Abstract
The size and surface area of the mammalian brain are thought to be critical determinants of intellectual ability. Recent studies show that development of the gyrated human neocortex involves a lineage of neural stem and transit-amplifying cells that forms the outer subventricular zone (OSVZ), a proliferative region outside the ventricular epithelium. We discuss how proliferation of cells within the OSVZ expands the neocortex by increasing neuron number and modifying the trajectory of migrating neurons. Relating these features to other mammalian species and known molecular regulators of the mouse neocortex suggests how this developmental process could have emerged in evolution.
Introduction
Evolution of the neocortex in mammals is considered to be a key advance that enabled higher cognitive function. However, neo-cortices of different mammalian species vary widely in shape, size, and neuron number (reviewed by Herculano-Houzel, 2009). These differences are presumably reflected in the organization and behavior of neural progenitor cells during embryonic development. Recent models of neocortical development have largely been based on cellular and molecular studies of the mouse and rat, whose neocortex exhibits many of the key features general to all mammals, including a six-layered organization and regionalization into sensory, motor, and association areas. However, because the rodent neocortex is small and nonfolded (lissencephalic), its ability to model or illuminate the developmental mechanisms of larger and highly folded (gyrencephalic) neocortex, such as that of Homo sapiens, is inherently limited.
Because the fossil record for soft tissues such as the brain is severely restricted, efforts to understand the evolution of the neocortex at a cellular level have been limited to comparisons of living species—an approach known as evo-devo (Gould, 1977). This approach assumes that related biological systems share inherent functional modularity, such that a small number of evolutionary changes to key regulators of these modules, if heritable and beneficial, can be positively selected and have major consequences for the species. Observed differences in the mechanisms of brain development across species may therefore be evolutionary variations of the same ancestral mechanisms.
Although our understanding of mouse development exceeds that of other species, recent observations of the developing neocortex in humans, nonhuman primates, carnivores, and marsupials begin to reveal how differences in neural progenitor cell populations can result in neocortices of variable size and shape. Increases in neocortical volume and surface area, particularly in the human, are related to the expansion of progenitor cells in the outer subventricular zone (OSVZ) during development (Smart et al., 2002; Hansen et al., 2010; Fietz et al., 2010). Therefore, it is of increasing importance to place these developmental mechanisms in the broader context of neocortical evolution and explore how features of human neocortical development could be explained by changes to known molecular regulators of the developing rodent neocortex.
Following an introduction to previous models of neocortical expansion, this Review will focus on how the observed proliferation of stem and transit-amplifying cells in the OSVZ functions to increase neuronal number and surface area of the neocortex. We hypothesize that OSVZ proliferation is contingent on evolutionary changes that permit the generation and maintenance of neural progenitor cells outside of the ventricular epithelium and look toward molecular studies of the rodent to understand how the intracellular signaling state of neural progenitor cells could be recapitulated in the OSVZ. Finally, we will compare OSVZ proliferation in different species and explore how the degree of OSVZ proliferation may be tuned to give rise to diversity in brain size and shape.
Historical Perspective
An appreciation that the structure and organization of the mature neocortex depends on proliferation of cells lining the ventricle of the neural tube began with the work of Wilhelm His, who from observations of the developing human neocortex, asserted several key principles: (1) that germinal cells divide rapidly in the ventricular epithelium, (2) that immature neuroblasts migrate from inner to outer zones, and (3) that a cell population known as spongioblasts forms a syncytium through which the neuroblasts migrate (Bentivoglio and Mazzarello, 1999). The subsequent work of Magini, Cajal, and Golgi collectively indicated that these spongioblasts near the ventricle were intermixed with nonradial “germinal” cells and substantiated the concept that these two cell types were lineally distinct, giving rise only to glia or neurons, respectively (Bentivoglio and Mazzarello, 1999). These spongioblasts, now universally known as radial glia (RG) (Rakic, 1971), have cell bodies situated near the ventricle and long fibers that extend to the pial surface. Through the observation of migrating neurons intimately apposed to these fibers, RG are now appreciated as the scaffolds for radial neuronal migration (Rakic, 1971, 1972).
The Radial Unit Hypothesis
Studies by Pasko Rakic that utilized a wide range of techniques to describe the development of monkey neocortex culminated in the influential concept known as the radial unit hypothesis (Rakic, 1988). This scheme formally integrated a number of key observations, proposing that: (1) the neuronal output of proliferative units (at the ventricle) is translated by the fiber guides of RG to the expanded cortex in the form of ontogenetic columns and (2) the proto-map formed by these proliferative units could be influenced by thalamic inputs and define cortical areas of variable size, cellular composition, and function. This provided a framework for the mechanics of neocortical development in the radial dimension, where neurons of the same ontogeny (progeny of a given ventricular neuronal progenitor) would tend to migrate on a continuous fiber to the cortical plate, form a radial column of cells with related function, and project a ventricular proto-map onto the developing cortex.
Neurogenesis by Radial Glia
The radial unit hypothesis described the events of neocortical development only in general terms and did not assume any lineage relationship between RG and neuronal progenitor cells in the ventricular zone (VZ). This conceptual separation was not resolved until a decade ago, as even modern studies reported that the primate VZ contains a heterogeneous population of progenitor cells of which only some express the glial marker GFAP (Levitt et al., 1981). Although evidence from the neurogenesis of songbirds first suggested and then demonstrated the neurogenic role of RG (Alvarez-Buylla et al., 1988; 1990), this concept was not fully appreciated until cell fate analysis in the developing rodent brain proved that RG cells give rise to neurons (Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2001). Time-lapse imaging of retrovirally labeled clones demonstrated that RG cells generate neurons by multiple rounds of self-renewing, asymmetric division and that newborn neurons often use the parent cell’s radial fiber to migrate to the cortical plate (Noctor et al., 2001, 2004, 2008).
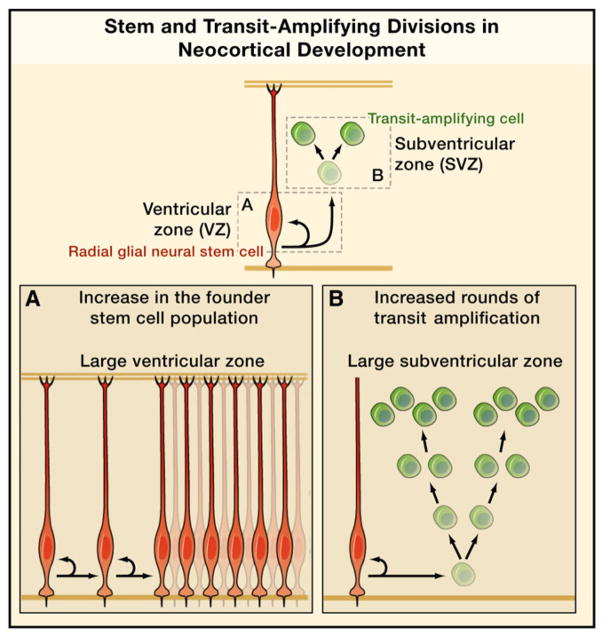
Further studies showed that, in the rodent, the vast majority of progenitor cells in the VZ have RG morphology and contact both the ventricular (apical) and pial (basal) surfaces of the neocortex (Hartfuss et al., 2001; Noctor et al., 2002). That RG cells were neuronal progenitor cells provided an explanation for the radial organization of the neocortex at a clonal level, with RG cells in the VZ forming an epithelial niche that gives rise to radial clones of excitatory neurons through repeated rounds of asymmetric division. This model also simplified the radial unit hypothesis, as the proliferative units were shown to be the same cells as the glial guides. Importantly, these data further suggested that evolutionary expansion of neocortical surface area could occur through expanding the RG founder population before the onset of neurogenesis (Figure 1A) (Rakic, 1995, 2009).
Figure 1. Progenitor Cell Expansion Can Underlie Neocortical Enlargement.
Neuronal number is a key determinant of neocortex size and shape. Neurons are produced from a lineage of radial glia (RG) stem cells (red) and transit-amplifying intermediate progenitor (IP) cells (green). Expansion in one or both cell populations has been proposed as potential mechanisms that underlie neocortical expansion. Expansion of the founder RG cell population prior to the onset of neurogenesis (A) predicts a large ventricular zone (VZ). Expansion in the number of transit-amplifying divisions (B) predicts a large subventricular zone (SVZ).
Transit Amplification by Intermediate Progenitor Cells
This model was oversimplified, as more detailed clonal analysis utilizing time-lapse imaging in the rodent showed that daughter cells of ventricular RG were often neuronal progenitor cells in their own right and would migrate superficially into the subventricular zone (SVZ) to divide (Noctor et al., 2004; Haubensak et al., 2004). Furthermore, whereas RG divisions were asymmetric and associated with self-renewal, the daughter progenitor cells (intermediate, or basal, progenitors) usually underwent one terminal symmetric division that produced two neurons and depleted the progenitor cell (Noctor et al., 2004). This partially reconciled the earlier observations of GFAP and non-GFAP expressing progenitor cells in the VZ because, at early stages prior to formation of a distinct SVZ, intermediate progenitor (IP) cells also divide in the VZ (Englund et al., 2005; Noctor et al., 2008; Kowalczyk et al., 2009). Others have reported that a third class of progenitor cells, termed short neural precursors (SNP), also resides in the VZ and divides at the ventricular surface (Gal et al., 2006; Stancik et al., 2010). These SNP cells are reported to have ventricular contact but only short basal processes that do not extend beyond the SVZ. Whether these are a distinct progenitor cell type or early IP cells that have not yet lost ventricular contact is controversial. At any rate, studies have clearly defined the SVZ as the major site of neurogenesis by IP cells that are the multipolar, nonepithelial daughters of RG (Noctor et al., 2004, 2008; Haubensak et al., 2004; Miyata et al., 2004). Analysis of RG versus IP cell divisions showed that, although RG cells outnumber IP cells at early stages, the majority of neuron-producing cell divisions are by IP cells during all stages (Kowalczyk et al., 2009), even before the SVZ is a distinct progenitor zone. This implies that a major role of RG cells in rodent neurogenesis is to make neuronally committed IP cells.
The identification of the VZ as a site of asymmetric divisions and the SVZ as a site of symmetric divisions altered hypotheses regarding neocortical expansion. Independently of RG divisions, the number of symmetric IP cell divisions could also affect neuron number and be a determinant of neocortical size. Because IP cell daughters have the same birth date and are predicted to occupy the same laminar position, an increase in their numbers would be more consistent with tangential expansion of neocortical surface area, whereas increasing the number of asymmetric RG cell divisions would be predicted to expand neocortical thickness. Furthermore, expansion of founder cells within the VZ can only account in part for the neuron number observed in gyrencephalic species, as neocortical surface area is disproportionately expanded compared to the ventricular surface area, which remains relatively modest. These observations could be reconciled if SVZ progenitor cells have a greater capacity for transit amplification in gyrencephalic animals, such that they undergo multiple rounds of cell division before generating neurons (Figure 1B) (Kriegstein et al., 2006). However, this scheme could exhaust the capacity of RG cells to guide neuronal migration, as the expanded population of immature neurons would greatly outnumber the restricted number of migratory guides.
Indeed, a human genetic study mapped the disease-causing gene in a family exhibiting congenital microcephaly to the homozygous silencing of TBR2 (EOMES) (Baala et al., 2007), a transcription factor shown in rodent studies to be a selective marker for IP cells (Englund et al., 2005) and functionally required for SVZ neurogenesis (Arnold et al., 2008; Sessa et al., 2008). This condition further supports the overall importance of SVZ progenitor cells in the control of neocortical size.
The Expanded Primate SVZ
The effect of progenitor cell expansion on a subsequent increase in neuron number is dependent on the natural counterbalancing relationship between rates of cell proliferation and death. Genetic deletion of key players in the apoptosis pathway in mice results in modest increases to neocortical size and widespread dysregulation of ventricular architecture (reviewed by Kuan et al., 2000). However, even though apoptosis plays a role in normal neocortical development, an evolutionary reduction in cell death during development is unlikely to be a factor that sufficiently explains the great magnitude of neocortical expansion in primates. Although mathematical modeling studies have predicted that cell death rates in developing primate neocortex may be increased compared to rodent (Gohlke et al., 2007), observations of the remarkable size of the primate SVZ region (Smart et al., 2002; Lukaszewicz et al., 2005) imply even greater increases in progenitor cell proliferation. We therefore turn our attention to recent studies that directly characterize the cells in the expanded SVZ of primates to illuminate mechanisms of neocortical expansion.
Human Neocortical Development
In the rodent, the great majority of RG are epithelial cells that reside in the VZ and make full apical-basal contacts (Noctor et al., 2002). However, it is becoming clear that RG are organized differently in the developing neocortex of other species. In developing monkey and human neocortex, cells with similar morphology to RG, but not necessarily spanning ventricle to pia, are found outside of the VZ. However, these cells were historically considered to be translocating RG transforming into astrocytes (Rakic, 1972; Schmechel and Rakic, 1979; Choi, 1986). Because RG were not yet appreciated to be neurogenic, nonventricular RG-like cells were also not considered to be neuron-producing cells.
Primate corticogenesis is distinguished by the appearance of a large SVZ that has an inner (ISVZ) and outer region (OSVZ), often split by a thin fiber layer (Smart et al., 2002; Zecevic et al., 2005; Fish et al., 2008). Furthermore, thymidine-labeling studies in primates collectively indicate that proliferation of cells within the OSVZ coincides with the major wave of cortical neurogenesis (Rakic, 1974; Lukaszewicz et al., 2005), suggesting that the OSVZ contributes to neuron production. In addition, cells in the OSVZ express RG markers such as nestin (NES), vimentin (VIM), PAX6, and GFAP, as well as the IP cell marker TBR2 (EOMES) (Zecevic et al., 2005; Bayatti et al., 2008; Mo and Zecevic, 2008). But how OSVZ cells expressing RG or IP cell markers related to the known progenitor cell types near the ventricle was not understood. It was hypothesized that the OSVZ contained progenitor cells that were either epithelial cells resembling RG (Fish et al., 2008) or transit-amplifying cells resembling IP cells (Kriegstein et al., 2006). Recent studies have begun to resolve these questions by demonstrating the cellular heterogeneity of the OSVZ, which includes both RG and IP cell types, and have highlighted their importance for neuron production during human fetal development.
Extending the Radial Glia Founder Population into the OSVZ
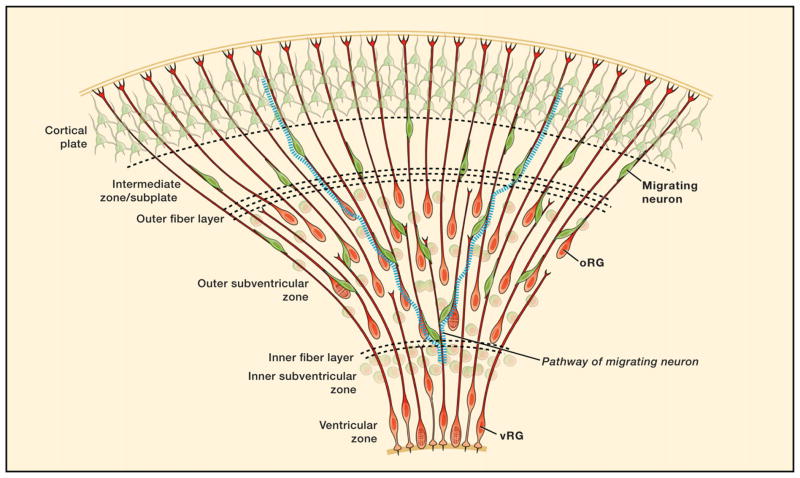
At the height of human OSVZ proliferation, the OSVZ contains roughly similar proportions of progenitor cells and postmitotic neurons (Figure 2A). The progenitor cell population is diverse in terms of morphology and marker expression. In particular, ~40% of OSVZ progenitor cells express nuclear and cytoplasmic markers typical of RG and also possess radial fibers. However, unlike RG in the ventricular zone (vRG cells), which are bipolar, the OSVZ radial glia-like cells (oRG cells) are unipolar, with a basal fiber that ascends toward the pia but without an apical fiber that descends toward the ventricle (Hansen et al., 2010; Fietz et al., 2010). Because some oRG cells contact the basal lamina, they can be classified as epithelial cells (Fawcett and Jensh, 2002). However, oRG cells lack key features of epithelial (apical-basal) polarity, as they are removed from the ventricular epithelium and do not express apically localized membrane constituents such as CD133 (PROM1), Par3 (PARD3), or aPKCλ (PRKCI) (Fietz et al., 2010). Immuno-staining for mitosis-specific cytoplasmic markers such as phosphorylated-vimentin demonstrates that oRG cells retain the basal fiber throughout the duration of M phase (Hansen et al., 2010), similar to vRG cells.
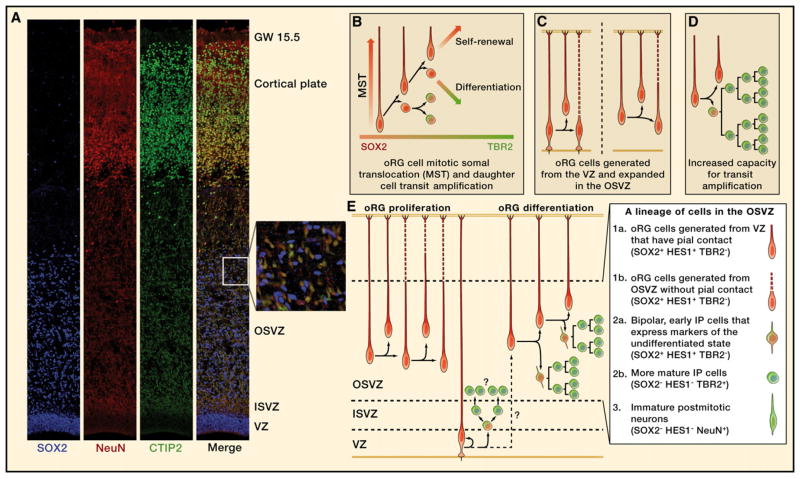
Figure 2. Features of Human Neocortical Development.
Cellular behaviors observed in the outer subventricular zone (OSVZ) show that the human neocortex uses expanded numbers of both stem and transit-amplifying cell types (radial glia [RG] and intermediate progenitor [IP] cells, respectively).
(A) Cells expressing the neuronal markers NeuN (RBFOX3, red) and CTIP2 (BCL11B, green) make up ~45% of the OSVZ population (gestational week [GW] 15.5) but never colabel with the progenitor cell marker SOX2 (blue, inset).
(B) The cell division and behavior of OSVZ radial glia-like (oRG) cells is illustrated.
(C) The RG population increases through the generation of oRG cells from the ventricular zone (VZ) and their expansion in the OSVZ. Dashed lines indicate the unknown length of newborn radial fibers.
(D) oRG daughter cells exhibit protracted differentiation and have an increased capacity for transit amplification.
(E) The differentiation of oRG daughter cells is marked by the loss of SOX2 expression and Notch activation (HES1), with a gain in TBR2 expression. Together, these observations explain how the combination of oRG proliferation and differentiation expands the OSVZ over the course of midgestation and gives rise to an increased number of neurons. The lineage and molecular signatures of cells that form the OSVZ are shown (inset).
Dynamic imaging of oRG cell divisions in cultured slices of fetal neocortex shows that oRG cells exhibit a distinctive behavior whereby the cell soma rapidly ascends along the radial fiber during the hour preceding cell division, a process termed mitotic somal translocation (Figure 2B) (Hansen et al., 2010). This is in contrast to vRG cells, which undergo interkinetic nuclear migration and descend to the ventricle to undergo mitosis. oRG cells, upon completing mitotic somal translocation, typically divide with a horizontal cleavage plane, with the more basal daughter cell always inheriting the basal fiber. oRG cells were observed to undergo multiple rounds of such divisions, suggesting that these divisions are self-renewing and asymmetric and push the boundary of the OSVZ outward. Cell fate analysis of oRG cell clones confirmed that self-renewed oRG cells remain undifferentiated, whereas oRG cell daughters continue to proliferate and sometimes express markers of neuronal commitment such as TBR2 or ASCL1 (Figure 2B). Although there is not yet formal evidence for oRG cells exhibiting long-term multipotency for different neural lineages, they can be considered neural stem cells based on their multiple rounds of self-renewing asymmetric division, which functionally define them as the building blocks for OSVZ-derived neurons and progenitor cells.
Although cortical neuron production begins by gestational week (GW) 6, the OSVZ does not arise until GW11. Over the following 6 weeks, the OSVZ expands dramatically to become the predominant germinal region in the neocortex. Importantly, the initial phase of OSVZ expansion does not occur at the expense of progenitor cells in the VZ/ISVZ, implying that the OSVZ is generated by proliferation rather than by delamination and migration of VZ progenitor cells. In cultured slices, oRG cells were sometimes observed to divide and produce another oRG cell (Figure 2C) (Hansen et al., 2010), suggesting that, though oRG cells likely originate from the VZ, they can also expand their numbers within the OSVZ.
The coexistence of oRG cells with vRG cells demonstrates that human RG consist of two major subclasses, each functioning as neural stem cells in their respective locations. Expanding the population of RG in a distinct germinal zone is a mechanism for increased neuron production that could be highly relevant for building a larger brain.
Enhanced Transit Amplification in oRG Daughter Cells
Although we have so far focused on oRG cells, they account for only ~40% of progenitor cells in the human OSVZ. oRG cells most commonly divide asymmetrically to self-renew and generate daughter cells that are bipolar. These bipolar cells tend to remain undifferentiated over additional transit-amplifying cell cycles and typically do not express markers of neuronal commitment, such as TBR2 (Sessa et al., 2008), until days after generation from an oRG cell (Hansen et al., 2010). Therefore, it is likely that each oRG cell functions as a founder cell for an extended lineage of transit-amplifying cells that lose SOX2 expression and gain TBR2 expression at some point during the ensuing cell cycles (Figures 2B, 2D, and 2E). This subset forms ~60% of the OSVZ progenitor cell population. In contrast, RG daughter cells in the rodent express TBR2 within hours after RG cell division (Noctor et al., 2008; Ochiai et al., 2009) and usually divide only once to produce two postmitotic neurons (Noctor et al., 2004, 2008). These observations suggest that increased transit amplification is highly relevant in human neocortical expansion. (For discussion of how the rates of neuron accumulation are impacted by different modes of progenitor cell division, see Figure 3.)
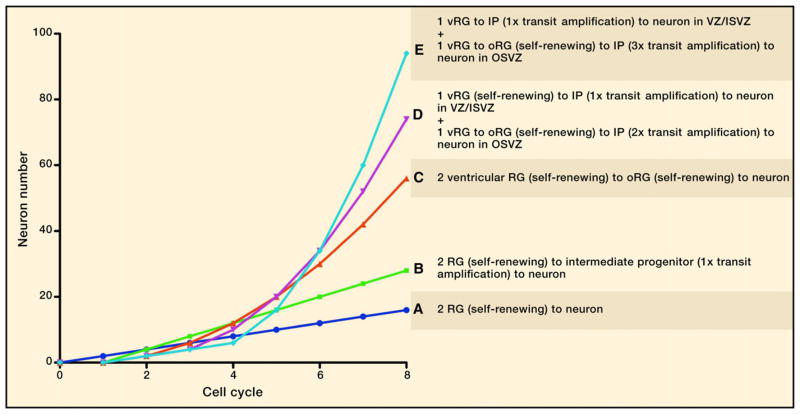
Figure 3. Different Rates of Neuron Accumulation as a Result of Different Modes of Progenitor Cell Division.
The cellular mechanisms of human corticogenesis suggest that the outer subventricular zone (OSVZ) contains OSVZ radial glia-like (oRG) cells and an extended lineage of transit-amplifying intermediate progenitor (IP) cells. To illustrate the effect of these progenitor cells on the rate of neurogenesis, we model and contrast the outcomes in neuronal number from developmental schemes that utilize OSVZ proliferation versus ones that do not. In every case, we start with two radial glial (RG) cells and plot neuron number over the course of eight cell cycles. We also assume no cell death.
(A) Neurons are directly born from RG through repeated rounds of asymmetric division. The rate of accumulation is linear at one neuron/cell cycle/RG cell (Rakic, 1988, 2009; Noctor et al., 2001).
(B) We take into account how RG cells give rise to IP cells that undergo one transit-amplifying division. This delays birth of the first neuron by one cycle but thereafter accumulates cells linearly at a rate of two neurons/cell cycle/RG cell. This is the model for rodent corticogenesis (Noctor et al., 2004, 2008; Haubensak et al., 2004).
(C) Transit amplification by IP cells is not taken into account, but ventricular RG (vRG) cells in the human are assumed to generate oRG cells by repeated rounds of asymmetric division. Once born, oRG cells divide repeatedly to generate one neuron per cell cycle. Because the number of oRG cells increases every cell cycle, this results in exponential growth of neuron number (Fietz et al., 2010; Fietz and Huttner, 2011).
(D and E) One vRG cell undergoes a developmental scheme resembling the rodent (B), whereas the other generates oRG cells over repeated cell cycles. In addition to the exponential growth attributed to oRG cell accumulation, neuronal production is further amplified by two (D) or three (E) transit-amplifying cycles by each oRG-derived IP cell (Hansen et al., 2010). These plots highlight the differential effects of stem cell accumulation and extended transit amplification. We hypothesize that (E) is the most accurate model thus far, although there are several unknown parameters that should change the model. First, the number of transit-amplifying divisions in humans is unknown. Based on how the proportion of oRG cells to IP cells is ~2:3, we predict that the number of IP divisions does not exceed 3–4, as IP cells would be observed to greatly outnumber oRG cells. Second, the rate of oRG generation is a combined effect of generation from the VZ and expansion in the OSVZ. These parameters are not understood but presumably dictate the relative amount of linear versus exponential neuron production in the human.
Gyrencephaly in the Ferret Neocortex
Gyrencephalic brain development is, of course, not limited to primates. Recent studies have examined whether oRG cells and OSVZ proliferation are shared features with ferrets (Mustela putorius furo) (Fietz et al., 2010; Reillo et al., 2010), which also exhibit gyrencephaly and have a disproportionate increase in cortical versus ventricular surface area (Smart and McSherry, 1986a, 1986b). Although cells in the ferret SVZ with oRG cell morphology had been reported previously, they were suggested to be translocating astrocytes (Voigt, 1989) or immature neurons undergoing somal translocation (Borrell et al., 2006). Immunolabeling for RG and IP cell markers showed that the ferret SVZ contained both types of progenitor cells, with many PAX6-positive cells also exhibiting oRG cell morphology (Fietz et al., 2010; Reillo et al., 2010). Thus, oRG cells (also called intermediate radial glial cells/IRGCs in ferret) and an enlarged SVZ (OSVZ) are not primate-specific features but are also present in carnivores.
The ideas that oRG cells expand in number within the OSVZ and that OSVZ proliferation correlates with gyrus formation are substantiated by experimental evidence in the ferret (Reillo et al., 2010). Green fluorescent protein (GFP)-expressing retrovirus was delivered to the OSVZ of early postnatal ferret kits by stereotactic injection, followed by morphological analysis of labeled cells 2 days later. Because retrovirus labels only one of the two daughter cells following the initial cell division and because more than half of the labeled cells were reported to have oRG cell morphology, this ratio suggested a predominance of divisions in the OSVZ that result in two oRG cells (Reillo et al., 2010). This is partially consistent with observations of oRG cell divisions in human tissue slices in which the oRG daughter cell that did not inherit the basal fiber occasionally remained unipolar and extended a new basal fiber of its own (Hansen et al., 2010). Together, these findings support the notion that oRG cells expand in number in the OSVZ (Figure 2C).
It had previously been proposed that regions of high and low amounts of SVZ proliferation would predict the future locations of gyri and sulci, respectively (Kriegstein et al., 2006). This connection between progenitor cell behavior during development and shape of the adult brain has recently been examined in ferret, cat, and human neocortex (Reillo et al., 2010). Indeed, regions of future gyral formation contain more proliferating cells in the SVZ during development, and in these areas, proliferation is more pronounced in the OSVZ than in the VZ or ISVZ. The authors of this study experimentally manipulated OSVZ proliferation in the ferret visual cortex by removing thalamic inputs, which resulted in local reduction in neocortical surface area. These findings confirm the importance of oRG cell proliferation in the tangential expansion of gyrencephalic neocortex and lead us to consider the mechanisms by which this occurs.
Addition of Radial Units and a Remodeled Migration Scaffold
The divisions of oRG cells coupled with mitotic somal translocation begin to explain the radial growth of the OSVZ, as we can infer that oRG cells originate from the VZ, pass through the ISVZ, and leave a trail of daughter progenitor cells as they translocate basally. Furthermore, the substantial addition of RG-like cells in a subventricular location increases the number of ontogenetic units active in generating neuronal clones at a given time. In addition to clear changes to the amount of neurogenesis, adding RG-like cells in the OSVZ must have been accompanied by complex rearrangements in the radial scaffold of the neo-cortex. That oRG cells continue to translocate toward the pial surface while their daughter cells undergo transit amplification makes it unlikely for an OSVZ-derived neuron to begin migration using the basal fiber of its parent oRG cell. However, the fibers provided by oRG cells are presumably still an important part of the scaffold for migrating neurons, functioning to guide cells part of the way toward the cortical plate.
The degree to which the basal fibers of oRG cells and vRG cells actually reach the pial surface and contact the basal lamina is unresolved. Dyes applied to the pial surface can diffuse into a proportion of oRG cells in the OSVZ through their basal fibers (Hansen et al., 2010). However, oRG cell fibers labeled from the OSVZ may not all reach the pia because cells born locally are unlikely to grow basal fibers that traverse such a great distance. The same may be true of vRG cells that initially gave rise to oRG cells but did not inherit the basal fiber. Dye and genetic labeling studies in the ferret have recently shown that radial fibers originating in the VZ diverge and form a fanned array by the time that they reach the pial surface. Because the density of fibers did not decrease at the same rate as the fibers were diverging, it was inferred that new fibers are added in basal locations, a property that is likely achieved by the expansion of oRG cells (Reillo et al., 2010). Although neuronal migration on oRG cell fibers has not been directly observed in either ferret or human, the addition of radial fibers by oRG cells is suggested to solve a topological problem—they provide guides for the migration of neurons that can then spread over a greater tangential surface.
Updated Views of Corticogenesis
Recent discoveries concerning the progenitor cell subtypes present in the OSVZ provide an updated view of neurogenesis, neuronal migration, and tangential expansion that revises the radial unit hypothesis and other previous models of human neocortical development (Figure 4).
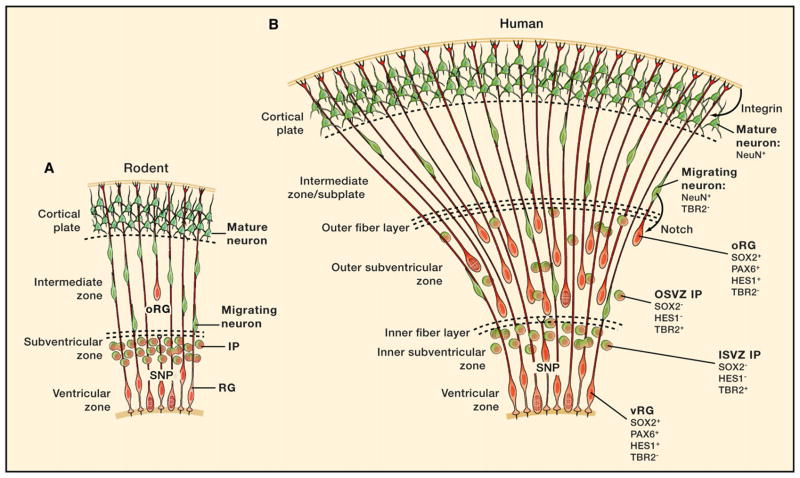
Figure 4. Contrasting Rodent and Human Neocortical Development.
(A) Current views of rodent corticogenesis are illustrated. Radial glial (RG) cells most often generate intermediate progenitor (IP) cells that divide to produce pairs of neurons. These neurons use RG fibers to migrate to the cortical plate. The historical view of neocortical development was that RG and neuronal progenitor cells were lineally distinct and that RG did not have a role in neurogenesis. Our current appreciation of the lineage relationship between RG cells, IP cells, and neurons has revised this view. The recent observation that small numbers of outer subventricular zone radial glia-like (oRG) cells exist in the mouse is also illustrated.
(B) We highlight the lineage of oRG cells, IP cells, and migrating neurons (red to green) present in the human outer subventricular zone (OSVZ) and the increased number of radial fibers that neurons can use to migrate to the cortical plate. The number of ontogenetic “units” is significantly increased with the addition of oRG cells over ventricular RG (vRG) cells. Maintenance of oRG cells by Notch and integrin signaling is shown. Short neural precursors (SNP), a transitional cell form between RG and IP cells, are also depicted in (A) and (B). For simplicity, we do not illustrate all of the cell types described in Figure 2E.
Neuronal Migration and Tangential Expansion
In contrast to the columnar organization of neuronal clones proposed by the radial unit hypothesis, several studies have shown considerable lateral dispersion of clonally related neurons in rodents (Walsh and Cepko, 1988; Austin and Cepko, 1990; Tan and Breen, 1993), ferrets (Ware et al., 1999; Reid et al., 1997), and primates (Kornack and Rakic, 1995). Though in rodents, this dispersion is relatively modest and regulated by EphA/ephrin-A signaling (Torii et al., 2009), the OSVZ-based radial scaffold may further contribute to the dispersion of neuronal clones in ferrets and primates. As a result of neuron production in the OSVZ, many newborn neurons begin migration in the OSVZ rather than the VZ. Instead of following the trajectory of a single radial glial fiber, neurons may follow a discontinuous relay of fibers, including numerous new ones that originate in the OSVZ from oRG cells (Figure 5). Discontinuity in the radial scaffold therefore provides ample opportunity for horizontal dispersion as neurons migrate from fiber to fiber. Studies that directly examine the extent to which the basal fibers of oRG cells support neuronal migration will be an important first step toward testing these concepts. The mature patterns of neocortical folding are likely the combined effect of these early mechanisms and others associated with neuronal differentiation and circuit formation that occur later, including the physical tension exerted by axonal fibers connecting closely associated cortical regions (Van Essen, 1997).
Figure 5. Remodeling of the Radial Migration Scaffold in the OSVZ.
We hypothesize that development of the outer subventricular zone (OSVZ) results in dramatic remodeling of the migration scaffold, where fibers no longer span the apical and basal surfaces. Radial glia (RG) fibers originate from both the ventricular zone (VZ) and OSVZ but may only extend part of the way to the pial surface, therefore forcing migrating neurons to switch fibers and disperse tangentially en route to the cortical plate. This mechanism may be important in the expansion of neocortical surface area observed in gyrencephalic species.
Increased Neurogenesis and Progenitor Cell Diversity
We propose that increases in neuron number in the human neocortex were achieved through three stages of progenitor cell expansion: (1) the population of founder cells increased in number in the form of nonventricular RG (oRG cells), (2) oRG cells undergo multiple rounds of asymmetric divisions to generate IP cells, and (3) IP cells acquired increased proliferative capacity to further amplify neuron production. Because the lineage from RG cell to immature neuron in the human OSVZ is punctuated by a greater number of intermediate steps than in the rodent, there may be considerable diversity in IP cell types (Hansen et al., 2010). Cells that constitute the OSVZ lineage of neurons (Figure 2E) can therefore be subdivided into: (1) oRG cells that either have pial contact and were initially derived from ventricular RG or oRG cells that do not have pial contact and were derived locally via proliferative divisions of other oRG cells, (2) IP cells that are bipolar and no longer have oRG cell morphology but still express markers of the undifferentiated state and Notch activation or more mature IP cells that are bipolar or multipolar but no longer have active Notch signaling, and (3) immature neurons that account for ~45% of the OSVZ cell population. Presumably, these neurons are derived both locally and from the VZ/ISVZ and are in the process of migrating to the cortical plate. This revises the radial unit hypothesis by highlighting the role of an important neurogenic region interposed between the ventricle and the cortical mantle. Neurogenesis in the human neocortex involves multiple stem and transit-amplifying cell divisions in two distinct locations.
Previous concepts about asymmetric versus symmetric divisions in neocortical development proposed that asymmetric divisions of RG generate neuronal diversity through the sequential production of distinct neuronal subtypes fated to occupy different laminar positions. Symmetric divisions of IP cells, on the other hand, act as the workhorse for increasing cell number by expanding the number of neurons produced per asymmetric RG division (Noctor et al., 2004; Kriegstein et al., 2006). These concepts are now extended to the OSVZ, as both RG and IP cell types are represented there. The major contribution of OSVZ progenitor cells to neurogenesis, distinct from neurogenesis in the VZ/ISVZ, could allow for differential ontogeny related to location of origin as a potential avenue for increased neuronal diversity. To begin testing this, it will be important to analyze the extent to which VZ/ISVZ proliferation contributes to neurogenesis independently of the OSVZ. Furthermore, because the number of neural progenitor cells in late versus early neurogenesis is so disparate due to OSVZ expansion, proliferation in this region may also be a mechanism to increase the number of later-born neurons, a feature that is consistent with observations that the upper cortical layers are particularly cell dense in the primate cerebral cortex (Marín-Padilla, 1992).
Future Directions and Challenges
Our new understanding of human neurogenesis describes the proliferative events within the OSVZ but leaves major questions unanswered, including: (1) the cell types generated from this region, (2) how the OSVZ is formed, and (3) how molecular regulators control OSVZ proliferation and distinguish it from what is observed in the rodent.
The significant overlap of cells expressing SOX2 with TBR2, a marker for commitment to the excitatory neuron lineage (Sessa et al., 2008), suggests that the human OSVZ contributes mostly to the generation of excitatory neurons. However, given that cell fate analysis of mature oRG cell progeny has not been performed in the human or ferret, the possibility remains that other cell lineages, including inhibitory neuron and glial precursor cells (Letinic et al., 2002; Jakovcevski and Zecevic, 2005; Reillo et al., 2010), may also be present. In particular, it has been suggested that, in contrast to the rodent neocortex, where inhibitory neurons are primarily derived from ventral sources (Anderson et al., 1997; Wonders and Anderson, 2006), the primate neocortex utilizes an additional dorsal source of inhibitory neuron progenitor cells to proportionately balance the increase in excitatory neurons (Letinic et al., 2002; Petanjek et al., 2009; Jakovcevski et al., 2010; Zecevic et al., 2011; Yu and Zecevic, 2011). These conclusions are primarily based on observations of proliferating cells in the dorsal VZ and SVZ that express ventral-associated markers relevant for the generation of inhibitory neurons, such as MASH1 (ASCL1), DLX2, NKX2-1, and calretinin (CALB2).
These concepts remain controversial, however, as human infants with severe ventral forebrain hypoplasia suffer almost complete depletion of NOS1-, NPY-, and SST-expressing inhibitory neuron subtypes, suggesting that these cell populations are unlikely to have a dorsal source (Fertuzinhos et al., 2009). The population of CALB2-positive inhibitory neurons was relatively spared in these patients, but the subpallial progenitor cells that are responsible for generating this subtype were also not dramatically affected by the disease. Inhibitory neuron lineage markers in the rodent may also not have the same significance in humans. ASCL1 expression, for example, has been observed at low levels in the dorsal proliferative zones of humans, where it appears to be associated with the production of excitatory neurons (Hansen et al., 2010). It is also possible that putative inhibitory neuron progenitor cells observed in the dorsal cortex are transit-amplifying cell types migrating from the ventral telencephalon. The notion that dorsal progenitor cells in primate neocortex generate most inhibitory neurons requires further investigation.
Our understanding of gyrencephalic brain development has greatly improved in recent years because of observations in human, nonhuman primate, and ferret tissues. However, the ethical and technical limitations of working in primate species continue to pose challenges in understanding the origin and progeny of OSVZ proliferation as well as other unexplored aspects of human corticogenesis. Future efforts to address these questions will benefit from a combination of new lineage tracing techniques and the employment of a model system that is more amenable to genetic manipulation, such as the ferret.
(For discussion of the OSVZ as a site of human disease and its implications for cell-based therapeutics, please see the Supplemental Information and Hansen et al., 2011).
Molecular Regulation of Neocortical Expansion
Understanding the molecular basis behind the cellular mechanisms in human corticogenesis is key to identifying the evolutionary changes that resulted in neocortical expansion. This section aims to bridge the gap from cellular to molecular mechanisms, drawing heavily on examples in rodent literature that inform how human RG cells may be regulated. We use two related perspectives to organize these studies.
First, we can infer that human corticogenesis involves: (1) the production of oRG cells from the VZ, (2) the maintenance of RG identity outside of the VZ, and (3) extensive transit amplification of oRG daughter cells before neuronal differentiation. These features depend critically on the production and maintenance of RG cells outside of the ventricular epithelium, so how might this be achieved at the molecular level? The manipulation of regulators involved in progenitor cell polarity, signaling, and cell-cell interaction reveal the factors and signaling pathways that are most important for maintaining the RG state in rodents. If these signaling requirements are conserved in humans, RG maintenance in the OSVZ must either rely more heavily on mechanisms that act through shared features of vRG cells and oRG cells, such as the basal fiber, or through novel forms of regulation that are specific to the OSVZ. In either case, knowing the molecular complexities of the RG state is essential as a starting point for understanding how oRG cells are maintained.
Second, loss- and gain-of-function studies in mice already recapitulate some of the features of human corticogenesis. Although it is unlikely that human evolution precisely recapitulated these changes at the molecular level, these results nevertheless highlight the types of pathways and mechanistic variations that could lead to formation of an OSVZ.
Regulation by Notch Signaling
The Notch signaling pathway is a highly conserved mechanism to create different signaling states between cells that make physical contact. This is achieved through lateral inhibition (Heitzler and Simpson, 1991; reviewed by Kageyama et al., 2008), whereby feedback loops that control gene expression levels operate in adjacent cells to amplify small, sometimes stochastic differences into larger ones. In the standard model, Notch ligands (Delta/DLL) are initially expressed at slightly different levels in a population of cells. Cells with higher expression levels stimulate Notch more potently in neighboring cells to induce downstream effectors such as HES1 and HES5, which repress the expression of Delta by transcriptional feedback, thereby diminishing the neighboring cell’s ability to stimulate Notch in the initiating cell. Over a number of iterations, cells are thought to stabilize into a salt and pepper pattern of mutually exclusive high and low signaling states.
In the context of neural development, HES1 and HES5 act as transcriptional repressors of proneural basic helix-loop-helix (bHLH) transcription factors such as NEUROG2 and ASCL1 (Ishibashi et al., 1995), therefore associating low Notch signaling with neuronal differentiation. However, the basic model of lateral inhibition is significantly complicated by the developmental dynamics of the neocortex, as cells enter the system through asymmetric division and leave through neuronal migration. In addition, RG cells also undergo interkinetic nuclear oscillations, and the extensive surface of their basal fibers that traverse the radial dimension of the developing neocortex could facilitate reception of Notch signals from distant sources and diverse cell types (Figure 6A). These observations suggest that the maintenance of Notch signaling in RG may be highly regulated in both space and time.
Figure 6. Molecular Mechanisms of Radial Glia Maintenance.
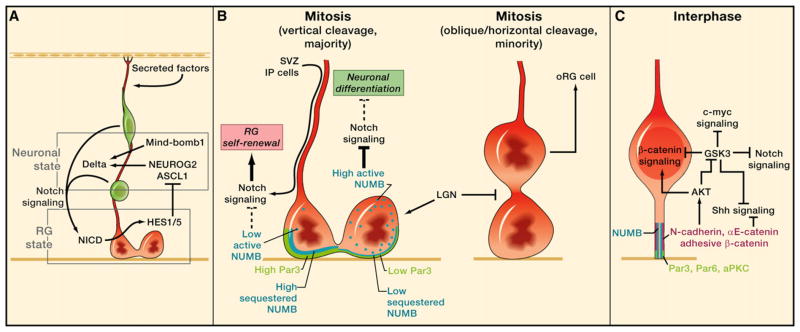
(A) The basal fiber of radial glia (RG) cells (red) is important for the reception of Notch signaling from neuronally committed cells in the subventricular zone (SVZ) and secreted factors such as retinoic acid from the meninges. In particular, we show how the reception of Notch signaling in RG results in activity of the Notch intracellular domain (NICD), which promotes HES1/5 expression and leads to repression of the neuronal state.
(B) We hypothesize that asymmetric distribution of Par3 (PARD3) and asymmetric inheritance of the basal fiber during RG mitosis result in differential Notch signaling and cell fate in the two daughters. Par3, a component of the apical complex, is distributed asymmetrically during mitosis and may contribute to asymmetric sequestration of NUMB, an inhibitor of Notch signaling, at cell-cell junctions. The daughter cell with less Par3 has higher levels of active NUMB that inhibits Notch signal, resulting in neuronal differentiation. Inheritance of the basal fiber may also help to enforce Notch signaling in only one of the daughter cells. We also highlight how removal of LGN (GPSM2) shifts vertical cleavages toward more oblique/horizontal ones, resulting in the production of outer subventricular zone radial glia-like (oRG) cells.
(C) Adherens junction components (pink) are critical for controlling multiple pathways that define the intracellular signaling state of RG cells.
Overexpression of the activated Notch intracellular domain (NICD) results in increased numbers of cells expressing RG markers, showing functionally that Notch activation is associated with the RG state (Gaiano et al., 2000). Loss-of-function studies showed the requirement of Notch signaling effectors HES1, HES3, and HES5 (Hatakeyama et al., 2004), either singly or in combination, as well as of the NICD signaling mediator CBF1 (RBPJ) (Mizutani et al., 2007; Imayoshi et al., 2010). Loss of these factors, in particular CBF1, promotes the depletion and premature differentiation of RG toward the neuronal lineage.
The expression patterns of Delta ligand (Campos et al., 2001) and Notch effector (Ohtsuka et al., 2006) suggested a scheme whereby Delta expressed on neuronally committed cells in the SVZ signals back to Notch receptors expressed on RG. This idea was tested by the removal of Mind-bomb1 (MIB1) (Yoon et al., 2008), an E3 ubiquitin ligase that is essential for generating functional Notch ligands (Itoh et al., 2003; Koo et al., 2005). MIB1 was expressed in neuronally committed TBR2-positive cells, and similar to studies of CBF1 deletion, its conditional knockdown in the neocortex also results in premature neuronal differentiation and depletion of RG cells. That isolated IP cells and postmitotic neurons stimulate HES1 activity in Notch-expressing neighboring cells in vitro further supports this idea. This study also raised the interesting possibility that the amount of time an IP cell pauses in the VZ before dividing or migrating away, a behavior that has been described as “sojourning” (Bayer and Altman, 1991; Noctor et al., 2004), could be related to its role in sustaining Notch activity in local RG cells.
Role of Notch Signaling Oscillations
In contrast to the standard model of Notch lateral inhibition, whereby cells are locked into high and low signaling states, Notch activation levels in rodent RG are dynamic and exhibit an oscillatory pattern (reviewed by Kageyama et al., 2008). An important insight came when HES1 expression was found to oscillate at 2–3 hr intervals in cultured cells due to negative autoregulation of Hes1 transcription (Hirata et al., 2002). Shimojo et al., (2008) discovered that HES1 control in RG is multifaceted, with the autonomous HES1 oscillatory circuit layered into a more pronounced level of regulation. HES1 protein was detected only during the phases of the cell cycle (late G1 through G2) when the cells were in the basal part of the VZ, but not during mitosis through early G1, when the cells were immediately adjacent to the ventricle. Furthermore, the oscillatory expression of HES1 in RG controls NEUROG2 and DLL1 expression and leads to their oscillation with inverse correlation. Therefore, RG are poised to generate neuronal progeny during periods of low HES1 expression. Consistent with previous reports, sustained overexpression of HES1 blocks expression of proneural genes, Notch ligands, and cell-cycle regulators—presumably locking the cells into a RG state but rendering them incapable of generating more differentiated progeny. Blockade of Notch signaling in RG has the opposite effect, whereby NEUROG2 and DLL1 switched from oscillatory to sustained expression, caused neuronal differentiation, and depleted the progenitor pool. These results show that, within the RG population, oscillatory expression of Notch-controlled genes is required for the simultaneous maintenance of progenitor identity and generation of neuronal progeny.
How might Notch oscillations be coordinated with asymmetric division? One possibility is that periods of low HES1 expression (M and early G1 phases) are permissive windows for neuronal differentiation. Directly after RG cell division, both daughter cells start with the same ground state of Notch activity and still possess neuroepithelial cell-cell interactions, having short apical fibers that contact the ventricle (Miyata et al., 2004). Why then does the self-renewed RG cell remain undifferentiated, whereas the daughter cell becomes neuronally committed? Because RG cells have long basal fibers, this could facilitate cell-cell interactions outside of the neuroepithelium. Retention of the basal fiber following division could be a critical determinant of a cell’s ability to sense signals from the SVZ and more basal cell types. In this case, because Notch ligands are highly expressed in the SVZ, the daughter cell in the VZ without the basal fiber will be predisposed toward sustained NEUROG2 expression and neuronal differentiation, whereas the cell with the basal fiber quickly receives Notch signal and preserves RG identity (Figures 6A and 6B).
Basal Fiber Inheritance
Inheritance of the basal fiber could also be a general mechanism that allows oRG and vRG cells to be regulated similarly. The Notch effector HES1 is expressed in oRG as well as vRG cells in fetal human neocortex, and pharmacological inhibition of Notch signaling in slice cultures induces neuronal differentiation of progenitor cells (Hansen et al., 2010). Therefore, Notch signaling restrains both types of RG from neuronal differentiation. The epithelial architecture in the VZ may facilitate Notch signaling from redundant sources, but oRG cells could rely more exclusively on neurons or IP cells attached to their basal fibers for Notch-dependent maintenance of the RG state (Figure 4B).
It has also been proposed that integrin signaling maintains oRG status in cells contacting the basal lamina via their basal fiber (Figure 4B) (Fietz et al., 2010; Fietz and Huttner, 2011). β3-integrin (ITGB3) expression has been observed in the basal fiber of oRG cells, and chemical or antibody-based inhibition of integrin-mediated signaling in ferret slice cultures causes a loss of PAX6-positive OSVZ cells, which include oRG cells. However, RG contact with the basal lamina in the rodent neocortex has been suggested to be dispensable for regulating cell fate and proliferation (Haubst et al., 2006), and not all oRG cells may contact the basal lamina. Therefore, further characterization of the precise requirements for integrin-mediated signaling in oRG cells will be important to substantiate this concept. Regulation of RG via the basal fiber is nevertheless an emerging idea that could be highly relevant to OSVZ proliferation.
Regulation by β-Catenin Signaling
β-catenin (CTNNB1) has also been established as a central regulator of proliferation versus differentiation in rodent neural progenitor cells. β-catenin protein lies functionally at a crossroad between tissue architecture and regulation of the transcriptional activity that modifies cell fate, having dual roles as a component in the adherens junctions that link neuroepithelial/RG cells and as a transcriptional coactivator in the presence of Wnt signal. Early studies using genetic deletion in the forebrain established a requirement for β-catenin in specifying dorsal telencephalic fate (Gunhaga et al., 2003; Backman et al., 2005), maintaining the structural integrity of the neuroepithelium and preventing progenitor cell apoptosis (Machon et al., 2003; Junghans et al., 2005), but did not distinguish whether the adhesive or transcriptional roles of β-catenin were primarily responsible for these effects. A later study tested the cell-autonomous role of β-catenin transcriptional activation by expressing ICAT or a dominant-negative version of TCF4, either of which blocks transcriptional activity by interfering with β-catenin/TCF4 complex formation (Woodhead et al., 2006). In this context, whereby neighboring cells provided a relatively normal radial scaffold, RG differentiated into neurons and migrated to the cortical plate, implying that β-catenin signaling suppresses neuronal differentiation and maintains progenitor cell proliferation independently of its role in adhesion. Observed increases in neuronal differentiation caused by β-catenin loss of function were preceded by increases in IP cell number, suggesting that β-catenin signaling restrains the development of IP cells from RG (Mutch et al., 2010).
Stabilizing β-Catenin Increases the Progenitor Pool
Consistent with these findings, transgenic mice expressing stabilized β-catenin in neural progenitor cells exhibited gross defects in neocortical development. Neuroepithelial cells in this mouse have a higher propensity to re-enter the cell cycle and undergo symmetric self-renewing divisions, resulting in a massively expanded population of VZ progenitors leading to folding of the cortical anlage (Chenn and Walsh, 2002). This study was the first experimental suggestion that expansion of the ventricular founder population could be the cellular basis for developing gyrencephaly, thus serving as an example of how progenitor cell expansion can underlie neocortical expansion (Figure 1A) (Rakic, 1995, 2009). However, this mode of expansion does not mimic the development of naturally gyrencephalic animals, which display vast increases in neocortical surface area without a proportional increase in ventricular surface area (for discussion, see Kriegstein et al., 2006).
Because the transgenic mice in this initial study did not live past birth, it was not possible to determine whether expansion of the neuroepithelial population would have lasting effects on neuronal number and neocortical size. Further work with mice that expressed lower amounts of stabilized β-catenin (Chenn and Walsh, 2003) indeed resulted in adult mice with enlarged forebrains and an increased number of neurons. However, the brains of these mice were disrupted in other ways, including an arrest of neuronal migration and disruption of cortical lamination. Thus, the example of stabilized β-catenin in neuroepithelial cells highlights how a relatively small genetic change can greatly enlarge the neocortex but also reveals that, unless such changes integrate with other developmental processes, the consequences will be deleterious.
How β-catenin functions in the developing human neocortex is an unexplored but interesting future direction. The ventricular epithelium in humans is capable of RG self-renewal and neurogenesis but also generates self-renewing oRG cells that presumably do not express adherens junction components. Therefore, understanding the relative roles of β-catenin expression in adherens junctions and in the nucleus in both proliferative zones of the human neocortex will be highly informative.
Regulation by Polarity Proteins
Adherens Junction Components
The participation of β-catenin in adherens junctions illustrates how proteins that are involved with RG polarity can regulate the progression of neurogenesis. Recent studies have highlighted that β-catenin signaling is affected by the expression of other adherens junction components such as cadherins and α-catenins, although not necessarily through mechanisms of β-catenin sequestration at cell-cell junctions. Reduction of N-cadherin (Cdh2) leads to a decrease in β-catenin signaling and results in a phenotype that is similar to direct removal of β-catenin—whereby knockdown cells exit the cell cycle and prematurely differentiate into neurons (Zhang et al., 2010). Remarkably, this effect is independent of the canonical Wnt pathway, as reducing N-cadherin did not alter the ability of Wnt to stimulate β-catenin signaling. Instead, N-cadherin function was linked to AKT activity, with AKT stimulating β-catenin activity through direct phosphorylation and perhaps also indirectly through GSK3 inhibition (Figure 6C).
Conditional genetic deletion of αE-catenin (Ctnna1) in the cortex disrupts the polarity of the epithelium, shortens cell-cycle length, and decreases neural progenitor cell apoptosis. Together, this results in a severely dysplastic phenotype, whereby progenitor cells disperse about the cortex and over-proliferate, eventually giving rise to a larger mature brain (Lien et al., 2006). Global gene expression comparisons of normal versus αE-catenin-deleted brains showed that targets of Sonic hedgehog (Shh) signaling were expressed at higher levels in mutants (Figure 6C). Chemical inhibition of Shh signaling rescues the overproliferation, but not the loss of cell polarity, in αE-catenin-deleted brains, implying a role for αE-catenin in suppressing Shh signaling in the developing cortex. Whether αE-catenin also regulates β-catenin signaling in RG has been controversial. A further study using αE-catenin conditional deleted mice found no evidence for changes in β-catenin nuclear activity using a number of assays, including quantification of protein levels, in vivo reporter activity, or quantification of downstream targets (Lien et al., 2008). However, another group reported that focal knockdown of αE-catenin phenocopied the removal of β-catenin signaling and promoted neuronal differentiation (Stocker and Chenn, 2009). The disparate findings between universal versus focal deletion of αE-catenin illustrate how tissue architecture can alter a cell-autonomous mutant phenotype and underscore how the RG intracellular signaling state is defined by both extrinsic and intrinsic factors.
NUMB and Par3 Link Cell Polarity with Notch Signaling
NUMB is a classic determinant of cell fate in dividing fly neuroblasts, where it localizes asymmetrically to the basal pole and promotes differentiation of the basal daughter cell by inhibiting the Notch pathway (Rhyu et al., 1994). In the context of vertebrate neural development, however, NUMB plays multiple roles in regulating RG cell polarity and differentiation. Though initially a matter of controversy (Zhong et al., 1996), mounting evidence supports the conserved role of NUMB as a determinant of neuronal fate in asymmetric RG cell divisions (Wakamatsu et al., 1999; Shen et al., 2002; Rasin et al., 2007). However, NUMB has an additional role in preserving apical-basal polarity within the mouse neuroepithelium (Rasin et al., 2007). NUMB, a known modulator of endocytosis, interacts and colocalizes with cadherins on the basolateral side of adherens junctions and with RAB11-positive endocytic vesicles. Combined loss-of-function experiments for NUMB and its homolog NUMBL showed disruption of epithelial integrity and RG that were displaced basally, implying a critical role for NUMB/NUMBL in regulating RG cell adhesion in the VZ. Although this displacement of RG resulted in the abnormal location of resulting neurons, the timing and sequence of neurogenesis was reasonably normal. Thus, RG can undergo the correct sequence of neurogenesis even in a disrupted tissue microenvironment.
In addition to the proteins that directly make up adherens junctions, other proteins that define the apical “identity” of RG are also critical for regulating the balance between proliferation and neurogenesis. Overexpression of Par3 (PARD3) or Par6 (PARD6A), core members of an apical protein complex that is important for epithelial polarity, increases the frequency of symmetric, proliferative RG cell divisions at the expense of asymmetric, neurogenic divisions, whereas small hairpin RNA-mediated knockdown has the opposite effect and promotes neuronal differentiation (Costa et al., 2008). Moreover, Par3 is segregated asymmetrically in RG cell divisions and has been found to exert its effects on neurogenesis through the Notch/Numb pathway (Bultje et al., 2009). Combined knockdown of NUMB and Par3 rescues the effects of loss of Par3 on neurogenesis, placing NUMB activity downstream of Par3. This effect depends on the physical interaction of Par3 and NUMB.
These studies point to cooperative roles for Par3 and NUMB in maintaining epithelial polarity but antagonistic roles in regulating Notch signaling and neurogenesis. Both proteins localize to the lateral membrane domain in ventricular endfeet during inter-phase and promote apical-basal polarity (Rasin et al., 2007; Bultje et al., 2009). However, the direct interaction of Par3 with NUMB is required for Par3 to potentiate Notch signaling, possibly through preventing NUMB from interacting with Notch. Asymmetric distribution of Par3 in RG divisions could therefore bias the amount of Notch signaling in each daughter by controlling the level of active NUMB in the next cell cycle. The daughter cell that does not inherit the apical complex will receive less Par3, have higher active NUMB levels, and better inhibit Notch activation, resulting in the upregulation of proneural bHLH transcription factors (Figure 6B). This scheme also explains the surprising observation that, when NUMB was knocked down, RG cells displaced outside of the ventricular epithelium remained functional as primary progenitor cells (Rasin et al., 2007). Loss of NUMB causes delamination of RG because of its polarity role but permits high enough levels of Notch signaling to maintain RG in their progenitor state outside of the VZ. Furthermore, these observations are consistent with the idea that oRG cells do not express apical constituents but are able to receive Notch signaling in the OSVZ.
Regulation by Multiple Integrated Pathways
The examples shown serve to highlight the tightly controlled balance of pathway activation and repression that is required for proper RG cell function. An important future direction will be to fully describe the mechanistic steps leading to aberrant pathway activity, as it is often not obvious why a particular pathway is downstream of a particular protein. We have emphasized the roles of Notch and β-catenin signaling for maintaining the RG progenitor state, but clearly, regulation by other pathways is also important. For example, FGF10 is expressed in neuroepithelial cells, and its loss extends the period of symmetric progenitor cell expansion and delays the onset of neurogenesis in the rostral cortex. However, RG cells in this mutant eventually differentiate in excess to overproduce neurons and IP cells, resulting in the tangential expansion of frontal areas and increased laminar thickness (Sahara and O’Leary, 2009). The RG signaling state is further complemented by signals from outside of the neuroepithelium. Mutant mice with disrupted meningeal development have increased self-renewing neuroepithelial divisions and decreased production of neurons and IP cells, resulting in a “gyrencephalic” appearance similar to that of the stabilized β-catenin mouse (Siegenthaler et al., 2009). This effect is rescued in vitro by meninges-derived conditioned media, suggesting the requirement of secreted factors from the meninges, most notably retinoic acid (RA), for normal neurogenesis. Although the importance of RA in this context has recently been called into question (Chatzi et al., 2011), this nevertheless underscores how regulation of the neuroepithelium could be due to the proximity of RG basal fibers with the meninges and other cell types (Figure 6A).
A recent study testing the function of GSK3 in neocortical development (Kim et al., 2009) illustrates the view that the RG state is tightly controlled by the intersection of multiple pathways. GSK3 is the kinase responsible for phosphorylating β-catenin and targeting it for degradation. Conditional genetic deletion of GSK3 leads to a phenotype very similar to that of the stabilized β-catenin mouse, as expected because GSK3 loss should result in stabilization of cytoplasmic β-catenin and increased signaling. This included an expanded ventricular surface composed of more SOX2-expressing RG-like cells, increased cell-cycle re-entry, increased β-catenin nuclear signaling, and a decrease in neuronal differentiation. However, the authors found that the overproliferation phenotype could also be attributed to overactive Notch, Shh, and c-Myc (MYC) signaling (Figure 6C). This demonstrates the widespread role of GSK3 in keeping multiple signaling pathways in check but also highlights the importance of having a comprehensive set of assays to fully disclose the regulatory matrix that underlies a mutant phenotype.
Maintenance of Radial Glia outside of the Neuroepithelium
Although the maintenance of RG is multifaceted, an emerging concept in rodent development is that the apical region is especially important because dysregulation most often leads to premature differentiation. However, this is not a rule, as neural progenitor cells isolated in culture recapitulate the proper timing and sequence of neuron production (Shen et al., 2006). Other studies have further substantiated the concept that RG can divide in a normal sequence despite having compromised polarity and location. In vivo studies show that loss of aPKCλ (PRKCI) (Imai et al., 2006) or its downstream substrate MARCKS (Weimer et al., 2009) disrupts RG polarity and placement in the cortical wall, but in neither case do they cause major defects in the sequence or degree of neurogenesis. Randomization of spindle orientation and cleavage plane angle by LGN (GPSM2) knockdown impairs the coinheritance of apical and basal contacts in RG, converting them to progenitor cells that are localized outside of the VZ/SVZ that still resemble RG in marker expression. Surprisingly, despite the aberrant location of these progenitor cells, the rate of neurogenesis and cell types produced were relatively normal (Konno et al., 2008). Further characterization of these LGN-deficient progenitor cells showed that they resemble oRG cells in morphology and are born in the VZ by RG divisions that have an oblique/horizontal cleavage plane. These divisions split the radial process into two daughter cells; the more basal daughter cell inherits the basal fiber, translocates to the SVZ, and remains undifferentiated (Figure 6B) (Shitamukai et al., 2011). Importantly, small numbers of these cells were found to be present in normal mice (Wang et al., 2011) and also required Notch signaling for their maintenance (Shitamukai et al., 2011). These observations suggest that oRG cells, although infrequent in some species, are part of a conserved developmental mechanism that can be studied in mice.
These studies are highly reminiscent of what is seen in human development and suggest that, even in the rodent, correct neurogenesis is not necessarily tied to cell polarity or location. It will be important for future studies to describe the signaling state of displaced rodent RG cells compared to their ventricular counterparts. Further comparison with vRG and oRG cells in the human will help to refine our ideas about the requirements and mechanisms for oRG cell maintenance in the OSVZ. Do oRG cells have the same signaling requirements but have them met through OSVZ niche-specific means, or is there a higher degree of developmental cell autonomy that does not exist in the rodent?
In addition to the maintenance of oRG cells in the OSVZ, their production and expansion must also involve distinct modes of cell division such that the total number of RG cells is increased. Rodent studies that lead to overproduction of RG, for example by constitutive expression of β-catenin (Chenn and Walsh, 2002), Fgf10 deletion (Sahara and O’Leary, 2009), or loss of meningeal secreted factors (Siegenthaler et al., 2009), could offer relevant insight. Alterations in one or more of these mechanisms could have biased the production of cells toward RG instead of neurons during the evolution of larger brains. For example, neocortical growth and the proposed discontinuity of oRG and vRG basal fibers in humans could negatively affect RA signaling from the meninges, thereby promoting RG cell expansion.
Analysis of Gene Expression in Human Brain
An understanding of the molecular basis of human brain development and function, although challenging, is beginning. Limitations in tissue collection have forced the majority of molecular studies of human brain development to use global profiling approaches to identify interesting distinguishing features. These efforts have proven to be effective, for instance, by the identification and correlation of brain development genes with regions of the genome that have undergone human-specific evolutionary acceleration (Pollard et al., 2006). Global transcriptome analysis of the developing human brain has successfully identified and categorized expression patterns, alternative splicing, and differential regulation of genes in major brain regions (Johnson et al., 2009). Analysis of gene coexpression relationships in human brain has been effective at extracting cell type-specific expression patterns from unbiased data sets of whole-brain tissue (Oldham et al., 2008). These studies will undoubtedly build an enormous number of hypotheses and candidate regulators for the study of human brain development.
The next challenge will be to apply this information to the cellular mechanisms in our evolving understanding of fetal brain development, particularly that of OSVZ proliferation. Testing the importance of candidate genes identified from molecular studies of the human will be a critical first step. However, with the development of techniques that can acutely and robustly alter gene expression, the extension of these studies to both gyrencephalic and lissencephalic species, as well as multiple experimental platforms, will be worthwhile.
The success of these experimental approaches and validity of interspecies comparisons depends on whether evolution of brain size and shape in different species is based on related mechanisms. In the next sections, we will explore the generality of OSVZ proliferation and attempt to place these features in the context of neocortical evolution.
Origins of the SVZ and Six-Layered Neocortex
From the examination of multiple species, a rough correlation can be made between the generation of three-layered, six-layered, and gyrencephalic neocortex and specific features of progenitor cell organization during development. The cortex of reptiles consists of three layers (Ulinski, 1974; Goffinet, 1983; Cheung et al., 2007) comparable to layers I, V, and VI in mammals, which are generated by a reptile homolog of RG, an ependymoradial glial cell (Weissman et al., 2003). Ependymoradial glia form a ventricular neuroepithelium, and there is no SVZ in the developing reptile dorsal cortex (Cheung et al., 2007).
In contrast to reptiles, mammalian species have a six-layered neocortex, and all species examined so far appear to have a SVZ during embryonic neocortical development. However, the view that SVZ proliferation is necessary to generate a six-layered cortex has recently been called into question from studies in two metatherian (marsupial) species, Monodelphis domestica (opossum) and Macropus eugenii (tammar wallaby). These animals exhibit a distinctive SVZ layer, including cells that express TBR2 (a marker of neuronal commitment often associated with IP cells) as well as sparse labeling of the mitotic marker phospho-H3, suggesting the existence of SVZ neurogenesis (Cheung et al., 2010). However, a subsequent study found that these TBR2-expressing cells never incorporate BrdU, implying that they do not form a transit-amplifying progenitor cell class but are likely to be immature postmitotic neurons (Puzzolo and Mallamaci, 2010). These observations need to be reconciled, but they suggest that development of a proliferative SVZ may have occurred after the eutherian-metatherian split and that this feature may be more strictly correlated with eutherians (placental mammals) than mammals at large.
The Human Neocortex: A Scaled-up Primate Brain
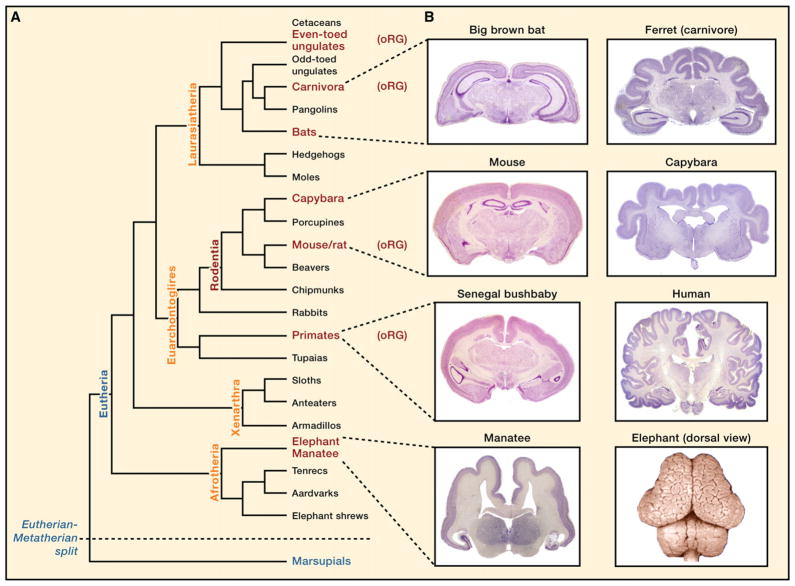
Studies of the human brain and comparisons of developmental proliferative zones between species may ultimately help to explain what makes the human brain unique. It is commonly thought that the exceptional cognitive abilities of humans are related to the large size of the neocortex. Recent evidence has shown that primates have greater neuronal density (neuron number/brain mass) compared to rodents of equal brain mass, a feature that is likely related to the topological differences in foldedness that could have been influenced by OSVZ proliferation. However, though the human brain is large by weight (1.5 kg) and neuron number (86 billion) (Azevedo et al., 2009; reviewed by Herculano-Houzel, 2009), this ratio does not deviate from what would be expected from a primate brain of similar mass, implying that, in terms of brain size and density, the human brain conforms to a scaled-up primate brain. Furthermore, developmental similarities between the human and ferret show that increased OSVZ proliferation and oRG cells are not primate-specific features (Figure 7A). The percentage of progenitor cells in the SVZ/OSVZ of rodents, carnivores, ungulates, and primates shows a remarkable positive correlation with the degree of neocortical gyrification (Reillo et al., 2010). Thus, development of OSVZ proliferation appears to be an important general feature for increasing neuronal number and neocortical surface area throughout Eutheria.
Figure 7. OSVZ Proliferation in the Context of Evolution.
(A and B) A phylogenetic tree (adapted from the Tree of Life project [http://tolweb.org] and WhoZoo Project [http://whozoo.org]) is shown to illustrate the diversity of brain size/shape in an evolutionary context. Eutherian (placental mammals) and metatherian (marsupial) species, the two major infraclasses of mammals, are included, whereas monotremes (egg-laying mammals), the other major order of extant mammals, are not. Species in which outer subventricular zone radial glia-like cells have been observed in embryonic tissues are labeled (oRG). Species that lack the oRG label have not yet been examined for the presence of these cells. Each eutherian superorder illustrated (A, orange) contains species that have lissencephalic (left) and gyrencephalic (right) brains (B, from the Comparative Mammalian Brain Collections [http://brainmuseum.org/]). Examples of both lissencephaly (armadillo) and gyrencephaly (sloth) also exist in the Xenarthra superorder (not shown). Thus, the evolution of gyrencephaly (or lissencephaly, as the case may be) was not an isolated event. oRG cells are present across multiple superorders, indicating that these features are not specific to a particular lineage of mammals. The degree of oRG-related proliferation and that of oRG-derived IP cells varies in different species and likely gives rise to diversity in neuronal number and brain shape. Images shown (B) are all coronal sections (not to scale) except for the elephant, which is a dorsal view
Diversity of Neocortical Foldedness
Whether an OSVZ exists in the developing neocortex of different mammalian species is difficult to address without direct observation of embryonic tissues, but topological comparisons of adult brains (Figure 7B) (Comparative Mammalian Brain Collections, http://brainmuseum.org) can be suggestive of developmental events. Interestingly, all eutherian superorders contain species that span the range of gyrencephalic to lissencephalic brains. For example, in the superorder Euarchontoglires, most members of Rodentia are lissencephalic and most primates are gyrencephalic. However, the capybara is a gyrencephalic rodent, and various primates, such as the marmoset, lemur, and particularly the Senegal bushbaby, are almost entirely lissencephalic. The next closest superorder, Laurasiatheria, includes a wide variety of lissencephalic (bats, shrews, and moles) and gyrencephalic (carnivores, ungulates, and cetaceans) species. Species within a third superorder, Afrotheria, also display a wide range of cortical configuration, from the extremely gyrencephalic elephant to its closest extant relative, the lissencephalic manatee.
These observations indicate that the development and evolution of gyrencephaly in Eutheria was not an isolated event but has occurred multiple times, probably with instances of both gain and loss of foldedness and likely through related cellular mechanisms that utilize SVZ proliferation in slightly different ways during development. For example, in ferret, the contiguous ISVZ and OSVZ contain similar proportions of progenitor cell types (~40% PAX6-positive cells and 45%–50% TBR2-positive cells) and are distinguished mainly by their cell density (Fietz et al., 2010; Reillo et al., 2010). By contrast, the human ISVZ consists mainly of TBR2-positive IP cells, with a limited number of new oRG cells en route to the OSVZ, which is separated from the ISVZ by an inner fiber layer (Smart et al., 2002; Bayatti et al., 2008; Hansen et al., 2010).
Beyond these anatomical differences, even the function of oRG cells has been proposed to be different between human and ferret. Evidence in human tissue suggests that oRG cells serve to augment both of the primary roles of ventricular RG—increasing neurogenesis and expanding the radial scaffolding for neuronal migration—and demonstrates a lineage relationship between oRG cells and neurogenic TBR2-expressing transit-amplifying cells (Hansen et al., 2010). In contrast, experiments in the ferret show little evidence for this neurogenic lineage in the OSVZ. Instead, oRG (or IRG) cells served mainly to expand the radial scaffold for neuronal migration and mostly gave rise to astrocytes (Reillo et al., 2010). If these observations are confirmed, the neurogenic and scaffolding roles of RG may need to be considered separately when connecting OSVZ proliferation with neocortical expansion in various species.
Despite potential differences in their function, the presence of oRG cells in the ferret, which is evolutionarily more distant from primates than are rodents, argues that this could be an ancestral feature that exists across different species, being utilized to facilitate neocortical expansion to variable extents. Indeed, the developing mouse neocortex contains rare instances of oRG cells (Shitamukai et al., 2011; Wang et al., 2011), which divide asymmetrically to generate neurons without transit amplification (Wang et al., 2011). Although these cells do not constitute a distinct proliferative zone, their sparse observation in a lissen-cephalic species supports the idea that oRG cells existed in the common ancestor of eutherians but that only in gyrencephalic species has the expansion of oRG cells and OSVZ proliferation been appreciably utilized as a means to generate bigger, more complex brains. That oRG cells appear to generate neurons directly in the mouse but indirectly through daughter IP cells in the human underscores how the evolutionary increase in neuron number is determined by a contribution of both these cell types.
Whether the common ancestor of eutherians possessed relatively abundant or rare numbers of oRG cells is unknown, but it is interesting to consider how evolution could have selected for either large or small brain size and that contexts wherein a smaller neocortex was beneficial could have led to an evolutionary loss of gyrencephaly. The degree to which oRG cells in different species undergo neurogenic versus gliogenic programs and the extent to which they support neuronal migration are therefore ways in which neural development can be finely tuned to achieve the diversity in brain size and shape that we observe today.
Conclusions
The studies summarized here suggest that an increase in sub-ventricular neurogenic divisions underlies the dramatic increase in neuron number in the evolutionary path to the human cerebral neocortex. In particular, oRG cells are the self-renewing building blocks of OSVZ proliferation and function to enhance both traditional roles of RG. They generate daughter transit-amplifying IP cells that expand neuron number, and they provide additional radial fiber guides for neuronal migration. Further identification of the mechanisms regulating large brain size will depend on a detailed description of cellular behaviors, signaling pathways, and gene expression patterns. This new understanding will shed further light on human neocortical development and evolution and provide a substrate for understanding neurodevelopmental diseases of neocortical function.
Supplementary Material
Acknowledgments
We thank Arturo Alvarez-Buylla, David Rowitch, and Daniel Lim for their critical reading of the manuscript and insightful comments. We also thank Stephanie Redmond for helping make the initial observation of immature neurons in the OSVZ (Figure 2A). Artwork in Figures 1, 2, 4, 5, and 6 is by Kenneth X. Probst. Due to space limitations, we apologize that many primary and historical publications have not been cited. This work was supported by grants from the NIH-NINDS, the Bernard Osher Foundation, and a California Institute for Regenerative Medicine Predoctoral Fellowship for J.H.L. (TG2-01153). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the California Institute for Regenerative Medicine or any other agency of the State of California.
Footnotes
Supplemental Information includes Extended Discussion and can be found with this article online at doi:10.1016/j.cell.2011.06.030.
References
- Alvarez-Buylla A, Theelen M, Nottebohm F. Mapping of radial glia and of a new cell type in adult canary brain. J Neurosci. 1988;8:2707–2712. doi: 10.1523/JNEUROSCI.08-08-02707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Inter-neuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Huang GJ, Cheung AF, Era T, Nishikawa S, Bikoff EK, Molnár Z, Robertson EJ, Groszer M. The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev. 2008;22:2479–2484. doi: 10.1101/gad.475408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CP, Cepko CL. Cellular migration patterns in the developing mouse cerebral cortex. Development. 1990;110:713–732. doi: 10.1242/dev.110.3.713. [DOI] [PubMed] [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Baala L, Briault S, Etchevers HC, Laumonnier F, Natiq A, Amiel J, Boddaert N, Picard C, Sbiti A, Asermouh A, et al. Homozygous silencing of T-box transcription factor EOMES leads to microcephaly with polymicrogyria and corpus callosum agenesis. Nat Genet. 2007;39:454–456. doi: 10.1038/ng1993. [DOI] [PubMed] [Google Scholar]
- Backman M, Machon O, Mygland L, van den Bout CJ, Zhong W, Taketo MM, Krauss S. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol. 2005;279:155–168. doi: 10.1016/j.ydbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JF, Lindsay S, Clowry GJ. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Neocortical Development. New York: Raven Press; 1991. [Google Scholar]
- Bentivoglio M, Mazzarello P. The history of radial glia. Brain Res Bull. 1999;49:305–315. doi: 10.1016/s0361-9230(99)00065-9. [DOI] [PubMed] [Google Scholar]
- Borrell V, Kaspar BK, Gage FH, Callaway EM. In vivo evidence for radial migration of neurons by long-distance somal translocation in the developing ferret visual cortex. Cereb Cortex. 2006;16:1571–1583. doi: 10.1093/cercor/bhj094. [DOI] [PubMed] [Google Scholar]
- Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos LS, Duarte AJ, Branco T, Henrique D. mDll1 and mDll3 expression in the developing mouse brain: role in the establishment of the early cortex. J Neurosci Res. 2001;64:590–598. doi: 10.1002/jnr.1111. [DOI] [PubMed] [Google Scholar]
- Chatzi C, Brade T, Duester G. Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. PLoS Biol. 2011;9:e1000609. doi: 10.1371/journal.pbio.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Cheung AF, Pollen AA, Tavare A, DeProto J, Molnár Z. Comparative aspects of cortical neurogenesis in vertebrates. J Anat. 2007;211:164–176. doi: 10.1111/j.1469-7580.2007.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AF, Kondo S, Abdel-Mannan O, Chodroff RA, Sirey TM, Bluy LE, Webber N, DeProto J, Karlen SJ, Krubitzer L, et al. The sub-ventricular zone is the developmental milestone of a 6-layered neocortex: comparisons in metatherian and eutherian mammals. Cereb Cortex. 2010;20:1071–1081. doi: 10.1093/cercor/bhp168. [DOI] [PubMed] [Google Scholar]
- Choi BH. Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study. J Neuropathol Exp Neurol. 1986;45:408–418. doi: 10.1097/00005072-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Costa MR, Wen G, Lepier A, Schroeder T, Götz M. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Jensh RP. Bloom & Fawcett: Concise Histology. 2. London: Hodder Arnold; 2002. [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Curr Opin Neurobiol. 2011;21:23–35. doi: 10.1016/j.conb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Fietz SA, Kelava I, Vogt J, Wilsch-Bräuninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- Fish JL, Dehay C, Kennedy H, Huttner WB. Making bigger brains-the evolution of neural-progenitor-cell division. J Cell Sci. 2008;121:2783–2793. doi: 10.1242/jcs.023465. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet AM. The embryonic development of the cortical plate in reptiles: a comparative study in Emys orbicularis and Lacerta agilis. J Comp Neurol. 1983;215:437–452. doi: 10.1002/cne.902150408. [DOI] [PubMed] [Google Scholar]
- Gohlke JM, Griffith WC, Faustman EM. Computational models of neocortical neuronogenesis and programmed cell death in the developing mouse, monkey, and human. Cereb Cortex. 2007;17:2433–2442. doi: 10.1093/cercor/bhl151. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Ontogeny and Phylogeny. Cambridge: Harvard University Press; 1977. [Google Scholar]
- Gunhaga L, Marklund M, Sjödal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Rubenstein JL, Kriegstein AR. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70:645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Götz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Götz M. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–3254. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science. 2002;298:840–843. doi: 10.1126/science.1074560. [DOI] [PubMed] [Google Scholar]
- Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 2005;25:10064–10073. doi: 10.1523/JNEUROSCI.2324-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Cereb Cortex. 2010. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Published online December 7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanović D, Geschwind DH, Mane SM, State MW, Sestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans D, Hack I, Frotscher M, Taylor V, Kemler R. Beta-catenin-mediated cell-adhesion is vital for embryonic forebrain development. Dev Dyn. 2005;233:528–539. doi: 10.1002/dvdy.20365. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat Neurosci. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat Cell Biol. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Koo BK, Lim HS, Song R, Yoon MJ, Yoon KJ, Moon JS, Kim YW, Kwon MC, Yoo KW, Kong MP, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Radial and horizontal deployment of clonally related cells in the primate neocortex: relationship to distinct mitotic lineages. Neuron. 1995;15:311–321. doi: 10.1016/0896-6273(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Noctor S, Martínez-Cerdeño V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat Rev Neurosci. 2006;7:883–890. doi: 10.1038/nrn2008. [DOI] [PubMed] [Google Scholar]
- Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. doi: 10.1016/s0166-2236(00)01581-2. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Levitt P, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultra-structural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Null M, Vasioukhin V. alphaE-catenin is not a significant regulator of beta-catenin signaling in the developing mammalian brain. J Cell Sci. 2008;121:1357–1362. doi: 10.1242/jcs.020537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Giroud P, Huissoud C, Berland M, Kennedy H, Dehay C. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuro-epithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- Marín-Padilla M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Comp Neurol. 1992;321:223–240. doi: 10.1002/cne.903210205. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M. Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development. 2004;131:3133–3145. doi: 10.1242/dev.01173. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Is Pax6 critical for neurogenesis in the human fetal brain? Cereb Cortex. 2008;18:1455–1465. doi: 10.1093/cercor/bhm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutch CA, Schulte JD, Olson E, Chenn A. Beta-catenin signaling negatively regulates intermediate progenitor population numbers in the developing cortex. PLoS ONE. 2010;5:e12376. doi: 10.1371/journal.pone.0012376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Wong WS, Clinton BK, Kriegstein AR. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai W, Nakatani S, Takahara T, Kainuma M, Masaoka M, Minobe S, Namihira M, Nakashima K, Sakakibara A, Ogawa M, Miyata T. Periventricular notch activation and asymmetric Ngn2 and Tbr2 expression in pair-generated neocortical daughter cells. Mol Cell Neurosci. 2009;40:225–233. doi: 10.1016/j.mcn.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Imayoshi I, Shimojo H, Nishi E, Kageyama R, McConnell SK. Visualization of embryonic neural stem cells using Hes promoters in transgenic mice. Mol Cell Neurosci. 2006;31:109–122. doi: 10.1016/j.mcn.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Oldham MC, Konopka G, Iwamoto K, Langfelder P, Kato T, Horvath S, Geschwind DH. Functional organization of the transcriptome in human brain. Nat Neurosci. 2008;11:1271–1282. doi: 10.1038/nn.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Berger B, Esclapez M. Origins of cortical GABAergic neurons in the cynomolgus monkey. Cereb Cortex. 2009;19:249–262. doi: 10.1093/cercor/bhn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KS, Salama SR, Lambert N, Lambot MA, Coppens S, Pedersen JS, Katzman S, King B, Onodera C, Siepel A, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443:167–172. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- Puzzolo E, Mallamaci A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural Dev. 2010;5:8. doi: 10.1186/1749-8104-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 1971;33:471–476. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, Sestan N. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- Reid CB, Tavazoie SF, Walsh CA. Clonal dispersion and evidence for asymmetric cell division in ferret cortex. Development. 1997;124:2441–2450. doi: 10.1242/dev.124.12.2441. [DOI] [PubMed] [Google Scholar]
- Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V. A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex. 2010;21:1674–1694. doi: 10.1093/cercor/bhq238. [DOI] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Sahara S, O’Leary DD. Fgf10 regulates transition period of cortical stem cell differentiation to radial glia controlling generation of neurons and basal progenitors. Neuron. 2009;63:48–62. doi: 10.1016/j.neuron.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat Embryol (Berl) 1979;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Hadjantonakis AK, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development. 2002;129:4843–4853. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Shitamukai A, Konno D, Matsuzaki F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J Neurosci. 2011;31:3683–3695. doi: 10.1523/JNEUROSCI.4773-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, McSherry GM. Gyrus formation in the cerebral cortex in the ferret. I Description of the external changes. J Anat. 1986a;146:141–152. [PMC free article] [PubMed] [Google Scholar]
- Smart IH, McSherry GM. Gyrus formation in the cerebral cortex of the ferret. II Description of the internal histological changes. J Anat. 1986b;147:27–43. [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF. Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci. 2010;30:7028–7036. doi: 10.1523/JNEUROSCI.6131-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker AM, Chenn A. Focal reduction of alphaE-catenin causes premature differentiation and reduction of beta-catenin signaling during cortical development. Dev Biol. 2009;328:66–77. doi: 10.1016/j.ydbio.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SS, Breen S. Radial mosaicism and tangential cell dispersion both contribute to mouse neocortical development. Nature. 1993;362:638–640. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461:524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulinski PS. Cytoarchitecture of cerebral cortex in snakes. J Comp Neurol. 1974;158:243–266. doi: 10.1002/cne.901580303. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385:313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Voigt T. Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol. 1989;289:74–88. doi: 10.1002/cne.902890106. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Jones SU, Weston JA. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron. 1999;23:71–81. doi: 10.1016/s0896-6273(00)80754-0. [DOI] [PubMed] [Google Scholar]
- Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsai JW, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware ML, Tavazoie SF, Reid CB, Walsh CA. Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cereb Cortex. 1999;9:636–645. doi: 10.1093/cercor/9.6.636. [DOI] [PubMed] [Google Scholar]
- Weimer JM, Yokota Y, Stanco A, Stumpo DJ, Blackshear PJ, Anton ES. MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development. 2009;136:2965–2975. doi: 10.1242/dev.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Koo BK, Im SK, Jeong HW, Ghim J, Kwon MC, Moon JS, Miyata T, Kong YY. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Yu X, Zecevic N. Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain. J Neurosci. 2011;31:2413–2420. doi: 10.1523/JNEUROSCI.5249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical sub-ventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Dev Neurobiol. 2011;71:18–33. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Woodhead GJ, Swaminathan SK, Noles SR, McQuinn ER, Pisarek AJ, Stocker AM, Mutch CA, Funatsu N, Chenn A. Cortical neural precursors inhibit their own differentiation via N-cadherin maintenance of beta-catenin signaling. Dev Cell. 2010;18:472–479. doi: 10.1016/j.devcel.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.