Abstract
Treatment for glioblastoma consists of radiotherapy and temozolomide-based chemotherapy. However, virtually all patients recur, leading to a fatal outcome. Receptor tyrosine kinase (RTK)-targeted therapy has been the focus of attention in novel treatment options for these patients. Here, we compared the efficacy of imatinib, sunitinib, and cediranib in glioblastoma models. In the present work, the biologic effect of the drugs was screened by viability, cell cycle, apoptosis, migration, and invasion in vitro assays or in vivo by chick chorioallantoic membrane assay. Intracellular signaling was assessed by Western blot and the RTK targets were identified using phospho-RTK arrays. The amplified status of KIT, PDGFRA, and VEGFR2 genes was assessed by quantitative polymerase chain reaction. In a panel of 10 glioblastoma cell lines, we showed that cediranib was the most potent. In addition, cediranib and sunitinib synergistically sensitize the cells to temozolomide. Cediranib efficacy was shown to associate with higher cytostatic and unique cytotoxic effects in vitro and both antitumoral and antiangiogenic activity in vivo, which could associate with its great capacity to inhibit mitogen-activated protein kinase (MAPK) and AKT pathways. The molecular status of KIT, PDGFRA, and VEGFR2 did not predict glioblastoma cell responsiveness to any of the RTK inhibitors. Importantly, phospho-RTK arrays revealed novel targets for cediranib and sunitinib therapy. In conclusion, the novel targets found may be of value as future biomarkers for therapy response in glioblastoma and lead to the rational selection of patients for effective molecular targeted treatment.
Introduction
Brain tumors represent the leading cause of cancer-related death in children and the fourth in middle-aged men [1]. Gliomas are the most frequent, accounting for approximately 70% of all primary brain tumors. According to the World Health Organization, gliomas can be divided in four grades of malignancy and three histologic subtypes, being astrocytoma grade IV (glioblastoma) the most common subtype [2,3]. Glioblastomas are among the most lethal tumors, with median survival of approximately 16 months, despite aggressive surgery, radiotherapy, and chemotherapy [4]. So far, temozolomide, a cytotoxic drug, remains the only anticancer agent that has improved outcome, but almost all glioblastoma patients eventually develop tumor recurrence, resulting in death [5]. Therefore, it is imperative to improve the treatment options for glioblastoma.
Currently, there are great expectations on the translation of glioma biology [2,3] to the development of agents that target key glioma players, such as receptor tyrosine kinases (RTKs) [6]. As previously demonstrated by us and others, epidermal growth factor receptor (EGFR) alterations are the most common genetic abnormalities in malignant gliomas, alterations of 4q12 region, namely the PDGFRA, KIT, and vascular endothelial growth factor receptor 2 (VEGFR2) (KDR) amplicon, which are also frequently upregulated [7–9]. Interestingly, several recent studies showed that gliomas, and particularly glioblastomas, exhibit intratumoral heterogeneity of RTK (EGFR, PDGFRA, and MET) alterations, with individual tumors displaying co-amplification of RTKs, yet, different cells showing amplification of a different RTK [10–12]. Thus, inhibition of multiple RTKs constitutes a rational approach for treatment of these cancers. Additionally, glioblastomas are among the most human vascularized tumors, and endothelial proliferation is pathognomonic of these tumors [13]. Hence, combination of antineoplastic with antiangiogenic therapies constitutes an attractive approach [14].
Small-molecule compounds, such as imatinib, that inhibit the kinase domain of specific kinase targets have recently changed clinical practice for several advanced cancers. It has been used in the context of chronic myeloid leukemia by inhibiting the BCR-ABL fusion protein [15] and for gastrointestinal stromal tumors by inhibition of the activating KIT mutations [16]. Other drugs have been also successfully used, such as sunitinib, which inhibits VEGFR and PDGFR in metastatic renal cell carcinoma [17], and sorafenib, which targets Raf, PDGFR, VEGFR, and KIT in advanced hepatocellular carcinoma [18].
The ability of imatinib to inhibit PDGFR signaling has suggested a potential therapeutic benefit in glioblastoma. However, imatinib clinical trials showed absence or minimal therapeutic activity [19,20]. Recently, single-target bevacizumab, a humanized monoclonal antibody against VEGF, was approved by the Food and Drug Administration (FDA) for treatment of recurrent glioblastoma [21]. Although bevacizumab prolonged progression-free survival, resistance to antiangiogenic (or anti-VEGF) therapy is expected [22]. Furthermore, pre-clinical models suggest that anti-VEGF therapy can induce a previously noninvasive glioma tumor to invade normal brain [23]. Therefore, single-agent activity is questionable in the treatment of glioblastomas, and it is currently believed that multi-kinase inhibitors, which target several RTKs simultaneously—pan-RTK inhibitors (RTKis)—may yield greater clinical efficacy [12,24].
Sunitinib malate (SU11248, Sutent by Pfizer, New York, NY) is a small orally bioavailable molecule that has been identified as an inhibitor of the PDGFR-α/β, VEGFR1-3, KIT, RET, FLT3, and CSF1R RTKs [25]. Sunitinib has demonstrated activity in glioma preclinical mouse models [26–31] and in phase II clinical trials [32]. Cediranib (AZD2171, Recentin by AstraZeneca, London, United Kingdom) is a potent inhibitor of VEGFRs, which also targets KIT and PDGFRA [33,34]. Cediranib showed promising results in gliomas, leading to 6-month progression-free survival in phase II clinical trials [35]. The efficacy of cediranib has been related to its antiangiogenic capability and ability to normalize tumor vasculature and alleviate edema in glioma patients [36–38]. Despite the promise that the abovementioned drugs hold, the identification of biomarkers for response prediction to those therapies is a major issue that remains to be addressed.
In the present work, we first aimed to compare the therapeutic and biologic impact of three main RTKis, cediranib, sunitinib, and imatinib, in in vitro and in vivo glioblastoma models and to identify the major RTK targets in glioblastomas cells, thereby fostering the identification of potential predictive biomarkers for therapy response to these RTKis in glioblastoma.
Materials and Methods
Cell Lines and Cell Culture
Eight immortalized glioblastoma cell lines were used: SW1088, SW1783, U-87 MG, and A172 were obtained from American Type Culture Collection (ATCC, Manassas, VA), SNB-19 and GAMG were obtained from DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany), and U251 and U373 were kindly provided by Professor Joseph Costello. All the cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM 1x, high glucose; Gibco, Invitrogen, Grand Island, NY) supplemented with 10% FBS (Gibco, Invitrogen) and 1% penicillin/streptomycin solution (DMEM-10), at 37°C and 5% CO2 [39]. Additionally, primary tumor cell cultures were derived from glioblastoma surgical biopsies obtained at the Neurosurgery Department of Barretos Cancer Hospital (São Paulo, Brazil). The study protocol was approved by the local Ethics Committee, and patients signed an informed consent form. Each tumor specimen was cut into small pieces, removing blood vessels, then resuspended in trypsin solution (Gibco, Invitrogen) and incubated at 37°C for 30 minutes. During the incubation, the suspension was pipetted up and down several times for total digestion of the tissues. The isolated cells were grown in DMEM-10. The established cell lines were characterized for the expression of glial fibrillary acidic protein (GFAP) and Nestin by immunocytochemistry (Figure W1).
Authentication of cell lines was performed by IdentiCell Laboratories [Department of Molecular Medicine (MOMA), Aarhus University Hospital Skejby, Århus, Denmark] in August 2011. Genotyping confirmed the complete identity of all cell lines, with the exception of U373, which was shown to be a subclone of the U251 cell line.
Drugs
Cediranib, sunitinib, and imatinib were obtained from Selleck Chemicals (Houston, TX) and temozolomide was obtained from Sigma-Aldrich (Sintra, Portugal). All the drugs were prepared as stock solutions in DMSO and stored at -20°C. The drugs were subsequently prepared as intermediate dilutions in DMSO to obtain an equal quantity of DMSO (1% final concentration) in each of the conditions studied. In all experimental conditions, the drugs were diluted in 0.5% FBS culture medium (DMEM-0.5) to a final concentration of 1% DMSO. Vehicle control (1% DMSO, final concentration) was also used in all experiments.
Cell Viability Assay
To determine the concentration at which 50% of the cell growth is inhibited by drug treatment (IC50 concentration), the cells were plated into 96-well plates at a density of 2 x 103 cells per well and allowed to adhere overnight in DMEM-10. Subsequently, the cells were treated with increasing concentrations of the drugs diluted in DMEM-0.5. After 72 hours, cell viability was quantified using CellTiter 96 Aqueous Cell Proliferation Assay (MTS) (Promega, Madison, WI). The results were expressed as mean viable cells relatively to DMSO alone (considered as 100% viability) ± SD. The IC50 concentration was calculated by nonlinear regression analysis using GraphPad Prism software. Combination studies with temozolomide were done using the same protocol, and the results were expressed as mean viable cells relatively to the condition of the fixed drug alone (considered as 100% viability) ± SD. Drug interactions were analyzed using CalcuSyn software. Determination of synergy was quantified by the combination index (CI).
To assess the effect of a fixed concentration of the drug in cellular viability over time, the cells were plated into 96-well plates at a density of 1 x 103 cells per well and allowed to adhere overnight in DMEM-10. Next, the viable cells were quantified using MTS assay and used as time 0 of the experience. Then, the cells were incubated with fixed concentrations of the drugs diluted in DMEM-0.5 during 24, 48, and 72 hours. At the end of each time point, cell viability was again assessed by MTS assay. The results were calibrated to the starting viability (time 0 hour, considered as 100% of viability) and expressed as the means ± SD. Both assays were done in triplicate at least three times.
Cell Cycle Analysis
The cells were plated in a six-well plate at a density of 2 x 105 cells per well and allowed to adhere overnight. After 6 hours of serum starvation, the cells were incubated for 48 hours with fixed concentrations of the drugs diluted in DMEM-0.5. Cells were trypsinized and fixed in 70% ethanol for at least 30 minutes and then stained during 1 hour at 50°C with propidium iodide (PI) solution [20 µg/ml PI and 250 µg/ml RNAse in a solution of 0.1% Triton X-100 in phosphate-buffered saline (PBS)]. Cell cycle analysis of the PI-stained cells was performed by flow cytometry (LSRII; BD Biosciences, San Jose, CA). The percentage of cells in each phase of the cell cycle was determined using FlowJo version 7.6.3, as described [39].
Wound Healing Migration Assay
The cells were seeded in six-well plates and cultured in DMEM-10 to at least 95% of confluence. Monolayer cells were washed with PBS, scraped with a plastic 200-µl pipette tip, and incubated with fixed concentrations of the drugs diluted in DMEM-0.5. The “wounded” areas were photographed by phase contrast microscopy after 0 and 48 hours. The relative migration distance was calculated by the following formula: percentage of wound closure (%) = 100(A - B)/A, where A is the width of cell wounds before incubation, and B is the width of cell wounds after incubation [39].
Matrigel Invasion Assay
Invasion of the cells was measured using BD BioCoat Matrigel invasion chambers (BD Biosciences), as indicated by the manufacturer. Briefly, 2.5 x 104 cells were plated in the matrigel-coated 24-well trans-well inserts in DMEM-0.5 containing fixed concentration of the drugs. DMEM-10 was used as chemoattractant. The cells were allowed to invade for 48 hours. The invasive cells, attached to the insert membrane, were fixed with methanol and stained with hematoxylin. The invasive cells were photographed at x40 magnification microscope and counted in all the fields of the membrane. The results were expressed in relation to the DMSO control (considered as 100% of invasion) as the mean percentage of invasion ± SD.
Chick Chorioallantoic Membrane Assay
To assess in vivo tumor proliferation and angiogenesis, we used the chorioallantoic membrane (CAM) assay, as previously described [39–41]. Fertilized chicken eggs were incubated at 37°C and 70% humidity, and on day 3 of development, a window was made into the shell and the eggs were returned to the incubator. On day 9, small plastic rings were placed on the CAM. For angiogenesis assessment, 20 µl of 0.5% FBS culture medium containing fixed concentration of the drugs was injected in the ring wall over the CAM on day 14. For tumor formation evaluation, 3 x 106 cells were resuspended in 20 µl of DMEM medium and injected in the rings over the CAM on day 10. On day 14, the tumors formed were photographed in ovo using a stereomicroscope (Olympus S2x16). Next, 20 µl of 0.5% FBS culture medium containing 5 µM cediranib or vehicle control was injected under the tumors. At day 17 (72 hours of incubation with the drug), the tumors were again photographed in ovo. The chickens were sacrificed at ?80°C for 10 minutes, and the tumors or CAM alone was fixed with paraformaldehyde at 4% and photographed ex ovo. The perimeter of the tumors was measured using Cell B software (Olympus) in ovo at days 14 and 17. The results were expressed as mean percentage of tumor growth for each group, from day 14 (considered as 0%) to day 17, ± SD. For blood vessel counting, photographs were taken at day 17 ex ovo, and the results were expressed as the mean of the vessels counted for each group of treatments ± SD.
Western Blot and Human RTK Arrays
To assess the effect of the drugs on the inhibition of intracellular signaling pathways and RTKs, the cells were cultured in DMEM-10 in T25 culture flasks, allowed to grow to 85% of confluence and then serum starved for 2 hours and incubated with the drugs, diluted in DMEM-0.5, by 2 hours. To assess apoptosis, cells were incubated with only one concentration of each drug and incubated for 12, 24, and48hours.Atthe indicatedtimepoints, thecells were washed and scraped in cold PBS and lysed in buffer containing 50 mM Tris (pH 7.6-8), 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 10 mM NaF, 10 mM sodium pyrophosphate, 1% NP-40, and 1/7 of protease cocktail inhibitors (Roche, Amadora, Portugal). Western blot analysis was done using standard 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, loading 20 µg of protein per lane. All the antibodies were used as recommended by the manufacturer (see Supplementary Materials and Methods for antibody information). Concerning the assessment of RTK phosphorylation, a proteome Profiler human phospho-RTK antibody array (ARY001; R&D Systems, Minneapolis, MN) was used according to the manufacturer's instructions. Briefly, a total of 500 µg of fresh protein lysates was incubated overnight at 4°C with nitrocellulose membranes dotted with duplicated spots for 42 anti-RTK and control antibodies. Bound phospho-RTKs were incubated with a pan anti-phosphotyrosine-HRP antibody for 2 hours at room temperature.
Blot detection was done by chemiluminescence (ECL Western Blotting Detection Reagents, RPN2109; GE Healthcare, Piscataway, NJ) in ImageQuant LAS 4000 mini (GE Healthcare) or using X100 Hyperfilm ECL (Amersham, GE Healthcare).
Statistical Analysis
Single comparisons between the different conditions studied were done using Student's t test, and differences between groups were tested using two-way analysis of variance. Statistical analysis was done using GraphPad Prism version 5. The level of significance in all the statistical analyses was set at P < .05.
Results
Efficacy of Cediranib, Sunitinib, and Imatinib in Glioblastoma Cells
To compare the in vitro efficacy of cediranib, sunitinib, and imatinib treatment in glioblastomas, we determined the half-maximal inhibitory concentrations (IC50) of each drug in a panel of eight glioblastoma immortalized cell lines and in two primary glioblastoma cell cultures. As shown in Table 1, cediranib was the most potent, displaying a mean IC50 of 1.71 ± 0.97 µM (range, 0.47–4.17 µM), whereas the mean IC50 for sunitinib was 2.92 ± 1.59 µM (range, 1.26–6.00 µM), and the least efficient was imatinib (mean IC50 of 13.01 ± 6.09 µM; range, 6.28–23.20 µM). Notably, cediranib was clearly the most potent of three RTKIs in the two primary culture glioblastoma cell lines analyzed (HCB2 and HCB7).
Table 1.
IC50 for Imatinib, Sunitinib, and Cediranib in Glioblastoma Cell Lines.
| Cell Line | Mean IC50 ± SD (µM)* | ||
| Imatinib | Sunitinib | Cediranib | |
| U251 | 8.3 ± 1.65 | 1.26 ± 0.21 | 1.36 ± 0.41 |
| SNB-19 | 16.93 ± 0.11 | 6.00 ± 0.35 | 2.05 ± 1.34 |
| U373 | 20.50 ± 0.71 | 3.70 ± 0.53 | 4.17 ± 0.75 |
| SW1783 | 8.94 ± 0.76 | 3.23 ± 0.32 | 1.55 ± 0.31 |
| U-87 MG | 23.20 ± 3.11 | 1.38 ± 0.45 | 1.96 ± 0.91 |
| GAMG | 6.28 ± 0.04 | 1.48 ± 0.49 | 0.47 ± 0.35 |
| SW1088 | 7.53 ± 2.16 | 1.68 ± 0.77 | 1.56 ± 0.86 |
| A172 | 8.94 ± 0.23 | 4.87 ± 1.18 | 1.40 ± 0.14 |
| HCB2 | 11.43 ± 0.61 | 3.05 ± 0.45 | 1.27 ± 0.29 |
| HCB7 | 18.04 ± 1.75 | 2.59 ± 1.44 | 1.27 ± 0.10 |
| Mean† | 13.01 ± 6.09 | 2.92 ± 1.59 | 1.71 ± 0.97 |
Mean of at least three independent experiments done in triplicate.
Mean of the IC50 concentration (µM) for all cell lines.
To evaluate the potential combinatorial value of these drugs in the context of standard glioblastoma therapy, we determined the IC50 of temozolomide when administrated alone or in combination with fixed doses of cediranib (1 µM to U251 and 2.5 µM to SNB-19), sunitinib (2.5 µM to U251 and 5 µM to SNB-19), and imatinib (5 µMto U251 and 15 µM to SNB-19). As exhibited in Table 2, we found that both cediranib and sunitinib synergistically sensitize glioblastoma cells to temozolomide treatment (CI < 1), whereas the effect of imatinib seems to be merely additive (CI ∼ 1).
Table 2.
IC50 of Temozolomide in Combination with Cediranib, Sunitinib, and Imatinib.
| U251 | SNB | |||
| TMZ IC50 (µM) | CI | TMZ IC50 (µM) | CI | |
| TMZ alone | 557.8 | - | 955.1 | |
| TMZ + CD | 164.0 | 0.838 | 448.7 | 0.985 |
| TMZ + SU | 309.4 | 0.837 | 485.0 | 0.866 |
| TMZ + IM | 406.2 | 1.176 | 515.4 | 1.033 |
CD, cediranib; SU, sunitinib; IM, imatinib.
Biologic Effect of Cediranib, Sunitinib, and Imatinib in Glioblastoma Cells
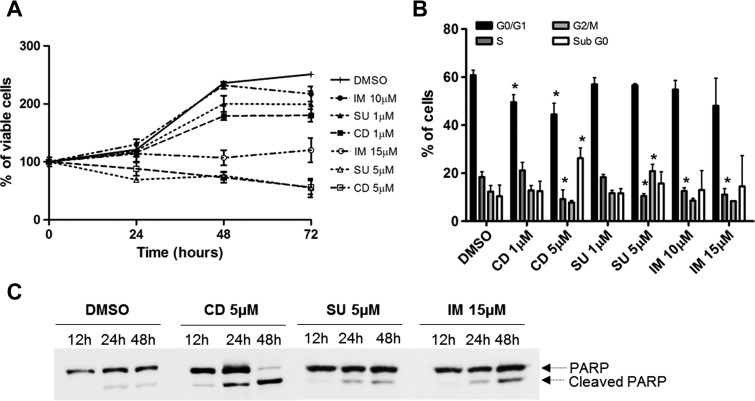
All drugs were able to reduce the viability of U251 cells over time, as assessed by MTS assay, and this effect was dose dependent (Figure 1A). Consistently, the effects of cediranib and sunitinib over time were the most pronounced, whereas imatinib displayed a somewhat milder inhibitory potency. To determine whether decreased viability was due to cytostatic or cytotoxic effects, we analyzed cell cycle distribution (Figure 1B) and poly(ADP-ribose) polymerase (PARP) cleavage (Figure 1C). We observed that all drugs were cytostatic, with a significant reduction of cells in S phase of the cell cycle, mainly for higher doses of treatment. Additionally, cell cycle arrest on the G2/M phase was observed after cells were treated with 5 µM sunitinib, and a significant reduction of cells in G0/G1 was observed after cediranib treatment. A statistically significant higher percentage of apoptotic cells (sub-G0) was detected only after treatment with 5 µMcediranib (Figure 1B). In agreement, a striking effect on PARP cleavage was detected only after high-dose cediranib treatment, whereas sunitinib and imatinib promoted only minor PARP cleavage (Figure 1C). Taken together, these results suggest that the high sensitivity that glioblastoma cells show to cediranib likely results from its concomitant cytostatic and cytotoxic effects.
Figure 1.
Effect of cediranib, sunitinib, and imatinib on U251 cellular survival, cell cycle, apoptosis, migration, and invasion. (A) Cellular viability was measured at 24, 48, and 72 hours by MTS. (B) Cell cycle analysis was done at a 48-hour time point by flow cytometric analysis of PI-stained cells. (C) Apoptosis we assessed by Western blot for PARP cleavage.
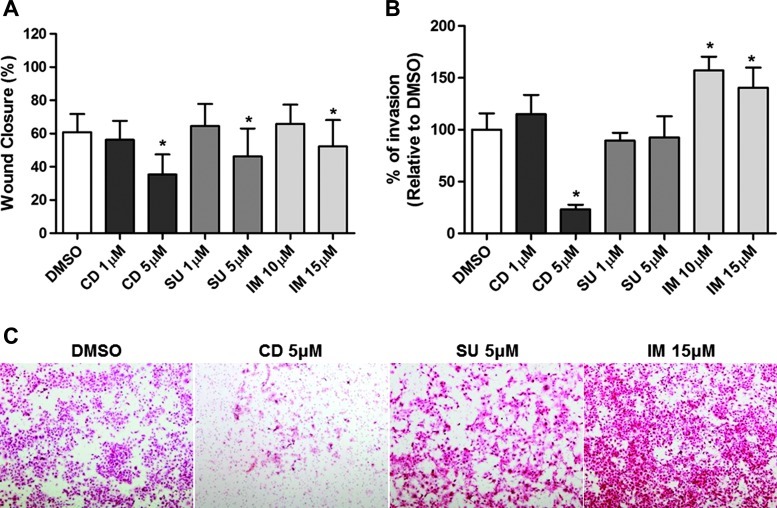
Next, we addressed whether cediranib, sunitinib, or imatinib could impair cell migration and invasion in vitro (Figure 2). Using a wound healing migration assay, we observed that all the drugs, at higher doses, significantly inhibited cell migration and that, similar to the other assays, cediranib was the most efficient drug at the concentrations analyzed (Figure 2A). In contrast, only cediranib was able to impair cell invasion in a matrigel invasion assay. Surprisingly, imatinib promoted the invasion of the cells (Figure 2B).
Figure 2.
Effect of cediranib, sunitinib, and imatinib on U251 cellular migration and invasion. (A) Migration was measured at 48 hours by wound healing migration assay. The relative migration distance was calculated as indicated in Materials and Methods section and the results are expressed as the means ± SD. (B) Invasion was assessed by Matrigel invasion assay. The results were expressed in relation to the DMSO control (considered as 100% of invasion) as the mean percentage of invasion ± SD. (C) Representative pictures of invasion assay (x40 magnification).
The biologic effects of cediranib on viability, cell cycle, apoptosis, migration, and invasion were confirmed in another cell line (SNB-19; Figure W2). The effects of sunitinib and imatinib on viability, cell cycle, and apoptosis were comparable in both cell lines analyzed, whereas the effects on migration and invasion appeared to be cell type specific, since, in contrast to the observations in U251 cells, imatinib had no effect on cellular migration and invasion, and sunitinib efficiently inhibited invasion of SNB-19 cells (Figure W2).
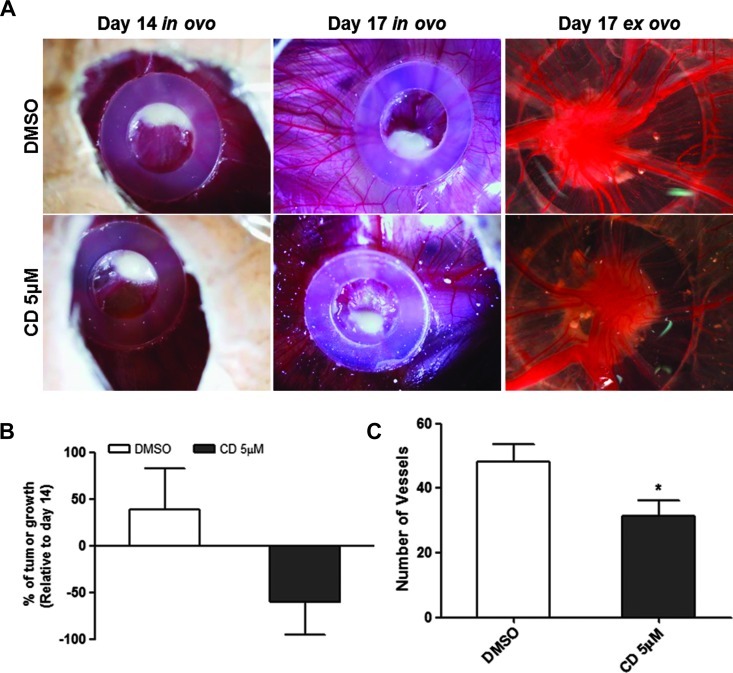
To evaluate whether the antineoplastic effect of cediranib also happens in vivo, we induced the formation of tumors by injection of U251 cells in the CAM (Figure 3). From day 14 to day 17 of embryo development, we observed a mean growth of 69.1 ± 40.1% in the tumors treated with DMSO. In contrast, tumors treated with 5 µM cediranib showed a reduction of 59.8 ± 32.1% of the tumor growth (Figure 3B). Additionally, the number of blood vessels recruited to the tumors treated with cediranib was significantly reduced compared to the tumors treated with DMSO alone (mean 33.3 ± 11.9 vs 48.3 ± 14.2 blood vessels, respectively; P < .05; Figure 3C). Moreover, we further confirmed that all the drugs are antiangiogenic, with a significant reduction (P < .05) on the number of blood vessels formed after CAM treatment with 5 µM cediranib, 5 µM sunitinib, and 15 µM imatinib when compared to the drug vehicle (Figure W3).
Figure 3.
In vivo effect of cediranib on glioblastoma growth and angiogenesis. (A) Representative pictures (x16 magnification) of CAM assay in ovo and ex ovo at days 14 and 17. (B) Tumor growth was measured as described in Materials and Methods section. The results were expressed as mean percentage of tumor growth from day 14 (considered as 0%) to day 17 of development ± SD. (C) Counting of the blood vessels ex ovo at day 17. The results were expressed as the mean number of vessels for each group of treatments ± SD. Differences with P < .05 on the Student's t test were considered statistically significant (*). A total of 12 eggs were used for tumor formation (six were treated with cediranib and six with DMSO alone).
Identification of Novel RTK Targets of Imatinib, Cediranib, and Sunitinib in Glioblastoma Cells
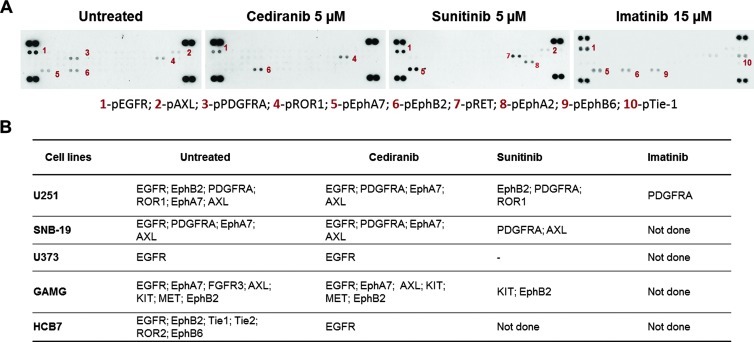
To characterize the molecular basis for the differential sensitivity of glioblastoma cells to the three drugs, we initially characterized the immortalized cell lines for the presence of genomic alterations of PDGFRA amplicon (4q12), in particular the PDGFRA, KIT, and VEGFR2 genes (see Supplementary Materials and Methods). No activating mutations were found in the hotspot exons of PDGFRA and KIT oncogenes. By quantitative polymerase chain reaction (qPCR), we found co-amplification of the three genes in U251, SNB-19, and U373 cell lines. SW1783 cell line has co-amplification of PDGFRA and KIT, and GAMG cell line has amplification of PDGFRA (Table W1). Subsequently, to identify the basal levels of RTK activation in the glioblastoma cell lines (including HCB7 primary cell line), we used a phospho-RTK array to assess the levels of 42 RTKs and observed that all the cell lines presented different basal levels of RTK phosphorylation (Table W1).
Next, to identify the RTKs that are targets of imatinib and mainly cediranib and sunitinib treatment in glioblastomas, we extended the RTK array analysis to some of the cell lines treated with each drug (Figures 4 and W4). As expected, we confirmed KIT and PDGFRA as common targets for both sunitinib and cediranib. Additionally, cediranib inhibited EGFR, EphA7, and AXL in all the cell lines tested that showed constitutive activation of these RTKs. MET was inhibited in the only cell line that showed basal activation of this particular RTK (GAMG cells), whereas EphB2 phosphorylation was downregulated in two of three cell lines (GAMG and HCB7). Sunitinib inhibited EphB2 in two of two cell lines (U251 and GAMG) and ROR1 in one of one (U251), whereas AXL was inhibited in SNB-19 cells but not in U251 or GAMG (Figure 4B). Furthermore, we observed that PDGFRA was the only target to imatinib in U251 cells (Figure 4).
Figure 4.
Cediranib, sunitinib, and imatinib targets on glioblastoma cells. (A) Representative pictures of phospho-RTK arrays for U251 cell line. Each RTK is represented in duplicate in the arrays (two spots side by side), and four pairs of phosphotyrosine positive controls are located in the corners of each array. (B) Other cell lines were treated with cediranib, sunitinib, and imatinib at the indicated concentrations, and the RTKs that were targeted after drug treatment are shown in the table.
In addition to RTK inhibition, we also observed activation of some RTKs after sunitinib and imatinib treatment. In U251 cells, EphB6 and Tie-1 were activated upon imatinib treatment. After sunitinib treatment, we found phosphorylation of EphA2 in U251 and GAMG cell line and also of RET in U251 cell line. We did not detect any significant increase in RTK phosphorylation after treatment with cediranib (Figures 4A and W4A).
To validate the arrays, we performed Western blot analysis for U251 cells with a specific antibody for EGFR phosphorylation (Figure W4B).
Effect of Cediranib, Sunitinib, and Imatinib in Intracellular Signaling Pathway Activation in Glioblastoma Cells
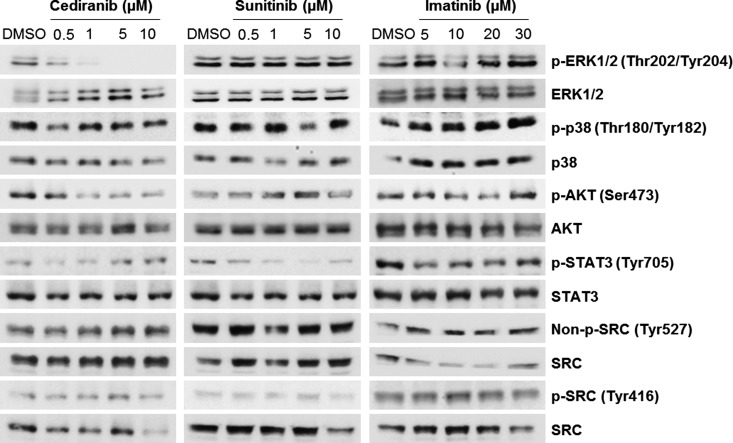
To further characterize the RTK inhibitors and assess whether their effects could correlate with differential activation of intracellular signaling pathways, we exposed U251 cells to increasing concentrations of the three drugs. By Western blot, we assessed the activation levels of some intermediates of the mitogen-activated protein kinase (MAPK), AKT, JAK/STAT, and SRC pathways (Figure 5). Cediranib reduced the activation levels of extracellular signal-regulated kinase 1/2 (ERK1/2) at low doses (0.5 and 1 µM) and completely inhibited the pathway starting from 5 µM. A dose-dependent reduction of AKT activation levels was also found in cells treated with cediranib. Moreover, the activation levels of STAT3 seem to be inhibited after sunitinib and imatinib treatment.
Figure 5.
Effect of cediranib, sunitinib, and imatinib on intracellular signaling pathway activation in U251 cells. The cells were incubated with increasing concentrations of the three drugs by 2 hours. By Western blot, we assess the activation levels of several intermediates of the MAPK (p-ERK1/2 and p-p38), AKT (p-AKT), JAK/STAT (p-STAT3), and SRC (non-p-SRC Tyr527 and p-SRC Tyr416) pathways. The levels of phosphorylation were compared with the corresponding total proteins.
Discussion
Molecular therapies that targeted RTKs are promising therapeutic strategies for glioblastoma tumors. However, the majority of preliminary results of clinical trials are unsatisfactory and failed to show outcome improvements, mainly because the predictive targets for therapy response in glioblastomas remain to be identified; hence, it is believed that those patients are not being properly selected for the therapy [24]. In the present study, we intended to identify the specific RTK targets of two RTKis (sunitinib and cediranib). Further, we aimed to determine, in vitro and in vivo, the efficacy of these drugs in comparison to imatinib.
Hitherto, the antiproliferative effect of these drugs in glioblastomas was unclear. The reports of the effect of imatinib on glioblastoma cell proliferation impairment and cell cycle arrest are contradictory [42–45], as well as its apoptosis or autophagy consequence effect [43,44]. Concerning sunitinib, only two studies addressed its in vitro effects in glioblastomas, using a single cell line (U-87 MG), and found that sunitinib impairs cell survival by apoptosis induction [28,46,47] and induces cell cycle arrest in G2/M [28]. Regarding cediranib, there are no studies reporting the effect of this drug in tumor cells in vitro.Two preclinical in vivo models showed an effect of cediranib in the reduction of xenografted tumors [48,49]. The clinical studies on glioblastoma patients showed that cediranib decreases the cell density in the central area of the tumor [50] and controls tumor growth by normalizing tumor vasculature in addition to alleviating edema [36,37]. Yet, one preclinical study with xenografted models showed that cediranib controls edema and prolongs survival but did not affect tumor growth [38].
In the present work, we found that all the drugs were effective against a panel of 10 glioblastoma cell lines (two of them being primary cultures), with cediranib being the most potent. Furthermore, we observed in U251 cells that all the drugs impair cellular survival over time and in a dose-dependent manner, and again, cediranib was the most effective, even in the less sensitive cell line (SNB-19). By cell cycle analysis, we observed that all the drugs are cytostatic and reduce the number of cells in S phase. In the cells treated with sunitinib, cell cycle arrest in G2/M phase was also found, as described before [28]. In contrast to the other two drugs, cediranib showed to be also cytotoxic—inducing cell death by apoptosis, as assessed by PARP cleavage. We further confirmed by in vivo assays that cediranib displays simultaneously antiangiogenic and antitumoral activity in glioblastomas.
Glioblastomas are highly invasive tumors and this feature influences glioma survival and response to therapy [51]. Overall, we observed in vitro that both sunitinib and cediranib inhibited cellular migration and invasion. At variance, imatinib leads to a slight increase of both migration and invasion in some cells. There are no in vitro studies in the literature using cediranib in glioblastoma cells, precluding any comparisons. Concerning sunitinib, previous works on U-87 MG cells showed that sunitinib treatment was also associated with migration and invasion reduction [28,46,47]. However, invasion inhibition was not proved in vivo [28]. In respect to imatinib, there are only migration studies, which also reported the antimigratory capabilities of the drug in some cell lines and the absence of effect in others [52]. The effect of imatinib in glioblastoma cell invasion, as far as we know, was never reported before. Here, we observed that at least for U251 cells, imatinib can actually promote invasion. This effect was described before for other antiangiogenic drugs, such as bevacizumab [23].
The aforementioned effects are regulated by the intracellular signaling cascades. In agreement with the higher and broader activity that cediranib exhibited against glioblastoma cells, we found that cediranib inhibited both MAPK and AKT pathways, as previously described [33].
Importantly, and in agreement with other studies [48,52], we found that cediranib, as well as sunitinib, synergistically sensitizes glioma cells to temozolomide. These findings suggest that combining temozolomide with RTK inhibitor drugs may improve tumor control.
Besides the discrepant results in the preclinical models using the three drugs we tested, it has been postulated by clinical trial results that cediranib is the most promising to prolong patients' survival [32,35]. Our study, comparing the three drugs for the first time, confirmed in vitro the results achieved in the clinical trials, showing cediranib as the most potent antiproliferative inhibitor. However, similar to the clinical trials, we observed heterogeneous responses of the cells to the same drugs, mainly in migration and invasion assays, suggesting that each drug may have specific targets in glioblastoma cells that have yet to be identified.
The main RTK targets for the pharmacological inhibition in glioblastomas have been EGFR, PDGFRA, KIT, and VEGFR2 [6]. PDGFRA, KIT, and VEGFR2 are reported to be overexpressed, resulting from gene amplification without activating mutations [7–9]. VEGFR2, PDGFRA, and KIT are located side by side in the same chromosomal region 4q12, and co-amplification of these three oncogenes has been observed in glioblastoma patients [8,9]. Thus, to identify the potential targets for imatinib, sunitinib, and cediranib in glioblastomas, we first characterized the cell lines for the molecular status (mutations and amplification) of KIT, PDGFRA, and VEGFR2. No associations were found between the presence of those alterations and the response of the cells to the drugs. Our results are in agreement with clinical studies that failed to find associations between the presence of genomic alterations in these loci and response to sunitinib and imatinib therapy [19,32]. Next, we assessed the phosphorylation levels of 42 different RTKs, before and after treatment, using a human phospho-RTK array. We found that glioblastoma cells have coactivation of several RTKs simultaneously, as previously suggested by others [53]. Recent studies suggested that RTK amplifications (mainly EGFR and PDGFRA) in gliomas can occur in mosaic being heterogeneously distributed within single tumors [10–12]. Overall, those findings reinforce the usage of RTK multitargeted therapies, such as cediranib, for glioblastoma treatment. In the present work, we found PDGFRA as the only target of imatinib in U251 cells, and both PDGFRA and KIT were targets for cediranib and sunitinib as expected. Importantly, additional and novel targets were identified in these cells, such as EGFR, EphA7, AXL, MET, and EphB2 for cediranib and EphB2, ROR1, and AXL for sunitinib. Phyllis and colleagues showed that tumors expressing high levels of EGFR exhibited greater sensitivity to cediranib [49]. Here, we found that EGFR is one of the targets of cediranib. Overall, those findings can suggest that EGFR expression/activation can potentially be used to predict response to cediranib therapy. The role of the remaining RTK targets that we identified in glioblastoma is unclear, and their predictive value for therapy response has to be further explored.
In addition to RTK inhibition, we also observed activation of EphB6 and Tie-1 after imatinib treatment and of EphA2 and RET after sunitinib treatment. These observations, including the identification of novel targets of cediranib and sunitinib in glioblastoma and the activation of particular RTKs in some cell lines upon sunitinib and imatinib, but not cediranib, treatment, might be clinically relevant. The role of these alterations in the modulation of therapy response has yet to be determined for glioblastomas. Usually, this is a phenomenon associated with resistance of other cancers to therapy [54,55].
In conclusion, our study constitute the first comparative study of the efficacy of imatinib, sunitinib, and cediranib in glioblastoma cells and found that cediranib, either alone or in combination with temozolomide, is the most effective drug not only through its antiangiogenic activity but also as a consequence of its greater antitumoral activity. Importantly, we identified the RTK targets of cediranib and sunitinib in glioblastomas, some of which are reported for the first time. This study constitutes a step forward in the identification of potential predictive biomarkers to anti-RTK therapies in glioblastomas that may allow, in the future, the rational selection of patients for specific targeted therapies.
Supplementary Materials and Methods
Molecular Characterization of PDGFRA, KIT, and VEGFR2
DNA isolation was performed using TRIzol reagent (Invitrogen) as indicated by the manufacturer. The screening of mutations in the hotspot exons of KIT (exons 9, 11, 13, and 17) and PDFGFRA (exons 12, 14 and 18) was done by PCR-single-strand conformation polymorphism (SSCP), as previously described [1,2]. Assessment of PDGFRA, KIT, and VEGFR2 gene amplification was performed by quantitative PCR, as previously described [1–3]. Primers and probes for PDGFRA and KIT amplification were previously described [1–3]. For VEGFR2, the primers and probes were the following: 5′-TGAAAGAGACACAGGAAATTACACTG-3′ (forward primer), 5′-CATAATAAATCTTGGGCAGAGAGG-3′ (reverse primer), 5′-CACAACCAGAGAGACCACATGGCTC-FL (donator probe), LC640-GCTTCTCCTTTGAAATGGGATTGGTAAGGA-3′ (acceptor probe).
Antibodies Used in the Western Blot and Array Validation
To assess the activation of intracellular signaling pathways, the antibodies used were the following: phospho-p44/42 MAPK (Thr202/Tyr204) (D13.14.4E); phospho-p38 MAPK (Thr180/Tyr182) (D3F9); phospho-Akt (Ser473) (D9E); phospho-Stat3 (Tyr705) (D3A7); non-phospho-Src (Tyr527); phospho-Src Family (Tyr416). The membranes were stripped and incubated with antibodies to detect the total protein levels as follows: p44/42 MAP kinase (137F5); p38 MAP kinase; Akt (pan) (C67E7); Stat3; Src (36D10). An antibody against PARP expression was used for apoptosis detection. When necessary, β-actin (dilution 1:300; Santa Cruz Biotechnology, Dallas, TX) was used as a loading control.
For array validation, Western blot was performed with the same lysates used for the arrays to detect EGFR phosphorylation with the specific antibody phospho-EGFR (Y1068) (D7A5) from Cell Signaling Technology (Danvers, MA). The antibody to detect total EGFR (31G7; Zymed Laboratories, San Francisco, CA) was used as controls.
Footnotes
This work was funded by Fundação para a Ciência e Tecnologia (FCT, Portugal; Project PTDC/SAU-TOX/114549/2009) and by Pfizer/Sociedade de Ciências Médicas de Lisboa with the award “Research in Oncology Diseases, Prof. Francisco Gentil.” Olga Martinho is a recipient of a PhD fellowship (SFRH/BD/36463/2007), and Vera Miranda-Gonçalves is a recipient of a research fellowship (SFRH/BI/33503/2008), both from FCT. André Lopes Carvalho has a Brazilian National Council for Scientific and Technological Development Scholarship (CNPq, 313181/2009-8).
This article refers to supplementary materials, which are designated by Table W1 and Figures W1 to W4 and are available online at www.transonc.com.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 4.Niyazi M, Siefert A, Schwarz SB, Ganswindt U, Kreth FW, Tonn JC, Belka C. Therapeutic options for recurrent malignant glioma. Radiother Oncol. 2011;98:1–14. doi: 10.1016/j.radonc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor GS, O'Rourke DM. Receptor tyrosine kinase signaling in gliomagenesis: pathobiology and therapeutic approaches. Cancer Biol Ther. 2003;2:330–342. doi: 10.4161/cbt.2.4.507. [DOI] [PubMed] [Google Scholar]
- 7.Gomes AL, Reis-Filho JS, Lopes JM, Martinho O, Lambros MB, Martins A, Schmitt F, Pardal F, Reis RM. Molecular alterations of KIT oncogene in gliomas. Cell Oncol. 2007;29:399–408. doi: 10.1155/2007/926274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinho O, Longatto-Filho A, Lambros MB, Martins A, Pinheiro C, Silva A, Pardal F, Amorim J, Mackay A, Milanezi F, et al. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br J Cancer. 2009;101:973–982. doi: 10.1038/sj.bjc.6605225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network, author. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ, Akhavanfard S, Cahill DP, Aldape KD, Betensky RA, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20(6):810–817. doi: 10.1016/j.ccr.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Little SE, Popov S, Jury A, Bax DA, Doey L, Al-Sarraj S, Jurgensmeier JM, Jones C. Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumor heterogeneity. Cancer Res. 2012;72:1614–1620. doi: 10.1158/0008-5472.CAN-11-4069. [DOI] [PubMed] [Google Scholar]
- 12.Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y, Ozawa T, Holland EC, Huse JT, Jhanwar S, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci USA. 2012;109:3041–3046. doi: 10.1073/pnas.1114033109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuettenberg J, Friedel C, Vajkoczy P. Angiogenesis in malignant glioma—a target for antitumor therapy? Crit Rev Oncol Hematol. 2006;59:181–193. doi: 10.1016/j.critrevonc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7:1152–1160. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- 15.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 17.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 18.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 19.Baruchel S, Sharp JR, Bartels U, Hukin J, Odame I, Portwine C, Strother D, Fryer C, Halton J, Egorin MJ, et al. A Canadian paediatric brain tumour consortium (CPBTC) phase II molecularly targeted study of imatinib in recurrent and refractory paediatric central nervous system tumours. Eur J Cancer. 2009;45:2352–2359. doi: 10.1016/j.ejca.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Wen PY, Yung WKA, Lamborn KR, Dahia PL, Wang YF, Peng B, Abrey LE, Raizer J, Cloughesy TF, Fink K, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 21.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. JNatl Compr Canc Netw. 2011;9:403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- 23.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Witt Hamer PC. Small molecule kinase inhibitors in glioblastoma: a systematic review of clinical studies. Neuro Oncol. 2010;12:304–316. doi: 10.1093/neuonc/nop068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377–389. doi: 10.2165/11318860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Navis AC, Hamans BC, Claes A, Heerschap A, Jeuken JW, Wesseling P, Leenders WP. Effects of targeting the VEGF and PDGF pathways in diffuse orthotopic glioma models. J Pathol. 2011;223:626–634. doi: 10.1002/path.2836. [DOI] [PubMed] [Google Scholar]
- 27.Chahal M, Xu Y, Lesniak D, Graham K, Famulski K, Christensen JG, Aghi M, Jacques A, Murray D, Sabri S, et al. MGMT modulates glioblastoma angiogenesis and response to the tyrosine kinase inhibitor sunitinib. Neuro Oncol. 2010;12:822–833. doi: 10.1093/neuonc/noq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boüard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, Guillamo JS. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9:412–423. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Guo P, Gallo JM. Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin Cancer Res. 2008;14:1540–1549. doi: 10.1158/1078-0432.CCR-07-4544. [DOI] [PubMed] [Google Scholar]
- 30.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 31.Schueneman AJ, Himmelfarb E, Geng L, Tan J, Donnelly E, Mendel D, McMahon G, Hallahan DE. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res. 2003;63:4009–4016. [PubMed] [Google Scholar]
- 32.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2010;103(3):491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 33.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 34.Brave SR, Ratcliffe K, Wilson Z, James NH, Ashton S, Wainwright A, Kendrew J, Dudley P, Broadbent N, Sproat G, et al. Assessing the activity of cediranib, a VEGFR-2/3 tyrosine kinase inhibitor, against VEGFR-1 and members of the structurally related PDGFR family. Mol Cancer Ther. 2011;10:861–873. doi: 10.1158/1535-7163.MCT-10-0976. [DOI] [PubMed] [Google Scholar]
- 35.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, Eichler AF, Drappatz J, Hochberg FH, Benner T, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28:2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batchelor TT, Duda DG, Di Tomaso E, Kozak KR, Zhang W, Ancukiewicz M, Loeffler JS, Wen PY, Sorensen GA, Jain RK. Normalization of tumor vasculature and alleviation of edema by AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor in glioblastoma patients. Int J Radiat Oncol Biol Phys. 2007;69:S50–S51. [Google Scholar]
- 37.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, di Tomaso E, Duda DG, Munn LL, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinho O, Granja S, Jaraquemada T, Caeiro C, Miranda-Goncalves V, Honavar M, Costa P, Damasceno M, Rosner MR, Lopes JM, et al. Downregulation of RKIP is associated with poor outcome and malignant progression in gliomas. PLoS One. 2012;7:e30769. doi: 10.1371/journal.pone.0030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva-Correia J, Miranda-Goncalves V, Salgado AJ, Sousa N, Oliveira JM, Reis RM, Reis RL. Angiogenic potential of Gellan-gum-based hydrogels for application in nucleus pulposus regeneration: in vivo study. Tissue Eng Part A. 2012;18(11–12):1203–1212. doi: 10.1089/ten.TEA.2011.0632. [DOI] [PubMed] [Google Scholar]
- 41.Moniz S, Martinho O, Pinto F, Sousa B, Loureiro C, Oliveira MJ, Moita LF, Honavar M, Pinheiro C, Pires M, et al. Loss of WNK2 expression by promoter gene methylation occurs in adult gliomas and triggers Rac1-mediated tumour cell invasiveness. Hum Mol Genet. 2013;22(1):84–95. doi: 10.1093/hmg/dds405. [DOI] [PubMed] [Google Scholar]
- 42.Hagerstrand D, Hesselager G, Achterberg S, Wickenberg BU, Kowanetz M, Kastemar M, Heldin CH, Isaksson A, Nister M, Ostman A. Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene. 2006;25:4913–4922. doi: 10.1038/sj.onc.1209497. [DOI] [PubMed] [Google Scholar]
- 43.Shingu T, Fujiwara K, Bogler O, Akiyama Y, Moritake K, Shinojima N, Tamada Y, Yokoyama T, Kondo S. Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. Int J Cancer. 2009;124:1060–1071. doi: 10.1002/ijc.24030. [DOI] [PubMed] [Google Scholar]
- 44.Ranza E, Mazzini G, Facoetti A, Nano R. In-vitro effects of the tyrosine kinase inhibitor imatinib on glioblastoma cell proliferation. J Neurooncol. 2010;96:349–357. doi: 10.1007/s11060-009-9975-4. [DOI] [PubMed] [Google Scholar]
- 45.Gross D, Bernhardt G, Buschauer A. Platelet-derived growth factor receptor independent proliferation of human glioblastoma cells: selective tyrosine kinase inhibitors lack antiproliferative activity. J Cancer Res Clin Oncol. 2006;132:589–599. doi: 10.1007/s00432-006-0109-5. [DOI] [PubMed] [Google Scholar]
- 46.Dimitropoulos K, Giannopoulou E, Argyriou AA, Zolota V, Petsas T, Tsiata E, Kalofonos HP. The effects of anti-VEGFR and anti-EGFR agents on glioma cell migration through implication of growth factors with integrins. Anticancer Res. 2010;30:4987–4992. [PubMed] [Google Scholar]
- 47.Giannopoulou E, Dimitropoulos K, Argyriou AA, Koutras AK, Dimitrakopoulos F, Kalofonos HP. An in vitro study, evaluating the effect of sunitinib and/or lapatinib on two glioma cell lines. Invest New Drugs. 2010;28:554–560. doi: 10.1007/s10637-009-9290-0. [DOI] [PubMed] [Google Scholar]
- 48.Wachsberger PR, Lawrence RY, Liu Y, Xia X, Andersen B, Dicker AP. Cediranib enhances control of wild type EGFR and EGFRvIII-expressing gliomas through potentiating temozolomide, but not through radiosensitization: implications for the clinic. J Neurooncol. 2011;105(2):181–190. doi: 10.1007/s11060-011-0580-y. [DOI] [PubMed] [Google Scholar]
- 49.Wachsberger PR, Lawrence YR, Liu Y, Daroczi B, Xu X, Dicker AP. Epidermal growth factor receptor expression modulates antitumor efficacy of vandetanib or cediranib combined with radiotherapy in human glioblastoma xenografts. Int J Radiat Oncol Biol Phys. 2010;82(1):483–491. doi: 10.1016/j.ijrobp.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 50.di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 52.Ren H, Tan X, Dong Y, Giese A, Chou TC, Rainov N, Yang B. Differential effect of imatinib and synergism of combination treatment with chemotherapeutic agents in malignant glioma cells. Basic Clin Pharmacol Toxicol. 2009;104:241–252. doi: 10.1111/j.1742-7843.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 53.Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 54.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 55.Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della CK, Wisner L, Iorio M, Shakalya K, Garewal H, et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–3919. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
Supplementary References
- 1.Gomes AL, Reis-Filho JS, Lopes JM, Martinho O, Lambros MB, Martins A, Schmitt F, Pardal F, Reis RM. Molecular alterations of KIT oncogene in gliomas. Cell Oncol. 2007;29:399–408. doi: 10.1155/2007/926274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinho O, Longatto-Filho A, Lambros MB, Martins A, Pinheiro C, Silva A, Pardal F, Amorim J, Mackay A, Milanezi F, et al. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br J Cancer. 2009;101:973–982. doi: 10.1038/sj.bjc.6605225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinho O, Goncalves A, Moreira MA, Ribeiro LF, Queiroz GS, Schmitt FC, Reis RM, Longatto-Filho A. KIT activation in uterine cervix adenosquamous carcinomas by KIT/SCF autocrine/paracrine stimulation loops. Gynecol Oncol. 2008;111:350–355. doi: 10.1016/j.ygyno.2008.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.