Hepatocellular carcinoma (HCC) is one of the leading causes of death among patients with cirrhosis and has an increasing incidence in the United States1. The prognosis for patients with HCC depends on tumor stage at the time of diagnosis, with curative options only available for patients diagnosed at an early stage2. Patients with early HCC achieve 5-year survival rates near 70% with resection and transplantation, whereas those with advanced HCC have a median survival of less than one year3, 4. HCC screening strives to detect HCC at an early stage and is recommended for patients with cirrhosis5.
HCC screening is a complex process, requiring several steps when implemented in clinical practice.6 First, providers must be knowledgeable about the benefits of HCC screening and for whom screening is recommended. Second, providers must be able to accurately identify patients with cirrhosis and refer these patients for appropriate screening tests. Third, patients must comply with these provider recommendations. The healthcare system must have sufficient capacity to schedule and complete/deliver the screening tests, and finally, providers and patients must complete clinically-indicated follow-up on any abnormal screening test results7. In addition to each of the above steps in the screening process, screening tests must remain effective in usual practice settings8, 9. Thus, the effectiveness of HCC screening may be reduced due to factors at the patient-level (e.g. insurance), provider-level (e.g. knowledge of guidelines), and system-level (e.g. availability of screening tests).
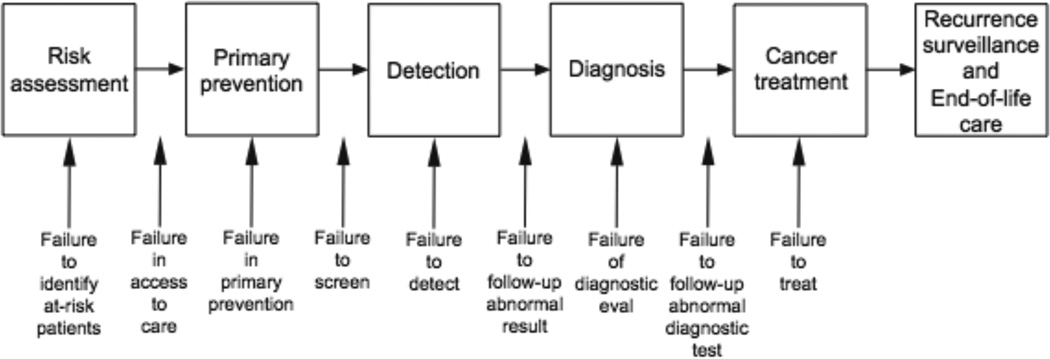
The Quality in the Continuum of Cancer Care (QCCC) conceptual framework (Figure 1), which has been successfully used for understanding screening processes in breast, cervical, and colorectal cancer10, 11, provides a useful model to highlight how aspects of the HCC screening process differ from other cancers and therefore may pose unique challenges to its effectiveness. While research on improving the HCC screening process is in its infancy stage, important lessons can be learned from prior colorectal (CRC) screening studies. Although CRC screening rates and outcomes still fall short of desirable levels, several successful interventions have led to substantial improvement over time12, 13. In fact, there has been a steady increase in CRC rates from 20–30% in 1997 to approximately 55% in 200814, 15. This improvement in CRC screening rates is in part related to lessons learned from experiences in breast and cervical cancer screening programs16. In contrast, HCC screening rates among patients with cirrhosis remain below 30% nationally, and the majority of tumors are still diagnosed at an advanced stage when curative therapies are no longer available17–20. In this commentary, we compare and contrast several steps in the cancer screening processes for CRC and HCC to illustrate issues that need to be addressed in the promotion and delivery of HCC screening.
Figure 1.
The Quality in the Continuum of Cancer Care (QCCC) conceptual framework
Step 1: Accurate provider identification of at-risk population
CRC screening requires providers to assess age- and family-related risk and recommend screening to at-risk patients. CRC screening is uniformly recommended to all average-risk patients at age 50 years. Early screening initiation is recommended in high-risk patients, such as those with a family history of colon cancer, which providers fail to adequately assess in up to one-third of patients21. However, given the relative ease of assessing age-related risk, identification of the at-risk population has not been regarded as a major barrier to effective CRC screening.
Risk assessment for HCC screening is likely to be more challenging compared to identification of those eligible for CRC screening based on age and family history. Providers must recognize the presence of underlying liver disease as well as the transition to cirrhosis, which can occur without overt clinical symptoms. Under-recognition of liver disease and cirrhosis substantially contributes to the underutilization of HCC screening; in fact, nearly 40% of patients present with HCC without having previously recognized liver disease and/or cirrhosis (Figure 2)22, 23. Although liver biopsy currently remains the gold standard for assessing stage of fibrosis, the increasing availability and accuracy of non-invasive markers of fibrosis may help improve the recognition of cirrhosis in the future24. Patients with non-alcoholic fatty liver disease (NAFLD) appear to be at the highest risk, with over 80% of patients having unrecognized liver disease at the time of HCC presentation. With the prevalence of NAFLD increasing and now approaching 50% of the population in the United States, this issue may become even more problematic in the future25.
The Centers for Disease Control and Prevention (CDC) recently recommended screening for hepatitis C virus (HCV) infection using a birth-cohort screening strategy (i.e. screening all patients born between 1945 and 1965) instead of the previously recommended risk-based strategy (i.e. screening only patients with known risk factors for HCV infection) to simplify risk assessment and increase HCV testing rates26. Although mass screening strategies are possible for certain liver diseases, such as viral hepatitis, this would not be possible for other etiologies, such as NAFLD given that it is a diagnosis of exclusion with no serologic markers. Therefore, education of primary care providers regarding the at-risk population for NAFLD and the necessity for high clinical suspicion is likely crucial.
There have not been any interventions to date that address the accurate identification of patients with cirrhosis for HCC screening. Liver biopsy remains the gold standard for assessing liver fibrosis but is often avoided given the potential for complications and lack of patient acceptance27. Despite significant advances in the accuracy of non-invasive markers for liver fibrosis, they have yet to be widely incorporated into routine clinical practice28. With the growing use of electronic medical records, incorporation of electronic prompts using applicable ICD-9 codes or non-invasive markers of fibrosis, such as AST to platelet ratio index (APRI), could potentially help providers accurately identify at-risk patients in the future23, 28–30. Overall, we anticipate optimizing screening will required concerted efforts to develop and implement interventions for identifying individuals at risk for HCC in usual practice.
Step 2: Provider recommendation and referral for screening
Despite over ten years of consistent United States Preventive Services Task Force (USPTF) and American Cancer Society (ACS) guidelines recommending CRC screening31, 32, providers are still not systematically referring all individuals eligible for screening15. In fact, lack of physician recommendation for screening remains the most powerful predictor for screening non-completion33, 34. In one analysis, 20% of patients not up-to-date with CRC screening reported lack of provider recommendation as a significant barrier14. Although CRC screening rates have consistently improved over this time period, there are still many missed opportunities for screening recommendations35. Physicians have reported multiple barriers to implementing cancer screening guidelines including inadequate levels of knowledge, provider forgetfulness, time constraints in clinic, provider fatigue, lack of financial incentive, and competing health problems36, 37
Successful strategies to bypass or increase provider recommendations for CRC screening rates have included organized screening efforts, patient-directed prompts, provider-focused reminders, and systematic mass screening programs38. Patient-directed prompts, such as postcards and one-on-one patient education, has been shown to be effective at improving CRC screening rates by 5% to 42% compared to usual care15, 39. Provider-focused intervention strategies, such as provider assessment and feedback or provider reminder and recall systems, are also effective and recommended by the Community Preventive Services Task Force40, 41. Interventions at the health system, such as public awareness campaigns and systematic mass screening programs which do not rely on provider-based referrals, may improve screening rates by 7% to 28%15, 39, 42
HCC screening has not been adopted into routine clinical practice and rates remain below 30% nationally, despite being standard of care in patients with cirrhosis18–20. Screening rates are higher among patients who receive subspecialty care; however, only 20–40% of cirrhotic patients are followed by gastroenterologists or hepatologists nationally23. Even among patients with recognized cirrhosis, providers fail to order HCC screening in nearly two-thirds of patients22. In a secondary analysis of the Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial, nearly one-third of patients followed by expert hepatologists in academic centers had inconsistent screening43. The strongest predictor for receipt of consistent screening in this study was the provider, after adjusting for differences in patient characteristics.
There have yet to be any interventions to increase HCC screening referrals among patients with cirrhosis. Future interventions should likely focus on optimizing provider referrals for HCC screening given that physician factors appear to be more important than patient-level factors in determining HCC screening rates. Potential provider-based interventions include provider education, electronic reminder systems, and provider feedback of screening rates. Although system-based screening invitations to patients with known cirrhosis could also be considered, issues of potential overuse among patients with Child Pugh C or poor functional status would need to be addressed. Given HCC screening is only performed in a targeted population of patients with cirrhosis, interventions such as public awareness campaigns are unlikely to be cost-effective; however targeted patient education programs about the importance of HCC screening may increase rates of patient demand and self-referral.
Step 3: Patient adherence to recommendations for screening
Despite provider recommendations, many patients do not complete CRC screening. In fact, nearly one-fourth of patients failed to adhere to provider recommendations to endoscopic CRC screening and over one-half failed to adhere to FOBT testing44, 45. Patient factors associated with screening non-completion include younger age, minority background, recent immigration, limited knowledge regarding the importance and effectiveness of screening, and low socioeconomic status15. The type of CRC screening test offered may also play a role, with significantly lower rates of screening completion among patients who were only offered colonoscopy compared to those who were given a choice between colonoscopy and other screening modalities46. Provision of client reminders, small media, one-on-one patient education, and reducing structural barriers are all effective ways of overcoming patient barriers to screening completion once it is offered40, 47–49.
Patient adherence does not currently appear to be a major barrier to HCC screening. Overall, over 95% of patients complete HCC screening once ordered by their provider22. At-risk patients have also demonstrated high levels of knowledge and reported high rates of acceptance for HCC screening, although this study was conducted among well-insured, highly-educated patients in a tertiary care setting50. In contrast to colonoscopy, whose uptake is limited by prep tolerance and patient perceptions, HCC screening primarily consists of an ultrasound, which is easy, painless, and without significant complications. Although younger age, minority race, and lower socioeconomic status are associated with lower HCC screening rates20, it is unknown if these associations are due to lack of access to medical care, providers not ordering HCC screening in these subgroups, or patient non-adherence. This will be crucial to understand in the future, given the populations at highest risk for HCC tend to be socially disadvantaged, such as immigrants and those of low socioeconomic status51.
Although interventions to increase patient adherence, such as patient education and nurse navigation programs, have been useful for increasing CRC screening rates, no studies have evaluated these intervention strategies for HCC screening interventions. However, the current high rates of patient adherence with HCC screening may simply reflect experiences with early adopters of screening or the “worried well”. Therefore, patient adherence will need to be monitored closely as HCC screening becomes more widely adopted. We anticipate that patient adherence rates may become suboptimal in the future, as broader populations are offered HCC screening, potentially requiring us to draw from lessons from interventions used to optimize CRC screening adherence at that time.
Step 4: Capacity of the health system to schedule tests
Although there are sufficient providers and resources to perform universal CRC screening with fecal occult blood testing, there is insufficient capacity for widespread CRC screening through colonoscopy. A study performed by the CDC reported 14.2 million colonoscopies were performed in 200252; given the current underuse of CRC screening, endoscopic capacity is not an issue at this time. In fact, endoscopic output could be increased by 8.2 million without requiring an increase in resources or personnel. This increase would provide sufficient endoscopic capacity for expanding FOBT testing to the 41.8 million unscreened portion of the US population. However, using flexible sigmoidoscopy and colonoscopy as initial CRC screening tests among the unscreened portion of the population would quickly overwhelm endoscopic capacity. Furthermore, this analysis did not account for repeat routine or post-polypectomy surveillance, which would even further limit endoscopic capacity. Overall, it is clear that providing screening and diagnostic colonoscopy for all patients eligible for screening remains a challenge14.
It is unknown if radiologic capacity is a significant barrier to HCC screening. In a single-center study, over 95% of patients were appropriately scheduled for HCC screening testing once ordered by their provider22. However, it is unknown if these results are generalizable, and radiologic capacity must still be assessed on a national level. Furthermore, radiologic capacity could become an issue if HCC screening rates improved and created a larger burden on the radiology scheduling system. This may be particularly difficult for community hospitals in rural areas and safety net hospitals, which often have limited resources53.
One key difference between HCC and CRC screening comes in the separation between screening and diagnostic tools. In CRC screening, colonoscopy is often used as both the screening and diagnostic tool. In HCC screening, ultrasound is used as the screening tool, requiring CT or MRI to confirm the diagnosis in any patients with a suspicious mass. Therefore, future studies of ability to deliver HCC screening should assess the national radiologic capacity for ultrasonography as well as CT and MRI.
Step 5: Appropriate and timely follow-up of abnormal screening tests
Evidence-based follow-up of abnormal screening results is critical for the effectiveness of any screening program. Nonetheless, in CRC screening, high variability in diagnostic colonoscopy completion rates after abnormal fecal occult blood test screening has challenged screening effectiveness. Indeed, diagnostic colonoscopy completion rates after abnormal fecal occult blood testing as low as 22% have been reported in the literature54–61. Importantly, focused attention to quality improvement has been shown to significantly improve clinically indicated follow up after abnormal CRC screening tests62.
Follow up after an abnormal US or AFP screening requires diagnostic imaging with contrast-enhanced CT or MRI to confirm the diagnosis. A secondary analysis of data from the HALT-C Trial suggests that follow-up of abnormal screening tests could be delayed more than six months in nearly one-fourth of patients43. These delays in follow-up are concerning given an approximate tumor doubling time of three months for HCC63. In fact, patients with tumors larger than 2 cm in diameter were significantly more likely to not have prior HCC screening and/or timely follow-up of abnormal tests than patients found with very early stage tumors. These screening process failures contributed to more advanced tumor stage in over one-third of HCC patients in HALT-C43. Further studies assessing the impact of delayed or lack of follow-up on HCC outcomes in clinical practice are still needed. If confirmed, several interventions that have been effective in CRC, including patient navigation, could potentially be applied to HCC screening.
Summary
In this commentary, the Quality in the Continuum of Cancer Care conceptual framework was used to highlight differences between the HCC and CRC screening processes and identify how HCC screening may pose unique challenges to its effectiveness. Although lack of provider recommendations is a significant barrier for both CRC and HCC screening, HCC screening appears to be limited by under-recognition of at-risk individuals with liver disease and cirrhosis. Future HCC screening interventions must help providers accurately identify at-risk patients as well as promote ordering of HCC screening among those with cirrhosis. On the other hand, patient adherence, a well-recognized barrier to CRC screening, does not appear to be a major issue in HCC screening at this time. However, adherence may become a more significant issue in the future, with expanded adoption of HCC screening, and must be monitored closely. Other steps in the screening process, including radiology capacity and timely follow-up, have been demonstrated as barriers for CRC screening, but further studies assessing their impact on HCC screening outcomes are still needed. Overall, many lessons learned from challenges to CRC screening can be applied to rapidly optimize HCC screening in usual practice. The QCCC framework can continue to be used as a guide for monitoring progress as well as to identify new barriers to HCC screening in the future.
Acknowledgments
Financial disclosures: This work was conducted with support from UT-STAR, NIH/NCATS Grant Number KL2 TR000453 and the ACG Junior Faculty Development Award awarded to Dr. Singal. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, UT Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
Abbreviations
- ACS
American Cancer Society
- APRI
AST to platelet ratio index
- CDC
Centers for Disease Control and Prevention
- CRC
colorectal cancer
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
- QCCC
Quality in the Continuum of Cancer Care
- USPTF
United States Preventive Services Task Force
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None of the authors have any conflicts of interest to disclose.
Author Contributions:
Amit Singal was involved in study concept and design, drafting of the manuscript, critical revision of the manuscript, and study supervision.
Jasmin Tiro was involved in critical revision of the manuscript.
Samir Gupta was involved in drafting of the manuscript and critical revision of the manuscript.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Singal AG, Marrero JA. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2010;26:189–195. doi: 10.1097/MOG.0b013e3283383ca5. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Bru C, Rodes J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010:3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 8.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, Conjeevaram HS, Volk ML, Fu S, Fontana RJ, Askari F, Su GL, Lok AS, Marrero JA. Effectiveness of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012;21:793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leyden WA, Manos MM, Geiger AM, Weinmann S, Mouchawar J, Bischoff K, Yood MU, Gilbert J, Taplin SH. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 11.Taplin SH, Ichikawa L, Yood MU, Manos MM, Geiger AM, Weinmann S, Gilbert J, Mouchawar J, Leyden WA, Altaras R, Beverly RK, Casso D, Westbrook EO, Bischoff K, Zapka JG, Barlow WE. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? J Natl Cancer Inst. 2004;96:1518–1527. doi: 10.1093/jnci/djh284. [DOI] [PubMed] [Google Scholar]
- 12.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph DA, King JB, Miller JW, Richardson LC. Prevalence of colorectal cancer screening among adults--Behavioral Risk Factor Surveillance System, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;(61 Suppl):51–56. [PubMed] [Google Scholar]
- 14.Klabunde CN, Brown M, Ballard-Barbash R, White MC, Thompson T, Plescia M. Cancer Screening - United States 2010. In: CDC, editor. MMWR. 2012. Volume 61. pp. 41–45. [Google Scholar]
- 15.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, Keating NL, Kim P, Lave JR, Laveist TA, Ness RB, Optican RJ, Virnig BA. National Institutes of Health state-of-the-science conference statement: Enhancing use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:663–667. doi: 10.7326/0003-4819-152-10-201005180-00237. [DOI] [PubMed] [Google Scholar]
- 16.Ransohoff DF. Colon cancer screening in 2005: status and challenges. Gastroenterology. 2005;128:1685–1695. doi: 10.1053/j.gastro.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 19.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of Hepatocellular Carcinoma Surveillance Among American Patients: A Systematic Review. J Gen Intern Med. 2012:861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroy PC, 3rd, Barrison AF, Ling BS, Wilson S, Geller AC. Family history and colorectal cancer screening: a survey of physician knowledge and practice patterns. Am J Gastroenterol. 2002;97:1031–1036. doi: 10.1111/j.1572-0241.2002.05624.x. [DOI] [PubMed] [Google Scholar]
- 22.Singal AG, Yopp A, Gupta S, Skinner CS, Halm EA, Okolo E, Nehra M, Lee WM, Marrero JA, Tiro JA. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res. 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–126. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 25.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 26.Tomaszewski KJ, Deniz B, Tomanovich P, Graham CS. Comparison of Current US Risk Strategy to Screen for Hepatitis C Virus With a Hypothetical Targeted Birth Cohort Strategy. Am J Public Health. 2012 doi: 10.2105/AJPH.2011.300488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szymczak A, Simon K, Inglot M, Gladysz A. Safety and effectiveness of blind percutaneous liver biopsy: analysis of 1412 procedures. Hepat Mon. 2012;12:32–37. doi: 10.5812/kowsar.1735143x.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 29.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 30.Nehra M, Ma Y, Clark CB, Amarasingham R, Rockey DC, Singal AG. Use of Administrative Claims Data for Identifying Patients with Cirrhosis. J Clin Gastroenterol. 2012 doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 32.Smith RA, Cokkinides V, Brooks D, Saslow D, Shah M, Brawley OW. Cancer screening in the United States, 2011: A review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2011;61:8–30. doi: 10.3322/caac.20096. [DOI] [PubMed] [Google Scholar]
- 33.Dulai GS, Farmer MM, Ganz PA, Bernaards CA, Qi K, Dietrich AJ, Bastani R, Belman MJ, Kahn KL. Primary care provider perceptions of barriers to and facilitators of colorectal cancer screening in a managed care setting. Cancer. 2004;100:1843–1852. doi: 10.1002/cncr.20209. [DOI] [PubMed] [Google Scholar]
- 34.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–944. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 35.Fenton JJ, Reid RJ, Baldwin LM, Elmore JG, Buist DS, Franks P. Influence of primary care use on population delivery of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2009;18:640–645. doi: 10.1158/1055-9965.EPI-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlandi MA. Promoting health and preventing disease in health care settings: an analysis of barriers. Prev Med. 1987;16:119–130. doi: 10.1016/0091-7435(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 37.Pommerenke FA, Dietrich A. Improving and maintaining preventive services, Part 2: Practical principles for primary care. J Fam Pract. 1992;34:92–97. [PubMed] [Google Scholar]
- 38.Levin TR, Jamieson L, Burley DA, Reyes J, Oehrli M, Caldwell C. Organized colorectal cancer screening in integrated health care systems. Epidemiol Rev. 2011;33:101–110. doi: 10.1093/epirev/mxr007. [DOI] [PubMed] [Google Scholar]
- 39.McPhee SJ, Bird JA, Jenkins CN, Fordham D. Promoting cancer screening. A randomized, controlled trial of three interventions. Arch Intern Med. 1989;149:1866–1872. doi: 10.1001/archinte.149.8.1866. [DOI] [PubMed] [Google Scholar]
- 40.Updated recommendations for client- and provider-oriented interventions to increase breast, cervical, and colorectal cancer screening. Am J Prev Med. 2012;43:92–96. doi: 10.1016/j.amepre.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Sabatino SA, Lawrence B, Elder R, Mercer SL, Wilson KM, DeVinney B, Melillo S, Carvalho M, Taplin S, Bastani R, Rimer BK, Vernon SW, Melvin CL, Taylor V, Fernandez M, Glanz K. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43:97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Tape TG, Campbell JR. Computerized medical records and preventive health care: success depends on many factors. Am J Med. 1993;94:619–625. doi: 10.1016/0002-9343(93)90214-a. [DOI] [PubMed] [Google Scholar]
- 43.Singal AG, Nehra M, Huet B, Marrero J, Lok AS, Lee WM. Failure Rates in a Surveillance Program for Hepatocellular Carcinoma among Patients in the HALT-C. Trial Gastroenterology. 2012;142:S1008. [Google Scholar]
- 44.Freedman JD, Mitchell CK. A simple strategy to improve patient adherence to outpatient fecal occult blood testing. J Gen Intern Med. 1994;9:462–464. doi: 10.1007/BF02599066. [DOI] [PubMed] [Google Scholar]
- 45.Turner BJ, Weiner M, Yang C, TenHave T. Predicting adherence to colonoscopy or flexible sigmoidoscopy on the basis of physician appointment-keeping behavior. Ann Intern Med. 2004;140:528–532. doi: 10.7326/0003-4819-140-7-200404060-00013. [DOI] [PubMed] [Google Scholar]
- 46.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 47.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 48.Lasser KE, Murillo J, Lisboa S, Casimir AN, Valley-Shah L, Emmons KM, Fletcher RH, Ayanian JZ. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171:906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 49.Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, Richter JM, Atlas SJ. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009;24:211–217. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singal A, Volk M, Rakoski M, Fu S, Su G, McCurdy H, Marrero J. Patient Involvement is Correlated with Higher HCC Surveillance in Patients with Cirrhosis. J Clin Gastroenterol. 2011;45:727–732. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]
- 51.Shebl FM, Capo-Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012;21:1330–1335. doi: 10.1158/1055-9965.EPI-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeff LC, Manninen DL, Dong FB, Chattopadhyay SK, Nadel MR, Tangka FK, Molinari NA. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 53.Goldman LE, Henderson S, Dohan DP, Talavera JA, Dudley RA. Public reporting and pay-for-performance: safety-net hospital executives' concerns and policy suggestions. Inquiry. 2007;44:137–145. doi: 10.5034/inquiryjrnl_44.2.137. [DOI] [PubMed] [Google Scholar]
- 54.Humphrey LL, Shannon J, Partin MR, O'Malley J, Chen Z, Helfand M. Improving the follow-up of positive hemoccult screening tests: an electronic intervention. J Gen Intern Med. 2011;26:691–697. doi: 10.1007/s11606-011-1639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jimbo M, Myers RE, Meyer B, Hyslop T, Cocroft J, Turner BJ, Weinberg DS. Reasons patients with a positive fecal occult blood test result do not undergo complete diagnostic evaluation. Ann Fam Med. 2009;7:11–16. doi: 10.1370/afm.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kistler CE, Kirby KA, Lee D, Casadei MA, Walter LC. Long-term outcomes following positive fecal occult blood test results in older adults: benefits and burdens. Arch Intern Med. 2011;171:1344–1351. doi: 10.1001/archinternmed.2011.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miglioretti DL, Rutter CM, Bradford SC, Zauber AG, Kessler LG, Feuer EJ, Grossman DC. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008;46:S91–S96. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powell AA, Gravely AA, Ordin DL, Schlosser JE, Partin MR. Timely follow-up of positive fecal occult blood tests strategies associated with improvement. Am J Prev Med. 2009;37:87–93. doi: 10.1016/j.amepre.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Powell AA, Nugent S, Ordin DL, Noorbaloochi S, Partin MR. Evaluation of a VHA collaborative to improve follow-up after a positive colorectal cancer screening test. Med Care. 2011;49:897–903. doi: 10.1097/MLR.0b013e3182204944. [DOI] [PubMed] [Google Scholar]
- 60.Rao SK, Schilling TF, Sequist TD. Challenges in the management of positive fecal occult blood tests. J Gen Intern Med. 2009;24:356–360. doi: 10.1007/s11606-008-0893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh H, Daci K, Petersen LA, Collins C, Petersen NJ, Shethia A, El-Serag HB. Missed opportunities to initiate endoscopic evaluation for colorectal cancer diagnosis. Am J Gastroenterol. 2009;104:2543–2554. doi: 10.1038/ajg.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh H, Kadiyala H, Bhagwath G, Shethia A, El-Serag H, Walder A, Velez ME, Petersen LA. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol. 2009;104:942–952. doi: 10.1038/ajg.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581–586. doi: 10.1023/a:1022505203786. [DOI] [PubMed] [Google Scholar]