Abstract
Purpose
To measure in vivo transverse relaxation times (T2) of gamma-aminobutyric acid (GABA) at 7T using the experimental spectral-editing method.
Materials and Methods
Experiments were performed at 7T in a 10 mM GABA phantom to determine the intrinsic TE-dependence of the edited signal. Then, the same method is applied with editing-based suppression of co-edited macromolecular signals to five healthy volunteers to determine T2 of GABA in vivo.
Results
From in vivo data acquired at multiple echo times, the in vivo GABA T2 relaxation time is estimated to be 63±19 ms.
Conclusion
We present a measurement of the T2 of edited GABA signal at 7T, by first using phantom measurements to determine the echo time-dependence of edited signal. The method is purely experimental and does not rely upon prior knowledge of coupling constants or simulation of realistic experiments.
Keywords: GABA, transverse relaxation, T2, editing, 7T, magnetic resonance spectroscopy
INTRODUCTION
Proton magnetic resonance spectroscopy (1H-MRS) can non-invasively measure the concentrations of metabolites in vivo in the human brain, provided that appropriate corrections can be made for transverse and longitudinal relaxation. While it has been possible to measure N-acetylaspartate (NAA), myo-inositol (mI), and choline- (Cho) and creatine-containing compounds (Cr) in the brain for over twenty years, quantitative measurements of gamma-aminobutyric acid (GABA) have been more elusive. This is mainly due to its lower concentration and coupled spin system, which results in lower intensity signals in the MR spectrum which overlap with larger signals from other, more abundant metabolites. For this reason, editing approaches are often applied to separate GABA from these stronger overlying signals (1–4).
Interest in measuring GABA arises from its role as the major inhibitory neurotransmitter in the brain (4) and GABA measurements may be valuable for the understanding of healthy brain function (5–9) and the pathogenesis of several neuropsychiatric disorders, including schizophrenia, anxiety disorders, epilepsy and drug abuse (10–16). To date, absolute concentrations of GABA are rarely measured and signal ratios to other metabolites are most widely used - either GABA/Cr (17), GABA/NAA (6) or GABA/Water. Absolute quantification is still not possible without knowledge of the longitudinal (T1) and transverse (T2) relaxation times of GABA. T1 and T2 relaxation times differ for different metabolites and at different magnetic field strengths (with T2 decreasing at higher field strength and T1 increasing (18)).
T2 relaxation time measurements can be achieved by performing localized spin-echo experiments at multiple echo times (TEs). For metabolites that give singlet signals in the MR spectrum, such as NAA, Cr and Cho, the acquired signal intensity decays exponentially with increasing echo time as a result of T2 relaxation. Integral data can therefore be fitted with a simple exponential model in order to calculate T2 relaxation times. However, for a complex spin system with multiplet resonances, such as GABA, the pattern of signal intensity decay is more complicated as both T2 relaxation and scalar coupling modulate the signal intensity as a function of echo time.
Ultra-high-field MRS promises theoretical increases in signal-to-noise ratio (SNR), improving the detectability of the small GABA signals (19). Higher spectral resolution can also be achieved at high field which allows unambiguous signal assignment and more precise quantification of the coupled metabolites (18–20). In the best-case scenario of narrow linewidths and excellent SNR, low-concentration metabolites including GABA may be directly estimated from the unedited spectra (19). However, editing approaches are still valuable in measuring GABA at 7T (21) - one commonly used method is J-difference editing using Point Resolved Spectroscopy with Mescher-Garwood editing (MEGA-PRESS) (2,21).
A method was recently proposed and demonstrated for the measurement of T2 relaxation times of GABA at 3T (22) using spectral-editing and multiple echo times. The aim of this manuscript is to extend this method to 7T and to report an estimate of GABA T2 at 7T. One particular refinement that is made possible by the increased chemical shift separation at 7T is the implementation of editing-based suppression of co-edited MM signal (23), not generally possible at 3T.
MATERIALS AND METHODS
All experiments were carried out on a Philips Achieva 7T scanner (Philips Medical Systems, Cleveland, OH, USA) equipped with a quadrature head transmit coil and a 32-channel phased-array receive coil.
The MEGA-PRESS sequence, a J-difference editing technique, was used, detecting the signal from the GABA β-CH2 spins at 3 ppm by applying frequency-selective editing pulses to the β-CH2 spins at 1.9 ppm. The two sub-experiments of MEGA-PRESS will be referred to as the ‘ON’ and ‘OFF’ experiments.
Provided that the editing pulses are sufficiently selective, the choice of editing frequency in the OFF experiment allows the suppression of the co-edited macromolecule signal in vivo whose coupled protons resonate at 1.7 ppm, by applying the editing pulse at 1.5 ppm as proposed by Henry et al (23). Thus, in the ON and OFF experiments the editing pulses are placed symmetrically around the macromolecule signal at 1.7 ppm, resulting in equal degrees of inversion of the MM signal, and therefore co-edited MM signal is suppressed in the DIFF spectrum.
Phantom Experiments
Experiments were performed in a two-litre cylindrical GABA phantom containing 10 mM GABA in phosphate-buffered aqueous solution. MEGA-PRESS experiments were acquired at TEs ranging from 50 to 210 ms in 10 ms increments. These relatively long TEs are required for GABA editing to allow the evolution of scalar couplings that is required for editing. The following acquisition parameters were used: TR = 3 s; 2048 datapoints; spectral width = 3 kHz; voxel size = 24×24×24 mm; 128 transients; CHESS water suppression (400 Hz bandwidth). Editing pulses were single-lobe sinc-Gaussian pulses with flip angle 180°, duration 6 ms (equivalent selectivity to 14 ms pulses commonly used at 3T), and full-width half maximum (FWHM) bandwidth 177 Hz, applied at 1.9 ppm (ON) and 7.46 ppm (OFF).
ON spectra and the subtraction of ON and OFF spectra (DIFF) were processed in MATLAB using exponential line broadening of 3 Hz and zero filling to 8k points. Baseline correction was applied to points ±0.18 ppm from the edited GABA signal at 3 ppm, and the GABA signal was integrated over the same range.
In Vivo Experiments
Five healthy volunteers (age 26.6 ± 3.1 years, 4 female) were studied with local institutional review board approval. Written informed consent was obtained from all subjects before the scan. MEGA-PRESS experiments were performed at TE = 70, 90 and 110 ms with the following parameters: TR = 3 s; data points = 2048; spectral width = 3 Hz; 352 transients. High-bandwidth amplitude-modulated slice-selective refocusing pulses with bandwidth of 1.3 kHz were used with a peak B1 field of 14 uT, resulting in a chemical shift displacement (CSD) of 25% between the 1.9 ppm and 3.0 ppm spins of GABA. VAPOR water suppression (400 Hz bandwidth) achieved sufficiently good levels of water suppression in vivo without power optimization. The acquired voxel was a 30×30×30 mm volume located medially in posterior cingulate cortex. The scan time was 18 minutes for each acquisition. More selective editing pulses were used in vivo in order to allow suppression of MM signals: pulse duration 14 ms, and FWHM bandwidth 76 Hz.
DIFF spectra were processed with 3 Hz exponential line broadening with zero filling to 16k datapoints, and a cubic-spline baseline correction was applied before the edited GABA signal was integrated from 2.85 to 3.15 ppm using in-house software (xxxxx).
T2 Determination
The T2 determination was performed with three-step approach, as described more fully in reference (22):
Determine T2 relaxation time of the phantom (T2,phantom) from ON spectra
Determine the TE-dependence of the edited signal κ(TE) from phantom DIFF spectra
Determine T2,in vivo from in vivo DIFF spectra performed at a range of TEs
T2,in vivo can be determined by using κ(TE) from Step 2 and fitting the in vivo integral data into the following model:
| [1] |
where A is a scalar amplitude factor.
The GABA signal at 3 ppm in the in vivo DIFF spectra was integrated at each TE and averaged over 5 participants’ data. The averaged integral data was fitted by a model as described in Equation 1, with A and T2,in vivo included as variable parameters. In order to determine the bounds of uncertainty on the measured value, a bootstrapping approach was used – 5 datapoints were randomly selected with replacement at each TE and re-analyzed with the same procedure (500 repeats). The standard deviation of the fitted in vivo T2 relaxation times for these 500 repeats is quoted as the error on the measurement.
In order to establish that the TEs sampled in vivo were able to capture T2 relaxation in the difference spectra, the integrals of the phantom difference spectra for TE = [70, 90, 110] ms were fitted using the same model.
RESULTS
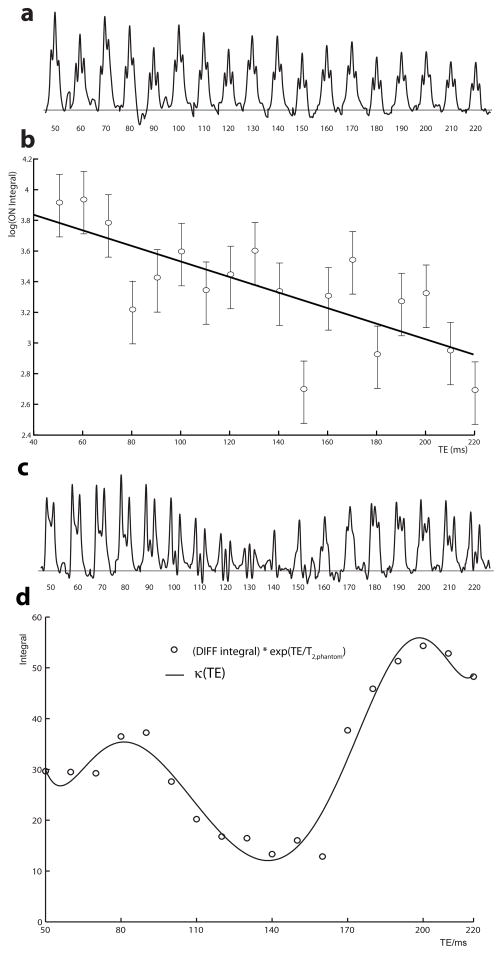
Figure 1a shows the phantom GABA signal at 3 ppm from the TE series of ON spectra, which decreases with increasing TE as a result of T2 decay and the signal retains the triplet-like lineshape at all echo times due to refocusing of coupling evolution by the editing pulses. Shimming was performed to a water linewidth (full-width at half maximum) of less than 5 Hz, resulting in the good multiplet resolution seen. The semi-log plot in Figure 1b shows a good fit with a linear model (Pearson’s R = −0.735), justifying the assumed mono-exponential behavior. T2,phantom is determined from the slope of the line (T2,phantom = −1/slope) to be 201 ms. Fitting the κ(TE) curve to the phantom data at TEs of 70 ms, 90 ms and 110 ms resulted in a T2 of 191 ms, in relatively good agreement.
Figure 1.
(a) The GABA signal at 3 ppm in the phantom ON spectra is plotted for TEs ranging from 50 to 210 ms. (b) A semi-log plot of the phantom ON integral against TE. T2,phantom can be determined from the slope of the linear model shown. Error bars shown represent the log limits for 20% integration errors. (c) The GABA signal at 3ppm in the phantom DIFF spectra is plotted for TEs from 50 to 210 ms. (d) A plot of DIFF integrals with T2-weighting removed (i.e. multiplied by exp(TE/T2,phantom) against the echo time. The κ(TE) function is characterized by fitting a polynomial function to this plot.
Figure 1c shows the phantom GABA signal in the DIFF spectra, where it can be seen that both the intensity and the form of the multiplet changes due not only to T2 relaxation but also to coupling modulation. Figure 1d shows the κ(TE) function, which is obtained from the curve fitting of the DIFF integral with exponential T2,phantom relaxation weighting removed (DIFF integral multiplied by exp(TE/T2,phantom)).
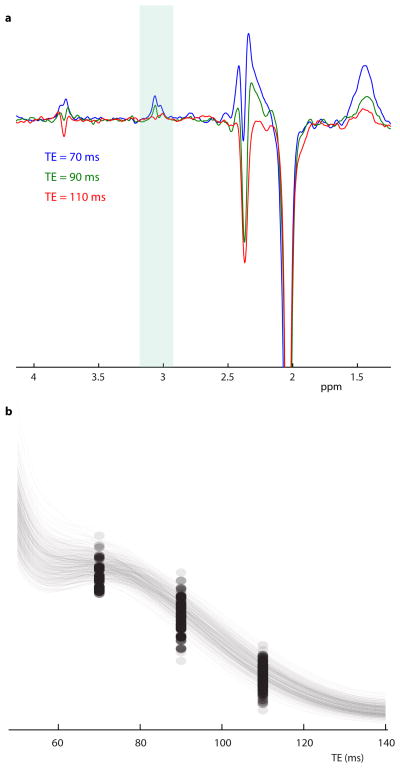
In vivo DIFF spectra are acquired at all three echo times from one participant is shown in Figure 2a. The GABA signal clearly decreases with TE due to both T2 relaxation and the κ(TE) modulation (which has a negative slope in this TE range). The residual water signal in in vivo SUM spectra had a linewidth (full-width at half maximum) of 12 ± 2 Hz. The in vivo relaxation time of the edited signal T2,in vivo is fit as 63±19 ms, as seen in Figure 2b.
Figure 2.
(a) In vivo DIFF spectra obtained from one subject at the TE of 70 ms (blue), 90 ms (green) and 110 ms (red). (b) Bootstrapping of in vivo DIFF integral data. For each of 500 repeats, the average integral at each TE and the optimized model is plotted to show the form and the range of the model of best fit.
DISCUSSION
This manuscript demonstrates a feasible, experimentally-based method to measure T2 of GABA at 7T using spectral editing with macromolecule suppression and requiring no additional pulse programming beyond the implementation of J-difference editing. This method exploits the fact that editing pulses refocus evolution of scalar couplings during TE, and therefore ON spectra are only modulated by T2 relaxation. It is thus relatively straightforward to calculate T2,phantom from a mono-exponential fit to data acquired at a range of echo times. Note that in order for this approach to work, the editing pulses must be separated by TE/2 so as to refocus coupling evolution over the entire duration of the echo time. As a second step, the coupling modulation function of the edited GABA signal κ(TE) is derived by effectively removing T2 relaxation effects from the integrals of DIFF spectra. This function can then be used to determine T2,in vivo.
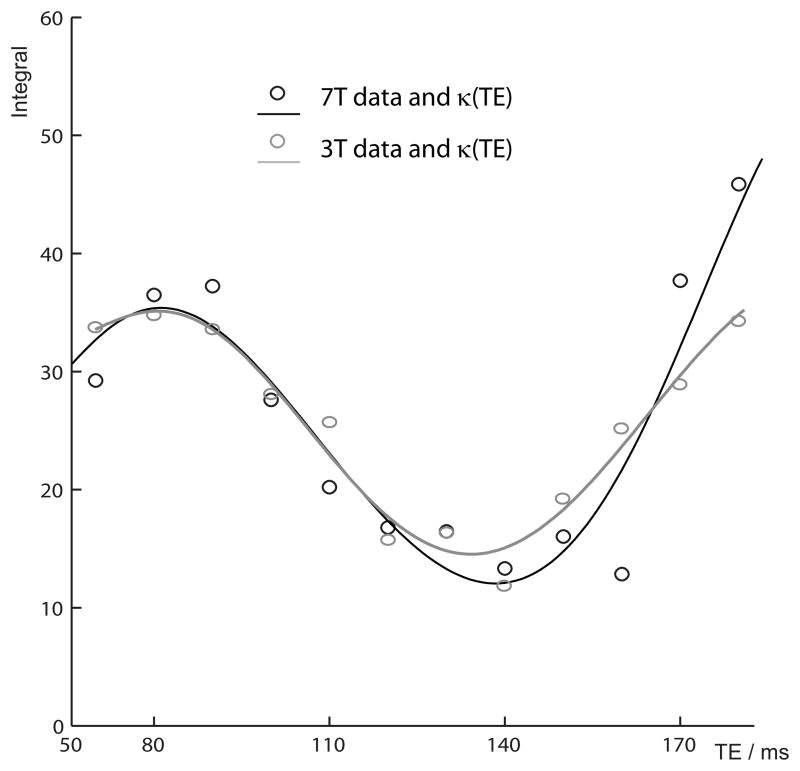
By measuring the κ(TE) experimentally rather than simulating it, this strategy sidesteps any errors that may be introduced by using an incorrect description of the GABA spin system, incompletely capturing spatial heterogeneity of coupling evolution, or other non-ideal experimental characteristics. Figure 3 reproduces the data from Figure 2d for comparison with the equivalent data from reference (22). In the ideal case, since the kappa function would be simply a function of the coupling constants and therefore field-independent, these curves would be expected to be identical. Indeed, there is reasonable agreement within the range shown; however at longer TEs the data diverge due to the increasing impact of differences in the spatial heterogeneity of coupling evolution and other experimental imperfections.
Figure 3.
Comparison of κ(TE) functions determined at 3T and 7T. Datapoints represent measured integrals with T2 relaxation removed. Solid lines represent the polynomial κ(TE) functions. The 3T data and function is taken from reference (22).
The measured in vivo transverse relaxation time of edited GABA signal (63 ms) is within the range of metabolite T2s reported in the literature at 7T (from 61 ms for occipital glutathione up to 191 ms for NAA) (19,24). It is also within the substantially smaller range previously reported for signals from J-coupled molecules; i.e. between 61 ms for occipital glutathione to 110 ms for aspartyl group of NAA in motor cortex (24).
The error in the measurement presented here, T2,in vivo = 63±19 ms, is approximately 30%. Given the relative homogeneity of our subject pool and the large size of the measurement voxel (which leads to relatively consistent voxel placement), it is likely that the majority of this variance arises from measurement errors rather than between-subject or between-tissue-type biological differences. The modest signal-to-noise ratio of edited spectra and artifacts arising from imperfect subtraction of Cr signals in vivo will both contribute to substantial uncertainty in the integral of in vivo difference spectra.
Metabolite transverse relaxation times reduce with increasing field strength and are around twice as short at 7T as at 3T (e.g. comparing references (19) and (25)). The measured T2 of GABA at 7T (63 ms) is shorter than the value at 3T from the recent study (88 ms) (22), but only by 30%, a smaller reduction than might perhaps be expected, based on the T2 values of other metabolites. Assuming a linear increase in SNR (in the absence of relaxation times changes) moving for 3T to 7T, these values suggest that GABA editing at 70 ms will have 70% higher SNR even once the shorter T2 at 7T has been accounted for.
One particular advantages of performing edited MRS at high field is the increased selectivity of editing pulses. At lower field strengths, it is common for edited GABA measurements to be contaminated by MM signal because the GABA signal at 1.9 ppm to which editing pulses are applied is insufficiently removed from MM signal at 1.7 ppm, and both signals are coupled to overlapping signals at 3 ppm. The selectivity for a given length of editing pulse increases at higher field because the chemical shift dispersion (in Hz) increases linearly with field strength. The achievable selectivity of an editing pulse is inversely proportional to its length, which in turn is usually determined by the optimal echo time for GABA editing of close to 70 ms (~1/2J, field independent). In this study, the increased separation between 1.7 ppm and 1.9 ppm resonances at high field allows us to suppress co-edited MM signal using the Henry method (23), which cannot be applied using MEGA-PRESS at 3T with B1 fields of ~14 μT (26,27).
Despite some advantages, high-field MRS may be limited by technical difficulties associated with magnetic field inhomogeneity, shorter T2 and a larger chemical shift displacement (CSD) artifact. The CSD artifact has been addressed recently with the introduction of MEGA-sLASER (28). Although the losses in edited signal that occur in MEGA-PRESS due to CSD (29) are substantial at 7T (at least 30% (28)), MEGA-PRESS was chosen for this study as it allows the sampling for shorter minimum TEs. The measurement of T2 presented acquires phantom data to determine κ(TE), using that function to model in vivo data, and therefore accommodates and accounts for editing efficiency losses due to CSD.
The principal limitation of our study is a narrow range of echo times sampled (70, 90 and 110 ms). The minimum achievable echo time is limited by the time required for editing pulses and slice-selective refocusing in the MEGA-PRESS experiment (shorter editing pulses could be used in phantom experiments as MM suppression was not required). It can be seen from the κ(TE) curve in Figure 1d that editing efficiency is very low between 120 and 160 ms, and at longer echo times signal loss from T2 relaxation prevents meaningful measurements being made in vivo. In pilot in vivo experiments, no measurable signal was detected at TE of 180 ms (chosen as a longer TE with high κ(TE)) in the phantom experiment. These limitations notwithstanding, the sampling of TE’s greater than the measured T2, may bias the measurement itself (30).
This experiment is also limited by the small number of echo times sampled. In order to maximize SNR for echo times with sub-optimal editing efficiency (i.e. 90 and 110 ms), each in vivo acquisition lasted 18 minutes so more echo times could not be sampled within a one-hour protocol. It is also doubtful how much additional information could be gained from sampling more densely within the very limited range of echo time accessible. It is possible that TEs chosen as a result of these restrictions result in a systematic bias in the estimate of T2 calculated.
In conclusion, we present a measurement of the T2 of edited GABA signal at 7T, by first using phantom measurements to determine the echo time J-modulation (κ(TE)).
Acknowledgments
Grant support: Supported in part by NIH P41RR015241
This work was supported by NIH P41 EB015909.
References
- 1.Waddell KW, Avison MJ, Joers JM, Gore JC. A practical guide to robust detection of GABA in human brain by J-difference spectroscopy at 3 T using a standard volume coil. Magn Reson Imaging. 2007;25(7):1032–1038. doi: 10.1016/j.mri.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Rothman DL, Petroff OA, Behar KL, Mattson RH. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A. 1993;90(12):5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puts NA, Edden RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol. 2011;21(6):480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boy F, Evans CJ, Edden RA, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20(19):1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53(2):392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13(7):825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 9.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31(46):16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CG, Bottiglieri T, Snead OC., 3rd GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003;54 (Suppl 6):S3–12. doi: 10.1002/ana.10696. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Cloak CC, Ernst T. Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry. 2003;64 (Suppl 3):7–14. [PubMed] [Google Scholar]
- 12.Novotny EJ, Jr, Fulbright RK, Pearl PL, Gibson KM, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54 (Suppl 6):S25–31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- 13.Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68(7):667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tayoshi S, Nakataki M, Sumitani S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117(1):83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Behar KL, Rothman DL, Petersen KF, et al. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156(6):952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- 16.Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):739–743. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004;161(2):368–370. doi: 10.1176/appi.ajp.161.2.368. [DOI] [PubMed] [Google Scholar]
- 18.Di Costanzo A, Trojsi F, Tosetti M, et al. High-field proton MRS of human brain. Eur J Radiol. 2003;48(2):146–153. doi: 10.1016/j.ejrad.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Tkac I, Andersen P, Adriany G, Merkle H, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at 7 T. Magn Reson Med. 2001;46(3):451–456. doi: 10.1002/mrm.1213. [DOI] [PubMed] [Google Scholar]
- 20.Hetherington HP, Pan JW, Chu WJ, Mason GF, Newcomer BR. Biological and clinical MRS at ultra-high field. NMR Biomed. 1997;10(8):360–371. doi: 10.1002/(sici)1099-1492(199712)10:8<360::aid-nbm477>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Terpstra M, Ugurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn Reson Med. 2002;47(5):1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 22.Edden RA, Intrapiromkul J, Zhu H, Cheng Y, Barker PB. Measuring T2 in vivo with J-difference editing: application to GABA at 3 Tesla. J Magn Reson Imaging. 2012;35(1):229–234. doi: 10.1002/jmri.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magn Reson Med. 2001;45(3):517–520. doi: 10.1002/1522-2594(200103)45:3<517::aid-mrm1068>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Marjanska M, Auerbach EJ, Valabregue R, Van de Moortele PF, Adriany G, Garwood M. Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T2 relaxation times and concentrations of cerebral metabolites. NMR Biomed. 2012;25(2):332–339. doi: 10.1002/nbm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaaraoui W, Fleysher L, Fleysher R, Liu S, Soher BJ, Gonen O. Human brain-structure resolved T(2) relaxation times of proton metabolites at 3 Tesla. Magn Reson Med. 2007;57(6):983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]
- 26.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31(1):204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 27.Near J, Simpson R, Cowen P, Jezzard P. Efficient gamma-aminobutyric acid editing at 3T without macromolecule contamination: MEGA-SPECIAL. NMR Biomed. 2011;24(10):1277–1285. doi: 10.1002/nbm.1688. [DOI] [PubMed] [Google Scholar]
- 28.Andreychenko A, Boer VO, Arteaga de Castro CS, Luijten PR, Klomp DW. Efficient spectral editing at 7T: GABA detection with MEGA-sLASER. Magn Reson Med. 2012;68(4):1018–25. doi: 10.1002/mrm.24131. [DOI] [PubMed] [Google Scholar]
- 29.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58(6):1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 30.Kreis R, Slotboom J, Hofmann L, Boesch C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magn Reson Med. 2005;54(4):761–768. doi: 10.1002/mrm.20673. [DOI] [PubMed] [Google Scholar]