Abstract
In addition to adipocytokines, estradiol (E2) and vitamin D have been reported to affect insulin sensitivity, glucose homeostasis and body weight. However, studies about the impact of E2 and vitamin D on metabolic syndrome (MetS) are still limited. The aim of this study is to clarify the roles of circulating E2 and vitamin D on the risk of MetS in middle-aged Taiwanese males. A total of 655 male volunteers, including 243 subjects with MetS (mean age: 56.7±5.8 years) and 412 normal controls (mean age: 55.1±3.6 years), were evaluated. Subjects with MetS had significantly lower circulating E2, 1,25(OH)2D3, and adiponectin, and higher leptin than those without MetS (P<0.001 for all comparisons). E2 and 1,25(OH)2D3 were significantly associated with 4 individual components of MetS; more than adiponectin and leptin that were only associated with 3 individual components. In multivariate regression analysis, E2 (beta = −0.216, P<0.001) and 1,25(OH)2D3 (beta = 0.067, P = 0.045) were still significant predictors of MetS independent of adiponectin and leptin. Further large studies are needed to confirm our preliminary results and elucidate the possible mechanism.
Introduction
Metabolic syndrome (MetS) is a collection of cardiometabolic risk factors, including obesity, insulin resistance, hypertension, and dyslipidemia. Both MetS and type 2 diabetes mellitus (T2DM) are closely related to the increasing risk of developing coronary heart disease and cardiovascular disease (CVD) [1], [2], [3], [4], [5], [6], [7] which could manifest severe and fatal consequences. Therefore, the prevention of MetS is essential.
Numerous factors, such as hormones, adipocytokines and vitamin D have been reported to be associated with the pathogenesis of MetS. Estradiol (E2) has been reported to be able to affect insulin sensitivity, glucose homeostasis, body weight and adiposity [8], [9]. In addition, adipocytokines like adiponectin and leptin are known to play important roles in the development of MetS. Low adiponectin status has been reported to be associated with obesity, MetS, and CVD [10], [11], [12], [13], while high leptin or leptin resistance status has been reported to be associated with obesity, MetS and CVD [13], [14], [15], [16].
The best model proposed, which links sex hormones with MetS, is polycystic ovary syndrome(POS). POS, a common disorder for women, is associated with a low estradiol-to-testosterone ratio in oligo-anovulatory cycles and atherogenic lipidic patterns resulting from the suppression of E2 production by potent endogenous aromatase inhibitors [17], [18], [19]. Recently, Saltiki et al reported that endogenous estrogen levels were related to endothelial function in males independent of lipid levels [20]. However, association studies between E2 and MetS in the middle-aged male population are still limited.
Vitamin D deficiency has been reported to be associated with increased risk of CVD, including hypertension, heart failure, and ischemic heart disease [21] in addition to MetS [22], [23], [24], [25]. A significant, positive correlation between 25-hydroxyvitamin D (25(OH)D) levels and adiponectin levels was also found in subjects with abnormal glucose tolerance and in a young Middle-Eastern population [26], [27]. 1alpha-hydroxylase, responsible for the final step in vitamin D activation, is associated with type 1 DM and is responsible for calcitriol-related complications and MetS [28], [29], [30], [31]. In contrast to 25(OH)D, calcitriol (the activated form of vitamin D also known as 1,25-dihydroxyvitamin D or 1,25(OH)2D3) works on cardiac muscle directly, regulates hormone secretion of the parathyroid gland, and affects the renin-angiotensin-aldosterone system, and immune system [32]. However, the associations between vitamin D (1,25(OH)2D3 or calcitriol) levels and the risk of Met or its individual components have not yet been completely clarified.
Therefore, the aim of this study was to evaluate the impact of the levels of E2 and 1,25(OH)2D3, beyond adipocytokines, on the risk of MetS and its individual components in a middle-aged Taiwanese male population.
Materials and Methods
Subjects and study protocol
The cross-sectional data of 694 Taiwanese males (age: 44–77 years) were collected from a free health screening held by a medical center in Kaohsiung city, Taiwan. Ethics approval following the Declaration of Helsinki was authorized by the Institutional Research Ethics Committee of Kaohsiung Medical University Hospital and informed written consent was obtained from each participant. Men who had previously been diagnosed with hypertension, DM, or hyperlipidemia (kept under control by regular medication) were included in the study, but men who were diagnosed as labile for hypertension, labile for diabetes, having current malignancy, advanced liver and/or renal disease or who were using hormones, antiandrogen treatment, antifungal drugs, or steroidal agents were excluded [3], [33].
A complete medical, surgical, and psychosexual history and the results from detailed physical examinations, including measurement of the body weight, height, and blood pressure, were recorded for each subject. Fasting blood samples were also taken for further biochemical analysis and hormone profiling. Body mass index (BMI) (kg/m2) was calculated as the ratio of the body weight and the square of body height. Subjects were classified as alcohol drinkers, cigarette smokers, or betel nut chewers if they had regularly consumed any alcoholic beverage ≥1 times per week, had smoked ≥10 cigarettes per week, or had chewed ≥7 betel quids per week respectively, for at least 6 months. Current users were those who were still using any of these substances within one year before the interview. Former users were defined as those who had stopped any of these habits for at least 1 year before interview [34], [35]. Hypertension was defined by a systolic blood pressure (SBP) of ≥140 mmHg or a diastolic blood pressure (DBP) of ≥90 mmHg, while hyperlipidemia was defined by a total cholesterol level of ≥200 mg/dL or a triglycerides level of ≥200 mg/dL [3], [36]. DM was diagnosed when the fasting blood glucose (FBG) was ≥126 mg/dL. An individual was diagnosed with MetS if he was positive for at least three of the five following criteria: (1) waist circumference (WC) ≥90 cm; (2) high density lipoprotein (HDL) cholesterol <40 mg/dL; (3) triglyceride (TG) ≥150 mg/dL; (4) blood pressure (BP) ≥130/85 mm Hg or diagnosed as hypertensive and on therapy; (5) fasting blood glucose (FBG) ≥100 mg/dL or diagnosed as type 2 diabetes mellitus (DM), in accordance with the modified criteria proposed by the Bureau of Health Promotion in Taiwan [3], [7], [37].
Biochemical analysis using radioimmunoassay
Peripheral venous blood samples after more than 8 hours fasting overnight were drawn into pyrogen-free tubes for analysis of serum glucose, lipid panels, and routine biochemical profiles. Through radioimmunoassay (RIA), E2 was measured using the Siemens' RIA kits (Los Angeles, USA; Inter-assay coefficient of variation (CV): 4.0%∼7.0%; Inter-assay CV: 4.2%∼8.1%), while 1,25(OH)2D3 was determined using the DiaSorin RIA kits (Northwestern Avenue Stillwater, USA; Intra-assay CV: 6.8%∼11.3%; Inter-assay CV:12.3%∼15.3%). Leptin and adiponectin were measured using Millipore's RIA kits (Missouri, USA; Intra-assay CV: 3.4%∼8.3% and 1.78%∼6.21% respectively; Inter-assay CV: 3.0%∼6.2% and 6.90%∼9.25% respectively). SHBG levels (detectable range: 0.2–180 nmol/L; inter-assay CV of 4.8%, and intra-assay CV of 3.5%) were determined using a DPC Immulite analyzer (Diamond Diagnostics, Holliston, MA). Free E2 was calculated based on mass action laws with Vermeulen's formula [38], [39].
Statistical analysis
Quantitative demographic and laboratory data were presented as mean ± standard deviation (SD). To quantify the differences between subjects with MetS and without MetS, qualitative variables were compared using the chi-square test and Fisher's exact test, while quantitative variables were compared using the Student's t-test. A one-way analysis of variance (ANOVA) with the LSD post-hoc test was used to compare the differences in the quantitative variables between the various components of MetS. Correlations between clinical characteristics, biochemical variables, and MetS were analyzed by Spearman's correlation with a correlation coefficient (r) of 0–0.25 indicating little to no correlation, 0.26–0.50 for a fair correlation, 0.51–0.75 for a moderate to good correlation, greater than 0.76–0.99 for a good to excellent correlation, and 1.00 for a perfect linear relationship. Any variables with significant association with the risk of MetS in the initial analyses were further examined in multivariate regression analyses to determine the independent risk factors for MetS. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Baseline characteristics
Among the 694 participants, 39 were excluded from the study because they failed to follow through with the complete biochemistry profile, thus, 655 men were included in this study. Participants were divided into two groups according to the presence or absence of MetS. The baseline characteristics and biochemical data of the subjects with MetS (n = 243) and without MetS (n = 412) are summarized in Table 1 . Subjects with MetS had significant increases in age, BMI, current habits of smoking, drinking, betel quid chewing, and prevalence of CVD when compared to those without MetS. The MetS group also had significantly higher level of leptin, but lower levels of adiponectin, E2, and 1,25(OH)2D3. However, free E2 level was not significantly different between the two groups.
Table 1. Means ± standard deviations of the baseline characteristics and biochemical variables in the subjects with and without MetS.
| Subjects without MetS (n = 412) | Subjects with MetS (n = 243) | P value | |
| Age (yrs) | 55.1±3.6 | 56.7±5.8 | <0.001* |
| BMI (Kg/m2) | 24.4±2.3 | 27.6±9.1 | <0.001* |
| Waist circumference (cm) | 83.4±5.6 | 90.6±6.8 | <0.001* |
| Hip circumference (cm) | 95.3±6.7 | 106.8±86.8 | 0.039* |
| Waist-hip ratio | 0.96±1.83 | 0.91±0.08 | 0.21 |
| SBP (mm-Hg) | 128.4±11.8 | 136.5±10.8 | <0.001* |
| DBP (mm-Hg) | 80.5±8.2 | 86.3±8.5 | <0.001* |
| DM n (%) | 16 (3.9) | 47 (19.3) | <0.001* |
| Hypertension, n (%) | 71 (17.2) | 115 (47.3) | <0.001* |
| Dyslipidemia history, n (%) | 40 (9.7) | 89 (36.6) | <0.001* |
| CVD history, n (%) | 16 (3.9) | 29 (11.9) | <0.001* |
| Smoking, n (%) | 0.002* | ||
| Nonsmokers | 315 (76.5) | 160 (65.8) | |
| Former Smokers | 57 (13.8) | 37 (15.2) | |
| Current Smokers | 40 (9.7) | 44 (18.1) | |
| Drinking, n (%) | 0.002* | ||
| Non-drinker | 356 (86.4) | 182(74.9) | |
| Former Drinkers | 6 (1.5) | 11 (4.5) | |
| Current Drinkers | 50 (12.1) | 50 (20.6) | |
| Betel quid, n (%) | 0.022* | ||
| Never Chewed | 404 (98.1) | 227 (93.4) | |
| Former Chewers | 6 (1.5) | 13 (5.3) | |
| Current Chewers | 1 (0.2) | 2 (0.8) | |
| Blood Biochemistry | |||
| Fasting glucose (mg/dl) | 94.0±14.1 | 109.1±24.8 | <0.001* |
| TG (mg/L) | 108.6±85.0 | 177.6±92.7 | <0.001* |
| Total cholesterol (mg/dl) | 189.5±33.9 | 190.3±31.6 | 0.77 |
| HDL-C (mg/dl) | 51.6±11.0 | 41.8±8.5 | <0.001* |
| Adiponectin (ng/ml) | 14.3±6.9 | 7.8±4.4 | <0.001* |
| Leptin (ng/ml) | 3.3±1.9 | 5.3±2.7 | <0.001* |
| 1,25(OH)2D3 (pg/ml) | 47.7±19.2 | 40.6±16.1 | <0.001* |
| E2 (pg/ml) | 26.3±8.1 | 19.7±9.1 | <0.001* |
| Free E2 (pg/ml) | 0.7±0.3 | 3.0±40.0 | 0.35 |
| SHBG(nmol/L) | 49.7±24.7 | 37.0±15.5 | <0.001* |
| Albumin(g/dl) | 4.4±0.2 | 4.5±0.2 | <0.001* |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; DM: diabetes mellitus; CVD: cardiovascular disease; TG: triglyceride; HDL: high density lipoprotein; CHOL(T): total cholesterol; E2: estradiol; SHBG: sex hormone–binding globulin;
: Significant difference (P<0.05).
Clinical characteristics and biochemical variables in subjects with various number of MetS components
The clinical characteristics and biochemical data of subjects with various numbers of MetS components are summarized in Table 2 . As the number of MetS components increased, age, BMI, and leptin level increased, but adiponectin, E2, and 1,25(OH)2D3 levels decreased significantly (P<0.001 for all comparisons). Furthermore, as the number of MetS components increased, adiponectin and 1,25(OH)2D3 decreased but leptin levels increased in a linear shape. Only E2 levels decreased in a ladder shape as the numbers of MetS components increased. ( Figure 1 )
Table 2. Means ± standard deviations of the clinical characteristics and biochemical variables in subjects with various numbers of metabolic syndrome (MetS) components.
| Subjects without MetS | Subjects with MetS | P value | |||||
| Numbers of MetS Components | 0 (n = 95) | 1 (n = 183) | 2 (n = 134) | 3 (n = 139) | 4 (n = 82) | 5 (n = 22) | |
| Age (yrs) | 54.8±3.0 | 55.1±3.0 | 55.3±4.6 | 56.3±5.2a b | 57.3±6.7a b c | 57.3±6.2a b | <0.001* |
| BMI (Kg/m2) | 23.3±2.1 | 24.3±2.1 | 25.1±2.4a | 26.2±2.3a b | 29.6±15.1a b c | 28.9±2.3a b c | <0.001* |
| Adiponectin (ng/ml) | 16.8±8.3 | 14.7±6.6a | 12.0±5.5a b | 8.4±4.6a b c | 7.2±4.2a b c | 5.6±3.1a b c d | <0.001* |
| Leptin (ng/ml) | 2.7±1.4 | 3.2±1.6a | 3.8±2.3a b | 4.7±2.3a b c | 6.1±3.2a b c d | 6.3±1.9a b c d | <0.001* |
| E2 (pg/ml) | 26.0±8.4 | 26.9±7.6 | 26.0±8.8 | 19.6±9.1a b c | 19.8±9.4a b c | 19.7±10.0a b c | <0.001* |
| 1,25(OH)2D3 (pg/ml) | 45.4±16.2 | 49.3±21.5 | 47.3±18.0 | 43.1±16.3b | 37.8±15.4a b c d | 35.1±15.8a b c | <0.001* |
BMI: body mass index; E2: estradiol;
: Significant difference (P<0.05);
P<0.05 as compared to the subjects without MetS components;
P<0.05 as compared to subjects with one of the MetS components;
P<0.05 as compared to subjects with two of the MetS components;
P<0.05 as compared to subjects with three of the MetS components;
e P<0.05 as compared to subjects with four of the MetS components.
Figure 1. Ensemble-averaged levels ± standard error of the adiponectin, leptin, E2, and 1,25(OH)2D3 as the numbers of the MetS components increased.
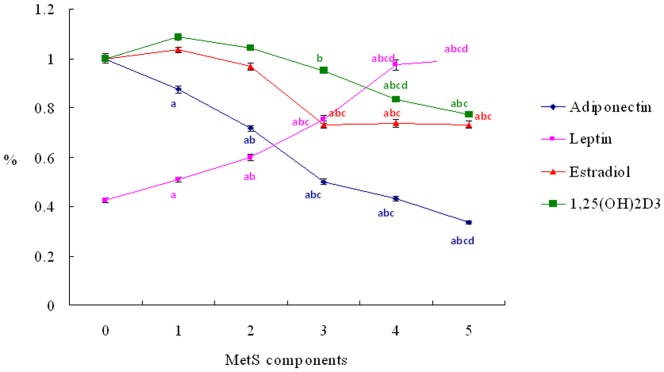
The reference points of “100 %” for the adiponectin, leptin, E2 and 1,25(OH)2D3 are 16.77 ng/ml, 6.28 ng/ml, 25.99 pg/ml and 45.36 pg/ml respectively. a P<0.05 as compared to the subjects without MetS components; b P<0.05 as compared to subjects with one of the MetS components; c P<0.05 as compared to subjects with two of the MetS components; d P<0.05 as compared to subjects with three of the MetS components; e P<0.05 as compared to subjects with four of the MetS components.
Correlations between biochemical variables and individual MetS components
Adiponectin was correlated with WC (r = 0.57, P<0.001), HDL (r = 0.46, P<0.001) and TG (r = −0.30, P<0.001). Leptin was correlated with HDL (r = −0.27, P<0.001), WC (r = 0.63, P<0.001), and DBP (r = 0.28, P<0.001); E2 level was correlated with WC (r = −0.13, P = 0.001), FBG (r = −0.16, P<0.001), TG (r = −0.11, P = 0.006), and HDL (r = −0.20, P<0.001). 1,25(OH)2D3 was correlated with WC (r = −0.12, P = 0.003), FBG (r = −0.13, P = 0.001), TG(r = −0.09, P = 0.02), and HDL(r = −0.11, P = 0.007). In total, adiponectin and leptin were significantly associated with 3 MetS components, but E2 and 1,25(OH)2D3 were significantly associated with 4 MetS components.
Multivariate regression analyses
All of the clinical and biochemical parameters which were not diagnostic parameters for MetS and were significantly different between subjects with and without MetS, were placed into a multivariate regression model. In model 1 with adjustment for age, BMI, and personal habits (smoking, alcohol drinking and betel quid chewing), adiponectin (beta = −0.421, P<0.001), leptin (beta = 0.111, P = 0.002), E2 (beta = −0.321, P<0.001) and 1,25(OH)2D3 (beta = −0.153, P<0.001) were all significantly independent predictors of MetS ( Table 3 ). In full adjusted model 2 after considering age, BMI, personal habits (smoking, alcohol drinking, and betel quid chewing), SHBG and above 4 factors (adiponectin, leptin, E2 and 1,25(OH)2D3), E2 (beta = −0.216, P<0.001) and 1,25(OH)2D3(beta = 0.067, P = 0.045) were still indepndent predictors of MetS, in addition to adiponectin (beta = −0.259, P<0.001) and leptin (beta = 0.086, P = 0.007) ( Table 3 ).
Table 3. Multivariate regression analyses for the associations of circulating adiponectin, E2, leptin, 1,25(OH)2D3 levels and metabolic syndrome (MetS).
| Variables | β (standardized coefficient) | SE | t | 95% Confidence Interval (CI) | P-value |
| Model 1: adjustment for age, BMI, and personal habits (smoking, alcohol drinking and betel quid chewing) | |||||
| Adiponectin | −0.421 | 0.002 | −12.510 | (−0.034∼−0.025) | <0.001* |
| E2 | −0.321 | 0.002 | −9.243 | (−0.021∼−0.014) | <0.001* |
| Leptin | 0.111 | 0.001 | 3.069 | (0.001∼0.006) | 0.002* |
| 1,25(OH)2D3 | −0.153 | 0.001 | −4.172 | (−0.006∼0.002) | <0.001* |
| (B) Model 2: adjustment for age, BMI, personal habits (smoking, alcohol drinking and betel quid chewing), SHBG and all of above 4 factors (adiponectin, E2, leptin, and 1,25(OH)2D3 levels)( R2 = 0.438). | |||||
| Adiponectin | −0.259 | 0.003 | −7.054 | (−0.023∼−0.013) | <0.001* |
| E2 | −0.216 | 0.002 | −6.397 | (−0.015∼−0.008) | <0.001* |
| Leptin | 0.086 | 0.001 | 2.335 | (0.001∼0.005) | 0.007* |
| 1,25(OH)2D3 | −0.067 | 0.001 | −2.010 | (−0.003∼0.000) | 0.045 |
BMI: body mass index; E2: estradiol; SHBG: sex hormone–binding globulin;
: Significant difference (P<0.05).
Discussion
In addition to adiponectin and leptin, this cross-sectional study found that circulating E2 and vitamin D3 levels were significantly associated with the risk of MetS and its individual components in middle-aged Taiwanese males. Subjects with MetS were found to have significantly lower circulating E2, 1,25(OH)2D3, and adiponectin levels as well as significantly higher leptin levels when compared to those without MetS (Table 1). With increasing numbers of MetS components, there were linear-shaped declines in 1,25(OH)2D3 and adiponectin; linear-shaped increases in leptin levels; and a ladder-shaped decline in E2 levels (Figure 1). In addition, correlation analyses also showed that E2 and 1,25(OH)2D3 were significantly associated with 4 individual components of MetS; more than adiponectin and leptin that were only associated with 3 individual components of MetS. The multivariate regression analyses also confirmed that E2 and 1,25(OH)2D3 were significant predictors of MetS independent of adiponectin and leptin (Table 3).
Previous studies have found that low E2 was associated with obesity and MetS in productive females with PCO, and adult males with the aromatase gene mutation. [17], [18], [19], [29], [31], [40], [41], [42], [43]. In our study, we also found that low E2 was significantly associated with MetS in middle-aged males. This is in contrast to findings reported by Maggio et al that found an independent association of increased E2 with MetS in an elderly male population [44]. Therefore, E2 might have contrary influences on MetS in middle-aged and elderly male populations. The result of low E2 with MetS in our study is consistent with the low estradiol-to-testosterone ratio seen in polycystic ovary syndrome with MetS, which is also associated with oligo-anovulatory cycles, atherogenic lipidic pattern, and insulin resistance [17], [18], [19], [45]. E2 and its receptor play important physiological and protective roles in the reproductive ages of both males and females. For males, E2 acts to prevent apoptosis of sperm cells [46] and works in vascular protection and modulation of inflammation [47], [48]. However, hormone replacement therapy (HRT) during menopause has turned out to be a harmful risk factor in that it increases the risk of stroke, venous thromboembolism, and coronary heart disease [49], [50]. This may be associated with the age-related decrease in estrogen receptor-mediated vascular relaxation [51] and may be used to elucidate the protective effects of E2 at reproductive ages and the contrary influences of increased E2 for the elderly or menopausal populations. Furthermore, E2 is also known to modulate insulin sensitivity and glucose homeostasis, and E2 supplementation has been shown to aid mice treated with a high fat diet in order to overcome central leptin resistance [8], [9], [52].
In addition, our study is the first to report an association between 1,25(OH)2D3 levels and the risk of MetS. Previous studies had reported that low 25(OH)D levels were significantly associated with insulin resistance and an increased risk of MetS [22], [23], [24], [26], [53], [54], [55], and was considered to be a risk factor for CVD [21]. Recently, 1,25(OH)2D3, the activated form of vitamin D, was suggested to play important roles in the insulin sensitivity and glucose metabolism [56] and was able to improve the weight-related inflammation and insulin resistence [57]. Studies on clinial intervention also found that vitamin D3 supplement can reduce the body fat, especially the visceral fat and lipid metabolism in subjects with obesity [58], [59], [60], [61], which may further decrease the risk of MetS. In our study, we found that 1,25(OH)2D3 levels displayed a linear decline as the numbers of MetS components increased in middle-aged males, which supported that a reduction in 1,25(OH)2D3 levels may be a contributing factor to an increased risk of developing MetS. However, an alternative explanation is that obesity and MetS can increase the inflmmation status [62], [63], which may further influence the level of 1,25(OH)2D3. Further studies are needed to evalute the real causal-relationships among 1,25(OH)2D3, inflammation and MetS.
In our study, we also found that adiponectin level was negatively correlated with the number of MetS components. Recently, Zhuo et al also reported that adiponectin concentrations decreased with increasing MetS components in older Chinese adults [64], which is consistent with our findings. HDL has been found to affect adipocyte metabolism and adiponectin expression [65]. Our study also found that adiponectin level was significantly corrected with HDL in our study population. In addition, previous studies also indicate that adiponectin and E2 are interactive. Adiponectin increases insulin-like growth factor I-induced estradiol secretion [66], influences adjacent epithelial function by estrogen receptor(ER) -dependent and ER-independent mechanisms to reduce breast cancer risk [67] and E2 suppresses the adiponectin-regulated OPG/RANKL expression to inhibit osteoclastogenesis-related inflammation and bone resorption [68]. Therefore, low adiponectin in MetS may disturb its regulation in E2 and increase the associated inflammation, which may also lead to the decrease of circulating E2 levels. Further studies are needed to evalute the real causal-relationships among adioponetin, E2 and MetS.
Our study also found that leptin level was positively correlated with the number of MetS components. Previous studies also reported that high leptin or leptin resistance status was associated with obesity, MetS and CVD [13], [14], [15], [16] , which is consistent with our results. In addition to smoking and alcohol drinking, our study also found that subjects with MetS had significantly higher prevalence of betel quid chewing than those without MetS. Arecoline, a major alkaloid in betel nuts, has been reported to have an impact on adipogenic differentiation (adipogenesis), lipolysis, and glucose uptake by fat cells [69] and contributes to the formation of MetS [70] and CAD [71]. Further large studies may be needed to elucidate the possible mechanisms of betel quid chewing on the risk of MetS.
There are some limitations in this study. First, our data was based on community-dwelling men participating in a free health screening. Although the screening was open to the general male population, some selection bias may have existed. Other large population-based studies may be needed to confirm our preliminary results. Second, this is a cross-sectional study that can only evaluate the possible associations among those factors, MetS and its individual components. However, we can not evaluate the real causal-relationships. Further prospective studies are needed to elucidate the possible effects of those factors on the risk of MetS and its individual components. Third, we did not evaluate the effect of total and free testosterone levels which may play important roles in the risk of MetS and circulating E2 levels via aromatization.
Conclusions
In addition to adiponectin and leptin, E2 and 1,25(OH)2D3 were significantly associated with the risk of MetS and its individual components in middle-aged males. With increasing numbers of MetS components, there were a linear-shaped decline in 1,25(OH)2D3 and a ladder-shaped decline in E2 levels. Further large studies are needed to confirm our preliminary results and elucidate the possible mechanism.
Acknowledgments
The authors thank Ms. Chao-Shih Chen for her help to hold health screening and perform data collection.
Funding Statement
This study was financially supported by grants from the Taiwan National Science Council (NSC98-2314-B-037-030-MY3; NSC99-2314-B-037-022-MY3) and Kaohsiung Medical University Hospital (KMUH100-0R45; KMUH101-1M43). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ballantyne CM, Hoogeveen RC, McNeill AM, Heiss G, Schmidt MI, et al. (2008) Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes (Lond) 32: S21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Assmann G, Schulte H, Seedorf U (2008) Cardiovascular risk assessment in the metabolic syndrome: results from the Prospective Cardiovascular Munster (PROCAM) Study. Int J Obes (Lond) 32: S11–16. [DOI] [PubMed] [Google Scholar]
- 3. Lee YC, Liu CC, Huang CN, Li WM, Wu WJ, et al. (2010) The Potential Impact of Metabolic Syndrome on Erectile Dysfunction in Aging Taiwanese Males. J Sex Med 7 ((9)): 3127–34. [DOI] [PubMed] [Google Scholar]
- 4. Ginsberg HN, MacCallum PR (2009) The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 4: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malik S, Wong ND (2009) Metabolic syndrome, cardiovascular risk and screening for subclinical atherosclerosis. Expert Rev Cardiovasc Ther 7: 273–280. [DOI] [PubMed] [Google Scholar]
- 6. Huang PL (2009) eNOS, metabolic syndrome and cardiovascular disease. Trends Endocrinol Metab 20: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang PL (2009) A comprehensive definition for metabolic syndrome. Dis Model Mech 2: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM (2008) Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 57: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matyšková R, Zelezná B, Maixnerová J, Koutová D, Haluzík M, et al. (2010) Estradiol supplementation helps overcome central leptin resistance of ovariectomized mice on a high fat diet. Horm Metab Res 42: 182–186. [DOI] [PubMed] [Google Scholar]
- 10. Frankel DS, Vasan RS, D'Agostino RB Sr, Benjamin EJ, Levy D, et al. (2009) Resistin, Adiponectin, and Risk of Heart Failure: The Framingham Offspring Study. J Am Coll Cardiol 53: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziemke F, Mantzoros CS (2010) Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr 91: 258S–261S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barseghian A, Gawande D, Bajaj M (2011) Adiponectin and Vulnerable Atherosclerotic Plaques. J Am Coll Cardiol 57: 761–770. [DOI] [PubMed] [Google Scholar]
- 13. Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, et al. (2008) Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 32: 268–274. [DOI] [PubMed] [Google Scholar]
- 14. Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor DA, et al. (2009) Leptin and Coronary Heart Disease: Prospective Study and Systematic Review. J Am Coll Cardiol 53: 167–175. [DOI] [PubMed] [Google Scholar]
- 15. Söderberg S, Colquhoun D, Keech A, Yallop J, Barnes EH, et al. (2009) Leptin, but not adiponectin, is a predictor of recurrent cardiovascular events in men: results from the LIPID study. Int J Obes (Lond) 33: 123–130. [DOI] [PubMed] [Google Scholar]
- 16. Conde J, Scotece M, Gómez R, Gómez-Reino JJ, Lago F, et al. (2010) At the crossroad between immunity and metabolism: focus on leptin. Expert Rev Clin Immunol 6: 801–808. [DOI] [PubMed] [Google Scholar]
- 17. Amato MC, Verghi M, Nucera M, Galluzzo A, Giordano C (2011) Low estradiol-to-testosterone ratio is associated with oligo-anovulatory cycles and atherogenic lipidic pattern in women with polycystic ovary syndrome. Gynecol Endocrinol 27: 579–586. [DOI] [PubMed] [Google Scholar]
- 18. Valkenburg O, Steegers-Theunissen RP, Smedts HP, Dallinga-Thie GM, Fauser BC, et al. (2008) A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab 93: 470–476. [DOI] [PubMed] [Google Scholar]
- 19. van Dam EW, Roelfsema F, Veldhuis JD, Hogendoorn S, Westenberg J, et al. (2004) Retention of estradiol negative feedback relationship to LH predicts ovulation in response to caloric restriction and weight loss in obese patients with polycystic ovary syndrome. Am J Physiol Endocrinol Metab 286: E615–620. [DOI] [PubMed] [Google Scholar]
- 20. Saltiki K, Papageorgiou G, Voidonikola P, Mantzou E, Xiromeritis K, et al. (2010) Endogenous estrogen levels are associated with endothelial function in males independently of lipid levels. Endocrine 37: 329–335. [DOI] [PubMed] [Google Scholar]
- 21. Judd SE, Tangpricha V (2009) Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci 338: 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, et al. (2007) Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr 26: 573–580. [DOI] [PubMed] [Google Scholar]
- 23. Kim MK, II Kang M, Won Oh K, Kwon HS, Lee JH, et al. (2010) The association of serum vitamin D level with presence of metabolic syndrome and hypertension in middle-aged Korean subjects. Clin Endocrinol (Oxf) 73: 330–8. [DOI] [PubMed] [Google Scholar]
- 24. Lu L, Yu Z, Pan A, Hu FB, Franco OH, et al. (2009) Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care 32: 1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martini LA, Wood RJ (2006) Vitamin D status and the metabolic syndrome. Nutr Rev 64: 479–486. [DOI] [PubMed] [Google Scholar]
- 26. Gannagé-Yared MH, Chedid R, Khalife S, Azzi E, Zoghbi F, et al. (2009) Vitamin D in relation to metabolic risk factors, insulin sensitivity and adiponectin in a young Middle-Eastern population. Eur J Endocrinol 160: 965–971. [DOI] [PubMed] [Google Scholar]
- 27. Nimitphong H, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B (2009) The association between vitamin D status and circulating adiponectin independent of adiposity in subjects with abnormal glucose tolerance. Endocrine 36: 205–210. [DOI] [PubMed] [Google Scholar]
- 28. Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, et al. (2007) Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 56: 2616–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones G (2007) Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin Dial 20: 316–324. [DOI] [PubMed] [Google Scholar]
- 30. Fichna M, Zurawek M, Januszkiewicz-Lewandowska D, Fichna P, Nowak J (2010) PTPN22, PDCD1 and CYP27B1 polymorphisms and susceptibility to type 1 diabetes in Polish patients. Int J Immunogenet 37: 367–372. [DOI] [PubMed] [Google Scholar]
- 31. Peterlik M, Cross HS (2009) Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr 63: 1377–1386. [DOI] [PubMed] [Google Scholar]
- 32. Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, et al. (2009) Vitamin D and cardiovascular disease. Pharmacotherapy 29: 691–708. [DOI] [PubMed] [Google Scholar]
- 33. Liu CC, Wu WJ, Lee YC, Wang CJ, Ke HL, et al. (2009) The prevalence of and risk factors for androgen deficiency in aging Taiwanese men. J Sex Med 6: 936–946. [DOI] [PubMed] [Google Scholar]
- 34. Liu CC, Huang SP, Wu WJ, Chou YH, Juo SH, et al. (2009) The impact of cigarette smoking, alcohol drinking and betel quid chewing on the risk of calcium urolithiasis. Ann Epidemiol 19: 539–545. [DOI] [PubMed] [Google Scholar]
- 35. Lee CH, Wu DC, Lee JM, Wu IC, Goan YG, et al. (2007) Anatomical subsite discrepancy in relation to the impact of the consumption of alcohol, tobacco and betel quid on esophageal cancer. Int J Cancer 120: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 36. Ponholzer A, Temml C, Rauchenwald M, Madersbacher S (2006) Vascular risk factors and erectile dysfunction in a cohort of healthy men. Int J Impot Res 18: 489–493. [DOI] [PubMed] [Google Scholar]
- 37. Hwang LC, Bai CH, Chen CJ (2006) Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc 105: 626–635. [DOI] [PubMed] [Google Scholar]
- 38. Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, et al. (2002) Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 11: 1065–1071. [PubMed] [Google Scholar]
- 39. Clapauch R, Mattos TM, Silva P, Marinheiro LP, Buksman S (2009) Total estradiol, rather than testosterone levels, predicts osteoporosis in aging men. Arq Bras Endocrinol Metabol 53: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 40. Arnal JF, Laurell H, Fontaine C, Billon A, Calippe B, et al. (2009) Estrogen receptor actions on vascular biology and inflammation: implications in vascular pathophysiology. Climacteric 12 Suppl 1: 12–17. [DOI] [PubMed] [Google Scholar]
- 41. Saunier EF, Vivar OI, Rubenstein A, Zhao X, Olshansky M, et al. (2011) Estrogenic plant extracts reverse weight gain and fat accumulation without causing mammary gland or uterine proliferation. PLoS One 6 ((12)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chang-Quan H, Bi-Rong D, Ping H, Zhen-Chan L, Xiao-Dong P (2008) Insulin resistance, renal injury, renal 1-alpha hydroxylase, and bone homeostasis in aged obese rats. Arch Med Res 39: 380–387. [DOI] [PubMed] [Google Scholar]
- 43. Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, et al. (2007) A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 67: 218–224. [DOI] [PubMed] [Google Scholar]
- 44. Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, et al. (2010) Estradiol and metabolic syndrome in older italian men: The InCHIANTI Study. J Androl 31: 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teede H, Deeks A, Moran L (2010) Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L (2000) Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab 85: 2057–2067. [DOI] [PubMed] [Google Scholar]
- 47. Xing D, Nozell S, Chen YF, Hage F, Oparil S (2009) Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol 29: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gilliver SC (2010) Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol 120: 105–115. [DOI] [PubMed] [Google Scholar]
- 49. Denti L (2010) The hormone replacement therapy (HRT) of menopause: focus on cardiovascular implications. Acta Biomed 81 Suppl 1: 73–76. [PubMed] [Google Scholar]
- 50. Shufelt CL, Bairey Merz CN (2009) Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 53: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qiao X, McConnell KR, Khalil RA (2008) Sex steroids and vascular responses in hypertension and aging. Gend Med 5 Suppl A S46–64. [DOI] [PubMed] [Google Scholar]
- 52. Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM (2008) Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 57: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ (2008) Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990–2000. Diabetes 57: 2619–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee DM, Rutter MK, O'Neill TW, Boonen S, Vanderschueren D, et al. (2009) Vitamin D, parathyroid hormone and the metabolic syndrome in middle-aged and older European men. Eur J Endocrinol 161: 947–954. [DOI] [PubMed] [Google Scholar]
- 55. Reis JP, von Mühlen D, Miller ER 3rd (2008) Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol 159: 41–48. [DOI] [PubMed] [Google Scholar]
- 56. Teegarden D, Donkin SS (2009) Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev 22: 82–92. [DOI] [PubMed] [Google Scholar]
- 57. Alkharfy KM, Al-Daghri NM, Yakout SM, Ahmed M (2013) Calcitriol attenuates weight-related systemic inflammation and ultrastructural changes in the liver in a rodent model. Basic Clin Pharmacol Toxicol 112 42–49. [DOI] [PubMed] [Google Scholar]
- 58. Zhu W, Cai D, Wang Y, Lin N, Hu Q, et al. (2013) Calcium plus vitamin D3 supplementation facilitated Fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutr J 12 ((1)) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rosenblum JL, Castro VM, Moore CE, Kaplan LM (2012) Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. Am J Clin Nutr 95: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, et al. (2012) A 12-week double-blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J 11: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Song Q, Sergeev IN (2012) Calcium and vitamin D in obesity. Nutr Res Rev 25: 130–141. [DOI] [PubMed] [Google Scholar]
- 62. Scarpellini E, Tack J (2012) Obesity and metabolic syndrome: an inflammatory condition. Dig Dis 30: 148–153. [DOI] [PubMed] [Google Scholar]
- 63. Nishimura S, Manabe I, Nagai R (2009) Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med 8: 55–60. [PubMed] [Google Scholar]
- 64. Zhuo Q, Wang ZQ, Fu P, Piao JH, Tian Y, et al. (2010) Association between adiponectin and metabolic syndrome in older adults from major cities of China. Biomed Environ Sci 23: 53–61. [DOI] [PubMed] [Google Scholar]
- 65. Van Linthout S, Foryst-Ludwig A, Spillmann F, Peng J, Feng Y, et al. (2010) Impact of HDL on adipose tissue metabolism and adiponectin expression. Atherosclerosis 210: 438–444. [DOI] [PubMed] [Google Scholar]
- 66. Chabrolle C, Tosca L, Ramé C, Lecomte P, Royère D, et al. (2009) Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells. Fertil Steril 92: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 67. Rahal OM, Simmen RC (2011) Paracrine-acting adiponectin promotes mammary epithelial differentiation and synergizes with genistein to enhance transcriptional response to estrogen receptor β signaling. Endocrinology 152: 3409–3421. [DOI] [PubMed] [Google Scholar]
- 68. Wang QP, Yang L, Li XP, Xie H, Liao EY, et al. (2012) Effects of 17β-estradiol on adiponectin regulation of the expression of osteoprotegerin and receptor activator of nuclear factor-κB ligand. Bone 51: 515–23. [DOI] [PubMed] [Google Scholar]
- 69. Hsu HF, Tsou TC, Chao HR, Shy CG, Kuo YT, et al. (2010) Effects of arecoline on adipogenesis, lipolysis, and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. Toxicol Appl Pharmacol 245: 370–377. [DOI] [PubMed] [Google Scholar]
- 70. Javed F, Al-Hezaimi K, Warnakulasuriya S (2012) Areca-nut chewing habit is a significant risk factor for metabolic syndrome: a systematic review. J Nutr Health Aging 16: 445–448. [DOI] [PubMed] [Google Scholar]
- 71. Tsai WC, Wu MT, Wang GJ, Lee KT, Lee CH, et al. (2012) Chewing areca nut increases the risk of coronary artery disease in Taiwanese men: a case-control study. BMC Public Health 12: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]


