Introduction
For many cancer types, American Indians and Alaska Natives (AI/AN) experience worse outcomes and lower chances of survival than other racial groups in the United States. Cancer is the third leading cause of death for AI/ANs of all ages, and the second leading cause of death for American Indians over the age of 45 [1]. AI/AN people have the poorest survival of any race for all cancer types combined and for eight of the ten leading types [2]. Although in the Northwest, the overall incidence of cancer is lower among AI/ANs, the cancer mortality rate is significantly higher for this group when compared to the non-Hispanic white population [3].
Research indicates that higher rates of mortality and morbidity among AI/ANs are mainly due to delays and obstacles in seeking and receiving cancer care. Funding limitations to Indian health care, low socioeconomic status, and a variety of cultural, social and geographic barriers to cancer care impede AI/AN patients’ ability to participate in screening, diagnosis, and treatment [4] [5] [6] [7]. Lower levels of health care literacy, mistrust in the health care delivery system and dissatisfaction with providers are further challenges specific to tribal communities [8] [9] [10] [11] [12].
In recent years there have been significant improvements in cancer screening techniques, and major developments in cancer treatments; however, not all communities benefit equally from these research advances. Evidence suggests that screening rates are comparatively low among AI/ANs and they often present with advanced stages of cancer. This is especially true for AI/ANs in the Northwest region and may be the primary cause of poor outcomes seen among this population [13] [14] [15] [9] [16] [17] [18] [19] [20] [21].
The main goal of cancer screening - to detect and treat disease early - is not achieved if patients fail to receive timely and appropriate follow-up diagnostic tests and treatments. Delays in follow-up after suspicious finding for cancer can lead to increased patient anxiety, diagnosis at an advanced stage, and ultimately result in poor prognosis and outcomes [14] [15] [8] [22] [23] [24] [17] [25] [26].
Recent studies have identified a number of patient, provider, and policy-level factors that have significant influence on timely follow-up after the detection of an abnormality. Patient compliance, anxiety or fear towards follow-up procedures, limited financial resources, lack of health care coverage, transportation, language barriers, lack of trust in modern health care delivery, and cultural beliefs and practices are known to be associated with failure to complete diagnostic evaluations after detecting cancer abnormalities [9] [27] [11] [28] [29] [30] [31].
The ‘gap’ between research findings, advances in knowledge and what is delivered is a critical determinant in cancer health disparities. One of the ideas attempting to bridge this ‘gap’ is to provide one person who works with patients to navigate the complex medical and financial systems, overcome barriers to care and assist with logistics. This idea was formalized and put into practice by Harold P. Freeman in 1990s and is now referred to as the “patient navigation model” [22]. During the past two decades several studies have demonstrated the importance of navigator services in cancer care, especially in improving time to diagnosis and treatment [8] [22] [23] [32]. In the limited funding setting of Indian Health Service, it is also worth noting that patient navigation has also been shown, in preliminary studies, to be cost-effective [33]. While it is beyond the scope and economic means of most community-based programs to solve the vast social, economic and health system problems that create barriers to screening and delay diagnostic resolution, patient navigation has proven to be effective in tackling some of these issues among minority populations [34] [35] [25] [36] [37] [38] [39] [40] [41] [42] [43] [44]. However, it has not been widely assessed and implemented in AI/AN communities.
Thus, through a grant from the Center to Reduce Cancer Health Disparities at the National Cancer Institute, a patient navigator program was developed for Northwest Tribal communities, one of nine sites in the Patient Navigation Research Program (PNRP). The PNRP served different populations nationally. The Northwest Tribal Cancer Navigator Program (NTCNP) was the only program among PNRP sites that was implemented in a rural setting and specifically served the AI/AN population, and included all types of cancer. The NTCNP was managed out of the Northwest Portland Area Indian Health Board (NPAIHB), a non-profit tribal advisory organization serving the 43 federally recognized tribes of Idaho, Oregon and Washington. The primary aim of the NTCNP was to help reduce barriers to cancer care, and improve clinical outcomes and quality of life for AI/AN cancer patients residing in Northwest region through providing navigator services.
The primary aim of this analysis was to estimate the effect of navigator services in reducing the time between suspicious cancer-related finding and definitive diagnosis.
Materials and Methods
Study Data Collection Method
Navigation services were provided between June 2006 and August 2010 at four Northwest Tribes and their respective clinics. Four additional Northwest Tribes and their respective clinics were recruited to serve as control clinics. Clinics were not randomly assigned to either group, as was the wish of the tribal communities in the Northwest; intervention sites were recruited first, and were chosen based on tribes which expressed an interest and need, which had sufficient user population size to provide a sample suitable for the study, and which varied in geography, administrative structure and patient base. Control sites were subsequently recruited with a focus on matching approximate user population size, and representing the same geographic and administrative structure of clinics. Thus (by IHS user population estimates for FY 2011) the total population of the intervention clinics and the control clinics only differed by about 2,000 patients. However, the results section will show that, despite this initial matching, the final study population was much lower for the intervention arm. This was primarily due to the fact that control patients were enrolled only through chart reviews, and never contacted directly. Enrollment through chart reviews posed no temporal limits, as they could be conducted at any time after their initial abnormal finding. Navigated patients had to be identified in a timely manner, successfully contacted, and had to consent to both services and involvement in the research portion of the study. As a result, fewer subjects were successfully enrolled in the intervention arm of the study. The study was approved by Portland Area Indian Health Service Institutional Review Board.
Patient Navigators
Navigators were employed at four intervention sites and encounters took place at the clinics, at the participant’s home, over the phone, or the navigator accompanied the participant to an appointment. Navigators were either registered nurses, community health representatives (CHR), or individuals with experience working in a medical setting. During the course of the project, a total of seven individuals served as navigators at the four tribes. Three of the navigators were American Indians, two from the Tribe at which they were employed, and one from another Tribe. The other four were non-native. Two had lived and worked in the local tribal community for many years, while the other two were new to the community. Each tribe recruited and hired their own navigators, and had the option to choose a candidate with the background they determined best suited the needs of their community. The impact of differences in navigators may also be examined in subsequent analyses of these data, although some studies have shown that navigation is effective without the need to match the navigator’s race or culture to that of the patient [32]. Navigators were placed at the tribal clinics as members of the clinic staff with full access to patient charts and the clinic’s medical database. They received standardized training at the NPAIHB, and national training from PNRP, along with appropriate continuing education.
The navigator’s primary responsibility was to assist patients who had an abnormal finding or cancer diagnosis in obtaining appropriate health care services. This included assisting participants and families to early resolution of the health issue, patient education, emotional and psychosocial support, coordination of resources, facilitation of services, and interaction with providers, family members, and community members. Secondary responsibilities included planning and attending health fairs, screening events, pow wows, fundraisers and luncheons in order to build trust among community members, encouraging study participation, and establishing community networks. Faced with participant needs that were not being met by the existing resources, navigators networked with local and national organizations and advocated to bring additional resources to their communities. Some examples include mobile mammography and transportation services. For indigenous communities, navigation was a natural fit because it reflects the traditional focus on caring for the health of the entire community.
The data for the study were collected from various sources. At navigated sites, clinical data were obtained from the clinical database and paper medical charts. Clinical data were collected through chart reviews at control clinics and no contact was made with participants in these communities. Every month de-identified data was emailed to research staff at the NPAIHB and a diagnostic program was run which identified and reported erroneous or missing data to navigators for correction. NTCNP collected demographic and clinical data on all navigated participants. The same data were collected for any participants meeting the eligibility criteria at control clinics. Trained research staff made multiple visits to each control clinic to collect these data. As noted previously, differences in sample size between navigated and control sites arose due to the fact that data were only collected for navigated subjects who were a) identified and contacted in a timely manner, b) agreed to receive navigation services and c) agreed to share their data for the research portion of the study. On the other hand, for control subjects, every patient who met the eligibility criteria was enrolled and received a chart review.
Study Eligibility Criteria
Any patient over the age of 18, who was eligible to be seen at one of the participating clinics and who had received a cancer diagnosis or abnormality suspicious for cancer was eligible for the study. At the navigation sites any patient with abnormal findings suggestive of cancer or a cancer diagnosis was referred for navigator services by their physicians, nurses or other health care workers. Navigators themselves were never the patient’s initial contact.
In the national PNRP model navigators were only to provide services to those who had abnormal findings or diagnosis related to breast, cervical, prostate or colorectal cancer during the study period. In small tribal communities where health resources are scarce and the need is overwhelming it was seen as unethical and exclusive to limit eligibility to specific cancers. Following formal tribal consultation and review of our pilot study [35], eligibility criteria were expanded to patients with any type of cancer related abnormalities. However, the primary research design was retained by keeping variable definitions and data collection methods consistent with those described by the larger study, and participants with cancer types not meeting the national eligibility criteria were held in separate tables during data collection. Participants received navigation services once the eligibility criteria were met and continued until resolution of the issue through non-cancerous diagnosis, completion of primary cancer treatment, death, loss to follow-up, or end of the study period.
Outcome Measures
The time interval between abnormal finding and definitive diagnosis (T1) was the primary outcome variable in the study and was dichotomized in the multivariate analysis to estimate the effect of navigation.
There still exist controversies over who should receive follow-up diagnostic tests, and which is the optimal timeline for follow-up tests after the initial abnormal screening tests [6]. Therefore, during the outcome analysis, three cutoff points were tested to categorize T1: cutoff points of 60 and 90 days were chosen to assess the effect of navigation on early diagnostic resolution, and a cutoff point of 365 days was used to assess the effect of navigation on extreme delays.
Prior to analysis, we reviewed the detailed notes on cases with long T1 times. It became apparent that participants who experienced extreme delays in obtaining definitive diagnosis were usually those who had been lost to follow-up with regard to the initial abnormal finding. Due to geographic isolation of these clinics, these patients returned to the clinic for issues unrelated to the initial screening and were given a routine screening or, less commonly, the old abnormal finding was discovered in their chart and a new referral for follow-up was issued. Because of the loss to follow up, these cases showed more similarities with a case that went unresolved than those which were resolved in the short-term through sustained patient contact. Therefore, the cases with extreme delays and the undiagnosed were combined. The cutoff point of 365 days was chosen for ease of interpretation, as one year was considered the point of “lost to follow-up” for the larger PNRP study.
Ten participants were enrolled with multiple concurrent abnormalities (defined as two abnormal findings or diagnoses within 30 days). During the analysis these participants were assigned to a single cancer type using the following criteria: where the date of definitive diagnosis was missing for one, the cancer type with complete data was assigned (n=4); where complete data was available for both, the cancer type with earliest date of abnormal finding was assigned (n=4).
Participants with abnormal findings and definitive diagnosis on the same day (T1 =0) were excluded since these participants would not have received any benefit from navigation as both screening and definitive tests were performed on the same day. Those with pre-existing cancer were also excluded. Pre-existing cancer was defined two ways: those who began treatment before enrollment and those who had a previous cancer diagnosis within five years or less of the eligible diagnosis. The former were excluded as they would not have received navigation services during the relevant time in their cancer journey. The latter were excluded to prevent biasing either study arm with participants who would have a relatively greater understanding of the system and how to navigate through it, having recently been through it themselves. In order to avoid an inflated proportion of undiagnosed participants enrolled toward the end of the study, all participants with an abnormal finding resulting in a recommended six-month follow-up (such as BIRADS 3 for mammogram or ASCUS on a pap test) were excluded if the date of that abnormal finding was less than six months from the final date of data collection.
Statistical Analysis
Participant characteristics were summarized using descriptive statistics. Since clustering effects for participants within each clinic were anticipated, we used a mixed effects logistic regression model to evaluate the effect of navigation while controlling for the clustering effects, as well as potential confounding variables. Covariates such as age, gender, body mass index (BMI), health care coverage, Charlson Co-morbidity Index (CCI), cancer type, cancer stage, metastasis, and severity of disease were adjusted for during the final analysis.
Univariate association was first assessed between each independent variable and the dichotomized time to definitive diagnosis. Independent variables with a univariate P-value ≤ 0.1 were considered for the multivariate model. The final model was then determined using the backward selection method including the intervention variable (navigated versus control) and covariates with P-values < 0.05. Effect modifiers were tested by including interaction terms.
The statistical software SPSS version 17.0 (SPSS Inc, Chicago IL) was used for the initial data preparation and descriptive analysis, and SAS version 9.2 package (SAS Institute Inc., Cary NC) was used for the outcome analysis. All the statistical tests were two sided and a p-value of <0.05 was considered significant.
Results
A total of 1236 subjects were enrolled between June 2006 and August 2010. Among them 32 subjects were excluded due to having diagnoses on the same day as abnormal findings (n=22) or pre-existing cancer diagnoses (n=10). For an additional 17 participants, data were missing which resulted in removal from the research sample. Of those excluded, thirty percent were from the control arm and seventy percent from the intervention arm. The final study sample included 1187 subjects: 151 who received navigator services (navigated) and 1036 who did not (controls).
Demographic and Clinical Characteristics
Demographic information is summarized in Table.1. Overall, the study had a higher proportion of women (79%) because more participants presented with breast and cervical cancer abnormalities. There were 400 (34%) with breast, 298 (25%) cervical, 234 (20%) colorectal, 70 (6%) prostate, and 185 (15%) other cancer abnormalities.
Table 1. Demographic characteristics of participants enrolled in the Northwest Tribal Cancer Navigator Study.
| Characteristics | Navigated Patients (N=151) |
Control Patients (N=1036) |
|||
|---|---|---|---|---|---|
|
| |||||
| Tribe | Count | Percent | Count | Percent | χ2 p value |
| Tribe 1 | 31 | 21.7% | – | – | |
| Tribe 2 | 49 | 31.6% | – | – | |
| Tribe 3 | 57 | 36.8% | – | – | |
| Tribe 4 | 14 | 9.9% | – | – | |
| Tribe 5 | – | – | 282 | 27.2% | |
| Tribe 6 | – | – | 119 | 11.7% | |
| Tribe 7 | – | – | 249 | 24.3% | |
| Tribe 8 | – | – | 386 | 36.9% | |
| Total | 151 | 100.00% | 1036 | 100.0% | |
|
| |||||
| Age | 0.01 | ||||
|
| |||||
| 46 | |||||
| Mean Age in Years (SDa) | 52 (16.3) | (17.7) | |||
| 18-29 Years | 20 | 13.2% | 250 | 24.2% | |
| 30-39 Years | 12 | 7.9% | 129 | 12.5% | |
| 40-49 Years | 33 | 21.9% | 178 | 17.2% | |
| 50-59 Years | 40 | 26.5% | 222 | 21.5% | |
| 60-69 Years | 24 | 15.9% | 151 | 14.6% | |
| > 70 Years | 22 | 14.6% | 103 | 10.0% | |
| Total | 151 | 100.0% | 1033 | 100.0% | |
|
| |||||
| Body Mass Index (BMI) | 0.004 | ||||
|
| |||||
| Mean BMI (SD) | 33 (9) | 32 (8) | |||
| Less than 18.5 (Underweight) | 6 | 4.1% | 10 | 1.0% | |
| 18.5 -24.9 (Normal weight) | 22 | 15.2% | 147 | 14.3% | |
| 25-29.9 (Overweight) | 27 | 18.6% | 287 | 27.9% | |
| 30-39.9 (Obese) | 64 | 44.1% | 443 | 43.1% | |
| 40 or Higher(Morbidly obese) | 26 | 17.9% | 140 | 13.6% | |
| Total | 145 | 100.0% | 1027 | 100.0% | |
|
| |||||
| Gender | 0.4 | ||||
|
| |||||
| Male | 36 | 23.8% | 216 | 20.8% | |
| Female | 115 | 76.2% | 820 | 79.2% | |
| Total | 151 | 100.0% | 1036 | 100.0% | |
|
| |||||
| Health Care Coverage | 0.688 | ||||
|
| |||||
| IHSb direct only | 18 | 12.0% | 114 | 11.0% | |
| IHS direct & CHSc only | 37 | 24.7% | 280 | 27.1% | |
| Other combinationsd | 37 | 24.7% | 285 | 27.5% | |
| Private insurance | 58 | 38.6% | 356 | 34.4% | |
| Total | 150 | 100.0% | 1035 | 100.0% | |
|
| |||||
| Disease Status at Enrollment | <0.0001 | ||||
|
| |||||
| Abnormal Findings | 94 | 62.3% | 1030 | 99.4% | |
| Cancer Diagnosis | 57 | 37.7% | 6 | 0.6% | |
| Total | 151 | 100.0% | 1036 | 100.0% | |
|
| |||||
| Charlson Comorbidity Index (CCI) | 0.001 | ||||
|
| |||||
| No Comorbidity | 94 | 62.3% | 779 | 75.2% | |
| Any CCI | 57 | 37.7% | 257 | 24.8% | |
| Total | 151 | 100.0% | 1036 | 100.0% | |
SD=Standard Deviation
IHS=Indian Health Service
CHS=Contract Health Service
Other Combinations= Medicare/Medicaid and Military health or other State sponsored health plans but exclude private insurance
Navigated participants (mean 52 yrs., sd 16.3) were older than those in the control group (mean 46 yrs., sd 17.7). BMI was similar between both the groups. The mean BMI was 32, which indicates obesity in many participants. Health care coverage was proportionately similar among navigated and control groups. While 38% of navigated participants had some type of comorbidity (as determined by CCI) only 25% of control participants had similar conditions. This may be partly explained by age differences between the groups.
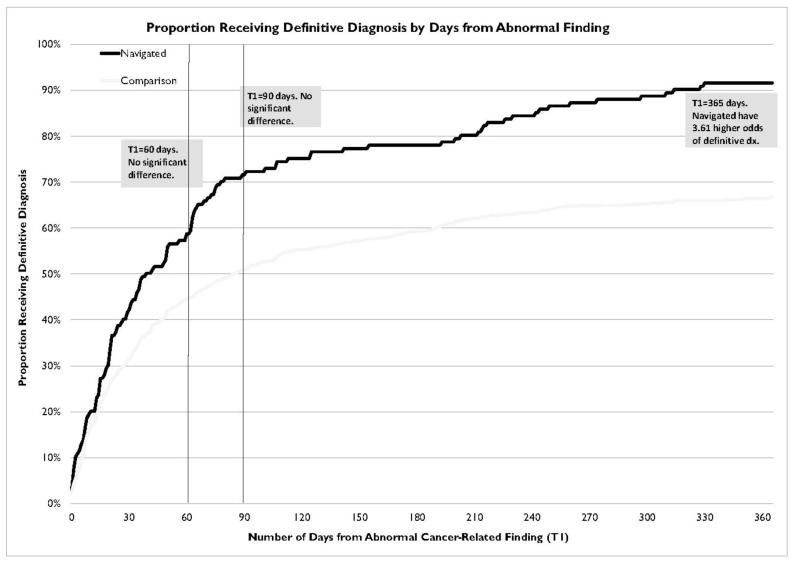
The median T1 for navigated and control participants was 37 and 42 days respectively. Figure 1 shows the cumulative proportion in each group who had received resolution over time. Note that this graph is not adjusted for other differences between the groups, and are simply descriptive, not inferential. These assessed and controlled for in the models described below.
Figure 1.
In the final adjusted model using the T1=60 day cutoff point as the outcome, two covariates were significantly associated with the outcome variable (type of cancer related abnormality and health care coverage); however the intervention variable (navigated versus control) was not significant (p=0.45). Similar results were obtained when we used a cutoff point of T1=90 days. In this model, three covariates were significantly associated with the outcome variable (health care coverage, type of cancer related abnormality and cancer stage); again, the intervention variable was not significantly associated with the outcome (p =0.21).
It should be noted that, for reasons discussed in detail in the conclusions, the final sample size of navigated subjects was much smaller than anticipated. We conducted a power analysis using the actual sample size and other parameters in our logistic regression model to determine the minimum detectable effect size that would result from this study. The sample size was adjusted down to account for design effect using an intra-cluster correlation coefficient of 0.004. The results showed that navigation would have needed to increase the odds of diagnosis by at least 1.99 times at 60 days and 2.01 times at 90 days to be detected in this study. Thus it is possible that navigated subjects experienced increased odds of diagnosis at T1=60 or T1=90 days, but this effect was not detected in our models. It is also possible that navigation had no effect at this point in the cancer continuum. Due to the small sample size and clustered design, these findings should be interpreted with caution, understanding that these issues preclude us from making strong conclusions for these time points.
When T1=365 days was used as the cutoff point, the intervention variable (navigated versus control) was significantly associated with outcome variable (p=0.013), along with health care coverage, type of cancer related abnormality and cancer stage were significantly associated with the outcome variable (Table. 2). None of the interaction terms tested were significant in the model.
Table 2. Factors associated with diagnostic resolution within 365 days in an adjusted Generalized Linear Mixed Model: Northwest Tribal Cancer Navigation Study.
| Variable | Referent | Median T1a in days (range) |
Adjusted ORb (95% CIc) |
p- value |
|---|---|---|---|---|
|
| ||||
| Study Arm | Control Participants (n=1036) | 42 (1 – 1240) | ||
|
| ||||
| Navigated Participants (n=151) | 37 (1 – 1002) | 3.61 (1.47, 8.88) | 0.013 | |
|
| ||||
| Cancer Site | Cervical Abnormality (n=298) | 107 (3 – 1240) | ||
|
| ||||
| Breast Abnormality (n=400) | 18 (1 – 998) | 4.19 (2.88, 6.10) | <.0001 | |
| Colorectal Abnormality (n=234) | 42 (1 – 1002) | 1.92 (1.29, 2.86) | 0.0024 | |
| Prostate Abnormality (n=70) | 75 (5 – 1078) | 0.77 (0.42, 1.44) | <.4065 | |
| Other Cancer Abnormality (n=185) | 34 (1 – 467) | 3.75 (2.13, 6.63) | <.0001 | |
|
| ||||
| Health Care Coverage | IHS Direct Only (n=132) | 68 (1 – 713) | ||
|
| ||||
| IHSd Direct and CHSe Only (n=317) | 35 (1 – 848) | 2.07 (1.26, 3.40) | 0.006 | |
| Other Combinationsf (n=322) | 41 (1 – 1240) | 1.96 (1.19, 3.24) | 0.011 | |
| Private Insurance (n=414) | 41 (1 – 1017) | 2.47 (1.52, 4.01) | 0.0008 | |
|
| ||||
| Cancer Stage | Non Cancer Cases (n=1019) | 42 (1 – 1240) | ||
|
| ||||
| Early Stage (No Metastasis) (n=75) | 47 (2 – 939) | 6.32 (2.34, 16.7) | 0.001 | |
| Regional/Distant Spread (n=93) | 24 (1 – 1002) | 11.3 (2.33, 55.0) | 0.005 | |
T1=0 and pre-cancerous patients are excluded in outcome analysis; subjects with no definitive diagnosis are included in more than one year group.
T1 is number of days from abnormal cancer related finding to definitive diagnosis
OR= Odds Ratio
CI=Confidence Interval
IHS =Indian Health service
CHS=Contract Health Service
Other Combinations include Medicare/Medicaid and Military health or other State sponsored health plans but exclude private insurance
In the final multivariate model, after adjusting for potential confounders, the odds of receiving a definitive diagnosis within 365 days of abnormal cancer related finding for navigated participants was 3.6 times (95% CI: 1.47, 8.88; p=0.01) the odds for controls.
Participants with cervical abnormalities had the longest wait to obtain definitive diagnosis, with a median time to definitive diagnosis of 107 days, while those with breast abnormalities waited a median of 18 days. In the adjusted model the odds of receiving definitive diagnosis was 4.2 times (95% CI: 2.9, 6.1, p<0.0001) for those with breast abnormalities, 1.9 times (95% CI: 1.3, 2.9, p=0.002) for those with colorectal abnormalities, and 3.8 times (95% CI: 2.1, 6.6, p<0.0001) for those with other cancer related abnormalities when compared to the odds for participants with cervical abnormalities.
Those who had both access to both Indian Health Programs (direct-care services) and Contract Health Services (CHS) waited a median of 35 days to obtain definitive diagnosis, and the odds of receiving definitive diagnosis within 365 days was twice (95% CI: 1.26. 3.4; p=0.006) that of participants who only had access to services provided at the tribal or IHS clinic. The odds were significantly higher for participants who had private insurance coverage (OR 2.47, 95% CI: 1.52, 4.01, p=0.0008) than those who only had access to services provided at the tribal or IHS clinic.
Among those who were diagnosed with cancer, those with metastases obtained definitive diagnosis in a median time of 24 days after the detection of cancer related abnormality, and the odds of obtaining definitive diagnosis within a year was 11.3 times (95% CI: 2.33, 55.0, p=0.005) the odds for participants who had a non-cancer resolution.
Discussion
The initial results of this study show that AI/AN patients who worked with a patient navigator had significantly higher odds of obtaining a definitive diagnosis than their counterparts who did not have navigation. This finding shows a real benefit for tribal communities which may have a positive impact on health disparities in Indian Country. There are, however, several limitations within the Northwest Tribal Cancer Navigator Program which should be noted.
Some of these limitations are germane to the nature of the community recommendations for enhancement of the study design noted in the approval process. For example the community opted not to randomize patients to navigation at the individual level, so that all members of a particular tribal community would be treated equitably. Thus, the study design called for assignment to a study arm at the community level rather than at the individual level, which is the model preferred by the Northwest Tribal communities. As a result, the data are vulnerable to lack of comparability between navigated and non-navigated groups. Our samples did show some disparate characteristics between disease status at enrollment, cancer type and severity of disease. For those confounders which had a significant relationship with the outcome variable, adjustments were made in the final model. Our assignment to study arms at the community level increased sample size requirements to determine true effects and was less efficient than individual randomized trials due to correlation within clinics.
Our sample size within the navigated arm was smaller than initial projections, mainly due to difficulty identifying and enrolling eligible patients early, prior to diagnosis. One of the primary hurdles faced in enrolling subjects early in the cancer journey was simply identifying those who had an abnormal test result or symptom. Although some test results are recorded in the medical database system used in tribal clinics, there is no dedicated module within this system for tracking cancer screening results. As a result, navigators and research staff had to develop methods to search for abnormal test results. In some cases, inconsistent and delayed data entry added to the challenge. A number of different search templates were tested and navigators were trained to perform these searches within the medical database. They became adept at scanning these reports for eligible patients, and the process improved towards the end of the study. However, finding time to carry out these searches remained a challenge for navigators who already had full case loads.
Another hurdle to early enrollment was obtaining test results. Most imaging tests, colonoscopies and biopsies took place outside of the tribal clinics. Navigators often found it difficult to obtain the results of these tests in a timely manner. They took on the task of calling outside providers to request test results be returned to the clinic. This proved time consuming work and required the navigator to track a large number of patients who were not part of their formal case load.
Another issue which emerged was the recruitment materials themselves, which created a barrier to early enrollment by positioning the program as a service for “cancer patients” rather than those who had an abnormal finding. Although changes were made to these materials once it was determined they were hindering recruitment, the communities and providers continued to view the navigators this way. Providers reported a reluctance to refer pre-diagnosis cases that were unlikely to progress to cancer, such as mild abnormalities on a pap test. Aware of the navigators’ already heavy case loads of high needs patients, providers attempted to shield the navigators from extra cases that they didn’t feel warranted such one-on-one attention.
The consent process for the study allowed for subjects to receive navigation services, but to opt out of sharing their clinical data for the purposes of the research portion of the study. This was a requirement of the participating tribes in designing the consent process. In three of the intervention communities this did not pose a problem and very few subjects chose this option. However, in one community this option was chosen more frequently by eligible patients. The navigator in this community reported a high level of distrust of research among the patient population. Thus the consent process further served to decrease the final sample size available for these analyses.
Due to the nature of referrals to patient navigation and the presentation of patients to the program, participants were not all navigated through the early parts of their journey – exposure was limited to later in their journey for many. Results of our study lead us to believe that early entry into patient navigation is beneficial. Thus the ideal experience with patient navigation would include navigation from the earliest points of entry into the health care system. It should be noted that those who were eligible, but not included in the navigated group, probably differ systematically from those who were included for the reasons outlined above – fewer patients with mild abnormalities were enrolled, and those who received an abnormal finding from a test occurring outside the tribal clinic were also less likely to be enrolled. These differences were apparent between the two groups, as seen the results section. Where they were significantly related to the outcome variable, they were adjusted for in the final model.
The participants in this study were from a variety of tribes in the Northwest. Service types and availability may differ from other regions of the United States. Results are likely generalizable. However, caution should be used and the results should be interpreted in the context of the particular community assessing the need for a patient navigator.
We tested three cutoff points for dichotomized time to definitive diagnosis, and we did not do an adjustment for such multiple testing, which may inflate type I error. However, for our results from this study, p-values from the final multivariable model were small (Table 2). Assessing these results using a Bonferroni adjustment with a p-value of 0.0167, all associations remainede significant.
Our findings indicate a broad appeal for this type of program and a “natural fit” for tribal communities. The model of a navigator walking alongside the patient throughout their interactions with the health care system parallels traditional values of caring present in the AI/AN community. In results published elsewhere, the NTCNP received highly favorable feedback from the clients, families and communities in which it was present.
Extreme delays were prevented by having a navigator, as seen when looking at those who were undiagnosed at the end of the study or who waited more than a year for diagnosis. The benefit of appropriate diagnostic resolution is clearly an asset in AI/AN communities, who experience disparities in both rates of and outcomes of cancer [2] [3]. Coming to a timely diagnostic resolution has the potential to assist in the elimination of disparities and the enhancement of diagnosis at earlier time points.
Clearly early resolution of abnormal diagnostic screening is important. Early diagnosis can reduce the burden and cost of treatment. The early resolution of abnormal screenings that conclude with benign findings can ease the burden of concern on the patient and their family.
Improved synergy between community events and clinical health care should enhance and maximize resource use. This synergy could include improved implementation of the navigation program to include enhancements of mechanisms within clinics and communities to identify patients earlier in their journey. Having one or more navigators available to community members to encourage screening and follow up would assist patients in being identified early and being followed closely throughout the course of their journey [41] [32]. The role of a navigator is still in transition, and for Northwest tribal communities the definition was tailored to each community. Studies have shown similar tailoring exists in ongoing patient navigator programs [45]. However, our study indicates that expanding navigation to include “wellness” roles such as health fairs to promote screenings, promotion of men’s and women’s routine health visits and involvement in the preventive health aspects of the community could lead to the early inclusion of the navigator on the health care team, should the need arise. This would appear to have beneficial outcomes in diagnostic resolution [32] [46].
Due to limited sample size it is difficult to detect differences between the two study arms, should they exist. Navigation would need to have had a large effect to show statistical significance. In addition, finding and enrolling patients early in their journey was a challenge throughout this study, resulting in many patients on the intervention side who were not exposed to navigation during the first 30, 60 or even 90 days following diagnosis. Thus, while early cut off points did not show a significant improvement in diagnosis for those with a navigator, it is possible that a future, more highly powered study in which participants were exposed to navigation earlier would come to a different conclusion. Future implementation of navigator programs in AI/AN communities would benefit from thoughtful strategies to position the program as a service for prevention of cancer, rather than a program for diagnosed cancer patients, which posed a barrier for this NTCNP. Recent work being conducted with other navigator programs in Indian Country has determined that bringing navigation services to the community’s attention through educational workshops can be effective, and resulted not only in increased requests for help with scheduling screening and other services through the navigator but also with patient knowledge of cancer topics [46].
Navigating the health care system can be overwhelming for individuals of all educational levels, regardless of type of health insurance or basic information they may have. AI/AN individuals can benefit from patient navigation to help them find, arrange and reach cancer screening, treatment and other services for which they may be eligible. Redwood et al. reported in their study conducted among Alaska Native population that with ongoing efforts and intervention with patient navigation they were able to increase screening rates from 29% in 2000 to 55% in 2010 [39]. Our study found that having a navigator gave patients a better chance at obtaining diagnostic resolution, indicating that navigators were successful in combating loss to follow-up and preventing patients from “falling through the cracks”. This observation suggests navigators improved continuity of care and patient communication with their primary care clinic. Their assistance appears to be crucial in preventing individuals from dropping out of the health care system. Within AI/AN populations, those at highest risk may include those who are older, fearful of the health care system, geographically isolated and with limited transportation. It appears that patient navigators can enhance engagement and follow through with cancer care.
The initial results of patient navigation in Indian Country appear promising. It would be beneficial to conduct additional studies employing navigation in the community and clinic to determine the effects of comprehensive navigator involvement on client outcomes. In particular, expanding navigation to earlier stages, including screening, seems a logical next step. Clearly the benefit of diagnostic resolution is one which has the potential to have significant impact on the cancer health disparities present in Indian Country.
Acknowledgments
Grant Support
The Northwest Tribal Cancer Navigator Program was funded by the National Cancer Institute’s Center to Reduce Cancer Health Disparities, and the Grant number is U01 CA116925-01.
Contributor Information
Victoria Warren-Mears, Northwest Tribal Epidemiology Center, Northwest Portland Area Indian Health Board, 2121 SW Broadway, Suite 300, Portland, Oregon 97201.
Jenine Dankovchik, Northwest Tribal Cancer Navigator Program & NW IDEA Project, Northwest Portland Area Indian Health Board, 2121 SW Broadway, Suite 300, Portland, OR 97201, Ph# 503-416-3265, Fax # 503-228-8182, jdankovchik@npaihb.org.
Meena Patil, Northwest Tribal Cancer Navigator Program, Northwest Portland Area Indian Health Board, 2121 SW Broadway, Suite 300, Portland, Oregon 97201, Ph# 503-416-3265, Fax # 503-228-8182, mpatil@npaihb.org.
Rongwei Fu, Department of Public Health and Preventive Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Road, Portland, OR 97239-3098, Ph# 503-494-6069, fur@ohsu.edu.
References
- [1].Burhansstipanov L, Gilbert A, LaMarca K, Krebs LU. An innovative path to improving cancer care in Indian country. Public health reports. 2001;116(5):424–33. doi: 10.1093/phr/116.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mahoney MA, M.C. The health status of American Indians and Alaska Natives: Lessons for cancer educators. Journal of cancer education. 1999;14(1):23–7. doi: 10.1080/08858199909528569. [DOI] [PubMed] [Google Scholar]
- [3].Northwest Portland Area Indian Health Board . Cancer Among Northwest American Indians and Alaska Natives. Northwest Tribal Epidemiology Center; Portland, OR: 2011. [Google Scholar]
- [4].Facing Cancer in Indian Country: The Yakama Nation and Pacific Northwest Tribes. US Dept of Health and Human Services, National Institutes of Health, National Cancer Institute; Washington, DC: 2003. President’s Cancer Panel Annual Report. [Google Scholar]
- [5].Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104(4):848–855. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- [6].Bastani R, Yabroff KR, Myers RE, Glenn B. Interventions to improve follow-up of abnormal findings in cancer screening. Cancer. 2004;101(5 Suppl):1188–200. doi: 10.1002/cncr.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Warne D, Kaur J, Perdue D. American Indian/Alaska Native cancer policy: systemic approaches to reducing cancer disparities. Journal of cancer education. 2012;27(Suppl 1):S18–23. doi: 10.1007/s13187-012-0315-6. [DOI] [PubMed] [Google Scholar]
- [8].Guadagnolo BA, Boylan A, Sargent M, Koop D, Brunette D, Kanekar S, Shortbull V, Molloy K, Petereit DG. Patient navigation for American Indians undergoing cancer treatment: utilization and impact on care delivery in a regional healthcare center. Cancer. 2011;117(12):2754–61. doi: 10.1002/cncr.25823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coughlin SS, Uhler RJ, Blackman DK. Breast and cervical cancer screening practices among American Indian and Alaska Native women in the United States, 1992-1997. Preventive medicine. 1999;29(4):287–95. doi: 10.1006/pmed.1999.0537. [DOI] [PubMed] [Google Scholar]
- [10].Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005;104(3):629–39. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- [11].McKee D. Improving the follow-up of patients with abnormal Papanicolaou smear results. Archives of family medicine. 1997;6(6):574–7. doi: 10.1001/archfami.6.6.574. [DOI] [PubMed] [Google Scholar]
- [12].Kanekar S, Petereit D. Walking forward: a program designed to lower cancer mortality rates among American Indians in Western South Dakota. South Dakota Medicine. 2009;62(4):151–159. [PMC free article] [PubMed] [Google Scholar]
- [13].“CDC,” [Accessed 11 10 2011]; [Online]. Available: http://www.cdc.gov/cancer/cervical/statistics/screening.htm.
- [14].Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska Natives-findings from the behavioral risk factor surveillance system, 2000-2006. Cancer. 2008;113(5 Suppl):1131–41. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- [15].Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Gafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA: a cancer journal for clinicians. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- [16].Kothari A, Fentiman IS. Diagnostic delays in breast cancer and impact on survival. International journal of clinical practice. 2003;57(3):200–3. [PubMed] [Google Scholar]
- [17].Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of internal medicine. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- [18].Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- [19].Petereit DG, Rogers D, Govern G, Coleman N, Osburn CH, Howard SP, et al. Increasing access to clinical cancer trials and emerging technologies for minority populations: the Native American Project. Journal of clinical oncology. 2004;22(22):4452–5. doi: 10.1200/JCO.2004.01.119. [DOI] [PubMed] [Google Scholar]
- [20].Call KT, McAlpine DD, Johnson PJ, Beebe TJ, McRae JA, Song Y. Barriers to care among American Indians in public health care programs. Medical care. 2006;44(6):595–600. doi: 10.1097/01.mlr.0000215901.37144.94. [DOI] [PubMed] [Google Scholar]
- [21].Paskett ED, Tatum D, Rushing J, Michielutte R, Bell R, Foley KL, et al. Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer. 2004;101(11):2650–9. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Practice. 1995;3(1):19–30. [PubMed] [Google Scholar]
- [23].Psooy BJ, Schreuer D, Borgaonkar J, Caines JS. Patient navigation: improving timeliness in the diagnosis of breast abnormalities. Canadian Association of Radiologists journal. 2004;55(3):145–50. [PubMed] [Google Scholar]
- [24].Guadagnolo BA, Cina K, Helbig P, Molloy K, Reiner M, Cook EF, et al. Medical mistrust and less satisfaction with health care among Native Americans presenting for cancer treatment. Journal of health care for the poor and underserved. 2009;20(1):210–26. doi: 10.1353/hpu.0.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. Journal of urban health: bulletin of the New York Academy of Medicine. 2007;85(1):114–24. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gabram SG, Lund BMJ, Gardner J, Hatchett N, Harvey LB, Okoli J, et al. Effects of an outreach and internal navigation program on breast cancer diagnosis in an urban cancer center with a large African-American population. Cancer. 2008;113(3):602–607. doi: 10.1002/cncr.23568. [DOI] [PubMed] [Google Scholar]
- [27].Lerman C, Hanjani P, Caputo C, Miller S, Delmoor E, Nolte S, et al. Telephone counseling improves adherence to colposcopy among lower-income minority women. Journal of clinical oncology. 1992;10(2):330–3. doi: 10.1200/JCO.1992.10.2.330. [DOI] [PubMed] [Google Scholar]
- [28].Kerner JF, Yedidia M, Padgett D, Muth B, Washington KS, Tefft M, et al. Realizing the promise of breast cancer screening: clinical follow-up after abnormal screening among Black women. Preventive medicine. 2003;37(2):92–101. doi: 10.1016/s0091-7435(03)00087-2. [DOI] [PubMed] [Google Scholar]
- [29].Melnikow J, Chan BK, Stewart GK. Do follow-up recommendations for abnormal Papanicolaou smears influence patient adherence? Archives of family medicine. 1999;8(6):510–4. doi: 10.1001/archfami.8.6.510. [DOI] [PubMed] [Google Scholar]
- [30].Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–26. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- [31].Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Preventive medicine. 2007;44(1):26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [32].Robinson-White S, Conroy B, Slavish KH, Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer nursing. 2010;33(2):127–140. doi: 10.1097/NCC.0b013e3181c40401. [DOI] [PubMed] [Google Scholar]
- [33].Markossian TW, Calhoun EA. Are breast cancer navigation programs cost-effective? Evidence from the Chicago Cancer Navigation Project. Health Policy. 2011;99(1):52–59. doi: 10.1016/j.healthpol.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Freeman HP. A Model Patient Navigation Program. Oncology Issues. 2004:44–46. [Google Scholar]
- [35].Lasser KE, Murillo J, Lisboa S, Casimir AN, Valley-Shah L, Emmons KM, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Archives of internal medicine. 2011;171(10):906–12. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- [36].Heckman BD, Fisher EB, Merbaum M, Ristvedt S, Bishop C. Coping and anxiety in women recalled for additional diagnostic procedures following an abnormal screening mammogram. Health psychology. 2004;23(1):42–8. doi: 10.1037/0278-6133.23.1.42. [DOI] [PubMed] [Google Scholar]
- [37].Wilson RT, Adams-Cameron M, Burhansstipanov L, Roubidoux MA, Cobb N, Lynch CF, et al. Disparities in breast cancer treatment among American Indian, Hispanic and non-Hispanic White Women Enrolled in Medicare. Journal of health care for the poor and underserved. 2007;18(3):648–64. doi: 10.1353/hpu.2007.0071. [DOI] [PubMed] [Google Scholar]
- [38].Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: results from the National Breast and Cervical Cancer Early Detection Program, 1991-1995. American journal of public health. 2000;90(1):130–4. doi: 10.2105/ajph.90.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Redwood D, Provost E, Perdue D, Haverkamp D, Espey D. The last frontier: innovative efforts to reduce colorectal cancer disparities among the remote Alaksa Native population. Gastrointestinal Endoscopy. 2012;75(3):474–480. doi: 10.1016/j.gie.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Raj A, Ko N, Battaglia TA, Chabner BA, Moy B. Patient Navigation for underserved patients diagnosed with breast cancer. The Oncologist. 2012;17(8):1006–1010. doi: 10.1634/theoncologist.2012-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Percac-Lima S, Milosavljevic B, Abernethy Oo S, Marable D, Bond B. Patient navigation to improve breast cancer screening in Bosnian refugees and immigrants. Journal of immigrant minority health. 2012;14(4):727–730. doi: 10.1007/s10903-011-9539-5. [DOI] [PubMed] [Google Scholar]
- [42].Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. Journal of general internal medicine. 2012;27(8):1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Weber JJ, Mascarenhas DC, Bellin LS, Raab RE, Wong JH. Patient navigation and the quality of breast cancer care: an analysis of the breast cancer care quality indicators. Annals of surgical oncology. 2012;19(10):3251–3256. doi: 10.1245/s10434-012-2527-8. [DOI] [PubMed] [Google Scholar]
- [44].Wong LJ, Valery PC, Beesley VL, Moore SP, Lokuge K, et al. Navigating the cancer journey: a review of patient navigator programs for indigenous cancer patients. Asia-Pacific journal of clinical oncology. doi: 10.1111/j.1743-7563.2012.01532.x. doi: 10.1111/j.1743-7563.2012.01532.x. [DOI] [PubMed] [Google Scholar]
- [45].Braun KL, Kagawa-Singer M, Holden AEC, Burhansstipanov L. Cancer patient navigator tasks across the cancer care continuum. Journal of health care for the poor and underserved. 2012;23(1):398–413. doi: 10.1353/hpu.2012.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Burhansstipanov L, Krebs LU, Watanabe-Galloway S, Petereit DG, Pingatore NL, et al. Preliminary lessons learned from the ”Native Navigators and the Cancer Continuum (NNACC) Journal of cancer education. 2012;27(Suppl 1):S57–S65. doi: 10.1007/s13187-012-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]