Abstract
A general method for determining the physical location of an origin of bidirectional DNA replication has been developed recently and shown to be capable of correctly identifying the simian virus 40 origin of replication (L. Vassilev and E. M. Johnson, Nucleic Acids Res. 17:7693-7705, 1989). The advantage of this method over others previously reported is that it avoids the use of metabolic inhibitors, the requirement for cell synchronization, and the need for multiple copies of the origin sequence. Application of this method to exponentially growing Chinese hamster ovary cells containing the nonamplified, single-copy dihydrofolate reductase gene locus revealed that DNA replication begins bidirectionally in an initiation zone approximately 2.5 kilobases long centered about 17 kilobases downstream of the DHFR gene, coinciding with previously described early replicating sequences. These results demonstrate the utility of this mapping protocol for identifying cellular origins of replication and suggest that the same cellular origin is used in both the normal and the amplified DHFR locus.
Full text
PDF
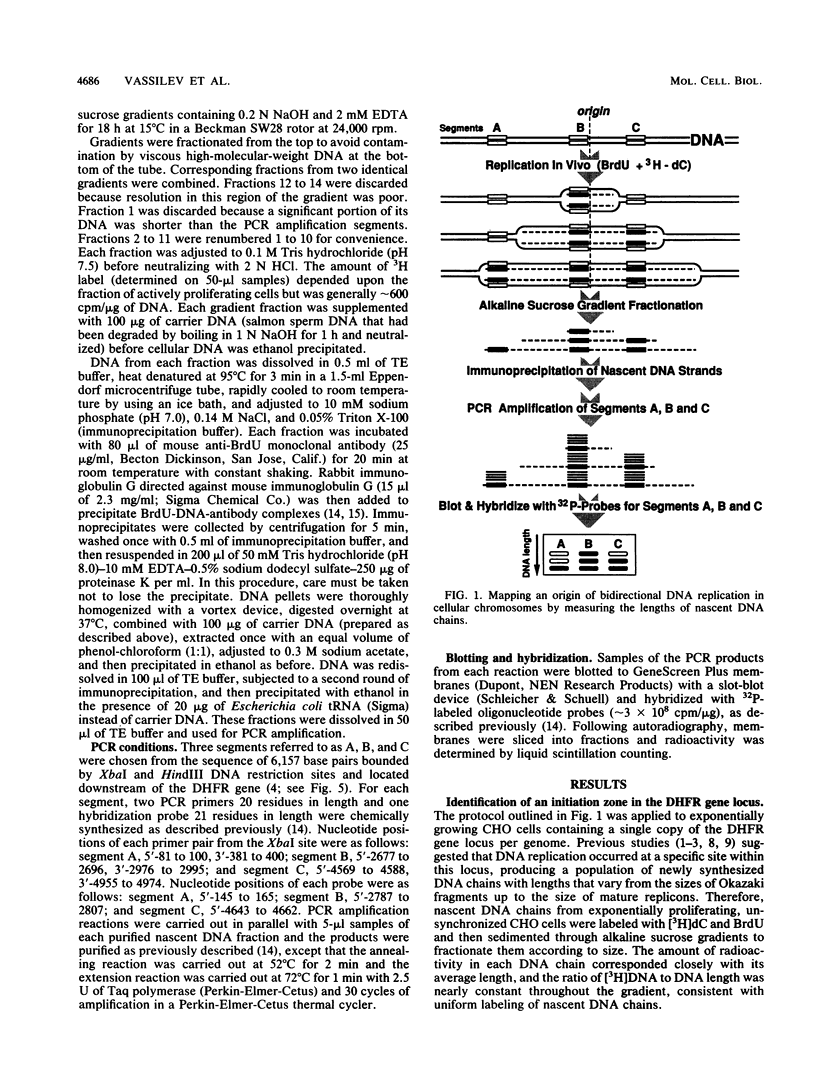
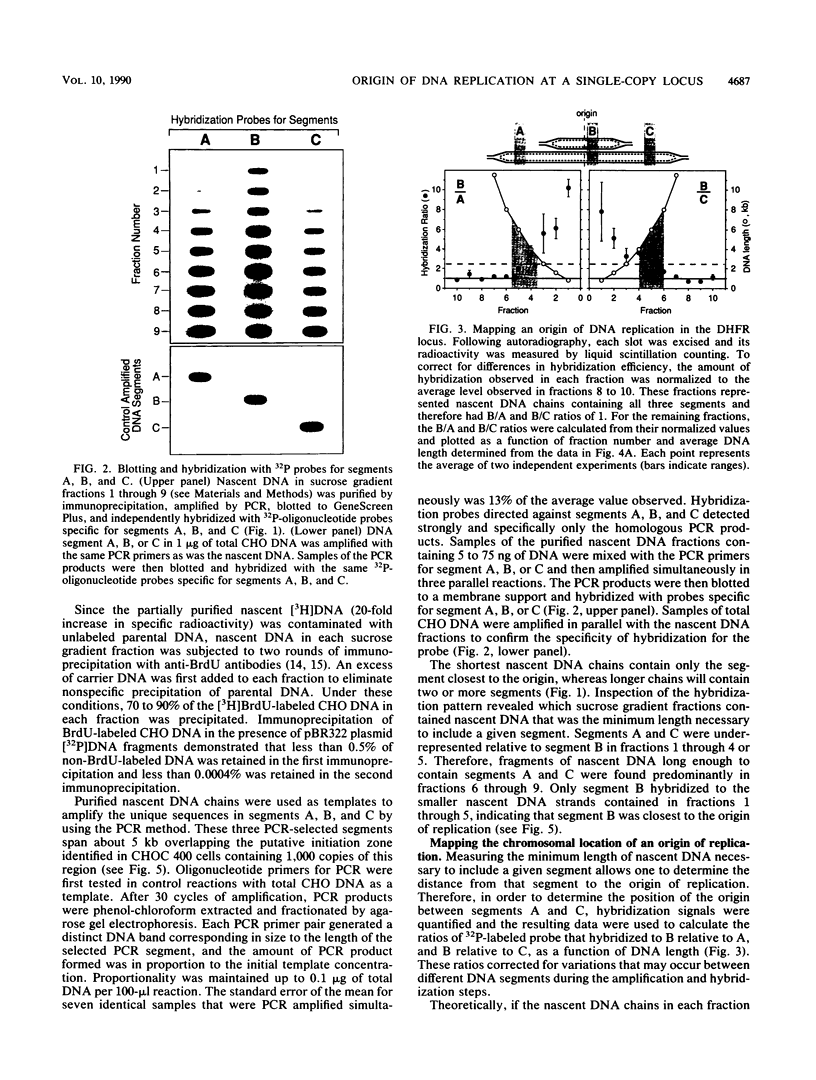
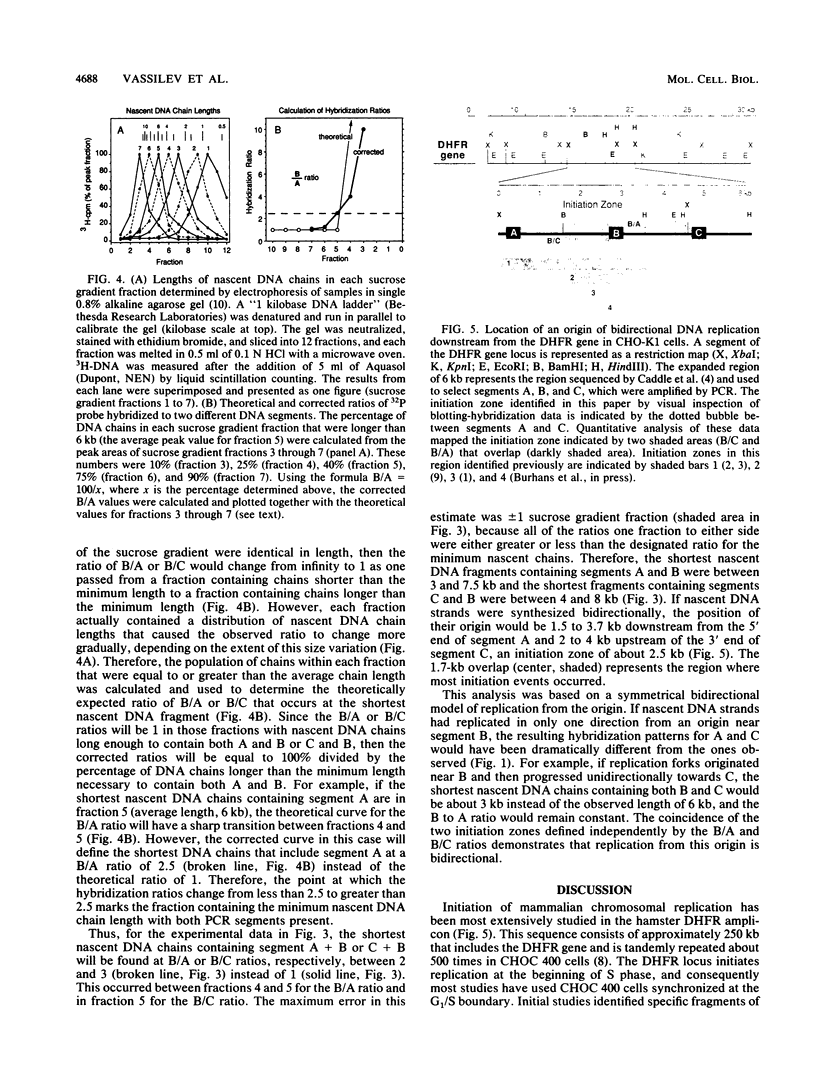

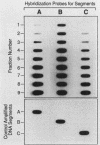
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anachkova B., Hamlin J. L. Replication in the amplified dihydrofolate reductase domain in CHO cells may initiate at two distinct sites, one of which is a repetitive sequence element. Mol Cell Biol. 1989 Feb;9(2):532–540. doi: 10.1128/mcb.9.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Isolation of the origin of replication associated with the amplified Chinese hamster dihydrofolate reductase domain. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7790–7794. doi: 10.1073/pnas.83.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhans W. C., Selegue J. E., Heintz N. H. Replication intermediates formed during initiation of DNA synthesis in methotrexate-resistant CHOC 400 cells are enriched for sequences derived from a specific, amplified restriction fragment. Biochemistry. 1986 Jan 28;25(2):441–449. doi: 10.1021/bi00350a025. [DOI] [PubMed] [Google Scholar]
- Caddle M. S., Lussier R. H., Heintz N. H. Intramolecular DNA triplexes, bent DNA and DNA unwinding elements in the initiation region of an amplified dihydrofolate reductase replicon. J Mol Biol. 1990 Jan 5;211(1):19–33. doi: 10.1016/0022-2836(90)90008-A. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Yamaguchi M., Possenti R., DePamphilis M. L. Initiation of simian virus 40 DNA replication in vitro: aphidicolin causes accumulation of early-replicating intermediates and allows determination of the initial direction of DNA synthesis. Mol Cell Biol. 1986 Nov;6(11):3815–3825. doi: 10.1128/mcb.6.11.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter-Gottlieb G., Kaufmann G. Aphidicolin arrest irreversibly impairs replicating simian virus 40 chromosomes. J Biol Chem. 1983 Mar 25;258(6):3809–3812. [PubMed] [Google Scholar]
- Handeli S., Klar A., Meuth M., Cedar H. Mapping replication units in animal cells. Cell. 1989 Jun 16;57(6):909–920. doi: 10.1016/0092-8674(89)90329-2. [DOI] [PubMed] [Google Scholar]
- Heintz N. H., Milbrandt J. D., Greisen K. S., Hamlin J. L. Cloning of the initiation region of a mammalian chromosomal replicon. 1983 Mar 31-Apr 6Nature. 302(5907):439–441. doi: 10.1038/302439a0. [DOI] [PubMed] [Google Scholar]
- Leu T. H., Hamlin J. L. High-resolution mapping of replication fork movement through the amplified dihydrofolate reductase domain in CHO cells by in-gel renaturation analysis. Mol Cell Biol. 1989 Feb;9(2):523–531. doi: 10.1128/mcb.9.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney C., Leffak M. Autonomous replication of a DNA fragment containing the chromosomal replication origin of the human c-myc gene. Nucleic Acids Res. 1990 Mar 11;18(5):1233–1242. doi: 10.1093/nar/18.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russev G., Vassilev L. Isolation of a DNA fraction from Ehrlich ascites tumour cells containing the putative origin of replication. J Mol Biol. 1982 Oct 15;161(1):77–87. doi: 10.1016/0022-2836(82)90279-0. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Linskens M. H., Kowalski D., Huberman J. A. New beginnings in studies of eukaryotic DNA replication origins. Biochim Biophys Acta. 1989 Jan 23;1007(1):1–14. doi: 10.1016/0167-4781(89)90123-1. [DOI] [PubMed] [Google Scholar]
- Vassilev L., Johnson E. M. An initiation zone of chromosomal DNA replication located upstream of the c-myc gene in proliferating HeLa cells. Mol Cell Biol. 1990 Sep;10(9):4899–4904. doi: 10.1128/mcb.10.9.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L., Johnson E. M. Mapping initiation sites of DNA replication in vivo using polymerase chain reaction amplification of nascent strand segments. Nucleic Acids Res. 1989 Oct 11;17(19):7693–7705. doi: 10.1093/nar/17.19.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L., Russev G. Purification of nascent DNA chains by immunoprecipitation with anti-BrdU antibodies. Nucleic Acids Res. 1988 Nov 11;16(21):10397–10397. doi: 10.1093/nar/16.21.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]