Abstract
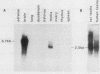
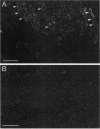
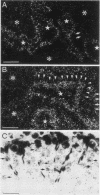
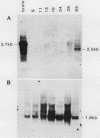
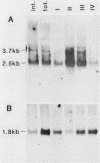
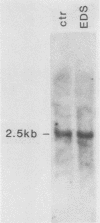
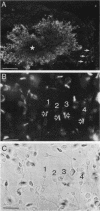
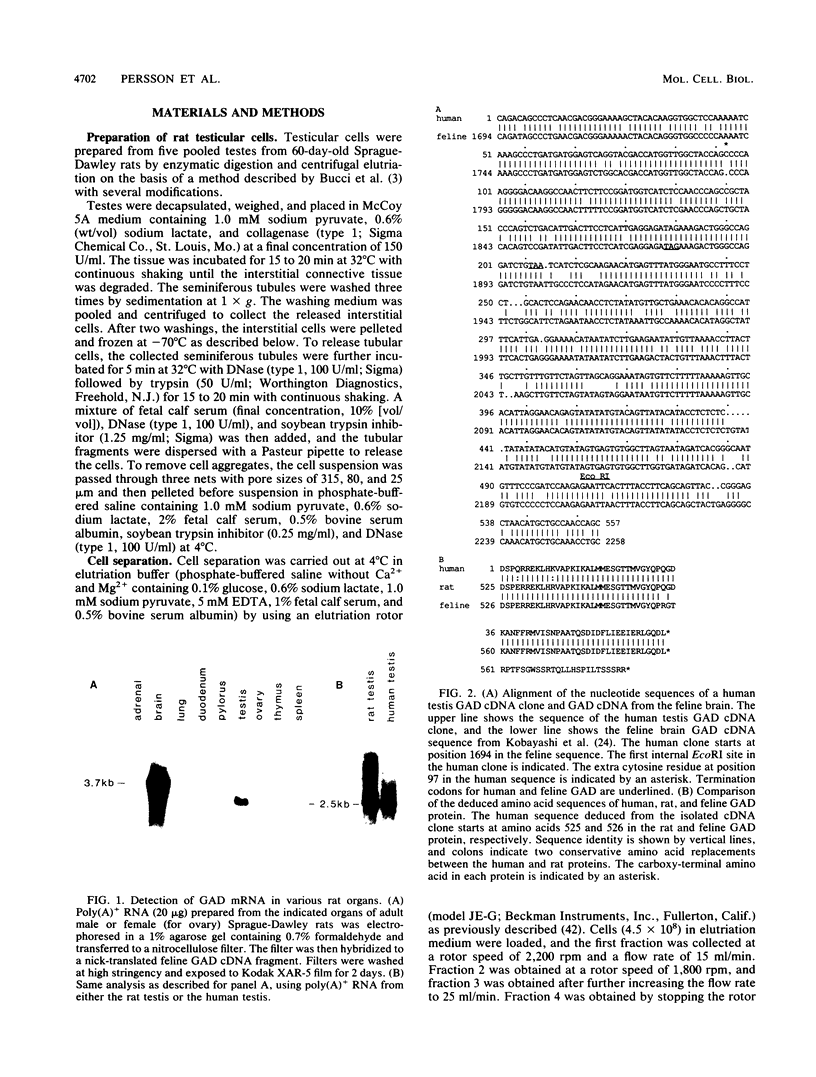
The gene encoding glutamic acid decarboxylase (GAD), the key enzyme in the synthesis of the inhibitory neurotransmitter gamma-aminobutyric acid, is shown to be expressed in the testis of several different species. Nucleotide sequence analysis of a cDNA clone isolated from the human testis confirmed the presence of GAD mRNA in the testis. The major GAD mRNA in the testis was 2.5 kilobases. Smaller amounts of a 3.7-kilobase mRNA with the same size as GAD mRNA in the brain was also detected in the testis. In situ hybridization using a GAD-specific probe revealed GAD mRNA expressing spermatocytes and spermatids located in the middle part of rat seminiferous tubules. Studies on the ontogeny of GAD mRNA expression showed low levels of GAD mRNA in testes of prepubertal rats, with increasing levels as sexual maturation is reached, compatible with GAD mRNA expression in germ cells. In agreement with this, fractionation of cells from the rat seminiferous epithelium followed by Northern (RNA) blot analysis showed the highest levels of GAD mRNA associated with spermatocytes and spermatids. Evidence for the presence of GAD protein in the rat testis was obtained from the demonstration of GAD-like immunoreactivity in seminiferous tubules, predominantly at a position where spermatids and spermatozoa are found. Furthermore, GAD-like immunoreactivity was seen in the midpiece of ejaculated human spermatozoa, the part that is responsible for generating energy for spermatozoan motility.
Full text
PDF
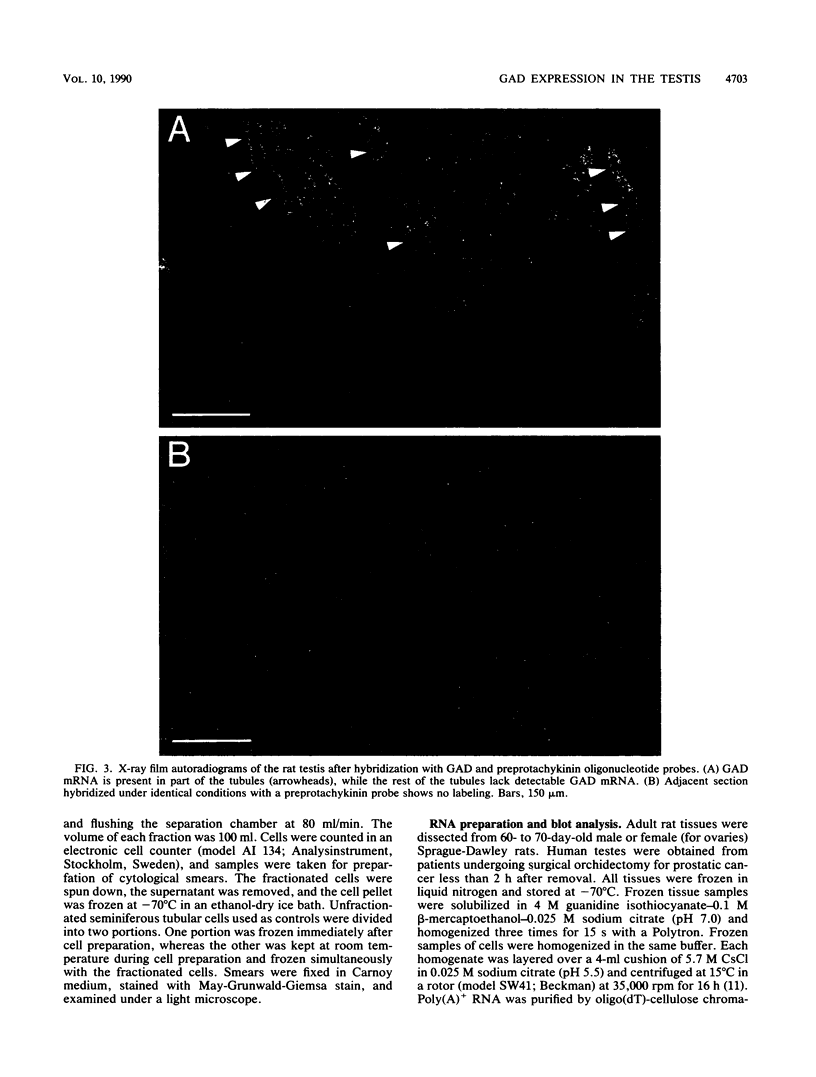
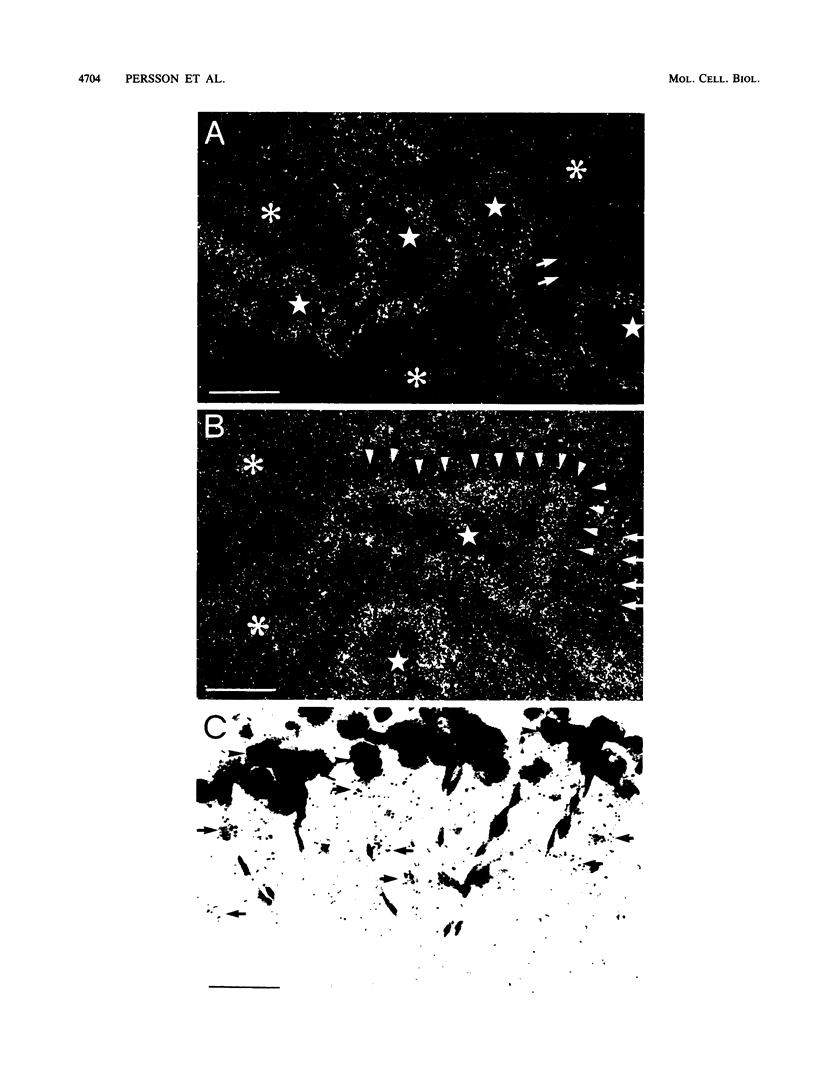
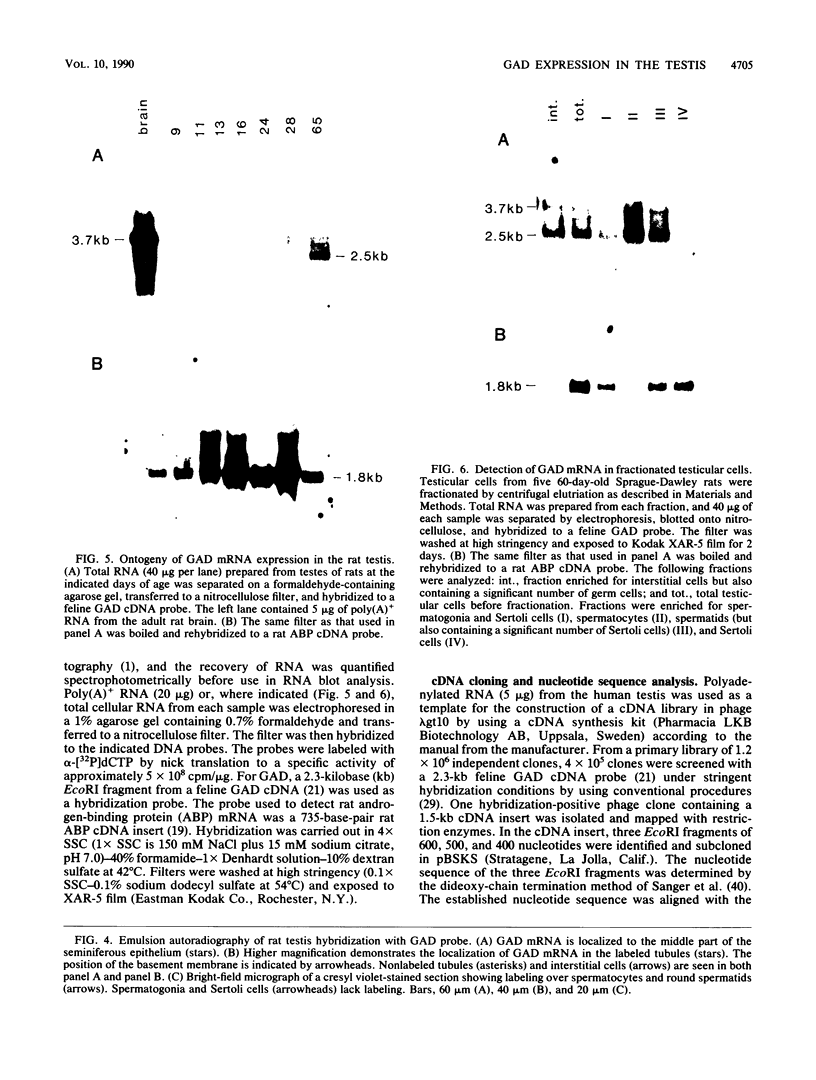

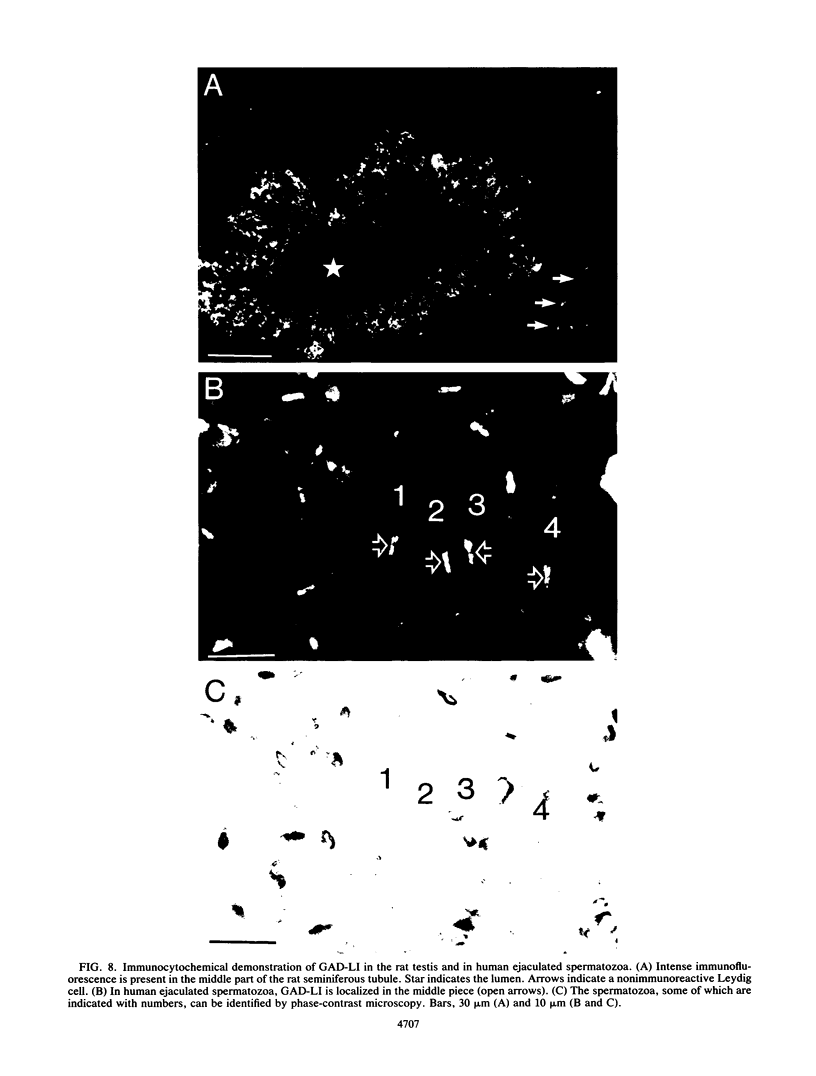




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci L. R., Brock W. A., Johnson T. S., Meistrich M. L. Isolation and biochemical studies of enriched populations of spermatogonia and early primary spermatocytes from rat testes. Biol Reprod. 1986 Feb;34(1):195–206. doi: 10.1095/biolreprod34.1.195. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn D. F., Homonnai Z. T., Paz G. F. The effect of anticonvulsant drugs on the development of male rats and their fertility. J Neurol Neurosurg Psychiatry. 1982 Sep;45(9):844–846. doi: 10.1136/jnnp.45.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Cox B., Quinn B., Civelli O., Herbert E. Expression of the prodynorphin gene in male and female mammalian reproductive tissues. Endocrinology. 1987 Feb;120(2):707–713. doi: 10.1210/endo-120-2-707. [DOI] [PubMed] [Google Scholar]
- Erdö S. L., Wolff J. R. gamma-Aminobutyric acid outside the mammalian brain. J Neurochem. 1990 Feb;54(2):363–372. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- Ericsson A., Schalling M., McIntyre K. R., Lundberg J. M., Larhammar D., Seroogy K., Hökfelt T., Persson H. Detection of neuropeptide Y and its mRNA in megakaryocytes: enhanced levels in certain autoimmune mice. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5585–5589. doi: 10.1073/pnas.84.16.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Hallbök F., Ebendal T., Shooter E. M., Radeke M. J., Misko T. P., Persson H. Developmental and regional expression of beta-nerve growth factor receptor mRNA in the chick and rat. Neuron. 1988 Dec;1(10):983–996. doi: 10.1016/0896-6273(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Geenen V., Legros J. J., Franchimont P., Baudrihaye M., Defresne M. P., Boniver J. The neuroendocrine thymus: coexistence of oxytocin and neurophysin in the human thymus. Science. 1986 Apr 25;232(4749):508–511. doi: 10.1126/science.3961493. [DOI] [PubMed] [Google Scholar]
- Gerendai I., Shaha C., Gunsalus G. L., Bardin C. W. The effects of opioid receptor antagonists suggest that testicular opiates regulate Sertoli and Leydig cell function in the neonatal rat. Endocrinology. 1986 May;118(5):2039–2044. doi: 10.1210/endo-118-5-2039. [DOI] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Wolgemuth D. J. Localization of mRNAs in mouse testes by in situ hybridization: distribution of alpha-tubulin and developmental stage specificity of pro-opiomelanocortin transcripts. Dev Biol. 1985 Oct;111(2):293–305. doi: 10.1016/0012-1606(85)90484-1. [DOI] [PubMed] [Google Scholar]
- Hagenäs L., Plöen L., Ekwall H., Osman D. I., Ritzén E. M. Differentiation of the rat seminiferous tubules between 13 and 19 days of age. Int J Androl. 1981 Apr;4(2):257–264. doi: 10.1111/j.1365-2605.1981.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Ivell R., Walther N., Morley S. Proopiomelanocortin cDNA sequences from the bovine ovary indicate alternative non-functional transcriptional initiation and a new polymorphism. Nucleic Acids Res. 1988 Aug 11;16(15):7747–7747. doi: 10.1093/nar/16.15.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. E., O'Leary P. C., Ayers M. M., de Kretser D. M. The effects of ethylene dimethane sulphonate (EDS) on rat Leydig cells: evidence to support a connective tissue origin of Leydig cells. Biol Reprod. 1986 Sep;35(2):425–437. doi: 10.1095/biolreprod35.2.425. [DOI] [PubMed] [Google Scholar]
- Jeannotte L., Burbach J. P., Drouin J. Unusual proopiomelanocortin ribonucleic acids in extrapituitary tissues: intronless transcripts in testes and long poly(A) tails in hypothalamus. Mol Endocrinol. 1987 Oct;1(10):749–757. doi: 10.1210/mend-1-10-749. [DOI] [PubMed] [Google Scholar]
- Joseph D. R., Hall S. H., French F. S. Identification of complementary DNA clones that encode rat androgen binding protein. J Androl. 1985 Nov-Dec;6(6):392–395. doi: 10.1002/j.1939-4640.1985.tb03301.x. [DOI] [PubMed] [Google Scholar]
- Julien J. F., Samama P., Mallet J. Rat brain glutamic acid decarboxylase sequence deduced from a cloned cDNA. J Neurochem. 1990 Feb;54(2):703–705. doi: 10.1111/j.1471-4159.1990.tb01928.x. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., McGinnis J. F., Krieger N. R., Tobin A. J. Brain glutamate decarboxylase cloned in lambda gt-11: fusion protein produces gamma-aminobutyric acid. Science. 1986 May 30;232(4754):1138–1140. doi: 10.1126/science.3518061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Rosenthal J. L. The proenkephalin gene is widely expressed within the male and female reproductive systems of the rat and hamster. Endocrinology. 1986 Jul;119(1):370–374. doi: 10.1210/endo-119-1-370. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kaufman D. L., Tobin A. J. Glutamic acid decarboxylase cDNA: nucleotide sequence encoding an enzymatically active fusion protein. J Neurosci. 1987 Sep;7(9):2768–2772. doi: 10.1523/JNEUROSCI.07-09-02768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J. E., Chirgwin J. M., Carter M. S., Xu Z. S., Hershey A. D. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci U S A. 1987 Feb;84(3):881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaze-Masmonteil T., de Keyzer Y., Luton J. P., Kahn A., Bertagna X. Characterization of proopiomelanocortin transcripts in human nonpituitary tissues. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7261–7265. doi: 10.1073/pnas.84.20.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindefors N., Brene S., Herrera-Marschitz M., Persson H. Region specific regulation of glutamic acid decarboxylase mRNA expression by dopamine neurons in rat brain. Exp Brain Res. 1989;77(3):611–620. doi: 10.1007/BF00249614. [DOI] [PubMed] [Google Scholar]
- Lindefors N., Brené S., Persson H. Increased expression of glutamic acid decarboxylase mRNA in rat substantia nigra after an ibotenic acid lesion in the caudate-putamen. Brain Res Mol Brain Res. 1990 Apr;7(3):207–212. doi: 10.1016/0169-328x(90)90029-d. [DOI] [PubMed] [Google Scholar]
- Margioris A. N., Liotta A. S., Vaudry H., Bardin C. W., Krieger D. T. Characterization of immunoreactive proopiomelanocortin-related peptides in rat testes. Endocrinology. 1983 Aug;113(2):663–671. doi: 10.1210/endo-113-2-663. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Gillo B., Sealfon S., Landau E. M. Activation of ionic currents in Xenopus oocytes by corticotropin-releasing peptides. Brain Res. 1988 Nov;464(3):201–205. doi: 10.1016/0169-328x(88)90026-5. [DOI] [PubMed] [Google Scholar]
- Pelto-Huikko M., Persson H., Schalling M., Rehfeld J. F., Hökfelt T. Immunocytochemical demonstration of cholecystokinin-like immunoreactivity in spermatozoa in monkey testis and epididymis. Acta Physiol Scand. 1989 Nov;137(3):465–466. doi: 10.1111/j.1748-1716.1989.tb08781.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Ericsson A., Schalling M., Rehfeld J. F., Hökfelt T. Detection of cholecystokinin in spermatogenic cells. Acta Physiol Scand. 1988 Dec;134(4):565–566. doi: 10.1111/j.1748-1716.1998.tb08534.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Rehfeld J. F., Ericsson A., Schalling M., Pelto-Huikko M., Hökfelt T. Transient expression of the cholecystokinin gene in male germ cells and accumulation of the peptide in the acrosomal granule: possible role of cholecystokinin in fertilization. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6166–6170. doi: 10.1073/pnas.86.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalling M., Dagerlind A., Brené S., Hallman H., Djurfeldt M., Persson H., Terenius L., Goldstein M., Schlesinger D., Hökfelt T. Coexistence and gene expression of phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and neuropeptide tyrosine in the rat and bovine adrenal gland: effects of reserpine. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8306–8310. doi: 10.1073/pnas.85.21.8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog S., Tribukait B., Sundius G. Energy metabolism and ATP turnover time during the cell cycle of Ehrlich ascites tumour cells. Exp Cell Res. 1982 Sep;141(1):23–29. doi: 10.1016/0014-4827(82)90063-5. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Blalock J. E. Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7530–7534. doi: 10.1073/pnas.78.12.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Seasholtz A. F., Herbert E. Rat corticotropin-releasing hormone gene: sequence and tissue-specific expression. Mol Endocrinol. 1987 May;1(5):363–370. doi: 10.1210/mend-1-5-363. [DOI] [PubMed] [Google Scholar]
- Tsong S. D., Phillips D., Halmi N., Liotta A. S., Margioris A., Bardin C. W., Krieger D. T. ACTH and beta-endorphin-related peptides are present in multiple sites in the reproductive tract of the male rat. Endocrinology. 1982 Jun;110(6):2204–2206. doi: 10.1210/endo-110-6-2204. [DOI] [PubMed] [Google Scholar]
- Westly H. J., Kleiss A. J., Kelley K. W., Wong P. K., Yuen P. H. Newcastle disease virus-infected splenocytes express the proopiomelanocortin gene. J Exp Med. 1986 Jun 1;163(6):1589–1594. doi: 10.1084/jem.163.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Matsuda T., Roberts E. Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem. 1973 May 10;248(9):3029–3034. [PubMed] [Google Scholar]
- Yoon D. J., Sklar C., David R. Presence of immunoreactive corticotropin-releasing factor in rat testis. Endocrinology. 1988 Feb;122(2):759–761. doi: 10.1210/endo-122-2-759. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]