Abstract
The daily light–dark cycle affects many aspects of normal physiology through the activity of circadian clocks. It emerges that the pancreas has a clock of its own, which responds to energy fluctuations.
The pancreas is a mosaic organ: its different cell types regulate sugar and fat metabolism through controlled production of digestive enzymes and hormones in response to food and physiological demand. To maintain normal blood glucose levels after a meal, for instance, β-cells in pancreatic islets produce insulin, which then stimulates glucose uptake and storage by the muscle and fat cells, and stops glucose production and secretion by the liver. It is increasingly being appreciated that circadian clocks — sets of genes through which organisms keep track of time — regulate these processes. But exactly how they function is not known. On page 627 of this issue, Marcheva et al.1 report that pancreatic islets have a circadian clock that regulates insulin secretion, and that disruption of this clock causes greatly increased blood glucose due to impaired insulin secretion, a hallmark of diabetes.
Circadian biological phenomena — from the daily movements of plant leaves to human sleep–wake cycles — have been recognized for centuries, but their underlying physical and biochemical mechanisms have remained mysterious. In 1972, a study2 demonstrated that a brain region in the hypothalamus called the suprachiasmatic nucleus, which sits just above the optic nerves, is required for daily rhythms in animal behaviour. This established a physical location for the generation of rhythmic behaviour in mammals. The suprachiasmatic nucleus receives light signals through the optic nerves, and so uses daylight cues to set the clock time and to couple light–dark transitions to behavioural outputs.
Subsequent genetic studies identified several genes that mediate rhythmic behaviour. Biochemical investigation of the proteins expressed by these genes led to the current model of the mammalian circadian clock. This clock is a molecular oscillator based on a negative-feedback loop in which the transcription factors CLOCK (or the related protein NPAS2) and BMAL1 work together to drive the expression of many genes, including those encoding their own inhibitors — the period (PER1, PER2 and PER3) and the cryptochrome (CRY1 and CRY2) proteins3.
Surprisingly, it emerged later that fruitflies have circadian clocks not only in the brain, but also in every cell4. Moreover, studies in mice showed that many mammalian organs harbour circadian clocks5. The question was: do clocks outside the suprachiasmatic nucleus have physiological roles?
Two sets of findings suggested that mammalian clocks outside the brain participate in metabolic regulation. First, expression of enzymes, transporters and receptors that regulate metabolism fluctuate robustly throughout the day6. Second — and unexpectedly — circadian clocks outside the suprachiasmatic nucleus are adjusted on the basis of feeding time rather than the light–dark schedule7,8. For example, the cellular energy sensor AMPK controls the stability of cryptochromes and may contribute to nutrient entrainment of the clock in the liver9.
General disruption of clock genes profoundly affects both locomotor activity and feeding behaviour, and so may indirectly alter metabolism. This problem can be overcome by organ-specific inactivation of genes such as Bmal1, which leaves general behavioural patterns intact. ‘Conditional’ ablation of Bmal1 in this way demonstrated a role for retinal circadian clocks in visual perception10 and for liver circadian clocks in glucose regulation11. Notably, liver-specific ablation of Bmal1 caused lowered blood glucose levels only during the times of day when mice naturally fast. This observation supports a role for mammalian circadian clocks outside the brain in predicting recurrent daily changes in metabolic demand — in this case11 leading to increased glucose production by the liver during times of expected fasting.
Despite these advances, many questions remain about the function of various circadian clocks. For instance, although disruption of Bmal1 — either in all cells or specifically in the liver — alters metabolism, the effects are mild and, depending on the nature of disruption, metabolic outcomes differ. Do clocks in other organs counteract some of the effects of the liver clock?
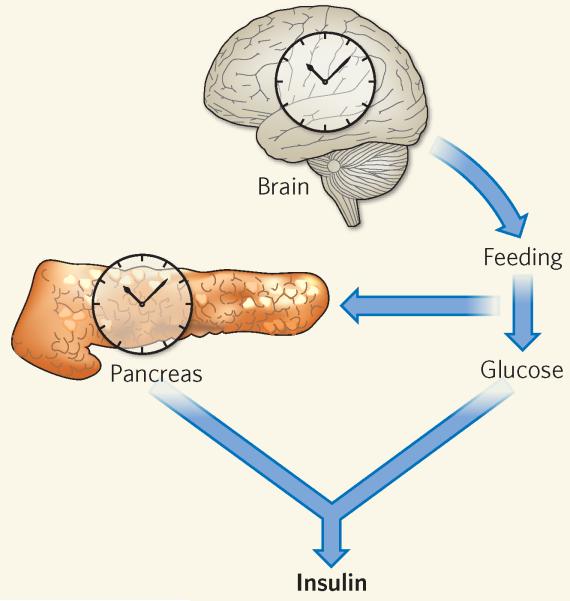
Marcheva et al.1 show that the mouse pancreas also harbours a functional circadian clock, with individual pancreatic islets having robust clock function even when outside their normal tissue environment. The islet clock seems to consist of the same components as other mammalian circadian clocks, and drives rhythmic expression of genes involved in insulin sensing, glucose sensing, and islet growth and development. These clocks are therefore crucial for the specific metabolic needs and functions of islet cells (Fig. 1).
Figure 1. Going by the local clock.
In response to the daily light–dark cycle, the clock in the suprachiasmatic nucleus of the brain regulates metabolism by affecting rhythmic behaviour such as feeding. Marcheva et al.1 show that the pancreas also has a local clock, which directly affects insulin secretion in response to high blood glucose levels.
The authors1 find that, compared with normal mice, mice in which circadian-clock function is generally disrupted produce less insulin, both under resting conditions and after a shot of glucose. Moreover, these animals’ pancreatic islets are smaller and less adept at insulin production than are those of normal mice. These results indicate that the islet clock directly regulates insulin production. But is reduced insulin secretion due to a loss of the islet clock specifically, or to indirect mechanisms associated with the loss of other circadian clocks? To answer this question, Marcheva et al. specifically disrupted the circadian clock in the pancreas by ablating Bmal1 there. They note that the resulting mice have profoundly elevated levels of blood glucose under resting conditions, as well as impaired insulin secretion in response to a dose of glucose.
Marcheva and co-workers’ observations should put to rest any doubts as to whether CLOCK and BMAL1 have crucial roles in the regulation of metabolism, independently of their roles in controlling behaviour. They also provide further evidence for the idea that mammalian circadian clocks outside the brain enable animals to synchronize physiological processes with recurring, and therefore predictable, changes in metabolic demand.
One enduring difficulty in the study of circadian clocks’ role in metabolism is whether disruption of CLOCK and BMAL1 leads to metabolic defects by affecting circadian rhythms or through activities unrelated to their clock function. Answering this question will require a technical breakthrough — for example, a small-molecule clock inhibitor — enabling selective disruption of clock function without ablating CLOCK or BMAL1 proteins entirely. Regardless of these semantic details, by linking CLOCK and BMAL1 activity to insulin production, Marcheva and co-workers’ data hint at a potential alternative strategy for the treatment of diabetes, through enhancing the activity of these proteins.
Contributor Information
Katja A. Lamia, Scripps Research Institute, Biological Studies, La Jolla, California 92037, USA, klamia@scripps.edu
Ronald M. Evans, Salk Institute for Biological Studies, La Jolla, California 92037, USA. evans@salk.edu
References
- 1.Marcheva B, et al. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephan FK, Zucker I. Proc. Natl Acad. Sci. USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allada R, Emery P, Takahashi JS, Rosbash M. Annu. Rev. Neurosci. 2001;24:1091–1119. doi: 10.1146/annurev.neuro.24.1.1091. [DOI] [PubMed] [Google Scholar]
- 4.Plautz JD, Kaneko M, Hall JC, Kay SA. Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 5.Yoo S-H, et al. Proc. Natl Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panda S, et al. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 7.Stokkan K-A, Yamazaki S, Tei H, Sakaki Y, Menaker M. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 8.Damiola F, et al. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamia KA, et al. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storch K-F, et al. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamia KA, Storch K-F, Weitz CJ. Proc. Natl Acad. Sci. USA. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]