Abstract
Background:
Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (EBUS-TBNA) is performed with a dedicated 22- or 21-gauge needle while suction is applied. Fine-needle sampling without suction (capillary sampling) has been studied for endoscopic ultrasound and for biopsies at various body sites and has resulted in similar diagnostic yield and fewer traumatic samples. However, the role of EBUS-guided transbronchial needle capillary sampling (EBUS-TBNCS) is still to be determined.
Methods:
Adults with suspicious hilar or mediastinal lymph nodes (LNs) were included in a single-blinded, prospective, randomized trial comparing EBUS-TBNA and EBUS-TBNCS. The primary end point was the concordance rate between the two techniques in terms of adequacy and diagnosis of cytologic samples. The secondary end point was the concordance rate between the two techniques in terms of quality of samples.
Results:
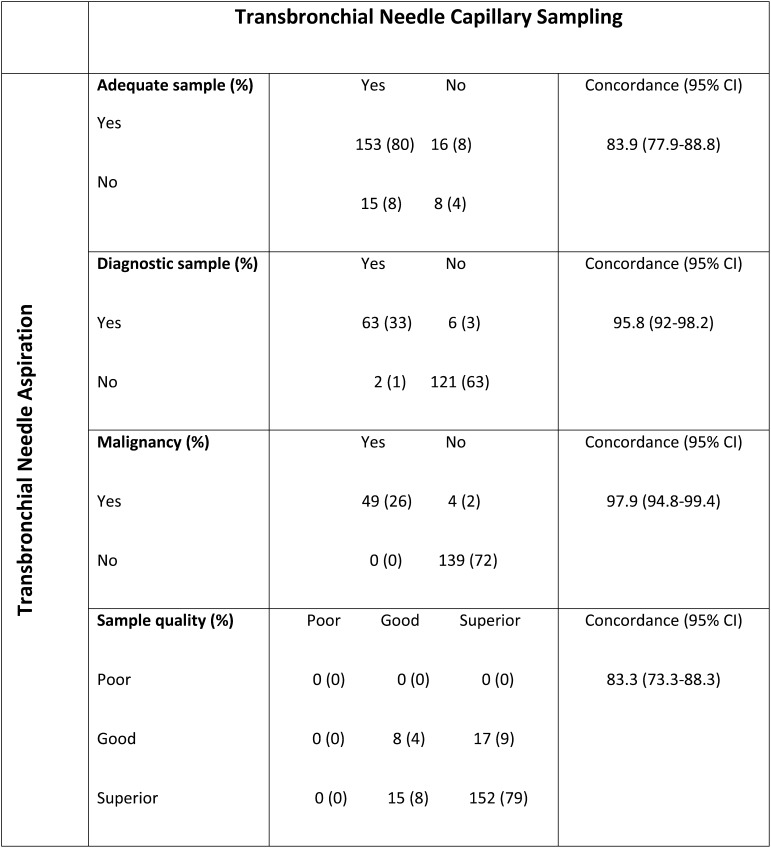
A total of 115 patients and 192 LNs were studied. Concordance between EBUS-TBNA and EBUS-TBNCS was high, with no significant difference in adequacy (88% vs 88%, respectively [P = .858]; concordance rate, 83.9% [95% CI, 77.9-88.8]); diagnosis (36% vs 34%, respectively [P = .289]; concordance rate, 95.8% [95% CI, 92-92.8]); diagnosis of malignancy (28% vs 26%, respectively [P = .125]; concordance rate, 97.9% [95% CI, 94.8-99.4]); or sample quality (concordance rate, 83.3% [95% CI, 73.3-88.3]). Concordance between EBUS-TBNA and EBUS-TBNCS was high irrespective of LN size (≤ 1 cm vs > 1 cm).
Conclusions:
Regardless of LN size, no differences in adequacy, diagnosis, or quality were found between samples obtained using EBUS-TBNA and those obtained using EBUS-TBNCS. There is no evidence of any benefit derived from the practice of applying suction to EBUS-guided biopsies.
Trial registry:
ClinicalTrials.gov; No.: NCT00886847; URL: www.clinicaltrials.gov
Endobronchial ultrasound (EBUS)-guided transbronchial needle aspiration (EBUS-TBNA) has become a valuable tool for diagnosing mediastinal and hilar lymphadenopathies because of its high diagnostic yield and favorable safety profile.1,2 In fact, EBUS-TBNA appears to be at least as effective as mediastinoscopy for mediastinal staging of non-small cell lung cancer and has a lower rate of complications, thereby making it the initial procedure of choice at many institutions.3 EBUS-TBNA is performed with a dedicated 22- or 21-gauge needle through which suction (aspiration) is applied while the needle is moved with a rapid stabbing motion inside the target lesion.1‐3 Fine-needle sampling without the use of suction (“capillary sampling”) has been studied at various sites (eg, breast, thyroid, peripheral lymph nodes [LNs]) and has resulted in a similar diagnostic yield as fine-needle aspiration with fewer traumatic samples.4‐6 A prospective randomized trial comparing endoscopic ultrasound (EUS)-guided needle capillary sampling with EUS-guided needle aspiration (including sampling of mediastinal LNs) revealed no difference in diagnostic yield but lower-quality samples, due to excessive blood, in the aspiration group.7 To date, however, the use of EBUS-guided transbronchial needle capillary sampling (EBUS-TBNCS) has not been studied. The purpose of our study was to compare EBUS-TBNA and EBUS-TBNCS of mediastinal and hilar LNs in terms of sample adequacy, diagnosis, and quality.
Materials and Methods
Subjects
Both outpatients and hospitalized patients older than 18 years of age with an indication for EBUS-guided needle biopsy based on suspicion of either benign or malignant disease in mediastinal or hilar LNs were enrolled in this study. The study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center (FWA No. IRB 5 IRB00006023), and written informed consent was obtained prior to enrollment.
Study Design
The study was a prospective randomized trial in which EBUS-TBNA and EBUS-TBNCS were compared. Cytologists were blinded to the technique used for biopsy. The primary end point was the concordance between the adequacy and diagnosis of cytologic samples of LNs obtained using EBUS-TBNA and the adequacy and diagnosis of cytologic samples of LNs obtained using EBUS-TBNCS. A sample size of 200 LNs was planned to allow us to estimate the degree of concordance between EBUS-TBNA and EBUS-TBNCS in terms of adequacy and diagnosis, with a 95% CI bound of 6.9%. We estimated an a priori agreement between the two techniques of at least 80%, which would yield a 95% CI bound of < 5.5%. The secondary end point was to compare the concordance rate in the quality of cytologic samples from LNs obtained using these two techniques. To perform this quality assessment, we used Mair’s5 score (e-Appendix 1 (342.9KB, pdf) ), values for which range from 0 to 10 and are classified as follows: 0 to 2 = poor; 3 to 6 = good; and 7 to 10 = superior. We tabulated the quality of samples obtained using EBUS-TBNA and EBUS-TBNCS and estimated with 95% CIs the proportions of samples that were of poor, good, and superior quality for each technique. Secondary stratified analyses were conducted based on LN size (≤ 1 cm vs > 1 cm in short-axis diameter) and type of stain (Romanowsky type vs Papanicoulau [Pap]).
Cytology results were categorized into one of the following five groups: inadequate material (defined as having a predominance of blood or bronchial epithelial cells), normal lymphoid tissue, granulomatous inflammation, necrosis, and malignancy. Samples were ranked from worst to best as inadequate material, normal lymphoid tissue, granulomatous inflammation, necrosis, and malignancy. For every LN, we selected the best sample for each sampling technique. For the outcome of sample adequacy, we dichotomized results as either “inadequate” (inadequate material) or “adequate” (normal lymphoid tissue, granulomatous inflammation, necrosis, or malignancy). To determine the concordance on diagnosis, we dichotomized results as either “diagnostic” (granulomatous inflammation or malignancy) or “nondiagnostic” (inadequate material, normal lymphoid tissue, or necrosis).
Study Procedures
EBUS-guided transbronchial needle biopsy was performed using a real-time ultrasound biopsy bronchoscope (XBF-UC260F-OL8; Olympus Ltd). A 7.5-MHz linear ultrasound transducer with a maximal penetration of 50 mm was linked to a processor (EU-60; Olympus Ltd). Transbronchial needle biopsies were performed using a dedicated 22-gauge needle (NA-201SX; Olympus Ltd). All patients were administered general anesthesia through a laryngeal mask airway. Only the first two LNs that were sampled in each patient were included in our study. Only LNs that were > 0.5 cm in short-axis diameter on EBUS were sampled. A total of two needle passes without suction (TBNCS) and two needle passes with suction (TBNA), applied with a 10-mL syringe, were performed at each LN. Each pass consisted of 20 to 30 needle thrusts within the LN. Patients were randomized using a computer to undergo TBNCS in passes 1 and 3 and TBNA in passes 2 and 4, or vice versa, to control for any “first-pass” bias. Passes 1 and 3 were done with one needle and passes 2 and 4 were done with a second needle to prevent cross-contamination. Two slides were prepared from each pass by an on-site cytotechnologist; one slide was stained using the Romanowsky technique, and the other was stained using the Pap technique. Only the initial four passes were included in the analysis; if these passes did not yield adequate material, additional passes were performed at the discretion of the EBUS operator but were not included in the study.
EBUS-guided endobronchial needle biopsy was performed by interventional pulmonologists (R. F. C., F. A. A., M. H. U., D. O., C. A. J., G. A. E., and R. C. M.). Cytology samples were reviewed initially by an on-site cytotechnologist, who assessed their adequacy, diagnosis, and quality. These slides and cell blocks were later reassessed by a single cytopathologist (G. A. S.). Both the cytotechnologist and the cytopathologist were blinded to the technique used to obtain the sample. The final reading used for analysis was that of the cytopathologist. The following data were also collected from the medical records of each patient: demographics, baseline malignancy, indication for EBUS (diagnostic, staging, or restaging), history of radiation therapy to the chest, and LNs sampled and their size (as assessed by EBUS).
Statistical Analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics of all patients in the study. The experimental unit was the LN, and all analyses were performed on a per-LN basis. We compared the quality (poor, good, or superior) of samples obtained using TBNA and TBNCS using McNemar test. Concordance between the TBNA and TBNCS with regard to adequacy, diagnosis, and quality of samples was estimated with 95% CIs. This analysis was repeated for slides stained using the Romanowsky or Pap technique and for LNs ≤ 1 cm or > 1 cm in short-axis diameter. As a sensitivity analysis, we also repeated these analyses for patients with only one LN sampled and for patients with two LN sampled. We used a two-sided P value of < .05 to define statistical significance. All analyses were performed using STATA software, version 11.2 (StataCorp).
Results
One hundred twenty patients were enrolled in the study. Two patients withdrew consent prior to the biopsy, and the biopsy was cancelled in three other patients. A total of 115 patients and 192 LNs were included in the analysis. The baseline characteristics of patients and LNs are summarized in Table 1. Figure 1 shows the adequacy, diagnosis, and quality of samples from all LNs (N = 192). Concordance between EBUS-TBNA and EBUS-TBNCS was high, and we found no significant difference between the two procedures in terms of the adequacy, diagnosis, or quality of samples. The percentage of adequate samples was 88% with both techniques (P = .858), with a concordance rate of 83.9% (95% CI, 77.9-88.8). A diagnosis (granulomatous inflammation or malignancy) was obtained in 36% and 34% of LNs using TBNA and TBNCS, respectively (P = .289), with a concordance rate of 95.8% (95% CI, 92-98.2). A diagnosis of malignancy was obtained in 28% and 26% of LNs using TBNA and TBNCS, respectively (P = .125), with a concordance rate of 97.9% (95% CI, 94.8-99.4). Assessment of sample quality using Mair’s5 score revealed no poor samples obtained using either technique, 13% and 12% good samples obtained using TBNA and TBNCS, respectively, and 87% and 88% superior samples obtained using TBNA and TBNCS, respectively (P = .724), with a concordance rate of 83.3% (95% CI, 73.3-88.3). Stratified analysis based on LN size (≤ 1 cm vs > 1 cm) revealed that concordance between EBUS-TBNA and EBUS-TBNCS remained high irrespective of LN size and revealed no significant difference between EBUS-TBNA and EBUS-TBNCS in terms of the adequacy, diagnosis, and quality of samples (see e-Appendix 1 (342.9KB, pdf) for details). Additional sensitivity analysis of patients who had one or two LNs sampled was done to assess bias due to the number of LNs sampled per patient. This analysis showed that the concordance between the two techniques remained high, regardless of whether one or two LNs were sampled. Concordance between EBUS-TBNA and EBUS-TBNCS was higher with the Pap technique than with the Romanowsky technique in terms of sample adequacy (80.2% vs 73.4%, P = .028), diagnosis (95.2% vs 89.6%, P = .012), diagnosis of malignancy (96.5% vs 92.2%, P = .007), and sample quality (81.8% vs 73.4%, P = .008).
Table 1.
—Baseline Characteristics of Patients and Lymph Nodes
| Characteristic | Value |
| No. patients | 115 |
| Age, median (range), y | 64 (27-86) |
| Sex | |
| Women | 60 (52) |
| Men | 55 (48) |
| Known baseline malignancy | |
| Yes | 87 (76) |
| No | 28 (24) |
| Procedure indication | |
| Diagnosis | 55 (48) |
| Staging | 28 (24) |
| Diagnosis and staging | 10 (9) |
| Restaging | 22 (19) |
| Previous chest irradiation | |
| Yes | 17 (15) |
| No | 98 (85) |
| Previous chemotherapy | |
| Yes | 31 (27) |
| No | 84 (73) |
| Lymph nodes, No. | 192 |
| Lymph node size, median (range), cm | 0.98 (0.47-4.02) |
| Lymph node station, No. | |
| 1R | 1 |
| 1L | 1 |
| 2R | 2 |
| 2L | 0 |
| 3 | 0 |
| 4R | 51 |
| 4L | 31 |
| 7 | 42 |
| 10R | 1 |
| 10L | 3 |
| 11R | 27 |
| 11L | 33 |
Data are presented as No. (%) unless indicated otherwise. L = left; R = right.
Figure 1.
Concordance between endobronchial ultrasound-guided transbronchial needle aspiration and endobronchial ultrasound-guided transbronchial needle capillary sampling in terms of the adequacy, diagnosis, and quality of samples from all sampled lymph nodes (N = 192).
Discussion
Our study showed a very high concordance rate in terms of the adequacy, diagnosis, and quality of samples obtained using EBUS-guided transbronchial needle biopsy with and without suction. Because EBUS-guided transbronchial needle biopsy has been established as a helpful tool for minimally invasive access to the mediastinum, it is important to evaluate and refine procedural techniques to enhance diagnosis and sample quality.
Since Paget8 first described fine-needle aspiration (performed on a breast tumor) in 1853, using suction has become a common practice for most pathologists, radiologists (for ultrasound- and CT scan-guided biopsies), and gastroenterologists (for EUS). However, the role of suction in fine-needle sampling remains somewhat controversial. Advocates argue that the cellularity of the samples is greater with suction, whereas opponents believe that suction draws more blood and fluid into the sample, thereby diluting it and decreasing its quality.9 Zajdela et al6 retrospectively compared fine-needle aspiration in 7,877 breast tumors with fine-needle capillary sampling in 635 breast tumors and found no difference in diagnostic yield or cellularity but less blood in samples obtained without the use of suction. Mair et al5 also found no difference in diagnostic yield between conventional fine-needle aspiration and fine-needle capillary sampling in a study of 100 superficial masses at various body sites. However, there was a trend toward better-quality samples with capillary sampling.
As mentioned previously, the use of fine-needle capillary sampling has been investigated in EUS-guided biopsy, which is more similar to EBUS-guided biopsy than breast or superficial biopsies. Wallace et al7 carried out a prospective randomized trial in which they compared EUS-guided biopsies (including those of mediastinal LNs) obtained with and without suction and found no difference in diagnostic yield. However, they did find that samples in the suction group were of lower quality because of excessive blood. Storch et al10 also prospectively studied EUS-guided biopsies obtained with and without suction in 53 patients and found no difference between the two procedures in terms of the quality or diagnostic accuracy of samples. Although we found a high concordance rate between EBUS-TBNA and EBUS-TBNCS in terms of diagnosis, EBUS-TBNCS did not yield superior-quality samples. Of note, our method of assessing sample quality was different from that of Wallace et al,7 and the amount of blood in the samples was only one of five components that were evaluated (see Mair’s score in e-Appendix 1 (342.9KB, pdf) ).
We hypothesized that when sampling smaller LNs with EBUS-guided needle biopsy, the needle would travel a shorter distance within the node and the specimens obtained might have less material if suction were not applied. However, when we compared EBUS-TBNA and EBUS-TBNCS for LNs ≤ 1 cm and > 1 cm in short-axis diameter, we found no difference in the adequacy, diagnosis, or quality of the samples. Despite traveling a shorter distance with the needle within the LN, suction does not seem to be required to guarantee diagnosis and a high-quality sample.
The concordance rate between EBUS-TBNA and EBUS-TBNCS was higher when slides were stained using the Pap technique and lower when slides were stained using the Romanowsky technique. We believe the concordance rate might have been different because slides were fixed with modified Carnoy solution before Pap staining but were air-dried only before Romanowsky staining. Hence, the RBCs aspirated with TBNA are not lysed when subjected to Romanowsky staining, and obscuring blood can be an issue.
The relatively low diagnostic yield documented in our study, especially for malignancy (28% and 26% using TBNA and TBNCS, respectively), can be explained partially by the fact that only the first two LNs sampled in each patient were included in this study. This led us to have many normal samples without cancer because we started staging systematically from N3 nodes if the suspected diagnosis was lung cancer. Our reason for including only the first two LNs for each patient in this study was to reduce the procedure time and risk to participants. Because the analysis was per LN and not per patient, we felt in our study design that it should not influence the results.
One limitation of our study was that we did not analyze the quality and number of cells in rinses (ie, tissues flushed from each needle pass once the two slides were prepared) obtained using each technique. A large number of cells is required for flow cytometry when non-Hodgkin’s lymphoma is suspected, and an adequate number of viable tumor cells is needed for molecular analysis such as epidermal growth factor receptor, anaplastic lymphoma kinase, and Kirsten rat sarcoma viral oncogene homolog mutations, which can help guide treatment of non-small cell lung cancer.
Another limitation could be related to the number of extensions/retractions of the needle within the LN that we used (20-30). Although there is no evidence regarding the ideal number, some authors could argue that the number we chose was high and could have led to more bloody samples, particularly with TBNA.
To our knowledge, this is the first randomized controlled study in which EBUS-guided transbronchial needle biopsy samples obtained with and without the use of suction were compared. In our randomized study, we found no differences between the two techniques in terms of the adequacy, diagnosis, and quality of samples, regardless of LN size.
Conclusions
EBUS-guided TBNA requires either the use of a syringe with a lock mechanism or an additional set of hands to apply and maintain suction during the biopsy, which can be obviated with the TBNCS technique. The authors recommend transbronchial needle capillary sampling as a simpler and equally effective technique for EBUS-guided biopsies of mediastinal and hilar LNs.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: Dr Casal takes full responsibility for the integrity of the work as a whole, from inception to published article.
Dr Casal: contributed to the study design, data collection, institutional review board application, EBUS performance, statistical analysis, data interpretation, and manuscript composition and revision.
Dr Staerkel: contributed to the study design, data collection, cytologic sample analysis, and manuscript revision.
Dr Ost: contributed to the study design, data collection, EBUS performance, statistical analysis, data interpretation, and manuscript composition and revision.
Dr Almeida: contributed to the EBUS performance, data collection, and manuscript revision.
Dr Uzbeck: contributed to the EBUS performance, data collection, and manuscript revision.
Dr Eapen: contributed to the study design, EBUS performance, data collection, and manuscript revision.
Dr. Jimenez: contributed to the study design, EBUS performance, data collection, data interpretation, and manuscript revision.
Ms Nogueras-Gonzalez: contributed to the statistical analysis, data interpretation, and manuscript revision.
Dr Sarkiss: contributed to the EBUS performance and manuscript revision.
Dr Morice: contributed to the study design, data collection, EBUS performance, statistical analysis, data interpretation, and manuscript composition and revision.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, collection and analysis of the data, or preparation of the manuscript.
Other contributions: We thank Mark Munsell, MS, from the Department of Biostatistics of The University of Texas MD Anderson Cancer Center for his statistical support. We also thank our Bronchoscopy Laboratory technicians and Betsy Jacobs, senior cytopathology technician, from the Department of Pathology and Laboratory Medicine for her contribution to the preparation and initial review of all cytology slides. Without their invaluable assistance, this study would not have been possible.
Additional information: The e-Appendix can be found in the “Supplemental Materials” area of the online article.
Abbreviations
- EBUS
endobronchial ultrasound
- EBUS-TBNA
endobronchial ultrasound-guided transbronchial needle aspiration
- EBUS-TBNCS
endobronchial ultrasound-guided transbronchial needle capillary sampling
- EUS
endoscopic ultrasound
- LN
lymph node
- Pap
Papanicoulau
For editorial comment see page 551
Funding/Support: This research was supported in part by the National Institutes of Health through a Cancer Center Support Grant [Grant CA016672] to The University of Texas MD Anderson Cancer Center.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61(9):795-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest. 2004;126(1):122-128 [DOI] [PubMed] [Google Scholar]
- 3.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3(6):577-582 [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Misra RK, Sharma SP, Singh HN, Chaturvedi AK. Aspiration vs nonaspiration technique of cytodiagnosis—a critical evaluation in 160 cases. Indian J Pathol Microbiol. 2000;43(2):107-112 [PubMed] [Google Scholar]
- 5.Mair S, Dunbar F, Becker PJ, Du Plessis W. Fine needle cytology—is aspiration suction necessary? A study of 100 masses in various sites. Acta Cytol. 1989;33(6):809-813 [PubMed] [Google Scholar]
- 6.Zajdela A, Zillhardt P, Voillemot N. Cytological diagnosis by fine needle sampling without aspiration. Cancer. 1987;59(6):1201-1205 [DOI] [PubMed] [Google Scholar]
- 7.Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54(4):441-447 [DOI] [PubMed] [Google Scholar]
- 8.Paget J. Lectures on Tumours. London, England: Longman; 1853 [Google Scholar]
- 9.Thomson HD. Thin needle aspiration biopsy. Acta Cytol. 1982;26(2):262-263 [PubMed] [Google Scholar]
- 10.Storch IM, Sussman DA, Jorda M, Ribeiro A. Evaluation of fine needle aspiration vs. fine needle capillary sampling on specimen quality and diagnostic accuracy in endoscopic ultrasound-guided biopsy. Acta Cytol. 2007;51(6):837-842 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement