Abstract
Background
Like fear conditioning, the acquisition phase of extinction involves new learning that is mediated by the amygdala. During extinction training, the conditioned stimulus is repeatedly presented in the absence of the unconditioned stimulus and the expression of previously learned fear gradually becomes suppressed. Our previous study revealed that chronic treatment with a selective serotonin reuptake inhibitor (SSRI) impairs the acquisition of auditory fear conditioning. To gain further insight into how SSRIs affect fear learning, we tested the effects of chronic SSRI treatment on the acquisition of extinction.
Methods
Rats were treated chronically (22 days) or subchronically (9 days) with the SSRI citalopram (10 mg/kg/day) before extinction training. The results were compared to those following chronic and subchronic treatment with tianeptine (10 mg/kg/day), an antidepressant with a different method of action. The expression of the NR2B subunit of the NMDA receptor in the amygdala was examined after behavioral testing.
Results
Chronic but not subchronic administration of citalopram impaired the acquisition of extinction and downregulated the NR2B subunit of the NMDA receptor in the lateral and basal nuclei of the amygdala. Similar behavioral and molecular changes were found with tianeptine treatment.
Conclusions
These results provide further evidence that chronic antidepressant treatment can impair amygdala-dependent learning. Our findings are consistent with a role for glutamatergic neurotransmission in the final common pathway of antidepressant treatment.
Keywords: fear conditioning, SSRI, citalopram, amygdala, NR2B subunit, extinction
INTRODUCTION
Selective serotonin reuptake inhibitors (SSRIs) are widely prescribed for treating a range of anxiety disorders (1–5). Although the known pharmacological actions of these drugs take place immediately (6), patients require several weeks of continuous treatment before experiencing their therapeutic effects (7). Despite extensive experimental work focused on understanding this delay in therapeutic onset, the relevant underlying mechanisms remain unknown.
Evidence indicates that the amygdala may be one site of action for long-term SSRI treatment (8–13). Our previous study in rats revealed that chronic SSRI treatment impairs the acquisition of auditory fear conditioning (14), a task that depends on the amygdala (15, 16). In fear conditioning, a neutral conditioned stimulus (CS), such as a tone, acquires the capacity to elicit fear-related behavioral responses after being associated with an aversive unconditioned stimulus (US), such as a footshock. When the CS is repeatedly presented in the absence of the US, conditioned fear responses diminish, a process called extinction.
Extinction does not destroy original fear memories, but instead involves learning new information about the relationship between the CS and US (17–19). Learning during both initial fear conditioning and extinction depends upon activation of N-methyl-D-aspartate receptors (NMDARs) in the lateral and basal nuclei of the amygdala. The finding that both types of learning are impaired by selective blockade of the NR2B subunit of the NMDAR in the amygdala, indicate a distinct role for this subunit in the underlying plasticity (20, 21).
The extinction phases of fear conditioning are thought to be important from a therapeutic standpoint. Extinction-based cognitive behavioral therapy (CBT) is widely used in the treatment of anxiety disorders (22, 23), during which patients systematically confront feared objects, situations, autonomic responses, or memories in the absence of an aversive event. The therapeutic value of targeting extinction is exemplified by studies using the drug d-cycloserine, which facilitates extinction in animals (24) and enhances the outcome of exposure therapy (25–30).
Given that antidepressants and extinction-based CBT are each effective in treating anxiety disorders (1–3, 23, 31), the expectation has been that combining these treatments would further enhance outcome. However, numerous studies reveal that combination treatment leads to few advantages when compared to either treatment modality alone (32–38), prompting the suggestion that medication may interfere with the beneficial effects of CBT (39, 40). Understanding how SSRIs affect the cognitive mechanisms underlying extinction-based therapies will provide important insight into how combination treatment affects clinical outcome.
We tested the effects of chronic treatment with the SSRI citalopram on the acquisition of fear extinction in rats. In contrast to our previous study, rats were fear conditioned drug-free and SSRI treatment began the next day, a protocol that better mimics the clinical setting. To evaluate the length of treatment time required for changes in extinction to be detected, the effects of subchronic citalopram treatment were also measured. In an effort to reveal some of the neuroadapative changes underlying SSRI-mediated modifications in extinction learning, NR2B protein levels in the amygdala were quantified upon completion of behavioral testing. The results were compared to those following chronic and subchronic treatment with tianeptine, an antidepressant with a different method of action. Unlike citalopram, which increases the synaptic availability of serotonin by binding to the serotonin transporter protein, tianeptine does not bind to any monoaminergic receptors or transporters (41, 42) and does not affect extracellular levels of serotonin (43). Instead, the effects of tianeptine have been linked to changes in the glutamatergic system (44, 45).
METHODS AND MATERIALS
Subjects
Adult male Sprague-Dawley rats (Hilltop Laboratories, Scottdale, Pennsylvania) weighing 280–310g were housed individually in clear plastic cages in a thermally controlled colony room with a 12-hour light/dark cycle, where food and water were provided ad libitum. All procedures were performed at New York University, were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals, and were approved by the New York University Animal Care and Use Committee.
Drugs
Citalopram hydrobromide (Sigma, St. Louis, MO) and tianeptine sodium salt (courtesy of Servier, Courbevoie, France) were dissolved in 0.9% sterile saline and injected intraperitoneally (i.p.) at a dose of 10 mg/kg (14). Based on studies measuring plasma drug levels in rats (46–48) and humans (49, 50), the human dose equivalence is 25 mg for citalopram and 37 mg for tianeptine. Drugs were made fresh daily in a volume of 1 mL and administered at approximately the same time each day. All animals were weighed daily so the appropriate dose could be calculated.
Behavioral Procedures
Apparatus
Fear conditioning took place in a brightly lit Plexiglas rodent conditioning chamber with a metal grid floor and three white house lights, which was enclosed within a sound-attenuating cubicle (ENV-001; Med Associates, Georgia, Vermont). The context of the chamber was changed during extinction training by covering the metal rods on the floor with a flat black Formica plate scented with peppermint soap and putting red lenses on two of the house lights (51, 52). An overhead camera videotaped behavior.
Auditory Fear Conditioning
Rats were habituated to handling and the conditioning context for 20 minutes. The next day, they were trained with a single conditioning trial involving two presentations of a tone CS (20 sec, 10 kHZ, 75 dB) that co-terminated with a footshock US (0.5 sec, 0.7 mA), with an inter-trial interval that varied randomly between 90 and 120 seconds. Freezing (see below) was scored during training so that natural variability in acquisition could be counterbalanced across treatment groups the next day.
Twenty-four hours after conditioning, separate groups of rats were weighed and injected with citalopram, tianeptine, or saline. Animals treated chronically were injected once/day for 22 consecutive days and animals treated subchronically were injected once/day for 9 consecutive days. There was a separate control group for each type of treatment (subchronic or chronic) and for each drug (citalopram or tianeptine). Rats received their final injection one hour before extinction training, and were on drug when extinction training began. Extinction training involved ten presentations of the CS tone in the absence of a US shock (Tones 1–10). The next day, drug was not administered and long-term memory of extinction was tested with 10 additional CS tones (Tones 11–20). The properties of the tones and the inter-trial interval were identical during fear conditioning and each extinction trial.
Freezing, defined as the cessation of all movement unrelated to respiration (53), was used as a measure of conditioned fear. An experimenter blind to treatment group watched the videotapes and measured the number of seconds rats froze during each 20-second tone with a digital timer. Freezing is reported as a percentage of total tone presentation time.
Determination of NR2B Protein in the Amygdala
Immediately following presentation of the final tone (Tone 20), a subset of rats in each condition were deeply anesthetized with Nembutal (120 mg/kg, i.p.) and decapitated. Brains were removed, frozen on dry ice and stored at −80°C until processed. A 1 mm punch tool (Fine Science Tools, Foster City, CA) was used to obtain the lateral and basal nuclei of the amygdala from 400 µm-thick sections cut on a freezing microtome. Portions of the lateral central nucleus and cortical tissue directly lateral to the external capsule may have been included in the punches. Tissue samples were homogenized in 50 µl of ice-cold buffer (0.35 M sucrose, 0.5 mM EGTA, 2 mM EDTA, 2 mM Na3VO4, and 1 mM PMSF, in 10 mM Tris-HCl, pH 7.4), centrifuged at 2000 X g, and the pellet was removed. The homogenate was sonicated for 10 mins, boiled for 5 mins with added sample buffer, electrophoresed on 7.5% SDS-polyacrylamide gels and blotted onto Immobilon-P membranes (Millipore, Bedford, MA). Western blots were blocked in 5% milk in TTBS buffer (50 mM Tris-HCl, pH 7.5, 150mM NaCl, and 0.05% Tween 20) and incubated with anti-NR2B (1:250; BD Biosciences). Blots were incubated with anti-mouse conjugated to horseradish-peroxidase (Jackson Immunoresearch, West Grove, PA) and visualized using chemiluminescence (Pierce, Rockford, IL). Actin was used as a loading control (1:1000; Sigma, St Louis, MO). To control for variance between gels, NR2B signal of each sample was divided by its actin signal and expressed as a percentage of that in the vehicle-treated group on the same blot.
Statistical Analysis
Data were analyzed with Student t test for independent samples or a one- or three-way ANOVA. A one-way ANOVA a priori test was used for planned mean comparisons on freezing during the first tone trial of extinction training. JMP Version 5 software (SAS Institute Inc., Cary, NC) was used for the analyses and significance was accepted for p<0.05; p-values > 0.05 but < 0.1 were considered trends.
RESULTS
During extinction training, freezing during the 20-second period before the first presentation of the tone (Pre-CS) was used as an indicator of the drug’s effects on motor activity. Freezing during the first presentation of the tone was a measure of the drug’s effects on fear expression and freezing during subsequent presentations of the tone indicated extinction learning. Data were analyzed with a three-way ANOVA with factors drug group (drug vs. saline), day (day 1 vs. day 2), and tone trial (1–20; repeated measures).
Chronic SSRI Treatment Impairs the Acquisition of Fear Extinction
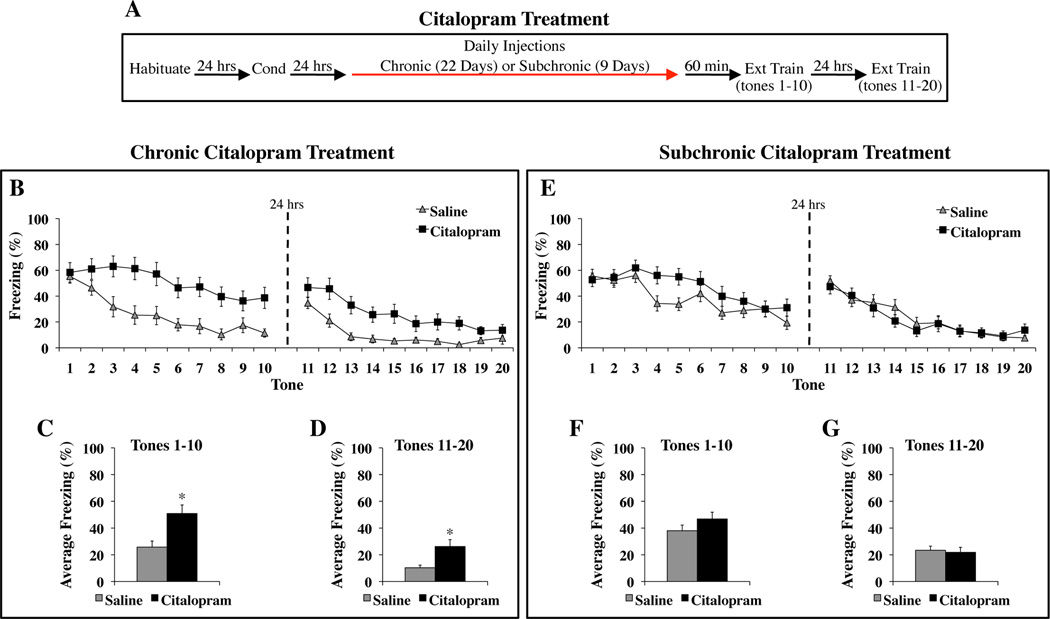
Rats treated chronically with citalopram or saline (Figure 1A) exhibited similar low levels of Pre-CS freezing (Cit: 5.67 ± 2.92 %; Sal: 1.79 ± 1.45 %) (F(1,27) = 1.35, p = 0.25) and similar CS-elicited freezing during the first trial of extinction training (F(1,27) = 0.10, p = 0.75). With subsequent tone presentations, saline-treated rats showed a gradual reduction in freezing, while citalopram-treated rats displayed sustained freezing, indicating impaired extinction learning (Figure 1B, left). The next day, vehicle-treated rats exhibited low levels of freezing, indicating successful retrieval of extinction learning. Freezing elicited by the citalopram-treated group was still higher than controls, but gradually diminished to control levels by the end of the session (Figure 1B, right).
Figure 1. Chronic but not subchronic citalopram treatment impairs the acquisition of fear extinction.
(A) General behavioral procedures: 24 hours after habituation, rats were fear conditioned with two tone-shock pairings. The next day, animals began receiving daily injections of citalopram (10 mg/kg, i.p.) or saline. Chronic treatment lasted for 22 consecutive days. Subchronic treatment lasted for 9 consecutive days. Extinction training began the same day the final injection was administered and involved 20 presentations of the tone alone over the course of two days. (B–D) The effects of chronic citalopram treatment on extinction learning. (B) Mean ± SEM percent freezing of citalopram-treated (n=15) and saline-treated (n=14) rats during each trial of extinction training. The average response of each group was not significantly different during tone 1, indicating that chronic citalopram treatment did not affect expression of the fear memory. (C) Mean ± SEM percent freezing of each group averaged across the first 10 tones, which were presented on the first day of extinction training. (D) Mean ± SEM percent freezing of each group averaged across the last 10 tones, which were presented on the second day of extinction training. (E–G) The effects of subchronic citalopram treatment on extinction learning. (E) Mean ± SEM percent freezing of citalopram-treated (n=19) and saline-treated (n=24) rats during each trial of extinction training. The average response of each group was not significantly different during tone 1, but was significantly different during tones 4 and 5 (p<0.05). (F) Mean ± SEM percent freezing of each group averaged across the first 10 tones, which were presented on the first day of extinction training. (G) Mean ± SEM percent freezing of each group averaged across the last 10 tones, which were presented on the second day of extinction training. *p<0.01 versus saline.
The three-way ANOVA reveled significant effects of drug group (F(1,27) = 11.32, p<0.01), day (F(1, 27) = 39.74, p<0.01), tone trial (F(9,243) = 28.94, p<0.01), and a significant drug group X tone trial interaction (F(9,243) = 2.59, p<0.01). When each day was analyzed separately, citalopram significantly enhanced freezing during the first ten tones (F(1,27) = 10.05, p<0.01) (Figure 1C) and the subsequent ten tones the following day (F(1,27) = 7.93, p<0.01) (Figure 1D). Citalopram enhanced freezing during tone 10 (t(27) = 3.02, p<0.01) but not tone 20 (t(27) = 0.96, p = 0.34). Since citalopram-treated rats were impaired through the end of the initial day of extinction training, the deficit detected during the second day only confirmed the impairment in extinction learning found the previous day and cannot be used to address the effects of drug treatment on the consolidation and/or retrieval of extinction. These findings indicate that chronic citalopram treatment impaired the acquisition of extinction across two days of training.
Subchronic SSRI Treatment Does Not Impair the Acquisition of Fear Extinction
Rats treated subchronically with citalopram or saline (Figure 1A) exhibited similar low levels of Pre-CS freezing (Cit: 5.00 ± 4.21 %; Sal: 2.29 ± 1.47 %) (F(1,41) = 0.44, p = 0.51) and similar CS-elicited freezing during the first trial of extinction training (F(1,41) = 0.17, p = 0.69). With subsequent tone presentations, both groups showed a gradual reduction in freezing, although citalopram transiently increased freezing during two tone trials in the middle of the training session (Figure 1E, left). The next day, both groups exhibited low levels of freezing (Figure 1E, right).
The three-way ANOVA revealed no significant effect of drug group (F(1,41) = 0.48, p=0.49). The effects of day (F(1,41) = 92.44, p<0.01), tone trial (F(9,369) = 37.86, p<0.01) and the drug group × day × tone trial interaction (F(9,369) = 2.18, p<0.05) were significant. When each day was analyzed separately, groups exhibited similar freezing responses to the first ten tones (F(1,41) = 1.86, p = 0.18) (Figure 1F) and the subsequent ten tones the next day (F(1,41) = 0.09, p=0.76) (Figure 1G). There were no differences between groups during tones 10 (t(41) = 1.41, p = 0.17) and 20 (t(41) = 1.24, p = 0.22). Significant group differences during tones 4 (t(41) = 2.46, p<0.05) and 5 (t(41) = 2.66, p<0.05) supported the impression that subchronic citalopram treatment led to a transient increase in freezing during extinction training.
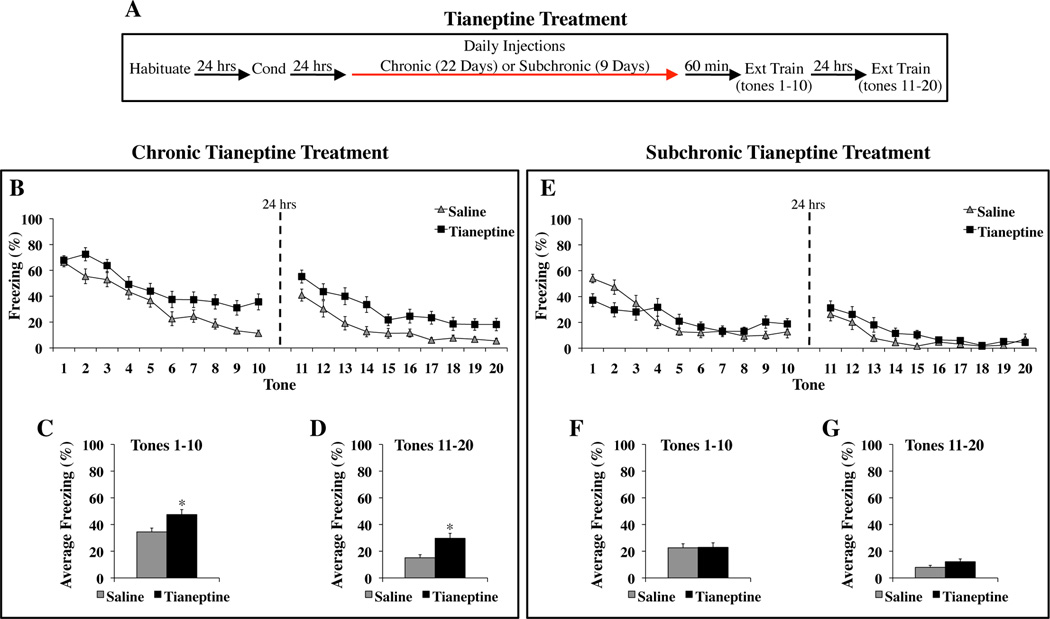
Chronic Tianeptine Treatment Impairs the Acquisition of Fear Extinction
Rats treated chronically with tianeptine or saline (Figure 2A) exhibited similar low levels of Pre-CS freezing (Tian: 9.17 ± 4.09 %; Sal: 9.35 ± 4.29 %) (F(1,45) = 0.0009, p = 0.98) and similar CS-elicited freezing during the first trial of extinction training (F(1,45) = 0.11, p = 0.74). With subsequent tone presentations, both groups showed a gradual decrease in freezing, although tianeptine-treated rats did not reach control levels by the end of the training session (Figure 2B, left). The next day, saline-treated rats exhibited low levels of freezing, while tianeptine-treated rats continued to freeze more than controls (Figure 2B, right).
Figure 2. Chronic but not subchronic tianeptine treatment impairs the acquisition of fear extinction.
(A) General behavioral procedures: 24 hours after habituation, rats were fear conditioned with two tone-shock pairings. The next day, animals began treatment with tianeptine (10 mg/kg, i.p.) or saline. Chronic treatment lasted for 22 consecutive days. Subchronic treatment lasted for 9 consecutive days. Extinction training began the same day the final injection was administered and involved 20 presentations of the tone alone over the course of two days. (B–D) The effects of chronic tianeptine treatment on extinction learning. (B) Mean ± SEM percent freezing of tianeptine-treated (n=24) and saline-treated (n=23) rats during each trial of extinction training. The average response of each group was not significantly different during tone 1, indicating that chronic tianeptine treatment did not affect expression of the fear memory. (C) Mean ± SEM percent freezing of each group averaged across the first 10 tones, which were presented on the first day of extinction training. (D) Mean ± SEM percent freezing of each group averaged across the last 10 tones, which were presented on the second day of extinction training. (E–G) The effects of subchronic tianeptine treatment on extinction learning. (E) Mean ± SEM percent freezing of tianeptine-treated (n=21) and saline-treated (n=24) rats during each trial of extinction training. Tianeptine-treated rats exhibited significantly less tone-elicited freezing than saline-treated rats during the first tone trial, indicating that subchronic tianeptine treatment reduced fear expression (p<0.01). (F) Mean ± SEM percent freezing of each group averaged across the first 10 tones, which were presented on the first day of extinction training. (G) Mean ± SEM percent freezing of each group averaged across the last 10 tones, which were presented on the second day of extinction training. *p<0.01 versus saline.
A three-way ANOVA revealed significant effects of drug group (F(1,45) = 10.44, p<0.01), day (F(1,45) = 115.54, p<0.01) and tone trial (F(9,405) = 48.62, p<0.01). There was no significant drug group × tone trial interaction (F(9, 405) = 0.58, p = 0.81). When each day was analyzed separately, tianeptine significantly enhanced freezing during the first ten tones (F(1,45) = 7.45, p<0.01) (Figure 2C) and the subsequent ten tones the following day (F(1,45) = 10.75, p<0.01) (Figure 2D). Tianeptine also increased freezing during tones 10 (t(45) = 3.60, p<001) and 20 (t(45) = 2.41, p<0.05). Since tianeptine-treated rats were impaired through the end of the initial day of extinction training, the deficit detected during the second day of training only confirmed the impairment in extinction learning found the previous day and cannot be used to address the effects of drug treatment on the consolidation and/or retrieval of extinction. These results indicate that like chronic citalopram treatment, chronic tianeptine treatment impaired extinction learning across two days of training.
Subchronic Tianeptine Treatment Does Not Impair the Acquisition of Fear Extinction
Rats treated subchronically with tianeptine or saline (Figure 2A) exhibited similar low levels of Pre-CS freezing (Tian: 0.71 ± 0.52 %; Sal: 1.25 ± 0.86 %) (F(1,43) = 0.26, p = 0.61). Subchronic tianeptine treatment reduced CS-elicited freezing during the first trial of extinction training (F(1,43) = 8.21, p<0.01), indicating reduced fear memory expression. With subsequent tone presentations, the saline group showed a gradual decrease in freezing, while freezing levels in the tianeptine group remained low (Figure 2E, left). The next day, both groups exhibited low levels of CS-elicited freezing (Figure 2E, right).
The three-way ANOVA revealed no significant effect of drug group (F(1,43) = 0.45, p =0.51). The effects of day (F(1,43) = 81.56, p<0.01), tone trial (F(9,387) = 32.78, p<0.01), and the drug group × day × tone trial interaction (F(9,387) = 4.33, p<0.01) were significant. When each day was analyzed separately, groups exhibited similar freezing responses to the first ten tones (F(1,43) = 0.005, p=0.94) (Figure 2F) and the subsequent ten tones the next day (F(1,43) = 2.84, p = 0.10) (Figure 2G). There were no group differences during tones 10 (t(43) = 0.97, p = 0.34) and 20 (t(43) = 0.54, p = 0.59). These results indicate that subchronic tianeptine treatment did not impair the acquisition of extinction.
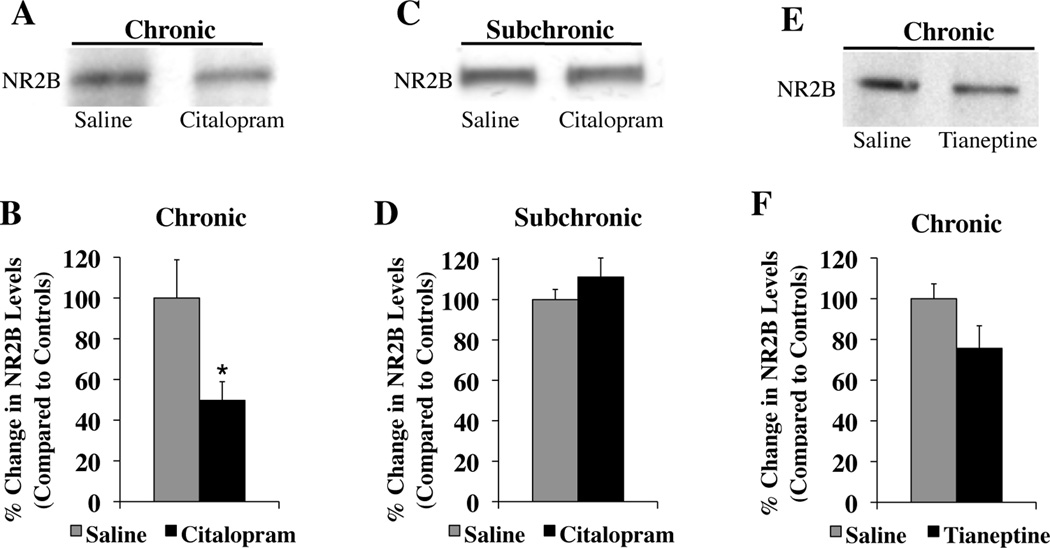
Chronic SSRI Treatment Decreases NR2B Expression Levels in the Amygdala
Rats treated chronically with citalopram had significantly less NR2B protein in the lateral and basal nuclei than animals treated chronically with saline (t(12) = 2.41, p<0.05) (Figure 3A, B). The amount of NR2B protein expression correlated negatively with the amount of freezing animals exhibited across two days of extinction training (r = −0.61). In contrast, animals treated subchronically with citalopram or saline had similar levels of NR2B protein (t(6) = 1.03, p = 0.34) (Figure 3C, D), indicating that downregulation of the NR2B subunit requires more than nine days of citalopram treatment. Chronic tianeptine treatment also appeared to reduce NR2B protein in the amygdala, but the decrease did not reach significance (t(21) = 1.87, p = 0.076) (Figure 3E, F). A different mechanism of action may underlie the impairment in extinction following chronic tianeptine treatment.
Figure 3. NR2B protein expression in the amygdala (A, C, & E).
Representative western blots of rat tissue containing the lateral and basal nuclei of the amygdala. (B & D) Bar graphs summarizing the effects of chronic (citalopram, n=7; saline, n=7) (B) and subchronic (citalopram, n=4; saline, n=4) (D) citalopram treatment on NR2B subunit expression. (F) Bar graph summarizing the effects of chronic (tianeptine, n=11; saline, n=12) tianeptine administration on NR2B subunit expression. All samples were normalized to actin levels and then compared to controls samples. *p<0.05 versus saline.
DISCUSSION
Main Findings
It has long been understood that extinction does not erase conditioned fear memories, but is instead a form of new learning (17, 18). Here we show that chronic but not subchronic administration of citalopram and tianeptine impairs the acquisition of fear extinction in rats. In a previous study, we showed that chronic but not acute administration of these same drugs impairs the acquisition of a conditioned fear memory (14). Hence, two drugs with proven antidepressant efficacy adversely affect two types of learning tested with auditory fear conditioning when administered chronically. In contrast, citalopram and tianeptine modulate fear circuits differently when administered subchronically. While subchronic citalopram treatment briefly impairs the acquisition of extinction, subchronic tianeptine treatment decreases expression of a fear memory. An investigation of the neuroadaptive changes that accompany chronic SSRI treatment reveals that the citalopram-induced impairment in extinction learning is associated with downregulation of the NR2B subunit of the NMDA receptor in the lateral and basal nuclei of the amygdala. The time course of these behavioral and molecular effects in rodents mirrors the delay in onset of therapeutic action found among clinical populations.
A Role for Amygdala NMDA Receptors in Mediating Antidepressant-Induced Impairments in Extinction Learning
Numerous studies implicate a role for the amygdala in extinction learning. The acquisition of extinction is blocked by inactivation of the basal nucleus of the amygdala (54) and impaired by intra-amygdala infusions of NMDAR antagonists (55–58). A subset of cells in the basal nucleus show an in increase in CS-evoked activity during extinction training (54). Interestingly, the amygdala is not required for extinguishing the association between two neutral stimuli (58).
NMDA receptors are heteromeric complexes comprised of the NR1 subunit and different NR2 subunits (59, 60), each of which confers distinct biophysical and pharmacological properties of the receptor (61, 62). Compared to the NR2A subunit, the NR2B subunit is important for learning and memory and associated synaptic plasticity (63–68). Selective blockade of the NR2B subunit in the lateral amygdala impairs both the acquisition of auditory fear conditioning and the acquisition of extinction, without affecting expression of the fear memory (20, 21). Here and in our previous study (14), we found the same pattern of learning impairments following chronic citalopram treatment. Similarly, chronic fluoxetine treatment impairs the reacquisition of fear responses (69), which is also dependent upon NMDA receptors in the amygdala. A direct role of the NR2B subunit in mediating these effects is suggested by our finding that impaired extinction learning was associated with downregulation of the NR2B subunit in the amygdala. Although it is not known how chronic SSRI treatment leads to this downregulation, studies in the prefrontal cortex implicate the involvement of the 5-HT1A and 5-HT2A receptors (70, 71). Our results are in line with earlier work showing that chronic antidepressant treatment modulates the structure and function of NMDA receptors (72–74) and indicate that a functional consequence of such a change is impaired fear extinction learning.
In contrast to the cognitive deficits that result from reduced glutamatergic neurotransmission, improvements in mood and anxiety are also found when glutamatergic transmission is antagonized. Inhibiting the glutamatergic system with the NMDA receptor antagonist ketamine or the presynaptic glutamate release inhibitor riluzole produces an antidepressant effect (75–77) and may improve symptoms of anxiety (78–80). Selectively blocking the NR2B subunit is sufficient to produce an antidepressant response (79, 81, 82) and anxiolytic-like effects in animals (79). These beneficial effects of reducing glutamatergic transmission, along with previous work identifying changes in NMDA and AMPA receptors with antidepressant treatment (42,72–74, 83), and our finding that chronic citalopram and tianeptine administration similarly downregulate the NR2B subunit, all support the hypothesis that adaptive changes in the glutamatergic system within different brain regions represent a final common pathway for the therapeutic action of antidepressants (84–86). Unfortunately, such adaptive changes block the facilitating effects of the partial NMDA agonist D-cycloserine on extinction (87), indicating that the opposing effects of glutamatergic neurotransmission on fear learning and mood/anxiety may pose a problem when combining different types of treatment.
The impairment in extinction learning that we find following twenty-two days of administration with two independent antidepressants contrasts with a recent study reporting that fourteen days of fluoxetine treatment facilitates learning to extinguish a conditioned response (88). One difference between these studies is that the latter was conducted in mice, whereas we used rats, which is the primary species used in Pavlovian fear conditioning studies (89–93). Rats and mice respond differently to several drugs (94, 95), including MDMA, which has a neurodegenerative effect on serotonin in rats but not mice (96). Species differences in baseline corticosterone levels (97), the expression of some serotonin, estrogen and corticosteroid receptors (98–100), and risk assessment (101) could contribute to a differential drug response. Alternatively, the discrepant results might be attributable to the use of different experimental conditions. In our study, rats were weighed and injected for twenty-two consecutive days and were habituated to the training context the day before fear conditioning. Consequently, they were handled significantly more than the mice used in the other study, which were given fluoxetine in the drinking water and were not habituated prior to training. While handling significantly elevates physiological correlates of stress in rats and mice (102), weighing a rat and administering seven daily injections decreases anxiety compared to non-handled controls (103). The potential relevance of an altered stress response is implicated by the finding that brief handling in conjunction with administration of the partial NMDA receptor agonist, D-cycloserine, enhances the consolidation of extinction learning (104), while a decrease in corticosterone impairs the acquisition of extinction tested with contextual fear conditioning (105). Although it is expected that daily handling of our rats decreased non-specific responses to the experimental procedure, it is possible that consequent changes in stress contributed to the effects we report. These and other methodological differences, including the use of different SSRIs for different periods of time, may account for the discrepant findings.
Clinical Implications
Numerous studies have investigated whether the combination of antidepressant treatment and extinction-based CBT is more effective than either monotherapy alone for treating anxiety disorders. While some find a modest benefit of combining treatment in the short-term (32–35), others report no short-term advantage (36–38, 106, 107). However there is evidence that combined treatment impedes the long-term benefits of exposure therapy. For example, when panic disorder patients were evaluated after treatment discontinuation, the outcome of those previously treated with CBT and imipramine (32) or alprazolam (33) was worse than patients who received CBT alone. Similarly, when social anxiety disorder patients were evaluated after treatment ended, the outcome of patients previously treated with both CBT and sertraline (108) was worse than patients who received CBT alone. It has been suggested that the limited improvements seen with combined treatment are the result of both the beneficial anxiolytic effects of antidepressants and their disruptive effects on exposure-based therapy (39, 40). One way in which the inclusion of antidepressant is thought to be disruptive is by creating distinct internal states during treatment and follow-up (40). Since extinction is context-dependent (109) and internal state is a contextual cue, fear that is extinguished on-drug could be renewed off-drug (110). Such a change in internal state provides one potential explanation for why any advantage of combined treatment is lost upon discontinuation of medication (40). Data presented here indicates that the blunted effects of medication on extinction learning during exposure-based CBT may also contribute to the disruptive effects of antidepressants on CBT found over time.
Summary
In summary, we found that chronic but not subchronic administration of two antidepressants with distinct pharmacological properties produces impaired extinction learning in rats. This impairment was associated with downregulation of the NR2B subunit of the NMDA receptor in the amygdala. These and our previous findings demonstrate that chronic antidepressant administration impairs amygdala-dependent learning. Our work supports a role for glutamatergic neurotransmission in the final common pathway of antidepressant treatment.
ACKNOWLEDGEMENTS
This work was supported by NIH grant MH58911 to J.E.L. and MH41256 to B.S.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE: None of the authors reported biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Baldwin DS, Ajel KI, Garner M. Pharmacological treatment of generalized anxiety disorder. Curr Top Behav Neurosci. 2010;2:453–467. doi: 10.1007/7854_2009_2. [DOI] [PubMed] [Google Scholar]
- 2.Irons J. Fluvoxamine in the treatment of anxiety disorders. Neuropsychiatr Dis Treat. 2005;1:289–299. [PMC free article] [PubMed] [Google Scholar]
- 3.Kent JM, Coplan JD, Gorman JM. Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry. 1998;44:812–824. doi: 10.1016/s0006-3223(98)00210-8. [DOI] [PubMed] [Google Scholar]
- 4.Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. J Clin Psychiatry. 2007;68:711–720. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- 5.Insitute of Medicine. Treatment of PTSD: An Assessment of the Evidence. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 6.Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 7.Schatzberg AF, Dessain E, O'Neil P, Katz DL, Cole JO. Recent studies on selective serotonergic antidepressants: trazodone, fluoxetine, and fluvoxamine. J Clin Psychopharmacol. 1987;7:44S–49S. [PubMed] [Google Scholar]
- 8.Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 10.Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 11.Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 12.Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U, et al. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage. 2010;49:1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 15.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Maren S. Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiol Learn Mem. 2001;76:268–283. doi: 10.1006/nlme.2001.4042. [DOI] [PubMed] [Google Scholar]
- 17.Bouton ME. Conditioning, Remembering, and Forgetting. J Exp Psychol Anim B. 1994;20:219–231. [Google Scholar]
- 18.Rescorla RA. Preservation of Pavlovian Associations through Extinction. The Quarterly Journal of Experimental Psychology. 1996;49B:245–258. [Google Scholar]
- 19.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. Journal of Neuroscience. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- 22.Barlow DH. Long-term outcome for patients with panic disorder treated with cognitive-behavioral therapy. J Clin Psychiatry. 1990;51(Suppl A):17–23. [PubMed] [Google Scholar]
- 23.Rothbaum BO, Schwartz AC. Exposure therapy for posttraumatic stress disorder. Am J Psychother. 2002;56:59–75. doi: 10.1176/appi.psychotherapy.2002.56.1.59. [DOI] [PubMed] [Google Scholar]
- 24.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 27.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 30.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- 31.Ougrin D. Efficacy of exposure versus cognitive therapy in anxiety disorders: systematic review and meta-analysis. BMC Psychiatry. 2011;11:200. doi: 10.1186/1471-244X-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA. 2000;283:2529–2536. doi: 10.1001/jama.283.19.2529. [DOI] [PubMed] [Google Scholar]
- 33.Marks IM, Swinson RP, Basoglu M, Kuch K, Noshirvani H, O'Sullivan G, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry. 1993;162:776–787. doi: 10.1192/bjp.162.6.776. [DOI] [PubMed] [Google Scholar]
- 34.Power KG, Simpson RJ, Swanson V, Wallace LA, Feistner ATC, Sharp D. A Controlled Comparison of Cognitive-Behavior Therapy, Diazepam, and Placebo, Alone and in Combination, for the Treatment of Generalized Anxiety Disorder. J Anxiety Disord. 1990;4:267–292. [Google Scholar]
- 35.van Apeldoorn FJ, van Hout WJ, Mersch PP, Huisman M, Slaap BR, Hale WW, 3rd, et al. Is a combined therapy more effective than either CBT or SSRI alone? Results of a multicenter trial on panic disorder with or without agoraphobia. Acta Psychiatr Scand. 2008;117:260–270. doi: 10.1111/j.1600-0447.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- 36.Foa EB, Franklin ME, Moser J. Context in the clinic: how well do cognitive-behavioral therapies and medications work in combination? Biol Psychiatry. 2002;52:987–997. doi: 10.1016/s0006-3223(02)01552-4. [DOI] [PubMed] [Google Scholar]
- 37.Franklin ME, Abramowitz JS, Bux DA, Zoellner LA, Feeny NC. Cognitive-behavioral therapy with and without medication in the treatment of obsessivecompulsive disorder. Prof Psychol-Res Pr. 2002;33:162–168. [Google Scholar]
- 38.van Balkom AJ, de Haan E, van Oppen P, Spinhoven P, Hoogduin KA, van Dyck R. Cognitive and behavioral therapies alone versus in combination with fluvoxamine in the treatment of obsessive compulsive disorder. J Nerv Ment Dis. 1998;186:492–499. doi: 10.1097/00005053-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Joss JD, Burton RM, Keller CA. Memory loss in a patient treated with fluoxetine. Ann Pharmacother. 2003;37:1800–1803. doi: 10.1345/aph.1D154. [DOI] [PubMed] [Google Scholar]
- 40.Otto MW, McHugh RK, Kantak KM. Combined Pharmacotherapy and Cognitive-Behavioral Therapy for Anxiety Disorders: Medication Effects, Glucocorticoids, and Attenuated Treatment Outcomes. Clin Psychol-Sci Pr. 2010;17:91–103. doi: 10.1111/j.1468-2850.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kato G, Weitsch AF. Neurochemical profile of tianeptine, a new antidepressant drug. Clin Neuropharmacol. 1988;11(Suppl 2):S43–S50. [PubMed] [Google Scholar]
- 42.Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M, et al. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci. 2007;26:3509–3517. doi: 10.1111/j.1460-9568.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- 43.Malagie I, Deslandes A, Gardier AM. Effects of acute and chronic tianeptine administration on serotonin outflow in rats: comparison with paroxetine by using in vivo microdialysis. Eur J Pharmacol. 2000;403:55–65. doi: 10.1016/s0014-2999(00)00486-6. [DOI] [PubMed] [Google Scholar]
- 44.McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci. 2007;25:3109–3114. doi: 10.1111/j.1460-9568.2007.05560.x. [DOI] [PubMed] [Google Scholar]
- 46.Kugelberg FC, Apelqvist G, Carlsson B, Ahlner J, Bengtsson F. In vivo steady-state pharmacokinetic outcome following clinical and toxic doses of racemic citalopram to rats. Br J Pharmacol. 2001;132:1683–1690. doi: 10.1038/sj.bjp.0704015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wikell C, Apelqvist G, Carlsson B, Hjorth S, Bergqvist PB, Kugelberg FC, et al. Pharmacokinetic and pharmacodynamic responses to chronic administration of the selective serotonin reuptake inhibitor citalopram in rats. Clin Neuropharmacol. 1999;22:327–336. [PubMed] [Google Scholar]
- 48.Couet W, Girault J, Latrille F, Salvadori C, Fourtillan JB. Kinetic profiles of tianeptine and its MC5 metabolite in plasma, blood and brain after single and chronic intraperitoneal administration in the rat. Eur J Drug Metab Pharmacokinet. 1990;15:69–74. doi: 10.1007/BF03190130. [DOI] [PubMed] [Google Scholar]
- 49.Bjerkenstedt L, Flyckt L, Overo KF, Lingjaerde O. Relationship between clinical effects, serum drug concentration and serotonin uptake inhibition in depressed patients treated with citalopram. A double-blind comparison of three dose levels. Eur J Clin Pharmacol. 1985;28:553–557. doi: 10.1007/BF00544066. [DOI] [PubMed] [Google Scholar]
- 50.Wagstaff AJ, Ormrod D, Spencer CM. Tianeptine: a review of its use in depressive disorders. CNS Drugs. 2001;15:231–259. doi: 10.2165/00023210-200115030-00006. [DOI] [PubMed] [Google Scholar]
- 51.Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5- HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–1118. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent on protein synthesis, PKA, and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- 53.McAllister W, McAllister D. Behavioral measurement of conditioned fear. In: Brush FR, Black AH, editors. Aversive conditioning and learning. New York: Academic Press; 1971. pp. 105–179. [Google Scholar]
- 54.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 55.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parkes SL, Westbrook RF. The basolateral amygdala is critical for the acquisition and extinction of associations between a neutral stimulus and a learned danger signal but not between two neutral stimuli. J Neurosci. 2010;30:12608–12618. doi: 10.1523/JNEUROSCI.2949-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 60.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 61.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and Regional Expression in the Rat-Brain and Functional-Properties of 4 Nmda Receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 62.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 63.Kojima N, Sakamoto T, Endo S, Niki H. Impairment of conditioned freezing to tone, but not to context, in Fyn-transgenic mice: relationship to NMDA receptor subunit 2B function. Eur J Neurosci. 2005;21:1359–1369. doi: 10.1111/j.1460-9568.2005.03955.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 65.Wong RW, Setou M, Teng J, Takei Y, Hirokawa N. Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:14500–14505. doi: 10.1073/pnas.222371099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshimura Y, Ohmura T, Komatsu Y. Two forms of synaptic plasticity with distinct dependence on age, experience, and NMDA receptor subtype in rat visual cortex. J Neurosci. 2003;23:6557–6566. doi: 10.1523/JNEUROSCI.23-16-06557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 68.Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 69.Deschaux O, Spennato G, Moreau JL, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology (Berl) 2011;215:231–237. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- 70.Bennett MR. Synapse regression in depression: the role of 5-HT receptors in modulating NMDA receptor function and synaptic plasticity. Aust N Z J Psychiatry. 2010;44:301–308. doi: 10.3109/00048670903555146. [DOI] [PubMed] [Google Scholar]
- 71.Yuen EY, Jiang Q, Chen P, Gu Z, Feng J, Yan Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. Journal of Neuroscience. 2005;25:5488–5501. doi: 10.1523/JNEUROSCI.1187-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. J Mol Neurosci. 1998;10:219–233. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- 73.Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive-Changes in the N-Methyl-D-Aspartate Receptor Complex after Chronic Treatment with Imipramine and 1-Aminocyclopropanecarboxylic Acid. J Pharmacol Exp Ther. 1993;265:1380–1386. [PubMed] [Google Scholar]
- 74.Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the NMethyl- D-Aspartate Receptor Complex Following Chronic Antidepressant Treatments. J Pharmacol Exp Ther. 1994;269:95–102. [PubMed] [Google Scholar]
- 75.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 76.Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- 77.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatmentresistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 78.Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 79.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathew SJ, Amiel JM, Coplan JD, Fitterling HA, Sackeim HA, Gorman JM. Open-label trial of riluzole in generalized anxiety disorder. Am J Psychiatry. 2005;162:2379–2381. doi: 10.1176/appi.ajp.162.12.2379. [DOI] [PubMed] [Google Scholar]
- 81.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 82.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 83.Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc Natl Acad Sci U S A. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca- Garcia E. New perspectives in glutamate and anxiety. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 87.Werner-Seidler A, Richardson R. Effects of D-cycloserine on extinction: consequences of prior exposure to imipramine. Biol Psychiatry. 2007;62:1195–1197. doi: 10.1016/j.biopsych.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 88.Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 90.LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 93.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 94.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–160. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 95.Goodwin GM, Green AR. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985;84:743–753. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Battaglia G, Yeh SY, De Souza EB. MDMA-induced neurotoxicity: parameters of degeneration and recovery of brain serotonin neurons. Pharmacol Biochem Behav. 1988;29:269–274. doi: 10.1016/0091-3057(88)90155-4. [DOI] [PubMed] [Google Scholar]
- 97.Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 98.Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, et al. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- 99.Pryce CR. Postnatal ontogeny of expression of the corticosteroid receptor genes in mammalian brains: inter-species and intra-species differences. Brain Res Rev. 2008;57:596–605. doi: 10.1016/j.brainresrev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 100.Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, et al. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 102.Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- 103.Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol. 1993;235:109–112. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- 104.Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, et al. D-cycloserine deters reacquisition of cocaine selfadministration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davidson JR, Foa EB, Huppert JD, Keefe FJ, Franklin ME, Compton JS, et al. Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry. 2004;61:1005–1013. doi: 10.1001/archpsyc.61.10.1005. [DOI] [PubMed] [Google Scholar]
- 107.Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151–161. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- 108.Haug TT, Blomhoff S, Hellstrom K, Holme I, Humble M, Madsbu HP, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry. 2003;182:312–318. doi: 10.1192/bjp.182.4.312. [DOI] [PubMed] [Google Scholar]
- 109.Bouton ME, Bolles RC. Contextual Control of the Extinction of Conditioned Fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- 110.Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]