Abstract
The incidence of opportunistic fungal infections has increased in recent decades due to the growing proportion of immunocompromised patients in our society. Candida krusei has been described as a causative agent of disseminated fungal infections in susceptible patients. Although its prevalence remains low among yeast infections (2–5%), its intrinsic resistance to fluconazole makes this yeast important from epidemiologic aspects. Non mammalian organisms are feasible models to study fungal virulence and drug efficacy. In this work we have used the lepidopteran Galleria mellonella and the nematode Caenorhabditis elegans as models to assess antifungal efficacy during infection by C. krusei. This yeast killed G. mellonella at 25, 30 and 37°C and reduced haemocytic density. Infected larvae melanized in a dose-dependent manner. Fluconazole did not protect against C. krusei infection, in contrast to amphotericin B, voriconazole or caspofungin. However, the doses of these antifungals required to obtain larvae protection were always higher during C. krusei infection than during C. albicans infection. Similar results were found in the model host C. elegans. Our work demonstrates that non mammalian models are useful tools to investigate in vivo antifungal efficacy and virulence of C. krusei.
Introduction
Fungal infections have emerged worldwide due to a growing population of immunosuppressed patients, including patients with cancer, AIDS, solid-organ and hematopoietic stem cell transplant recipients, premature neonates, and patients recovering from major surgery [1]–[5]. These infections have significant morbidity and mortality rates and are difficult to prevent, diagnose and treat [6]–[8].
Candida spp are commensal yeasts responsible for different clinical manifestations, from mucocutaneous overgrowth to blood stream infections [1], [9]–[12]. Candida albicans is still the major cause of invasive fungal disease. However, a growing number of infections produced by non-albicans Candida spp has been reported in the last years [1], [13]–[15]. Among them, there are some species that are intrinsically resistant or have reduced susceptibility to antifungals. The massive use of antifungals in prophylaxis, such as fluconazole, has facilitated the selection of pathogenic fungi resistant to these agents [16]–[19].
Candida krusei is an opportunistic pathogen which presents intrinsic resistance to fluconazole. The infection is associated with the prophylactic or therapeutic use of this antifungal agent [20]–[23]. Two mechanisms of azole resistance in C. krusei have been described: overexpression of drug efflux pumps [24] and diminished sensitivity of the target enzyme, the cytochrome P450 sterol 14-demethylase (encoded by the CYP51 gene) [25]. Diseases caused by C. krusei have high associated mortality (30–60%) [26], [27]. Despite the intrinsic resistance to fluconazole, C. krusei is usually susceptible to voriconazole in vitro, which correlates with the binding of this drug to the target enzyme [21].
Antifungal resistance in vitro does not always correlate with clinical resistance. The best correlation between in vitro and clinical efficacy is found in HIV-positive patients with oropharyngeal candidiasis [22], [28], [29]. In contrast, although C. parapsilosis shows reduced in vitro susceptibility to echinocandins, these antifungals have been shown to be effective in the treatment of invasive candidiasis caused by this species [30].
The use of invertebrate hosts has recently emerged and facilitated the study of fungal pathogenesis. Among these non-mammalian hosts, amoebae (Acanthamoeba castellanii, Dictyostellium discoideum), nematodes (Caenorhabditis elegans) and insects (Drosophila melanogaster, Galleria mellonella) have been successfully used to study the virulence of some fungi [31]–[35]. Moreover, some aspects of the innate response are conserved between these hosts and mammals [36]. Galleria mellonella is a lepidopteran (Pyralidae) that provides important advantages as host model. The larvae can be incubated in a range of temperature between 25 to 37°C, so it is possible to simulate the natural fungal habitat and the infection conditions in mammals. In addition, as in mammalian models, it is possible to introduce by injection exact doses of pathogens to the larvae, which poses a technical improvement over other non-conventional hosts. Galleria mellonella has six types of phagocytic cells that play an important role in the defense system [37], [38]. The density of these cells in the haemolymph is not constant, and changes during infection can be easily measured and used as a parameter of the response of the larvae after exposure to pathogens [39]. The viability of the larvae can be easily recorded by the lack of movement and the massive melanization induced by G. mellonella in response to infection [40]–[42]. Another organism that is used as model host is the soil nematode Caenorhabditis elegans, which feeds on microorganisms, but is susceptible to bacterial and fungal pathogens [33], [43]–[45]. Caenorhabditis elegans has been used to study virulence, filamentation and antifungal efficacy of antifungal drugs [44], [46].
In this study, we initially aimed to characterize the interaction between G. mellonella and C. krusei with two purposes: 1) To get insights about virulence traits of this pathogenic yeast, and 2) to investigate if antifungal efficacy in vivo correlates with the susceptibility profile shown by C. krusei in vitro. Furthermore, we have complemented our studies with C. elegans, and observed similar behaviors, indicating that non-conventional models can be used to investigate C. krusei virulence and antifungal efficacy.
Materials and Methods
Strains and media
Candida albicans SC5314 [47], C. krusei ATCC 6258 and two clinical isolates (CL8053 and CL80317) from the Yeast Collection of the Mycology Reference Laboratory of the Spanish National Centre for Microbiology and Cryptococcus neoformans variety grubii (H99 strain, ATCC 20882) were used in this study. The yeasts were grown overnight in liquid Sabouraud medium (Difco, BD, USA) at 30°C with shaking. Escherichia coli OP50 strain was obtained from the Caenorhabditis Genetics Center (University of Minnesota) and was maintained on LB agar plates at 37°C.
Antifungal susceptibility testing (AFST)
Minimum inhibitory concentration (MIC) values were determined using the EUCAST protocol [48], [49]. For AFST, 10 different clinical isolates of C. albicans and 10 clinical isolates of C. krusei were obtained from the yeast collection of the Mycology Reference Laboratory of the Spanish National Centre for Microbiology. Data were expressed as geometric mean, mode, range (minimum-maximum) and MIC frequency distribution.
Insect larvae manipulation and incubation conditions
Galleria mellonella larvae (0.3–0.5 g, R.J. Mous Livebait, The Netherlands) were placed in Petri dishes and incubated at 37°C in the dark the night before the experiments. Larvae with color alterations (i.e. dark spots or with apparent melanization) were excluded. Antifungals and yeast suspensions were injected in the haemocele through the last left pro-leg of the larvae using a 10 µL Hamilton syringe (Hamiltion, USA). The pro-leg had been previously cleaned with 70% ethanol. A total of 10 µL were injected in each larva. Larvae death was monitored by visual inspection of the color (brown-dark brown) and by lack of movement after touching them with forceps. For each condition, a total of 20 larvae were used, and each experiment was repeated at least twice. After infection, larvae were incubated at 25, 30 or 37°C.
Survival assay
Yeasts were grown overnight in liquid Sabouraud, washed with PBS, and suspended in the same buffer. Cell density was estimated with an Automatic Cell Counter TC10 (Bio Rad). For survival assays, larvae were inoculated with 107, 5×106 and 2.5×106 cells/larva of C. krusei and 106, 5×105 and 105 cells/larva of C. albicans. The inocula were prepared in PBS plus 20 mg/L of ampicillin to prevent bacterial contamination. The infected larvae were incubated at 25°C, 30 or 37°C, and the death was daily monitored during 7 days.
Growth curve at different temperatures
Yeast strains were grown overnight and diluted in fresh Sabouraud liquid medium at 103 cells/mL. Two hundred microliters of this suspension were placed in 96-well microdilution plates, and incubated at 25, 30 or 37°C in a Labsystems IEMS Reader MF spectrophotometer. Optical density (OD) was determined at 530 nm every hour during 72 hrs.
In vivo phagocytosis assay
Yeast cells were stained with 10 µg/mL Calcofluor white (Sigma, St. Louis, MO) for 30 min at 37°C. Then, these cells were injected into G. mellonella larvae (107 cells/larva, 5 per group), and phagocytosis was analyzed after 3 hrs of incubation at 25 and 37°C. Haemolymph was collected in 1.5 mL tubes and diluted 1∶1 in IPS buffer (Insect Physiological Saline: 150 mM sodium chloride, 5 mM potassium chloride, 10 mM Tris-HCl pH 6.9, 10 mM EDTA and 30 mM sodium citrate) to avoid coagulation and melanization of the haemolymph. Haemocytes were placed on a slide and phagocytosis was visually quantified using a Leica DMI 3000B microscope. One hundred haemocytes from each larva were counted in each case, and the percentage of haemocytes containing yeasts was calculated and plotted. Cryptococcus neoformans H99 strain was used as control. Phagocytosis was also analyzed in larvae infected in the same way, but treated with 64 mg/kg fluconazole or 4 mg/kg amphotericin B.
Determination of haemocyte density
Groups of five G. mellonella were infected with 107 yeast cells/larvae and incubated at 37°C for 3 hrs. The haemolymph of each larva was collected in 1.5 mL tubes and diluted 1∶10 in IPS buffer. The cells were counted using a haemocytometer.
Measurement of in vivo filament formation
Galleria mellonella was infected with 107 cells/larva of C. albicans and C. krusei strains. The larvae were incubated at 37°C for 24 hours. Larvae were macerated in 100 µm nylon cell strainers (Falcon, BD, USA) with 1 mL of IPS. The liquid was then collected, centrifuged and suspended in 1 mL of the same buffer. Samples were stained with Calcofluor white (Sigma, St. Louis, MO), as described above, and yeast morphology was observed with a Leica DMI 3000B fluorescence microscope.
Melanization quantification
Larvae were infected with PBS, 5×105, 106 and 5×106 cells/larva of C. krusei. Then, the haemolymph of each larva was collected after 3 and 24 hrs in 1.5 mL tubes and diluted 1∶10 with IPS buffer. The samples were placed in 96 well microdilution plates. To quantify melanin levels, we took advantage of existing protocols that quantify laccase activity by detecting the final product of the reaction by measuring the OD in the visible range (400–500) [50], [51]. In our conditions, we observed that 405 nm was an optimal OD to quantify larval melanin and to correlate the results with the visualization of the dark compound. So the OD at 405 nm was measured using a Labsystems IEMS Reader MF spectophotometer. Melanization of larvae infected with C. albicans (5×105 cells/larva) and C. krusei (5×106 cells/larva) and treated with 64 mg/kg fluconazole and 4 mg/kg amphotericin B was also evaluated.
Treatment with antifungal drugs
Infected larvae were treated with amphotericin B (1, 2 or 4 mg/kg, Sigma Aldrich Quimica, Madrid, Spain), fluconazole (128, 64, 32, 12, or 4 mg/kg, Pfizer SA, Madrid, spain), voriconazole (7.5 or 10 mg/kg, Pfizer SA, Madrid, Spain) or caspofungin (1, 2 or 4 mg/kg Merck & Com, Inc, NJ, USA). In some experiments, a combination of fluconazole and amphotericin B was also used. Antifungals were applied immediately after the infection. Groups of 10 larvae were treated with the antifungals alone to test the toxicity.
Fungal burden determination
Infected larvae were selected at different times of infection, washed with 70% ethanol and cut into small pieces with a scalpel. Two mL of PBS-ampicillin were added and the mix was homogenized gently with a vortex and glass beads for 10 seconds. The mix was finally suspended in 9 mL of PBS-ampicillin. Different dilutions were made for each sample and 50 µL from these dilutions were placed on Sabouraud-cloramphenicol agar plates (Oxoid). The plates were incubated at 37°C for 48 h, and the number of colony forming units (CFUs) was determined.
Histology
Three larvae from different groups (uninfected, infected and/or treated with antifungals) were collected on different days of the infection. The larvae were preserved in 70% ethanol and longitudinal incisions were made with a scalpel in the dorsal part. The samples were fixed with 10% buffered formaline for 24 hrs. Then, the samples were dehydrated with increasing concentrations of ethanol, rinsed with xylol, and embedded in paraffin. Tissue sections (5 microns) were stained with periodic acid Schiff (PAS) solution and observed with a Leica DMI3000 microscope.
Caenorhabditis elegans strain and infection conditions
The following C. elegans mutant strain, obtained from CGC, was used in all experiments: AU37 (glp-4(bn2) I; sek-1(km4) X). This strain was grown on agar plates seeded with E. coli OP50 and incubated at 15°C according to standard procedures [52]. This strain is usually chosen for virulence and antifungal efficacy assays because glp-4 mutants are sterile at 25°C. This allows to easily following up the survival of the individual animals from the beginning to the end of the experiment and avoids mixing with their progeny [33], [44]. The sek-1 gene encodes a mitogen-activated protein kinase which is important for the defense of C. elegans against microbial infections [33], [44]. Therefore worms defective for sek-1 are more susceptible to infection and die earlier than wild-type C. elegans animals. Candida strains were cultivated in liquid Sabouraud medium (Difco, BD, USA) at 35°C with shaking. One hundred µL from this culture were inoculated on solid BHI media (Difco) containing kanamycin (90 µg/mL) and ampicillin (200 µg/mL) and incubated at 30°C for 24 hours. Synchronized worms in the L4 stage were added to the center of the agar plates inoculated with the yeast strains lawns and incubated for three hours at 25°C. In parallel, L4 worms were placed on agar plates containing lawns of E. coli OP50 strain. After the three hours incubation, worms were washed with M9 and transferred to 12-well plates with 1 mL 60% M9 buffer [45], 40% BHI, 10 µg/mL cholesterol in ethanol, 200 µg/mL ampicilin and 90 µg/mL kanamycin. Around 20–30 worms were placed in each well. For antifungal efficacy, amphotericin B (1 and 2 µg/mL), fluconazole (12 µg/mL), voriconazole (0.25, 7.5 and 10 µg/mL), caspofungin (2, 4 and 6 µg/mL), or a combination of amphotericin B (1 µg/mL) plus fluconazole (12 µg/mL) were added to the media. Plates were incubated at 25°C and individual worm survival was monitored daily. Nematodes were considered dead when they did not respond to touching. A minimum of two independent experiments was carried out for each treatment. Images were captured with a video camera (JVC KY-F550) attached to a dissecting microscope (Leica MZ7.5).
Statistics
Graphs and Statistics analyzes were performed with Graph Pad Prisma 5 (La Jolla CA, USA). Survival curves were analyzed by Log-rank (Mantel-Cox) Test and phagocytosis assays, haemocyte density, melanization quantification and fungal burden were analyzed using t-Test.
Results
Candida krusei killed G. mellonella in a dose dependant manner
We first investigated if C. krusei killed G. mellonella. Our results showed that G. mellonella is susceptible to C. krusei infection (Figure 1A). The death rate of the larvae depended on the yeast dose injected. Most reproducible results were found when larvae were infected with 5×106 C. krusei cells. However, C. krusei was less virulent than other fungi, such as C. albicans, which killed G. mellonella at lower doses (5×105, Figure 1B). To confirm that the death was not a consequence of a shock due to big amounts of yeast injected in the larvae, we inoculated a group of larvae with yeast inactivated by incubation in 4% paraformaldehyde. As shown in Figure 1C, inactivated yeast did not kill G. mellonella, confirming that larvae death was dependent on living yeast.
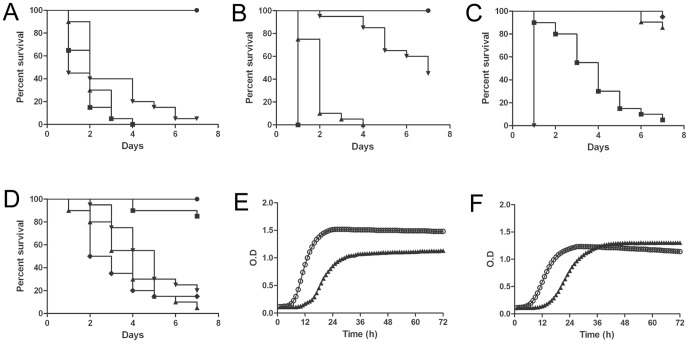
Figure 1. Comparison of the virulence of C. krusei and C. albicans in G. mellonella.
(A) Survival curve of G. mellonella infected with different inocula of C. krusei ATCC 6258 • PBS; ▪ 107 cells/larva; ▴ 5×106 cells/larva;▾ 2.5×106 cells/larva incubated at 37°C (B). Survival curve of G. mellonella infected with different inocula of C. albicans SC5314 • PBS; ▪ 106 cells/larva; ▴ 5×105 cells/larva; ▾105 cells/larvae (C) Survival of G. mellonella infected with inactivated yeast. Control dead cells • PBS; ▪ C. krusei ATCC 6258 5×106 cells/larva; ▴ C. krusei ATCC 6258 5×106 cells/larva (dead); ▾ C. albicans SC5314 106 cells/larva; ♦ C. albicans SC5314 106 cells/larva (dead) (D); Effect of the incubation temperature on the virulence of C. albicans and C. krusei. • PBS; ▴ C. krusei ATCC 6258 (37°C); ▾C. krusei ATCC 6258 (30°C); ♦ C. albicans SC5314 (37°C); ▪C. albicans SC5314 (30°C); Growth curves of C. albicans (E) and C. krusei (F) at different temperatures. ○ 37°C; ▴ 30°C.
To verify if C. krusei virulence in G. mellonella depended on the temperature at which the larvae are incubated, we compared virulence of C. krusei and C. albicans at different temperatures (25, 30 or 37°C). Candida albicans was more virulent at 37°C than at 30°C. In contrast, no statistical difference was observed in the survival of G. mellonella infected with C. krusei and incubated at the different temperatures, indicating that C. krusei virulence does not depend on temperature (Figure 1D). Similar findings were obtained with C. krusei clinical isolates (result not shown). We also studied the virulence of these two species at environmental temperature (25°C). In agreement with the previous data, we found that C. krusei was virulent at 25°C, while C. albicans virulence was significantly decreased at this temperature (data not shown). To confirm these results, we investigated if C. krusei growth was affected by temperature in a similar manner as C. albicans. So we performed growth curves of both species at 30 and 37°C. As shown in Figure 1E and F, C. albicans grew better at 37°C compared to 30°C (two-fold reduction in generation time). In contrast, C. krusei grew similarly at both temperatures (0.85 fold decrease in generation time when the cells were grown at 37°C compared to 30°C). We found similar results at 25°C (data not shown), supporting that C. krusei growth is not affected by the incubation temperature. The final OD reached at the stationary phase at different temperatures was different with both species. Candida albicans reached higher OD at 37°C, which differed from the situation found in C. krusei, where the final OD at the stationary phase was almost identical at 30 and 37°C. Latency period was longer at 30°C, but the same trend was observed in both species (Figures 1E and 1F).
Yeast inoculation caused early melanization of the larvae
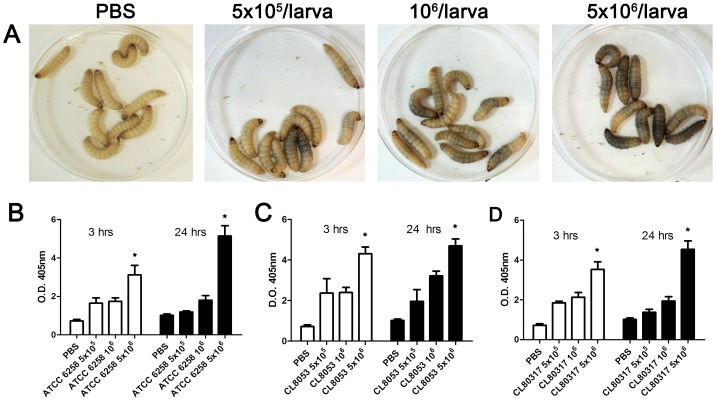
Galleria mellonella larvae appeared melanized after a few minutes of C. krusei injection (Figure 2A). To quantify this phenomenon, we collected the haemolymph and measured its optical density at 405 nm. When larvae were infected with 5×106 C. krusei cells, there was a significant accumulation of melanin in the haemolymph (4.3 times compared to the non-infected larvae), and this melanization increased over time (5 times at 24 hrs, Figure 2B). Clinical isolates showed a similar behavior (Figures 2C and D). We evaluated if C. krusei induced melanization of G. mellonella at lower temperatures, and we found that this phenomenon also occurred at 25°C (data not shown).
Figure 2. Melanization of G. mellonella infected with C. krusei.
(A) Visual appearance of G. mellonella larvae infected with different C. krusei doses. (B, C and D) Optical Density (OD) of the haemolymph of G. mellonella infected with C. krusei ATCC 6258 (B), clinical isolate CL8053 (C) and CL80317 (D) with 5×105, 106, 5×106 cells/larva. The different size inoculum reveals dose-response melanization (* p<0.05). All the experiments in this figure were performed at 37°C.
Phagocytosis and effect of C. krusei on haemocyte density
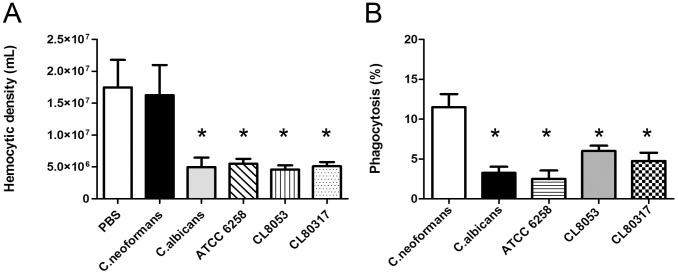
We examined if different C. krusei strains had any effect on haemocyte density. As shown in Figure 3A, C. krusei produced a decrease in haemocyte density in a similar manner to C. albicans.
Figure 3. Interaction between C. krusei and haemocytes.
(A) Changes in haemocyte density during C. krusei infection. The haemolymph of infected larvae with C. neoformans, C. albicans SC5314, C. krusei ATCC 6258, CL8053 and CL80317 clinical isolates and PBS was collected and the concentration of haemocytes was estimated using a haemocytometer (B). Phagocytosis percentage of C. neoformans, C. albicans SC5314, C. krusei ATCC 6258, CL8053 and CL80317 clinical isolates. Asterisks denote differences statistically significant (p<0.05).
We then investigated if C. krusei cells were phagocytosed by G. mellonella haemocytes. We compared the phagocytosis of this pathogen to the one measured with C. albicans and C. neoformans. The phagocytosis for all Candida strains (albicans and krusei) was significantly lower to the phagocytosis observed with C. neoformans (Figure 3B). The same result was found when phagocytosis was assessed at 25°C (data not shown).
Candida krusei can filament in vitro, so we investigated if this change also took place during infection in G. mellonella. We included C. albicans in these experiments as control, since it has been reported that this yeast can form hyphae in this model host [53]. As expected, C. albicans efficiently produced filaments in the larvae. Candida krusei also produced filaments, and in G. mellonella crude extracts they were frequently found in clumps of fat body of dark color, which we believe that are composed mainly of insect melanin. This fact may explain the fast melanization of G. mellonella when infected with C. krusei.
Antifungal efficacy during C. krusei infection in G. mellonella
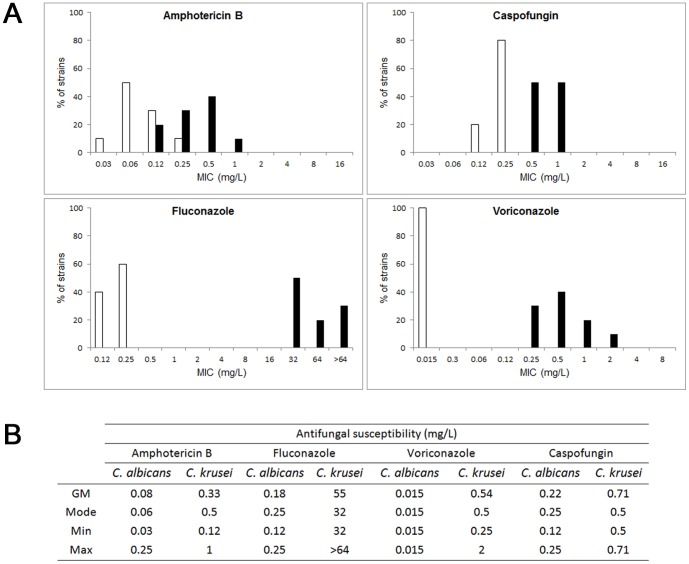
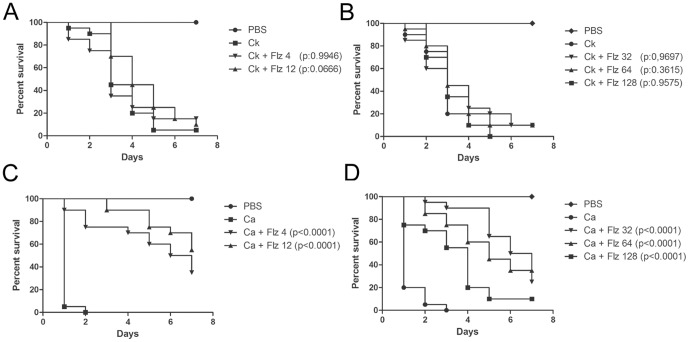
One of the main features for C. krusei is its in vitro susceptibility profile. As shown in Figure 4, C. krusei is less susceptible to amphotericin B, voriconazole and caspofungin than C. albicans, and intrinsically resistant to fluconazole. So we studied if this phenotype correlated with a lack of response to the antifungal during infection in G. mellonella. For this purpose, we infected G. mellonella with C. krusei or C. albicans, and treated the larvae with different antifungals (fluconazole, voriconazole, amphotericin B and caspofungin). In the case of larvae infected with C. krusei, treatment with fluconazole, even at very high doses (32 or 64 mg/kg) did not increase the survival (Figures 5A and B). At higher concentrations (128 mg/kg), there was a decrease in the survival, which is explained by the toxicity of the antifungal at this high concentration, which induced 25% of death after 7 days of treatment (data not shown). When the same experiments were performed with C. albicans, treatment with all the fluconazole concentrations produced significant survival (Figures 5C and 5D). Concerning other azoles, C. krusei is considered susceptible to voriconazole, although it presents higher MIC values to this antifungal than C. albicans (see Figure 4). So we studied the efficacy of voriconazole during infection in G. mellonella. We found that both voriconazole concentrations tested (7.5 and 10 mg/kg) protected larvae from C. albicans infection (Figure 6A). In contrast, larvae infected with C. krusei were only protected with higher voriconazole concentrations (Figure 6B). Lower doses did not have any effect on survival.
Figure 4. Antifungal susceptibility profile of C. krusei and C. albicans.
A) Distribution of MIC values (n = 10) to amphotericin B, caspofungin, fluconazole and voriconazole of C. albicans (white bars) and C. krusei (black bars). B) Description of antifungal susceptibility of C. albicans and C. krusei to different antifungals. N = 10. The geometric mean (GM), mode, minimum (Min) and maximum (Max) are shown.
Figure 5. Efficacy of fluconazole during G. mellonella infection with C. krusei or C. albicans.
Effect of fluconazole during infection of larvae with 5×106 cells of C. krusei (ATCC 6258) per larvae (A and B) and 5×105 cells of C. albicans cells (SC5314) per larva (C and D) in G. mellonella. Fluconazole treatment with 4 or 12 mg/kg (A and C); 32, 64 or 128 mg/kg (B and D).
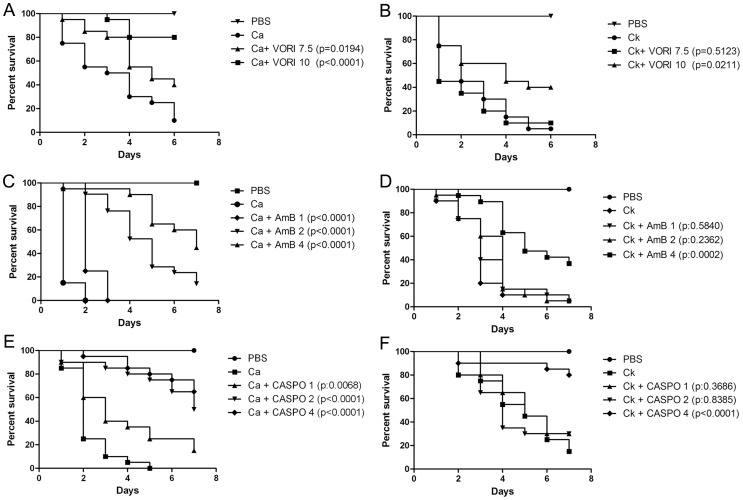
Figure 6. Efficacy of voriconazole, amphotericin B or caspofungin during C. krusei and C. albicans infection in G. mellonella.
A and B) Voriconazole treatment efficacy (7 and 10 mg/kg) in G. mellonella infected with C. albicans SC5314 (A) or C. krusei ATCC 6258 (B). C and D) Amphotericin B treatment efficacy (1, 2, 4 mg/kg) in G. mellonella infected with C. albicans SC5314 (C) or C. krusei ATCC 6258 (D). E and F) Caspofungin treatment efficacy (1, 2, 4 mg/kg) in G. mellonella infected with C. albicans SC5314 (E) or Candida krusei ATCC 6258 (F). In all the cases, the larvae were infected with 5×105 C. albicans cells/larva and 5×106 C. krusei cells/larva.
Amphotericin B (4 mg/kg) prolonged survival of larvae infected with C. albicans at all the concentrations tested (Figure 6A). In contrast, amphotericin B only protected larvae infected with C. krusei at the highest dose (4 mg/kg), which produced a 60% survival at the fourth day (Figure 6B). In a similar way, caspofungin was effective during C. albicans infection at all the doses tested (Figure 6C), while it only protected larvae inoculated with C. krusei at the highest dose (4 mg/kg) (Figure 6D). We also used an antifungal combination with fluconazole (12 or 4 mg/kg) and amphotericin B at a sub-therapeutic dose in G. mellonella (1 mg/kg), but we found no synergic effect between the antifungals (data not shown).
Fungal burden determination and histopathology
The fungal burden was determined by recovering the yeast cells from the larvae infected with C. albicans or C. krusei and treated with fluconazole (12 mg/kg) or amphotericin B (4 mg/kg). The number of CFUs increased in larvae infected with both pathogens with the time of infection (Figure 7). Treatment of larvae infected with C. albicans with fluconazole or amphotericin B decreased the number of CFUs by 1000-fold (Figure 7A). In larvae infected with C. krusei, amphotericin B reduced the fungal burden by 10-fold. Curiously, fluconazole also reduced the initial fungal burden, although it did not have an effect after longer times (5 days, Figure 7B).
Figure 7. Effect of antifungal treatment on fungal burden in G. mellonella infected with C. albicans or C. krusei.
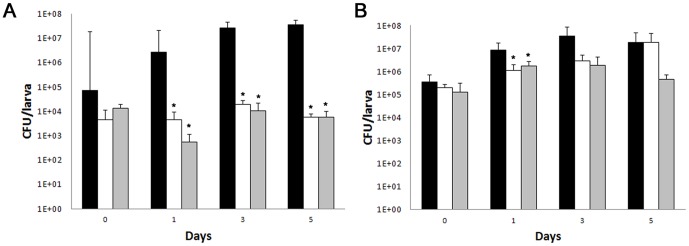
Galleria mellonella larvae were infected with C. krusei ATCC 6258 (A, 5×106 cells/larva) or C. albicans SC5314 (B, 5×105 cells/larva) and CFUs recovered from G. mellonella. Black bars, no treatment, white bars, fluconazole (12 mg/kg), grey bars, amphotericin B (4 mg/kg).
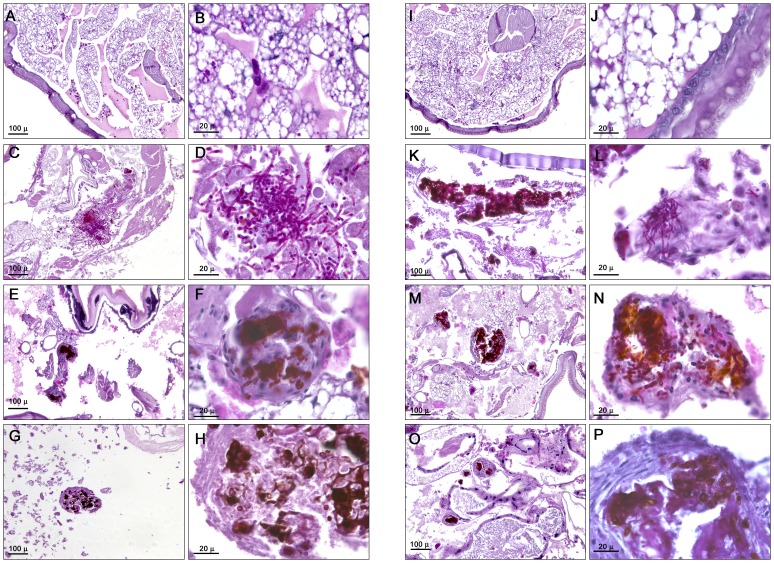
To complement these studies, we performed histopathology of infected and treated larvae. Candida albicans (Figure 8C and 8D) and C. krusei (Figure 8K and 8L) were found both in yeast and filament forms. The antifungal treatment with fluconazole (12 mg/kg) in larvae infected with C. albicans or C. krusei decreased the number of yeasts. Moreover, the fungi were mainly found in defined structures surrounded by G. mellonella cells (Figure 8E, F, M, N). Amphotericin B (4 mg/kg) had the same effect as fluconazole, although fewer yeast cells were found with this treatment (Figure 8G, H, O, P). The antifungals did not have a different effect on larvae infected with C. albicans or C. krusei. Treatment with the antifungals alone did not have any effect on the histopathology of the larvae (result not shown).
Figure 8. Histopathology of G. mellonella infected with C. krusei and C. albicans and treated with different antifungals.
Galleria mellonella was infected with 5×105 cells/larva of C. albicans SC5314 (C–H), or with 5×106 cells/larva of C. krusei ATCC 6258 (K–P). After 96 hours of infection, larvae were processed for histopathology as described in Material and Methods. (A, B, I, J), uninfected controls; (C, D, K and L), untreated controls; (E, F, M and N), larvae treated with fluconazole (12 mg/kg); (G, H, O and P), larvae treated with amphotericin B (4 mg/kg). (A, C, E, G, I, K, M, O), low magnification; (B, D, F, H, J, L, N and P), high magnification.
Effects of amphotericin B and fluconazole on the physiology of G. mellonella during C. albicans and C. krusei infection
Antifungals have immunomodulatory properties in mammals and in G. mellonella [54]–[56]. We studied the effect of Amphotericin B (4 mg/kg) and fluconazole (12 and 64 mg/kg) on haemocyte density, melanization and phagocytosis during G. mellonella infection by C. krusei and C. albicans. None of the antifungal treatments influenced the haemocyte density of C. krusei infected larvae. However, fluconazole (64 mg/kg) reduced the haemocyte density in larvae infected with C. albicans by two fold (p = 0.017, Figure 9A).
Figure 9. Effect of antifungal treatment of haemocyte density and melanization of G. mellonella infected with C. krusei or C. albicans.
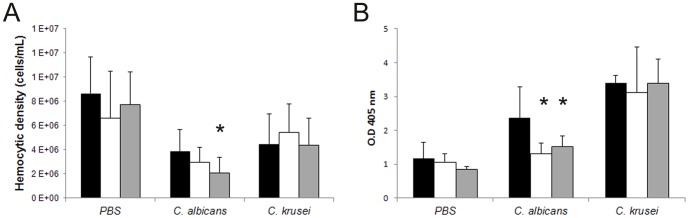
(A) Hemocytic density of G. mellonella infected with C. albicans SC5314 or C. krusei ATCC 6258 treated with amphotericin B (4 mg/kg) or fluconazole (64 mg/kg). (B) Optical Density (OD) of the haemolymph of G. mellonella infected with C. albicans or C. krusei treated with amphotericin B (4 mg/kg) or with fluconazole (64 mg/kg). Black bars, no treatment; grey bars, fluconazole; white bars, amphotericin B. * p<0.05.
None of the antifungals had a significant effect on the melanization of larvae infected with C. krusei. In contrast, antifungal treatment of larvae infected with C. albicans reduced melanization after 24 hours of infection. Fluconazole (64 mg/kg) and amphotericin B (4 mg/kg) reduced the melanization of these larvae by 1.8 (p = 0.0139) and 1.5 fold, respectively (p = 0.003, Figure 9B). No differences were observed in melanization or phagocytosis after 3 hours of infection with C. albicans or C. krusei. Antifungal drugs alone did not cause any effect in G. mellonella on the parameters analyzed.
Virulence and antifungal efficacy in C. elegans model
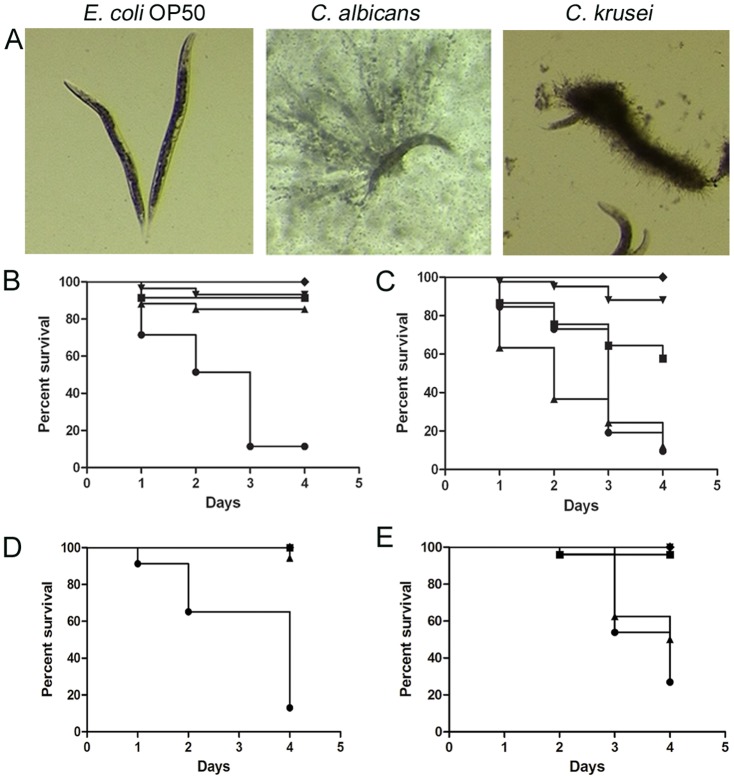
The nematode C. elegans is another non mammalian model that has been used as a host to study microbial virulence in this study. We also used this model to evaluate the in vivo protection of antifungals during C. krusei infection such as amphotericin B, fluconazole, voriconazole, caspofungin, and a combination of amphotericin B plus fluconazole. Candida albicans and C. krusei both killed C. elegans worms. In both Candida strains, worm death was associated with filamentation of the yeast in the worms (Figure 10A). When we investigated the protection of the different antifungal treatments, we found that all the antifungals protected during C. albicans infection at all the concentrations tested (Figure 10B). In contrast, in nematodes infected with C. krusei, the behavior of the antifungals was different: amphotericin B only protected at concentrations ≥2 µg/mL and fluconazole was not protective at any of the concentrations used (Figure 10C). Caspofungin showed similar protection as the one observed when the worms were infected with C. albicans (Figure 10C). The antifungal combination of fluconazole (12 µg/mL) and amphotericin B (1 µg/mL) did not show any synergistic effect in this model (result not shown). We also studied how voriconazole protected the worms from infection. As shown in Figure 10D, all the concentrations used (0.25, 7.5 and 10 mg/L) protected larvae from infection by C. albicans. However, only the higher doses (7.5 and 10 mg/L) showed efficacy during C. krusei infection, while the lowest dose (0.25 mg/L) was not protective.
Figure 10. Virulence of C. krusei and C. albicans in C. elegans and antifungal efficacy.
Caenorhabditis elegans was infected as described in material and methods with C. krusei (ATCC 6258), C. albicans (SC5314) and E. coli OP50. (A) Visual appearance of infected worms (50× magnification). (B) Antifungal efficacy in C. elegans infected with C. albicans. ♦ OP50, • C. albicans, ▪ C. albicans and treated with 2 µg/mL amphotericin B (p<0.0001), ▴ Fluconazole 12 µg/mL (p<0.0001); ▾Caspofungin 2 µg/mL (p<0.0001). (C) Antifungal efficacy during C. krusei infection ♦ OP50; • C. krusei; ▪ C. krusei treated with amphotericin B 2 µg/mL; (p<0.0001); ▴Fluconazole 12 µg/mL (p = 0.1207); ▾Caspofungin 2 µg/mL (p<0.0001). (D) Effect of voriconazole on survival of C. elegans worms infected with C. albicans (•, C. albicans, ▴, voriconazole 0.25 mg/L (p<0.0001); ▪, voriconazole, 7.5 mg/L (p<0.0001); ▾ voriconazole 10 mg/L (p<0.0001)). (E), Efficacy of voriconazole during infection of C. elegans by C. krusei (•C. krusei; ▴voriconazole 0.25 mg/L (p = 0.1217); ▾ voriconazole 7.5 mg/L (9<0.0001); ▪ voriconazole 10 mg/L (p<0.0001)).
Discussion
The use of invertebrate hosts to study the virulence of microbial pathogens presents advantages over conventional mammals. Amoebae, nematodes and insect hosts are good models to study virulence and to elucidate host–pathogen interaction. Ethical issues, cost and faster results are other benefits of these models [41], [42], [57]. During evolution, non vertebrate animals have developed immunity against microbial pathogens [42], and for this reason, there are functional and structural similarities between the innate immune system of mammals and insects. So, these models can be used to study immune responses [57].
In this work, we have used two different hosts, G. mellonella and C. elegans, to investigate virulence of C. krusei and antifungal efficacy. Compared to other non-conventional models, G. mellonella allows the use of precise pathogen doses by injection, low cost and evaluation of survival at different temperatures. The virulence of five C. albicans strains with mutations in genes related to filamentation was evaluated in G. mellonella and it was demonstrated that this model is useful as a filamentation assay [53]. In the case of C. neoformans, the virulence of different isolates, morphogenesis and antifungal treatments in G. mellonella showed good correlation with mammalian system [57], [58]. Previous work demonstrated that C. elegans is susceptible to different Candida species. For that reason, this host has been used to look for new compounds with antifungal activity [44], [46]. Besides, the available C. elegans mutant animals defective in signaling pathways involved in the immune system allows the study of the molecular mechanisms of host-pathogen interaction [59]. However, there are also some cases in which there is no correlation between virulence in mammals and G. mellonella [60], so further studies are required to validate the use of these models.
For these reasons, C. krusei offers a good model to validate the use of invertebrate models. This yeast shows reduced virulence in mammalian systems and fungal burden is significantly lower in animals infected with C. krusei than in animals infected with other fungal pathogens, such as C. albicans [61], so it is possible to compare its virulence with other highly virulent yeasts. Moreover, C. krusei is intrinsically resistant to fluconazole, so it offers an excellent model to correlate antifungal efficacy in vitro and in vivo.
Previous work showed that G. mellonella infected with 2×106 cells/larva of C. krusei killed 20% of the larvae after 72 hrs [62]. In our work, we have reproduced the model and observed that larvae killing can be faster by increasing the pathogen concentration. However, C. krusei was less virulent than C. albicans because the amount of yeast required to cause 100% death on the fourth day was 10 times higher. This is also in agreement with the reduced virulence of C. krusei in mammalian models [63], [64], but also indicates that G. mellonella offers a simple method to study virulence traits of C. krusei. This finding is of particular interest, since not every microorganism (i.e., Pneumocystis murina) can cause disease in G. mellonella [65].
The possibility to incubate G. mellonella at different temperatures is one of the best advantages of this model because it permits to study virulence at both environment and mammalian temperatures. The virulence of some pathogenic microorganisms (such as Cryptococcus neoformans, Fusarium spp and Acinetobacter baumannii) in G. mellonella is affected by the incubation temperature of the larvae after inoculation [66], [67]. In contrast, no statistical difference in the virulence of C. krusei was observed between the two temperatures. This correlates with the growth rate of C. krusei at both temperatures. In contrast to C. albicans, C. krusei growth was less affected by the temperature. Interestingly, G. mellonella seems to better tolerate environmental temperature than physiological temperature, and it would be expected that immunity is impaired at high temperature. However, our data indicate that in the case of fungi with reduced virulence, immunity at high temperature can control infection, and virulence of the fungus is more dependent on virulence traits of the yeast. Candida krusei and C. albicans produced filaments in G. mellonella, although the morphology was different. Candida krusei tended to form cell aggregates with melanization, characteristic of encapsulation. This result indicates that G. mellonella differentially recognizes pathogenic yeasts, which can be related to the different virulence exhibited by these two Candida species.
Decrease in the amount of haemocytes has been associated with increased susceptibility to fungal infections [39]. Candida krusei induced a reduction in the proportion of haemocytes in a similar way as C. albicans. This result suggests a mechanism of phagocytosis avoidance by which Candida species induce killing of G. mellonella, but does not explain the difference in virulence shown by the different Candida spp. This reduction might be a consequence of haemocyte explosion after filamentation of these yeasts after internalization. Interestingly, Cryptococcus neoformans does not cause a reduction in the number of hemocytes in the first two hours post-infection [58], [66], which might correlate with the fact that this fungus is an intracellular pathogen and can survive in phagocytic cells without affecting their viability [68], [69]. In addition, haemocytes are recruited at infection sites to form clumps or nodules [38], [70], so it is also possible that a proportion of the haemocytes migrate from the haemolymph to the infection sites. In agreement with our findings, it has been described that C. albicans induced a reduction in the concentration of haemocytes. In contrast, larvae infected with S. cerevisiae showed higher survival and haemocytic concentration [39]. Moreover, the compound [Ag2 (mal) (phen3)] increased the survival of larvae infected with C. albicans, and also increased the haemocytic concentration [71]. Phagocytosis of C. krusei and C. albicans was also lower compared to other fungi, such as C. neoformans. There are several mechanisms that could account for this phenomenon. Candida spp might be poorly phagocytosed due to impaired pathogen recognition by insect haemocytes. In addition to the reduction of haemocyte density and haemocyte explosion after filament formation discussed above could also explain the reduced phagocytosis observed. The future development of in vitro models to study yeast-haemocyte interaction will be of great help to fully characterize these phenomena.
Melanization is a humoral response of the insect that is catalyzed by the enzyme phenoloxidase, and the reaction occurs through the formation of capsules that surround foreign particles [72]. We observed a fast melanization process after infection with C. krusei. The degree of melanization depended on the inoculum concentration, but not on the viability of the cells, indicating that melanization is an unspecific process that depends on the presence of foreign particles.
One of the main findings of our work is the correlation between the in vivo efficacy of antifungals during C. albicans and C. krusei infection and their in vitro susceptibility profiles. Fluconazole did not have any protective effect during C. krusei infection in both G. mellonella and C. elegans models. Similar findings have been obtained with protection in immunosuppressed mice [63], [73], which validates the use of non-mammalian models to study antifungal efficacy. Due to the simplicity of these models, we believe that these hosts offer suitable and reliable systems to evaluate antifungal efficacy in vivo. In this sense, C. elegans has been successfully used to perform high-throughput assays to evaluate fungal susceptibility to different types of compounds [46], [74]–[76]. However, more information with resistant strains is required to fully validate their use. We also noticed differences in the protection between C. albicans and C. krusei in vivo after treatment with voriconazole, amphotericin B and caspofungin. During C. krusei infection, the caspofungin and amphotericin B concentrations required for protection were always higher than during C. albicans infection. Although C. krusei is considered susceptible to the three drugs, it has reduced susceptibility to caspofungin and amphotericin B compared to C. albicans [20], [77], [78]. So our data are again in agreement with the different susceptibility profile shown by these species in vitro. While several articles suggest molecular mechanisms for the resistance to fluconazole exhibited by C. krusei, it is not known why this species is less susceptible to amphotericin B and caspofungin than C. albicans. The survival experiments correlated with the fungal burden observed in the larvae. Reduction of the fungal burden was very significant during C. albicans with all the antifungals. In contrast, in larvae infected with C. krusei, fluconazole had no effect on CFUs and the effect of amphotericin B was less pronounced than in larvae inoculated with C. albicans. These data indicate that larvae response is less dynamic during C. krusei infection, which poses a limitation to perform pharmacodynamic studies in this infection model. Similar findings have been found in mammalian models. In immunosuppressed mice, fluconazole does not protect during C. krusei infection and amphotericin B had a partial effect, while anidulafungin treatment resulted in almost full survival of the animals [79]. In agreement, in another study, fluconazole had a very moderate effect in reducing fungal burden in neutropenic mice compared to other azoles, such as isavuconazole [64]. The use of antifungal combinations has not been sufficiently explored to study the pharmacodynamics response during C. krusei infection, and we believe that non mammalian models might offer a simple and easy procedure to evaluate this important issue.
Caenorhabditis elegans is also useful to test antifungal efficacy against several pathogenic fungi, including Candida spp and Fusarium spp [44], [80]. We found very similar results with C. elegans, and these results are comparable with the ones found in G. mellonella. This finding is important in the context of our work, because we have been able to reproduce very similar results using two different and independent host models. Despite the differences in the immune responses between nematodes and insects, C. krusei and C. albicans were virulent in both hosts. These results strongly support the use of these hosts as screening models. Interestingly, we could not find significant differences in the virulence of these species in C. elegans, in contrast to the results found in G. mellonella, where C. albicans was more virulent than C. krusei. We believe that this difference between the behavior of the different yeast species in G. melonella and C. elegans is the temperature at which the virulence experiments are performed, which is significantly lower in C. elegans.
Understanding fungal pathogenesis and the antifungal discovery are key aspects in medical mycology. Non-conventional models represent an important alternative for in vivo studies, even in the case of organisms that present low virulence in mammalian systems, such as C. krusei. Our results also demonstrate the feasibility of non-mammalian models to identify new antifungal compounds against resistant species. The correlation of the virulence of pathogenic fungi in mammals and non-mammalian models is still unclear. However, there is increasing evidence that some virulence phenotypes are reproduced in invertebrate models. For this reason, we believe that more studies to validate the full use of these hosts are required in the future.
Acknowledgments
We thank Raquel Pérez Tavárez for the histology experiments.
Funding Statement
O.Z. is funded by grant SAF2011-25140 from the Spanish Ministry for Economics and Competitiveness. L.S. is funded by a fellowship from the Agencia Española para la Cooperación Internacional y Desarrollo. C. elegans strain AU37 was provided by the CGC (Caenorhabditis Genetics Center), which is funded by National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1. Pfaller MA, Diekema DJ (2007) Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20: 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, et al. (2005) Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J Clin Microbiol 43: 1829–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen S, Slavin M, Nguyen Q, Marriott D, Playford EG, et al. (2006) Active surveillance for candidemia, Australia. Emerg Infect Dis 12: 1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labelle AJ, Micek ST, Roubinian N, Kollef MH (2008) Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36: 2967–2972. [DOI] [PubMed] [Google Scholar]
- 5. Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36: 1–53. [DOI] [PubMed] [Google Scholar]
- 6. Colombo AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M (2011) Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol 49: 785–798. [DOI] [PubMed] [Google Scholar]
- 7. Warnock DW (2006) Fungal diseases: an evolving public health challenge. Med Mycol 44: 697–705. [DOI] [PubMed] [Google Scholar]
- 8. Shorr AF, Gupta V, Sun X, Johannes RS, Spalding J, et al. (2009) Burden of early-onset candidemia: analysis of culture-positive bloodstream infections from a large U.S. database. Crit Care Med 37: 2519–2526; quiz 2535. [DOI] [PubMed] [Google Scholar]
- 9. Eggimann P, Garbino J, Pittet D (2003) Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 3: 685–702. [DOI] [PubMed] [Google Scholar]
- 10. Colombo AL, Nucci M, Park BJ, Nouer SA, Arthington-Skaggs B, et al. (2006) Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol 44: 2816–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao PL, Huang LM, Hsueh PR (2007) Recent advances and challenges in the treatment of invasive fungal infections. Int J Antimicrob Agents 30: 487–495. [DOI] [PubMed] [Google Scholar]
- 12. Pemán J, Salavert M (2012) General epidemiology of invasive fungal disease. Enferm Infecc Microbiol Clin 30: 90–98. [DOI] [PubMed] [Google Scholar]
- 13. Arendrup MC (2010) Epidemiology of invasive candidiasis. Curr Opin Crit Care 16: 445–452. [DOI] [PubMed] [Google Scholar]
- 14. Peman J, Canton E, Quindos G, Eraso E, Alcoba J, et al. (2012) Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J Antimicrob Chemother 67: 1181–1187. [DOI] [PubMed] [Google Scholar]
- 15. Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP (2002) Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis 35: 627–630. [DOI] [PubMed] [Google Scholar]
- 16. Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Nagy E, et al. (2008) Candida krusei, a multidrug-resistant opportunistic fungal pathogen: geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J Clin Microbiol 46: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, et al. (2009) Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 18. Miceli MH, Díaz JA, Lee SA (2011) Emerging opportunistic yeast infections. Lancet Infect Dis 11: 142–151. [DOI] [PubMed] [Google Scholar]
- 19. Rodríguez-Tudela JL, Arendrup MC, Cuenca-Estrella M, Donnelly JP, Lass-Flörl C (2010) EUCAST breakpoints for antifungals. Drug News Perspect 23: 93–97. [DOI] [PubMed] [Google Scholar]
- 20. Abbas J, Bodey GP, Hanna HA, Mardani M, Girgawy E, et al. (2000) Candida krusei fungemia. An escalating serious infection in immunocompromised patients. Arch Intern Med 160: 2659–2664. [DOI] [PubMed] [Google Scholar]
- 21. Fukuoka T, Johnston DA, Winslow CA, de Groot MJ, Burt C, et al. (2003) Genetic basis for differential activities of fluconazole and voriconazole against Candida krusei . Antimicrob Agents Chemother 47: 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, et al. (2004) Guidelines for treatment of candidiasis. Clin Infect Dis 38: 161–189. [DOI] [PubMed] [Google Scholar]
- 23. Spanakis EK, Aperis G, Mylonakis E (2006) New agents for the treatment of fungal infections: clinical efficacy and gaps in coverage. Clin Infect Dis 43: 1060–1068. [DOI] [PubMed] [Google Scholar]
- 24. Venkateswarlu K, Denning DW, Manning NJ, Kelly SL (1996) Reduced accumulation of drug in Candida krusei accounts for itraconazole resistance. Antimicrob Agents Chemother 40: 2443–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orozco AS, Higginbotham LM, Hitchcock CA, Parkinson T, Falconer D, et al. (1998) Mechanism of fluconazole resistance in Candida krusei . Antimicrob Agents Chemother 42: 2645–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pemán J, Cantón E, Orero A, Viudes A, Frasquet J, et al. (2002) Epidemiology of candidemia in Spain - Multicenter study. Rev Iberoam Micol 19: 30–35. [PubMed] [Google Scholar]
- 27. Muñoz P, Sánchez-Somolinos M, Alcalá L, Rodríguez-Créixems M, Peláez T, et al. (2005) Candida krusei fungaemia: antifungal susceptibility and clinical presentation of an uncommon entity during 15 years in a single general hospital. J Antimicrob Chemother 55: 188–193. [DOI] [PubMed] [Google Scholar]
- 28. Rogers TR (2006) Antifungal drug resistance: limited data, dramatic impact? Int J Antimicrob Agents 27 Suppl 1: 7–11. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez-Tudela JL, Alcazar-Fuoli L, Cuesta I, Alastruey-Izquierdo A, Monzon A, et al. (2008) Clinical relevance of resistance to antifungals. Int J Antimicrob Agents 32 Suppl 2: S111–113. [DOI] [PubMed] [Google Scholar]
- 30. Colombo AL, Ngai AL, Bourque M, Bradshaw SK, Strohmaier KM, et al. (2010) Caspofungin use in patients with invasive candidiasis caused by common non-albicans Candida species: review of the caspofungin database. Antimicrob Agents Chemother 54: 1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steenbergen JN, Shuman HA, Casadevall A (2001) Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci U S A 98: 15245–15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A (2003) Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum . Infect Immun 71: 4862–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mylonakis E, Ausubel FM, Perfect JR, Heitman J, Calderwood SB (2002) Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc Natl Acad Sci U S A 99: 15675–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glavis-Bloom J, Muhammed M, Mylonakis E (2012) Of model hosts and man: using Caenorhabditis elegans, Drosophila melanogaster and Galleria mellonella as model hosts for infectious disease research. Adv Exp Med Biol 710: 11–17. [DOI] [PubMed] [Google Scholar]
- 35. Alarco AM, Marcil A, Chen J, Suter B, Thomas D, et al. (2004) Immune-deficient Drosophila melanogaster: a model for the innate immune response to human fungal pathogens. J Immunol 172: 5622–5628. [DOI] [PubMed] [Google Scholar]
- 36. Fallon AM, Sun D (2001) Exploration of mosquito immunity using cells in culture. Insect Biochem Mol Biol 31: 263–278. [DOI] [PubMed] [Google Scholar]
- 37. Boman HG, Hultmark D (1987) Cell-free immunity in insects. Annu Rev Microbiol 41: 103–126. [DOI] [PubMed] [Google Scholar]
- 38. Kavanagh K, Reeves EP (2004) Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 28: 101–112. [DOI] [PubMed] [Google Scholar]
- 39. Bergin D, Brennan M, Kavanagh K (2003) Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella . Microbes Infect 5: 1389–1395. [DOI] [PubMed] [Google Scholar]
- 40. Fuchs BB, O'Brien E, Khoury JB, Mylonakis E (2010) Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1: 475–482. [DOI] [PubMed] [Google Scholar]
- 41. Desalermos A, Fuchs BB, Mylonakis E (2012) Selecting an invertebrate model host for the study of fungal pathogenesis. PLoS Pathog 8: e1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fuchs BB, Mylonakis E (2006) Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol 9: 346–351. [DOI] [PubMed] [Google Scholar]
- 43. Mahajan-Miklos S, Tan MW, Rahme LG, Ausubel FM (1999) Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96: 47–56. [DOI] [PubMed] [Google Scholar]
- 44. Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, et al. (2007) Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog 3: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tampakakis E, Okoli I, Mylonakis E (2008) A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat Protoc 3: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 46. Okoli I, Coleman JJ, Tampakakis E, Tempakakis E, An WF, et al. (2009) Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS One 4: e7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gillum AM, Tsay EY, Kirsch DR (1984) Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet 198: 179–182. [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez-Tudela JL, Arendrup M, Barchiesi F, Bille J, Chryssanthou E, et al. (2008) EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin Microbiol Infect 14: 398–405. [DOI] [PubMed] [Google Scholar]
- 49. Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W (2012) EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18: E246–247. [DOI] [PubMed] [Google Scholar]
- 50. Alvarado-Ramirez E, Torres-Rodriguez JM, Sellart M, Vidotto V (2008) Laccase activity in Cryptococcus gattii strains isolated from goats. Rev Iberoam Micol 25: 150–153. [DOI] [PubMed] [Google Scholar]
- 51. Williamson PR (1994) Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol 176: 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sulston J, Hodgkin J (1988) The Nematode Caenorhabditis elegans. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 53. Fuchs BB, Eby J, Nobile CJ, El Khoury JB, Mitchell AP, et al. (2010) Role of filamentation in Galleria mellonella killing by Candida albicans . Microbes Infect 12: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ben-Ami R, Lewis RE, Kontoyiannis DP (2008) Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis 47: 226–235. [DOI] [PubMed] [Google Scholar]
- 55. Kelly J, Kavanagh K (2011) Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J Med Microbiol 60: 189–196. [DOI] [PubMed] [Google Scholar]
- 56. Mesa-Arango AC, Scorzoni L, Zaragoza O (2012) It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Frontiers in Microbiology 3: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mylonakis E, Casadevall A, Ausubel FM (2007) Exploiting amoeboid and non-vertebrate animal model systems to study the virulence of human pathogenic fungi. PLoS Pathog 3: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. García-Rodas R, Casadevall A, Rodríguez-Tudela JL, Cuenca-Estrella M, Zaragoza O (2011) Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One 6: e24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pukkila-Worley R, Ausubel FM, Mylonakis E (2011) Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog 7: e1002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jackson JC, Higgins LA, Lin X (2009) Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella . PLoS One 4: e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arendrup M, Horn T, Frimodt-Moller N (2002) In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30: 286–291. [DOI] [PubMed] [Google Scholar]
- 62. Cotter G, Doyle S, Kavanagh K (2000) Development of an insect model for the in vivo pathogenicity testing of yeasts. FEMS Immunol Med Microbiol 27: 163–169. [DOI] [PubMed] [Google Scholar]
- 63. Karyotakis NC, Anaissie EJ, Hachem R, Dignani MC, Samonis G (1993) Comparison of the efficacy of polyenes and triazoles against hematogenous Candida krusei infection in neutropenic mice. J Infect Dis 168: 1311–1313. [DOI] [PubMed] [Google Scholar]
- 64. Majithiya J, Sharp A, Parmar A, Denning DW, Warn PA (2009) Efficacy of isavuconazole, voriconazole and fluconazole in temporarily neutropenic murine models of disseminated Candida tropicalis and Candida krusei . J Antimicrob Chemother 63: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fuchs BB, Bishop LR, Kovacs JA, Mylonakis E (2011) Galleria mellonella are resistant to Pneumocystis murina infection. Mycopathologia 171: 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mylonakis E, Moreno R, El Khoury JB, Idnurm A, Heitman J, et al. (2005) Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun 73: 3842–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Coleman JJ, Muhammed M, Kasperkovitz PV, Vyas JM, Mylonakis E (2011) Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol 115: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Feldmesser M, Tucker S, Casadevall A (2001) Intracellular parasitism of macrophages by Cryptococcus neoformans . Trends Microbiol 9: 273–278. [DOI] [PubMed] [Google Scholar]
- 69. Garcia-Rodas R, Zaragoza O (2012) Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans . FEMS Immunol Med Microbiol 64: 147–161. [DOI] [PubMed] [Google Scholar]
- 70. Mesa-Arango AC, Forastiero A, Bernal-Martinez L, Cuenca-Estrella M, Mellado E, et al. (2012) The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol [DOI] [PubMed] [Google Scholar]
- 71. Rowan R, Moran C, McCann M, Kavanagh K (2009) Use of Galleria mellonella larvae to evaluate the in vivo anti-fungal activity of [Ag2(mal)(phen)3]. Biometals 22: 461–467. [DOI] [PubMed] [Google Scholar]
- 72. Bidla G, Hauling T, Dushay MS, Theopold U (2009) Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innate Immun 1: 301–308. [DOI] [PubMed] [Google Scholar]
- 73. Anaissie EJ, Karyotakis NC, Hachem R, Dignani MC, Rex JH, et al. (1994) Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J Infect Dis 170: 384–389. [DOI] [PubMed] [Google Scholar]
- 74. Coleman JJ, Ghosh S, Okoli I, Mylonakis E (2011) Antifungal activity of microbial secondary metabolites. PLoS One 6: e25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Desalermos A, Muhammed M, Glavis-Bloom J, Mylonakis E (2011) Using C. elegans for antimicrobial drug discovery. Expert Opin Drug Discov 6: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, et al. (2010) Characterization of plant-derived saponin natural products against Candida albicans . ACS Chem Biol 5: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Drago M, Scaltrito MM, Morace G (2004) In vitro activity of voriconazole and other antifungal agents against clinical isolates of Candida glabrata and Candida krusei . Eur J Clin Microbiol Infect Dis 23: 619–624. [DOI] [PubMed] [Google Scholar]
- 78. Munoz P, Sanchez-Somolinos M, Alcala L, Rodriguez-Creixems M, Pelaez T, et al. (2005) Candida krusei fungaemia: antifungal susceptibility and clinical presentation of an uncommon entity during 15 years in a single general hospital. J Antimicrob Chemother 55: 188–193. [DOI] [PubMed] [Google Scholar]
- 79. Ostrosky-Zeichner L, Paetznick VL, Rodriguez J, Chen E, Sheehan DJ (2009) Activity of anidulafungin in a murine model of Candida krusei infection: evaluation of mortality and disease burden by quantitative tissue cultures and measurement of serum (1,3)-beta-D-glucan levels. Antimicrob Agents Chemother 53: 1639–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Muhammed M, Fuchs BB, Wu MP, Breger J, Coleman JJ, et al. (2012) The role of mycelium production and a MAPK-mediated immune response in the C. elegans-Fusarium model system. Med Mycol 50: 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]