Summary
Maltreated foster children often exhibit alterations in diurnal hypothalamic-pituitary-adrenal (HPA) axis activity that are characterized by lower cortisol levels upon waking and smaller declines in morning-to-evening cortisol levels. Previous research has shown that this dysregulated pattern is associated with high caregiver stress levels over the course of foster care placements. In contrast, therapeutic interventions that emphasize consistent and responsive caregiving have been associated with more regulated cortisol rhythms. In this paper, two related issues were explored: whether placement changes (i.e., moving between foster homes or from a foster home to a permanent placement) were associated with more blunted daily cortisol rhythms and whether a caregiver-based intervention exerted a protective effect in this context. Because the intervention program has components specifically designed to prepare foster children for placement changes and to maintain consistent parenting techniques despite them, a prevention effect on HPA axis dysregulation during placement changes was hypothesized. The results of linear mixed modeling analyses showed that placement changes predicted dysregulation in cortisol rhythms in the regular foster care group but not in the intervention foster care group. These findings are discussed in terms of implications for child welfare policy and practice.
Keywords: diurnal cortisol, dysregulation, foster care, intervention, preschool
Introduction
Placement changes are a common occurrence for foster children (Connell, 2006), who move from one home to another at least once (i.e., initial entry into foster care). In addition, many foster children experience further placement changes: moving between foster homes, entering permanent placements (e.g., reunifications or adoptions), or reentering care after failed permanent placement attempts. As many as two thirds of all foster placements disrupt within the first 2 years (Wulczyn et al., 2003), and close to half of all new foster placements disrupt within the first 6 months (Wulczyn et al., 2007). Given that there are over half a million foster children in the United States in any given year (National Clearinghouse on Child Abuse and Neglect Information, 2005), this is a problem of considerable public health significance.
There is a growing body of research documenting the negative effects of placement changes on foster children. Rubin et al. (2004) found that foster children with multiple placement changes had up to 63% higher risk for behavior problems than foster children who did not experience such instability. Similarly, Newton et al. (2000) found higher rates of internalizing and externalizing behaviors among children with unstable placement histories. Stovall and Dozier (1998) suggested that placement changes negatively impact a child’s ability to form attachments with caregivers, an effect that could prove extremely problematic in the long term.
In addition to observing the psychosocial effects of placement instability, researchers have found associations between placement instability and a foster child’s brain development. For example, Pears et al. (2008) examined the performance of a group of preschool-aged foster children on neuropsychological tasks involving self-regulation and behavioral control, which are known to activate prefrontal cortical circuitry. They reported a negative association between the number of unique caregivers with whom a child had lived and positive performance on these tasks (i.e., having more caregivers was associated with poorer performance). Similarly, the number of foster caregiver changes has been shown to be negatively correlated with a foster child’s performance on neuropsychological tasks measuring executive functioning (Fisher et al., 2006; Lewis et al., 2007).
Given the negative effects of placement instability, reducing placement changes should be a focus of foster care policy and programs. Providing additional social and mental health services for foster children and families might play a role in this area, as might increasing the availability of training and consultation to foster caregivers and reducing the caseloads for child welfare workers. Even with policy changes aimed at reducing placement instability, placement changes will undoubtedly occur in some circumstances. Thus, it is also important to develop strategies for limiting the impact of placement changes—for example, family-based interventions that can be deployed systematically on a wide-scale basis in public sector service settings. In this study, we examined whether one such intervention, previously documented to increase placements stability during and after foster care (Fisher et al., 2009), might also prevent hypothalamic-pituitary-adrenal (HPA) axis dysregulation specifically associated with placement changes in foster care.
HPA Axis Dysregulation in Foster Children
The HPA axis plays a central role in regulating an individual’s responses to stressful and arousing events. HPA axis activity, which can be assessed via salivary cortisol levels, is activated in response to real or perceived threat to the physical and social self and in response to uncontrollability and unpredictability (Mason, 1968). HPA axis activation involves a hormonal cascade, beginning with the release of corticotrophin releasing hormone (CRH) in the hypothalamus, which in turn stimulates the secretion of adrenocorticotropin releasing hormone (ACTH) in the anterior pituitary. ACTH in the bloodstream leads to the release of the glucocorticoid hormone, cortisol, by the adrenal cortex. Cortisol regulates its own production via negative feedback at the level of the hippocampus and hypothalamus, slowing and ultimately stopping the release of CRH. Glucocorticoids also act on a number of bodily stress response systems, metabolizing stored energy and stimulating the immune system. In addition, the HPA axis is structurally and functionally connected to a number of other key neural structures involved in the regulation of stress, including the prefrontal cortex and the amygdala, via the presence of glucocorticoid receptors in these regions.
The HPA axis exhibits a diurnal rhythm, with peak cortisol levels around the time of morning waking that decline over the day to reach near zero levels around the onset of nighttime sleep. There is increasing evidence that chronic stress blunts the diurnal pattern of cortisol production, particularly lowering the peak production of cortisol early in the morning (Heim et al., 2000; Roy et al., 2003). Such diurnal cortisol rhythmicity has been associated with high levels of behavioral and emotional problems in childhood (Shirtcliff and Essex, 2008), immunosuppression (Shirtcliff et al., 2009), and increased mortality in adult cancer patients (Sephton et al., 2000).
This blunted diurnal cortisol rhythm has also been associated with severe early life stress—for example, children living in orphanages/institutions in Russia and Romania (Gunnar and Vazquez, 2001) and severely neglected preschoolers entering new foster placements (Bruce et al., 2009). Moreover, among school-aged maltreated children, this dysregulated rhythm was observed in children with high levels of internalizing problems who had experienced severe physical and sexual abuse prior to age 5 years (Cicchetti et al., 2010).
The specific psychobiological mechanisms of diurnal cortisol dysregulation in children following early stress are not thoroughly understood, although a number of hypotheses have been proposed (Gunnar and Vazquez, 2001; Fisher and Gunnar, in press). This phenomenon might represent decreased neuroendocrine activity associated with a downregulation of the HPA axis, which might be an adaptive response to an early caregiving environment that lacks a responsive caregiver to buffer the infant from stress and negative arousal. Alternatively, it might result from enhanced negative feedback associated with high concentrations of glucocorticoid receptors in the hypothalamus and other brain regions, might be associated with disrupted sleep patterns (Tininenko et al., in press), or might result from a combination of these and other processes.
Improved Behavioral Adjustment and HPA Axis Regulation via Intervention
Although foster children are clearly at high risk for adverse developmental and psychosocial outcomes (Landsverk et al., 2002), only recently has evidence emerged of the potential to systematically reduce this risk through preventive interventions. The interventions that have provided the most robust evidence of effectiveness, via randomized efficacy trials, have typically focused on providing services to support the caregiver–child relationship (Dozier et al., 2002; Fisher et al., 2006).
The Multidimensional Treatment Foster Care Program for Preschoolers (MTFC-P; Fisher et al., 1999) intervention involves multiple components, including in-home consultation, foster caregiver support groups, and child therapeutic playgroups for 6–9 months. It emphasizes the use of consistent, positive parenting strategies to promote child psychosocial development and behavioral/emotional regulation. In the context of a longitudinal randomized trial, a number of positive results have been reported, including increases in foster child attachment security (Fisher and Kim, 2007) and increased successful permanent placements (Fisher et al., 2005). Notably, the intervention’s positive impact on successful permanent placements is particularly pronounced among children with histories of placement instability (Fisher et al., 2009).
In addition, a number of prior studies have highlighted the effects of the MTFC-P intervention on children’s HPA axis functioning. Fisher et al. (2007) examined whether the intervention stimulated more optimal diurnal cortisol patterns. The 3- to 6-year-old children in this study were followed for 12 months beginning soon after entering new foster placements; morning and evening saliva samples for cortisol determination were taken over 2 days every month. The children in regular foster care exhibited increasingly dysregulated diurnal cortisol patterns over time due to lower early morning cortisol levels. In contrast, the intervention foster children and a group of nonabused community comparison children showed no dissolution of the typical diurnal cortisol pattern over the 12-month period.
Fisher and Stoolmiller (2008) reported that one predictor of children’s lower early morning cortisol levels was how much stress foster caregivers reported managing their child’s behavior problems. If a foster caregiver reported being highly stressed by the child’s behavior on a given day, the child had lower cortisol levels the following morning relative to his or her levels on other days. Notably, the MTFC-P intervention appeared to reduce caregiver stress caused by child misbehavior. That is, the intervention caregivers appeared to respond quickly to the training and support to manage behavior problems when difficulties arose. Consequently, they exhibited a drop in caregiver stress in response to child misbehavior that was fairly stable across the study period. In contrast, the caregivers of regular foster care children exhibited no decrease in parenting stress across time. Rather, their stress levels remained significantly higher than those of the intervention caregivers and were significantly associated with child lower morning cortisol levels over time.
Additional evidence points to increased behavioral and HPA axis regulation in the context of therapeutic interventions for foster children. For example, Bakermans-Kranenburg et al. (2008) developed the Video-Feedback Intervention to Promote Positive Parenting and Sensitive Discipline, which focuses on improving outcomes by recording and showing caregivers samples of positive caregiver–child interaction. The approach is designed for children up to age 3 years. In the context of a randomized efficacy trial to examine the effects of this approach among foster children, the intervention children exhibited more typical diurnal cortisol levels.
Dozier et al. (2006, 2008) developed the Attachment and Biobehavioral Catch-Up intervention for foster and adopted infants and toddlers. This intervention involves a 10-session curriculum focusing on the caregiver’s ability to provide a nurturing environment that facilitates the development of the child’s self-regulatory capabilities. In the context of a randomized efficacy trial, Dozier et al. reported more typical diurnal cortisol patterns (2006) and lower cortisol reactivity during a laboratory stressor task (2008) in the intervention children versus the comparison children.
Goals of the Present Study: Exploring the Dysregulating Effects of Placement Changes
Although the aforementioned research findings demonstrate the potential of therapeutic interventions to increase foster children’s diurnal cortisol rhythm over time, no studies to date have explored the role of placement changes on diurnal HPA axis regulation or whether therapeutic interventions have the potential to mediate such dysregulation. We examined these issues using data from the MTFC-P randomized trial. We hypothesized that the regular foster care children who had experienced placement changes within the first 6 months following a new placement would show low morning cortisol levels immediately following the placement change, whereas this effect would be mediated in the intervention group.
Methods
Several clarifying comments are required prior to describing the methods employed in this study. First, because we were interested in the effects of any placement change, we included all transitions that occurred during the first 6 months following entry into the study: foster placements (between foster homes or returning from a permanent placement), reunifications with biological parents, and adoptions. Although different placement changes might produce different effects, any caregiver change is likely to be stressful. Moreover, we did not have a sufficient sample size to examine the differential effects of specific placement change types. However, as we describe below, the proportions of different placement change types were not significantly different between groups.
Second, some participants in the MTFC-P randomized trial did not experience a placement change during the study period or had multiple placement changes too quickly to examine postplacement effects. Consequently, this study contains only a subsample of the original randomized trial participants. As such, the intervention effects discussed here can only be generalized to children who have experienced placement changes after entering care.
Participants
Of the 117 foster care children in the MTFC-P randomized clinical trial, 71 (61%) experienced a placement change during the first 6 months following study entry and did not experience a subsequent placement change for at least 6 months after the first. Of the foster children in the overall randomized trial who were not included in this study, 14 experienced no placement changes during the sampling period, 6 experienced secondary placement changes within 4 months of the first, and 3 had no preplacement data (see Analysis Plan); all of these children were excluded from the current analyses. The remaining 23 cases were excluded from the present study due to attrition; typically, this involved the caregivers in new placements declining study participation.
The 71 children (41 boys; 30 girls) in the current study included 36 MTFC-P children and 35 regular foster care (RFC) children with an average age of 4.47 years (SD = 0.77) at study entry. The ethnicity breakdown was as follows: 62 Caucasian, 5 Latino, 1 African American, 1 Native American, 1 mixed race, and 1 other. The excluded individuals did not differ from the present subsample in terms of age, F(1, 115) = .24, ns, sex, χ2(1) = 1.11, ns, race (Caucasian vs. non-Caucasian), χ2(1) = .77, ns, or intervention condition, χ2(1) = .29, ns.
We examined placement change types to verify that no differences existed between groups. In the MTFC-P group, there were 11 foster placement failures, 17 reunifications with biological parents, and 8 adoptions. In the RFC group, there were 13 foster placement failures, 15 reunifications with biological parents, and 7 adoptions. The results from a chi-square test confirmed that the proportions of placement change types were not significantly different between groups.
MTFC-P Design
To be selected for the larger randomized trial study, each child had to be between 3- and 5-years old and entering a foster placement under the care of the Lane County Branch of the Oregon Department of Human Services, Child Welfare Division. The sample included children new to foster care, reentering care, and moving between foster placements. To be eligible for the clinical trial, the current placement had to be expected to last for 3 or more months. The eligible children were randomly assigned to the MTFC-P or RFC conditions (see information on the recruitment process in Fisher and Stoolmiller, 2008).
MTFC-P Group Procedures
MTFC-P is a caregiver-based intervention designed to address the developmental and social-emotional needs of preschool-aged foster children. It is delivered via a treatment team approach (Fisher et al., 1999; Fisher and Chamberlain, 2000). Services are provided to the foster children, their foster caregivers, and their permanent placement resources (biological parents or adoptive parents). Prior to placement, the foster caregivers complete 12 hr of intensive training. After placement, the foster caregivers receive support and supervision via daily telephone contacts, weekly group meetings, and 24-hr staff availability. These services are intended to facilitate the maintenance of a warm, responsive, consistent environment in which positive behavior is encouraged and problem behavior is limited and to reduce caregiver stress in managing child problem behavior. The children receive individualized treatment with child therapists to facilitate the acquisition of prosocial skills and to improve functioning in preschool, daycare, and home settings. The children also participate in weekly therapeutic playgroup sessions focused on facilitating school readiness, emphasizing social-emotional functioning and early literacy skills. To ensure treatment fidelity for all MTFC-P components, progress notes and checklists regarding services received are completed by the clinical staff and are monitored by the research team.
The MTFC-P model is also designed to facilitate consistency between caregiving contexts during placement changes. First, the program emphasizes a set of consistent and practical parenting management training techniques that have been shown to support positive behavior and limit negative behavior (Chamberlain, 2003). These techniques are taught to the foster caregivers via training and ongoing consultation and are employed in family therapy with the biological or adoptive parents prior to and after permanent placements are attempted. Thus, although some aspects of family environments undoubtedly vary before and after placement changes, the intervention is designed to ensure that the central parenting processes are retained. Second, the MTFC-P intervention is designed so that attention is devoted to the transition process itself. Whereas placement changes can be abrupt, traumatic, and destabilizing for RFC children, the MTFC-P children visit with their future caregivers, beginning with brief, in-office visits and increasing to home visits and overnight stays. Unless there is a need to move a child out of a particular foster home quickly (which rarely occurs because of the frequency of contact between the foster caregivers and the staff members), the transition process lasts 2 weeks to 2 months, giving children time to adjust to the new family contexts.
RFC Group Procedures
In this county child welfare system, the typical services include monthly or more frequent contact with caseworkers to monitor progress and to identify issues in need of attention. Additional services can include weekly individual psychotherapy to address trauma and/or behavioral issues, medication prescribed by a primary care physician or child psychiatrist for extreme behavioral and emotional problems, and developmental screening and early childhood special education services as necessary. See Fisher and Kim (2007) and Fisher and Stoolmiller (2008) for the more detail regarding the services specific to the RFC group in this study.
Measures
Placement changes
Each child’s placement data was obtained via computer reports from the state child welfare agency database. These reports list the types of service provided (e.g., short duration shelter care, regular foster care, and foster placement with a relative; listed chronologically), the arrival and/or departure dates for each placement, the service disposition codes indicating the reason for each placement change, and the names and numbers for each foster caregiver and service provider. Once a child’s caseworker and foster caregiver(s) consented to participation, a project staff member requested the child’s placement record. Updated records were requested every 6 months during the study. The number of placement changes for the 117 children in the clinical trial ranged from one to nine (M = 2.62, SD = 1.28). The number of placement changes for the subsample of children included in this analysis ranged from two to nine (M = 2.70, SD = 1.18), which was not significantly different from excluded children, t(115) = −.81, ns.
Salivary cortisol
Monthly salivary cortisol samples were collected twice daily on 2 consecutive days. The initial assessments occurred 3–5 weeks after a placement change. This allowed the children to adjust to the foster homes, allowed the foster caregivers to get to know the children, and limited unnecessary stress during a potentially fragile period.
The first collection (AM) occurred at 30 min after the child awoke and before eating or drinking, a time when cortisol has been shown to peak in response to awakening (Schmidt-Reinwald et al., 1999). The second collection (PM) occurred at 30 min before bedtime. The caregivers were trained by research staff members to complete saliva collection at home following procedures described in Schwartz et al. (1998). For each collection, the child chewed a piece of Trident Original Flavor Gum (Cadbury Adams USA, Plano, TX) for 1 min to stimulate saliva flow. The child then spat the gum out, and the caregiver tipped a Salivette (Sarstedt, Newton, NC) absorbent roll from a protective plastic tube into the child’s mouth without touching the roll. The child kept the roll in his/her mouth for 1 min and was instructed not to touch it with his/her fingers. The caregiver then assisted the child in returning the roll to the protective tube before recording the date and time of the collection on the tube label and completed a brief questionnaire regarding sampling time and the child’s eating and sleeping behavior that day. Certain medications, general health, food intake, and sleep patterns have been shown to affect cortisol levels (de Kloet, 1991). Thus, children who used steroid-based medications (e.g., steroidal asthma inhalers) on a regular basis and other medications known to affect cortisol levels were excluded from the study. Each caregiver was instructed not to sample on days when the child was took a steroid-based medication or had a fever.
The saliva samples were stored in the participants’ freezers until being collected for assay by research staff members. The samples were assayed using the High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics, State College, PA). All samples from a particular child were included in the same assay batch to minimize within-subject variability. The samples were assayed in duplicate and were averaged. Duplicates varying by more than 15% were reassayed. The intraassay and interassay coefficients of variance were 2.69% and 10.98%, respectively.
Analysis Plan
For each child’s monthly salivary cortisol samples, AM cortisol level and PM cortisol level were computed by averaging the two AM and two PM samples, respectively; the morning-to-evening cortisol decrease (i.e., diurnal cortisol level decrease) was calculated by subtracting the PM cortisol level from the AM cortisol level. When examining the impact of placement changes, we used the first 6 months of morning-to-evening cortisol decrease figures after each child’s first placement. To ensure that we were not simply detecting individual differences in the set point of the HPA axis, we averaged the previous 3 months of morning-to-evening cortisol decrease figures prior to the placement change to establish a baseline value for each child. We averaged all available morning-to-evening cortisol decrease figures if less than 3 months of data were available. We then subtracted this baseline value from all morning-to-evening cortisol decrease values after the placement change: a zero value would indicate no change, a negative value would indicate a smaller decrease, and a positive value would indicate a larger decrease.
We chose to aggregate cortisol data across morning-to-evening and across 3-month intervals for several reasons. We employed this strategy to achieve the most parsimonious analysis possible given the research question. Multivariate statistical modeling allows for the inclusion of disaggregated data that can then be examined at multiple levels to consider more fine-grained effects; however, we have not found this approach to be useful when investigating hypothesis-driven research questions. For example, because there was extremely little variation in PM cortisol level in our sample, including AM and PM cortisol levels separately and then considering slope or change scores would add limited new information, increase the complexity of the models examined, and introduce redundancy into the results (e.g., AM cortisol levels and morning-to-evening cortisol decreases were virtually identical). Thus, we have found it more straightforward to include only the morning-to evening values. Similarly, in terms of aggregating across months, from the perspective of the ecological context of foster care, we were primarily interested in establishing a defensible interval before and after placement changes that would provide a relatively stable measure of diurnal cortisol. Including individual months in the models might be interesting in examining variation in diurnal cortisol across months prior to and after a placement change, but this was beyond the scope of our investigation.
To investigate the impact of MTFC-P on morning-to-evening cortisol decreases, we employed linear mixed modeling (LMM; Fitzmaurice et al., 2004). LMM and other growth curve modeling techniques enable the specification of a mean growth curve based upon a polynomial equation. Subsequently, variation around this curve can be modeled in terms of static or dynamic covariates. This approach enables modeling of the average start point and the average rate of change across the population as well as the factors that can cause an individual to deviate from these averages. We initially tested for the effects of sex, age, and time before the first placement; none of these effects were significant. We also controlled for each child’s wake time on measurement days and time between waking and the AM collection.
In our analyses, we examined whether the MTFC-P children exhibited a significantly different growth curve across the first 6 months postplacement. We first fit a polynomial equation to describe the mean growth curve and removed nonsignificant higher order terms. Next, we inserted an interaction term between intervention condition (i.e., a dummy code for MTFC-P participation) and each polynomial term in the model to examine the highest order interaction (e.g., Dummy × Quadratic) in the model for significance; if the interaction was not significant, we removed it, reran the model, and examined the next–highest order interaction (e.g., Dummy × Linear). We continued this process until a significant interaction was found or all interactions between the model terms and the dummy variable had been removed. In each case, we tested only the highest order term for significance.
Results
Preplacement Morning-to-Evening Cortisol Decrease
To determine whether the MTFC-P and RFC children differed in their preplacement morning-to-evening cortisol decrease values, we examined aggregated cortisol data for each child across monthly intervals following entry into the study but prior to a subsequent placement change. The results demonstrated the typical diurnal pattern involving a significant linear decrease from morning to evening, B = −.16, SE(B) = .06, p < .01. There were no significant differences between the MTFC-P and RFC children in the intercept or the linear term; thus, the groups were comparable on this variable.
Postplacement Morning-to-Evening Cortisol Decrease
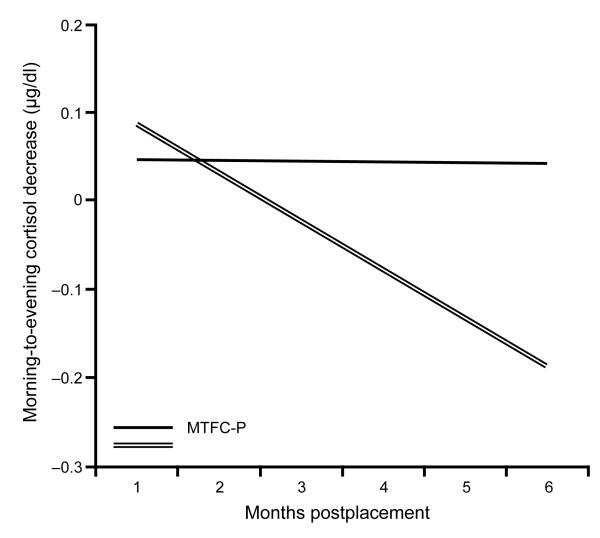
We initially fit a quadratic curve to the postplacement model, but this term was not significant. When it was removed, we found a significant negative linear term, B = −.31, SE(B) = .08, p < .001 (see overall means in Table 1). When intervention condition was added to the model, the interaction between intervention condition and time was significant (see Table 2). As is shown in Figure 1, the MTFC-P children did not show any postplacement changes from their preplacement morning-to-evening cortisol decrease values, whereas the RFC children showed significantly more blunted morning-to-evening cortisol levels following placement changes.
Table 1.
Magnitude of Postplacement Morning-to-Evening Cortisol Decreases
| Month | N | M | SD |
|---|---|---|---|
| 1 | 71 | .09 | .29 |
| 2 | 61 | .03 | .34 |
| 3 | 51 | −.02 | .34 |
| 4 | 54 | .02 | .35 |
| 5 | 49 | −.09 | .30 |
| 6 | 45 | −.08 | .42 |
Table 2.
Results of Linear Mixed Modeling with Intervention Condition Included
| Term | Estimate | SE |
|---|---|---|
| Intercept | .42 | .20 |
| Linear | −.52 | .12 |
| Intervention Condition × Intercept | −.06 | .07 |
| Intervention Condition × Linear | .40* | .16 |
p < .05.
Figure 1.
Morning-to-evening cortisol decreases for the first 6 months postplacement among MTFC-P and RFC children. Note. MTFC-P = Multidimensional Treatment Foster Care for Preschoolers; RFC = regular foster care.
Discussion
Our results are noteworthy in several respects. First, the increasingly blunted morning-to-evening cortisol decrease values following a placement change for RFC children highlight the overall vulnerability of diurnal HPA axis functioning in foster children in the context of environmental stressors. In contrast to prior cross-sectional studies comparing clinical and typical populations, which have employed principally mean-level comparisons between groups, our results suggest that changes in diurnal HPA axis activity in the presence of significant stressors might be an equally important characteristic to examine in high-risk groups. Given the well-documented associations between HPA axis dysregulation and many psychological and health problems, this finding underscores the need for foster care services that decrease and limit the impact of stressors.
Our results also provide evidence that MTFC-P mitigates the negative effects of placement changes on diurnal HPA axis activity. In prior studies (Fisher et al., 2007; Fisher and Stoolmiller, 2008), we reported that, in the overall sample of children participating in the randomized clinical trial, the MTFC-P children showed more consistent morning-to-evening cortisol decreases across the months of the study protocol, whereas the RFC children showed smaller morning-to-evening cortisol decreases over the same period. However, the mechanisms of these effects are not clear.
In the present study, we examined only the subgroup of children from the randomized trial who experienced a placement change during the study period. The RFC children showed relatively stable morning-to-evening cortisol decreases in the months prior to the placement transition but diminishing morning-to-evening cortisol decreases postplacement. In contrast, the MTFC-P children showed stable and typical morning-to-evening cortisol decreases before and after placement changes, suggesting less disruption to their HPA axis functioning.
Our results suggest that MTFC-P mitigates the dysregulating effects of placement changes on children’s diurnal HPA axis activity. This effect was hypothesized because the intervention emphasizes the use of specific parenting techniques and provides extensive support to help ensure that the caregivers use these techniques. In addition, placement changes that occur in the MTFC-P program are typically planned, giving staff members and foster caregivers time to facilitate the transition. MTFC-P children usually visit their new homes several times, which allows them to get to know the new environment and caregiver(s) before the move, and staff members work to facilitate consistency in daily schedules and routines between homes. These experiences are in contrast to the placement changes that often occur in regular foster care, especially in the context of placement failures, which can be abrupt and in which there might be no emphasis on maintaining consistency between homes. In the absence of the support provided in MTFC-P, placement changes might prove to be highly stressful events capable of disrupting diurnal HPA axis regulation.
In the present study, we did not examine whether the MTFC-P intervention strategies specific to placement changes alone are sufficient to prevent diurnal HPA axis dysregulation or whether the additional intervention elements are needed to achieve the effects observed. The MTFC-P intervention is fairly intensive in nature and is delivered throughout the course of a child’s placement, typically lasting 6–9 months. These services appear to be important to achieving other intervention effects, including increased attachment security (Fisher and Kim, 2007) and decreased placement disruptions (Fisher et al., 2005, 2009). However, in relation to focus of the present study—the prevention of diurnal HPA axis dsyregulation specifically associated with placement changes—perhaps the support and services delivered to children and families around placement changes would prove sufficient to achieve this effect. This question remains for future research.
Overall, our results have several implications for policy and practice. Consistent with prior evidence documenting the negative effects of placement instability on developmental, behavioral, and neurobiological functioning, our results suggest that placement changes in regular foster care have the potential to disrupt the diurnal regulation of a key neuroendocrine system. Given the widespread evidence of altered HPA axis functioning in relation to anxiety disorders, affective disorders, and disruptive behavior disorders, this finding is cause for concern. Taken together with the prior research findings in this area, it underscores the importance of implementing system-wide reforms in child welfare to prevent unnecessary placement changes and lessen the impact of necessary placement changes.
On the positive side, it appears that systematic efforts to intervene in the context of placement changes have the potential to limit diurnal HPA axis dysregulation. Additionally, the impact of these intervention efforts appears to function independent of maltreatment severity. This is promising because interventions often have their greatest impact on only a subgroup of the target population (e.g., the lowest risk children).
MTFC-P is focused on preschool-aged children, but other versions of the intervention have been employed with samples of older children and adolescents (see Fisher and Chamberlain, 2000). The results from a number of randomized clinical trials to evaluate this approach show consistent positive effects of the intervention on psychosocial functioning (Leve & Chamberlain, 2007; Leve, Fisher, & Chamberlain, 2009). However, these studies have not examined HPA axis activity or other areas of neurobiological functioning. Whether this intervention, or others that involve the family context, show effects on older children’s neurobiology is an important question for future research.
Our findings may also have implications outside of foster care. Many other children experiences challenging transitions, including starting school and experiencing a parental divorce, bereavement, or a family relocation. Our findings suggest that interventions designed to promote consistency and predictability in the family context may have an impact on maintaining HPA regulation during such transitions. It would be interesting for interventions targeting these experiences to examine their potential effects on young children’s HPA functioning.
Study Limitations
Two study limitations should be noted. First, it was not clear which intervention components were most effective at stabilizing morning-to-evening cortisol decreases. Perhaps the support specifically around the placement change was the most impactful, or perhaps the more comprehensive services delivered throughout the intervention period were necessary to produce the observed effects. Additional research will be necessary to disentangle these intervention effects and to determine how these effects impact long-term outcomes. Such research is an important step in determining what services are most effective and for whom.
Second, we had a relatively small sample size. The reduced statistical power resulting from this led to only marginal significance for potentially important effects. Moreover, it made it impossible to examine whether different placement types produce different patterns of HPA axis dysregulation. Although small samples are understandable given the sensitivity of the foster care population and the challenges of conducting randomized trials in this context, they limit the potential reach of the findings. Replicating these results with a larger sample would be desirable and may be possible given the ongoing randomized trials of MTFC-P in public sector settings.
Summary
Despite the study limitations, our results advance knowledge in several areas. First, they suggest that early adversity can lead to stable but smaller morning-to-evening cortisol decreases and might indicate a greater likelihood of the environment adversely impacting the HPA axis in response to subsequent stressors. Second, they support emerging evidence that the HPA axis maintains plasticity (at least the diurnal component of the HPA axis) following early stress and is amenable to environmental interventions. These intervention effects appear to be concordant with improved outcomes for foster children in important domains. Whether the intervention effects extend further in time and reduce (or delay) the onset of psychopathology remains to be seen; this is one of the primary emphases of our ongoing program of research.
Acknowledgements
Support for this research was provided by the following grants: R01 MH059780 and R21 MH065046, NIMH, U.S. PHS; R01 HD045894, NICHD, U.S. PHS; and R01 DA021424 and P30 DA023920, NIDA, U.S. PHS. The authors thank the staff and families of the Multidimensional Treatment Foster Care for Preschoolers program, Kristen Greenley for project management, and Matthew Rabel for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Philip A. Fisher, University of Oregon, Oregon Social Learning Center, and Center for Research to Practice.
Mark J. Van Ryzin, Oregon Social Learning Center, 10 Shelton McMurphey Boulevard, Eugene, OR 97401; 541-485-2711 (Phone); 541-485-7087 (Fax); markv@oslc.org
Megan R. Gunnar, Institute of Child Development, 51 East River Parkway, Minneapolis, MN 55455; 612-624-2846 (Phone); 612-624-6373 (Fax); gunnar@umn.edu
References
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Mesman J, Alink LRA, Juffer F. Effects of an attachment-based intervention on daily cortisol moderated by dopamine receptor D4: a randomized control trial on 1- to 3-year-olds screened for externalizing behavior. Dev. Psychopathol. 2008;20:805–820. doi: 10.1017/S0954579408000382. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Dev. Psychobiol. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain P. Treating Chronic Juvenile Offenders: Advances Made Through the Oregon Multidimensional Treatment Foster Care Model. American Psychological Association; Washington, DC: 2003. [Google Scholar]
- Cicchetti D, Rogosch FA, Gunnar MR, Toth SL. The differential impacts of early physical and sexual abuse and internalizing problems on daytime cortisol rhythm in school-aged children. Child Dev. 2010;81:252–269. doi: 10.1111/j.1467-8624.2009.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell CM. Changes in placement among children in foster care: a longitudinal study of child and case influences. Soc. Serv. Rev. 2006;80:398–419. doi: 10.1086/505554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Frontiers Neuroendocrinol. 1991;12:95–164. [Google Scholar]
- Dozier M, Albus K, Fisher PA, Sepulveda S. Interventions for foster parents: implications for developmental theory. Dev. Psychopathol. 2002;14:843–860. doi: 10.1017/s0954579402004091. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children’s diurnal production of cortisol: an exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Dozier M, Pelosoa E, Lewisa E, Laurenceaua J-P, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Dev. Psychopathol. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Burraston BO, Pears KC. The Early Intervention Foster Care program: permanent placement outcomes from a randomized trial. Child Maltreatment. 2005;10:61–71. doi: 10.1177/1077559504271561. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Chamberlain P. Multidimensional Treatment Foster Care: a program for intensive parent training, family support, and skill building. J. Emotional Behav. Disord. 2000;8:155–164. [Google Scholar]
- Fisher PA, Gunnar M, Dozier M, Bruce J, Pears KC. Effects of a therapeutic intervention for foster children on behavior problems, caregiver attachment, and stress regulatory neural systems. Ann. N. Y. Acad. Sci. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Ellis BH, Chamberlain P. Early Intervention Foster Care: a model for preventing risk in young children who have been maltreated. Child. Serv.: Soc. Policy Res. Pract. 1999;2:159–182. [Google Scholar]
- Fisher PA, Gunnar MR. Early life stress as a risk factor for disease in adulthood. In: Vermetten E, Lanius R, Pain C, editors. The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; Cambridge, UK: in press. [Google Scholar]
- Fisher PA, Kim HK. Intervention effects on foster preschoolers’ attachment-related behaviors from a randomized trial. Prev. Sci. 2007;8:161–170. doi: 10.1007/s11121-007-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Kim HK, Pears KC. Effects of Multidimensional Treatment Foster Care for Preschoolers (MTFC-P) on reducing permanent placement failures among children with placement instability. Child Youth Serv. Rev. 2009;31:541–546. doi: 10.1016/j.childyouth.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M. Intervention effects on foster parent stress: associations with child cortisol levels. Dev. Psychopathol. 2008;20:1003–1021. doi: 10.1017/S0954579408000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinol. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; Hoboken, NJ: 2004. [Google Scholar]
- Gunnar MR, Vasquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DK. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinol. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Landsverk J, Garland AF, Leslie LK. Mental health services for children reported to child protective services. In: Meyers JEB, Berliner L, Briere JN, Hendrix CT, Reid TA, Jenny CA, editors. APSAC Handbook on Child Maltreatment. 2nd ed Sage; Thousand Oaks, CA: 2002. pp. 487–507. [Google Scholar]
- Leve LD, Chamberlain P. A randomized evaluation of Multidimensional Treatment Foster Care: Effects on school attendance and homework completion in juvenile justice girls. Research on Social Work Practice. 2007;17:657–663. doi: 10.1177/1049731506293971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leve LD, Fisher PA, Chamberlain P. Multidimensional treatment foster care as a preventive intervention to promote resiliency among youth in the child welfare system. Journal of Personality. 2009;77:1869–1902. doi: 10.1111/j.1467-6494.2009.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis EE, Dozier M, Ackerman J, Sepulveda-Kozakowski S. The effect of placement instability on adopted children’s inhibitory control abilities and oppositional behavior. Dev. Psychol. 2007;43:1415–1427. doi: 10.1037/0012-1649.43.6.1415. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the pituitary-adrenal cortical system. Psychosom. Med. 1968;30:576–607. [PubMed] [Google Scholar]
- National Clearinghouse on Child Abuse and Neglect Information [Retrieved July 6, 2010];Child Maltreatment 2003: Summary of Key Findings. 2005 from http://library.softgenx.com/Children/Abuse/canstats.pdf.
- Newton RR, Litrownik AJ, Landsverk JA. Children and youth in foster care: disentangling the relationship between problem behaviors and number of placements. Child Abuse Negl. 2000;24:1363–1374. doi: 10.1016/s0145-2134(00)00189-7. [DOI] [PubMed] [Google Scholar]
- Pears KC, Kim HK, Fisher PA. Psychosocial and cognitive functioning of children with specific profiles of maltreatment. Child Abuse Negl. 2008;32:958–971. doi: 10.1016/j.chiabu.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Kirschbaum C, Steptoe A. Intraindividual variation in recent stress exposure as a moderator of cortisol and testosterone levels. Ann. Behav. Med. 2003;26:194–200. doi: 10.1207/S15324796ABM2603_04. [DOI] [PubMed] [Google Scholar]
- Rubin DM, Alessandrini EA, Feudtner C, Localio AR, Hadley T. Placement changes and emergency department visits in the first year of foster care. Pediatr. 2004;114:e354–e360. doi: 10.1542/peds.2003-0594-F. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schürmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel H. Diurnal cortisol rhythm as a predictor of breast cancer survival. J. Nat. Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc. Nat. Acad. Sci. USA. 2009;106:2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev. Psychbiol. 2008;50:690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovall KC, Dozier M. Infants in foster care: an attachment theory perspective. Adoption Q. 1998;2:55–58. [Google Scholar]
- Tininenko JR, Fisher PA, Bruce J, Pears KC. Sleep disruption in young foster children. Child Psychiatry Hum. Dev. doi: 10.1007/s10578-010-0177-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulczyn F, Hislop K, Chen L. Foster Care Dynamics 2000-2005: A Report From the Multistate Foster Care Data Archive. Chapin Hall Center for Children at the University of Chicago; Chicago, IL: 2007. [Google Scholar]
- Wulczyn F, Kogan J, Harden BJ. Placement stability and movement trajectories. Soc. Serv. Rev. 2003;77:212–236. [Google Scholar]