Abstract
Rationale
Oxidative myofibers in the skeletal muscles express high levels of angiogenic factors, have dense vasculature, and promptly revascularize during ischemia. Estrogen-related receptor-gamma (ERRγ) activates genes that govern metabolic and vascular features typical to oxidative myofibers. Therefore, ERRγ-dependent remodeling of the myofibers may promote neoangiogenesis and restoration of blood perfusion in skeletal muscle ischemia.
Objective
To investigate the muscle fiber type remodeling by ERRγ and its role in the vascular recovery of ischemic muscle.
Methods and Results
Using immunohistology, we show that skeletal muscle-specific transgenic overexpression of ERRγ increases the proportions of oxidative and densely vascularized type IIA and IIX myofibers and decreases glycolytic and less vascularized type IIB myofibers. This myofiber remodeling results in a higher basal blood flow in the transgenic skeletal muscle. By applying unilateral hind limb ischemia to transgenic and wild-type mice, we found accelerated revascularization (fluorescent microangiography), restoration of blood perfusion (laser Doppler flowmetry), and muscle repair (Evans blue dye exclusion) in transgenic compared to wild-type ischemic muscles. This ameliorative effect is linked to enhanced neoangiogenesis (CD31 staining and microfil perfusion) by ERRγ. Using cultured muscle cells in which ERRγ is inactivated, we show that the receptor is dispensable for the classical hypoxic response of transcriptional upregulation and secretion of vascular endothelial growth factor A. Rather, the ameliorative effect of ERRγ is linked to the receptor-mediated increase in oxidative myofibers that inherently express and secrete high levels of angiogenic factors.
Conclusions
The ERRγ is a hypoxia-independent inducer of neoangiogenesis that can promote reparative revascularization.
Keywords: estrogen-related receptors, hypoxia, ischemia, neoangiogenesis, skeletal muscle
Ischemic damage to the skeletal muscle attributable to compromised blood supply is a common complication in cardiovascular and metabolic diseases such as heart failure, atherosclerosis, obesity, and diabetes.1–3 Because of limited effective treatment options, which include invasive procedures such as endovascular reconstruction or surgical revascularization, muscle ischemia often leads to limb amputation in alarmingly large numbers of patients.4 Noninvasive treatment with pharmacological agents directed toward restoring vascular function by inducing therapeutic angiogenesis in ischemic skeletal muscle are underdeveloped. Although targeting of some of the angiogenic factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor has shown functional revascularization of ischemic muscle in preclinical studies, success in the clinical setting has been limited.5–8 Because neoangiogenesis is a complex phenomenon involving a plethora of angiogenic factors,9 alternative strategies that can “switch-on” a comprehensive vascular program rather than individual factors are warranted for promoting functional neoangiogenesis in the ischemic skeletal muscle.7
One potential strategy to treat muscle ischemia might be to enhance the inherent ability of skeletal muscles to secrete angiogenic factors and to recruit new blood vessels. In this regard, skeletal muscle beds rich in oxidative myofibers, such as type I and IIX, as well as the oxidative/glycolytic fibers IIA, express high levels of angiogenic factors and therefore are densely vascularized.10–13 Interventions such as regular exercise that boost oxidative myofiber phenotype are beneficial in the management of ischemia by increasing capillary density, collateralization, and microcirculation in the skeletal muscle.13,14 The ameliorative effect of exercise might be linked to the ability of oxidative muscles to induce higher angiogenic response compared with glycolytic muscles during ischemia.15 Several recent reports suggest that transcriptional regulators of oxidative myofiber type can stimulate neoangiogenesis and reverse muscle ischemic damage in exercise-independent fashion. For instance, nuclear receptor coactivator PGC-1α, a key regulator of oxidative fiber type, can induce angiokine expression and neoangiogenesis in ischemic skeletal muscle.16 Likewise, nuclear receptor PPARδ has been shown to trigger oxidative myofiber remodeling and to promote angiogenic gene expression in skeletal muscle.17,18 Therefore, transcriptional regulators of oxidative myofibers that also can induce the angiogenic program are attractive candidates that can be targeted for promoting vascular recovery in skeletal muscle.
Estrogen-related receptor-γ (ERRγ), a constitutively active orphan nuclear receptor, is highly expressed in skeletal muscles enriched in oxidative and densely vascularized myofibers.19–21 Using receptor transgenesis in the skeletal muscle, we and others recently showed that ERRγ drives a transcriptional program leading to the activation of genes linked to fatty acid metabolism, mitochondrial biogenesis, as well as angiogenesis that governs the “metabovascular” features of the oxidative myofibers.20,21 However, exactly which myofiber types (type I, IIA, IIX, or IIB) are regulated by ERRγ in increasing oxidative phenotype and whether the receptor can promote vascular recovery in ischemic skeletal muscle remain unknown. Therefore, we investigated the effect of ERRγ on the expression of different fiber types and its ability to induce neoangiogenesis and reperfusion in ischemic skeletal muscles using a murine model of hind limb vascular occlusion. We show that transgenic overexpression of ERRγ increases the proportion of type IIA and IIX myofibers, simultaneously decreasing glycolytic type IIB myofibers. This fiber type remodeling results in both higher angiogenic factor expression and basal blood flow in the transgenic skeletal muscle. More importantly, in a murine model of hind limb vascular occlusion, muscle-specific ERRγ overexpression induces neoangiogenesis, triggers revascularization, accelerates restoration of blood perfusion, and reverses myofiber damage in the ischemic skeletal muscle. We provide additional evidence that the receptor is dispensable for the classical hypoxic response of Vegfa induction, and the ameliorative effect of ERRγ in ischemia is linked to the receptor-mediated transformation of the transgenic skeletal muscle to one that inherently expresses high levels of proangiogenic factors. These findings reveal ERRγ as a potential therapeutic target for treating ischemic disease particularly in the skeletal muscle.
Methods
Detailed Methods are provided in the Online Supplement.
Animal Husbandry
We previously reported the generation of transgenic mice overexpressing ERRγ specifically in the skeletal muscle.20 Twelve to 16-week-old male mice were used in all the experiments.
Hind Limb Ischemia and Tissue Collection
Hind limb ischemia was achieved in wild-type and transgenic mice by unilateral femoral occlusion, adapting previously described protocols22,23 such that left hind limb was ischemic and the contralateral right hind limb served as the control. Hind limb muscles such as tibialis anterior (TA), extensor digitorum longus, gastrocnemius, soleus, and plantaris were harvested at various time points after the induction of ischemia and processed. All the measurements were made in contralateral wild-type, ischemic wild-type, contralateral transgenic, and ischemic transgenic muscles of the hind limbs.
Laser Doppler Blood Flow Measurement
Blood flow was measured in both the contralateral nonischemic and ischemic muscles from wild-type and transgenic mice with a deep tissue laser Doppler probe (Vasamedics Laserflo BPM2). Blood flow in the ischemic muscles is reported as the percent of the blood flow to contralateral nonischemic hind limb (ischemic/contralateral×100).
Vascular Mapping
For imaging of intact vasculature in TA, fluorescence microangiography was performed on wild-type and transgenic mice subjected to unilateral hind limb ischemia using fluorescent microspheres (Invitrogen). Transverse cryosections of the TA were processed and subjected to fluorescent microscopy to image and quantify skeletal muscle vasculature.
Additionally, neoangiogenesis was measured by whole-mount visualization of vascular branching in TA muscles of wild-type and transgenic mice subjected to unilateral hind limb ischemia using intracardiac microfil perfusion.
Muscle Damage
Muscle damage was determined in both the cryosections of TA and the extensor digitorum longus using the Evans blue dye exclusion test.
Immunohistochemistry
Serial transverse cryosections of the TA (and other muscles) isolated from contralateral and ischemic hind limbs of wild-type and transgenic mice were immunohistologically stained for endothelial cells (CD31) and fiber type (myosin heavy chain type I, IIA, IIX, and IIB). Details regarding capture and quantification of digital images of the muscle cryosections after immunostaining or fluorescence microangiography are also provided in the Online Supplement.
Mouse Angiogenesis Array and VEGFA Enzyme-Linked Immunosorbent Assay
Muscle lysates from plantaris were prepared and subjected to mouse angiogenesis array (R&D Systems ARY015) and sandwich VEGFA enzyme-linked immunosorbent assay using the DuoSet enzyme-linked immunosorbent assay development system (R&D Systems DY493) according to the manufacturer’s instructions.
Cell Culture
Measurements of Vegfa gene expression and secretion by muscle C2C12 cells stably expressing empty vector or dominant-negative ERRγ (ERRGDN) under normoxic or hypoxic conditions were performed.
Gene Expression
Gastrocnemius muscles from both the contralateral and ischemic limbs were used for studying gene expression by quantitative real-time polymerase chain reaction, as described previously.20
Chromatin Immunoprecipitation and Reporter Gene Assay
Chromatin immunoprecipitation and luciferase reporter assays to determine activation of Vegfa promoter by ERRγ are described in the Online Supplement.
Statistical Analysis
Data are shown as mean±standard deviation. Significant differences among groups for dependent variables were detected by using two-way (genotype [wild-type versus transgenic]×treatment [contralateral limb versus ischemic limb]) analysis of variance. Comparison between two groups was performed by Student t test for independent variables. Significant differences were considered for P<0.05.
Results
Remodeling of Muscle Fiber Type by ERRγ
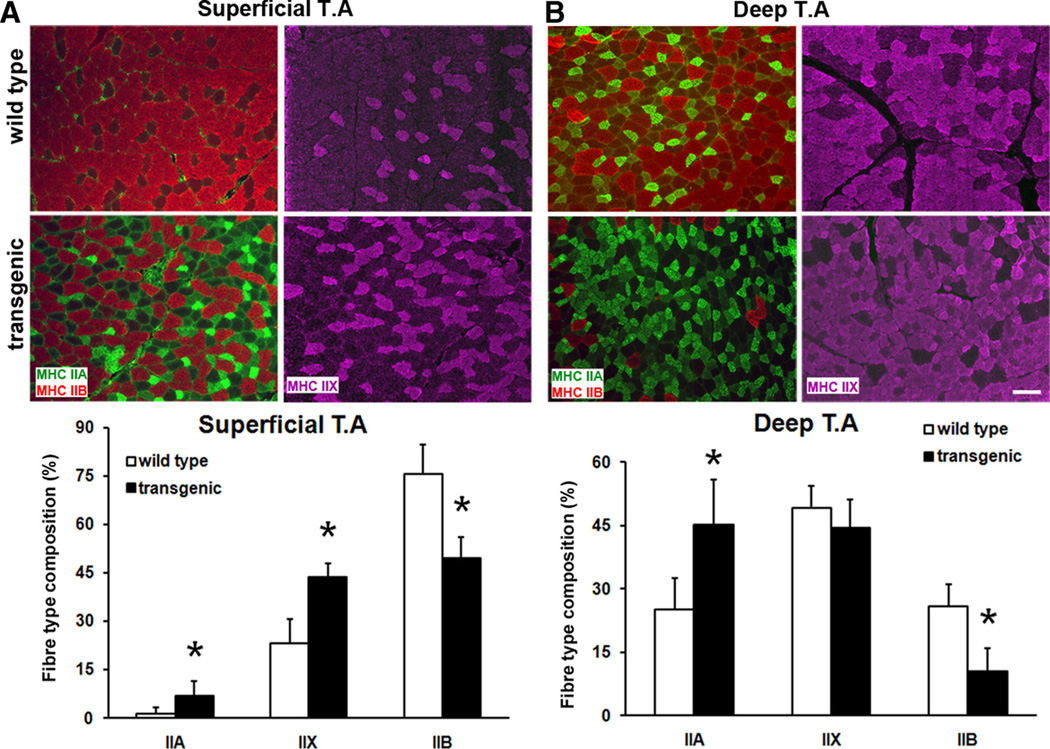
We and others previously showed that ERRγ transcribes a gene program encoding the metabolic and vascular features typical of oxidative myofibers.20,21 Therefore, in this study, we first examined the effects of ERRγ overexpression on changes in myofiber type and also on basal blood flow in the skeletal muscles. Skeletal muscle myofibers are classified into types I, IIA, IIX, and IIB based on the expression of myosin heavy chain isoforms. Therefore, fiber type analysis was performed by immunohistochemically staining skeletal muscles (isolated from wild-type and muscle-specific ERRγ-overexpressing transgenic mice20) for myosin heavy chain isoforms I, IIA, IIX, and IIB. We found that muscle-specific ERRγ overexpression triggered a muscle fiber type transformation in both the superficial (Figure 1A) and deep (Figure 1B) regions of TA in the transgenic mice. Specifically, the expression of the type IIA (stained green) and type IIX (stained purple) oxidative myofibers were increased, whereas the expression of type IIB (stained red) glycolytic myofibers was suppressed. The basal expression of type I myofibers was sparse in TA, and their expression was not further affected by ERRγ (data not shown). Similar changes also were observed in other muscles such as extensor digitorum longus and gastrocnemius (data not shown). Because soleus is a muscle that expresses significant levels of type I myofibers, we examined whether ERRγ has any effect on type I myofiber expression in this muscle. Type I myofibers remained unchanged in ERRγ overexpressing soleus (Online Figure I).
Figure 1. Regulation of muscle fiber type by estrogen-related receptor-γ (ERRγ).
A, Representative micrographs (upper panel) of superficial tibialis anterior (TA) muscle stained for type IIA (green), type IIB (red), and type IIX (purple) myofibers in wild-type and transgenic mice. Quantification of different fiber types (lower panel) in superficial TA (N=8 mice). B, Representative micrographs (upper panel) of deep TA stained for different myofibers. Quantification of different fiber types (lower panel) in deep TA (N=8 mice). *Statistically significant difference between wild-type and transgenic mice (P≤0.001, unpaired Student t test). Scale, 100 µm.
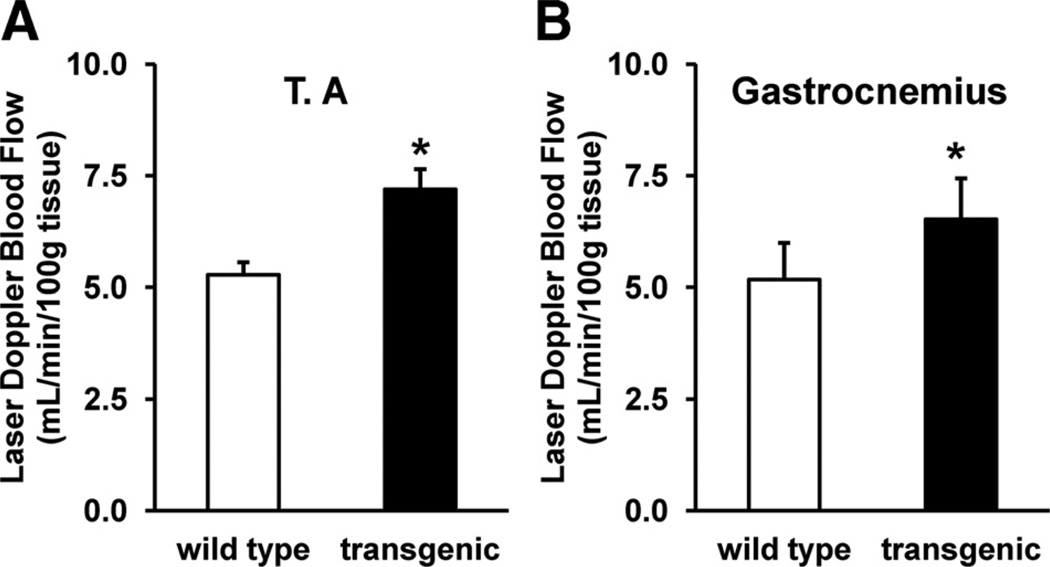
Because oxidative myofibers such as IIA and IIX are enriched in angiogenic factors, which can recruit vasculature and in turn increase blood flow,10–12,15 we measured the basal blood flow in the ERRγ-transformed muscle. Laser Doppler measurements in both the TA (Figure 2A) and gastrocnemius (Figure 2B) showed that the blood flow to the skeletal muscles is higher in the ERRγ transgenic compared to the wild-type muscles. These findings demonstrate that oxidative transformation of muscle by ERRγ involves increases in the proportions of type IIA and IIX myofibers with a concomitant decrease in type IIB myofibers. This ERRγ-mediated fiber type switch might be responsible for the ability of the receptors to improve blood flow to the skeletal muscle.
Figure 2. Regulation of basal blood flow by estrogen-related receptor-γ (ERRγ).
Basal blood flow in (A) tibialis anterior (TA) and (B) gastrocnemius was measured using laser Doppler deep tissue probe and presented as mL/min/100 g tissue (N=14 mice). *Statistically significant difference between wild-type and transgenic mice (P<0.001, unpaired Student t test).
ERRγ Rescues Ischemic Muscle Pathology
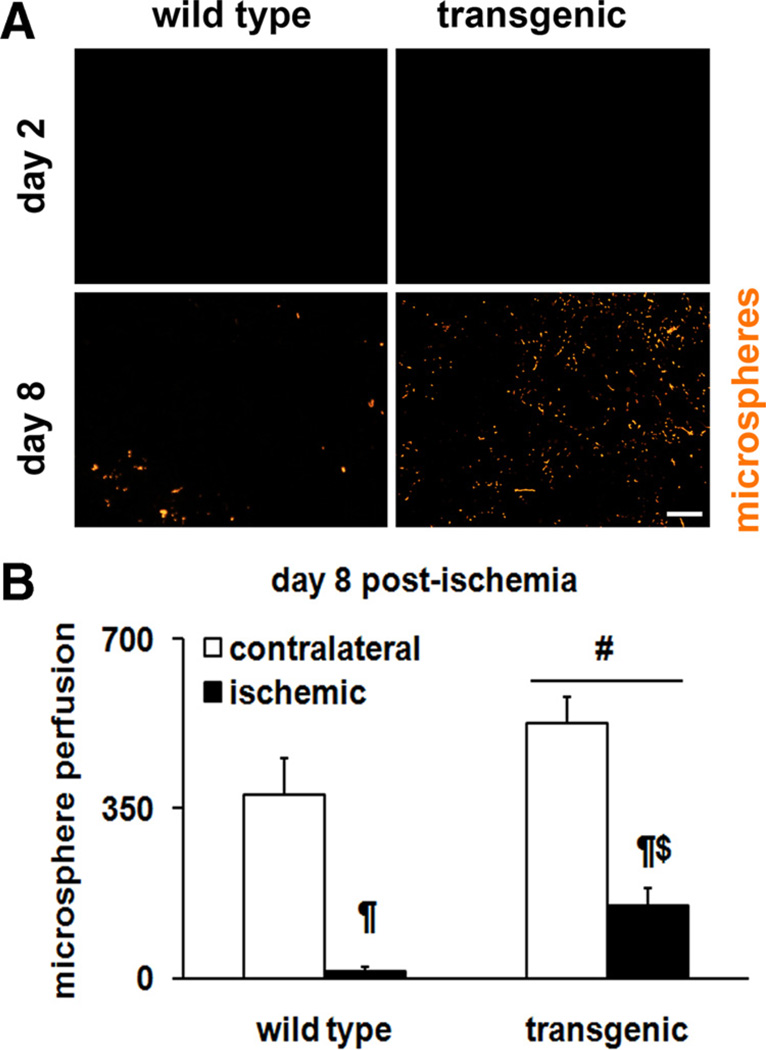
Because increasing oxidative and highly vascularized myofibers might be beneficial in skeletal muscle ischemia, we asked the question whether ERRγ can reverse ischemic muscle pathology. To study skeletal muscle ischemia, we used a murine model of unilateral hind limb vascular occlusion. Ischemia was surgically induced in the left hind limb, whereas the contralateral hind limb served as the control. This procedure was applied to both the muscle-specific ERRγ overexpresser and wild-type littermate mice to investigate the effects of the receptor on vascular recovery in the skeletal muscle. The day of surgery was considered as day 0. Vascular recovery was first measured by fluorescent microsphere angiography. Briefly, fluorescent microspheres were perfused by intracardiac route in both groups. Fluorescent microangiography in cryosections of TA at day 2 postsurgery showed that blood supply is completely blocked in ischemic muscles (Figure 3A) in both the wild-type and transgenic mice. In a similar examination of ischemic TA, but on day 8 postsurgery, we detected vascular recovery in the transgenic compared to wild-type littermate animals (Figure 3A, B). Microsphere perfusion in the contralateral nonischemic hind limbs of both the wild-type and transgenic mice are shown (Online Figure II).
Figure 3. Fluorescence microangiography in ischemic muscle.
A, Representative images for microsphere perfusion on day 2 (note absence of perfusion in both genotypes) and on day 8 in ischemic tibialis anterior (TA) cryosections from wild-type and transgenic mice. Scale, 100 µm. B, Quantification of microsphere perfusion in ischemic TA on day 8 postischemia (N=8 mice). #Statistically significant difference between wild-type and transgenic mice (P<0.001). ¶Statistically significant difference between contralateral and ischemic limb (P=0.001). $Significantly different from the ischemic wild-type limb (P=0.04).
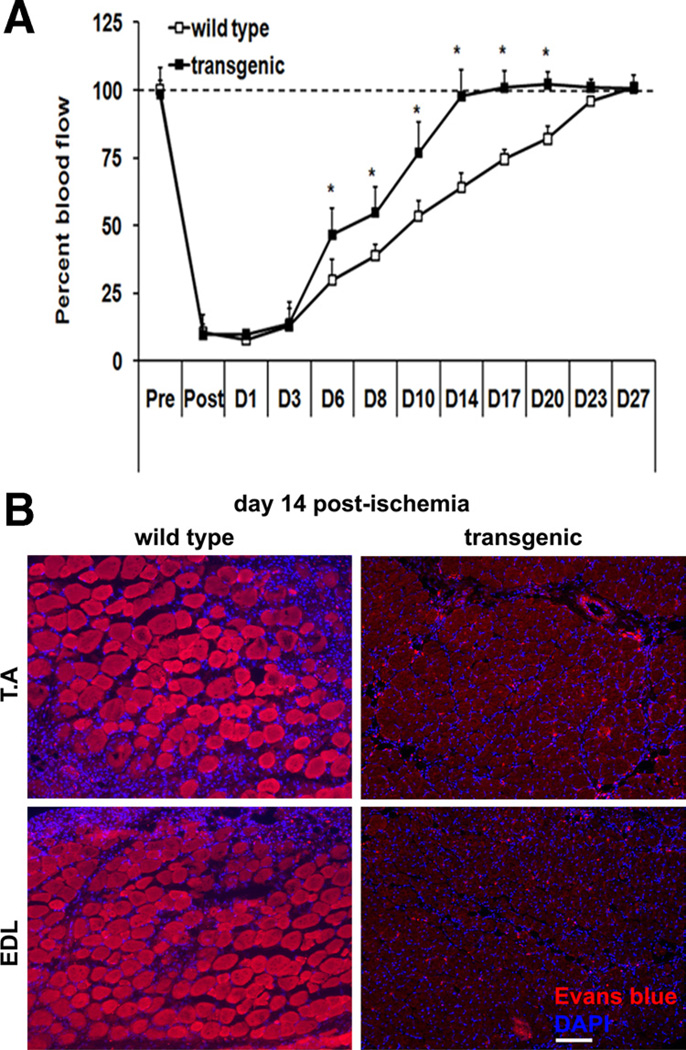
To obtain a physiological insight into revascularization of the ischemic muscle, we used a deep tissue laser Doppler probe to measure blood flow over the course of 4 weeks after surgery in TA from both the wild-type and the transgenic mice. In agreement with the lack of microsphere perfusion on day 2 postsurgery, blood flow to TA in the ischemic hind limb was 90% lower than that in contralateral nonischemic hind limb in both the wild-type and the transgenic mice (Figure 4A). This measurement further confirms that hind limb vascular occlusion has been equally applied to the wild-type and the transgenic mice. Starting on day 6, blood flow in the ischemic hind limbs returned to the normal levels. Strikingly, the blood flow measurements revealed an accelerated recovery in the ischemic TA from the transgenic mice compared to those from the wild-type mice. Although the blood flow in the ischemic TA from transgenic mice fully recovered within 14 days after surgery, the wild-type littermates required 23 to 27 days for the complete recovery of blood flow. Taken together, fluorescent microsphere angiography and laser Doppler blood flow measurements reveal an accelerated revascularization and restoration of blood perfusion in transgenic compared to wild-type ischemic muscles.
Figure 4. Time course (A) of blood perfusion.
Blood perfusion in both the ischemic and contralateral tibialis anterior (TA) was measured using laser Doppler probe. Blood perfusion in ischemic TA muscle is calculated as the percent of perfusion in the contralateral control muscle (N=14). *Statistically significant difference between wild-type and transgenic mice (P<0.001, two-way analysis of variance with repeated measurements on time). B, Representative immunofluorescent images depicting Evans blue dye staining in the ischemic TA and extensor digitorum longus (EDL) muscles from wild-type and transgenic mice on day 14 postischemia. Inclusion of Evans blue dye within a fiber is indicative of sarcolemmal damage and myofiber degeneration. Scale, 100 µm. Similar results were obtained from N=5 animals per group.
Does accelerated revascularization of ischemic muscle by ERRγ result in reversal of muscle damage? To answer this question, we measured the extent of skeletal muscle damage using Evans blue dye exclusion test. In this experiment, infiltration of the dye into the myofibers is indicative of skeletal muscle damage. As shown in Figure 4B, day 14 postischemic muscles from the wild-type mice shows extensive Evans blue dye infiltration demonstrating extensive muscle damage. In contrast, the day 14 postischemic muscles from the transgenic mice showed complete exclusion of Evans blue dye from the myofibers. This result shows that ERRγ-mediated revascularization of the skeletal muscle promotes a striking recovery from ischemic damage.
ERRγ Increases Neoangiogenesis in Ischemic Skeletal Muscle
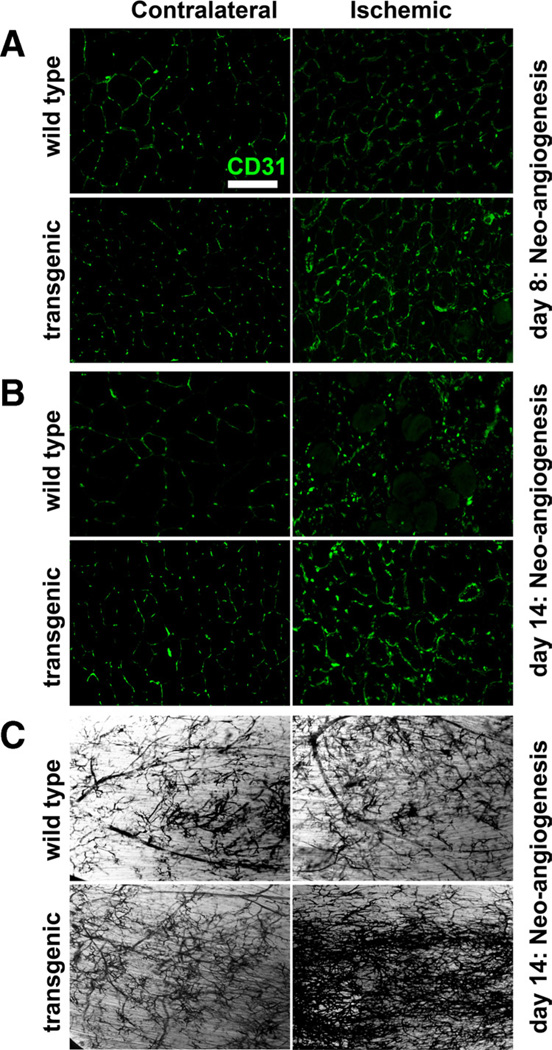
We determined whether the beneficial effects of ERRγ observed involve stimulation of neoangiogenesis by the receptor. First, we assessed neoangiogenesis in the TA on day 8 after the induction of hind limb ischemia as a function of CD31-positive capillary structures in both the contralateral and ischemic muscles (Figure 5A). In the wild-type ischemic TA, we did not detect any change in capillary structures compared to the nonischemic contralateral TA. However, we detected increase in the capillary structures in the ERRγ transgenic ischemic TA compared with the nonischemic contralateral TA. In similar measurements performed on day 14 (Figure 5B), we detected neoangiogenesis in the ischemic wild-type TA, which nevertheless remained comparatively higher in the transgenic ischemic TA.
Figure 5. Neoangiogenesis in the ischemic muscle.
Representative images for the CD31 stained capillaries in contralateral and ischemic tibialis anterior (TA) cryosections from wild-type and transgenic mice on day 8 (A) and day 14 (B) postischemia. Scale, 100 µm. C, Microfil pigment vascular mapping in whole-mounted TA muscles from wild-type and transgenic mice on day 14 postischemia indicating neoangiogenesis. Similar results were obtained from N=3 to 8 animals per group.
Neoangiogenesis in the TA was additionally determined using microfil perfusion and whole-mount visualization for vascular branching, as previously described.22 At day 14 postischemia, neoangiogenesis was modestly induced in the ischemic compared to nonischemic TA in the wild-type mice (Figure 5C, upper panel). At the same time point, we detected a tremendous increase in neoangiogenesis in the ischemic TA of the transgenic mice compared with the nonischemic TA (Figure 5C, lower panel). These findings demonstrate that ERRγ-induced revascularization involves neoangiogenesis.
Regulation of Angiogenic Factors by ERRγ
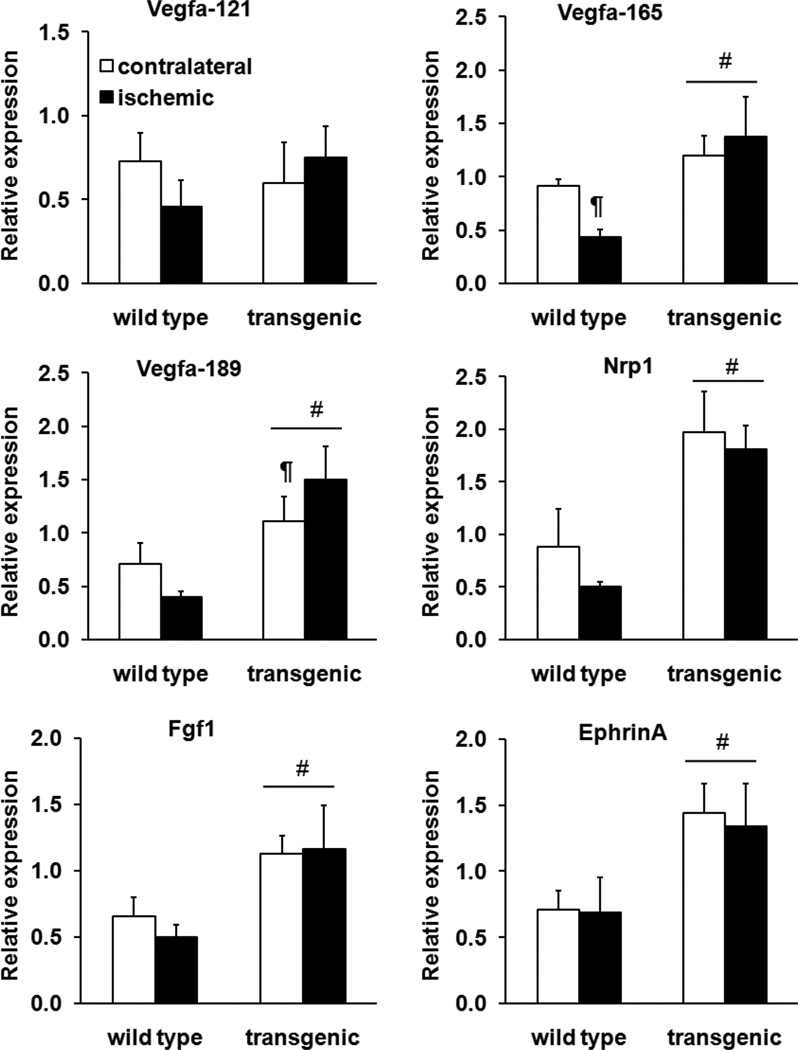
To identify the transcriptional bases of the reparative effects of ERRγ, we measured the expression of typical angiogenic factors24–26 in the contralateral and ischemic muscle from wild-type and transgenic mice. We found that the expression of most of these factors was substantially higher in TA of transgenic compared to wild-type mice (Figure 6) independent of ischemia. Among these genes, only Vegfa-189 was further induced by ischemia in the transgenic muscle. The gene expression pattern was also supported by protein analysis using an angiogenesis protein array panel (Online Figure III). We found that the angiogenic regulators that are typically induced during ischemic revascularization are already highly expressed in the transgenic muscle. Ischemia did not further increase the expression of these regulators in the transgenic skeletal muscle. Nevertheless, the ischemic induction of some of these angiogenic regulators was enhanced in the transgenic mice. To confirm this, we measured the accumulation of VEGFA protein in plantaris muscle from wild-type and transgenic mice by enzyme-linked immunosorbent assay. Transgenic plantaris had higher VEGFA levels compared with wild-type muscle in absence of ischemia (Online Figure IV). The VEGFA protein levels were further induced in the ischemic transgenic plantaris (Online Figure IV). These findings suggest that generally higher levels of angiogenic transcripts achieved in the ERRγ transgenic muscle might afford sustained protein levels of the angiokines in ischemia, thus accelerating vascular repair. However, the ERRγ transcriptional program, as such, may not be further enhanced under ischemic conditions.
Figure 6. Angiogenic gene expression in ischemia.
Relative expression of angiogenic genes on day 8 postischemia (two-way analysis of variance). #P<0.001, significantly different from wild-type. ¶P<0.05, significantly different from transgenic contralateral limb. N=8 mice per genotype.
ERRγ Is Dispensable for Hypoxic Response in Skeletal Muscles
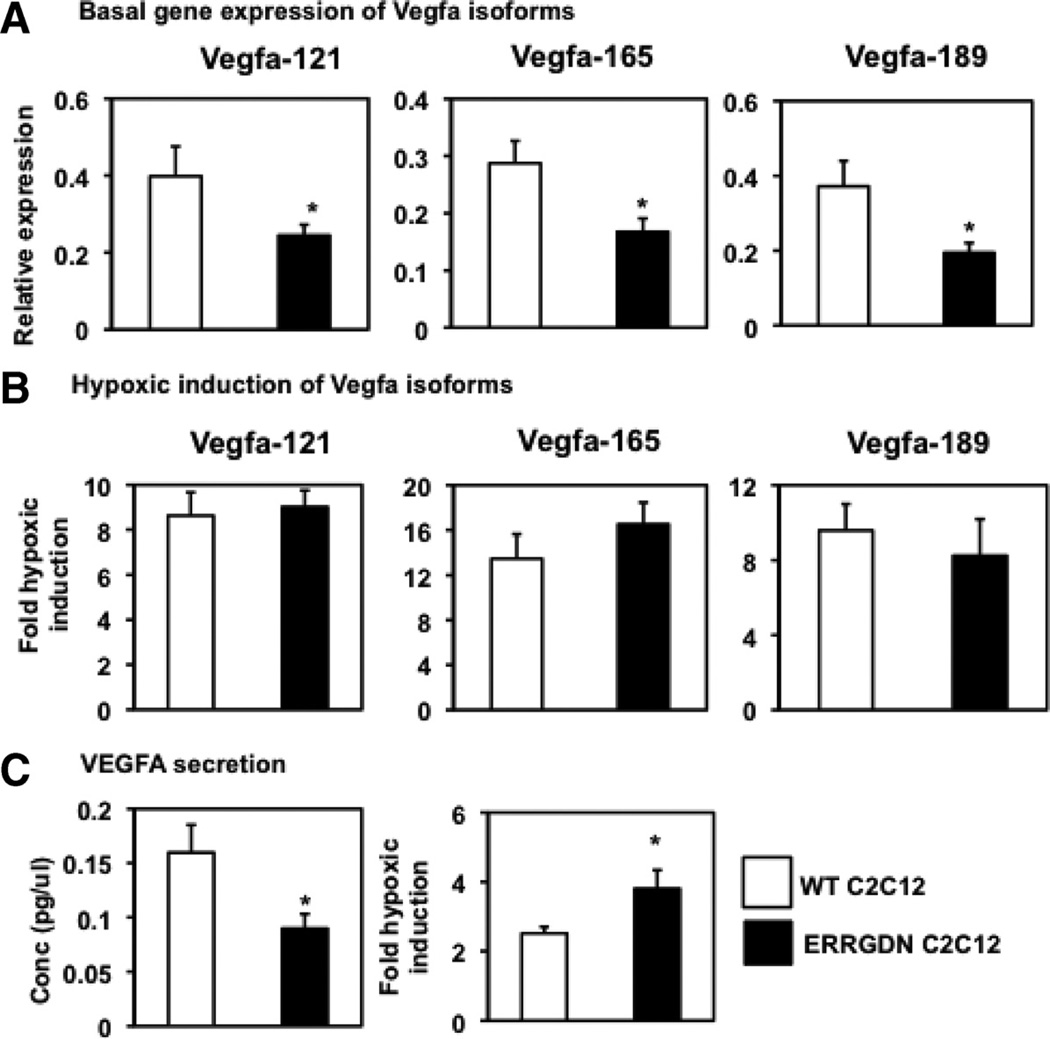
To further define the regulation of angiogenic genes by ERRγ and to determine a potential role for the receptor in hypoxic response typical in ischemia, we used cultured muscle cells subjected to normoxic or hypoxic conditions. We generated stable muscle C2C12 cells overexpressing either control vector (WT C2C12) or ERRGDN (ERRGDN C2C12), and focused on hypoxic regulation of Vegfa induction and secretion in these cells.27 The ERRGDN is a mutant form lacking the AF2 domain, which blocks the transcriptional activity of the endogenously expressed receptor. In these cells, we found that blocking ERRγ signaling in C2C12 cells suppresses the basal gene expression of Vegfa isoforms such as Vegfa-121, Vegfa-165, and Vegfa-189 (Figure 7A). The Vegfa gene expression was induced in WT C2C12 cells subjected to 24 hours of hypoxia. Despite the observed ameliorative effect of ERRγ in ischemic muscle, we found that hypoxic induction of Vegfa gene expression in ERRGDN C2C12 cells was comparable to that in WT C2C12 cells (Figure 7B). Similarly, although ERRGDN overexpression suppressed basal Vegfa secretion, it did not affect hypoxic increase in the angiokine secretion in C2C12 cells (Figure 7C). In alternative experiments, endogenous ERRγ expression was knocked down in primary muscle cells, as we previously described.20 Similar to the ERRGDN studies, siRNA knockdown of ERRγ resulted in downregulation of Vegfa isoform expression. Knockdown of the receptor, however, did not affect the hypoxic response of Vegfa isoform induction in primary muscle cells (Online Figure V).
Figure 7. Hypoxic response in C2C12 cells.
Measurements were made in wild-type (open bars) and dominant-negative estrogen-related receptor-γ (ERRGDN) overexpressing (black bars) C2C12 cells. A, Basal expression of Vegfa121, Vegfa165, and Vegfa189 genes in C2C12 cells. B, Fold induction of Vegfa121, Vegfa165, and Vegfa189 gene expression in C2C12 cells by 24 hours of hypoxia (N=4 per group). C, Basal levels of Vegfa secretion by C2C12 cells in culture medium and fold induction in Vegfa secretion by C2C12 cells subjected to 24 hours of hypoxia. Values are represented as mean±standard deviation from n=4 experiments. *Statistically significant difference between WT C2C12 and ERRGDN C2C12 cells (P<0.05, unpaired Student t test).
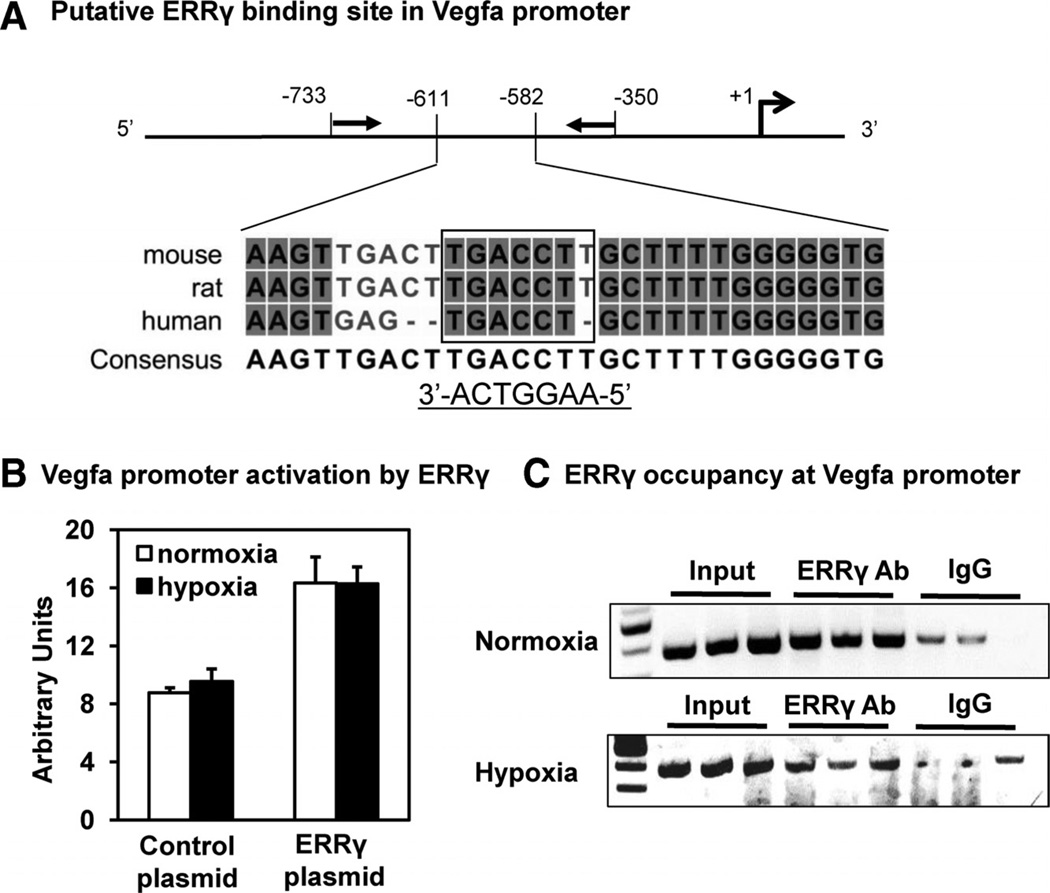
To achieve mechanistic insight into transcriptional regulation of Vegfa by ERRγ in normoxia and hypoxia, we performed reporter gene assay and chromatin immunoprecipitation. A conserved ERRγ binding site has been identified in the promoter of Vegfa gene (Figure 8A).16,20 As we previously showed,20 ERRγ transcriptionally activated a luciferase reporter driven by the Vegfa promoter under normoxic conditions (Figure 8B). This transcriptional activation of the Vegfa promoter by ERRγ was not further affected by hypoxia in ERRγ-transfected HEK 293T cells. Chromatin immunoprecipitation using primers flanking the conserved ERRγ binding site in the Vegfa promoter (Figure 8A) revealed that the receptor similarly occupies the predicted site under both normoxia and hypoxia (Figure 8C). Collectively, these results exclude a role for ERRγ in hypoxic response of skeletal muscle cells, at least in terms of Vegfa regulation. Further, ERRγ induces the angiogenic genes such as Vegfa by direct promoter occupancy in a hypoxia-independent fashion.
Figure 8. Estrogen-related receptor-γ (ERRγ) activation of the Vegfa promoter.
A, Multiple sequence alignment of mouse, rat, and human Vegfa promoter regions (from −582 to −611 bps) containing ERRγ binding site (box; 5′-TGACCT-3′ and 3′-ACTGGAA-5′). Highly conserved nucleotides are shaded. A consensus sequence is also shown at the bottom. Closed arrows show the locations of polymerase chain reaction primers flanking the conserved site used in chromatin immunoprecipitation polymerase chain reaction. Open arrow shows transcription start site (+1). The map is not drawn to scale. B, Luciferase reporter assay showing activation of the Vegfa promoter by ERRγ in transfected 293T cells subjected to normoxia or hypoxia for 24 hours. C, Chromatin immunoprecipitation polymerase chain reaction demonstrating ERRγ occupancy at the conserved binding site in promoter region of the endogenous Vegfa gene in differentiated C2C12 cells overexpressing the receptor under normoxia and hypoxia.
In summary, we show that the overexpression of ERRγ facilitates vascular recovery and reperfusion in ischemic skeletal muscle. This effect of ERRγ is linked to its ability to remodel the muscle fiber type to one expressing high and sustained levels of proangiogenic factors that can facilitate neoangiogenesis in skeletal muscle ischemia.
Discussion
Despite its prevalence, there is currently no effective noninvasive treatment for skeletal muscle ischemia, warranting discovery of newer pathways or strategies to reverse ischemia. One potential treatment strategy might be to increase the proportion of oxidative myofibers that inherently express higher levels of angiogenic factors and therefore recruit more blood vessels. Here, we show that nuclear hormone receptor ERRγ increases type IIA and IIX oxidative myofibers and enhances basal blood flow to the skeletal muscle. This ERRγ-mediated remodeling, by virtue of higher myofiber angiokine expression, enhances the ability of the skeletal muscle to mount reparative neoangiogenesis, revascularization, and rapid reperfusion of ischemic tissue. This ERRγ-mediated vascular recovery also promotes recovery from ischemic muscle damage. Quite surprisingly, however, we found muscle ERRγ to be dispensable for hypoxic response in the skeletal muscle. Therefore, ERRγ is a hypoxia-independent inducer of neoangiogenesis and revascularization that can reverse muscle ischemia.
Myofibers such as types I, IIA, and IIX, which are characteristically oxidative, express higher levels of angiogenic factors, are decorated with a rich network of capillaries, and promptly revascularize in ischemic conditions.10–12,15 Moreover, interventions such as exercise that increase the proportions of oxidative myofibers also promote vascularization of skeletal muscle.28–30 However, specific targeting of transcriptional regulators of myofiber type as therapeutic strategy in muscle ischemia has not been fully explored. We recently described that orphan nuclear receptor ERRγ drives a transcriptional program that would typically encode highly oxidative and vascularized myofibers,20 raising the exciting possibility that the receptor increases the proportions of type I, IIA, or IIX oxidative myofibers in the skeletal muscle and, in turn, promotes revascularization during ischemia. To test this, in the current study, we first subjected the skeletal muscles from the previously described20 muscle-specific ERRγ overexpresser mice and wild-type littermates to systematic immunohistological evaluation of the muscle fiber type. This examination revealed that ERRγ robustly remodels the skeletal muscle to an oxidative phenotype by increasing type IIA and IIX myofibers in a predominantly fast-twitch and typically glycolytic muscles. It should be noted that a complete fiber type shift of a muscle to type I, although anticipated from our previous genetic study,20 did not occur, because such hind limb muscles predominantly expressing type I fibers are few (eg, the soleus). The ERRγ-mediated oxidative remodeling takes places at the expense of type IIB fibers, because we observed a significant downregulation of type IIB glycolytic myofibers. As mentioned, oxidative myofibers such as types I, IIA, and IIX are rich in angiokines and highly vascularized compared to glycolytic type IIB myofibers. In agreement, we found that ERRγ transformation of myofiber type resulted in higher protein accumulation of angiogenic factors such as VEGFA, as well as a basal increase in blood flow in the transgenic compared to wildtype muscles. In the context of our findings, it is of interest that two known nuclear receptor coactivators, PGC-1α and PGC-1β, also have been recently shown to promote oxidative fiber type remodeling and angiogenesis in the skeletal muscle. 16,31 These coactivators also activate ERRγ;32,33 consequently, some of their “metabovascular” effects in the skeletal muscle might be mediated by this receptor. Along the same lines and speculatively, corepressors of ERRγ, such as RIP140, which are negative regulators of oxidative myogenesis, perhaps may repress neoangiogenesis.34,35 Our results in collaboration with the aforementioned reports on nuclear receptor coactivators of ERRγ strengthen the notion that direct targeting of transcriptional regulators that increase oxidative myofibers also can enhance vascularization and, hence, blood flow to the skeletal muscle.
To test the potential reparative role of ERRγ in muscle ischemia, we used a murine model of hind limb vascular occlusion, which has been used preclinically to identify signaling pathways/mechanisms that can accelerate and decelerate revascularization of ischemic muscle.23,36–39 On application of hind limb femoral occlusion to wild-type and ERRγ transgenic mice, we found that recovery of blood perfusion was accelerated in the ischemic muscle overexpressing ERRγ in comparison to the wild-type ischemic muscle. We propose that this rapid reperfusion is linked to ERRγ-facilitated neoangiogenesis that results in formation of new functional blood vessels in the ischemic muscle. This proposition is based on the following observations. First, an increase in CD31-positive capillary density (a measure of neoangiogenesis40) was observed in ischemic compared with the contralateral muscle as early as 8 days after the induction of ischemia in transgenic mice. However, at the same time point, there was no major change in the ischemic muscles of the wild-type mice. Second, microfil perfusion showed enhanced vascular branching in ischemic transgenic compared with either ischemic wild-type or transgenic contralateral muscle. Third, fluorescent microangiography revealed extensive perfusion of fluorescent microspheres in the transgenic compared with wild-type ischemic skeletal muscles. Because the microfil as well as microspheres are impermeable through intact vessel walls, these observations indicate formation of new functional and nonleaky vessels in the transgenic ischemic skeletal muscle. Importantly, these ERRγ-induced changes rescued ischemic myofiber phenotype.
A potent stimulant of revascularization in the ischemic tissue is low oxygen tension (hypoxia). Hypoxia induces revascularization via the HIF1α–Vegfa pathway.41 Interestingly, members of the ERR subfamily participate in HIF-induced transcription in cultured cells via protein–protein interaction.42 Therefore, we sought to determine whether ERRγ mediates the typical angiogenic response of the skeletal muscle to hypoxia, particularly focusing on Vegfa activation. Our cell culture data indicate that hypoxic induction of Vegfa gene expression and protein secretion were comparable in both the wild-type C2C12 cells and the ones expressing ERRGDN. Alternative inactivation of ERRγ using siRNA knockdown also yielded similar results. Therefore, ERRγ may not be involved in hypoxia-driven angiokine induction. It should be noted that the lack of effect of ERRγ inactivation on hypoxic response might be linked to compensation by other ERR isoforms, ERRα and ERRβ. In this context, blockage of total ERR activity might still affect the hypoxic response. Nevertheless, our hypothesis regarding ERRγ is mechanistically strengthened by the observation that ERRγ activates Vegfa promoter by occupying a conserved binding site independent of hypoxia. Additionally, in vivo support is obtained from our gene expression data in which the expression of several angiogenic factors are generally upregulated in the ERRγ overexpressing skeletal muscle independent of ischemia, as would be expected by oxidative transformation of the muscle. Consequently, the therapeutic effect of ERRγ in ischemic muscle might be linked to the programming of muscle cells to a fiber type primed to express and secrete more angiogenic factors rather than to its direct involvement in hypoxic pathway. We perceive that the hypoxia-independent reparative angiogenesis by ERRγ has potential implication for muscle ischemia, particularly in diabetic or older patients in whom activation of the Hif1α–Vegfa pathway is impaired.43,44
Skeletal muscle ischemia is prevalent in cardiovascular and metabolic disorders,45 but pharmaceutical treatment for ischemia is underdeveloped because of incomplete understanding of vasculogenic molecular pathways in the skeletal muscle, especially those that are drug-targetable. In our study, we demonstrate that nuclear receptor ERRγ is one such “master switch” that enhances the intrinsic ability of the skeletal muscles to revascularize in response to ischemia by remodeling the myofibers to one that expresses high levels of secretable angiogenic factors. Nuclear hormone receptors such as ERRγ are specialized transcriptional factors in a sense that each one of these receptors contains a unique ligand binding pocket, which can bind selective hormones or synthetic drugs that can, in turn, regulate the receptor transcriptional activity.46 Consequently, nuclear receptors have proved to be excellent pharmaceutical targets. Whereas the endogenous hormone that activates ERRγ is yet unknown and the ligand chemistry is still in its infancy, recent reports have emerged showing that the transcriptional activity of this receptor can be pharmacologically regulated by certain synthetic drugs.47–49 Therefore, in light of our findings that ERRγ can promote neoangiogenesis and revascularization, it is potentially an excellent target for treating skeletal muscle ischemia with small molecules targeting the receptor. It is noteworthy that ERRγ is also highly expressed in heart, brain, and kidney,19,50,51 which are highly vascularized organs that are often prone to ischemia leading to myopathy, stroke, and renal failure, respectively. ERRγ may also prove to be an excellent antiischemic target in these organs.
In summary, we show that ERRγ remodels the skeletal muscle to increase the proportion of oxidative myofibers that express high levels of angiogenic factors and increases basal blood flow. This remodeling by ERRγ promotes rapid neoangiogenesis, revascularization, and reperfusion of ischemic skeletal muscle, a phenomenon that is hypoxia-independent.
Supplementary Material
Novelty and Significance.
What Is Known?
Ischemic skeletal muscle damage occurs because angiogenesis induced by the activation of the classical hypoxia-dependent Hif1α pathway during chronic vascular occlusion is insufficient to overcome the overall deficit in tissue perfusion.
Therapeutic approaches with individual factors such as Vegfa, although successful in preclinical models, have so far failed in human clinical trials.
A molecular strategy to enhance the inherent ability of skeletal muscle to trigger a comprehensive angiogenic program, such as by increasing key angiogenic factors and densely vascularized myofibers, might benefit ischemic recovery.
What New Information Does This Article Contribute?
Estrogen-related receptor-γ (ERRγ) increases the proportion of highly vascularized myofibers (types IIa and IIX) and suppresses the expression of sparsely vascularized myofibers (type IIb), thus increasing the overall blood perfusion in the normoxic skeletal muscle.
ERRγ induces functional neoangiogenesis, rapid reperfusion, and tissue repair in ischemic skeletal muscle.
ERRγ is dispensable for a typical hypoxic response; instead, its reparative effects are linked to transcriptional remodeling to a muscle type with enhanced angiokine expression.
Skeletal muscle ischemia is a prevalent problem in diseases such as obesity, diabetes, and atherosclerosis. Yet, few pharmaceutical strategies have been developed to treat this skeletal muscle ischemia. In this study, we show that transgenic activation of nuclear receptor ERRγ in the skeletal muscle enhanced neoangiogenesis and revascularization. It also promoted muscle repair during ischemic damage. Despite this reparative effect and previously known interaction with Hif1α, the ERRγ pathway is not required for a typical hypoxic response in the skeletal muscle. Therefore, ERRγ is a hypoxia-independent transcriptional factor that can rescue ischemic muscle damage. Notably, nuclear receptors–including ERRγ–contain evolutionarily conserved ligand-binding domains that can be targeted to regulate transcriptional activity. Our study highlights the potential for synthetically targeting ERRγ for treating ischemic damage in muscle, and potentially in other organs susceptible to ischemia, such as heart, kidney, and brain, in which ERRγ is highly expressed.
Acknowledgments
The authors thank Dr Mark Entman for assistance with the hind limb ischemia model, Dr Jarek Aronowski for assistance with laser Doppler measurements, and Drs Perry Bickel and Ali Marian for critically reading the manuscript.
Sources of Funding
This study was supported by UTHealth intramural funds, American Heart Association, and Muscular Dystrophy Association.
Nonstandard Abbreviations and Acronyms
- ERRγ
estrogen-related receptor-γ
- ERRGDN
dominant-negative estrogen-related receptor-γ
- TA
tibialis anterior
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.112.266478/-/DC1.
Disclosures
None.
References
- 1.Varu VN, Hogg ME, Kibbe MR. Critical limb ischemia. J Vasc Surg. 2010;51:230–241. doi: 10.1016/j.jvs.2009.08.073. [DOI] [PubMed] [Google Scholar]
- 2.Chi YW, Osinbowale O, Milani R. Genetic association studies in peripheral arterial disease. J La State Med Soc. 2011;163:30–39. [PubMed] [Google Scholar]
- 3.Baumgartner I, Schainfeld R, Graziani L. Management of peripheral vascular disease. Annu Rev Med. 2005;56:249–272. doi: 10.1146/annurev.med.56.082103.104649. [DOI] [PubMed] [Google Scholar]
- 4.Conrad MF, Crawford RS, Hackney LA, Paruchuri V, Abularrage CJ, Patel VI, Lamuraglia GM, Cambria RP. Endovascular management of patients with critical limb ischemia. J Vasc Surg. 2011;53:1020–1025. doi: 10.1016/j.jvs.2010.10.088. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Hong A, Schulten H, Post MJ. Update on therapeutic neovascularization. Cardiovasc Res. 2005;65:639–648. doi: 10.1016/j.cardiores.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 6.van Weel V, van Tongeren RB, van Hinsbergh VW, van Bockel JH, Quax PH. Vascular growth in ischemic limbs: A review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg. 2008;22:582–597. doi: 10.1016/j.avsg.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–871. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- 8.Lekas M, Lekas P, Latter DA, Kutryk MB, Stewart DJ. Growth factor-induced therapeutic neovascularization for ischaemic vascular disease: Time for a re-evaluation? Curr Opin Cardiol. 2006;21:376–384. doi: 10.1097/01.hco.0000231409.69307.d2. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am J Physiol. 1998;274:H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 11.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 12.Ripoll E, Sillau AH, Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch. 1979;380:153–158. doi: 10.1007/BF00582151. [DOI] [PubMed] [Google Scholar]
- 13.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- 14.Yang HT, Prior BM, Lloyd PG, Taylor JC, Li Z, Laughlin MH, Terjung RL. Training-induced vascular adaptations to ischemic muscle. J Physiol Pharmacol. 2008;59(Suppl 7):57–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Cherwek DH, Hopkins MB, Thompson MJ, Annex BH, Taylor DA. Fiber type-specific differential expression of angiogenic factors in response to chronic hindlimb ischemia. Am J Physiol Heart Circ Physiol. 2000;279:H932–H938. doi: 10.1152/ajpheart.2000.279.3.H932. [DOI] [PubMed] [Google Scholar]
- 16.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 17.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudel C, Schwartz C, Giordano C, Abumrad NA, Grimaldi PA. Pharmacological activation of PPARbeta promotes rapid and calcineurindependent fiber remodeling and angiogenesis in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E297–E304. doi: 10.1152/ajpendo.00581.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giguere V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 20.Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM. Exercise and PGC- 1alpha-independent synchronization of type i muscle metabolism and vasculature by ERRgamma. Cell Metab. 2011;13:283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangwala SM, Wang X, Calvo JA, Lindsley L, Zhang Y, Deyneko G, Beaulieu V, Gao J, Turner G, Markovits J. Estrogen-related receptor gamma is a key regulator of muscle mitochondrial activity and oxidative capacity. J Biol Chem. 2010;285:22619–22629. doi: 10.1074/jbc.M110.125401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4:1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 23.Bonauer A, Carmona G, Iwasaki M, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 24.Hershey JC, Corcoran HA, Baskin EP, Gilberto DB, Mao X, Thomas KA, Cook JJ. Enhanced hindlimb collateralization induced by acidic fibroblast growth factor is dependent upon femoral artery extraction. Cardiovasc Res. 2003;59:997–1005. doi: 10.1016/s0008-6363(03)00522-4. [DOI] [PubMed] [Google Scholar]
- 25.Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert- Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 26.Uniewicz KA, Cross MJ, Fernig DG. Exogenous recombinant dimeric neuropilin-1 is sufficient to drive angiogenesis. J Biol Chem. 2011;286:12–23. doi: 10.1074/jbc.M110.190801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehne N, Kerkweg U, Otto T, Fandrey J. The HIF-1 response to simulated ischemia in mouse skeletal muscle cells neither enhances glycolysis nor prevents myotube cell death. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1693–R1701. doi: 10.1152/ajpregu.00892.2006. [DOI] [PubMed] [Google Scholar]
- 28.Barak S, Stopka CB, Archer Martinez C, Carmeli E. Benefits of lowintensity pain-free treadmill exercise on functional capacity of individuals presenting with intermittent claudication due to peripheral arterial disease. Angiology. 2009;60:477–486. doi: 10.1177/0003319708322388. [DOI] [PubMed] [Google Scholar]
- 29.Pena KE, Stopka CB, Barak S, Gertner HR, Jr, Carmeli E. Effects of low-intensity exercise on patients with peripheral artery disease. Phys Sportsmed. 2009;37:106–110. doi: 10.3810/psm.2009.04.1689. [DOI] [PubMed] [Google Scholar]
- 30.Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–C1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 31.Rowe GC, Jang C, Patten IS, Arany Z. PGC-1beta regulates angiogenesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E155–E163. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hentschke M, Susens U, Borgmeyer U. PGC-1 and PERC, coactivators of the estrogen receptor-related receptor gamma. Biochem Biophys Res Commun. 2002;299:872–879. doi: 10.1016/s0006-291x(02)02753-5. [DOI] [PubMed] [Google Scholar]
- 33.Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 34.Seth A, Steel JH, Nichol D, Pocock V, Kumaran MK, Fritah A, Mobberley M, Ryder TA, Rowlerson A, Scott J, Poutanen M, White R, Parker M. The transcriptional corepressor RIP140 regulates oxidative metabolism in skeletal muscle. Cell Metab. 2007;6:236–245. doi: 10.1016/j.cmet.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castet A, Herledan A, Bonnet S, Jalaguier S, Vanacker JM, Cavailles V. Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol Endocrinol. 2006;20:1035–1047. doi: 10.1210/me.2005-0227. [DOI] [PubMed] [Google Scholar]
- 36.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi K, Ouchi N, Higuchi A, Shaw RJ, Walsh K. LKB1 deficiency in Tie2-Cre-expressing cells impairs ischemia-induced angiogenesis. J Biol Chem. 2010;285:22291–22298. doi: 10.1074/jbc.M110.123794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol. 2009;29:3487–3499. doi: 10.1128/MCB.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–32811. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al Haj Zen A, Oikawa A, Bazan-Peregrino M, Meloni M, Emanueli C, Madeddu P. Inhibition of delta-like-4-mediated signaling impairs reparative angiogenesis after ischemia. Circ Res. 2010;107:283–293. doi: 10.1161/CIRCRESAHA.110.221663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semenza GL. Hydroxylation of hif-1: Oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 42.Ao A, Wang H, Kamarajugadda S, Lu J. Involvement of estrogen-related receptors in transcriptional response to hypoxia and growth of solid tumors. Proc Natl Acad Sci U S A. 2008;105:7821–7826. doi: 10.1073/pnas.0711677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bento CF, Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54:1946–1956. doi: 10.1007/s00125-011-2191-8. [DOI] [PubMed] [Google Scholar]
- 44.Semenza GL. Regulation of vascularization by hypoxia-inducible factor 1. Ann N Y Acad Sci. 2009;1177:2–8. doi: 10.1111/j.1749-6632.2009.05032.x. [DOI] [PubMed] [Google Scholar]
- 45.Sneider EB, Nowicki PT, Messina LM. Regenerative medicine in the treatment of peripheral arterial disease. J Cell Biochem. 2009;108:753–761. doi: 10.1002/jcb.22315. [DOI] [PubMed] [Google Scholar]
- 46.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 47.Chao EY, Collins JL, Gaillard S, Miller AB, Wang L, Orband-Miller LA, Nolte RT, McDonnell DP, Willson TM, Zuercher WJ. Structure-guided synthesis of tamoxifen analogs with improved selectivity for the orphan ERRgamma. Bioorg Med Chem Lett. 2006;16:821–824. doi: 10.1016/j.bmcl.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci U S A. 2001;98:8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuercher WJ, Gaillard S, Orband-Miller LA, Chao EY, Shearer BG, Jones DG, Miller AB, Collins JL, McDonnell DP, Willson TM. Identification and structure-activity relationship of phenolic acyl hydrazones as selective agonists for the estrogen-related orphan nuclear receptors ERRbeta and ERRgamma. J Med Chem. 2005;48:3107–3109. doi: 10.1021/jm050161j. [DOI] [PubMed] [Google Scholar]
- 50.Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRrgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: Tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- 51.Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274:22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.