Abstract
We previously identified TD-60 (RCC2) as a mitotic centromere-associated protein that is necessary for proper completion of mitosis. We now report that TD-60 is an essential regulator of cell cycle progression during interphase. siRNA suppression blocks progression of mammalian G₁/S phase cells and progression of G₂ cells into mitosis. Prolonged arrest occurs both in non-transformed cells and in transformed cells lacking functional p53. TD-60 associates with Rac1 and Arf6 and has recently been demonstrated to be an element of α5β1 integrin and cortactin interactomes. These associations with known elements of cell cycle control, together with our data, suggest that TD-60 is an essential component of one or more signaling pathways that drive cell cycle progression. During mitosis, TD-60 is required for correct assembly of the mitotic spindle and activation of key mitotic proteins. In contrast, in interphase TD-60 promotes cell cycle progression through what must be distinct mechanisms. TD-60 thus appears to be one of the growing categories of proteins that “moonlight,” or have more than one distinct cellular function.
Keywords: microtubules, checkpoint, RCC2, G1 phase, G2 phase
Introduction
After mammalian cells pass the restriction point in the G1 phase of the cell cycle, they are committed to complete the cell cycle and pass into mitosis.1 Cell cycle arrest after the restriction point can be imposed by checkpoints that respond to DNA damage, failure to complete S phase or failure to decatenate DNA strands in G2. Checkpoint controls after the restriction point normally involve transient arrest by activation of the Atm/Atr control pathway, or more durable response by activation of p53 and p21, which suppress Cdk cyclin-dependent kinase activity.2 Typically, tumor cells with compromised or inactivated p53 exhibit only transient arrest in response to interphase DNA damage checkpoint activation.
We had previously purified and cloned TD-60 (RCC2) and demonstrated that it was an RCC1 homolog that binds Rac1. We further showed that it binds microtubules and is required for proper spindle assembly in mitosis.3 Antibodies revealed that it localized in mitosis with passenger proteins,4,5 a group of interacting proteins that associate with the inner centromere in prometaphase and metaphase and transfer to the midzone of the cell in anaphase and telophase.6 We also demonstrated that TD-60 is required for recruitment of the passenger proteins to the centromere and for proper spindle function.3 In its absence, mitotic cells arrested indefinitely in prometaphase. TD-60 binds and activates the passenger protein Aurora B, and is required for the activation of another centromeric protein kinase, haspin.7
The homolog of TD-60, RCC1, is important to proper cell cycle progression both in interphase and in mitosis.8,9 However, its role in spindle assembly in mitosis is quite distinct from its role suppressing mitosis before DNA replication completes in interphase.10 Recent evidence indicated TD-60 was a key component in interactomes involved in cell signaling and interphase cell cycle progression, including α5β1 integrins11 and cortactin,12 suggesting it might, like RCC1, have a distinct function in interphase. We thus addressed whether TD-60 has a regulatory effect on the interphase cell cycle.
We now report that TD-60 plays an important role in interphase cell cycle progression. Following siRNA suppression of TD-60, mammalian cells cease to proliferate and arrest either in G1/S or G2 phases of the cell cycle. Our data suggest that TD-60 plays a functionally important role in regulating the signaling pathways that drive cell cycle progression.
Results
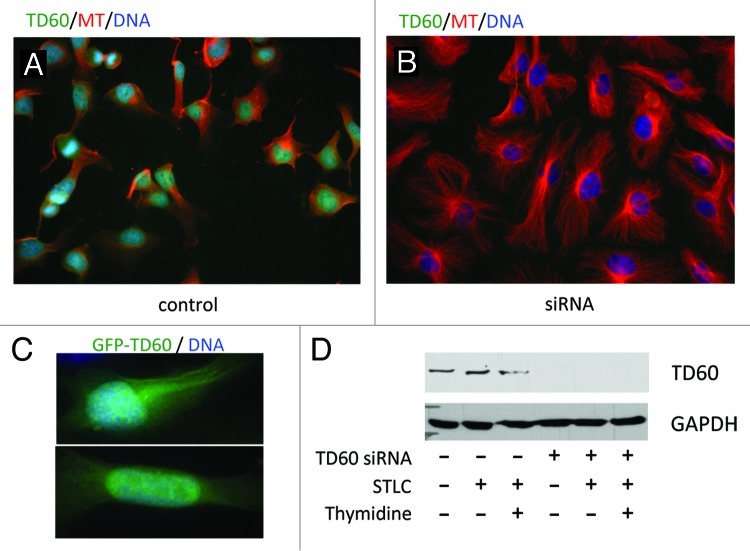
Transfection of human cells with siRNA to TD-60 is highly effective, blocking expression in the entire cell population as assayed by immunofluorescence microscopy at 72 h after transfection (Fig. 1A and B). Suppression of TD-60 provokes multiple effects in transfected HeLa cells. There is a striking increase in interphase cell spreading and a marked increase in the abundance and length of microtubules compared with control cells (Figs. 1 and 2), sometimes filling cell extensions that do not occur in controls (see for example Fig. 2B). GFP-TD-60 preferentially localizes to the nucleus and to the microtubules of interphase cells (Fig. 1C). The siRNA effectively suppresses TD-60, as assayed by western blot (Fig. 1D).
Figure 1. Knockdown of TD-60 is effective. (A) Control sham transfected HeLa cells, stained for TD-60, show a clear nuclear TD-60 stain and normal HeLa morphology. (B) HeLa cells transfected with siRNA to TD-60 and examined 72 h later have lost TD-60 stain at the same microscope settings and show augmented cell size and hyperabundant microtubules. Cells were stained with anti-tubulin and anti-TD-60 polyclonal antibodies and DNA was stained with Hoechst. (C) Ectopically expressed GFP-TD-60 localizes to the nucleus and to cytoplasmic microtubule arrays. Counterstain was Hoechst for DNA. (D) Immunoblot analysis of TD-60 in siRNA-treated HeLa cells indicates effective suppression in different assay conditions. GAPDH is a loading control. Times after transfection and drug treatment are as in Figure 3
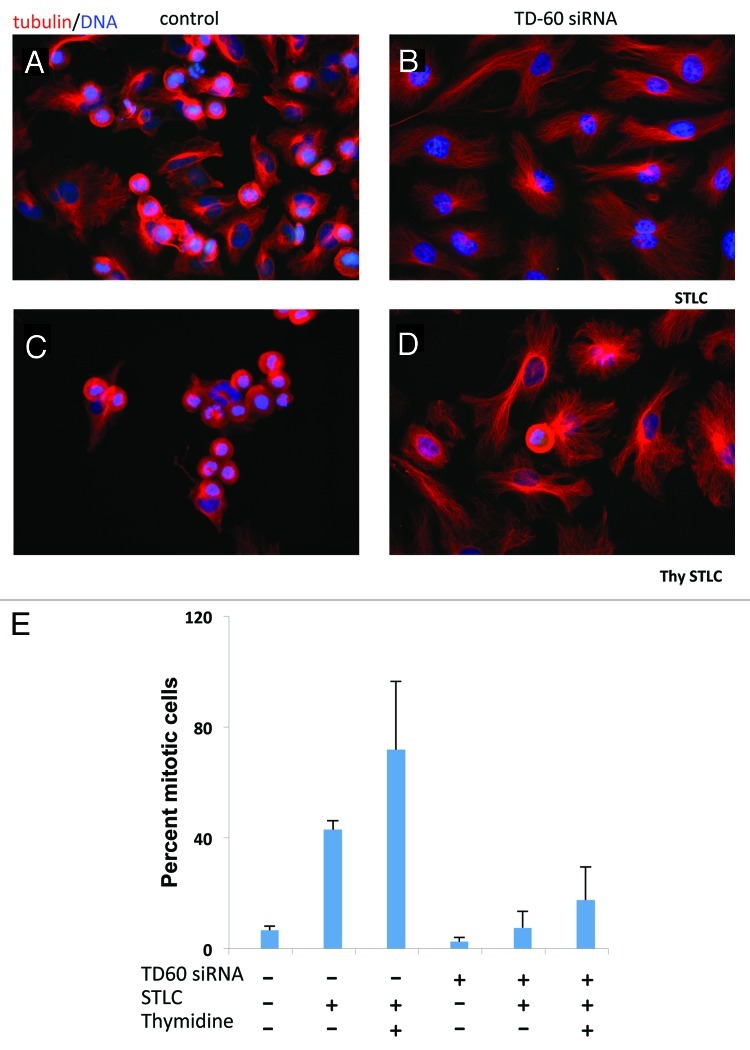
Figure 2. TD-60 knockdown suppresses mitotic entry. (A–D) HeLa cells were transfected with TD-60 siRNA or sham transfected. After 48 h, cells were treated with STLC (10 μM) for 14 h (A and B). Alternatively, after 24 h, cells were presynchronized in G1/S phase with 2 mM thymidine (18 h), and released. Six hours after release STLC (10 μM) was added for 14 h (C and D). Cells were fixed and stained with anti-tubulin and Hoechst. Control sham transfected cells (A and C) arrest abundantly in mitosis while TD-60 knockdown cells (B and D) remain in interphase. (E) Quantitation of mitotic entry. Cells were treated as in (A–D) and fixed and stained with anti-MPM2 and Hoechst. Cells with condensed chromosomes and MPM2 positive signal were scored as mitotic cells. Results are mean ± SD of two experiments.
We noted that the mitotic figures that are frequent in sham-transfected controls are virtually absent in transfected cells by 48 to 72 h after transfection (Fig. 1). We therefore assayed for the capacity of TD-60 siRNA transfected HeLa cells to enter mitosis. The result was striking. Control cells exposed for 14 h to S-trityl-L-cysteine (STLC), an Eg5 microtubule motor protein inhibitor that blocks cells in mitosis with a single aster,13 yielded abundant mitotic arrest, with cell rounding, chromosome condensation and formation of a spindle aster (Fig. 2A). In contrast, few of the TD-60 siRNA-transfected cells exposed to STLC were mitotic (Fig. 2B). To enhance mitotic arrest, we presynchronized cells by G1/S arrest with thymidine and then released the cells into STLC. In this case also, the doubly synchronized controls were largely mitotic (Fig. 2C), while TD-60 siRNA-transfected cells remained in interphase (Fig. 2D).
Control cells were approximately 43% mitotic after STLC exposure alone and 72% mitotic after thymidine presynchronization followed by STLC (Fig. 2E). In contrast, TD-60 siRNA-transfected cells were less than 10% mitotic (STLC) and 17% mitotic with thymidine followed by STLC. siRNA suppressed TD-60 under all synchronization protocols (Fig. 1D).
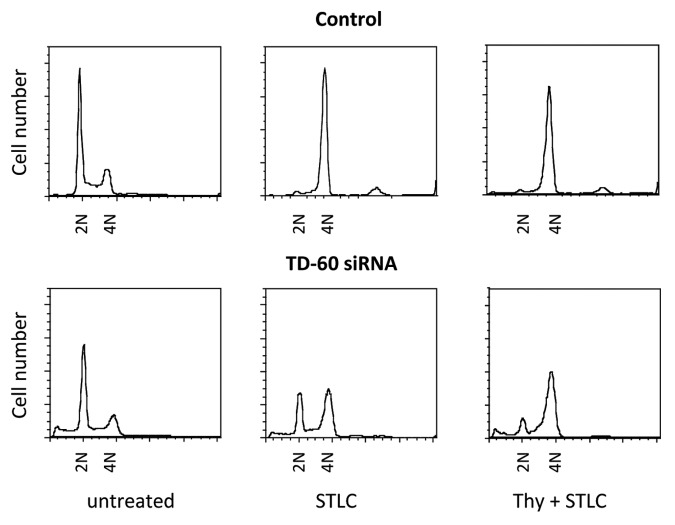
Flow cytometry of cells treated with either STLC alone, or presynchronized, showed that control cells arrested in 4N, as expected of mitotically arrested cells (Fig. 3). TD-60 siRNA-transfected cells arrest with 4N status with thymidine presynchronization, then STLC exposure (Fig. 3), although they are not mitotic (Fig. 2). Microscopic evidence shows that the cells have not proceeded through mitosis to a following G1, as their interphase nuclei are not fragmented, which would be indicative of mitotic catastrophe. On exposure to STLC alone the TD-60 siRNA-treated population is approximately equally divided between 2N and 4N arrested cells (Fig. 3). These results show that siRNA-suppressed cells may be blocked either at G1/S or G2, depending on their position in the cell cycle when TD-60 expression is lost. The ungated flow cytometry data also reveal the presence of a small but reproducible amount of cell death, as indicated by the < 2N population. In accord with cell cycle arrest, apparent in both microscopic and flow cytometry data, the siRNA population rapidly ceases to proliferate after transfection (Fig. 4A).
Figure 3. TD-60 knockdown suppresses cell cycle progression both in G1/S and G2. HeLa cells were transfected with TD-60 siRNA and assayed by flow cytometry. Samples were treated either with STLC alone or with 2 mM thymidine for 18 h, released for 6 h and then with STLC for 14 h. Untreated controls show normal cell cycle distribution. Controls synchronized with STLC alone, or thymidine then STLC, show a 4N peak. TD-60 knockdown without synchronization (lower left) yields largely 2N cells. TD-60 knockdown followed by STLC synchronization shows a substantial 2N population, as well as 4N cells. Tandem thymidine and STLC treatment yields a mixed 2N/4N population.
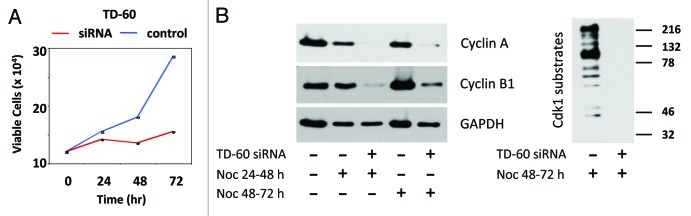
Figure 4. TD-60 knockdown suppresses proliferation and mitotic markers. (A) Quantitative assay of HeLa cells following siRNA transfection shows the cell population ceases proliferation and remains blocked for 3 days. Viable cells were counted relative to time 0 (time of transfection). The control was a sham transfection. (B) Immunoblot analysis of Cyclin B1 and Cyclin A of TD-60 siRNA-transfected cells treated with nocodazole (0.04 μg/ml) from 24 to 48 h or 48 h to 72 h (left), and of Cdk1 phospho-substrates after nocodazole treatment from 48 h to 72 h (right). The blots confirm absence of mitotic markers and Cdk1 phospho-substrates after TD-60 depletion.
Consistent with the low number of mitotic cells recorded, cyclin A1 and cyclin B, two proteins that are required for mitotic entry,14 were substantially suppressed in transfected cells exposed to nocodazole, a microtubule inhibitor that was used to create a mitotic trap (Fig. 4B, left). Similarly, mitotic Cdk1 protein kinase phospho-substrates were suppressed compared with mitotic controls (Fig. 4B, right). This ladder of Cdk1 substrates occurs only in cells that are in mitosis, as previously demonstrated.15
The above assays were performed in HeLa cells. Parallel suppression of the cell cycle is evident in non-transformed human fibroblasts. Movies of siRNA-transfected cells indicate that mitosis, evident in controls (Vid. S1), is virtually absent in transfected cells (Vid. S2) recorded over a 24 h period.
Discussion
To summarize, using analysis from a variety of approaches, our results have revealed that TD-60 has a substantial interphase role in cell cycle progression in mammalian cells. In the absence of TD-60 expression, cells cease to proliferate and arrest either in the G1 or G2 phase of the cell cycle.
The reason for the cell cycle arrest in interphase is unknown. The reported interactions of TD-60 with several proteins and protein interactomes offer possible mechanisms that remain to be tested. Among the reported interactions we, and others, have found that it binds to the Rho family GTPase Rac13,11 and to the small G-protein Arf6,11 that it binds and activates the mitotic kinase Aurora B,7 and that it associates with microtubules in vitro.3,7
In addition, recent interactome assays have demonstrated that TD-60 associates with two important components of focal adhesion plaques,16 α5β1 integrin and cortactin.11,12 Its association with α5β1 integrin and regulation of both Rac1 and Arf6 led to speculation that it was a key nodal protein that integrates two distinct cell regulatory circuits that are regulated by either Rac1 or Arf6.11
Thus, TD-60 is linked to protein complexes involved in cell signaling and cell cycle progression.11,12 Our evidence clearly links TD-60 to cell cycle control, and these interactions suggest one or more of these pathways may be important to its interphase function. Its association with both α5β1 integrin and Rac1 is particularly interesting, as α5β1 integrin has been linked to Rac1-dependent activation of cell cycle progression.17-19
We previously discovered that suppression of TD-60 blocked mitotic cells indefinitely in prometaphase.3 Combined with the present results, it thus appears that absence of TD-60 freezes cell cycle progression at multiple junctures, and that it thus plays a functionally important role throughout the cell cycle. Based on the evidence that TD-60 is important both in mitosis and at focal adhesions in interphase, it has previously been noted that it might exhibit multifunctional “moonlighting” behavior.20 Proteins that moonlight, or have more than one discrete function in the cell, can switch functions through change in intracellular localization, change of binding partners or post-translational modification.21 Importantly, the close paralog of TD-60, RCC1, is also a moonlighting protein that controls DNA replication10 and nucleocytoplasmic transport in interphase22-24 and regulates bipolar spindle assembly by creating a GTP-Ran gradient in the mitotic cell.9,25 Both RCC1 and TD-60 regulate the interphase cell cycle and mitotic spindle assembly. It will be interesting to know if the two paralogs have additional similarities.
We will, in future work, address which of the known regulatory protein interactions with TD-60 underpin the profound effect that it has on cell cycle progression and on cell proliferation.
Materials and Methods
Materials, cell culture and transfection
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and Trypsin-EDTA were purchased from Mediatech. Lipofectamine 2000, Lipofectamine RNAiMAX, Hoechst 33258 and Alexa fluor-conjugated secondary antibodies were purchased from Life Technologies. Rabbit monoclonal anti-TD-60 and rabbit polyclonal anti-phospho-serine CDK substrate antibodies were purchased from Cell Signaling. Rabbit polyclonal anti-GAPDH was from Abcam. Rabbit anti-cyclin A and mouse monoclonal anti-cyclin B1 were from Upstate. TD-60 coding region in pcDNA-TD-603 was amplified and cloned into GFP-pIC113 vector.26 Eight-chamber glass slides were from Labtek (Thermo Fisher). All other chemicals were from Sigma-Aldrich unless otherwise noted. HeLa cells and primary human foreskin fibroblasts (HFF) were cultured in DMEM with 10% FBS in a humidified incubator (37°C, 5% CO2). All siRNA (Qiagen) transfections used 30 nM siRNA (final) with Lipofectamine RNAiMax. TD-60 siRNA sequence was AACAGCAAGCTGCTTACCGCA. For transfection, Lipofectamine 2000 was mixed with 2 μg DNA per well of a 6-well plate according to manufacturer’s instructions. For all fixed cell microscopy, coverslips were pre-coated with poly-L-lysine.
Cell synchronization
Cells were seeded (5 × 104 per well) in 6-well plates and allowed to adhere for 24 h then transfected with siRNA, then either were harvested directly after transfection or after synchronization, as specified in figure legends. For G1/S phase synchronization, cells were incubated with thymidine (2 mM, 18 h), released for 6 h in drug-free medium, and then STLC (10 μM) was added for 14 h.
Cell proliferation assay
After siRNA transfection, cells were collected at 24, 48 and 72 h, and viable cells were counted by trypan blue exclusion.
Immunofluorescence
HeLa cells were seeded on coverslips. After transfection and/or synchronization, coverslips were fixed using 3.7% paraformaldehyde in PBS for 20 min, and then were permeabilized and blocked overnight at 4°C with 1% goat serum, followed by incubation in primary antibody for 1 h at room temperature. Primary antibodies including mouse anti-α-tubulin, and rabbit anti-TD-60 were used at 1:500 and anti-MPM-2 at 1:100. After washing, cells were incubated with Alexa Fluor secondary antibodies for 1 h at room temperature. Hoechst (10 μg/mL) was used to stain DNA and coverslips were mounted with Clarion mounting media. Stained cells were examined with a Delta Vision Deconvolution microscope (Applied Precision). To observe GFP-TD-60, HeLa cells were transfected with GFP-pIC113-TD-60 and after 72 h, cells were fixed as described above.
Mitotic index
Cells grown on coverslips were transfected with TD-60 siRNA, and synchronized as described above. Cells were then fixed and immunostained using anti-MPM-2 antibody and Hoechst. Cells positive for MPM-2 and containing condensed chromosomes were counted as mitotic.
Flow cytometry
HeLa cells were treated with TD-60 siRNA and synchronized as described above. Synchronized cells were harvested using trypsin-EDTA, washed and fixed in ice-cold methanol. Cells were permeabilized and stained with propidium iodide, as described,15 and analyzed by FACS (FACSort, Becton Dickinson) using FloJo software.
Immunoblotting
Cell lysates (25 μg/well) were resolved by PAGE, transferred to PVDF membranes and detected using chemiluminescence (Thermo Scientific Pierce). All primary antibodies were used at 1:1,000, except anti-GAPDH, which was used at 1:2,000. Appropriate secondary HRP conjugated antibodies were used at 1:10,000.
Video microscopy
TD-60 was knocked down in HFF seeded in 6-well plates. Forty-eight h after transfection, cells were replated in fibronectin coated 8-well glass chambers (1.0 × 104 cells/well), allowed to adhere (24 h), and then recorded with a 20× objective. The Delta Vision microscope stage was maintained at 37°C with 5% CO2. Videos were recorded at 10 min intervals for 24 h.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01GM068107 and R01GM088716 (R.L.M.) and R01CA108947 (R.F.).
Glossary
Abbreviations:
- STLC
S-trityl-L-cysteine
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/cc/article/23821
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/23821
References
- 1.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–8. doi: 10.1016/S0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 2.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–96. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 3.Mollinari C, Reynaud C, Martineau-Thuillier S, Monier S, Kieffer S, Garin J, et al. The mammalian passenger protein TD-60 is an RCC1 family member with an essential role in prometaphase to metaphase progression. Dev Cell. 2003;5:295–307. doi: 10.1016/S1534-5807(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 4.Andreassen PR, Palmer DK, Wener MH, Margolis RL. Telophase disc: a new mammalian mitotic organelle that bisects telophase cells with a possible function in cytokinesis. J Cell Sci. 1991;99:523–34. doi: 10.1242/jcs.99.3.523. [DOI] [PubMed] [Google Scholar]
- 5.Martineau-Thuillier S, Andreassen PR, Margolis RL. Colocalization of TD-60 and INCENP throughout G2 and mitosis: evidence for their possible interaction in signalling cytokinesis. Chromosoma. 1998;107:461–70. doi: 10.1007/s004120050330. [DOI] [PubMed] [Google Scholar]
- 6.Adams RR, Carmena M, Earnshaw WC. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/S0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- 7.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–72. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 8.Nishitani H, Ohtsubo M, Yamashita K, Iida H, Pines J, Yasudo H, et al. Loss of RCC1, a nuclear DNA-binding protein, uncouples the completion of DNA replication from the activation of cdc2 protein kinase and mitosis. EMBO J. 1991;10:1555–64. doi: 10.1002/j.1460-2075.1991.tb07675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature. 1999;400:178–81. doi: 10.1038/22133. [DOI] [PubMed] [Google Scholar]
- 10.Dasso M, Nishitani H, Kornbluth S, Nishimoto T, Newport JW. RCC1, a regulator of mitosis, is essential for DNA replication. Mol Cell Biol. 1992;12:3337–45. doi: 10.1128/mcb.12.8.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, et al. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;2:ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigera PR, Ma L, Borgman CA, Pinto AF, Sherman NE, Parsons JT, et al. Mass spectrometric analysis identifies a cortactin-RCC2/TD60 interaction in mitotic cells. J Proteomics. 2012;75:2153–9. doi: 10.1016/j.jprot.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBonis S, Skoufias DA, Lebeau L, Lopez R, Robin G, Margolis RL, et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol Cancer Ther. 2004;3:1079–90. [PubMed] [Google Scholar]
- 14.Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–91. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 15.Skoufias DA, Indorato RL, Lacroix F, Panopoulos A, Margolis RL. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J Cell Biol. 2007;179:671–85. doi: 10.1083/jcb.200704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Liu Y, Liao K. Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility. BMC Cell Biol. 2011;12:49. doi: 10.1186/1471-2121-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6:725–40. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–60. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 19.Jeanes AI, Wang P, Moreno-Layseca P, Paul N, Cheung J, Tsang R, et al. Specific β-containing integrins exert differential control on proliferation and two-dimensional collective cell migration in mammary epithelial cells. J Biol Chem. 2012;287:24103–12. doi: 10.1074/jbc.M112.360834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byron A, Humphries JD, Humphries MJ. Alternative cellular roles for proteins identified using proteomics. J Proteomics. 2012;75:4184–5. doi: 10.1016/j.jprot.2012.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeffery CJ. Molecular mechanisms for multitasking: recent crystal structures of moonlighting proteins. Curr Opin Struct Biol. 2004;14:663–8. doi: 10.1016/j.sbi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Tachibana T, Imamoto N, Seino H, Nishimoto T, Yoneda Y. Loss of RCC1 leads to suppression of nuclear protein import in living cells. J Biol Chem. 1994;269:24542–5. [PubMed] [Google Scholar]
- 23.Kuersten S, Ohno M, Mattaj IW. Nucleocytoplasmic transport: Ran, beta and beyond. Trends Cell Biol. 2001;11:497–503. doi: 10.1016/S0962-8924(01)02144-4. [DOI] [PubMed] [Google Scholar]
- 24.Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG. Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B. Science. 2001;292:1540–3. doi: 10.1126/science.292.5521.1540. [DOI] [PubMed] [Google Scholar]
- 25.Li HY, Zheng Y. Phosphorylation of RCC1 in mitosis is essential for producing a high RanGTP concentration on chromosomes and for spindle assembly in mammalian cells. Genes Dev. 2004;18:512–27. doi: 10.1101/gad.1177304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheeseman IM, Desai A. A combined approach for the localization and tandem affinity purification of protein complexes from metazoans. Sci STKE. 2005;2005:pl1. doi: 10.1126/stke.2662005pl1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.