608 ± 233 | 149 ± 2 | 607 ± 95 | 101 ± 43 | 468 ± 88 |
| 5-FU + Plerixafor (100 µM) | 516 ± 56 | 102 ± 9 | 398 ± 93 | 120 ± 37 | 301 ± 73 |
| 5-FU + Plerixafor (10 µM) | 474 ± 35 | 89 ± 12 | 365 ± 56 | 155 ± 34 | |
| 5-FU + Plerixafor (1 µM) | 462 ± 45 | 90 ± 12 | 305 ± 26 | 166 ± 18 | |
| Irinotecan | 60 ± 12 | 39 ± 5 | 206 ± 52 | 74 ± 16 | |
| Irinotecan + SDF-1α | 45 ± 6 | 39 ± 6 | 199 ± 25 | 102 ± 21 | |
| Irinotecan + Plerixafor (100 µM) | 52 ± 3 | 35 ± 3 | 103 ± 24 | 53 ± 15 | |
| Irinotecan + Plerixafor (10 µM) | 41 ± 12 | 30 ± 7 | 62 ± 7 | 33 ± 7 | |
| Irinotecan + Plerixafor (1 µM) | 38 ± 10 | 29 ± 8 | 70 ± 9 | 37 ± 3 | |
| Oxaliplatin | 53 ± 5 | 32 ± 9 | 80 ± 13 | 16 ± 5 | |
| Oxaliplatin + SDF-1α | 32 ± 7 | 16 ± 6 | 92 ± 1 | 12 ± 2 | |
| Oxaliplatin + Plerixafor (100 µM) | 21 ± 6 | 10 ± 3 | 37 ± 12 | 6 ± 3 | |
| Oxaliplatin + Plerixafor (10 µM) | 16 ± 6 | 6 ± 1 | 35 ± 3 | 7 ± 2 | |
| Oxaliplatin + Plerixafor (1 µM) | 24 ± 4 | 9 ± 3 | 33 ± 8 | 7 ± 3 |
Results have been determined by the medium dose-effect relationship [29] of at least three independent experiments.
Expression of CXCR4 was associated with increased chemosensitivity in all cell lines, independent of the cytostatic drug used (P < .043; Figure 5 and Table 1). Exposure of CXCR4-expressing cells to 5-FU plus SDF-1α resulted in a significant decrease of IC50 values compared to 5-FU alone (P = .049 for HT-29high cells and P = .001 for SW480CXCR4 cells, respectively; Table 1). This effect of SDF-1α was also observed in HT-29high cells exposed to oxaliplatin compared to oxaliplatin alone (P = .038) but not in SW480CXCR4 cells. A similar effect of SDF-1α was observed in oxaliplatin-treated HT-29low cells (P = .017) and in 5-FU-treated SW480EGFP cells (P = .045). SDF-1α showed no effect on irinotecan-treated cell lines.
Plerixafor (1, 10, and 100 µM) reduced the survival rate of CXCR4-overexpressing cells in combination with 5-FU and oxaliplatin (P < .011 for HT-29high cells and P < .001 for SW480CXCR4 cells; Figure 5 and Table 1). This Plerixafor effect was also seen in HT-29low cells in combination with oxaliplatin and in SW480EGFP cells in combination with all three drugs (Figure 5 and Table 1). The pronounced sensitization of SW480EGFP cells to all three cytostatic drugs by addition of Plerixafor was emphasized by the dose-response curves given in Figure 5, D to F.
Lentiviral knockdown of CXCR4 in SW480CXCR4 cells [23] decreased their sensitivity to 5-FU significantly (P = .003). In these SW480CXCR4sh cells, SDF-1α and Plerixafor had also a sensitizing effect toward 5-FU (Table 1).
Altogether, CXCR4 expression increased chemosensitivity of colon cancer cell lines. In no case, the combination of cytostatic drugs with SDF-1α or Plerixafor hampered the cytostatic effect but rather increased chemosensitivity.
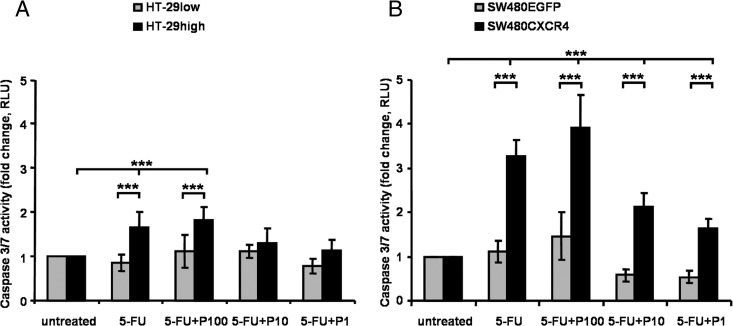
CXCR4 Expression and 5-FU Induced Apoptosis
To elucidate an impact of CXCR4 expression on apoptosis after chemotherapy, caspase 3/7 activity was measured following chemotherapy with 5-FU ± Plerixafor, using the respective IC50 values of 5-FU. Apoptosis was significantly induced by 5-FU exposure 1.8-fold (P < .001) in HT-29high cells (Figure 6A) and 3.3-fold (P < .001) in SW480CXCR4 cells (Figure 6B) compared to untreated CXCR4-expressing cells, whereas no effect was seen in HT-29low and SW480EGFP cells. Combination of 5-FU with Plerixafor resulted in a stronger induction of apoptosis than monotherapy of CXCR4-expressing cells (HT-29high only for 100 µM Plerixafor: 1.1-fold increase vs 5-FU alone; SW480CXCR4 for all Plerixafor concentrations used: 1.2-fold increase vs 5-FU alone). An increased rate of apoptosis following combination treatment with 5-FU plus Plerixafor versus 5-FU alone was also observed in HT-29low and SW480EGFP cells (Figure 6).
Figure 6.
Apoptosis of CXCR4-expressing HT-29 (A) and SW480 (B) cells. Cells were treated with the IC50 dose of 5-FU in the respective cell line [calculated according the results of the MTT assays; Table 1 and Figure 3, A (201 µg/ml) and D (266 µg/ml), respectively] ± 1, 10, and 100 µM Plerixafor. Results are presented as the mean ± SD of luminescence signal-derived relative light units of four independent measurements. See text for exact P values.
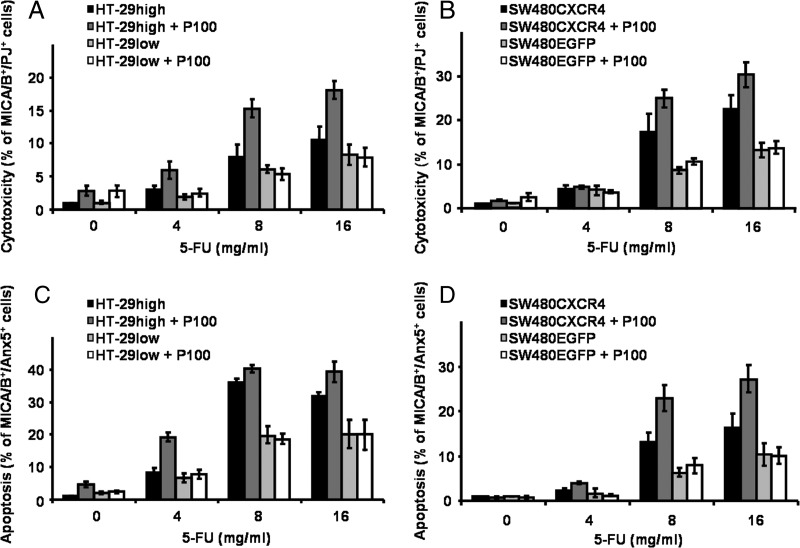
CXCR4 Expression and Stroma-Dependent Cytotoxicity/Apoptosis
To mimic drug-induced cytotoxicity and apoptosis in a stromal environment, murine SDF-1α-producing stromal FBMD-1 cells were cocultured with CXCR4-expressing colon cancer cells. Chemosensitivity (cytotoxicity) of colon cancer cells and apoptosis (expression of Annexin V) were determined after treatment with 5-FU (4, 8, and 16 mg/ml) and Plerixafor (1, 10, and 100 µM). Necrotic human colon cancer cells were determined by FACS analyses (MIC A/B+/PI+ staining; Figure 7, A and B).
Figure 7.
Stroma-dependent cytotoxicity (A, B) and apoptosis (C, D) of HT-29 (A, C) and SW480 (B, D) cells. Colon cancer cells were seeded on an FBMD-1 feeder layer and treated with 5-FU in increasing concentrations (4–16 mg/ml) ± 100 µM Plerixafor. After 48 hours of incubation, cells were analyzed by FACS. Chemotherapeutic cytotoxicity and apoptosis were determined by PI and Annexin V staining, respectively. Human colon cancer cells were characterized with anti-human MIC A/B. Results are presented as the mean ± SD of three independent measurements. See text for exact P values.
Dose escalation of 5-FU increased cytotoxicity in all cell lines. Treatment with 4, 8, and 16 mg/ml 5-FU significantly increased cytotoxicity in CXCR4-expressing HT-29high and SW480CXCR4 cells compared to the respective untreated controls (P < .043). Cytotoxicity of 5-FU—albeit less pronounced—was also observed in HT-29low and SW480EGFP cells.
Addition of Plerixafor to 5-FU increased chemosensitivity of CXCR4-expressing HT-29high cells significantly (P = .012; Figure 7A). In SW480CXCR4 cells, the combination was marginally superior to 5-FU alone (Figure 7B). Plerixafor did not sensitize HT-29low and SW480EGFP cells to 5-FU (Figure 7, A and B). Noteworthy, stroma-dependent growth necessitated about 10-fold concentrations of 5-FU compared to the MTT assay. Combination regimens of 5-FU plus 1 or 10 µM Plerixafor gave comparable results (data not shown).
Apoptosis, quantified by the percentages of MIC A/B+/Annexin V+ colon cancer cells, also showed a dose-response relationship to 5-FU (Figure 7, C and D) and was more pronounced in CXCR4-expressing cells compared to the respective CXCR4 low-expressing counterparts (HT-29low and SW480EGFP). Plerixafor (100 µM) significantly increased apoptosis in 5-FU-treated cells compared to 5-FU alone (P < .025 for HT-29high and SW480CXCR4 cells). This increase was not observed in HT-29low or SW480EGFP cells. Lower Plerixafor doses (1 and 10 µM) gave comparable results (data not shown).
Thus, CXCR4 expression increased sensitivity to chemotherapy as assessed by the induction of necrosis and apoptosis in colon cancer cells in a stromal environment. Coculture with SDF-1-expressing feeder cells required higher drug concentrations compared to MTT measurements (without SDF-1 supplement). Plerixafor increased 5-FU-induced cytotoxicity and apoptosis.
Discussion
This is the first study to show that expression of CXCR4 enhances chemosensitivity and that Plerixafor—besides its mobilization effect—might chemosensitize tumor cells as well. Tumor cells are embedded into a stromal microenvironment, including fibroblasts, inflammatory cells, and vascular cells [34], which provide signals either arresting or promoting tumor progression. Adhesion to stromal cells was shown to confer resistance by inhibiting drug-induced cell death of tumor cells and, therefore, was termed cell adhesion-mediated drug resistance [35,36]. Tumor cells that adhere to stromal cells through CXCR4 are therefore, at least partially, protected from the effects of cytotoxic chemotherapy. Stromal fibroblasts constitutively secrete SDF-1 into the tumor microenvironment [11,12], establishing a chemotactic gradient for cells evading the primary tumor. Beyond that, microenvironmental factors might protect metastatic tumor cells against drug cytotoxicity by maintaining dormancy [14–17] or quiescence of tumor stem cells [37].
In this study, we established endogenously and lentivirally CXCR4-expressing colon cancer cell lines and investigated intracellular signaling, cell proliferation, invasion, and chemosensitivity to different cytostatic drugs with, and without, addition of SDF-1α or Plerixafor.
We showed that treatment of lentivirally CXCR4-overexpressing SW480 cells with SDF-1α activated ERK1/2 signaling, while pretreatment with Plerixafor impaired ERK1/2 activation and, thus, gave evidence for the functionality of the lentivirally expressed CXCR4. CXCR4 signaling stimulates the MAPK/MEK/ERK cascade [31]. This signaling pathway is also known to affect proliferation and invasion of CRC cells [32]. We observed that colon cancer cell proliferation was stimulated by SDF-1α. This mitogenic effect was most pronounced in CXCR4-overexpressing cells and might be a prognosticmeasure since CXCR4 is a commonly found chemokine receptor in human cancers [38]. This observation underlines previous results [39,40] on the impact of the SDF-1/CXCR4 axis for proliferation of colon cancer tumor cells.
On the basis of a chemotactic principle [41,42], the secretion of SDF-1 by various organs [8,43] could be associated with CRC tumor growth [40] and metastasis. In our previous work [23], we demonstrated that masking of the SDF-1α binding site at the CXCR4 receptor by Plerixafor blocked chemotaxis and invasion of several CRC cell lines [23]. In the present investigation, CXCR4 antagonism by Plerixafor reduced tumor cell proliferation and invasion (Figures 3 and 4).
CXCR4 has been identified as an HSC marker [44], and its expression has been linked to chemoresistant phenotypes [45]. In the present experiments, however, CXCR4 overexpression marked chemosensitivity, while lower chemosensitivity was found in low or marginally CXCR4-expressing cells.
The stroma-dependent cytotoxicity assay showed that embedding of colon cancer cells in a stromal feeder layer protected them against drug cytotoxicity; an approximately 10-fold dose of anticancer drugs—compared to unembedded colon cancer cells—resulted in similar reduction of the tumor cell number.
Mobilization of dormant tumor cells from the protective microenvironment might be a quantum leap in overcoming minimal residual disease and improve the patients' prognosis significantly. In a recent approach in multiple myeloma, it was demonstrated that mobilization by Plerixafor resulted in an increased sensitivity of multiple myeloma cells to chemotherapy [46], and thus, an interruption of the CXCR4/SDF-1α axis and detachment from their niches by Plerixafor sensitized tumor cells to chemotherapy. In an in vitro approach, direct contact with stromal cells protected chronic lymphocytic leukemia B cells from chemotherapy-induced apoptosis. Blockade of CXCR4 signaling antagonized stroma-mediated interactions and restored chronic lymphocytic leukemia chemosensitivity [45]. In accordance with these results, we showed that Plerixafor mobilized CXCR4-expressing colon cancer cells from the feeder layer and increased their chemosensitivity compared to cells without Plerixafor treatment.
Increased chemosensitivity after CXCR4 signaling might be a result of the down-regulation of genes or proteins involved in DNA damage repair mechanisms or drug efflux pumps. SDF-1/CXCR4 signaling has been described to induce the transactivation of epidermal growth factor receptor (EGFR) whereas treatment with Plerixafor has hampered mitogenic signaling [47]. Concordant with these findings, we observed a distinct EGFR up-regulation in the CXCR4-overexpressing cells presented here (Heckmann et al., unpublished data) that might be a marker for chemosensitivity as shown for monoclonal antibody-induced sensitivity [48].
In summary, we showed that both high endogenous and lentiviral expression of CXCR4 in colon cancer cells increased SDF-1α-dependent proliferation and chemosensitivity to anticancer drugs. Plerixafor increased the sensitivity of different colon cancer cell lines to cytostatic drugs significantly; it mobilized colon cancer cells from stroma embedment, paving the way for increased chemotherapeutic sensitivity. Altogether, these results support the view that—in an adjuvant setting—the use of Plerixafor to interrupt the interaction of CXCR4 with SDF-1α has the potential to overcome tumor cell dormancy and drug resistance. Our data suggest that, in addition to chemosensitization of tumor cells by mobilization from stromal niches (indirect effect), a combination of chemotherapy plus Plerixafor might further directly improve the therapeutic effect of anticancer drugs on the mobilized tumor cells.
Acknowledgments
The technical assistance of Hans-Jürgen Engel and Philipp Münzer is gratefully acknowledged.
Footnotes
This work was supported by grant M39.1 of the H.W.& J. Hector Foundation, grant 0315-452-C of the Federal Ministry of Education and Research (BMBF; H.A., S.L., and S.F.), and partly by grant 10-2089-FI 1 of Deutsche Krebshilfe/Dr Mildred-Scheel-Stiftung. H.A. was supported by the Alfried Krupp von Bohlen und Halbach Foundation (Award for Young Full Professors, Essen, Germany), Hella-Buhler-Foundation (Heidelberg, Germany), Dr Ingrid zu Solms Foundation (Frankfurt/Main, Germany), the Walter Schulz Foundation (Munich, Germany), Deutsche Krebshilfe (Bonn, Germany), the German-Israeli Cooperation DKFZ-MOST and the Wilhelm Sander Foundation (Munich, Germany). The authors declare no competing financial interests.
References
- 1.Compton CC. Colorectal carcinoma: diagnostic prognostic and molecular features. Mod Pathol. 2003;16:376–388. doi: 10.1097/01.MP.0000062859.46942.93. [DOI] [PubMed] [Google Scholar]
- 2.Becouarn Y, Senesse P, Thezenas S, Boucher E, Adenis A, Cany L, Jacob JH, Cvitkovic F, Montoto-Grillot C, Ychou M. A randomized phase II trial evaluating safety and efficacy of an experimental chemotherapy regimen (irinotecan + oxaliplatin, IRINOX) and two standard arms (LV5 FU2 + irinotecan or LV5 FU2 + oxaliplatin) in first-line metastatic colorectal cancer: a study of the Digestive Group of the Fédération Nationale des Centres de Lutte Contre le Cancer. Ann Oncol. 2007;18:2000–2005. doi: 10.1093/annonc/mdm379. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, Schulz J, Richards D, Soufi-Mahjoubi R, Wang B, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol. 2007;25:4779–4786. doi: 10.1200/JCO.2007.11.3357. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–213. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 6.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gonner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi: 10.1158/1078-0432.CCR-04-1195. [DOI] [PubMed] [Google Scholar]
- 7.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 10.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 11.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 13.Pinto MP, Badtke MM, Dudevoir ML, Harrell JC, Jacobsen BM, Horwitz KB. Vascular endothelial growth factor secreted by activated stroma enhances angiogenesis and hormone-independent growth of estrogen receptor-positive breast cancer. Cancer Res. 2010;70:2655–2664. doi: 10.1158/0008-5472.CAN-09-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SA, Dave MA, Murthy RG, Helmy KY, Rameshwar P. Metastatic breast cancer cells in the bone marrow microenvironment: novel insights into oncoprotection. Oncol Rev. 2011;5:93–102. doi: 10.1007/s12156-010-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 16.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 17.Riethmuller G, Klein CA. Early cancer cell dissemination and late metastatic relapse: clinical reflections and biological approaches to the dormancy problem in patients. Semin Cancer Biol. 2001;11:307–311. doi: 10.1006/scbi.2001.0386. [DOI] [PubMed] [Google Scholar]
- 18.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix CW, Collier AC, Lederman MM, Schols D, Pollard RB, Brown S, Jackson JB, Coombs RW, Glesby MJ, Flexner CW, et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37:1253–1262. doi: 10.1097/01.qai.0000137371.80695.ef. [DOI] [PubMed] [Google Scholar]
- 20.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44:1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 22.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia. 2009;23:43–52. doi: 10.1038/leu.2008.299. [DOI] [PubMed] [Google Scholar]
- 23.Heckmann D, Laufs S, Maier P, Zucknick M, Giordano FA, Veldwijk MR, Eckstein V, Wenz F, Zeller WJ, Fruehauf S, et al. A lentiviral CXCR4 overexpression and knockdown model in colorectal cancer cell lines reveals Plerixafor-dependent suppression of SDF-1α-induced migration and invasion. Onkologie. 2011;34:502–508. doi: 10.1159/000332390. [DOI] [PubMed] [Google Scholar]
- 24.De Clercq E. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem Pharmacol. 2009;77:1655–1664. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Breems DA, Blokland EA, Ploemacher RE. Stroma-conditioned media improve expansion of human primitive hematopoietic stem cells and progenitor cells. Leukemia. 1997;11:142–150. doi: 10.1038/sj.leu.2400530. [DOI] [PubMed] [Google Scholar]
- 26.Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, Grez M, Thrasher AJ. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 27.Mudduluru G, Vajkoczy P, Allgayer H. Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol Cancer Res. 2010;8:159–169. doi: 10.1158/1541-7786.MCR-09-0326. [DOI] [PubMed] [Google Scholar]
- 28.Maier P, Spier I, Laufs S, Veldwijk MR, Fruehauf S, Wenz F, Zeller WJ. Chemoprotection of human hematopoietic stem cells by simultaneous lentiviral overexpression of multidrug resistance 1 and O6-methylguanine-DNA methyltransferaseP140K. Gene Ther. 2010;17:389–399. doi: 10.1038/gt.2009.133. [DOI] [PubMed] [Google Scholar]
- 29.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Dillmann F, Veldwijk MR, Laufs S, Sperandio M, Calandra G, Wenz F, Zeller WJ, Fruehauf S. Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib. Leuk Lymphoma. 2009;50:1676–1686. doi: 10.1080/10428190903150847. [DOI] [PubMed] [Google Scholar]
- 31.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang YM, Qin SX, Newman W, Groopman JE. The α-chemokine, stromal cell-derived factor-1α, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–23175. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 32.Brand S, Dambacher J, Beigel F, Olszak T, Diebold J, Otte JM, Goke B, Eichhorst ST. CXCR4 and CXCL12 are inversely expressed in colorectal cancer cells and modulate cancer cell migration, invasion and MMP-9 activation. Exp Cell Res. 2005;310:117–130. doi: 10.1016/j.yexcr.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75:1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 34.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 36.Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev. 2006;20:333–342. doi: 10.1016/j.blre.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Essers MA, Trumpp A. Targeting leukemic stem cells by breaking their dormancy. Mol Oncol. 2010;4:443–450. doi: 10.1016/j.molonc.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Li CL, Yu WB, Yin HP, Zhang GY, Zhang LF, Li S, Hu SY. Influence of CXCR4/SDF-1 axis on E-cadherin/β-catenin complex expression in HT29 colon cancer cells. World J Gastroenterol. 2011;17:625–632. doi: 10.3748/wjg.v17.i5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeelenberg IS, Ruuls-Van SL, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839. [PubMed] [Google Scholar]
- 41.Kollmar O, Rupertus K, Scheuer C, Junker B, Tilton B, Schilling MK, Menger MD. Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis. Neoplasia. 2007;9:862–870. doi: 10.1593/neo.07559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gassmann P, Haier J, Schluter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, et al. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia. 2009;11:651–661. doi: 10.1593/neo.09272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 44.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2mnull mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 45.Buchner M, Brantner P, Stickel N, Prinz G, Burger M, Bar C, Dierks C, Pfeifer D, Ott A, Mertelsmann R, et al. The microenvironment differentially impairs passive and active immunotherapy in chronic lymphocytic leukaemia—CXCR4 antagonists as potential adjuvants for monoclonal antibodies. Br J Haematol. 2010;151:167–178. doi: 10.1111/j.1365-2141.2010.08316.x. [DOI] [PubMed] [Google Scholar]
- 46.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pattarozzi A, Gatti M, Barbieri F, Wurth R, Porcile C, Lunardi G, Ratto A, Favoni R, Bajetto A, Ferrari A, et al. 17β-Estradiol promotes breast cancer cell proliferation-inducing stromal cell-derived factor-1-mediated epidermal growth factor receptor transactivation: reversal by gefitinib pretreatment. Mol Pharmacol. 2008;73:191–202. doi: 10.1124/mol.107.039974. [DOI] [PubMed] [Google Scholar]
- 48.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, Gambacorta M, Siena S, Bardelli A. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to anti-EGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]