Abstract
Pituitary adenomas are one of the most frequent intracranial tumors and occur with a prevalence of approximately 1:1000 in the developed world. Pituitary adenomas have a serious disease burden, and their management involves neurosurgery, biological therapies, and radiotherapy. Early diagnosis of pituitary tumors while they are smaller may help increase cure rates. Few genetic predictors of pituitary adenoma development exist. Recent years have seen two separate, complimentary advances in inherited pituitary tumor research. The clinical condition of familial isolated pituitary adenomas (FIPA) has been described, which encompasses the familial occurrence of isolated pituitary adenomas outside of the setting of syndromic conditions like multiple endocrine neoplasia type 1 and Carney complex. FIPA families comprise approximately 2% of pituitary adenomas and represent a clinical entity with homogeneous or heterogeneous pituitary adenoma types occurring within the same kindred. The aryl hydrocarbon receptor interacting protein (AIP) gene has been identified as causing a pituitary adenoma predisposition of variable penetrance that accounts for 20% of FIPA families. Germline AIP mutations have been shown to associate with the occurrence of large pituitary adenomas that occur at a young age, predominantly in children/adolescents and young adults. AIP mutations are usually associated with somatotropinomas, but prolactinomas, nonfunctioning pituitary adenomas, Cushing disease, and other infrequent clinical adenoma types can also occur. Gigantism is a particular feature of AIP mutations and occurs in more than one third of affected somatotropinoma patients. Study of pituitary adenoma patients with AIP mutations has demonstrated that these cases raise clinical challenges to successful treatment. Extensive research on the biology of AIP and new advances in mouse Aip knockout models demonstrate multiple pathways by which AIP may contribute to tumorigenesis. This review assesses the current clinical and therapeutic characteristics of more than 200 FIPA families and addresses research findings among AIP mutation-bearing patients in different populations with pituitary adenomas.
Introduction
-
Familial Isolated Pituitary Adenomas (FIPA)
Historical background
The emergence and characterization of FIPA
Clinical characteristics of FIPA
-
Pituitary Adenoma Predisposition due to Aryl Hydrocarbon Receptor Interacting Protein (AIP) Gene Mutations
Genome-wide studies and the discovery of the role of AIP mutations
AIP mutations in specific populations
Founder AIP mutations
AIP mutation screening: current status
Treatment outcomes in AIP-mutated pituitary adenomas
Role of AIP mutations in other tumor types
-
Mouse Models and in Vitro Studies of AIP in Pituitary Tumor Biology
Phenotypes of Aip knockout mouse models
Molecular and pathological characteristics of AIP/Aip-associated tumors
Analysis of the biological functions of AIP
-
Genetic Testing for AIP in Selected Pituitary Adenoma Populations
Defining the ideal testing populations
Disease penetrance
Toward integrated genetic screening in pituitary adenomas
Follow-up in the setting of FIPA and AIP mutation carriers
Future Directions
I. Introduction
Pituitary adenomas are one of the most frequent intracranial tumors, particularly in young patients. Clinically relevant pituitary adenomas were shown to occur in 1:1064 of the population in Liège, Belgium, which has been confirmed in similar cross-sectional studies in the United Kingdom and Switzerland, giving an overall prevalence of 78–94 cases per 100,000 population (1–3). Their classically benign histology belies their medical impact. Due to their position close to vital local structures and the potent biological effects of pituitary hormone hypersecretion or deficiency, pituitary adenomas can cause some of the most severe classical conditions in endocrine practice, including acromegaly and Cushing disease. Diagnosis and management of pituitary adenomas often involves a multidisciplinary approach that combines endocrine, neurosurgical, and radiological specialists. Pituitary adenomas can also present late with already locally expansive and invasive disease. Although multiple effective modalities are available, the challenges of aggressive disease behavior remain and provide an impetus for research.
The study of pituitary adenoma pathophysiology can take a number of directions. Some groups have highlighted the molecular and signaling abnormalities within human and animal pituitary adenomas at a somatic level. These somatic molecular genetic abnormalities are numerous due to the complexity of the pituitary gland itself (4–6). Some of these discoveries have led to the experimental use of novel therapies, such as tyrosine kinase inhibitors in this setting (7–10). Another direction of research is into the realm of inherited endocrine neoplasia syndromes, such as multiple endocrine neoplasia type 1 (MEN1) and Carney complex (CNC) (11–13). These challenging, protean clinical syndromes are caused by germline genetic mutations that impact many tissues and cause multiple endocrine tumors, including pituitary adenomas as a characteristic feature (14, 15). Study of these disrupted genes and the impact of absent or abnormal protein on cellular signaling and regulation can also provide information about normal pituitary physiology and the pathophysiology of pituitary adenomas occurring outside these specific genetic settings. These diseases provide an important clinical opportunity, namely, to allow the early diagnosis of at-risk carriers by germline genetic sequencing.
While MEN1 and CNC have been widely studied, numerous patients and families with apparently inherited endocrine neoplasia have been identified that have no genetic abnormalities in causative genes such as MEN1 and PRKAR1A. Interest in these cases has led to the identification of new conditions, such as MEN4, that are only beginning to be studied. In particular, familial isolated pituitary adenoma (FIPA), consisting of kindreds with two or more related members having pituitary adenomas in the absence of known genetic causes, was identified and characterized over the last decade, and research interest in FIPA has led to hundreds of new kindreds being identified worldwide. In parallel, a new gene, aryl hydrocarbon receptor interacting protein (AIP), was found to cause an inheritable propensity for pituitary adenomas, which have since been proven to represent an important cause of pituitary adenomas in young patients. Together, these two complimentary clinical and genetic projects have highlighted novel findings that illustrate a group of more aggressive pituitary adenomas and a molecular pathway through AIP that opens a new understanding of pituitary adenoma pathophysiology.
II. Familial Isolated Pituitary Adenomas (FIPA)
A. Historical background
The medical literature on pituitary adenomas before the naming of acromegaly by Pierre Marie was relatively sparse (16). The promulgation of Marie's report and de Souza Leite's subsequent follow-up series led to a wellspring of clinical cases of acromegaly in the literature (17). Focus on the signs and symptoms of acromegaly, allied with interest in the surgical and pathological findings, quickly led to the accumulation of a large body of fundamental literature on pituitary adenomas in general, including work from leading lights such as Harvey Cushing (18). Within these historical works are found the first scientific descriptions of pituitary tumors in a familial setting. The genetic etiology of these early reports is uncertain, with some, like the report by Erdheim in 1903 of pituitary and parathyroid adenomas, being strongly suggestive of MEN1 (19). Indeed, the clinical reports of syndromic pituitary adenomas (i.e., those occurring in association with other clinical abnormalities) led Wermer (20) to characterize MEN1 clinically (as Wermer syndrome), and he suggested correctly the inheritance mode. Like Wermer, Carney also undertook a meticulous clinicopathological characterization of his eponymous syndrome. CNC was defined as a syndrome of myxomas, spotty pigmentation, and endocrine dysregulation, with acromegaly seen in about 10% of the original case series (21, 22).
Although syndromic pituitary adenomas in the historical literature can likely be ascribed to MEN1 or other diseases, some reports noted the occurrence of isolated pituitary adenomas occurring in a familial setting. Rare cases of familial gigantism from the press and advertising materials of the 17th to 19th centuries have been well documented by de Herder (23, 24). An early but controversial scientific description that was later attributed to familial acromegaly is Friedreich's report (25) of the Hagner brothers. Ostensibly, this report was one of bone and joint deformities, and a plate illustrating one of the brothers does not appear classically affected by acromegaly. A monograph on acromegaly by the Austrian physician Sternberg (26) (aided by Atkinson's 1899 translation) served as a detailed examination of the etiological and clinical aspects of the disease. Here, initial cases of familial acromegaly were discussed, such as cases reported by Schwoner (27). Fraenkel et al. (28) later described the case of a 50-yr-old male (“Herr Gleiche”) who presented in 1898 and had first-degree relatives with acromegalic features (father and two siblings), and they also discussed a three-member familial acromegaly kindred. The two Hugo brothers were a very well-recognized familial pituitary tumor kindred with acrogigantism whose media fame crossed over into the scientific realm. While traveling to New York, one of the brothers died of a fulminant infection. On autopsy, the familial pituitary etiology was strongly supported by the finding of a pituitary macroadenoma larger than 40 mm in diameter (29).
In 1925, Bailey and Davidoff (30) reported the clinical features and tumor pathology of a series of patients treated by Cushing at the Peter Bent Brigham Hospital in Boston, Massachusetts. Case III was a 25-yr-old man who had onset of acromegalic features as an adolescent and, as the authors note, “came of a family of tall people…. His paternal great uncle was 7 feet 1 1/2 inches tall (217 cm) …. the “Kentucky giant.” One could speculate that this was the same Kentucky giant (Jim Porter) whom Charles Dickens encountered at Portland, Kentucky, in 1842 and described in the book American Notes for General Circulation. Dickens' description of Mr. Porter is both sympathetic and medically suggestive: “He had a weakness in the region of the knees, and a trustfulness in his long face …. He was only 25 years old, he said, and had grown recently …. (he) went bobbing down the cabin, among men of six feet high and upwards, like a lighthouse walking among lamp-posts” (31). In 1937, Gray (32) outlined the clinical history of a middle-aged male patient with acrogigantism who had been a silent movie actor and performer and noted also a reputed history of familial gigantism in his grandfather. These descriptions represent individual patients or relatively small case series. In contrast, Atkinson (the translator of Sternberg's monograph in 1899) revisited the subject of acromegaly in a monograph of his own in 1932 (33). This publication is an exhaustive review of the literature up to the end of 1930 in which Atkinson reviewed and codified 1319 cases of acromegaly, most being derived from individual case reports. The review is, even today, one of the most extensive on the presentation and clinical symptomatology of acromegaly. Among the listings of cases, Atkinson notes eight in which acromegaly was reported to be familial in first-degree relatives (seven in parents and one in a grandparent).
Kindreds with familial acromegaly continued to accrue over the mid-to-late 20th century. The advent of immunoassays permitted the definitive linking of acromegaly to excess GH secretion, and probably the first case of familial acromegaly with defined hypersecretion of GH was described by Levin et al. (34). Thereafter, a variety of other individual case reports of familial acromegaly and acrogigantism were reported in the literature (35–42). The genetic causes of both MEN1 and CNC were discovered in the 1990s. The MEN1 gene was initially localized to a specific region chromosome of 11q13 (43). The MEN1 gene was ultimately cloned by researchers at the National Institutes of Health (NIH) in 1997 (44). Mutations in the protein kinase A Iα regulatory subunit gene (PRKAR1A) on chromosome 17q were discovered to be related to CNC in 1998 and subsequently have been implicated in over 70% of cases (45, 46).
Therefore, by the end of the 1990s, the concept of familial acromegaly that was unrelated to existing conditions could be both clinically and genetically delineated. Before the identification of the MEN1 gene, familial acromegaly kindreds without genetic linkage to the MEN1 locus had been described initially by Benlian et al. (47) (three members— two living, one historical) and later by Yamada et al. (48) (three living members). These studies noted linkage to chromosome 11q13 and loss of heterozygosity (LOH) on chromosome 11q13 in tumor samples. Subsequent to the identification of the MEN1 gene, a series of studies demonstrated multiple kindreds with familial acromegaly and no MEN1 mutation (49–53). Such kindreds included families with more than three affected members, indicating a very strong likelihood of a common genetic cause. Most families had only somatotropinomas, but kindreds with prolactinomas alone or in conjunction with somatotropinomas were reported in Japan and Belgium (54–56). An early, very extensive Australian kindred of five affected persons was reported by Pestell et al. (38) in 1989. Although acromegaly predominated, one subject had a prolactinoma, whereas another had likely co-secretion of GH and prolactin from the pituitary tumor. A subsequent study of three new kindreds from Liège, Belgium, reviewed the clinical findings of these and 17 other reported kindreds (45 cases) (57). An autosomal dominant inheritance pattern with reduced, age-dependent penetrance was the most parsimonious model to explain the recurrent pattern (57). Kindreds were usually small (two affecteds), and in 50% of cases only siblings were affected. An early onset of disease was noted (puberty through 30 yr of age), and tumors—usually somatotropinomas, but also somatomammotrope and plurihormonal cases—were frequently large and invasive. These findings are mirrored by those of Gadelha et al. (58), also in 1999, who used the term “isolated familial somatotropinoma” to describe these seemingly acromegaly-only kindreds. In addition to the characteristics of large tumor size and early age at diagnosis, they also noted a male preponderance and frequent gigantism among their studied population.
B. The emergence and characterization of FIPA
In contrast to the clear evidence for familial occurrence of acromegaly-gigantism, the older historic scientific literature lacks firm reports of other familial pituitary tumor types. It was not until the 1980s that viable studies of kindreds with other pituitary tumor types came to light. Three kindreds with familial Cushing disease were published; two of the cases involved second-degree relatives (aunt/nephew, aunt/niece), and one case was of Cushing disease in two sisters (59–61). Berezin and Karasik (61) reported three prolactinoma-only kindreds from Israel. From Japan, there was a description of nonfunctioning pituitary adenoma (NFPA) occurring in a familial setting (62). Many of these kindreds were reported before the advent of MEN1 gene mutation screening, but in those cases, the clinical descriptions suggest a low likelihood of MEN1.
When taken together with data from other families noted in the previous section (38, 54–57), it became apparent that pituitary adenomas other than somatotropinomas could occur in a familial setting. To investigate this, a specific research program was undertaken at the University of Liège to identify kindreds with two or more pituitary adenomas (not limited a priori to somatotropinomas) in which MEN1 or CNC did not play a role. We defined this condition as FIPA to describe the clinical findings accurately and to use terminology in concordance with analogous conditions in hereditary endocrine neoplasia (e.g., familial isolated hyperparathyroidism, familial medullary thyroid carcinoma). That study identified 27 FIPA patients with somatotropinomas, prolactinomas, and nonsecreting pituitary adenomas among a group of 1500 patients (63). Patients within the same family could have the same pituitary tumor type in all affected members (homogeneous FIPA), or different pituitary tumor types could exist within the same family (heterogeneous FIPA). To confirm that the phenotype of FIPA was seen more generally, we performed an international case-finding study from 2000–2004 that identified new FIPA kindreds at 22 European and North American centers (64). Negative MEN1 genetic screening was available in at least one affected member of each FIPA kindred, and MEN1 was also ruled out clinically. For CNC, the situation was more focused, given the more limited repertoire of pituitary tumor types associated with this disease (normal PRKAR1A gene sequencing was available in at least one affected member of each homogeneous somatotropinoma kindred); all FIPA kindreds were clinically screened to exclude other features suggestive of CNC.
The clinical characteristics of FIPA patients (n = 138) and how they compared statistically with those of matched sporadic nonfamilial pituitary adenoma cases (n = 288) were first described by Daly et al. (65) in 2006. In about 75% of that cohort, the relationship between affected members was sibling/filial in nature (first degree). In that group, a mean of 15.4 individuals per family were assessed, and families with two, three, and four affected members were seen, whereas subsequent families of as many as seven or eight persons have since been classified as FIPA kindreds (66, 67). However, FIPA remains predominantly a condition of two to three, usually closely related persons per kindred, albeit kindreds are usually relatively small due to limited familial anamnesis in many reported families. In certain FIPA kindreds where extensive familial study has been possible, second- and third-degree relationships can be noted, particularly when photographs and other historical documents (old medical records) of deceased individuals are available for assessment. In general, FIPA is present in a minority of cases of pituitary adenomas overall, with Daly et al. (65) finding FIPA in approximately 2% of cases from two reference centers in one study. Multicenter epidemiological studies on the true prevalence of FIPA in large groups of pituitary adenoma patients are needed.
C. Clinical characteristics of FIPA
FIPA patients are diagnosed on average 4 yr before patients with sporadic pituitary adenomas (65). In families with vertical relationships between affecteds, patients in the later generations are diagnosed with pituitary adenomas at a statistically significantly younger age as compared with their parents or grandparents (on average 20 yr before). This earlier disease diagnosis in multigenerational families is particularly pronounced for homogeneous FIPA kindreds irrespective of the tumor phenotype (65). This is probably due to improvements over time in the availability of diagnostic modalities [e.g., magnetic resonance imaging (MRI)] and improved awareness of pituitary disease in the general medical community. Also, the sensitizing effect of having one family member with a pituitary adenoma already on improved recognition of pituitary related symptoms in another member should not be underestimated.
The rank order of frequency of pituitary adenoma subtypes in individual patients from our FIPA cohort is: prolactinoma (37.5%), somatotropinoma (35.0%), NFPAs (14.5%), somatolactotropinomas (6.4%), Cushing disease (2.9%), gonadotropinomas (2.0%), plurihormonal tumors (1.2%), and thyrotropinomas (0.5%) (65, 68, 69), and unpublished observation of A. Beckers and A. F. Daly. Therefore, as compared with the frequencies of pituitary adenoma subtypes in the general epidemiological population, the proportion of FIPA patients with prolactinoma is conspicuously lower (66 vs. 37.5%), although they remain the most frequent overall. The proportion of patients with somatotropinomas is consequently much higher in the setting of FIPA than in the general epidemiological data (35.0 vs. 13%) (1, 65, 68, 69). Prolactinomas in FIPA are most frequently microadenomas that occur in premenopausal females; this does not differ from general characteristics of sporadic prolactinomas. Also reflecting the case with sporadic prolactinomas, males with prolactinomas in the FIPA setting comprise a minority of cases, but frequently present with macroadenomas. However, when prolactinomas occur in the setting of heterogeneous FIPA kindreds, they appear to have more aggressive characteristics than sporadic adenomas, being significantly more frequently invasive and extending toward the optic chiasm (65). This term “aggressive” is used in a relative sense of tumors that are more difficult to control therapeutically or larger tumor size. There has only been one pituitary carcinoma (a malignant prolactinoma) that was subsequently found to occur in a heterogeneous two-member FIPA kindred (a sibling had a large nonmalignant NFPA) (70). In our experience, somatotropinomas in FIPA are almost equally divided between homogeneous and heterogeneous FIPA kindreds (65, 69, 71). Patients from FIPA kindreds with homogeneous acromegaly correspond to the previous terminology of isolated familial somatotropinoma and share their features, having a larger adenoma diameter and tumors that have an earlier age of onset (57, 58, 72, 73). NFPA in FIPA mainly occur in heterogeneous families and have a significantly younger age at onset than their sporadic counterparts (mean, 8 yr) (65). FIPA-associated NFPA are significantly more frequently invasive than sporadic NFPA (84.6 vs. 59.6%, respectively). NFPA in FIPA may be true null-cell adenomas or can be silent gonadotrope-positive, silent GH positive, silent corticotrope (type II), or plurihormonal tumors (65, 69, 74, 75). FIPA families with Cushing disease, TSH adenomas, and secreting gonadotropinomas are too rare to compare reliably against the characteristics of sporadic groups.
III. Pituitary Adenoma Predisposition due to Aryl Hydrocarbon Receptor Interacting Protein (AIP) Gene Mutations
A. Genome-wide studies and the discovery of the role of AIP mutations
In sporadic somatotropinomas, LOH at 11q13 has been detected in a variable proportion of pituitary tumors (57, 76–78). As noted above, studies of familial acromegaly kindreds during the 1990s pointed to the involvement of a region of chromosome 11q13 that was separate from the MEN1 locus (47, 48, 50, 55). These studies gave rise to important genetic linkage work that further refined the specific region involved to an area between microsatellite markers D11S956 and D11S527 (79, 80). By 2005, Soares et al. (81) had narrowed the region in question down to a 2.21-Mb stretch of DNA on 11q13.3, but targeted sequencing of potential candidate genes within that region proved negative. Among these acromegalic patients, macroadenomas were relatively common, gigantism was reported, and patients were relatively young at disease onset (57, 73, 82).
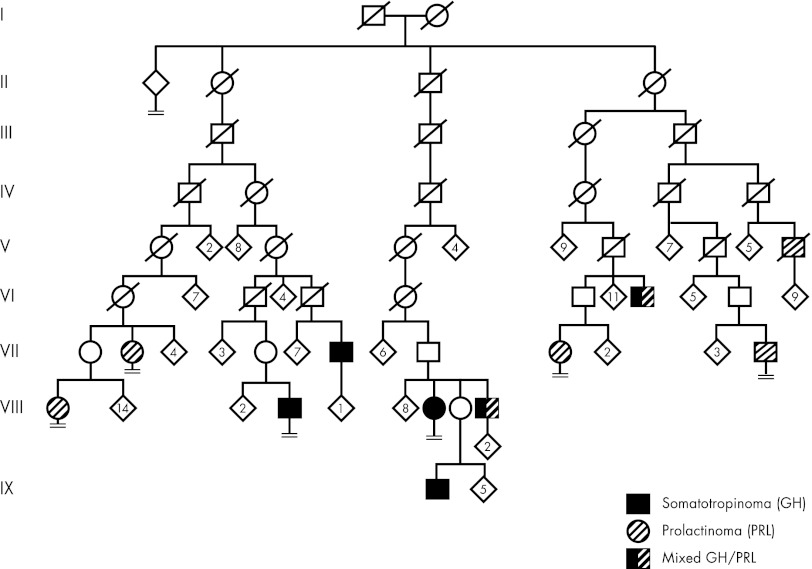
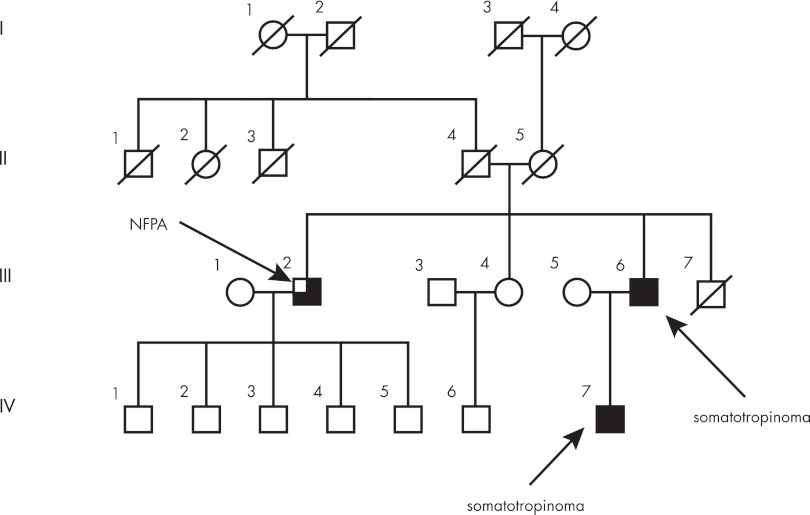
The hypothesis of a possible pituitary adenoma predisposition (PAP) gene in Finland was developed independently when three clusters of MEN1-negative families segregating acromegaly/gigantism and prolactinomas were detected in northern Finland. This familial occurrence of isolated pituitary adenomas not limited to acromegaly and without features of known syndromes prompted consideration of a previously uncharacterized form of low-penetrance PAP. Pedigree genealogy reaching back to the 1700s was available by combining information from the Finnish population register and parish registers. The genealogy analysis established a link between two families, whereas the third cluster appeared to be separate. The two linked clusters were found to have a common ancestor couple born in 1763 and 1770. Names in the pedigree genealogy were then combined with data from a previously characterized, population-based cohort of 54 acromegaly patients, diagnosed with GH-secreting adenomas between 1980 and 1999 in Oulu University Hospital, northern Finland (83). This enabled the construction of a complete pedigree with affected status (Fig. 1). Altogether, 11 affected individuals were identified. This genealogy was not limited to acromegaly only because there were five members with prolactinoma, four with somatotropinoma, and two with mixed GH/prolactin-secreting pituitary adenoma. The disease predisposition locus was identified in these families using whole genome single-nucleotide polymorphism genotyping. This, together with fine mapping of the most prominent candidate region on chromosome 11q12–13 (LOD score, 7.1), provided unambiguous evidence for susceptibility locus identification (84). The locus and pathology were termed “pituitary adenoma predisposition” (PAP) (OMIM no. 102200). The linked, more than 7-Mb long, founder haplotype segregated perfectly with the acromegaly phenotype in both pedigrees. Because the disease-associated haplotype was extremely gene rich, containing 295 genes, a novel approach was needed to select candidate genes. For this purpose, gene expression profiles were generated from peripheral blood samples of patients/obligatory carriers and healthy controls. In the gene expression experiment, we sought genes that were underexpressed, based on the earlier literature supporting the inactivation of a tumor suppressor gene in this chromosomal area locus (50, 51, 53). In the linked region, 172 probe sets fulfilled the criteria, and of these, 27 reached a P value ≤0.05. Two probe sets representing the AIP gene occupied the first two positions, with P values of 0.00026 and 0.00114. Therefore, AIP was chosen as the primary candidate gene. Direct genomic DNA sequencing in both families revealed an early stop codon mutation, c.40C>T/p.Q14X, in the first exon of AIP. The mutation was not detected in 209 population-matched healthy controls. The change segregated perfectly in patients with somatotropinoma and somatolactotrope type of adenoma and was also present in three prolactinoma patients. Interestingly, two other prolactinoma patients with microadenomas did not share the founder haplotype harboring the AIP mutation, thus representing phenocopies. The existence of such phenocopies is unsurprising given that prolactin-secreting microadenomas are the most frequently seen pituitary adenoma in the general population (1). Mutation screening of a northern Finland population-based group of 45 apparently sporadic acromegaly patients revealed the Finnish founder mutation p.Q14X in six patients and a splice-acceptor site (IVS3-1G>A) mutation in one patient. Overall, these two mutations accounted for 16% of all patients diagnosed with GH-secreting adenomas and for 40% of patients younger than 35 yr of age at diagnosis, indicating that the young age at onset is a useful indicator for the PAP caused by AIP mutations. The identification of AIP as a novel PAP gene was further confirmed when a late stop codon mutation in exon 6 (c.910C>T/p.R304X) was identified in Italian siblings affected with somatotropinoma at the age of 18 yr (previously reported in Ref. 53). In all available tumor samples from the mutation carriers in Finland and Italy (including somatotropinomas, mixed GH/prolactin-secreting tumors, and prolactinomas), the wild-type allele was lost. This biallelic inactivation of AIP in the tumors strengthened the assumption that AIP is likely to act as a tumor suppressor (84).
Figure 1.
Pedigree of original Finnish family with pituitary adenoma due to a Q14X founder mutation in AIP. Generations are indicated with Roman numerals. Generation I is from the 18th century. Numbers within diamonds indicate number of children. Circles, Females; squares, males; diagonal line, deceased. Pedigree has been modified for confidentiality.
B. AIP mutations in specific populations
Studies have examined the prevalence of AIP mutations in FIPA kindreds, in unselected populations of sporadic pituitary adenomas, and in focused populations of pituitary adenoma patients, such as young patients, and in other nonpituitary tumors. Together, these studies have confirmed and extended the initial findings and helped to delineate the characteristics and epidemiology of pituitary adenomas associated with AIP mutations. For clarity, sporadic populations mentioned below refer to populations that had no known family history of pituitary adenomas. After genetic testing, pituitary adenoma patients with AIP mutations and no known family history of pituitary tumors (irrespective of whether they have unaffected mutation carriers as family members) are referred to as “simplex” cases by some authors (85)
1. AIP mutations in FIPA
After the report of Vierimaa et al. (84) describing AIP as a gene associated with a predisposition to pituitary adenomas in a familial setting, the role of AIP mutations in the pathogenesis of FIPA was investigated. Daly et al. studied an international cohort of 73 FIPA families (n = 156 patients) from Europe and the Americas (71). Ten different germline AIP mutations were noted in that study; all but one were novel (the p.R304X mutation was noted in another Italian family). One of the changes, R16H, although highly conserved, has since been reclassified as probably being a variant of no pathogenic effect that does not map with the FIPA phenotype in some families (86–88). This study illustrated some of the characteristics of AIP mutation-positive FIPA patients. AIP mutations with pathogenic effects explained only a minority of FIPA families (<20%). A missense mutation, p.R271W, involving an important, conserved residue was found to cause different tumor patterns in two unrelated families, with acromegaly/gigantism in one kindred (described initially in Ref. 47) and somatotropinoma/prolactinoma in the second; this indicated that the same mutation in AIP could give rise to varying pituitary adenoma phenotypes in different kindreds. The p.K241E missense mutation, also in an important conserved residue, was associated with a two-sibling FIPA family (gonadotrope and α-subunit-positive NFPA patient and a prolactinoma patient), demonstrating that AIP mutations are not invariably related to somatotropinoma and prolactinoma-expressing kindreds only, and also included NFPA. In terms of demographic characteristics, the mean age at diagnosis was significantly lower in FIPA subjects with AIP mutations than those FIPA patients without mutations, and the mean maximal diameter of pituitary adenomas in the AIP mutation-bearing patients was significantly larger as compared with those without AIP mutations. Family screening also identified asymptomatic AIP mutation carriers. Because the PAP associated with AIP mutations is a condition with incomplete penetrance, asymptomatic mutation carriers are relatively common (67, 71, 84, 86, 89, 90).
The role of AIP mutations in FIPA families has also been studied by other groups, both as individual kindreds (89, 91–94) and as collaborative studies of multiple kindreds (67, 86, 90, 95–97). Two studies led by the Korbonits group in London have accrued and assessed the characteristics of 64 FIPA kindreds (160 patients) worldwide and studied the role of AIP mutations in the population (67, 86). These FIPA studies are of particular interest also because they identified AIP mutations as the cause of many previously identified familial acromegaly families reported by Frohman's and Gadelha's research groups, Pestell et al., and others (38, 39, 51, 79, 81). In their series, the mean age at diagnosis in the AIP mutation-positive FIPA cases was also significantly lower than in the AIP mutation-negative FIPA cases (approximately 16 yr). The Korbonits group found that families with AIP mutations had a significantly higher number of affected (3.2 ± 1.8 cases) than AIP mutation-negative FIPA families (2.2 ± 0.4 cases) (86).
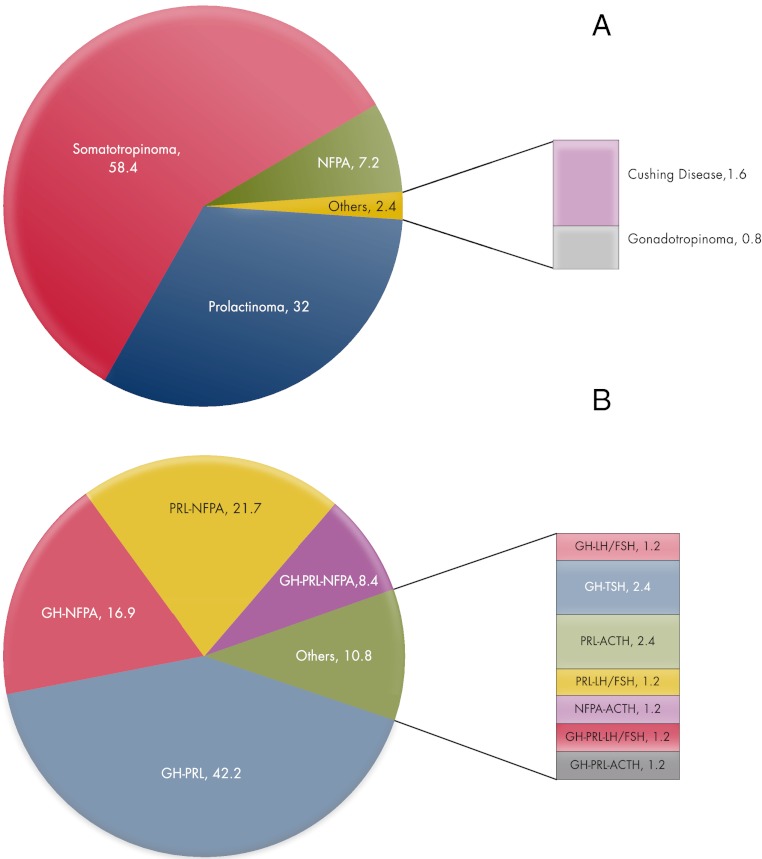
A combined analysis of the published results on FIPA cohorts, including 45 novel, genetically characterized, previously unreported FIPA kindreds, shows that a total of 211 FIPA families have been described in a manner that permits data analysis. These subdivide into 127 homogeneous families (60.2%) and 84 heterogeneous families. As shown in Fig. 2A, among homogeneous FIPA kindreds, somatotropinoma families (including those with somatolactotrope tumors) and prolactinoma families make up 90% of the total, with homogeneous NFPA (7.2%) being the only other frequent familial type. The heterogeneous FIPA cohort (Fig. 2B) contains 11 different combinations of pituitary adenomas, although subtypes such as somatotropinoma-prolactinoma and combinations of somatotropinomas and/or prolactinomas with NFPA account for nearly 90% of cases. However, FIPA families with heterogeneous presentation of Cushing disease, thyrotropinomas, and gonadotropinomas also have been identified. Among the 211 FIPA families reported comprehensively in the literature or studied by the authors, 43 AIP mutation-bearing FIPA kindreds have been identified. Thus, AIP mutations appear to explain only a minority of FIPA kindreds (20.4%): 29 homogeneous families (28 somatotropinoma, one NFPA), and 14 heterogeneous families (10 somatotropinoma-prolactinoma, one somatotropinoma-NPFA, two prolactinoma-NFPA, and one prolactinoma-Cushing disease family). There was a higher proportion of AIP mutation positivity among homogeneous FIPA families (22.8%) as compared with heterogeneous FIPA kindreds (16.7%), which is largely due to AIP-positive homogeneous acromegaly kindreds. The proportion of homogeneous acromegaly kindreds explained by AIP mutations was 36.1%. It is important to note that to date no homogeneous prolactinoma (n = 40), Cushing disease (n = 2) or gonadotropinoma (n = 1) FIPA kindreds have been shown to be AIP mutation positive in our analysis or in those from the other large international collaborative group (67, 86, 98).
Figure 2.
Proportions of 211 FIPA kindreds with homogeneous (A) or heterogeneous (B) presentation of pituitary adenomas within the same family. GH, Somatotropinoma (includes also somatolactotrope tumors); PRL, prolactinoma; ACTH, Cushing disease; LH/FSH, gonadotropinomas; TSH, thyrotropinomas.
2. AIP mutations in unselected sporadic populations
A germline AIP mutation was initially identified in 16% of seemingly sporadic acromegaly patients without a known family history of pituitary adenoma from the same geographical region of Finland (84). Studies of unselected sporadic pituitary adenoma patients have shown a low rate of AIP mutations. A general population-based study of 460 pituitary adenoma patients representing genetically heterogeneous populations from Europe and North America had an overall prevalence of AIP mutations of less than 2% (99). Using sequencing and multiplex ligation-dependent probe amplification (MLPA) for AIP mutations in 148 patients (germline and somatic DNA), Barlier et al. (100) noted an even lower prevalence (0.7%). More recently, Cazabat et al. (101) performed a large, single-center screening approach in 443 patients with sporadic pituitary adenomas. This study noted that AIP mutations account for no more than 3.6% of unselected pituitary adenomas overall (4.5% of prolactinoma patients, 4.1% of acromegalic patients, 6.8% of Cushing disease patients, and <1% of NFPA patients). Taking these and other studies in general sporadic pituitary adenoma populations together, AIP mutations occur at a relatively low frequency of less than 4%, meaning that unselected screening efforts are probably not a highly efficient method for identifying AIP mutation-positive cases in the general, nonfamilial pituitary tumor patient population (67, 91, 92, 95, 100–104).
3. AIP mutations in young adult patients
In the original study on AIP mutations and pituitary tumors, the Finnish founder mutation p.Q14X and the splice site mutation IVS3–1G>A accounted for a significant fraction of population-based acromegaly patients, especially the ones diagnosed at an early age (six of 15 aged <35 yr at diagnosis) (84). Since then it has been established that young age at onset/diagnosis is a characteristic feature of AIP-related pituitary adenomas. Among large heterogeneous international populations of pituitary adenoma patients, AIP mutations tend to occur rarely (0–3.6%) when unselected populations are studied (67, 92, 95, 100, 101, 105, 106). However, those cases that are identified are almost invariably found in younger patients. For example, Georgitsi et al. (99) reported that AIP-related pituitary adenomas occurred in 5.5 and 7.4% of those aged less than 45 and less than 40 yr of age at diagnosis, respectively. Although in unselected cases the prevalence of AIP mutations fell to 0–1.8%, all but three cases had disease onset before the age of 30, and one patient was an 8 yr old child. Similarly, Barlier et al. (100) found that only one of 148 (0.7%) unselected cases from Marseille/Liège bore an AIP mutation: a somatotropinoma in a male aged 24 yr at diagnosis. In the large Parisian cohort of unselected pituitary adenoma patients noted above, AIP mutations were detected in 3.6% (16 of 443) of cases, of which seven were diagnosed before the age of 18 yr (107). Occhi et al. (95) reported a similar overall rate of deleterious AIP changes in Italian patients (3.1%), although the age at diagnosis was higher with all four patients age 30 yr or older at diagnosis. Studies in FIPA kindreds from various groups have shown that AIP mutation-bearing patients are generally young at diagnosis (67, 71, 86) and significantly younger than FIPA cases without AIP mutations (67, 71, 86). We performed a specific screening study that was limited to sporadic patients with two of the most characteristic features of AIP mutation-related pituitary adenomas: young age (<30 yr of age) and macroadenoma at diagnosis (108). Germline AIP mutations were found to occur in 19 of 163 patients (11.7%), rising to 20.5% of pediatric/adolescent patients (aged <18 yr at diagnosis). Overall, 13% of somatotropinomas, 11.5% of prolactinomas, and one of 16 (6.3%) NFPA had germline AIP mutations. Although these patients had no known history of FIPA, six of seven families of affected patients that permitted testing revealed AIP mutation carriers (two asymptomatic microadenomas were diagnosed in carriers).
4. AIP mutations in pediatric and adolescent patients
The occurrence of pituitary tumors among children and adolescent patients is rare, and approximately 2–6% of all surgically treated pituitary adenomas occur in young patients—prolactin- and ACTH-secreting adenomas being the most common types. Pediatric somatotropinomas are usually more aggressive than those in adults (106, 109, 110). Studies have examined AIP mutation prevalence among populations that specifically included children and adolescents (i.e., those aged <18 yr at diagnosis or disease onset). A screening study was performed by Georgitsi et al. (104) in a specific, sporadic, non-FIPA pediatric population (n = 36) aged less than 18 yr at diagnosis or at the time of first signs/symptoms of a pituitary adenoma. Two patients were found to have pathological AIP mutations; one was a male with gigantism due to a large somatotropinoma, and the other had a NFPA at the age of 15 yr, giving an overall prevalence in this pediatric series of 5.6%. Subsequently, Stratakis et al. (109) reported a series of patients from the NIH Clinical Center in the United States. This was a diverse population of patients with and without familial or associated syndromic disease features. One of 74 pediatric patients (1.4%) with isolated sporadic Cushing disease had an AIP mutation; this patient was diagnosed at age 6 yr with a microadenoma that recurred postsurgically and required radiotherapy to achieve control, resulting in panhypopituitarism. Two pediatric patients with non-FIPA sporadic somatotropinomas (n = 1) or prolactinomas (n = 1) had AIP mutations; both were macroadenomas, and one was a de novo mutation, which remains the only case reported to date. Finally, one 11-yr-old patient from a heterogeneous FIPA family with a somatolactotrope macroadenoma had an AIP mutation, which was poorly responsive to somatostatin analogs and required three operations and radiotherapy. Interestingly, that patient's tumor had zones of hyperplasia on pathological analysis, a novel feature that was later reported in fraternal twin sisters with silent somatotrope adenomas and a separate AIP mutation (75). In the latter study, it was noted that while the adenoma tissue had LOH for AIP, as would be expected by the Knudson two-hit hypothesis, both the hyperplastic and normal pituitary tissues did not have LOH for AIP. This suggests that if the hyperplastic and adenomatous tissue were pathologically part of the same process, then loss of the wild-type allele may be a relatively late event in tumorigenesis in AIP-mutated FIPA kindreds. Overall in the NIH series, eight of 88 (9.1%) patients had mutations in either AIP or MEN1 genes, of which AIP comprised four of 88 cases (4.5%), although this population was predominantly Cushing disease patients, in which the rate of AIP mutations is low. A higher rate of AIP mutations appears to be present in pediatric and adolescent populations with macroadenomas, because Tichomirowa et al. (108) reported 20.5% of patients aged less than 18 yr at diagnosis had a germline AIP mutation. Similar evidence has been noted in a French series of 443 sporadic pituitary adenoma cases (101); of these cases, 30 were aged less than 18 yr at diagnosis, and 23.3% were noted to have germline AIP mutations. Pediatric/adolescent cases of AIP mutation-related pituitary adenomas, like their adult counterparts, are generally somatotropinomas, prolactinomas, mixed GH/prolactin-secreting adenomas, although Cushing disease cases are also seen.
C. Founder AIP mutations
The p.Q14X mutation has been described in the Finnish population and from haplotype and genealogical data is considered a founder mutation. A patient from Estonia with the same mutation has been noted and is possibly due to migration of this patient's ancestors from Finland (111). Founder mutations for a number of diseases (often recessive) have been described in Finland due to specific patterns of geographically delimited genetic drift in original founding populations after the last Ice Age, to which gene flow from Scandinavian populations to the west and south has created some striking differences between southern/western and northern/eastern Finland. We cannot speculate on when the founding AIP mutation occurred, in fact, although Finnish genealogical and parish information provide an excellent database. Historical evidence does point to many relevant cases coming from the same region of Finland. Daniel Cajanus, a northern Finnish man from Paltamo (modern province of Kainuu) was affected by gigantism in the early 18th century, and his sister and cousin also reputedly suffered from tall stature and enlarged extremities, respectively. Another man with gigantism from Tornio, northern Finland, was also noted some 70 yr previously (112), whereas other well-known patients with acrogigantism either came from or had family from Paltamo (Väinö Myllyrinne) or the nearby Puolanka [Lauri (Louis) Moilanen]. All of these cases came from or had their origin in a limited geographical area separated by no more than 200 km and also very close to Oulu, where many of the p.Q14X cases are currently cared for.
The p.R304X mutation has also been shown to be a founder mutation. The first published evidence of this came from Occhi et al. (96) who compared the microsatellite marker pattern around the loci for the AIP and MEN1 genes in three Italian families, two of which were previously described (71, 84). Haplotyping showed that two of the families shared alleles that suggest a common ancestor, probably in the Lazio region from which they originated. Subsequently, Chahal et al. (90) connected a historic case of acrogigantism to four FIPA families from Northern Ireland, via a common p.R304X mutation of AIP with a common ancestor between approximately 375 and 3750 yr ago. Among patients with gigantism, the case of Charles Byrne has probably been the best documented in the popular and scientific literature (113, 114). He was born in County Derry in the Irish Province of Ulster, and following his death in 1783, his skeleton has been on public display, currently at the Hunterian Museum in London. Extracting DNA from the teeth, Chahal et al. (90) demonstrated that Charles Byrne's gigantism (and enlarged pituitary fossa as shown by Cushing a century before) was also due to a p.R304X AIP mutation and had a shared haplotype with the four modern-day FIPA families. The p.R304 residue of AIP is a hot spot for truncating mutations (c.910 C>T) and also for a missense p.R304Q mutation (c.911 G>A), due to its being a CpG site; multiple FIPA families and simplex cases with these mutations have been described across the globe (Table 1). Similarly, other pathological mutations, such as p.R271W, p.K241E/p.K241X, and p.R81X also have been shown to act as hot spots in multiple kindreds (71, 109, 115–117).
Table 1.
AIP mutations identified in patients with pituitary adenomas and the main associated clinical/demographic features
| Mutation/variant | Protein prediction | Affected patients (n) | FIPA, sporadic, familial/simplex cases | Pituitary tumor type | Gender | Age at diagnosis | Macroadenoma/microadenoma (n) | Gigantism | Country (no. of patients) |
|---|---|---|---|---|---|---|---|---|---|
| Truncating mutations | |||||||||
| c.40C>T | Q14X | 19 | FIPA (13), sporadic (4), familial (1), N/A (1) | Somatotropinoma (11), somatolactotropinoma (2), NFPA (3), prolactinoma (2), N/A (1) | M (12), F (6), N/A (1) | 11, 14, 17, 18, 19, 21, 22, 23 (2), 24, 28, 29, 31, 34, 35, 41, 42, 48, N/A (1) | Macroadenoma (17), Microadenoma (1), N/A (1) | 5 | Finland (18), Estonia (1) |
| c.64C>T | R22X | 2 | Familial (2 separate) | Somatotropinoma (2) | M (2) | 11, 22 | Macroadenoma (2) | 1 | France (1), Spain (1) |
| c.70G>T | E24X | 7 | FIPA (7) | Somatotropinoma (3), somatolactotropinoma (1), N/A (3) | M (3), F (4) | 13, 15, 16, 17 (2), 18, 24 | Macroadenoma (3), microadenoma (1), N/A (3) | 5 | Brazil (7) |
| c.241C>T | R81X | 4 | FIPA (4) | Somatotropinoma (4) | M (2), F (2) | 14, 25, 34, 36 | Macroadenoma (4) | 1 | United States (2), Brazil (2) |
| c.424C>T | Q142X | 3 | FIPA (3) | Somatotropinoma (3) | M (2), F (1) | 17 (2), 29 | Macroadenoma (3) | 1 | Italy (3) |
| c.490C>T | Q164X | 2 | FIPA (2) | Somatotropinoma (2) | M (1), F (1) | 20, 23 | Macroadenoma (2) | 2 | Germany (2) |
| c.550C>T | Q184X | 1 | Familial | Somatotropinoma (1) | M (1) | 21 | Macroadenoma (1) | 1 | Italy |
| c.601A>T | K201X | 2 | Sporadic (2) | Somatotropinoma (2) | M (1), F (1) | 24, 27 | Macroadenoma (2) | France (2) | |
| c.646G>T | E216X | 2 | FIPA (2) | NFPA (silent somatotrope; 2) | F (2) | 12, 17 | Macroadenoma (2) | France (2) | |
| c.649C>T | Q217X | 2 | FIPA (2) | Somatotropinoma (1), somatolactotropinoma (1) | M (1), F (1) | 23, 28 | Macroadenoma (1), microadenoma (1) | Belgium | |
| c.662dupC | E222X | 2 | FIPA (2) | Somatotropinoma (2) | M (2) | 24, 28 | Macroadenoma (2) | United Kingdom | |
| c.715C>T | Q239X | 2 | FIPA (2) | Somatotropinoma (2) | M (2) | 14, 15 | Macroadenoma (2) | 2 | France (2) |
| c.721A>T | K241X | 1 | Sporadic (de novo) | Prolactinoma | M (1) | 18 | Macroadenoma (1) | United States (1) | |
| c.783C>G | Y261X | 2 | Sporadic (2) | Somatotropinoma (1), somatolactotropinoma (1) | M (2) | 17, 28 | Macroadenoma (2) | 2 | France (2) |
| c.804A>C | Y268X | 3 | FIPA (2), familial (1) | Somatotropinoma (2), prolactinoma (1) | M (1), F (2) | 23, 24, 29 | Macroadenoma (3) | Brazil (3) | |
| Q285X | 1 | Familial | Somatotropinoma (1) | M (1) | 60 | Macroadenoma (1) | The Netherlands | ||
| c.910C>T | R304X | 35 | FIPA (28), sporadic (2), familial (2), N/A (3) | Somatotropinoma (27), somatolactotropinoma (2), prolactinoma (3), N/A (3) | M (16), F (16), N/A (3) | 6, 8, 13, 16 (2), 17 (4), 18 (2), 19 (3), 20 (3), 21, 24 (2), 25 (2), 26, 27, 32 (2), 42, 62, N/A (7) | Macroadenoma (29), Microadenoma (1), N/A (5) | United Kingdom (19), Italy (7), New Zealand (2), Romania (2), Belgium (1), France (1), India (1), United States (1) | |
| Initiation codon mutation | 7 | Australia (1) | |||||||
| c.2T>C | M1? | 1 | Familial | Somatotropinoma (1) | M (1) | 8 | Macroadenoma (1) | 1 | France |
| Mutations leading to frameshift and truncation | |||||||||
| c.3_4insC | R2fsX43 | 4 | FIPA (4) | Somatotropinoma (2), NFPA (silent somatotrope; 2) | M (4) | 8, 12, 41, N/A (1) | Macroadenoma (1), microadenoma (1), empty sella (1), N/A (1) | 1 | Greece |
| c.88_89del GA | D30TfsX14 | 1 | Sporadic (1) | NFPA | M (1) | 19 | Macroadenoma | France | |
| c.74_81delins7 | L25PfsX130 | 5 | FIPA (5) | Somatotropinoma (3), somatolactotropinoma (1), prolactinoma (1) | M (3), F (2) | 13, 25, 27, 32, 38 | Macroadenoma (4), microadenoma (1) | 1 | Australia |
| c.244_248delGAAGG | E82fsX7 | 1 | Sporadic (1) | Somatotropinoma (1) | M (1) | 15 | Macroadenoma (1) | Bulgaria | |
| c.249G>T | G83AfsX15 | 3 | FIPA (3) | Somatotropinoma (1), prolactinoma (2) | M (3) | 14, 60, N/A | Macroadenoma (2), N/A (1) | 1 | United Kingdom |
| c.286_287delGT | V96PfsX32 | 3 | FIPA (3) | Somatotropinoma (3) | M (3) | 22, 26, 52 | Macroadenoma (2), empty sella (1) | 2 | Japan |
| c.350delG | E117AfsX39 | 3 | Sporadic (3) | Somatotropinoma (1), prolactinoma (2) | M (2), F (1) | 16, 18, 30 | Macroadenoma (3) | France | |
| c.338insACCC | P114fsX | 1 | Sporadic (1) | Somatotropinoma (1) | F (1) | 12 | Macroadenoma (1) | 1 | United States |
| c.404delA | H135LfsX21 | 1 | Sporadic (1) | Somatotropinoma (1) | M (1) | 14 | Macroadenoma (1) | 1 | France |
| c.500delC | P167HfsX3 | 6 | FIPA (6) | N/A (6) | M (2), F (4) | N/A | N/A (6) | Malaysia | |
| c.517_521delGAAGA | E174fsX47 | 3 | FIPA (3) | Somatotropinoma (2), somatolactotropinoma (1) | M (1), F (2) | 17, 25, 35 | Macroadenoma (2), microadenoma (1) | 1 | Brazil |
| c.543delT | L181fsX13 | 6 | FIPA (6) | Somatotropinoma (2), prolactinoma (1), NFPA (1), N/A (2) | M (4), F (2) | 16, 17, 32, 48, 65, N/A (1) | Macroadenoma (4), microadenoma (1), empty sella (1) | 1 | Spain |
| c.752delT | L251RfsX52 | 1 | Sporadic (1) | Cushing disease (1) | F (1) | 25 | Macroadenoma (1) | France | |
| c.824_825insA | H275QfsX12 | 1 | Sporadic (1) | Somatotropinoma (1) | M (1) | 8 | N/A (1) | United States | |
| c.854_857delAGGC | Q285fsX16 | 2 | FIPA (2) | Somatotropinoma (2) | M (2) | 20, 33 | Macroadenoma (2) | 1 | Italy |
| c.919insC | Q307fsX104 | 2 | FIPA (2) | Somatotropinoma (1), prolactinoma (1) | M (1), F (1) | 11, N/A (1) | Macroadenoma (2) | United States | |
| Inframe duplication | |||||||||
| c.805_825 dup | F269_H275dup | 4 | FIPA (3), sporadic (1) | Somatotropinoma (3), Somatolactotropinoma (1) | M (4) | 14, 15 (2), 29 | Macroadenoma (3), N/A (1) | 3 | United Kingdom, (3), France (1) |
| bp Substitutions | |||||||||
| c. (-270_-269CG>AA) and c.(220G>A) | Reduced promoter activity | 2 | FIPA (2) | Somatotropinoma (2) | F (2) | 10, 14 | Macroadenoma (2) | 2 | Japan |
| Inframe deletions | |||||||||
| c.66_71delAGGAGA | delG23_E24 | 1 | Sporadic (1) | Somatotropinoma (1) | M (1) | 20 | N/A (1) | Germany | |
| c.138_161del24 | delG47_R54 | 2 | FIPA (2) | Somatotropinoma (2) | M (2) | 25, 28 | Macroadenoma (2) | Argentina | |
| c.742_744delTAC | delY248 | 1 | Familial | Somatotropinoma (1) | M (1) | 19 | Macroadenoma (1) | 1 | Italy |
| Extensive deletions | |||||||||
| c.878_879AG>GT and c.880_891delCTGGACCCAGCC | E293G; and del L294_A297 | 2 | FIPA (2) | Somatotropinoma (2) | M (1), N/A (1) | 20, N/A (1) | N/A (2) | Germany | |
| c.1104_-109_279 + 578 | Exon 1 and 2 deletion | 2 | FIPA (2) | Somatotropinoma (2) | M (1), F (1) | 24, 46 | Macroadenoma (1), N/A (1) | Germany | |
| c.100–1025_279 + 357del | delA34_K93 (exon 2 deletion) | 4 | FIPA (4) | NFPA (2), somatolactotropinoma (1), plurihormonal tumor (GH, prolactin, gonadotrope; 1) | M (2), F (2) | 17, 18 (2), 29 | Macroadenoma (2), microadenoma (2) | 1 | United Kingdom |
| c.1-?_993+?del | Full gene (exon 1–6) deletion | 3 | FIPA (3) | Somatotropinoma (3) | M (3) | 19, 23, 35 | Macroadenoma (3) | United Kingdom | |
| Full gene deletion | Exon 1–6 deletion | 2 | FIPA (2) | Somatotropinoma (2) | M (1), F (1) | 10, 20 | Macroadenoma (2) | 2 | Serbia |
| Missense mutations | |||||||||
| c.26G>A | R9Q | 2 | Sporadic (2) | Cushing disease (1), prolactinoma (1) | F (2) | 14, 39 | Macroadenoma (1), Microadenoma (1) | France | |
| c.166C>A | R56C | 1 | Sporadic (1) | Prolactinoma | M (1) | 26 | Macroadenoma (1) | Bulgaria | |
| c.174G>C | K58N | 2 | Sporadic (2) | Prolactinoma (1), gonadotropinoma (1) | M (1), F (1) | 20, 32 | Macroadenoma (2) | France | |
| L70M | 2 | FIPA (2) | Somatolactotropinoma (1), prolactinoma (1) | M (1), F (1) | 22, 60 | Macroadenoma (2) | Italy | ||
| c.250G>A | E84K | 1 | Sporadic (1) | Somatolactotropinoma | F (1) | 22 | Macroadenoma (1) | Ukraine | |
| c.308A>G | K103R | 1 | Sporadic (1) | Cushing disease | M (1) | 6 | Microadenoma (1) | United States | |
| c.509T>C | M170T | 1 | Sporadic (1) | Somatotropinoma | M (1) | 32 | Macroadenoma (1) | 1 | France |
| c.563G>A | R188Q | 1 | Sporadic (1) | Prolactinoma | F (1) | 24 | Microadenoma (1) | France | |
| c.584T>C | V195A | 1 | Familial | Prolactinoma | M (1) | 12 | Macroadenoma (1) | Brazil | |
| c.713G>A | C238Y | 3 | FIPA (3) | Somatotropinoma (3) | M (3) | 19, 21, 23 | Macroadenoma (3) | Mexico | |
| c.718T>C | C240R | 3 | FIPA (3) | Somatotropinoma (3) | M (3) | 13, 15, 25 | Macroadenoma (3) | 1 | France |
| c.721A>G | K241E | 2 | FIPA (2) | Prolactinoma (1), NFPA (1) | M (1), F (1) | 39, 53 | Macroadenoma (2) | Belgium | |
| c.769A>G | I257V | 1 | Familial | Thyrotropinoma | M (1) | 39 | Macroadenoma (1) | Spain | |
| c.803A>G | Y268C | 1 | Familial | Prolactinoma | M (1) | 28 | Macroadenoma (1) | Belgium | |
| c.811C>T | R271W | 10 | FIPA (9), sporadic (1) | Somatotropinoma (8), Somatolactotropinoma (1), prolactinoma (1) | M (8), F (2) | 10, 12, 16, 18, 21, 22, 29, 42, N/A (2) | Macroadenoma (8), microadenoma (2) | France (4), New Zealand (3), India 3 (2), Italy (1) | |
| c.829G>C | A277P | 1 | Familial | Somatolactotropinoma (1) | M (1) | 12 | Macroadenoma (1) | 1 | Italy |
| c.871G>A | V291M | 1 | Sporadic (1) | Somatolactotropinoma (1) | F (1) | 30 | N/A (1) | Italy | |
| c.872T>A | V291E | 1 | Sporadic (1) | Somatolactotropinoma (1) | M (1) | 21 | Macroadenoma (1) | 1 | France |
| c.911G>A | R304Q | 10 | FIPA (2), familial (2), sporadic (6) | Somatotropinoma (6), prolactinoma (3), Cushing disease (1) | M (2), F (7), N/A (1) | 15, 17, 25, 26, 30, 37, 38, 67, N/A (2) | Macroadenoma (7), microadenoma (2), N/A (1) | Italy (3), Romania (2), The Netherlands (1), Poland (1), Belgium (1), France (2) | |
| c.974G>A | R325Q | 1 | Sporadic (1) | Prolactinoma | F (1) | 18 | Macroadenoma (1) | France | |
| Silent/synonymous splice site mutations | |||||||||
| c.591G>A | E197E | 2 | Sporadic (2) | Somatotropinoma (2) | F (2) | 23, 66 | Macroadenoma (2) | Italy (1), Lebanon (1) | |
| c.807C>T | F269F | 5 | FIPA (2), sporadic (3) | Somatotropinoma (3), prolactinoma (1), NFPA (1) | M (3), F (2) | 10, 47, 58, N/A (2) | Macroadenoma (4), N/A (1) | United Kingdom (2), Belgium (1), Bulgaria (1), Italy (1) | |
| Intronic mutations | |||||||||
| IVS3–2A>G | 2 | Sporadic (2) | Somatolactotropinoma (1), prolactinoma (1) | M (2) | 16, 40 | Macroadenoma (2) | France (2) | ||
| IVS3 + 1G>A | 1 | Sporadic (1) | Somatotropinoma | F (1) | 62 | Microadenoma (1) | Italy | ||
| IVS3–1G>A | 1 | Sporadic (1) | Somatolactotropinoma | F (1) | 26 | Microadenoma (1) | Finland | ||
| IVS2–1G>C | 1 | Sporadic (1) | Somatotropinoma | M (1) | 17 | Macroadenoma (1) | United States |
The predicted effect on protein, where known, is included. Missense mutations are included based on having demonstrable effects in vitro, in silico, or occurring in highly conserved domains within the AIP molecule. M, Male; F, female; N/A, not available.
D. AIP mutation screening: current status
Based on a review of the published literature, online resources such as GenBank and the authors' own unpublished screening results, a total of 215 patients with AIP mutations and pituitary adenomas have been reported. As shown in Table 1, to date, 70 different AIP mutations have been identified. Mutations in AIP occur relatively evenly throughout the coding region of the gene. All of these mutations have been germline mutations, and no somatic AIP mutations have been found in pituitary tumors. Different mutation types include nonsense, missense, splice site, insertion, deletion, frameshift, and promoter region mutations, as well as heterozygous deletion of most of or the entire AIP gene. Nonsense mutations and frameshifts leading to truncations account for half of reported AIP mutations. The missense mutations shown in Table 1 involve residues that are highly conserved and/or have been shown to correlate with altered in vitro activity in the published literature. Similarly, a variety of groups have used various in silico and in vitro methods to verify the pathogenicity of many intronic/splicing mutations. However, in cases where clear deleterious effects or correlates of genetic variants are not clearly present, caution should be used before labeling them as mutations, and a more conservative terminology such as “a variant of unknown significance” may be employed.
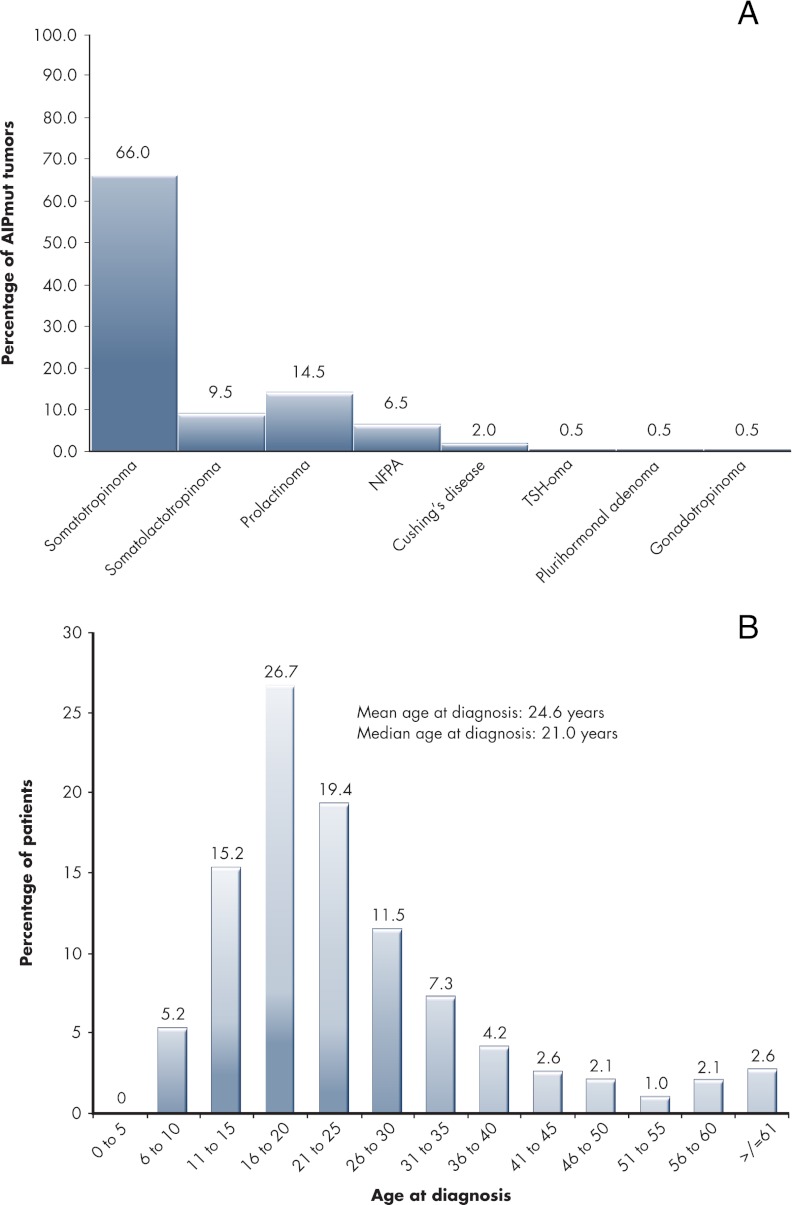
The most common mutations of the AIP gene are p.R304X (n = 35 patients), p.Q14X (n = 19 patients), p.R271W (n = 10 patients), and p.R304Q (n = 10 patients). As screening procedures have expanded globally, other mutations such as p.R81X appear to be growing in frequency. As seen in Fig. 3A and Table 1, the pituitary adenomas definitively diagnosed in association with AIP mutations consist of 132 somatotropinomas, 19 somatolactotropinomas, 29 prolactinomas, 13 NFPAs, four Cushing disease, one thyrotropinoma, one gonadotropinoma, and one plurihormonal tumor (tumor type not available; n = 15). The population remains predominantly male (61.2%), and the majority of patients present with macroadenomas (88.3%). Most cases present as FIPA (68.2%). Other cases may be pure simplex cases in which only one known AIP mutation-positive pituitary adenoma patient exists among a family of AIP mutation carriers (7.6%). So-called sporadic cases (24.2%) are likely to be a mix of classifications. Many will be cases in which familial screening for pituitary disease and/or AIP mutations was not offered, was declined, or is not reported, and as such, many could represent either unknown FIPA kindreds or simplex cases. Only one de novo mutation in AIP has been reported in a sporadic patient (109). Figure 3B shows the distribution of age at diagnosis among the known cases of AIP-associated pituitary adenomas. This confirms the consistent impression since the original studies on AIP that young age at diagnosis is a clear feature of this disease (71, 84, 99). A total of 78.0% of patients were diagnosed at or before the age of 30, and only a further 11.5% were diagnosed between 30 and 40 yr of age. Three patients had empty sella and a distant history of headache and acute illness that suggested apoplexy. As noted by Igreja et al. (86) in their series, apoplexy was a presenting feature in 8% of their 63 cases and has been noted in acute presentation by others in the FIPA setting (75, 86, 90, 118). Specific studies on series of patients presenting with pituitary tumor apoplexy may confirm this to be a suggestive feature of AIP mutation carriage. Gigantism occurred in more than one third of somatotropinoma cases (36.0%) and accounts for one fourth of all cases of AIP mutations reported to date. This confirms the finding from a specific comparison of AIP mutation-related somatotropinomas vs. sporadic AIP-intact cases that gigantism is significantly more common in the setting of AIP mutations and represents a characteristic phenotypic feature of the disease (115).
Figure 3.
A, Percentage of patients with different pituitary adenoma types seen in patients (n = 215) with germline AIP mutations. B, Distribution of AIP mutation-positive pituitary adenoma population by age at diagnosis (divided into 5 yr cohorts).
Most of the AIP mutations noted in Table 1 were identified by sequencing approach. Some patients harbor large germline AIP deletions, which are undetectable by traditional sequencing methods (86, 105). To identify such germline mutations, the use of the MLPA technique is needed and has proven useful in the demonstration of AIP genetic abnormalities, although in a relatively limited number of cases (86, 100, 105), and in numerous large studies no mutations were revealed by this method (95, 101, 108). MLPA studies in populations that are negative for AIP mutations on standard sequencing are, however, useful to identify those rarer instances of gene deletions (85).
E. Treatment outcomes in AIP-mutated pituitary adenomas
The characteristics of AIP-mutated pituitary adenomas as being large and expansive tumors occurring at a young age suggest a negative impact on treatment outcomes. Also, early evidence pointed to a possibility for AIP-mutated somatotropinomas to be relatively resistant to the effects of somatostatin analogs (67, 89). These and other characteristics were studied specifically in a series of 96 patients with AIP mutations and pituitary adenomas to determine whether larger tumor size translated into difficulties in disease control (115). Among the group, somatotropinomas were the most frequent type (n = 75), and these patients were compared with a control group of 232 acromegaly patients without AIP mutations (Table 2). The AIP mutation group had significantly more males than control acromegaly patients. As expected from previous studies, AIP mutation-related tumors were significantly larger and more frequently had extrasellar extension at diagnosis. Fifty-two percent of acromegaly cases in the AIP mutation group had first symptoms before the age of 18 as compared with less than 5% of controls. Gigantism was significantly more common among the AIP mutation group vs. controls, and all cases of gigantism in that group occurred in males. The increased tumor size was associated with higher GH secretion at diagnosis in the AIP-mutated group vs. controls, whereas prolactin co-secretion was present in 56 and 29% of the AIP-mutated and control populations, respectively. These comparisons were all statistically significant (115).
Table 2.
Clinical characteristics of 75 AIPmut-associated and 232 control non-AIPmut somatotropinoma (n = 232) patients
| Somatotropinoma groups |
P value | ||
|---|---|---|---|
| AIPmut | Control | ||
| n | 75 | 232 | |
| Percentage males | 61.3% | 46.5% | 0.027 |
| Age at diagnosis (yr) | 22.0 (8.0–60.0) | 43.0 (16.0–72.0) | <0.000001 |
| Age at first symptoms (yr) | 17.5 (4.0–50.0) | 38.0 (14.0–70.0) | <0.000001 |
| Maximum tumor diameter (mm) | 22.5 (7.0–60.0) | 16.0 (3.0–48.0) | 0.00026 |
| Macroadenoma | 93.1 | 80.8 | 0.026 |
| Extrasellar extension (%) | 65.1 | 49.8 | 0.018 |
| Invasion (%) | 51.7 | 38.8 | 0.11 |
| GH level at diagnosis (ng/ml) | 28.5 (3.3–183.0) | 17.4 (1.7–180.0) | 0.00068 |
| IGF-I level at diagnosis (%ULN) | 217.0 (116.0–1090.0) | 210.5 (20.0–550.0) | 0.48 |
| Prolactin co-secretion (%) | 56.1 | 28.9 | 0.00023 |
| Gigantism (%) | 32.0 | 6.5 | <0.000001 |
Extrasellar extension represents superior or lateral extension of the tumor beyond the sella on MRI/computed tomography or at surgery. Invasion represents evidence of the presence of pituitary tumor tissue invading or penetrating the normal border of the pituitary gland. Age at diagnosis, age at first symptoms, maximum tumor diameter, and GH and IGF-I levels at diagnosis are presented as median (range). ULN, Upper level of normal. [Adapted from A. F. Daly et al.: Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab 95:E373–E383, 2010 (115), with permission. © The Endocrine Society.].
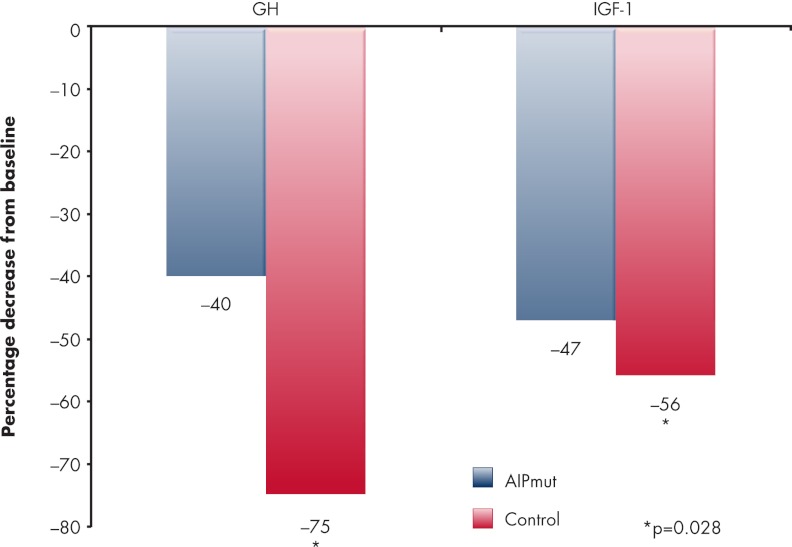
After a follow-up of at least 1 yr, acromegaly patients with AIP mutations had a similar overall rate of disease (70.4%) as the controls (80.5%). Specifically, patients with somatotropinomas in the setting of AIP mutations required a second or third neurosurgical intervention significantly more frequently than controls (22 vs. 6%, respectively). Although radiotherapy was employed more frequently in the AIP mutation group (41%) as compared with controls (25%), this did not reach statistical significance. In patients with AIP mutations treated with somatostatin analogs, the median percentage decreases in GH and IGF-I secretion were significantly lower than in the acromegalic controls (Fig. 4). These differences were unaffected when preoperative, primary, and postoperative somatostatin analog use was assessed separately. In parallel, a smaller degree of tumor shrinkage was noted with somatostatin analogs in the AIP mutation group vs. control patients. Four AIP mutation-bearing patients had complete resistance to somatostatin analogs and tumor growth while on treatment.
Figure 4.
Relative resistance to somatostatin analogs in AIP mutation-related somatotropinomas vs. controls. Patients treated with somatostatin analogs for acromegaly who had germline AIP mutations (n = 75) had a statistically significantly lower percentage decrease from baseline in serum GH and serum IGF-I concentration as compared with 232 wild-type AIP control patients that were matched for age, sex, and decade of diagnosis. [Derived from A. F. Daly et al.: Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab 95:E373–E383, 2010 (115), with permission. © The Endocrine Society.].
Similar to the case with somatotropinomas, patients with AIP mutation-related prolactinomas (n = 13) also displayed features that suggested relative resistance to treatment. More than three fourths of patients were males who were at a young age at the time of first symptoms (50% were younger than 18 yr). As with somatotropinomas, tumors were also large at diagnosis (only one was a microadenoma), and invasion of local structures had already occurred at diagnosis in nine of 13 cases. All but one case received dopamine agonist treatment, and overall efficacy was relatively poor (five of 12 cases controlled initially), leading to a need for surgery in seven patients, of whom one had four neurosurgical interventions and three underwent two transsphenoidal operations each. Importantly, relatively poor responses to dopamine agonists were seen, including cases of primary and secondary resistance. The reason for this characteristic remains unclear because dopamine D2 receptor studies were not performed on the tumor tissues from these patients. Information on NFPA and other tumor types was based on small patient numbers, and conclusions are difficult to draw from the limited data.
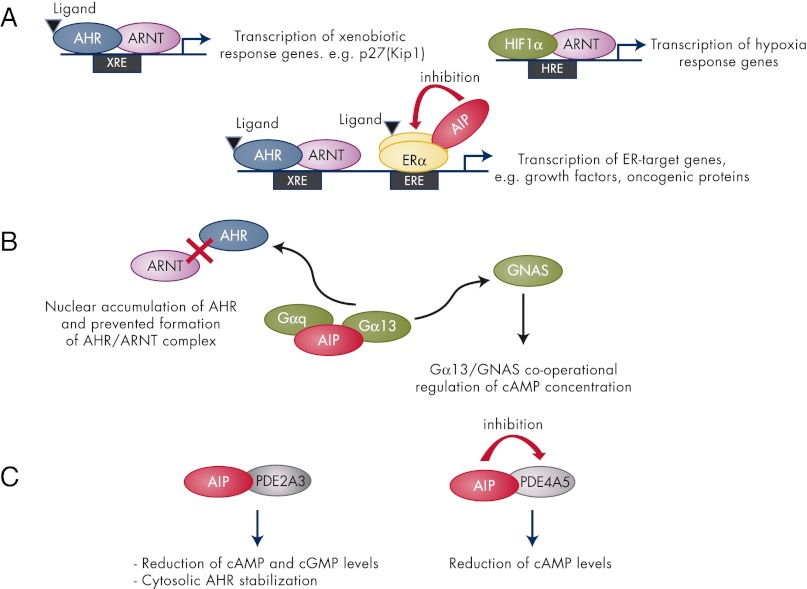
Overall, patients with AIP mutation-related pituitary adenomas usually have somatotropinomas/somatolactotropinomas or prolactinomas; in the course of clinical management, these tumors appear to have relatively poor medical therapy responses and require more frequent reoperation. The basis for the reduced response to somatostatin analogs in terms of hormonal reduction and tumor size changes is unknown. Further study of important determinants, such as tumor expression of somatostatin receptors 2 and 5 and particularly the truncated form of somatostatin receptor subtype 5 or other factors that determine octreotide responses is needed (119–125). Recent work from Chahal et al. (126) points to a potential mechanism via the zinc finger transcription factor ZAC1. ZAC1 is highly expressed in normal pituitary but is down-regulated in pituitary adenomas (123, 125); importantly, AIP expression is increased in tumors from patients previously treated with somatostatin analogs before surgery (126).
F. Role of AIP mutations in other tumor types
To date, the only tumors that have been found to unambiguously associate with AIP mutations are pituitary adenomas, which contrasts with MEN1, MEN4, and CNC, where several other tumor types are found among the typical manifestations. Mutation screening in a total of 499 colorectal, breast, and prostate cancers was performed (127). These tumors, apart from being the most prevalent among men and women worldwide, have been associated with acromegaly, particularly colorectal neoplasia. In this material, no somatic AIP mutations were identified. The occurrence of somatic AIP mutations has been studied also in nonpituitary endocrine tumors and familial nonmedullary thyroid cancer (128, 129). Altogether, 79 sporadic tumors of endocrine system, including thyroid, adrenal, and parathyroid lesions, carcinoids and adenocarcinoids, paragangliomas, and pancreatic tumors were screened with negative results; no germline AIP mutations were detected in familial nonmedullary thyroid cancers either.
Interestingly, loss of wild-type allele was recently reported in an adrenocortical carcinoma of an acromegaly patient with a germline AIP mutation (p.R81X) (130). However, the 11q13 LOH, in the region where the MEN1 gene is also located, could also be accompanied by a germline defect in another, as yet unidentified, tumor suppressor gene at 11q13. The existence of such a gene related to adrenocortical tumorigenesis has been suggested previously (12, 131). Although other groups have also noted nonpituitary tumors in patients with AIP mutations, no consistent pattern has emerged (67), and recently an association between meningioma and AIP mutation was discounted in a patient with a pituitary adenoma (117). Identification of rare tumor associations with AIP, which is already a quite low penetrance gene, may require further long-term follow-up of large numbers of affected individuals and mutation carriers. Hibernomas are benign neoplasms displaying cytogenetic rearrangements involving chromosome band 11q13. A recent study of Nord et al. (132) suggested MEN1 and AIP deletion to be involved in the pathogenesis of these brown fat tumors, but these tumors have, as yet, not been identified in carriers of germline AIP mutations.
IV. Mouse Models and in Vitro Studies of AIP in Pituitary Tumor Biology
Mouse models have been widely used to study pituitary development, function, and disease to gain important insight into the role of particular genes in different pathways and the nature of tumor development in the pituitary gland. Moreover, if the mouse model recapitulates the phenotype of human disease, it can offer a platform in which to test new drugs or targeted therapies for patients.
A. Phenotypes of Aip knockout mouse models
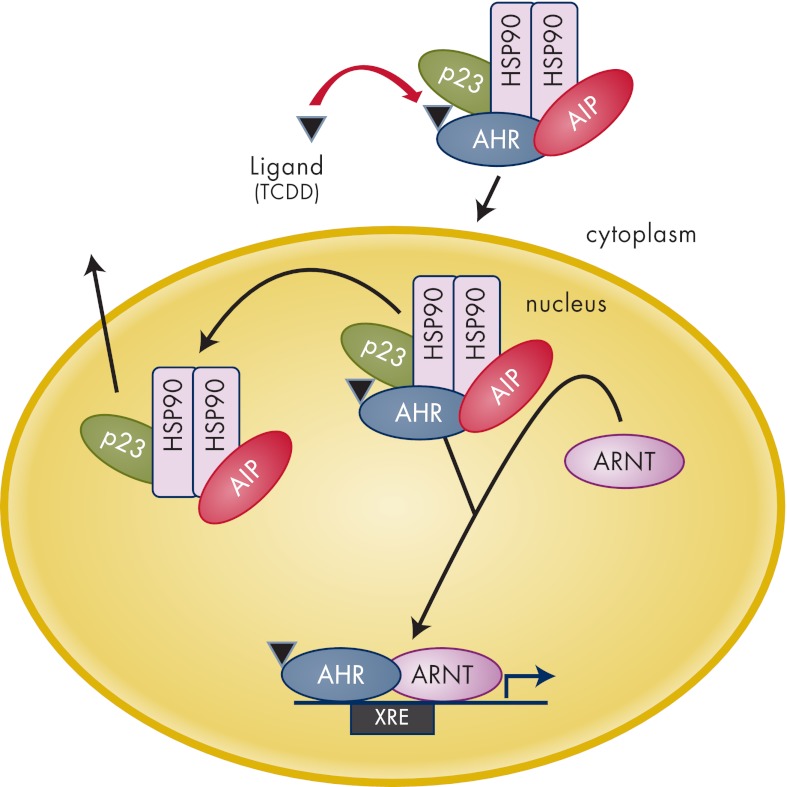
The first Aip mouse model was published by Lin et al. (133). Homozygous Aip loss was associated with embryonic lethality due to the congenital cardiovascular abnormalities such as a double-outlet right ventricle, ventricular septal defects, and pericardial edema at an embryonic age of 10.5–14.5 d. In this same study, heterozygous (Aip+/−) mice were phenotypically normal and fertile. Moreover, Lin et al. (134) created a hypomorphic Aip mouse model, which displayed a reduced Aip expression. Hypomorphic Aip mice showed a patent ductus venosus resulting in reduced liver size (134). Interestingly, failure of ductus venosus closure has also been detected in aryl hydrocarbon receptor (Ahr) and Ahr nuclear translator (Arnt) mouse models (135, 136), suggesting that AIP plays an important role in the AHR-mediated developmental pathway.
A conditional Aip mouse model where the Aip gene was deleted in hepatocytes showed that AIP deficiency leads to reduction of functional cytosolic AHR in the liver and eliminates/reduces dioxin-induced hepatotoxicity (137). AIP seemed to be required for the expression of AHR response genes, albeit that some of the dioxin-response genes were not dependent upon AIP coexpression. This differential dependence on AIP demonstrates that mammalian genome appears to contain more than one class of AHR-response genes and AIP may have a significant role in the up-regulation of a subset of these (137).
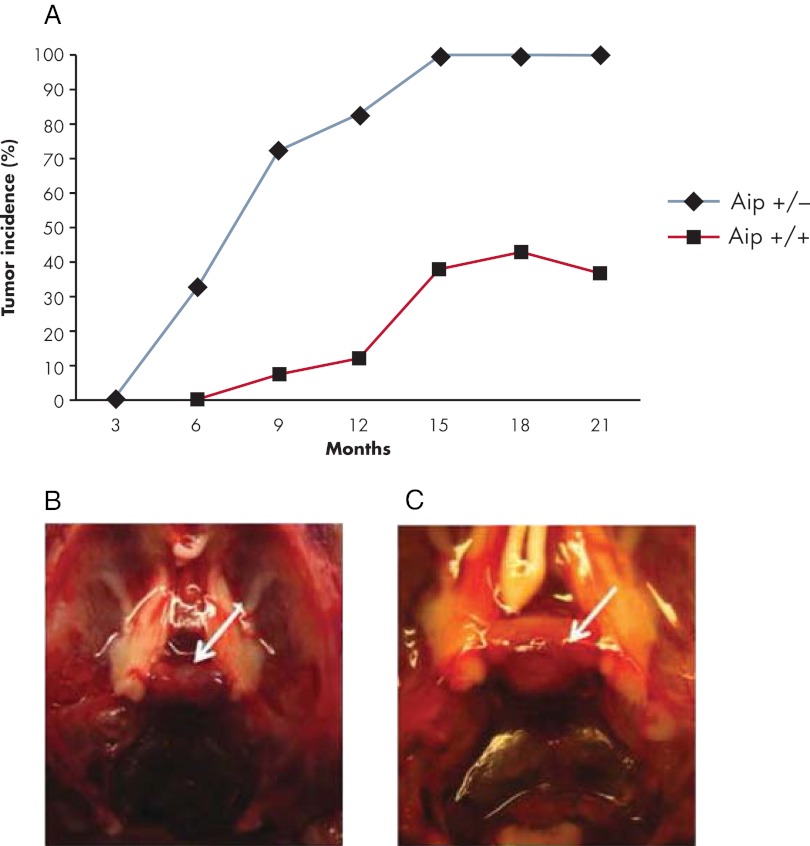
Possible tumor predisposition was not a focus of the above-mentioned Aip mouse model studies. To model the PAP caused by germline Aip mutations and to clarify the tumor spectrum, Raitila et al. (138) created a conventional Aip mouse model. The mouse model was generated by inserting a gene trap vector construct into an intronic region of Aip, which produced a truncated AIP protein. Homozygous knockout (Aip−/−) mice died during embryogenesis, this result being consistent with the earlier study of Lin et al. (133). Heterozygous Aip mice were highly prone to pituitary adenomas. Aip+/− mice developed pituitary tumors localized in the pars distalis, which corresponds to the human anterior pituitary, at the age of 6 months. No tumors were detected at 3 months, which could be explained by the true rarity of pituitary adenomas in this age group or possibly by the lesions being too small to be detected with routine immunostaining. AIP immunohistochemistry, as well as LOH screening, showed biallelic inactivation of AIP. Complete penetrance of pituitary adenomas was reached at the age of 15 months (Fig. 5), emphasizing the fundamental importance of Aip for pituitary tumorigenesis. The majority of mice developed somatotropinomas (88%), although mixed GH/prolactin, prolactinomas, and ACTH-positive adenomas were also detected; some adenomas grew to a very large size (Fig. 5, B and C) (138). Aip+/− mice with somatotropinomas had significantly elevated IGF-1 (Igf-1) expression levels and evidence of increased internal organ weight. The incidence of pituitary lesions in heterozygous Aip mice is extremely high as compared with known prevalence of these tumors in mice. However, no clear excess of any other tumor types was detected, although a slight excess of hyperplasia of adrenal glands was seen.
Figure 5.
Aip mouse model phenotype. A, Pituitary adenoma prevalence in heterozygous (Aip+/−) and wild-type (Aip+/+) mice. B, Normal pituitary gland of wild-type mouse. C, Macroadenoma of Aip+/− mouse. Pituitary glands are depicted by white arrows.
In humans, AIP-associated tumors can appear already in childhood or early adulthood. In the Aip mouse model, the first lesions were detected at the age of 6 months, thus in adulthood. The other main difference between the human and mouse AIP disease phenotype was the complete penetrance of pituitary adenomas in the Aip mouse model. Despite genomic conservation between the species (139), many biological functions differ, and any given response in humans may not occur in precisely the same way in mice (140). It is possible that such differences can explain these phenotypic discrepancies observed between human and mouse pituitary tumor onset and penetrance. Nevertheless, this conventional Aip mouse model greatly resembles human disease, displaying a pituitary tumor phenotype with a predominance of somatotropinomas. This suggests that the factors underlying AIP tumorigenesis are similar in mice and humans. Therefore, this mouse model provides an extremely useful tool to further study the AIP-associated pituitary tumorigenesis, and it is potentially a valuable platform for testing new therapeutic strategies for management of patients with treatment-resistant pituitary adenomas.
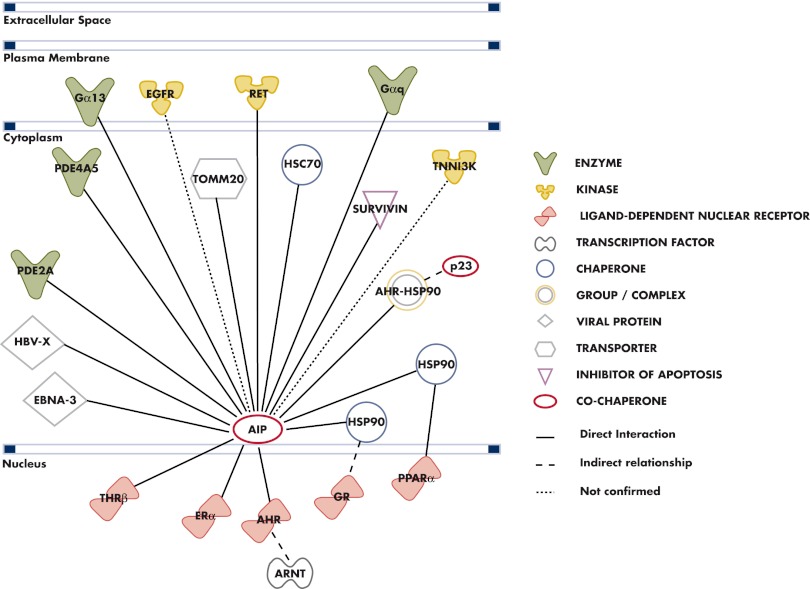
B. Molecular and pathological characteristics of AIP/Aip-associated tumors
The inactivating nature of the germline mutations, the loss of the normal allele in tumors, as well as recent functional evidence imply that the AIP gene acts as a tumor suppressor gene (67, 84, 138, 141). Wild-type AIP expression was shown to reduce cell proliferation in a rat somatomammotroph pituitary adenoma (GH3) cell line, and human fibroblast (HEK293 and TIG 3) cells and the mutant form of AIP protein lost the ability to block cell proliferation (67). This finding was echoed in the subsequent work of Heliövaara et al. (141), which found that Aip small interfering RNA silencing leads to a clear increase of cell proliferation rates in the GH3 cell line.
Human AIP-associated pituitary tumors have more aggressive features as compared with AIP mutation-negative tumors (115), and elevated staining for Ki-67, a marker of cell proliferation, has been demonstrated in some human AIP mutation-related pituitary adenomas (93, 142). To assess the aggressiveness of Aip-related tumors, the proliferation rates were evaluated in a set of mouse Aip-deficient pituitary tumors using Ki-67 immunohistochemistry. The Aip-associated tumors had significantly higher proliferation rates compared with Aip-proficient tumors. In Aip mutation-positive somatotropinomas and prolactinomas, the proliferation rates were 6.1 and 10.1%, respectively. In wild-type prolactinomas, the fraction of Ki-67-positive tumor cells was 3.6%. Hence, this result supports the view of a more aggressive disease profile of AIP mutation-positive tumors (138).
In a study of the histopathological characteristics of normal pituitary and adenoma tissues, Leontiou et al. (67) showed that in normal pituitary, AIP was present in GH- and prolactin-positive cells but not in other cell types. In sporadic pituitary adenomas without AIP mutations, AIP immunostaining was present in all adenomas irrespective of hormonal subtype; however, subcellular colocalization of AIP and hormone was only seen with GH in somatotropinomas. In AIP mutation-positive tumors from FIPA families (F269_H275dup and R304X mutations), double immunofluorescence staining showed that AIP colocalized with GH. On electron microscopy of normal pituitary, Leontiou et al. (67) showed that immunogold staining for AIP occurred only in GH- and prolactin-positive cells, and this staining was localized to the secretory granules. Whereas in sporadic somatotropinomas immunogold staining for AIP was also localized to the GH secretory vesicles, in sporadic prolactinomas this association between AIP and prolactin in secretory vesicles did not exist (AIP immunogold staining was cytoplasmic, as in corticotropinomas and nonfunctioning adenomas). Sparse and dense granulation patterns on electron microscopy have long been noted to associate with relatively poor and good responses to somatostatin analogs, respectively (143–145). Furthermore, cytokeratin staining of somatotropinomas in dot and perinuclear patterns mirror the electron microscopic classifications of sparse and dense granulation, respectively. Interestingly, dot pattern cytokeratin staining somatotropinomas tend to be larger sized, to occur in younger individuals, and to have poorer responses to test doses/treatment with somatostatin analogs (146). This combination of features echoes the findings of AIP-mutated somatotropinomas, suggesting an overlapping pathological relationship, which is further strengthened by a high frequency of sparsely granulated adenomas in somatotropinoma patients with AIP mutation in FIPA kindreds (67).
Recently, it has been suggested that AIP may be a factor in tumorigenesis and treatment responses of somatotropinomas without AIP mutations. Jaffrain-Rea et al. (142) demonstrated in a large series of AIP mutation-positive and -negative tumors that AIP mRNA levels and immunostaining intensities correlated with tumor phenotype and aggressiveness. Somatotropinomas, independently of their germline mutation status, showed lower AIP protein levels in invasive tumors as compared with noninvasive tumors. Supporting this notion, Gadelha's group (147) in Brazil recently noted that AIP immunostaining may be a more useful marker of invasiveness than Ki-67 labeling. Generally, patients with AIP-associated somatotropinoma have poor response to the somatostatin analog therapy (115). Kasuki et al. (148) studied whether the AIP protein expression could act as a predictor of treatment response to the somatostatin analog, octreotide, in AIP mutation-negative somatotropinomas. They reported that 22% of patients with low AIP levels in tumor were controlled with octreotide, whereas 65% of patients with high AIP protein expression were controlled. When both high AIP and somatostatin receptor 2 expression were used as predictors, control was achieved in 79% of patients. They concluded that the AIP protein expression alone is a good predictor of treatment response octreotide.
Because the regulatory actions of somatostatin analogs are mediated via five different somatostatin receptors, Chahal et al. (126) studied whether the reduced expression of the somatostatin receptor 1–3 and 5 proteins could explain the poor response to somatostatin analogs in AIP mutation-positive tumors. They did not detect reduction of somatostatin receptor subtypes, although the subtype 5 receptor had a somewhat higher expression in AIP-mutated tumors as compared with sporadic somatotropinomas (126). Importantly, AIP expression in tumors was increased in patients that were pretreated with somatostatin analogs before surgery. In that study, the effects of a somatostatin analog on AIP expression were examined in the GH3 rat somatomammotrope pituitary cell line. Octreotide treatment increased expression of AIP and ZAC1. Furthermore, overexpression of wild-type AIP was found to increase ZAC1 expression, whereas silencing of AIP reduced ZAC1 mRNA levels. Previously it was shown that Zac1 acts downstream of somatostatin receptor subtype 2, and knockdown of Zac1 (via RNA interference) prevented the effects of somatostatin analogs. Also, immunoreactivity for ZAC1 in somatotropinomas has previously been shown to significantly correlate with IGF-I normalization and tumor shrinkage with somatostatin analogs (123–125). These observations suggest that in somatotropinomas AIP may be involved in the regulation of the action of somatostatin analogs via the ZAC1 pathway. Furthermore, this interplay between the AIP and ZAC1 genes might also explain the statistically significantly reduced response to somatostatin analogs in somatotropinoma patients with AIP mutations (115).
C. Analysis of the biological functions of AIP
The AIP gene [also called the hepatitis B virus X-associated protein 2 (XAP2) (149), aryl hydrocarbon receptor-activated protein 9 (ARA9) (150), or FK506-binding protein 37 (FKBP37) (151)] contains six exons, and it encodes a cytoplasmic protein of 330 amino acids (37 kDa). The AIP protein was originally identified by its interaction and inhibition of the hepatitis B virus X protein 2 (152, 153). AIP displays structural similarity to AIP-like 1 and the immunophilin co-chaperones, FK506-binding protein (FKBP) 51 and FKBP52 (149, 150, 154). Despite homology with immunophilins, AIP is not considered a true immunophilin because it does not bind and mediate effects of immunosuppressive drugs (155). AIP contains three tetratricopeptide repeats and an α-helix in the C-terminal region, and this consensus tetratricopeptide repeat motif mediates various intra- and intermolecular protein interactions (156, 157). AIP can also self-associate and form a multimeric complex (158), and these AIP complexes might act as a reservoir for monomeric AIP that can be further used in the formation of AHR complexes.
AIP is considered as being ubiquitously expressed, but its expression levels vary considerably among different tissues (149, 153, 159). In normal pituitary, the AIP protein is expressed in somatotrophs and lactotrophs, where it associates with cytoplasmic secretory vesicles (67). Scattered AIP-expressed cells are detected also in the pars intermedia (142). In sporadic pituitary adenomas, AIP is expressed in all tumor types. In sporadic somatotropinomas, AIP colocalizes with GH in the secretory vesicles, similar to normal somatotrophs. In sporadic prolactinomas, corticotropinomas, and nonfunctioning adenomas, AIP resides in the cytoplasm (67). At present, the function of the AIP protein in normal pituitary or in pituitary adenoma cells is not known. Although it is generally acknowledged that AIP germline mutations predispose to pituitary adenomas, little is known about the molecular mechanisms leading to pituitary tumorigenesis. AIP has multiple cellular interaction partners (Fig. 6), and thus, AIP inactivation has the potential to interfere with a wide spectrum of cellular and environmental signals.
Figure 6.
AIP interaction partners. Nodes represent proteins, with their shape indicating the functional class of the protein. The protein-protein interaction network was generated with Ingenuity Pathway Analysis (IPA) software (www.ingenuity.com).
1. Xenobiotic signaling
The best-characterized AIP binding partner is AHR, also known as the dioxin receptor. AHR is a ligand-activated transcription factor belonging to the basic-helix-loop-helix (bHLH)/PER-ARNT-SIM homology region (PAS) family. In the cytoplasm, AIP forms a complex with AHR, two 90-kDa heat-shock proteins (HSP90), and the co-chaperone p23 (159–163), although Hollingshead et al. (158) suggested in their work that increased AIP levels can displace the HSP90-associated p23 from the AHR complex. AIP is involved in the cytoplasmic retention of AHR and decreases its proteosomal degradation by protecting it against ubiquitination (152, 160, 164, 165). Upon ligand binding, the HSP90 dimer is released from the complex, and AHR translocates into the nucleus, where it undergoes a conformational change and interacts with ARNT, also known as HIF1β. AHR-ARNT complex regulates the transcription of detoxification enzymes by binding to the xenobiotic response elements (XREs) (Fig. 7) (166, 167). This association is essential for transcriptional activation of these genes in the presence of environmental contaminants such as 2,3,7,8-tetrachlorodibenzo-p-dioxin and polycyclic aromatic hydrocarbons. There are conflicting data about the role of AIP in regulating the activity of AHR. Some studies indicate that AIP appears to facilitate the transcriptional activity of AHR (149, 150, 168), whereas others suggest that AIP interaction inhibits AHR activation (169–171). The reason for the controversy regarding the effect of AIP interaction on AHR function may arise from species differences, tissue-specific effects, and the presence or absence of a ligand.
Figure 7.
A schematic figure of the xenobiotic signaling. AHR exists in a dormant state in cytoplasm in association with a complex of HSP90, AIP, and the co-chaperone p23. Upon ligand binding, AHR is activated through conformational change and translocates to the nucleus. It forms a heterodimer with ARNT. The heterodimer binds to the XRE and alters expression of genes involved in the metabolism of xenobiotic agents.
Exogenous AHR ligands, especially dioxin, are known to inhibit cell proliferation and induce cell cycle arrest (172), and therefore, the role of AHR/ARNT signaling was studied in AIP-mediated tumorigenesis (141). Immunostaining revealed that ARNT protein expression was significantly reduced in human AIP-associated tumors. In line with this finding, the Aip mouse model revealed the total lack of either ARNT or ARNT2 protein, but not both, in Aip-related pituitary lesions. Both proteins were always present in Aip-proficient pituitary tumors (138). Also, a trend toward increased nuclear expression of AHR was detected. Supporting the nuclear accumulation of AHR in the absence of functional AIP, Nakata et al. (168) demonstrated that small interfering RNA silencing of Aip caused a weak nuclear accumulation of AHR in ARNT-deficient mouse hepatoma cell line. In contrast, in the work of Jaffrain-Rea et al. (142), no nuclear expression of AHR was detected in AIP-associated pituitary tumors. Such a discrepancy might arise from antibody epitope differences and the type of mutations studied.
Down-regulation of ARNT proteins in AIP mutation-positive adenomas may disturb the AHR/ARNT complex formation and lead to aberrant expression of xenobiotic response target genes (Fig. 8A). To study this aspect, expression of p27(Kip1), was studied in human AIP-deficient pituitary tumors (141). The expression of p27(Kip1) protein was, however, found to be equal in AIP-deficient and -proficient pituitary tumors (141). Although germline mutations in CDKN1B gene encoding p27(Kip1) cause MEN4 syndrome, such mutations are not associated with pituitary tumorigenesis in FIPA (173–175). Thus, it is not likely that p27(Kip1), acting via xenobiotic signaling would contribute to AIP-associated tumorigenesis. Nevertheless, dioxin-related compounds interfere with the body's endocrine system and may produce adverse developmental, reproductive, neurological, and immune effects (176). In the study of Pesatori et al. (177), where the authors analyzed the occurrence of pituitary adenomas in the Seveso population in Italy, after a severe 2,3,7,8-tetrachlorodibenzo-para-dioxin exposure accident in 1976, no statistically significant increase in the prevalence of pituitary tumors was found. However, an increased prevalence of acromegaly has been observed in highly polluted industrial areas (178), leading to the suggestion that such pollutants may act in some way to induce or promote pituitary tumorigenesis.
Figure 8.
A, Role of AHR-ARNT heterodimer in transcriptional regulation of xenobiotic, hypoxia, and estrogen signaling. B, Role of AIP in regulation of cAMP signaling via Gα-proteins and C, via PDEs. HRE, Hypoxia response element; ERE, estrogen response element.
2. Other AHR-regulated signaling cascades
Although the best-described AHR signaling pathway is the xenobiotic response, recent findings suggest that AHR is a multifunctional protein involved in the regulation of other signaling cascades and undertakes cross talk with several other pathways. Perhaps the best-characterized cross talk with the AHR pathway concerns steroid hormone receptors. AHR is involved in the regulation of estrogen response signaling, mediated by estrogen receptors α and β (ERα and ERβ) (179, 180). Also, the heterodimerization partners of AHR, ARNT and ARNT2, coactivate both ERα and ERβ (181). The cross talk between the AHR/ARNT and ER signaling is interesting because the estrogen receptor signaling pathway is known to act in the biosynthesis and secretion of hormones of the anterior pituitary and to stimulate the proliferation of lactotropes and gonadotropes (182). Moreover, estrogen-induced transcriptional targets include growth factors [e.g., IGF, epidermal growth factor (EGF), TGF, and vascular endothelial-derived growth factor], and several oncogenic proteins (c-myc, c-fos, erb, c-myb, pttg) (183). More recently, it has been demonstrated that AIP acts as a negative regulator of estrogen signaling through the interaction with ERα (Fig. 8A) (184). Thus, AIP may have a role in preventing cell proliferation in ERα-dependent tumors.
Apart from their AHR interaction, ARNT and ARNT2 are also binding partners of hypoxia-inducible factor 1α (HIF1α). The HIF1α-ARNT complex binds to hypoxia-responsive elements and activates the transcription of hypoxia response target genes (Fig. 8A) (185). This complex is involved in the adaptive response to oxygen deprivation in tumor cells, and it controls the up-regulation of a number of factors that are important for solid tumor expansion (185–189). To study the impact of the AIP tumorigenesis-associated ARNT protein imbalance on estrogen and hypoxia responses, expression of ERα and HIF1α was studied in human and mouse AIP-deficient pituitary adenomas (138, 141). Immunostaining showed that the ERα and HIF1α protein intensities were uniform in AIP mutation-positive and -negative tumors. These findings suggest that estrogen and hypoxia responses seem to be functional and that these signaling cascades are not necessarily involved in AIP-mediated tumorigenesis (138, 141).
Both ARNT proteins interact also with the single-minded 1 and 2 homolog (SIM1, SIM2) proteins (190, 191). SIM 1 and 2 belong to the basic helix-loop-helix/Per-Arnt-Sim homology (bHLH/PAS) protein family and require heterodimerization with either ARNT or ARNT2 to function. In the absence of Sim1 function, the paraventricular nucleus and supraoptic nucleus of the hypothalamus fail to develop. Both the paraventricular and supraoptic nuclei play important roles in modulating hormone secretion of the pituitary to maintain homeostasis (192). The Sim2 gene is required to produce a full complement of anterior hypothalamic cells expressing TRH and somatostatin (193, 194). Thus, it is possible that aberrant ARNT/ARNT2 function might have an impact on SIM1/2-controlled development and regulation of the pituitary/hypothalamic axis in AIP-associated pituitary tumorigenesis.
Of late, it has become evident that AHR also has a role in controlling the cell cycle. AHR appears to act as both a pro-proliferative and an anti-proliferative gene, depending on the cell type. Some studies indicate that AHR can promote cell cycle progression in the absence of exogenous ligands (195, 196), whereas more recent studies are suggesting that AHR may function as a tumor suppressor gene that becomes silenced during the tumor formation, e.g., in prostate and liver tumorigenesis (197, 198). This controversy regarding the role of AHR in tumorigenesis (pro-proliferative vs. anti-proliferative) may arise from tissue specificity and the absence or presence of ligand (199). For the most part, the exact role of AHR in signaling cascade responsible for modulating the cell cycle is unknown. One mechanism by which AHR has anti-proliferative potential is through the interaction with the retinoblastoma (RB1) protein. In the presence of mitogenic signals, AHR interacts with RB1 and cooperates in repressing cell cycle progression, particularly in the G1 to S phase transition (200). It is noteworthy that loss of the RB1 chromosomal region or promoter hypermethylation of the gene appears to be related to aggressive pituitary adenomas and carcinomas (201, 202).
Although it has been shown that low levels of AIP correlate with low levels of AHR in pituitary tumors (132), relatively little is known about the function of AHR in pituitary tissue. However, the AHR-ARNT imbalance associated with AIP-related tumorigenesis, as well as the role of AHR in the regulation of cell cycle progression, makes AHR an interesting candidate to be involved in the AIP-mediated tumorigenesis. Moreover, AHR has been found to be sensitive to cAMP signaling (169, 203). The ability of cAMP to modulate the biological function of AHR is very intriguing because cAMP is known to play a central role in the tumorigenesis of somatotropinomas.
3. Role of AIP in the regulation of cAMP signaling
cAMP signaling is a large network that generates interactions between different pathways and integrates signals from distinct receptors (204). In certain tissues, such as in thyroid, adrenal cortex, and pituitary somatotroph cells, cAMP stimulates cell proliferation, and aberrant cAMP signaling is directly implicated in several diseases including the genesis of somatotropinoma (205).
Guanine nucleotide-binding proteins (G proteins) form a large family of signal-transducing molecules. They are heterotrimeric proteins formed by Gα-, Gβ-, and Gγ-subunits. Ligand-receptor binding switches G protein to an active state and permits Gα activation of second messenger signaling cascades. G proteins communicate signals from many hormones, neurotransmitters, and other signaling factors and have an essential role in the regulation of cAMP levels. Alteration of the cAMP-protein kinase A pathway is known to be involved in somatotrope tumorigenesis via oncogenic somatic mutations of the α-subunit of the stimulatory guanine nucleotide-binding protein (GNAS, also known as Gαs) (MIM: 102200). It has been estimated that up to 40% of sporadic somatotropinomas harbor a somatic mutation in GNAS (206–210). High cAMP levels have also been linked to the pathogenesis of other syndromic conditions with pituitary adenomas such as McCune-Albright syndrome (MIM: 174800) and CNC (MIM: 160980) (211, 212). Although G proteins are known to be involved in the cAMP response regulation and share highly conserved primary structure, at present GNAS is the only G protein gene that has been identified as a target for mutations that are unequivocally associated with pituitary tumorigenesis (205).
AIP interacts with two Gα proteins, Gα13 and Gαq. These interactions were shown to inhibit the interaction between AHR and AIP (168). The Gα13 signal activation was also found to cause ligand-independent nuclear translocation of AHR (Fig. 8B), and similar to cAMP-mediated nuclear AHR, the Gα13-induced nuclear AHR adopts a structure that prevented a formation of active transcriptional complex with ARNT (168, 169). Additionally, it has been demonstrated that Gα13 is able to regulate cAMP concentration by cooperating with GNAS (213, 214), thereby providing a possible mechanism for pituitary tumorigenesis related to AIP germline mutations.
Phosphodiesterases (PDEs) are a large family of enzymes that degrade and deactivate cAMP. Thus, the finding that AIP interacts with PDE2A and PDE4A5 provides an additional mechanism by which AIP might contribute to the genesis of somatotropinomas (169, 170, 215). PDE2A hydrolyzes both cAMP and cGMP (216). The interaction of PDE2A with AIP is intriguing because the AIP-PDE2A interaction has been shown to inhibit cAMP-induced nuclear translocation of AHR (Fig. 8C) and to reduce the expression of AHR-regulated target genes (169, 215). Thus, it is possible that the lack of AIP can lead to an aberrant expression of AHR target genes through elevated cAMP levels, a hallmark of somatotropinomas. MacKenzie et al. (217) found that GH-mediated differentiation of 3T3-F442A cells was accompanied by increased cAMP PDE activity by a specific PDE4A isoform that they called PDE4A5. AIP binding to the cAMP-specific PDE4A5 inhibited its enzymatic activity (Fig. 8C) and attenuated the ability of cAMP-dependent protein kinase to phosphorylate PDE4A5 (170). As a method to assess the functional validity of the effects of specific germline mutation of AIP seen in FIPA families and simplex patients, many mutations completely abolished or significantly attenuated the interaction of AIP with PDE4A5 (67, 86). However, because elevated cAMP levels are associated with pituitary tumorigenesis, it is unclear how AIP could exert its tumor suppressor action through PDE4A5.
4. Other AIP-associated proteins and implications in AIP-mediated tumorigenesis
The rearranged during transfection (RET) proto-oncogene is a transmembrane tyrosine kinase. In the absence of ligand, RET induces apoptosis (218). Gain-of-function mutations of RET are associated with MEN2A and MEN2B (MIM: 171400 and 162300), and familial medullary thyroid carcinoma (MIM: 155240). Loss-of-function mutations of RET predispose to Hirschsprung's disease (MIM: 142623) (219–221). In the pituitary, RET is expressed in somatotropes where it is associated with apoptosis and differentiation and stimulates expression of pituitary transcription factor-1 and p53 (218). Among pituitary tumors, RET is expressed in somatotropinomas and a subset of corticotropinomas (222). AIP was recently found to interact with the proapoptotic domain of RET (223). However, pathogenic AIP and RET mutations that were introduced into cells did not impair the AIP-RET interaction. In addition, no RET mutations have been found in somatotropinomas or FIPA families (223, 224). Survivin belongs to the family of inhibitors of apoptosis. The survivin-AIP-HSP90 complex stabilizes survivin, but in the presence of RET, AIP is unable to bind and protect survivin from degradation, leading to enhanced apoptosis (223, 225). Although there is cross talk between RET and survivin and both of the proteins are involved in the regulation of apoptosis, the relevance of these proteins in pituitary tumorigenesis remains uncertain.
Trivellin et al. (226) recently highlighted yet another manner in which AIP may be involved in pituitary tumor pathology, namely via regulation by the micro-RNA-107 (miR-107). They noted that AIP was a target of miR-107 via a 6-mer site 65–70 bp downstream of the AIP stop codon on the 3′ untranslated region of AIP. This microRNA was capable of inhibiting AIP expression, and it was suggested that the overexpression of miR-107 in pituitary adenomas could explain the decreased AIP expression seen particularly in aggressive somatotropinomas. This effect may differ in other pituitary tumor subtypes, such as nonfunctioning tumors, where AIP expression levels are possibly less important in determining pathological behavior.
The EGF receptor (EGFR) is a transmembrane glycoprotein, a member of the protein kinase superfamily. AIP was reported to interact with EGFR in a large-scale interaction screen (227). However, the AIP-EGFR interaction status is uncertain because this interaction has not been further validated. EGFR is activated by binding of its specific ligands, including EGF and TGFα. Both of these ligands act as mitogens in endocrine pituitary cells. Recent work from Melmed's group has built a body of significant evidence that indicates that targeting EGFR is a valuable potential therapy for pituitary adenomas (8–10, 228). It is also noteworthy that dioxin-activated AHR can trigger sequential activation of EGFR and ERK, leading to the increased expression of TGFα (229). Thus, the confirmation of AIP-EGFR interaction would be important, given the aggressive phenotype and relative therapeutic resistance associated with AIP mutation-related pituitary adenomas (115).
AIP is involved in various nuclear receptor signaling pathways. In addition to AHR and ERα, other nuclear receptors capable of binding AIP include peroxisome proliferation-activated receptor α (PPARα), thyroid hormone receptor β1 (TRβ1), and glucocorticoid receptor (GR). PPARα regulates the expression of genes involved in fatty acid β-oxidation and is a major regulator of energy homeostasis. The cytosolic PPARα-HSP90-AIP complex has a repressor effect on PPARα (230, 231). TRβ1 is a nuclear hormone receptor and mediates the biological activity of thyroid hormone. AIP silencing is known to abolish the TRβ1-mediated thyroid hormone expression (232). GR interacts with AIP through HSP90. The effect of AIP on the GR signaling is inhibitory because AIP delays the nuclear accumulation of GR. In the nucleus, GR regulates genes controlling development, metabolism, and immune response. LOH has been observed at the GR gene in about one third of ACTH tumors, suggesting a possible role of GR at least in corticotrope tumorigenesis (233, 234).
AIP was first identified as a partner of the X antigen of the hepatitis B virus, a human DNA virus causing acute and chronic hepatitis. AIP seems to act as a negative regulator of the X protein, and the interaction may have a role in the hepatitis B virus pathology (149). Another viral protein that interacts with AIP is Epstein-Barr virus encoded nuclear antigen-3 (235). It has been suggested that the AHR pathway can be involved in virus-induced cell transformation (236). AIP has been reported to interact with the translocase of the outer membrane of mitochondria 20, a subunit of a translocator complex that imports mitochondrial pre-proteins into mitochondria (237). In addition to HSP90 binding, AIP can also interact with another heat-shock protein, the heat-shock cognate protein 70 (165). AIP interaction with the cardiac troponin I-interacting kinase (238) is intriguing because a recent study by Lin et al. (133, 134) showed that Aip knockout mice died during embryogenesis due to cardiac malformations.
As described above, AIP has multiple interaction partners (Fig. 6). Through these proteins, alterations in normal AIP function has the potential to affect a large number of different pathways and signaling cascades. It appears that a number of these interacting partners are potential candidates to promote AIP mutation-related pituitary tumorigenesis, particularly the role of AIP in the regulation of cAMP levels and the cross talk between AHR and cAMP pathways. Work based on the Aip knockout mouse model and human pituitary tumors is under way to determine the proteins and molecular mechanisms underlying genesis of AIP-associated pituitary tumorigenesis. Characterization of these proteins and signaling cascades might reveal novel therapeutic opportunities for the patients with these relatively treatment-resistant pituitary tumors.
V. Genetic Testing for AIP in Selected Pituitary Adenoma Populations
A. Defining the ideal testing populations
The main aim of genetic testing for AIP germline mutations is to identify those at risk of potentially aggressive pituitary adenomas and permit early diagnosis of such adenomas at the microadenoma and noninvasive stage, where treatment is more likely to be effective or curative (239). While AIP mutation-related pituitary adenomas appear to be associated with a decreased rate of control with medical therapies (somatostatin analogs and dopamine agonists), not enough is known about the molecular pathways involved in AIP mutation-related pituitary tumorigenesis to permit specific choices of medications to be recommended. Therefore, AIP mutation-positive pituitary adenomas should be managed according to current guidelines for acromegaly, prolactinoma, etc. (240–242).
Published results to date provide firm evidence that AIP mutation screening in unselected general pituitary adenoma populations is not strictly justifiable to identify affected patients. Two large studies in more than 400 subjects each have shown overall rates of AIP germline mutations of about 4% of unselected sporadic cases (99, 101). However, taking together these results and those from subgroup studies focused on acromegaly, pediatric studies in sporadic and syndromic cases and the clinical characteristics of large cohorts of AIP mutation-positive FIPA cases, some approaches to effective AIP mutation screening become apparent (67, 71, 75, 84, 86, 89, 90, 93, 95–101, 104–109, 115, 116, 118, 127, 142, 243, 244). The highest likelihood of identifying AIP mutation-positive cases is among patients with gigantism, who comprise 25% of those with AIP mutations overall and a third of AIP-mutated somatotropinomas. In particular, those patients with gigantism in a familial setting of gigantism have the highest probability of having an AIP mutation (85). The PAP associated with AIP mutations leads to the early development of large pituitary adenomas, usually somatotropinomas, so consistent excessive gain in height in children and adolescents when a pituitary adenoma is suspected should lead the clinician to consider AIP testing. More practically, for AIP mutation-positive children from families with a known AIP mutation, monitoring abnormal gain in height should be an integral part of clinical surveillance.
Up to 20.5% of pediatric patients (those aged less than 18 yr at diagnosis) with a macroadenoma have been reported to be AIP mutation positive (108). Macroadenomas occurring in young adults (<30 yr old) also appear to be a valuable indicator of a possible AIP germline mutation (11%) and may in the future represent a condition in which routine screening may be effective. The next readily identifiable group in which focused AIP genetic screening could be beneficial is among FIPA cohorts. Combining the results of major national and international collaborations, about 20% of FIPA kindreds are AIP mutation positive, with the overall rate being only slightly higher in homogeneous vs. heterogeneous kindreds (22.8 vs. 16.7%). Many different mixtures of pituitary tumor types are now associated with AIP mutations in the FIPA setting, although the majority of patients with AIP mutations come from families with somatotropinomas, somatolactotropinomas, prolactinomas, and NFPA.
B. Disease penetrance
As originally described, germline AIP mutations were associated with a low penetrance of pituitary adenomas in affected families in Finland (84). Since then, a large number of kindreds with AIP mutations have been reported (67, 71, 86), and some particularly large families have been studied genetically and clinically (84, 89, 90). In general, the larger and more completely studied kindreds have a penetrance that is in the low range (20% penetrance approximately). From the studies of FIPA cohorts together, the penetrance rate is more variable. Based on initial studies on selected families, a penetrance rate of up to 50% or more was suggested (67, 69). Based on a calculation that took into account affected patients, obligate carriers, and half of the subjects with a 50% risk of inheriting a mutation, Igreja et al. (86) reported a mean penetrance rate of pituitary tumors in AIP mutation-positive FIPA families of 42 ± 21%. These figures varying from 20–66% can be explained by a number of potential factors. First, many FIPA kindreds were initially described as clusters of closely related individuals, and AIP sequencing in these families also tended to discover clusters of affecteds and carriers that led to relatively high penetrance rates. Expansion of kindred studies to second- and third-degree relatives can serve to lower penetrance rates. In contrast, certain FIPA kindreds with large numbers of affected patients (five or more) have been described (67, 86). Such kindreds show that there appears to be an inherent variability in the penetrance, with some families having many affected subjects and a low number of carriers, whereas other families with similar truncating AIP mutations have a low number of affecteds among a wealth of unaffected adult carriers. In addition, numerous cases of familial mutations with only a single affected patient have been discovered (“simplex” cases), whereas other unrelated families with the same mutation (R304X, R271W) have multiple members with aggressive, early-onset macroadenomas. These findings can be explained at least in part by ascertainment bias; the families with striking occurrence of a disease enter genetic studies much more often than more subtle clusters. At this time, the penetrance of AIP mutations in the FIPA setting can be considered as low to perhaps moderate penetrance. The variability raises the possibility that, in addition to simple chance, some unknown endogenous genetic factors (i.e., other gene variants/mutations or modulators/repressors) or extraneous environmental effects (i.e., toxins, dioxin, etc.) related to or separate from AIP and its molecular pathway play a role in determining which mutation carriers develop pituitary adenomas.
One important feature of AIP mutation-positive FIPA kindreds is that of phenocopy in which a member of a FIPA kindred has a pituitary adenoma but has a normal AIP sequence, unlike the other affected members of the family that have an AIP mutation (73, 87). Phenocopies are of particular importance in the setting of FIPA because in the general population pituitary adenomas occur in about 1:1000 people, whereas incidental tumors (without clinical correlates) occur in more than 15% of individuals (1, 245). Hence, the genetic investigation of AIP mutation-positive FIPA kindreds can be confounded by the presence of such phenocopies and can lead to unnecessary study and follow-up of nonmutation carriers. The challenges are illustrated in Fig. 9, in which a kindred presented as a three-member heterogeneous FIPA family, with somatotropinomas in father and son (cases III-6 and IV-7, respectively) and an NFPA in case III-2, the brother of case III-6. The tumor size at diagnosis in case III-6 in the 1960s was unknown, although the patient was relatively young (29 yr old) at diagnosis and had suffered from symptoms since adolescence. His son was diagnosed in his early 40s with acromegaly due to a microadenoma. Case III-2 developed a pituitary macroadenoma in his 60s. Although both father and son with somatotropinomas were found to have the p.R271W AIP mutation, repeated sequencing of the AIP gene in case III-2 was normal. He was diagnosed as having a phenocopy NFPA, thereby markedly curtailing the screening process in generation IV.
Figure 9.
Phenocopy NFPA in the setting of an AIP mutation-positive FIPA kindred with acromegaly.
C. Toward integrated genetic screening in pituitary adenomas
Although AIP mutations are associated with isolated pituitary tumors, genetic testing cannot be viewed in isolation from syndromic forms of endocrine neoplasia. Indeed, investigation of the patient with a pituitary adenoma should always take account of the possibility of a genetic background and related disease in other tissues. Multiple germline gene mutations are associated with an increased predisposition to pituitary tumor development, but these are usually part of a syndrome displaying additional diagnostic features. The occurrence of multiple individuals with isolated pituitary adenomas in the setting of familial MEN1 and CNC in the absence of other cardinal skin, vascular, or neoplastic signs after a thorough clinical anamnesis is likely to be very rare. Indeed, only 2% of MEN1 patients only had pituitary adenomas, and familial MEN1 cases with only pituitary adenomas are, to our knowledge, unknown (14). However, it is important to note that because approximately 17% of MEN1 patients present with pituitary adenomas, specific efforts must be made to clinically and biochemically search for other disease manifestations. As such, an initial division of pituitary adenoma patients into those with or without any history of syndromic features or family history is a practical first step before considering genetic testing (Fig. 10). Surveillance for MEN1 can follow already agreed guidelines and recommendations, which have been recently and very comprehensively updated to take into account discrimination between MEN1 and other conditions like FIPA (246–248). In familial cases of MEN1, pituitary adenomas are significantly more commonly seen than in sporadic MEN1 (59 vs. 34%). As noted above, it is only in exceptional cases that MEN1 is characterized by only a pituitary adenoma in the absence of other cardinal syndromic features (e.g., parathyroid disease). Both FIPA and MEN1 have a large proportion of females with prolactinomas. Pituitary tumors in MEN1 differ significantly from sporadic tumors (including prolactinomas) in terms of more frequently being macroadenomas (85 vs. 42%) (14). Probably related to this larger size, pituitary tumors in MEN1 are also significantly more likely than sporadic tumors to cause symptoms and signs due to local tumor effects. In FIPA, patients are much more likely to present with somatotropinomas (14, 65, 69).
Figure 10.
Schematic of suggested clinical decision tree to integrate AIP genetic testing into existing testing strategies. The decision tree is based on the presence or absence of typical syndromic features that suggest known diseases such as MEN1, CNC, and McCune Albright syndrome (MAS), which have established genetic testing for known causative genes. Point 1, Established syndromes like MEN1 and CNC are being joined by newer associations of pituitary adenomas with other endocrine tumor types, such as pheochromocytoma in the setting of succinate dehydrogenase subtype gene mutations (250). Ongoing advances in this field will clarify the relative frequency of such associations and the need to integrate testing into standard clinical investigation. Point 2, In CNC, a proportion of patients are negative for PRKAR1A mutations, and another locus on chromosome 2 has been suggested (252). Point 3, MEN4 due to CDKN1B mutations is a rare but emerging condition with pituitary adenomas as part of the spectrum. CDKN1B testing in patients with pituitary adenomas should be limited in the clinical setting to those with associated syndromic features of endocrine or other tumors and negative sequencing for MEN1 mutations (174, 244). Other rare mutations in cyclin-dependent kinases have also been noted infrequently in MEN1-like conditions, but the study of these remains in the research realm (253). Point 4, In the setting of FIPA, the PAP due to AIP germline mutations accounts for about 20% of kindreds. For FIPA kindreds that are AIP mutation negative on sequencing, MLPA should be considered to detect more extensive deletions. To date, other genes have not been identified to cause FIPA. Syndromic conditions like MEN1 and CNC do not frequently present as isolated pituitary adenomas in the absence of other features such as hyperparathyroidism. An exception may be young patients with apparently sporadic pituitary macroadenomas, with recent information from Cuny et al (254) suggesting that MEN1 gene sequencing is a valuable investigation in that population. Therefore, in the verified FIPA setting and in younger patients with aggressive pituitary adenomas, AIP testing may be considered as the first genetic test to be discussed, as long as MEN1 and CNC are ruled out clinically and by simple biochemical testing (e.g., absence of hypercalcemia or cortisol secretion abnormalities).
CNC is associated with a typical spectrum of syndromic features, and particular attention should be paid to these, while recalling that pituitary adenomas occur in only a minority of CNC cases, and familial presentation limited only to acromegaly is not known in this condition at this time (46). For patients with pituitary adenomas with other MEN1-like features, but who do not have a MEN1 mutation, CDKN1B mutation screening should be considered (249). However, in the setting of FIPA, CDKN1B does not play a clinically relevant role (175). New associations have been made between pituitary adenomas and endocrine neoplasia genes previously not thought to be involved in pituitary tumorigenesis, such as SDHD as recently reported by Xekouki et al. (250). Investigation of this and other potential associations may expand the number and complexity of testing in the setting of endocrine neoplasia syndromes.
Specifically focusing on the investigation of FIPA, AIP testing should be considered in all kindreds, irrespective of pituitary tumor types in the family. In FIPA kindreds that are negative on AIP sequencing, deletions have been demonstrated using MLPA, and this method should be considered as a second line of screening. For AIP mutation-negative FIPA families (80% of cases), academic research projects are currently under way to discover novel genetic causes, but no current candidates can be recommended for further study in the clinical setting at present. For patients without FIPA or syndromic features suggestive of MEN1, CNC, etc., genetic testing for AIP mutations can be considered in a number of situations. Because AIP mutations are seen in up to 11% of pituitary macroadenomas diagnosed before the age of 30 and about 20% of those with macroadenomas under the age of 18, AIP sequencing should be strongly considered in this readily defined subgroup (108). Also, gigantism is common in AIP-mutated patients, and AIP testing would seem clinically reasonable in such patients with established gigantism and also in young patients with excessive height gain in association with a pituitary adenoma. There has also been a suggestion that pituitary apoplexy is a feature of AIP-mutated pituitary adenomas, reflecting their large size and potentially rapid size expansion; a strict association between an increased risk of apoplexy and AIP mutation status remains to be demonstrated. Finally, among patients with somatotropinomas, resistance to medical therapy has been reported for AIP mutation-related adenomas (67), which leads to statistically significantly lower hormonal and tumor size responses as compared with AIP intact controls (115). Prolactinomas, although less frequent than somatotropinomas in this setting, also appear to be large and relatively resistant to dopamine agonists (115). In patients with sporadic pituitary macroadenomas, resistance to therapy may be another suggestive feature to guide offering genetic testing for AIP mutations, although specific AIP mutation prevalence studies in resistant populations are awaited.
It is important to note that in cases where an AIP mutation is diagnosed in a sporadic case, many families that agree to genetic testing display asymptomatic mutation carriers. The variable low to moderate penetrance of pituitary adenomas among AIP mutation carriers complicates the process of screening because clinically affected patients are in the minority and currently most of our experience is with patients that have already developed macroadenomas. The issue of when to begin clinical and genetic surveillance in relatives of known carriers/affecteds with AIP mutations is one that must be informed by the fact that presentation in childhood/adolescence is a typical feature of the illness (90, 101, 104, 106, 108, 115). A patient as young as 6 yr has been diagnosed with an AIP mutation and pituitary macroadenoma, with additional signs and symptoms for the preceding 12–24 months (90). Although screening to date in mutation carriers is still relatively scant, work in larger families has identified carriers with pituitary microadenomas or other abnormalities of maturation (89, 115). These patients remain under surveillance, but information on the rate and characteristics of pituitary tumor evolution is limited and remains an important unanswered clinical question for the future.
For those individuals that are at risk for carrying an AIP mutation that has been diagnosed in a relative, the ideal step is to offer genetic screening after appropriate counseling and explanation of the benign nature of pituitary adenomas, the possible benefits of early diagnosis, and the current understanding of the variable penetrance. Genetic screening for a familial AIP mutation has the benefit of being able to rule out noncarriers definitively, thereby avoiding unnecessary stress. Given that more than 75% of cases have been diagnosed before the age of 30, screening efforts in new kindreds should ideally be weighted toward children and young adults in the first instance. Mutation carriers or potential carriers that decline initial genetic testing should be offered a thorough clinical assessment by an endocrinologist, accompanied by basal testing of GH/IGF-I and prolactin. Carriers with any signs or symptoms suggestive of a pituitary adenoma or those that express an interest in undergoing imaging should have a baseline pituitary MRI performed. Mutation carriers that are without clinical signs or hormonal abnormalities and have a normal MRI should be followed up, although no minimum period can be recommended based on a lack of evidence. The investigation of potential pediatric/adolescent AIP mutation carriers is of particular importance because these patients have added complications in terms of interpreting normal growth spurts and pubertal changes while actively surveying for suggestive endocrine abnormalities. Should genetic testing be permitted by a parent/guardian, this has been performed in children as young as 2 yr of age, with the benefit of being able to reassure parents if the familial mutation is not found. For carriers or those not wishing to have genetic testing performed in their child, care should be taken to actively seek out clinical signs/symptoms suggestive of somatotrope or lactotrope overactivity or tumor impingement on local structures (vision, recurrent headache) and to carefully examine growth velocity. In those with symptoms suggestive of a pituitary adenoma, imaging and hormonal testing should be performed as soon as possible because tumors as large as 35–38 mm have been diagnosed in patients aged 10 or younger.
D. Follow-up in the setting of FIPA and AIP mutation carriers
There are no established guidelines to outline the recommended follow-up for unaffected AIP mutation carriers. For those with no symptoms or evidence of pituitary abnormality, it is important to inform the individual of symptoms that may be suggestive of a new endocrine abnormality and to encourage the subject to return should such symptoms occur. It is probably wise to review the subject after a year, and in the absence of new clinical features, the subject can be returned to their regular physician's care. For pediatric and adolescent carriers, follow-up with the pediatrician for growth or other endocrine disorders is recommended, and rapid referral back to the pediatric endocrinologist upon the appearance of relevant symptoms should be emphasized.
The more challenging situation is the AIP mutation carrier that has a microadenoma on MRI and has no clinical signs/symptoms and normal hormonal screening. It is difficult in such cases to determine whether this patient has an incidentaloma that is stable or has an AIP-related growing pituitary adenoma. In adult patients, in the absence of new symptoms or hormonal abnormalities, there is probably no need to repeat an MRI until 12 months have passed since the baseline scan. Given the usual early appearance of these tumors, often in childhood/adolescence, and the lack of knowledge about their growth potential, in young patients a follow-up MRI may be more advisable 6 months after diagnosis in AIP mutation carriers. Should the tumor remain as a stable microadenoma over that period, then clinical and hormonal follow-up can probably remain in place on an annual or biannual basis as long as no change in endocrine status occurs. In such cases, the usual balance of definitive surgical cure over medical therapy would have to be made according to the individual tumor type and the clinical status of the patient. Follow-up of and decisions regarding intervention in patients with diagnosed AIP mutation-related pituitary adenomas does not differ from that of sporadic pituitary adenoma patients.
For FIPA patients without AIP mutations, the follow-up recommendations are relatively more difficult to codify in the absence of a causative gene. Efforts should be made to clearly identify patients within the kindred who may be harboring a pituitary adenoma, based on family history and careful anamnesis in collaboration with patients and relatives. Those individuals with specific symptoms or signs of a pituitary adenoma should, like any patient with suggestive symptomatology, undergo relevant hormonal and/or imaging studies. Follow-up management of AIP-negative subjects with pituitary adenomas in the setting of FIPA does not at this time differ from those with sporadic tumors and should, where possible, be guided by international consensus guidelines.
VI. Future Directions
The advent of FIPA and the discovery of AIP as a gene involved in inherited pituitary adenomas have refocused attention on the role of familial and genetic factors in the pituitary adenoma population. However, despite the advances made, approximately 80% of FIPA families remain without a genetic explanation. Ongoing work in AIP-negative FIPA families at a genomic level may highlight novel loci and eventually causative genes. In parallel, there may be value in studying the potential role of other endocrine neoplasia-related genes, such as the SDHx genes, to discover whether their newly reported links to pituitary tumorigenesis play a role in causation of the FIPA phenotype (250, 251). Because FIPA comprises 2–3% of pituitary adenomas at certain tertiary referral centers (65), improved questioning about family history of pituitary adenomas and other endocrine cancers will be helped by greater awareness of FIPA and the role of AIP in causation of pituitary adenomas in families and young patients. With respect to AIP, improved understanding of the penetrance related to the PAP induced by AIP mutations will be necessary to permit accurate counseling of patients and to improve genetic diagnosis of mutation carriers when tumors are still relatively small. Also, penetrance studies will help to answer whether rare tumor associations seen in FIPA families with AIP mutations occur by chance or form a predictable but infrequent association.
Study of the evolution of pituitary adenomas in association with AIP mutations from their early stage is necessary to determine what pathway they follow (for example, a hypothetical hyperplasia-microadenoma-macroadenoma progression) and the rate of tumor growth. In this, the potential role of other existing or acquired genetic mutations as cofactors in permitting AIP mutation-related tumor development will need to be explored. Of great interest will be the further exploration of Aip mutation-related tumorigenesis via the knockout mouse models available, particularly because these studies may relate to identifying the main causative pathway(s) among the legion of current possibilities (dioxin, AHR, PDEs, etc.). Such advances may allow for specific therapies to be suggested that could impact positively upon the relatively poor therapeutic responses in these patients.
Search Strategy
Articles were selected from a PubMed, EMBASE, Web of Science, Google Scholar, and GenBank search for keywords including, e.g., AIP, XAP2, ARA9, FIPA, IFS, pituitary tumorigenesis, somatotropinoma, PAP, and protein interaction. Reference lists in selected articles were also used to broaden the search, and abstract books of recent national and international endocrinology congresses were also consulted.
Acknowledgments
The authors express their gratitude to the many clinicians and researchers who have provided ample patient details, undertook familial studies, collected biological specimens, and sent insightful suggestions during the investigations and research that have helped to describe FIPA and to discover AIP as a PAP gene.
This work was supported by Fonds d'Investissement pour la Recherche Scientifique du Centre Hospitalier Universitaire de Liège 2008–2011 the Academy of Finland (grants 250345, 1263226), European Research Council (No 268648 - NGG), and The Novo Nordisk Foundation (grant 14582).
Disclosure Summary: The authors have no conflict of interest to declare.
Footnotes
- AHR
- Aryl hydrocarbon receptor
- AIP
- aryl hydrocarbon receptor interacting protein
- ARNT
- Ahr nuclear translator
- CNC
- Carney complex
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- ERα
- estrogen receptor α
- FIPA
- familial isolated pituitary adenoma
- GNAS
- α-subunit of the stimulatory guanine nucleotide-binding protein
- GR
- glucocorticoid receptor
- HIF1α
- hypoxia-inducible factor 1α
- HSP90
- 90-kDa heat-shock protein
- LOH
- loss of heterozygosity
- miR-107
- micro-RNA-107
- MLPA
- multiplex ligation-dependent probe amplification
- MRI
- magnetic resonance imaging
- NFPA
- nonfunctioning pituitary adenoma
- PAP
- pituitary adenoma predisposition
- PDE
- phosphodiesterase
- PPARα
- peroxisome proliferation-activated receptor α
- RB1
- retinoblastoma
- RET
- rearranged during transfection
- TRβ1
- thyroid hormone receptor β1
- XRE
- xenobiotic response element.
References
- 1. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. 2006. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 91:4769–4775 [DOI] [PubMed] [Google Scholar]
- 2. Fontana E, Gaillard R. 2009. Epidemiology of pituitary adenoma: results of the first Swiss study. Rev Med Suisse 5:2172–2174 [PubMed] [Google Scholar]
- 3. Fernandez A, Karavitaki N, Wass JA. 2010. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf) 72:377–382 [DOI] [PubMed] [Google Scholar]
- 4. Melmed S. 2011. Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266 [DOI] [PubMed] [Google Scholar]
- 5. Melmed S. 2009. Acromegaly pathogenesis and treatment. J Clin Invest 119:3189–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asa SL, Ezzat S. 2009. The pathogenesis of pituitary tumors. Annu Rev Pathol 4:97–126 [DOI] [PubMed] [Google Scholar]
- 7. Yu R, Melmed S. 2010. Pathogenesis of pituitary tumors. Prog Brain Res 182:207–227 [DOI] [PubMed] [Google Scholar]
- 8. Cooper O, Vlotides G, Fukuoka H, Greene MI, Melmed S. 2011. Expression and function of ErbB receptors and ligands in the pituitary. Endocr Relat Cancer 18:R197–R211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukuoka H, Cooper O, Ben-Shlomo A, Mamelak A, Ren SG, Bruyette D, Melmed S. 2011. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest 121:4712–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukuoka H, Cooper O, Mizutani J, Tong Y, Ren SG, Bannykh S, Melmed S. 2011. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol 25:92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xekouki P, Azevedo M, Stratakis CA. 2010. Anterior pituitary adenomas: inherited syndromes, novel genes and molecular pathways. Expert Rev Endocrinol Metab 5:697–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal SK, Ozawa A, Mateo CM, Marx SJ. 2009. The MEN1 gene and pituitary tumours. Horm Res 71(Suppl 2):131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vezzosi D, Vignaux O, Dupin N, Bertherat J. 2010. Carney complex: clinical and genetic 2010 update. Ann Endocrinol (Paris) 71:486–493 [DOI] [PubMed] [Google Scholar]
- 14. Vergès B, Boureille F, Goudet P, Murat A, Beckers A, Sassolas G, Cougard P, Chambe B, Montvernay C, Calender A. 2002. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab 87:457–465 [DOI] [PubMed] [Google Scholar]
- 15. Kirschner LS. 2010. PRKAR1A and the evolution of pituitary tumors. Mol Cell Endocrinol 326:3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marie P. 1886. Sur deux cas d'acromegalie. Hypertrophe singuliere no congénitale des extréméties supérieures, inférieures et cephalique. Révue Médicale Française 6:297–333 [Google Scholar]
- 17. Marie P, de Souza Leite JD. 1891. Essays on acromegaly. London: The New Sydenham Society [Google Scholar]
- 18. Cushing H. 1912. The pituitary body and its disorders, clinical states produced by disorders of the hypophysis cerebri. Philadelphia, London: J. B. Lippincott Company [Google Scholar]
- 19. Erdheim J. 1903. Zur normalen und pathologischen histologic der glandula thyreoidea, parathyreoidea und hypophysis. Beitr z path Anat u z allg Path 33:158–233 [Google Scholar]
- 20. Wermer P. 1954. Genetic aspects of adenomatosis of endocrine glands. Am J Med 16:363–371 [DOI] [PubMed] [Google Scholar]
- 21. Carney JA, Hruska LS, Beauchamp GD, Gordon H. 1986. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc 61:165–172 [DOI] [PubMed] [Google Scholar]
- 22. Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. 1985. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 64:270–283 [DOI] [PubMed] [Google Scholar]
- 23. de Herder WW. 2009. Acromegaly and gigantism in the medical literature. Case descriptions in the era before and the early years after the initial publication of Pierre Marie (1886). Pituitary 12:236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Herder WW. 2004. [Giantism. A historical and medical view]. Ned Tijdschr Geneeskd 148:2585–2590 [PubMed] [Google Scholar]
- 25. Friedreich N. 1868. Hyperostose des gesammten Skelettes. Virchows Archiv 43:83–87 [Google Scholar]
- 26. Sternberg M. 1899. Acromegaly. London: The New Sydenham Society [Google Scholar]
- 27. Schwoner F. 1897. Ueber heretidäre Akromegalie. Zeitsch f klin Med XXXII:S202 [Google Scholar]
- 28. Fraenkel A, Stadelmann E, Benda C. 1901. Klinische und anatomische Beiträge zur Lehre von Akromegalie. Part I. Zwei Fälle von Akromegalie. Deutsche Medizinische Wochenschrift 27:513–517 [Google Scholar]
- 29. Symmers D. 1917. Acromegalic giantism. Interstate Med J 24:1013–1015 [Google Scholar]
- 30. Bailey P, Davidoff LM. 1925. Concerning the microscopic structure of the hypophysis cerebri in acromegaly (based on a study of tissues removed at operation from 35 patients). Am J Pathol 1:185–208.19 [PMC free article] [PubMed] [Google Scholar]
- 31. Dickens C. 1842. American notes for general circulation. London: Chapman and Hall [Google Scholar]
- 32. Gray H. 1937. The Minneapolis giant. Ann Intern Med 10:1669–1682 [Google Scholar]
- 33. Atkinson F. 1932. Acromegaly. London: John Bale and Sons [Google Scholar]
- 34. Levin SR, Hofeldt FD, Becker N, Wilson CB, Seymour R, Forsham PH. 1974. Hypersomatotropism and acanthosis nigricans in two brothers. Arch Intern Med 134:365–367 [PubMed] [Google Scholar]
- 35. Himuro H, Kobayashi E, Kono H, Jinbo M, Kitamura K. 1976. [Familial occurrence of pituitary adenoma (author's translation)]. No Shinkei Geka 4:371–377 [PubMed] [Google Scholar]
- 36. Jones MK, Evans PJ, Jones IR, Thomas JP. 1984. Familial acromegaly. Clin Endocrinol (Oxf) 20:355–358 [DOI] [PubMed] [Google Scholar]
- 37. Abbassioun K, Fatourehchi V, Amirjamshidi A, Meibodi NA. 1986. Familial acromegaly with pituitary adenoma. Report of three affected siblings. J Neurosurg 64:510–512 [DOI] [PubMed] [Google Scholar]
- 38. Pestell RG, Alford FP, Best JD. 1989. Familial acromegaly. Acta Endocrinol (Copenh) 121:286–289 [DOI] [PubMed] [Google Scholar]
- 39. McCarthy MI, Noonan K, Wass JA, Monson JP. 1990. Familial acromegaly: studies in three families. Clin Endocrinol (Oxf) 32:719–728 [DOI] [PubMed] [Google Scholar]
- 40. Tamburrano G, Jaffrain-Rea ML, Grossi A, Lise A, Bulletta C. 1992. [Familial acromegaly. Apropos of a case. Review of the literature]. Ann Endocrinol (Paris) 53:201–207 [PubMed] [Google Scholar]
- 41. Links TP, Monkelbaan JF, Dullaart RP, van Haeften TW. 1993. Growth hormone-, α-subunit and thyrotrophin-cosecreting pituitary adenoma in familial setting of pituitary tumour. Acta Endocrinol (Copenh) 129:516–518 [DOI] [PubMed] [Google Scholar]
- 42. Izumi T, Kanazawa Y, Ishibashi M, Yamaji T, Kosaka K. 1982. [Pituitary gigantism and acromegaly in identical twin brothers]. Nihon Naika Gakkai Zasshi 71:1586–1590 [PubMed] [Google Scholar]
- 43. Lemmens I, Van de Ven WJ, Kas K, Zhang CX, Giraud S, Wautot V, Buisson N, De Witte K, Salandre J, Lenoir G, Pugeat M, Calender A, Parente F, Quincey D, Gaudray P, De Wit MJ, Lips CJ, Höppener JW, Khodaei S, Grant AL, Weber G, Kytölä S, Teh BT, Farnebo F, Thakker RV. 1997. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet 6:1177–1183 [DOI] [PubMed] [Google Scholar]
- 44. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. 1997. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276:404–407 [DOI] [PubMed] [Google Scholar]
- 45. Casey M, Mah C, Merliss AD, Kirschner LS, Taymans SE, Denio AE, Korf B, Irvine AD, Hughes A, Carney JA, Stratakis CA, Basson CT. 1998. Identification of a novel genetic locus for familial cardiac myxomas and Carney complex. Circulation 98:2560–2566 [DOI] [PubMed] [Google Scholar]
- 46. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Benlian P, Giraud S, Lahlou N, Roger M, Blin C, Holler C, Lenoir G, Sallandre J, Calender A, Turpin G. 1995. Familial acromegaly: a specific clinical entity—further evidence from the genetic study of a three-generation family. Eur J Endocrinol 133:451–456 [DOI] [PubMed] [Google Scholar]
- 48. Yamada S, Yoshimoto K, Sano T, Takada K, Itakura M, Usui M, Teramoto A. 1997. Inactivation of the tumor suppressor gene on 11q13 in brothers with familial acrogigantism without multiple endocrine neoplasia type 1. J Clin Endocrinol Metab 82:239–242 [DOI] [PubMed] [Google Scholar]
- 49. Fukino K, Kitamura Y, Sanno N, Teramoto A, Emi M. 1999. Analysis of the MEN1 gene in sporadic pituitary adenomas from Japanese patients. Cancer Lett 144:85–92 [DOI] [PubMed] [Google Scholar]
- 50. Teh BT, Kytölä S, Farnebo F, Bergman L, Wong FK, Weber G, Hayward N, Larsson C, Skogseid B, Beckers A, Phelan C, Edwards M, Epstein M, Alford F, Hurley D, Grimmond S, Silins G, Walters M, Stewart C, Cardinal J, Khodaei S, Parente F, Tranebjaerg L, Jorde R, Salmela P. 1998. Mutation analysis of the MEN1 gene in multiple endocrine neoplasia type 1, familial acromegaly and familial isolated hyperparathyroidism. J Clin Endocrinol Metab 83:2621–2626 [DOI] [PubMed] [Google Scholar]
- 51. Gadelha MR, Prezant TR, Une KN, Glick RP, Moskal SF, 2nd, Vaisman M, Melmed S, Kineman RD, Frohman LA. 1999. Loss of heterozygosity on chromosome 11q13 in two families with acromegaly/gigantism is independent of mutations of the multiple endocrine neoplasia type I gene. J Clin Endocrinol Metab 84:249–256 [DOI] [PubMed] [Google Scholar]
- 52. Ackermann F, Krohn K, Windgassen M, Buchfelder M, Fahlbusch R, Paschke R. 1999. Acromegaly in a family without a mutation in the menin gene. Exp Clin Endocrinol Diabetes 107:93–96 [DOI] [PubMed] [Google Scholar]
- 53. De Menis E, Prezant TR. 2002. Isolated familial somatotropinomas: clinical features and analysis of the MEN1 gene. Pituitary 5:11–15 [DOI] [PubMed] [Google Scholar]
- 54. Tanaka C, Yoshimoto K, Yamada S, Nishioka H, Ii S, Moritani M, Yamaoka T, Itakura M. 1998. Absence of germ-line mutations of the multiple endocrine neoplasia type 1 (MEN1) gene in familial pituitary adenoma in contrast to MEN1 in Japanese. J Clin Endocrinol Metab 83:960–965 [DOI] [PubMed] [Google Scholar]
- 55. Poncin J, Stevenaert A, Beckers A. 1999. Somatic MEN1 gene mutation does not contribute significantly to sporadic pituitary tumorigenesis. Eur J Endocrinol 140:573–576 [DOI] [PubMed] [Google Scholar]
- 56. Poncin J, Abs R, Velkeniers B, Bonduelle M, Abramowicz M, Legros JJ, Verloes A, Meurisse M, Van Gaal L, Verellen C, Koulischer L, Beckers A. 1999. Mutation analysis of the MEN1 gene in Belgian patients with multiple endocrine neoplasia type 1 and related diseases. Hum Mutat 13:54–60 [DOI] [PubMed] [Google Scholar]
- 57. Verloes A, Stevenaert A, Teh BT, Petrossians P, Beckers A. 1999. Familial acromegaly: case report and review of the literature. Pituitary 1:273–277 [DOI] [PubMed] [Google Scholar]
- 58. Gadelha MR, Kineman RD, Frohman LA. 1999. Familial somatotropinomas: clinical and genetic aspects. Endocrinologist 9:277–285 [Google Scholar]
- 59. Cameron FJ, Warne GL. 1997. Familial Cushing's disease with severe weight loss occurring in late childhood. J Paediatr Child Health 33:74–77 [DOI] [PubMed] [Google Scholar]
- 60. Salti IS, Mufarrij IS. 1981. Familial Cushing disease. Am J Med Genet 8:91–94 [DOI] [PubMed] [Google Scholar]
- 61. Berezin M, Karasik A. 1995. Familial prolactinoma. Clin Endocrinol (Oxf) 42:483–486 [DOI] [PubMed] [Google Scholar]
- 62. Yuasa H, Tokito S, Nakagaki H, Kitamura K. 1990. Familial pituitary adenoma—report of four cases from two unrelated families. Neurol Med Chir (Tokyo) 30:1016–1019 [DOI] [PubMed] [Google Scholar]
- 63. Valdes-Socin H, Poncin J, Stevens V, Stevenaert A, Beckers A. 2000. Familial isolated pituitary adenomas not linked to somatic MEN-1 mutations. Follow-up of 27 patients. Ann Endocrinol (Paris) 61:301 [Google Scholar]
- 64. Beckers A. 2004. Familial isolated pituitary adenomas. The Ninth International Workshop on Multiple Endocrine Neoplasia (MEN2004). J Intern Med 255:696–730 [Google Scholar]
- 65. Daly AF, Jaffrain-Rea ML, Ciccarelli A, Valdes-Socin H, Rohmer V, Tamburrano G, Borson-Chazot C, Estour B, Ciccarelli E, Brue T, Ferolla P, Emy P, Colao A, De Menis E, Lecomte P, Penfornis F, Delemer B, Bertherat J, Wémeau JL, De Herder W, Archambeaud F, Stevenaert A, Calender A, Murat A, Cavagnini F, Beckers A. 2006. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab 91:3316–3323 [DOI] [PubMed] [Google Scholar]
- 66. Khoo SK, Pendek R, Nickolov R, Luccio-Camelo DC, Newton TL, Massie A, Petillo D, Menon J, Cameron D, Teh BT, Chan SP. 2009. Genome-wide scan identifies novel modifier loci of acromegalic phenotypes for isolated familial somatotropinoma. Endocr Relat Cancer 16:1057–1063 [DOI] [PubMed] [Google Scholar]
- 67. Leontiou CA, Gueorguiev M, van der Spuy J, Quinton R, Lolli F, Hassan S, Chahal HS, Igreja SC, Jordan S, Rowe J, Stolbrink M, Christian HC, Wray J, Bishop-Bailey D, Berney DM, Wass JA, Popovic V, Ribeiro-Oliveira A, Jr, Gadelha MR, Monson JP, Akker SA, Davis JR, Clayton RN, Yoshimoto K, Iwata T, Matsuno A, Eguchi K, Musat M, Flanagan D, Peters G, Bolger GB, Chapple JP, Frohman LA, Grossman AB, Korbonits M. 2008. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab 93:2390–2401 [DOI] [PubMed] [Google Scholar]
- 68. Daly AF, Petrossians P, Beckers A. 2005. An overview of the epidemiology and genetics of acromegaly. J Endocrinol Invest 28:67–69 [PubMed] [Google Scholar]
- 69. Beckers A, Daly AF. 2007. The clinical, pathological, and genetic features of familial isolated pituitary adenomas. Eur J Endocrinol 157:371–382 [DOI] [PubMed] [Google Scholar]
- 70. Petrossians P, de Herder W, Kwekkeboom D, Lamberigts G, Stevenaert A, Beckers A. 2000. Malignant prolactinoma discovered by D2 receptor imaging. J Clin Endocrinol Metab 85:398–401 [DOI] [PubMed] [Google Scholar]
- 71. Daly AF, Vanbellinghen JF, Khoo SK, Jaffrain-Rea ML, Naves LA, Guitelman MA, Murat A, Emy P, Gimenez-Roqueplo AP, Tamburrano G, Raverot G, Barlier A, De Herder W, Penfornis A, Ciccarelli E, Estour B, Lecomte P, Gatta B, Chabre O, Sabaté MI, Bertagna X, Garcia Basavilbaso N, Stalldecker G, Colao A, Ferolla P, Wémeau JL, Caron P, Sadoul JL, Oneto A, Archambeaud F, Calender A, Sinilnikova O, Montañana CF, Cavagnini F, Hana V, Solano A, Delettieres D, Luccio-Camelo DC, Basso A, Rohmer V, Brue T, Bours V, Teh BT, Beckers A. 2007. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab 92:1891–1896 [DOI] [PubMed] [Google Scholar]
- 72. Frohman LA. 2003. Isolated familial somatotropinomas: clinical and genetic considerations. Trans Am Clin Climatol Assoc 114:165–177 [PMC free article] [PubMed] [Google Scholar]
- 73. Frohman LA, Eguchi K. 2004. Familial acromegaly. Growth Horm IGF Res 14(Suppl A):S90–S96 [DOI] [PubMed] [Google Scholar]
- 74. Villa C, Magri F, Morbini P, Falchetti A, Scagnelli P, Lovati E, Locatelli D, Canevari FR, Necchi V, Gabellieri E, Guabello G, Chiovato L, Solcia E. 2008. Silent familial isolated pituitary adenomas: histopathological and clinical case report. Endocr Pathol 19:40–46 [DOI] [PubMed] [Google Scholar]
- 75. Villa C, Lagonigro MS, Magri F, Koziak M, Jaffrain-Rea ML, Brauner R, Bouligand J, Junier MP, Di Rocco F, Sainte-Rose C, Beckers A, Roux FX, Daly AF, Chiovato L. 2011. Hyperplasia-adenoma sequence in pituitary tumorigenesis related to aryl hydrocarbon receptor interacting protein gene mutation. Endocr Relat Cancer 18:347–356 [DOI] [PubMed] [Google Scholar]
- 76. Thakker RV, Pook MA, Wooding C, Boscaro M, Scanarini M, Clayton RN. 1993. Association of somatotrophinomas with loss of alleles on chromosome 11 and with gsp mutations. J Clin Invest 91:2815–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhuang Z, Ezzat SZ, Vortmeyer AO, Weil R, Oldfield EH, Park WS, Pack S, Huang S, Agarwal SK, Guru SC, Manickam P, Debelenko LV, Kester MB, Olufemi SE, Heppner C, Crabtree JS, Burns AL, Spiegel AM, Marx SJ, Chandrasekharappa SC, Collins FS, Emmert-Buck MR, Liotta LA, Asa SL, Lubensky IA. 1997. Mutations of the MEN1 tumor suppressor gene in pituitary tumors. Cancer Res 57:5446–5451 [PubMed] [Google Scholar]
- 78. Farrell WE, Simpson DJ, Bicknell J, Magnay JL, Kyrodimou E, Thakker RV, Clayton RN. 1999. Sequence analysis and transcript expression of the MEN1 gene in sporadic pituitary tumours. Br J Cancer 80:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gadelha MR, Une KN, Rohde K, Vaisman M, Kineman RD, Frohman LA. 2000. Isolated familial somatotropinomas: establishment of linkage to chromosome 11q13.1-11q13.3 and evidence for a potential second locus at chromosome 2p16–12. J Clin Endocrinol Metab 85:707–714 [DOI] [PubMed] [Google Scholar]
- 80. Luccio-Camelo DC, Une KN, Ferreira RE, Khoo SK, Nickolov R, Bronstein MD, Vaisman M, Teh BT, Frohman LA, Mendonça BB, Gadelha MR. 2004. A meiotic recombination in a new isolated familial somatotropinoma kindred. Eur J Endocrinol 150:643–648 [DOI] [PubMed] [Google Scholar]
- 81. Soares BS, Eguchi K, Frohman LA. 2005. Tumor deletion mapping on chromosome 11q13 in eight families with isolated familial somatotropinoma and in 15 sporadic somatotropinomas. J Clin Endocrinol Metab 90:6580–6587 [DOI] [PubMed] [Google Scholar]
- 82. Soares BS, Frohman LA. 2004. Isolated familial somatotropinoma. Pituitary 7:95–101 [DOI] [PubMed] [Google Scholar]
- 83. Kauppinen-Mäkelin R, Sane T, Reunanen A, Välimäki MJ, Niskanen L, Markkanen H, Löyttyniemi E, Ebeling T, Jaatinen P, Laine H, Nuutila P, Salmela P, Salmi J, Stenman UH, Viikari J, Voutilainen E. 2005. A nationwide survey of mortality in acromegaly. J Clin Endocrinol Metab 90:4081–4086 [DOI] [PubMed] [Google Scholar]
- 84. Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA. 2006. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 312:1228–1230 [DOI] [PubMed] [Google Scholar]
- 85. Korbonits M, Storr H, Kumar AV. 2012. Familial pituitary adenomas. Who should be tested for AIP mutations? Clin Endocrinol (Oxf) 77:351–356 [DOI] [PubMed] [Google Scholar]
- 86. Igreja S, Chahal HS, King P, Bolger GB, Srirangalingam U, Guasti L, Chapple JP, Trivellin G, Gueorguiev M, Guegan K, Stals K, Khoo B, Kumar AV, Ellard S, Grossman AB, Korbonits M. 2010. Characterization of aryl hydrocarbon receptor interacting protein (AIP) mutations in familial isolated pituitary adenoma families. Hum Mutat 31:950–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guaraldi F, Salvatori R. 2011. Familial isolated pituitary adenomas: from genetics to therapy. Clin Transl Sci 4:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zatelli MC, Torre ML, Rossi R, Ragonese M, Trimarchi F, Degli Uberti E, Cannavo S. Should aip gene screening be recommended in family members of FIPA patients with R16H variant? Pituitary. 2012 Aug 23; doi: 10.1007/s11102-012-0409-5. doi: 10.1007/s11102-012-0409-5. [DOI] [PubMed] [Google Scholar]
- 89. Naves LA, Daly AF, Vanbellinghen JF, Casulari LA, Spilioti C, Magalhães AV, Azevedo MF, Giacomini LA, Nascimento PP, Nunes RO, Rosa JW, Jaffrain-Rea ML, Bours V, Beckers A. 2007. Variable pathological and clinical features of a large Brazilian family harboring a mutation in the aryl hydrocarbon receptor-interacting protein gene. Eur J Endocrinol 157:383–391 [DOI] [PubMed] [Google Scholar]
- 90. Chahal HS, Stals K, Unterländer M, Balding DJ, Thomas MG, Kumar AV, Besser GM, Atkinson AB, Morrison PJ, Howlett TA, Levy MJ, Orme SM, Akker SA, Abel RL, Grossman AB, Burger J, Ellard S, Korbonits M. 2011. AIP mutation in pituitary adenomas in the 18th century and today. N Engl J Med 364:43–50 [DOI] [PubMed] [Google Scholar]
- 91. Toledo RA, Lourenço DM, Jr, Liberman B, Cunha-Neto MB, Cavalcanti MG, Moyses CB, Toledo SP, Dahia PL. 2007. Germline mutation in the aryl hydrocarbon receptor interacting protein gene in familial somatotropinoma. J Clin Endocrinol Metab 92:1934–1937 [DOI] [PubMed] [Google Scholar]
- 92. Iwata T, Yamada S, Mizusawa N, Golam HM, Sano T, Yoshimoto K. 2007. The aryl hydrocarbon receptor-interacting protein gene is rarely mutated in sporadic GH-secreting adenomas. Clin Endocrinol (Oxf) 66:499–502 [DOI] [PubMed] [Google Scholar]
- 93. Naves LA, Jaffrain-Rea ML, Vêncio SA, Jacomini CZ, Casulari LA, Daly AF, Beckers A. 2010. Aggressive prolactinoma in a child related to germline mutation in the ARYL hydrocarbon receptor interacting protein (AIP) gene. Arq Bras Endocrinol Metabol 54:761–767 [DOI] [PubMed] [Google Scholar]
- 94. Raverot G, Arnous W, Calender A, Trouillas J, Sassolas G, Bournaud C, Pugeat M, Borson-Chazot F. 2007. Familial pituitary adenomas with a heterogeneous functional pattern: clinical and genetic features. J Endocrinol Invest 30:787–790 [DOI] [PubMed] [Google Scholar]
- 95. Occhi G, Trivellin G, Ceccato F, De Lazzari P, Giorgi G, Demattè S, Grimaldi F, Castello R, Davì MV, Arnaldi G, Salviati L, Opocher G, Mantero F, Scaroni C. 2010. Prevalence of AIP mutations in a large series of sporadic Italian acromegalic patients and evaluation of CDKN1B status in acromegalic patients with multiple endocrine neoplasia. Eur J Endocrinol 163:369–376 [DOI] [PubMed] [Google Scholar]
- 96. Occhi G, Jaffrain-Rea ML, Trivellin G, Albiger N, Ceccato F, De Menis E, Angelini M, Ferasin S, Beckers A, Mantero F, Scaroni C. 2010. The R304X mutation of the aryl hydrocarbon receptor interacting protein gene in familial isolated pituitary adenomas: mutational hot-spot or founder effect? J Endocrinol Invest 33:800–805 [DOI] [PubMed] [Google Scholar]
- 97. Pinho LK, Vieira Neto L, Wildemberg LE, Moraes AB, Takiya CM, Frohman LA, Korbonits M, Gadelha MR. 2010. Familial isolated pituitary adenomas experience at a single center: clinical importance of AIP mutation screening. Arq Bras Endocrinol Metabol 54:698–704 [DOI] [PubMed] [Google Scholar]
- 98. Chahal HS, Chapple JP, Frohman LA, Grossman AB, Korbonits M. 2010. Clinical, genetic and molecular characterization of patients with familial isolated pituitary adenomas (FIPA). Trends Endocrinol Metab 21:419–427 [DOI] [PubMed] [Google Scholar]
- 99. Georgitsi M, Raitila A, Karhu A, Tuppurainen K, Mäkinen MJ, Vierimaa O, Paschke R, Saeger W, van der Luijt RB, Sane T, Robledo M, De Menis E, Weil RJ, Wasik A, Zielinski G, Lucewicz O, Lubinski J, Launonen V, Vahteristo P, Aaltonen LA. 2007. Molecular diagnosis of pituitary adenoma predisposition caused by aryl hydrocarbon receptor-interacting protein gene mutations. Proc Natl Acad Sci USA 104:4101–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Barlier A, Vanbellinghen JF, Daly AF, Silvy M, Jaffrain-Rea ML, Trouillas J, Tamagno G, Cazabat L, Bours V, Brue T, Enjalbert A, Beckers A. 2007. Mutations in the aryl hydrocarbon receptor interacting protein gene are not highly prevalent among subjects with sporadic pituitary adenomas. J Clin Endocrinol Metab 92:1952–1955 [DOI] [PubMed] [Google Scholar]
- 101. Cazabat L, Bouligand J, Salenave S, Bernier M, Gaillard S, Parker F, Young J, Guiochon-Mantel A, Chanson P. 2012. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients. J Clin Endocrinol Metab 97:E663–E670 [DOI] [PubMed] [Google Scholar]
- 102. Buchbinder S, Bierhaus A, Zorn M, Nawroth PP, Humpert P, Schilling T. 2008. Aryl hydrocarbon receptor interacting protein gene (AIP) mutations are rare in patients with hormone secreting or non-secreting pituitary adenomas. Exp Clin Endocrinol Diabetes 116:625–628 [DOI] [PubMed] [Google Scholar]
- 103. Cazabat L, Guillaud-Bataille M, Bertherat J, Raffin-Sanson ML. 2009. Mutations of the gene for the aryl hydrocarbon receptor-interacting protein in pituitary adenomas. Horm Res 71:132–141 [DOI] [PubMed] [Google Scholar]
- 104. Georgitsi M, De Menis E, Cannavò S, Mäkinen MJ, Tuppurainen K, Pauletto P, Curtò L, Weil RJ, Paschke R, Zielinski G, Wasik A, Lubinski J, Vahteristo P, Karhu A, Aaltonen LA. 2008. Aryl hydrocarbon receptor interacting protein (AIP) gene mutation analysis in children and adolescents with sporadic pituitary adenomas. Clin Endocrinol (Oxf) 69:621–627 [DOI] [PubMed] [Google Scholar]
- 105. Georgitsi M, Heliövaara E, Paschke R, Kumar AV, Tischkowitz M, Vierimaa O, Salmela P, Sane T, De Menis E, Cannavò S, Gündogdu S, Lucassen A, Izatt L, Aylwin S, Bano G, Hodgson S, Koch CA, Karhu A, Aaltonen LA. 2008. Large genomic deletions in AIP in pituitary adenoma predisposition. J Clin Endocrinol Metab 93:4146–4151 [DOI] [PubMed] [Google Scholar]
- 106. Personnier C, Cazabat L, Bertherat J, Gaillard S, Souberbielle JC, Habrand JL, Dufour C, Clauser E, SainteRose C, Polak M. 2011. Clinical features and treatment of pediatric somatotropinoma: case study of an aggressive tumor due to a new AIP mutation and extensive literature review. Horm Res Paediatr 75:392–402 [DOI] [PubMed] [Google Scholar]
- 107. Cazabat L, Libè R, Perlemoine K, René-Corail F, Burnichon N, Gimenez-Roqueplo AP, Dupasquier-Fediaevsky L, Bertagna X, Clauser E, Chanson P, Bertherat J, Raffin-Sanson ML. 2007. Germline inactivating mutations of the aryl hydrocarbon receptor-interacting protein gene in a large cohort of sporadic acromegaly: mutations are found in a subset of young patients with macroadenomas. Eur J Endocrinol 157:1–8 [DOI] [PubMed] [Google Scholar]
- 108. Tichomirowa MA, Barlier A, Daly AF, Jaffrain-Rea ML, Ronchi C, Yaneva M, Urban JD, Petrossians P, Elenkova A, Tabarin A, Desailloud R, Maiter D, Schürmeyer T, Cozzi R, Theodoropoulou M, Sievers C, Bernabeu I, Naves LA, Chabre O, Montañana CF, Hana V, Halaby G, Delemer B, Aizpún JI, Sonnet E, Longás AF, Hagelstein MT, Caron P, Stalla GK, Bours V, Zacharieva S, Spada A, Brue T, Beckers A. 2011. High prevalence of AIP gene mutations following focused screening in young patients with sporadic pituitary macroadenomas. Eur J Endocrinol 165:509–515 [DOI] [PubMed] [Google Scholar]
- 109. Stratakis CA, Tichomirowa MA, Boikos S, Azevedo MF, Lodish M, Martari M, Verma S, Daly AF, Raygada M, Keil MF, Papademetriou J, Drori-Herishanu L, Horvath A, Tsang KM, Nesterova M, Franklin S, Vanbellinghen JF, Bours V, Salvatori R, Beckers A. 2010. The role of germline AIP, MEN1, PRKAR1A, CDKN1B and CDKN2C mutations in causing pituitary adenomas in a large cohort of children, adolescents, and patients with genetic syndromes. Clin Genet 78:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Keil MF, Stratakis CA. 2008. Pituitary tumors in childhood: update of diagnosis, treatment and molecular genetics. Expert Rev Neurother 8:563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Korbonits M. 2012. Pituitary tumourigenesis: tracing back a gene's influence. Endocr Abstr 29:S22.2 [Google Scholar]
- 112. Comte De Buffon Leclerc GL. 1777. Histoire naturelle, générale et particulièr. [Google Scholar]
- 113. Mantel H. 1998. The giant, O'Brien: a novel. New York: Henry Holt and Co [Google Scholar]
- 114. Doyal L, Muinzer T. 2011. Should the skeleton of “the Irish giant” be buried at sea? BMJ 343:d7597. [DOI] [PubMed] [Google Scholar]
- 115. Daly AF, Tichomirowa MA, Petrossians P, Heliövaara E, Jaffrain-Rea ML, Barlier A, Naves LA, Ebeling T, Karhu A, Raappana A, Cazabat L, De Menis E, Montañana CF, Raverot G, Weil RJ, Sane T, Maiter D, Neggers S, Yaneva M, Tabarin A, Verrua E, Eloranta E, Murat A, Vierimaa O, Salmela PI, Emy P, Toledo RA, Sabaté MI, Villa C, Popelier M, Salvatori R, Jennings J, Longás AF, Labarta Aizpún JI, Georgitsi M, Paschke R, Ronchi C, Valimaki M, Saloranta C, De Herder W, Cozzi R, Guitelman M, Magri F, Lagonigro MS, Halaby G, Corman V, Hagelstein MT, Vanbellinghen JF, Barra GB, Gimenez-Roqueplo AP, Cameron FJ, Borson-Chazot F, Holdaway I, Toledo SP, Stalla GK, Spada A, Zacharieva S, Bertherat J, Brue T, Bours V, Chanson P, Aaltonen LA, Beckers A. 2010. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab 95:E373–E383 [DOI] [PubMed] [Google Scholar]
- 116. Jennings JE, Georgitsi M, Holdaway I, Daly AF, Tichomirowa M, Beckers A, Aaltonen LA, Karhu A, Cameron FJ. 2009. Aggressive pituitary adenomas occurring in young patients in a large Polynesian kindred with a germline R271W mutation in the AIP gene. Eur J Endocrinol 161:799–804 [DOI] [PubMed] [Google Scholar]
- 117. Guaraldi F, Corazzini V, Gallia GL, Grottoli S, Stals K, Dalantaeva N, Frohman LA, Korbonits M, Salvatori R. 2012. Genetic analysis in a patient presenting with meningioma and familial isolated pituitary adenoma (FIPA) reveals selective involvement of the R81X mutation of the AIP gene in the pathogenesis of the pituitary tumor. Pituitary 15(Suppl 1):61–67 [DOI] [PubMed] [Google Scholar]
- 118. Bilbao Garay I, Alvarez Coca M, Aramburu M, Garcia C, Yoldi A, Matteucci T, Egaña N, Socias C, Goena M, Beckers A. Nueva mutacion de AIP en adenomas hipofisarios familiars (FIPA). Proc 14th Congress of the Sociedad de endocrinología, diabetes y nutrición de Euskadi (SEDyNE), Bilbao, Spain, 2011 [Google Scholar]
- 119. Durán-Prado M, Gahete MD, Hergueta-Redondo M, Martínez-Fuentes AJ, Córdoba-Chacón J, Palacios J, Gracia-Navarro F, Moreno-Bueno G, Malagón MM, Luque RM, Castaño JP. 2012. The new truncated somatostatin receptor variant sst5TMD4 is associated to poor prognosis in breast cancer and increases malignancy in MCF-7 cells. Oncogene 31:2049–2061 [DOI] [PubMed] [Google Scholar]
- 120. Córdoba-Chacón J, Gahete MD, Durán-Prado M, Luque RM, Castaño JP. 2011. Truncated somatostatin receptors as new players in somatostatin-cortistatin pathophysiology. Ann NY Acad Sci 1220:6–15 [DOI] [PubMed] [Google Scholar]
- 121. Durán-Prado M, Saveanu A, Luque RM, Gahete MD, Gracia-Navarro F, Jaquet P, Dufour H, Malagón MM, Culler MD, Barlier A, Castaño JP. 2010. A potential inhibitory role for the new truncated variant of somatostatin receptor 5, sst5TMD4, in pituitary adenomas poorly responsive to somatostatin analogs. J Clin Endocrinol Metab 95:2497–2502 [DOI] [PubMed] [Google Scholar]
- 122. Durán-Prado M, Gahete MD, Martínez-Fuentes AJ, Luque RM, Quintero A, Webb SM, Benito-López P, Leal A, Schulz S, Gracia-Navarro F, Malagón MM, Castaño JP. 2009. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab 94:2634–2643 [DOI] [PubMed] [Google Scholar]
- 123. Theodoropoulou M, Zhang J, Laupheimer S, Paez-Pereda M, Erneux C, Florio T, Pagotto U, Stalla GK. 2006. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res 66:1576–1582 [DOI] [PubMed] [Google Scholar]
- 124. Pagotto U, Arzberger T, Theodoropoulou M, Grübler Y, Pantaloni C, Saeger W, Losa M, Journot L, Stalla GK, Spengler D. 2000. The expression of the antiproliferative gene ZAC is lost or highly reduced in nonfunctioning pituitary adenomas. Cancer Res 60:6794–6799 [PubMed] [Google Scholar]
- 125. Theodoropoulou M, Tichomirowa MA, Sievers C, Yassouridis A, Arzberger T, Hougrand O, Deprez M, Daly AF, Petrossians P, Pagotto U, Beckers A, Stalla GK. 2009. Tumor ZAC1 expression is associated with the response to somatostatin analog therapy in patients with acromegaly. Int J Cancer 125:2122–2126 [DOI] [PubMed] [Google Scholar]
- 126. Chahal HS, Trivellin G, Leontiou CA, Alband N, Fowkes RC, Tahir A, Igreja SC, Chapple JP, Jordan S, Lupp A, Schulz S, Ansorge O, Karavitaki N, Carlsen E, Wass JA, Grossman AB, Korbonits M. 2012. Somatostatin analogs modulate AIP in somatotroph adenomas: the role of the ZAC1 pathway. J Clin Endocrinol Metab 97:E1411–E1420 [DOI] [PubMed] [Google Scholar]
- 127. Georgitsi M, Karhu A, Winqvist R, Visakorpi T, Waltering K, Vahteristo P, Launonen V, Aaltonen LA. 2007. Mutation analysis of aryl hydrocarbon receptor interacting protein (AIP) gene in colorectal, breast, and prostate cancers. Br J Cancer 96:352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Raitila A, Georgitsi M, Bonora E, Vargiolu M, Tuppurainen K, Mäkinen MJ, Vierimaa O, Salmela PI, Launonen V, Vahteristo P, Aaltonen LA, Romeo G, Karhu A. 2009. Aryl hydrocarbon receptor interacting protein mutations seem not to associate with familial non-medullary thyroid cancer. J Endocrinol Invest 32:426–429 [DOI] [PubMed] [Google Scholar]
- 129. Raitila A, Georgitsi M, Karhu A, Tuppurainen K, Mäkinen MJ, Birkenkamp-Demtröder K, Salmenkivi K, Orntoft TF, Arola J, Launonen V, Vahteristo P, Aaltonen LA. 2007. No evidence of somatic aryl hydrocarbon receptor interacting protein mutations in sporadic endocrine neoplasia. Endocr Relat Cancer 14:901–906 [DOI] [PubMed] [Google Scholar]
- 130. Toledo RA, Mendonca BB, Fragoso MC, Soares IC, Almeida MQ, Moraes MB, Lourenço DM, Jr, Alves VA, Bronstein MD, Toledo SP. 2010. Isolated familial somatotropinoma: 11q13-loh and gene/protein expression analysis suggests a possible involvement of aip also in non-pituitary tumorigenesis. Clinics Sao Paulo 65:407–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kjellman M, Roshani L, Teh BT, Kallioniemi OP, Höög A, Gray S, Farnebo LO, Holst M, Bäckdahl M, Larsson C. 1999. Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. J Clin Endocrinol Metab 84:730–735 [DOI] [PubMed] [Google Scholar]
- 132. Nord KH, Magnusson L, Isaksson M, Nilsson J, Lilljebjörn H, Domanski HA, Kindblom LG, Mandahl N, Mertens F. 2010. Concomitant deletions of tumor suppressor genes MEN1 and AIP are essential for the pathogenesis of the brown fat tumor hibernoma. Proc Natl Acad Sci USA 107:21122–21127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lin BC, Sullivan R, Lee Y, Moran S, Glover E, Bradfield CA. 2007. Deletion of the aryl hydrocarbon receptor-associated protein 9 leads to cardiac malformation and embryonic lethality. J Biol Chem 282:35924–35932 [DOI] [PubMed] [Google Scholar]
- 134. Lin BC, Nguyen LP, Walisser JA, Bradfield CA. 2008. A hypomorphic allele of aryl hydrocarbon receptor-associated protein-9 produces a phenocopy of the AHR-null mouse. Mol Pharmacol 74:1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Walisser JA, Bunger MK, Glover E, Harstad EB, Bradfield CA. 2004. Patent ductus venosus and dioxin resistance in mice harboring a hypomorphic Arnt allele. J Biol Chem 279:16326–16331 [DOI] [PubMed] [Google Scholar]
- 136. Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA. 2005. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol 67:714–720 [DOI] [PubMed] [Google Scholar]
- 137. Nukaya M, Lin BC, Glover E, Moran SM, Kennedy GD, Bradfield CA. 2010. The aryl hydrocarbon receptor-interacting protein (AIP) is required for dioxin-induced hepatotoxicity but not for the induction of the Cyp1a1 and Cyp1a2 genes. J Biol Chem 285:35599–35605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Raitila A, Lehtonen HJ, Arola J, Heliövaara E, Ahlsten M, Georgitsi M, Jalanko A, Paetau A, Aaltonen LA, Karhu A. 2010. Mice with inactivation of aryl hydrocarbon receptor-interacting protein (Aip) display complete penetrance of pituitary adenomas with aberrant ARNT expression. Am J Pathol 177:1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, et al. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562 [DOI] [PubMed] [Google Scholar]
- 140. Gharib WH, Robinson-Rechavi M. 2011. When orthologs diverge between human and mouse. Brief Bioinform 12:436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Heliövaara E, Raitila A, Launonen V, Paetau A, Arola J, Lehtonen H, Sane T, Weil RJ, Vierimaa O, Salmela P, Tuppurainen K, Mäkinen M, Aaltonen LA, Karhu A. 2009. The expression of AIP-related molecules in elucidation of cellular pathways in pituitary adenomas. Am J Pathol 175:2501–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jaffrain-Rea ML, Angelini M, Gargano D, Tichomirowa MA, Daly AF, Vanbellinghen JF, D'Innocenzo E, Barlier A, Giangaspero F, Esposito V, Ventura L, Arcella A, Theodoropoulou M, Naves LA, Fajardo C, Zacharieva S, Rohmer V, Brue T, Gulino A, Cantore G, Alesse E, Beckers A. 2009. Expression of aryl hydrocarbon receptor (AHR) and AHR-interacting protein in pituitary adenomas: pathological and clinical implications. Endocr Relat Cancer 16:1029–1043 [DOI] [PubMed] [Google Scholar]
- 143. Ezzat S, Kontogeorgos G, Redelmeier DA, Horvath E, Harris AG, Kovacs K. 1995. In vivo responsiveness of morphological variants of growth hormone-producing pituitary adenomas to octreotide. Eur J Endocrinol 133:686–690 [DOI] [PubMed] [Google Scholar]
- 144. Horvath E, Kovacs K. 2006. Pathology of acromegaly. Neuroendocrinology 83:161–165 [DOI] [PubMed] [Google Scholar]
- 145. Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J. 2012. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endocrinol (Oxf) 76:96–102 [DOI] [PubMed] [Google Scholar]
- 146. Bakhtiar Y, Hirano H, Arita K, Yunoue S, Fujio S, Tominaga A, Sakoguchi T, Sugiyama K, Kurisu K, Yasufuku-Takano J, Takano K. 2010. Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur J Endocrinol 163:531–539 [DOI] [PubMed] [Google Scholar]
- 147. Kasuki Jomori de Pinho L, Vieira Neto L, Armondi Wildemberg LE, Gasparetto EL, Marcondes J, de Almeida Nunes B, Takiya CM, Gadelha MR. 2011. Low aryl hydrocarbon receptor-interacting protein expression is a better marker of invasiveness in somatotropinomas than Ki-67 and p53. Neuroendocrinology 94:39–48 [DOI] [PubMed] [Google Scholar]
- 148. Kasuki L, Vieira Neto L, Wildemberg LE, Colli LM, de Castro M, Takiya CM, Gadelha MR. 2012. AIP expression in sporadic somatotropinomas is a predictor of the response to octreotide LAR therapy independent of SSTR2 expression. Endocr Relat Cancer 19:L25–29 [DOI] [PubMed] [Google Scholar]
- 149. Kuzhandaivelu N, Cong YS, Inouye C, Yang WM, Seto E. 1996. XAP2, a novel hepatitis B virus X-associated protein that inhibits X transactivation. Nucleic Acids Res 24:4741–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Carver LA, Bradfield CA. 1997. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem 272:11452–11456 [DOI] [PubMed] [Google Scholar]
- 151. Cox MB, Smith DF. 2007. Functions of the Hsp90-binding FDKBP immunophilins. In: Blatch GL, ed. Networking of chaperones by co-chaperones. Austin, TX: Landis Bioscience [Google Scholar]
- 152. Petrulis JR, Perdew GH. 2002. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact 141:25–40 [DOI] [PubMed] [Google Scholar]
- 153. Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. 1998. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol 18:978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ma Q, Whitlock JP., Jr 1997. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem 272:8878–8884 [PubMed] [Google Scholar]
- 155. Schiene C, Fischer G. 2000. Enzymes that catalyse the restructuring of proteins. Curr Opin Struct Biol 10:40–45 [DOI] [PubMed] [Google Scholar]
- 156. Goebl M, Yanagida M. 1991. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci 16:173–177 [DOI] [PubMed] [Google Scholar]
- 157. Blatch GL, Lässle M. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays 21:932–939 [DOI] [PubMed] [Google Scholar]
- 158. Hollingshead BD, Petrulis JR, Perdew GH. 2004. The aryl hydrocarbon (Ah) receptor transcriptional regulator hepatitis B virus X-associated protein 2 antagonizes p23 binding to Ah receptor-Hsp90 complexes and is dispensable for receptor function. J Biol Chem 279:45652–45661 [DOI] [PubMed] [Google Scholar]
- 159. Carver LA, LaPres JJ, Jain S, Dunham EE, Bradfield CA. 1998. Characterization of the Ah receptor-associated protein, ARA9. J Biol Chem 273:33580–33587 [DOI] [PubMed] [Google Scholar]
- 160. Kazlauskas A, Poellinger L, Pongratz I. 2000. The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem 275:41317–41324 [DOI] [PubMed] [Google Scholar]
- 161. Kazlauskas A, Poellinger L, Pongratz I. 2002. Two distinct regions of the immunophilin-like protein XAP2 regulate dioxin receptor function and interaction with hsp90. J Biol Chem 277:11795–11801 [DOI] [PubMed] [Google Scholar]
- 162. Kazlauskas A, Sundström S, Poellinger L, Pongratz I. 2001. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol Cell Biol 21:2594–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kazlauskas A, Poellinger L, Pongratz I. 1999. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (Aryl hydrocarbon) receptor. J Biol Chem 274:13519–13524 [DOI] [PubMed] [Google Scholar]
- 164. Meyer BK, Perdew GH. 1999. Characterization of the AhR-hsp90-XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry 38:8907–8917 [DOI] [PubMed] [Google Scholar]
- 165. Pollenz RS, Dougherty EJ. 2005. Redefining the role of the endogenous XAP2 and C-terminal hsp70-interacting protein on the endogenous Ah receptors expressed in mouse and rat cell lines. J Biol Chem 280:33346–33356 [DOI] [PubMed] [Google Scholar]
- 166. Reyes H, Reisz-Porszasz S, Hankinson O. 1992. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256:1193–1195 [DOI] [PubMed] [Google Scholar]
- 167. Ramadoss P, Perdew GH. 2005. The transactivation domain of the Ah receptor is a key determinant of cellular localization and ligand-independent nucleocytoplasmic shuttling properties. Biochemistry 44:11148–11159 [DOI] [PubMed] [Google Scholar]
- 168. Nakata A, Urano D, Fujii-Kuriyama Y, Mizuno N, Tago K, Itoh H. 2009. G-protein signalling negatively regulates the stability of aryl hydrocarbon receptor. EMBO Rep 10:622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F. 2005. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci USA 102:9218–9223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bolger GB, Peden AH, Steele MR, MacKenzie C, McEwan DG, Wallace DA, Huston E, Baillie GS, Houslay MD. 2003. Attenuation of the activity of the cAMP-specific phosphodiesterase PDE4A5 by interaction with the immunophilin XAP2. J Biol Chem 278:33351–33363 [DOI] [PubMed] [Google Scholar]
- 171. Pollenz RS, Buggy C. 2006. Ligand-dependent and -independent degradation of the human aryl hydrocarbon receptor (hAHR) in cell culture models. Chem Biol Interact 164:49–59 [DOI] [PubMed] [Google Scholar]
- 172. Kolluri SK, Weiss C, Koff A, Göttlicher M. 1999. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev 13:1742–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, Samson E, Bink K, Höfler H, Fend F, Graw J, Atkinson MJ. 2006. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA 103:15558–15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Georgitsi M, Raitila A, Karhu A, van der Luijt RB, Aalfs CM, Sane T, Vierimaa O, Mäkinen MJ, Tuppurainen K, Paschke R, Gimm O, Koch CA, Gündogdu S, Lucassen A, Tischkowitz M, Izatt L, Aylwin S, Bano G, Hodgson S, De Menis E, Launonen V, Vahteristo P, Aaltonen LA. 2007. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab 92:3321–3325 [DOI] [PubMed] [Google Scholar]
- 175. Tichomirowa MA, Lee M, Barlier A, Daly AF, Marinoni I, Jaffrain-Rea ML, Naves LA, Rodien P, Rohmer V, Faucz FR, Caron P, Estour B, Lecomte P, Borson-Chazot F, Penfornis A, Yaneva M, Guitelman M, Castermans E, Verhaege C, Wémeau JL, Tabarin A, Fajardo Montañana C, Delemer B, Kerlan V, Sadoul JL, Cortet Rudelli C, Archambeaud F, Zacharieva S, Theodoropoulou M, Brue T, Enjalbert A, Bours V, Pellegata NS, Beckers A. 2012. Cyclin dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenomas (FIPA) kindreds. Endocr Relat Cancer 19:233–241 [DOI] [PubMed] [Google Scholar]
- 176. Pitt JA, Buckalew AR, House DE, Abbott BD. 2000. Adrenocorticotropin (ACTH) and corticosterone secretion by perifused pituitary and adrenal glands from rodents exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicology 151:25–35 [DOI] [PubMed] [Google Scholar]
- 177. Pesatori AC, Baccarelli A, Consonni D, Lania A, Beck-Peccoz P, Bertazzi PA, Spada A. 2008. Aryl hydrocarbon receptor-interacting protein and pituitary adenomas: a population-based study on subjects exposed to dioxin after the Seveso, Italy, accident. Eur J Endocrinol 159:699–703 [DOI] [PubMed] [Google Scholar]
- 178. Cannavò S, Ferraù F, Ragonese M, Curtò L, Torre ML, Magistri M, Marchese A, Alibrandi A, Trimarchi F. 2010. Increased prevalence of acromegaly in a highly polluted area. Eur J Endocrinol 163:509–513 [DOI] [PubMed] [Google Scholar]
- 179. Swedenborg E, Pongratz I. 2010. AhR and ARNT modulate ER signaling. Toxicology 268:132–138 [DOI] [PubMed] [Google Scholar]
- 180. Safe S, Wormke M, Samudio I. 2000. Mechanisms of inhibitory aryl hydrocarbon receptor-estrogen receptor crosstalk in human breast cancer cells. J Mammary Gland Biol Neoplasia 5:295–306 [DOI] [PubMed] [Google Scholar]
- 181. Brunnberg S, Pettersson K, Rydin E, Matthews J, Hanberg A, Pongratz I. 2003. The basic helix-loop-helix-PAS protein ARNT functions as a potent coactivator of estrogen receptor-dependent transcription. Proc Natl Acad Sci USA 100:6517–6522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Pereira-Lima JF, Marroni CP, Pizarro CB, Barbosa-Coutinho LM, Ferreira NP, Oliveira MC. 2004. Immunohistochemical detection of estrogen receptor α in pituitary adenomas and its correlation with cellular replication. Neuroendocrinology 79:119–124 [DOI] [PubMed] [Google Scholar]
- 183. Heaney AP, Fernando M, Melmed S. 2002. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest 109:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Cai W, Kramarova TV, Berg P, Korbonits M, Pongratz I. 2011. The immunophilin-like protein XAP2 is a negative regulator of estrogen signaling through interaction with estrogen receptor α. PLoS One 6:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hirose K, Morita M, Ema M, Mimura J, Hamada H, Fujii H, Saijo Y, Gotoh O, Sogawa K, Fujii-Kuriyama Y. 1996. cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the aryl hydrocarbon receptor nuclear translocator (Arnt). Mol Cell Biol 16:1706–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Rankin EB, Giaccia AJ. 2008. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15:678–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Dougherty EJ, Pollenz RS. 2008. Analysis of Ah receptor-ARNT and Ah receptor-ARNT2 complexes in vitro and in cell culture. Toxicol Sci 103:191–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Damert A, Ikeda E, Risau W. 1997. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J 327:419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Keith B, Adelman DM, Simon MC. 2001. Targeted mutation of the murine aryl hydrocarbon receptor nuclear translocator 2 (Arnt2) gene reveals partial redundancy with Arnt. Proc Natl Acad Sci USA 98:6692–6697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Michaud JL, DeRossi C, May NR, Holdener BC, Fan CM. 2000. ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech Dev 90:253–261 [DOI] [PubMed] [Google Scholar]
- 191. Moffett P, Pelletier J. 2000. Different transcriptional properties of mSim-1 and mSim-2. FEBS Lett 466:80–86 [DOI] [PubMed] [Google Scholar]
- 192. Michaud JL, Rosenquist T, May NR, Fan CM. 1998. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev 12:3264–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Michaud JL, Boucher F, Melnyk A, Gauthier F, Goshu E, Lévy E, Mitchell GA, Himms-Hagen J, Fan CM. 2001. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum Mol Genet 10:1465–1473 [DOI] [PubMed] [Google Scholar]
- 194. Goshu E, Jin H, Lovejoy J, Marion JF, Michaud JL, Fan CM. 2004. Sim2 contributes to neuroendocrine hormone gene expression in the anterior hypothalamus. Mol Endocrinol 18:1251–1262 [DOI] [PubMed] [Google Scholar]
- 195. Ma Q, Whitlock JP., Jr 1996. The aromatic hydrocarbon receptor modulates the Hepa 1c1c7 cell cycle and differentiated state independently of dioxin. Mol Cell Biol 16:2144–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Elizondo G, Fernandez-Salguero P, Sheikh MS, Kim GY, Fornace AJ, Lee KS, Gonzalez FJ. 2000. Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Mol Pharmacol 57:1056–1063 [PubMed] [Google Scholar]
- 197. Fritz WA, Lin TM, Peterson RE. 2008. The aryl hydrocarbon receptor (AhR) inhibits vanadate-induced vascular endothelial growth factor (VEGF) production in TRAMP prostates. Carcinogenesis 29:1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y, Puga A. 2010. The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res 70:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Peng L, Mayhew CN, Schnekenburger M, Knudsen ES, Puga A. 2008. Repression of Ah receptor and induction of transforming growth factor-β genes in DEN-induced mouse liver tumors. Toxicology 246:242–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Puga A, Tomlinson CR, Xia Y. 2005. Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol 69:199–207 [DOI] [PubMed] [Google Scholar]
- 201. Donangelo I, Marcos HP, Araújo PB, Marcondes J, Filho PN, Gadelha M, Chimelli L. 2005. Expression of retinoblastoma protein in human growth hormone-secreting pituitary adenomas. Endocr Pathol 16:53–62 [DOI] [PubMed] [Google Scholar]
- 202. Simpson DJ, Hibberts NA, McNicol AM, Clayton RN, Farrell WE. 2000. Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res 60:1211–1216 [PubMed] [Google Scholar]
- 203. McMillan BJ, Bradfield CA. 2007. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol Pharmacol 72:487–498 [DOI] [PubMed] [Google Scholar]
- 204. Baker DA, Kelly JM. 2004. Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol Microbiol 52:1229–1242 [DOI] [PubMed] [Google Scholar]
- 205. Boikos SA, Stratakis CA. 2007. Molecular genetics of the cAMP-dependent protein kinase pathway and of sporadic pituitary tumorigenesis. Hum Mol Genet 16(Spec No. 1):R80–R87 [DOI] [PubMed] [Google Scholar]
- 206. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. 1989. GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340:692–696 [DOI] [PubMed] [Google Scholar]
- 207. Lania A, Spada A. 2009. G-protein and signalling in pituitary tumours. Horm Res 71(Suppl 2):95–100 [DOI] [PubMed] [Google Scholar]
- 208. Spada A, Mantovani G, Lania A. 2005. Inactivating and activating mutations of the Gs α gene. Ann Endocrinol (Paris) 66:258–263 [DOI] [PubMed] [Google Scholar]
- 209. Spada A, Arosio M, Bassetti M, Vallar L, Clementi E, Bazzoni N. 1991. Mutations in the α subunit of the stimulatory regulatory protein of adenylyl cyclase (Gs) in human GH-secreting pituitary adenomas. Biochemical, clinical, and morphological aspects. Pathol Res Pract 187:567–570 [DOI] [PubMed] [Google Scholar]
- 210. Picard C, Silvy M, Gerard C, Buffat C, Lavaque E, Figarella-Branger D, Dufour H, Gabert J, Beckers A, Brue T, Enjalbert A, Barlier A. 2007. Gs α overexpression and loss of Gs α imprinting in human somatotroph adenomas: association with tumor size and response to pharmacologic treatment. Int J Cancer 121:1245–1252 [DOI] [PubMed] [Google Scholar]
- 211. Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. 1991. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med 325:1688–1695 [DOI] [PubMed] [Google Scholar]
- 212. Schwindinger WF, Francomano CA, Levine MA. 1992. Identification of a mutation in the gene encoding the α subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci USA 89:5152–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Jiang LI, Collins J, Davis R, Fraser ID, Sternweis PC. 2008. Regulation of cAMP responses by the G12/13 pathway converges on adenylyl cyclase VII. J Biol Chem 283:23429–23439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Jiang LI, Collins J, Davis R, Lin KM, DeCamp D, Roach T, Hsueh R, Rebres RA, Ross EM, Taussig R, Fraser I, Sternweis PC. 2007. Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282:10576–10584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. de Oliveira SK, Hoffmeister M, Gambaryan S, Müller-Esterl W, Guimaraes JA, Smolenski AP. 2007. Phosphodiesterase 2A forms a complex with the co-chaperone XAP2 and regulates nuclear translocation of the aryl hydrocarbon receptor. J Biol Chem 282:13656–13663 [DOI] [PubMed] [Google Scholar]
- 216. Bender AT, Beavo JA. 2006. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520 [DOI] [PubMed] [Google Scholar]
- 217. MacKenzie SJ, Yarwood SJ, Peden AH, Bolger GB, Vernon RG, Houslay MD. 1998. Stimulation of p70S6 kinase via a growth hormone-controlled phosphatidylinositol 3-kinase pathway leads to the activation of a PDE4A cyclic AMP-specific phosphodiesterase in 3T3–F442A preadipocytes. Proc Natl Acad Sci USA 95:3549–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Cañibano C, Rodriguez NL, Saez C, Tovar S, Garcia-Lavandeira M, Borrello MG, Vidal A, Costantini F, Japon M, Dieguez C, Alvarez CV. 2007. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. EMBO J 26:2015–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kääriäinen H. 1994. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature 367:377–378 [DOI] [PubMed] [Google Scholar]
- 220. Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, Love DR, Mole SE, Moore JK, Papi L, Ponder MA, Telenius H, Tunnacliffe A, Ponder BAJ. 1993. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363:458–460 [DOI] [PubMed] [Google Scholar]
- 221. Arighi E, Borrello MG, Sariola H. 2005. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 16:441–467 [DOI] [PubMed] [Google Scholar]
- 222. Japón MA, Urbano AG, Sáez C, Segura DI, Cerro AL, Diéguez C, Alvarez CV. 2002. Glial-derived neurotropic factor and RET gene expression in normal human anterior pituitary cell types and in pituitary tumors. J Clin Endocrinol Metab 87:1879–1884 [DOI] [PubMed] [Google Scholar]
- 223. Vargiolu M, Fusco D, Kurelac I, Dirnberger D, Baumeister R, Morra I, Melcarne A, Rimondini R, Romeo G, Bonora E. 2009. The tyrosine kinase receptor RET interacts in vivo with aryl hydrocarbon receptor-interacting protein to alter survivin availability. J Clin Endocrinol Metab 94:2571–2578 [DOI] [PubMed] [Google Scholar]
- 224. Heliövaara E, Tuupanen S, Ahlsten M, Hodgson S, de Menis E, Kuismin O, Izatt L, McKinlay Gardner RJ, Gundogdu S, Lucassen A, Arola J, Tuomisto A, Mäkinen M, Karhu A, Aaltonen LA. 2011. No evidence of RET germline mutations in familial pituitary adenoma. J Mol Endocrinol 46:1–8 [DOI] [PubMed] [Google Scholar]
- 225. Kang BH, Altieri DC. 2006. Regulation of survivin stability by the aryl hydrocarbon receptor-interacting protein. J Biol Chem 281:24721–24727 [DOI] [PubMed] [Google Scholar]
- 226. Trivellin G, Butz H, Delhove J, Igreja S, Chahal HS, Zivkovic V, McKay T, Patócs A, Grossman AB, Korbonits M. 2012. MicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitro. Am J Physiol Endocrinol Metab 303:E708–E719 [DOI] [PubMed] [Google Scholar]
- 227. Deribe YL, Wild P, Chandrashaker A, Curak J, Schmidt MH, Kalaidzidis Y, Milutinovic N, Kratchmarova I, Buerkle L, Fetchko MJ, Schmidt P, Kittanakom S, Brown KR, Jurisica I, Blagoev B, Zerial M, Stagljar I, Dikic I. 2009. Regulation of epidermal growth factor receptor trafficking by lysine deacetylase HDAC6. Sci Signal 2:ra84. [DOI] [PubMed] [Google Scholar]
- 228. Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. 2008. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res 68:6377–6386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Cheon H, Woo YS, Lee JY, Kim HS, Kim HJ, Cho S, Won NH, Sohn J. 2007. Signaling pathway for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced TNF-α production in differentiated THP-1 human macrophages. Exp Mol Med 39:524–534 [DOI] [PubMed] [Google Scholar]
- 230. Sumanasekera WK, Tien ES, Davis JW, 2nd, Turpey R, Perdew GH, Vanden Heuvel JP. 2003. Heat shock protein-90 (Hsp90) acts as a repressor of peroxisome proliferator-activated receptor-α (PPARα) and PPARβ activity. Biochemistry 42:10726–10735 [DOI] [PubMed] [Google Scholar]
- 231. Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH. 2003. Evidence that peroxisome proliferator-activated receptor α is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem 278:4467–4473 [DOI] [PubMed] [Google Scholar]
- 232. Froidevaux MS, Berg P, Seugnet I, Decherf S, Becker N, Sachs LM, Bilesimo P, Nygård M, Pongratz I, Demeneix BA. 2006. The co-chaperone XAP2 is required for activation of hypothalamic thyrotropin-releasing hormone transcription in vivo. EMBO Rep 7:1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Laenger A, Lang-Rollin I, Kozany C, Zschocke J, Zimmermann N, Rüegg J, Holsboer F, Hausch F, Rein T. 2009. XAP2 inhibits glucocorticoid receptor activity in mammalian cells. FEBS Lett 583:1493–1498 [DOI] [PubMed] [Google Scholar]
- 234. Huizenga NA, de Lange P, Koper JW, Clayton RN, Farrell WE, van der Lely AJ, Brinkmann AO, de Jong FH, Lamberts SW. 1998. Human adrenocorticotropin-secreting pituitary adenomas show frequent loss of heterozygosity at the glucocorticoid receptor gene locus. J Clin Endocrinol Metab 83:917–921 [DOI] [PubMed] [Google Scholar]
- 235. Kashuba E, Kashuba V, Pokrovskaja K, Klein G, Szekely L. 2000. Epstein-Barr virus encoded nuclear protein EBNA-3 binds XAP-2, a protein associated with hepatitis B virus X antigen. Oncogene 19:1801–1806 [DOI] [PubMed] [Google Scholar]
- 236. Kashuba EV, Gradin K, Isaguliants M, Szekely L, Poellinger L, Klein G, Kazlauskas A. 2006. Regulation of transactivation function of the aryl hydrocarbon receptor by the Epstein-Barr virus-encoded EBNA-3 protein. J Biol Chem 281:1215–1223 [DOI] [PubMed] [Google Scholar]
- 237. Yano M, Terada K, Mori M. 2003. AIP is a mitochondrial import mediator that binds to both import receptor Tom20 and preproteins. J Cell Biol 163:45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Zhao Y, Meng XM, Wei YJ, Zhao XW, Liu DQ, Cao HQ, Liew CC, Ding JF. 2003. Cloning and characterization of a novel cardiac-specific kinase that interacts specifically with cardiac troponin I. J Mol Med Berl 81:297–304 [DOI] [PubMed] [Google Scholar]
- 239. Nomikos P, Buchfelder M, Fahlbusch R. 2005. The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure.’ Eur J Endocrinol 152:379–387 [DOI] [PubMed] [Google Scholar]
- 240. Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK. 2011. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Acromegaly–2011 update: executive summary. Endocr Pract 17:636–646 [DOI] [PubMed] [Google Scholar]
- 241. Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D, Clemmons D, Chanson P, Laws E, Schlechte J, Vance ML, Ho K, Giustina A. 2009. Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94:1509–1517 [DOI] [PubMed] [Google Scholar]
- 242. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA. 2011. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 96:273–288 [DOI] [PubMed] [Google Scholar]
- 243. Fajardo-Montañana C, Daly AF, Riesgo-Suárez P, Gómez-Vela J, Tichomirowa MA, Camara-Gómez R, Beckers A. 2009. [AIP mutations in familial and sporadic pituitary adenomas: local experience and review of the literature]. Endocrinol Nutr 56:369–377 [DOI] [PubMed] [Google Scholar]
- 244. Igreja S, Chahal HS, Akker SA, Gueorguiev M, Popovic V, Damjanovic S, Burman P, Wass JA, Quinton R, Grossman AB, Korbonits M. 2009. Assessment of p27 (cyclin-dependent kinase inhibitor 1B) and aryl hydrocarbon receptor-interacting protein (AIP) genes in multiple endocrine neoplasia (MEN1) syndrome patients without any detectable MEN1 gene mutations. Clin Endocrinol (Oxf) 70:259–264 [DOI] [PubMed] [Google Scholar]
- 245. Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. 2004. The prevalence of pituitary adenomas: a systematic review. Cancer 101:613–619 [DOI] [PubMed] [Google Scholar]
- 246. Newey PJ, Thakker RV. 2011. Role of multiple endocrine neoplasia type 1 mutational analysis in clinical practice. Endocr Pract 17(Suppl 3):8–17 [DOI] [PubMed] [Google Scholar]
- 247. Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA, Jr, Marx SJ. 2001. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86:5658–5671 [DOI] [PubMed] [Google Scholar]
- 248. Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. 2012. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 97:2990–3011 [DOI] [PubMed] [Google Scholar]
- 249. Marinoni I, Pellegata NS. 2011. p27kip1: A new multiple endocrine neoplasia gene? Neuroendocrinology 93:19–28 [DOI] [PubMed] [Google Scholar]
- 250. Xekouki P, Pacak K, Almeida M, Wassif CA, Rustin P, Nesterova M, de la Luz Sierra M, Matro J, Ball E, Azevedo M, Horvath A, Lyssikatos C, Quezado M, Patronas N, Ferrando B, Pasini B, Lytras A, Tolis G, Stratakis CA. 2012. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab 97:E357–E366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Xekouki P, Stratakis CA. 2012. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocr Relat Cancer 19:C33–C40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Rothenbuhler A, Stratakis CA. 2010. Clinical and molecular genetics of Carney complex. Best Pract Res Clin Endocrinol Metab 24:389–399 [DOI] [PubMed] [Google Scholar]
- 253. Agarwal SK, Mateo CM, Marx SJ. 2009. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab 94:1826–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Cuny T, Pertuit M, Sahnoun-Fathallah M, Daly AF, Occhi G, Odou MF, Tabarin A, Nunes ML, Delemer B, Rohmer V, Desailloud R, Kerlan V, Chabre O, Sadoul JL, Cogne M, Caron P, Cortet C, Lienhardt-Roussie A, Raingeard I, Guedj AM, Brue T, Beckers A, Weryha G, Enjalbert A, Barlier A. 15 January 2013. Genetic analysis in young patients with sporadic pituitary macroadenomas: Beside AIP don't forget MEN1 genetic analysis. Eur J Endocrinol doi: 10.1530/EJE-12-0763 [DOI] [PubMed] [Google Scholar]