Abstract
Background
Circulating adrenal steroids rise during the menopausal transition (MT) in most mid-aged women and may contribute to differences in between-woman symptoms as well as ultimate health outcomes. However, the mechanism(s) for this shift in adrenal steroid production in mid-aged women is not known.
Objective
To determine if hormone replacement therapy (HT) for one year can modulate adrenal androgen production.
Method
Younger (9.8 +/− 0.4 y/o, n=20) and older (22.7+/−0.4 y/o, n=37) female laboratory macaques were ovariectomized (OVX), and then each group was treated with different regimens of HT for up to one year. Changes in adrenal histology and circulating adrenal androgens were monitored following estradiol treatment alone (E) or estrogen plus progesterone (E+P), and these changes were compare to the same measures in similar aged animals given vehicle (V).
Results
Zona reticularis (ZR) area and serum dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) were higher in younger compared to older V-treated animals (P< 0.02). Both E and E+P treatments decreased circulating DHEAS in the younger group (P<0.05). While E also decreased DHEAS in the older group, this was not statistically significant. In contrast, E+P treatment in the older group resulted in a rise in DHEAS over V, which was significantly higher than the results of E alone (p< 0.01). Circulating concentrations of DHEA exhibited similar trends but these changes did not reach statistical significance.
Conclusion
These data demonstrate that intervention with ovarian steroids can modulate adrenal androgen production in female higher primates and that both animal age and type of HT regimen determines the adrenal response.
Keywords: Adrenal, Steroids, Hormone Therapy, Replacement
INTRODUCTION
Recent studies of women monitored longitudinally have identified a previously unrecognized increase in adrenal androgen secretion in the perimenopausal period, reversing the long age-related decline of the decades (1;2), and thereby implicating adrenal function as a component of the menopausal transition. The menopausal transition begins in the late reproductive period in women who may still exhibit normal cyclicity but experience menopause-associated symptoms including vasomotor instability, insomnia and dysphoria (3). The underlying patho-physiology underlying menopausal symptoms and response to therapy remains unclear (4). For instance, recent prospective studies failed to find any specific cycle characteristics related to the most consistent perimenopausal complaints, hot flashes and night sweats, or hormonal patterns, that predicted with much certainty the final progression to the menopause (5). Although symptomatic women are most often treated with some form of estrogen replacement (6;7), estradiol (E2) concentrations are normal or even elevated in perimenopausal women (8), and are seldom low in fact. Furthermore, the lack of symptoms in women who have low estrogen even several years after menopause suggests that E2 deficiency alone is not the cause, and estrogen therapy comes with obvious health risks (9). The most common adrenal androgens, dehydroepiandrosterone (DHEA) and its sulpho-conjugate (DHEAS), though potential substrates for estrogen and biopotent androgen synthesis, have not proven to be of benefit in the treatment of menopausal symptoms (10). However, other androgens from the adrenal gland such as androst-5-ene-3β, 17β-diol (Adiol) circulate in women (11) at levels capable of providing estrogenic stimulation when E2 concentrations are very low (12). Therefore, adrenal androgens may modulate estrogenic status and help explain the incongruence between menopausal symptoms and circulating E2 concentrations. The rhesus macaque has proven useful as a model of human menopause (13–16), as it has for fetal adrenal androgen secretion (17;18), adrenal development in infancy (19–22) and adrenal aging (23–25). Studies in rhesus monkeys may shed light on the variety and variability among adrenal androgens through the transition, and thereby suggest the possible value of alternative replacement therapies for perimenopausal and postmenopausal women.
Combined oral contraceptives suppress DHEAS concentrations in women (26–33)and have been used in part to lower androgen concentrations in patients with polycystic ovarian syndrome (34–35). Exactly how, mechanistically, steroidal treatment influences adrenal androgen secretion remains unknown. Although rhesus monkeys have been used to examine the cognitive and neural benefits of HT (36), the effects of estrogen and progesterone (P) on adrenal androgen secretion have not been investigated in this model. Since age affects the ability of estrogen alone to improve cognition in older but not younger females, the current study was conducted to determine how different HT regimens affect adrenal steroid production. We hypothesize that HT would modulate adrenal androgen in production the absence of ovaries, but that the response would change with age. We tested this hypothesis by examining the effects of HT in younger and older ovariectomized female rhesus monkeys.
METHODS
Animals
The study utilized ovariectomized younger female rhesus macaques (Macaca mullata) averaging 9.8 ± 0.4 years of age (n=15) and ovariectomized older females averaging 22.7 ± 0.4 (n=22) years of age in the treatment experiment. Animal assignments were randomized by the animal technician and the investigators were blinded to those assignment codes.
An older age intact group (25.1 ± 0.4 years of age, n=7) were used for an age control reference point. All animals were either group-housed during early social development or wild caught and then maintained in rooms where animals have visual, olfactory, and auditory contact with each other. The experimental history of each animal was reviewed to exclude animals previously used for behavioral research, studies involving potentially neurotoxic agents, and any studies that may have had an effect on reproductive function. All monkeys were pair-housed for a minimum of 6 months prior to entering the protocol, remaining with their cage mate during the day and separated at night (to facilitate monitoring of food consumption and urine collection). Prior to inclusion to the research project all animals received thorough physical exams including CBC, complete serum chemistry including fasting blood glucose, screen for Type D simian retrovirus, ophthalmologic exam, and assessment of physical capability to perform behavioral tasks, e.g., exclusion of advanced arthritis. Animals infected with Type D retrovirus (positive PCR result), eye lesions such as cataracts, or metabolic disorders, such as diabetes were also excluded from the project.
Individual animals were selected for entry into the study based on confirmation of ovarian function by hormonal analysis. Ovarian function tests in candidate animals were performed not only to confirm reproductive status but also to monitor the effectiveness of OVX and hormone exposures treatments. Daily, overnight urine samples were collected and frozen until analyzed for the major estrogen (E1C) and progesterone metabolites (PdG) normalized to creatinine. A second category of endocrine evaluation was conducted to verify the efficacy of treatment regimens. Each hormone regimen resulted in characteristic hormone levels that were easily distinguishable and comparable to the serum levels that were expected to result as a consequence of the respective treatments. The treatments consisted of continuous estradiol (E) and E plus P (E+P). Young females (n=5/group) were treated for a total of 216 days. Old females were treated on average for 564 days with either vehicle (n=8), E2 (n=8) or E+P (n=6). E2 was administered using subcutaneous silastic capsules to achieve approximately 150 pg/ml concentrations in serum. Silastic implants were placed subcutaneously between the shoulder blades under ketamine (10 mg/kg) and medetomidine (0.2–0.4 mg/kg) sedation, with atipamezole (0.2–0.4 mg/kg) given at the end of the procedure to reverse the medetomidine. The dose of micronized P was 10 to 20 mg per day given orally for a 10 day interval once each month to reach circulating levels of 4 to 8 ng/mL. The study was reviewed and approved by the UC Davis Institutional Animal Care and Use Committee.
Hormone Assays
Blood was collected by venipuncture, allowed to clot and sera were collected after centrifugation and stored frozen until assayed. DHEAS was measured by competitive chemiluminescent immunoassay on a Bayer Diagnostic ACS-180 automated analyzer as reported previously (2). For the DHEAS assay, intra- and inter-assay coefficients of variation (CV) were 2.9 and 6.9%, respectively. The limit of detection was 1.0 ug/dL. Androstenedione (Adione), testosterone (T), DHEA and Adiol were analyzed in serum by radioimmunoassay with preceding organic solvent extraction and Celite column partition chromatography steps. The assay sensitivities are 0.03 ng/mL, 0.15 ng/mL, 0.2 ng/mL and 0.03 ng/mL, respectively, with interassay CVS ranging from 8 to 13%.
Histology
Animals were euthanized and fixed by perfusion under deep anesthesia after completing the treatment protocol. Animals were anesthetized with ketamine (10 mg/kg) and pentobarbital (60 mg/kg) and then perfused transcardially with 1% paraformaldehyde (PF) for 1 minute, followed by 4% PF in 0.1 M phosphate buffer for 12 minutes at a flow rate of 225–250 ml/min. Adrenal glands were dissected and processed for routine histology. Tissues were embedded carefully to allow uniform sectioning along the long axis of the gland, and 5 μm sections were cut and stained with hemotoxylin and eosin from which the zona glomerulosa (ZG), zona fasciculata (ZF), zona reticularis (ZR) and medulla could be identified. Images were captured using a Zeiss Lumar, V12 stereo motorized microscope, with a Zeiss AxioCam HRc Rev3 color camera, and NeoLumar S 1.5x lens with total magnification (200x) that allowed visualization of whole adrenal cross-sections in one picture. The cortical zones were defined based on staining intensity using Image-Pro Plus 5.0 analysis software (Media Cybernetics, Silver Spring, MD), and zonal areas were estimated (Figure 1). Areas of each zone were calculated and expressed as a percentage of total cortical area.
Figure 1.
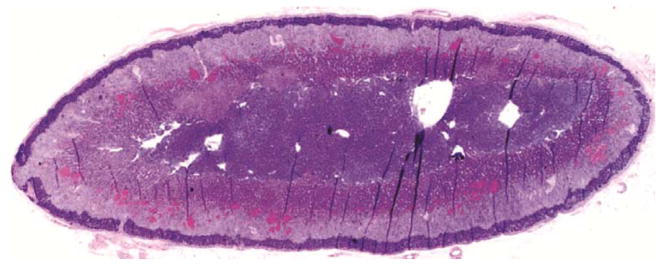
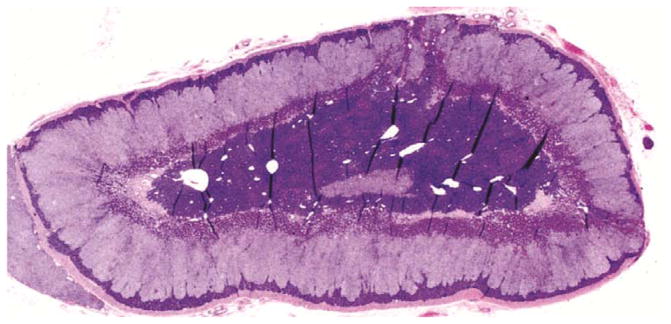
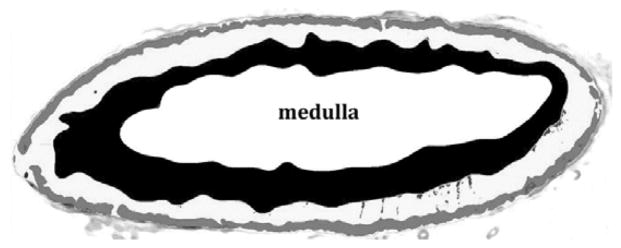
Histological sections through rhesus adrenal glands. A. Hemotoxylin/eosin stained section of old female adrenal. B. Hemotoxylin/eosin stained section of young female adrenal. C. Processed image of adrenal section in B, showing delineation of the areas comprising the zona reticularis (black), zona glomerulosa (gray), intervening zona fasciculata (white) and central medulla (labeled).
Statistics
Data were analyzed by one-way analysis of variance using the general linear models function of SAS (version 9.3 SAS Institute, Cary, NC). Values were log transformed where indicated by existing heterogeneity of variance. Main effects of age and treatment and their interaction were included in the model statement. Comparisons among means were examined using the Tukey’s HSD multiple range test, and Pearson’s correlation coefficients were calculated among the androgens and between androgens and the zona reticularis area.
RESULTS
Aged intact and aged vehicle-treated OVX females had similar concentrations of DHEAS, DHEA, and Adiol. DHEAS concentrations were 10-fold higher that DHEA, and DHEA was approximately 3-fold higher than Adiol which was still in the ng/ml range (Table 1). Adione and T concentrations averaged less than 1 ng/ml. DHEAS concentrations were significantly higher in young (before OVX) than in older intact females [12.63 ± 1.92 (n=20) vs 8.03 ± 0.94μg/dL (n=37), respectively, P<0.02]. DHEAS remained higher in younger than in older females after OVX (15.59 ± 2.32 vs 4.63 ± 0.60 μg/dL, respectively), as was unconjugated DHEA (16.69 ± 4.55 vs 6.21 ± 1.70 ng/ml, P<0.05) in animals receiving vehicle, but there were no statistically significant differences in Adiol, Adione or T (Table 1) although the mean Adiol was twice as high in OVX younger animals. All androgen concentrations in younger and older OVX females were positively correlated with one another (range of Pearson correlation coefficients, 0.34–0.81, P<0.05) with two exceptions. DHEAS was not correlated with either Adione (P>0.5) or with T (P>0.05). The highest correlations found were between DHEAS and DHEA (r=0.70, P<0.001) and DHEA and Adiol (r=0.81, P<0.001).
Table 1.
Circulating hormone levels in treatment groups.
Androgen concentrations (Mean +/− SD) in intact (n=7) aged, and ovariectomized aged (n=8, OVX) and young (n=5, OVX) vehicle-treated female rhesus monkeys. Shown are concentrations of dehydroepiandrosterone sulphate (DHEAS, μg/dL), dehydroepiandrosterone (DHEA, ng/ml), androstenedione (Adione, pg/ml), testosterone (Testost, pg/ml) and androst-5-ene-3β, 17β-diol (Adiol, ng/ml). Level of significance (P) of comparisons between concentrations of each steroid in aged and young females is shown in the right column.
| AGED | YOUNG | P (aged v young ovx) | ||
|---|---|---|---|---|
| Intact (n=7) | OVX (n=8) | OVX (n=5) | ||
| DHEAS (μg/dL) | 5.34 ± 1.05 | 4.63 ± 0.60 | 15.59 ± 2.32 | <0.002 |
| DHEA (ng/ml) | 5.13 ± 1.52 | 6.21 ± 1.70 | 16.69 ± 4.55 | <0.03 |
| Adione (pg/ml) | 614 ± 264 | 273 ± 27.3 | 300.8 ± 43.70 | >0.5 |
| Testost (pg/ml) | 238 ± 43 | 190 ± 34 | 175.2 ± 22.33 | >0.7 |
| Adiol (ng/ml) | 1.83 ± 0.33 | 1.96 ± 0.55 | 3.97 ± 0.79 | <0.06 |
HT intervention had a significant effect on concentrations of DHEAS in both younger and older ovariectomized females (Figure 2). E alone and E+P treatments decreased DHEAS concentrations to a similar degree in young females. In old females, DHEAS concentrations were similar between vehicle and E-treated subjects. DHEAS concentrations in females receiving E+P were marginally higher than in vehicle-treated females (P<0.06) but significantly elevated over those of the E-treated females (P<0.01). The difference in response to E+P with age, suppressing DHEAS in young but tending to stimulate it in old females, was supported statistically by a significant interaction between age and treatment (P<0.01). Despite the high correlations between DHEAS and both DHEA and Adiol, these treatment effects did not reach statistical significance.
Figure 2.
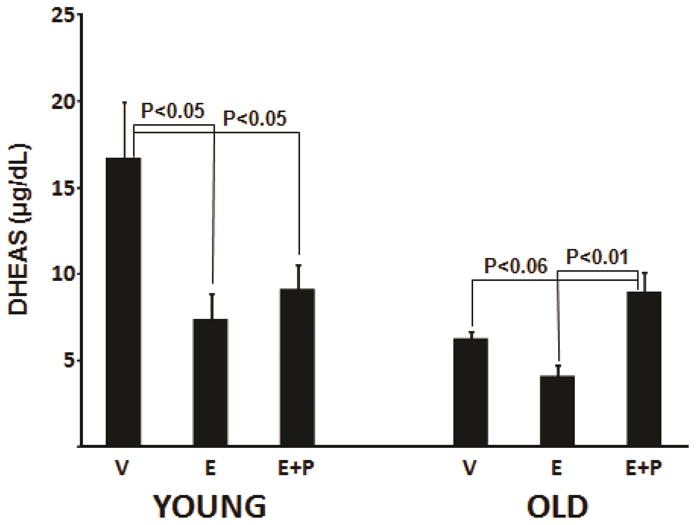
Dehydroepiandrosterone sulphate concentrations (μg/dL) in younger (left panel) and older (right panel) ovariectomized rhesus monkeys treated with vehicle (control), estradiol (E2) and E2 plus progesterone (E2+P). Levels of significance between treatment means within young and old females are indicated above each pair-wise comparison.
Comparisons between the younger (n=5) and older (n=8) vehicle-treated subjects (Figure 1) by image analysis of adrenal histology indicated that the zona reticularis (ZR) area as a percentage of total cortical area was greater in the younger than in the aged females (46.8±4.9 vs 29.0±4.3%, respectively, P<0.025), but there was no difference by age in area of the zona fasciculata (ZF)(38.3±4.2 vs 48.8±3.7%, respectively, P>0.05). Histological data were not available for a sufficient number of adrenal specimens from younger females in the HT groups to allow valid analysis, but there was no detectable effect of HT on ZR or ZF area in older females (P>0.1). However, circulating DHEAS levels were positively correlated with ZR area across young and old females (r=0.47, P<0.01) and negatively correlated with ZF area (r=−0.37, P<0.05). There were no statistically significant correlations found between ZR area and the other androgens.
DISCUSSION
The current study is the first to examine the concentrations of various adrenal androgens in younger and older rhesus monkeys, uncomplicated by any contribution from the gonads, and to examine the effects of extended HT. As expected from numerous previous studies (22;37–39), DHEAS concentrations were 3-fold higher in young than in aged females, DHEA in young females was more than twice that in old females and Adiol was marginally higher in young rhesus but T and Adione did not differ by age. DHEAS was positively correlated with ZR area and as a percentage of cortical area was higher in younger than older females, indicating that the age-related decline in DHEAS secretion is likely associated with shrinking of the ZR. This is consistent with the reduction in width of the ZR (56)(40), as well as in the expression of enzymes in the ZR involved in the synthesis of DHEAS (41). The relative concentrations of DHEAS and unconjugated DHEA in younger and older female primayes differed somewhat from those in women. As adults, rhesus monkeys have lower DHEAS but higher DHEA concentrations than humans (42). The ratios of conjugated to unconjugated DHEA do not differ substantially by age or treatment between humans and rhesus macaques. However, this analysis provides evidence that ovariectomized rhesus monkeys have circulating levels of Adiol that are considerably higher than those found in women (11) and would be expected to transduce signals through estrogen receptors (12). In this study we use circulating DHEAS as a surrogate for adrenal steroids which may influence symptoms and health outcome of women during the menopausal transition.
Of additional interest were the observed effects of HT on adrenal androgen concentrations, and the possible differences in response between younger and older OVX females. E inhibited DHEAS concentrations in younger females and marginally so in older females, as numerous studies in humans have demonstrated (27–34), but the addition of P to E appeared to have a different effect in old compared to the young subjects. Specifically, treatment of old females with E+P reversed the inhibition that E alone exerted. Though not reaching significance, females treated with E+P also had marginally (P<0.06) higher concentrations of DHEAS than vehicle-treated controls. No rescue of DHEAS occurred in young females treated with E+P, which inhibited DHEAS concentrations as effectively as E alone. This may simply reflect the much shorter period of treatment in younger compared with older females, though they were still treated for over 7 months. The differential effects of E versus E+P in stimulating DHEAS in the older females can clearly not be ascribed to effects on the ovaries themselves which, in postmenopausal women with intact ovaries, would be difficult to discern even if such data were at hand. In the absence of the ovaries, an effect of aging at the hypothalamus, as others have observed in non-human primates (25), must be suspected. Alternatively, a change in receptor expression in the adrenal cortex itself might alter adrenal sensitivity to trophic stimulation. Ovariectomy alone would be expected to increase gonadotropin concentrations but not those of pro-opiomelanocortin (POMC) (43). It must be noted that not all studies have reported inhibition as a consistent response to E2 in women (44–45). Slayden et al. failed to find a change in adrenal androgen concentrations, and no change in adrenal responsiveness to ACTH stimulation (46), but this was after only 3 months of therapy. The mechanism by which the addition of P to E alone in older females reverses the inhibition of DHEAS by E alone remains unknown at the present time.
These findings may also shed some light on the mechanism(s) by which the acceleration in adrenal androgen production in mid-aged women might contribute to their endocrine milieu (12). The age-related decline in adrenal androgen is reversed in a majority of women during the early perimenopause when FSH is also beginning to rise. This timing suggests that the decline in some ovarian component is the trigger or release of inhibition on adrenal androgen secretion. The increase in adrenal androgens in women continues through the menopausal transition then plateaus at or after menopause, suggesting that some non- ovarian component is providing a stimulus (2). In this study we use circulating DHEAS as a surrogate for adrenal steroids which may influence symptoms and health outcome of women during the menopausal transition. Although no similar spontaneous age-related rise and plateau in adrenal androgens was identified in the current study of aged rhesus monkeys, ovarian activity was confirmed in all animals at enrollment and therefore none were post-menopausal when the study began. Shideler et al. investigated circulating DHEAS levels in similarly aged female rhesus stratified by ovarian status (25) and when compared with normally cyclic females, those with abnormal cycles and those that were anovulatory had elevated levels of FSH, lower inhibin B levels and numerically higher circulating concentrations of DHEAS. Thus, the older animals in this study were apparently functionally younger that the animals Shideler et al (25) identified as having increased adrenal androgens and functionally younger than women during the menopausal transition (2).
In the present study, E replacement alone accelerated the decline in adrenal androgen production in younger females and tended to also do so in older females also (Figure 1) in the absence of ovaries. In contrast, E+P was not as effective in the younger animals in suppressing DHEAS and maintained it at pre-OVX levels in the older animals. Thus, while cyclic ovarian function is not essential for the increase in adrenal androgens following ovariectomy in women (47), it is apparently able to prevent the continuous fall that would occur in its absence in this animal model, with treatment extending over a year and a half in older females. This observation suggests that different HT regimens may provide different benefits to perimenopausal and postmenopausal women by the degree of support they offer to adrenal androgen synthesis.
Taken together these data reveal that different HT regimens have different effects on adrenal steroidogenesis in adult female primates and may have clinical relevance to women during the MT in regard to neurological changes and breast cancers. Estrogen alone decreases adrenal androgens as shown previously in women (44–45) and this suppression of adrenal androgen production may explain the lack of non-neoplastic adverse effects of estrogen-only compared to combination replacement regimens. In contrast, estrogen plus progesterone replacement may increase the full complement of adrenal androgens including DHEA, Adiol and, most importantly, shift the circulating ER alpha-to-ER beta ligand balance in favor of ER beta with several potential implications (48). First, the increase in ZR thickness associated with E+P indicates a shift in adrenal steroidogenesis from the delta five pathway to the delta four pathway, thereby increasing the need for additional ACTH stimulation to keep circulating cortisol at minimally normal levels. Such a “resetting” of the HPA axis has been associated with depression in mid-aged women but never sufficiently explained (49). The shift in the capacity of the adrenal cortex to respond to metabolic and environmental stressors may explain why there is a paradoxical increase in ACTH in such disorders during the MT in women. Second, ER beta agonists such as Adiol have been associated with changes in spine density and glutaminergic signaling that are opposite to the positive effects of those reported for E2 (50). Thus, the cyclic nature of E2 during the MT in association with increasing Adiol may explain discrepancies in the failure to associate various symptoms with the measurement of circulating E2. Third, several recent reports have associated estrogen plus progestogen replacement therapies with an increase in breast cancer in postmenopausal women (51). More importantly, tissue levels of DHEA and Adiol in tumor tissues have been detected at levels considered effective for Adiol to support cell proliferation in ER alpha positive cells in the absence of E2 (52). Since Adiol does not require aromatization for its ER-signaling activity, increased circulating levels, as promoted by combination HT, could support some types of breast cancer growth
The extreme variability of symptoms and underlying endocrinology of women in the menopausal transition remains a mystery. The late reproductive period is marked most consistently by increasing FSH concentrations and decreasing inhibin B (54). Despite having E2 concentrations similar to younger women, some perimenopausal women have a longer follicular phase and an unusually thick endometrium in the luteal phase (55), which is consistent with excessive estrogen-stimulated uterine growth. In any event, the perimenopause does not appear to be a period of estrogen deficiency, and though estrogen replacement is often prescribed, it is rarely given with regard to a verifiable estrogen deficit. Results of recent longitudinal studies on large numbers of women enrolled in the Study of Women Across America (SWAN) indicate that the MT involves a previously unrecognized but significant involvement of androgen secreted by the ZR. Examination of longitudinal hormone data from SWAN reveal that most women (≈85%) traversing the MT experienced a transient increase in the Δ5 adrenal steroids, namely Adiol and DHEA (12). This increase in Δ5 C-19 steroid secretion represents a major hormone dynamic during the MT that may have clinical relevance. Adiol has both androgenic and estrogenic bioactivities (12) and reaches effective circulating concentrations in some women (11). Its weak ER alpha potential may well contribute positively to circulating estrogenicity particularly when circulating estradiol levels are low (12), but its ER beta activity may contribute a wide range of effects depending on tissue concentrations of specific receptors.
Summary
The present data demonstrate that the ZR of female macaques undergoes a diminution in both size and androgen synthetic capacity during the second decade of life. Additionally, these results show a striking similarity between macaques and mid-aged women in terms of relative circulating concentration and source of adrenal androgens. A modest ovarian contribution of DHEAS and a more robust amount of Adione to circulating levels is indicated for the macaque following OVX of older animals similar to that shown for women (12). Intervention with E alone in OVX macaques results in an acceleration in the decline of ZR function in both young and older females. In contrast, intervention with E+P restored and tended to enhance adrenal androgen concentrations after a year and a half of treatment in older animals.
Conclusion
Female macaques provide an appropriate animal model for investigating the age-related changes in ZR function that has been observed in mid-aged women. Intervention with typical HT regimens can modulate ZR structure and function with potentially different effects in younger compared to older animals.
Acknowledgments
Support: Funded in part by P51 RR00169, the Base Grant of the California National Primate Center and John Morrison’s P01 AG01675-06A1
Footnotes
Disclosures: JHM: Industrial- Chemicon, Pharmington, Zymed
Academic Lectures: U Penn, Cornell, U Pitt
References
- 1.Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 2.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 4.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 5.Skurnick JH, Weiss G, Goldsmith LT, Santoro N, Crawford S. Longitudinal changes in hypothalamic and ovarian function in perimenopausal women with anovulatory cycles: relationship with vasomotor symptoms. Fertil Steril. 2009;91:1127–1134. doi: 10.1016/j.fertnstert.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11:11–33. doi: 10.1097/01.GME.0000108177.85442.71. [DOI] [PubMed] [Google Scholar]
- 7.Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106. doi: 10.1016/j.ajog.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 9.Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17:242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 10.Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96:1642–1653. doi: 10.1210/jc.2010-2888. [DOI] [PubMed] [Google Scholar]
- 11.McConnell DS, Stanczyk FZ, Sowers MR, Randolph JF, Jr, Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause. 2012 doi: 10.1097/gme.0b013e31823fe274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasley BL, Chen J, Stanczyk FZ, El Khoudary SR, Gee NA, Crawford S, McConnell DS. Androstenediol complements estrogenic bioactivity during the menopausal transition. Menopause. 2012 doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgen GD, Goodman AL, O’Connor A, Johnson DK. Menopause in rhesus monkeys: model for study of disorders in the human climacteric. Am J Obstet Gynecol. 1977;127:581–584. doi: 10.1016/0002-9378(77)90352-0. [DOI] [PubMed] [Google Scholar]
- 14.Walker ML. Menopause in Female Rhesus-Monkeys. Am J Primatol. 1995;35:59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RL, Kapsalis E. Menopause in free-ranging rhesus macaques: Estimated incidence, relation to body condition, and adaptive significance. International Journal of Primatology. 1998;19:751–765. [Google Scholar]
- 16.Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod. 2008;79:398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 18.Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ. The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology. 2009;150:1748–1756. doi: 10.1210/en.2008-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen AD, Mapes SM, Corbin CJ, Conley AJ. Morphological adrenarche in rhesus macaques: development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J Endocrinol. 2008;199:367–378. doi: 10.1677/JOE-08-0337. [DOI] [PubMed] [Google Scholar]
- 22.Conley AJ, Plant TM, Abbott DH, Moeller BC, Stanley SD. Adrenal Androgen Concentrations Increase During Infancy in Male Rhesus Macaques (Macaca mulatta) Am J Physiol Endocrinol Metab. 2011 doi: 10.1152/ajpendo.00200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing Dev. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 24.Goncharova ND, Lapin BA. Age-related endocrine dysfunction in nonhuman primates. Ann N Y Acad Sci. 2004;1019:321–325. doi: 10.1196/annals.1297.054. [DOI] [PubMed] [Google Scholar]
- 25.Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- 26.Bulbrook RD, Herian M, Tong D, Hayward JL, Swain MC, Wang DY. Effect of steroidal contraceptives on levels of plasma androgen sulphates and cortisol. Lancet. 1973;1:628–631. doi: 10.1016/s0140-6736(73)92198-3. [DOI] [PubMed] [Google Scholar]
- 27.Rance TA, Park BK. Effect of an oral contraceptive on plasma dehydroepiandrosterone concentrations. Experientia. 1977;33:1239–1240. doi: 10.1007/BF01922353. [DOI] [PubMed] [Google Scholar]
- 28.Madden JD, Milewich L, Parker CR, Jr, Carr BR, Boyar RM, Mac Donald PC. The effect of oral contraceptive treatment on the serum concentration of dehydroisoandrosterone sulfate. Am J Obstet Gynecol. 1978;132:380–384. doi: 10.1016/0002-9378(78)90771-8. [DOI] [PubMed] [Google Scholar]
- 29.Tazuke S, Khaw KT, Barrett-Connor E. Exogenous estrogen and endogenous sex hormones. Medicine (Baltimore) 1992;71:44–51. doi: 10.1097/00005792-199201000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Wiegratz I, Jung-Hoffmann C, Kuhl H. Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception. 1995;51:341–346. doi: 10.1016/0010-7824(95)00098-u. [DOI] [PubMed] [Google Scholar]
- 31.Casson PR, ElkindHirsch KE, Buster JE, Hornsby PJ, Carson SA, Snabes MC. Effect of postmenopausal estrogen replacement on circulating androgens. Obstetrics and Gynecology. 1997;90:995–998. doi: 10.1016/s0029-7844(97)00538-3. [DOI] [PubMed] [Google Scholar]
- 32.Aden U, Jung-Hoffmann C, Kuhl H. A randomized cross-over study on various hormonal parameters of two triphasic oral contraceptives. Contraception. 1998;58:75–81. doi: 10.1016/s0010-7824(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 33.Carlstrom K, Karlsson R, Von SB. Diurnal rhythm and effects of oral contraceptives on serum dehydroepiandrosterone sulfate (DHEAS) are related to alterations in serum albumin rather than to changes in adrenocortical steroid secretion. Scand J Clin Lab Invest. 2002;62:361–368. doi: 10.1080/00365510260296519. [DOI] [PubMed] [Google Scholar]
- 34.Nader S, Diamanti-Kandarakis E. Polycystic ovary syndrome, oral contraceptives and metabolic issues: new perspectives and a unifying hypothesis. Hum Reprod. 2007;22:317–322. doi: 10.1093/humrep/del407. [DOI] [PubMed] [Google Scholar]
- 35.Yildiz BO. Oral contraceptives in polycystic ovary syndrome: risk-benefit assessment. Semin Reprod Med. 2008;26:111–120. doi: 10.1055/s-2007-992931. [DOI] [PubMed] [Google Scholar]
- 36.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koritnik DR, Laherty RF, Rotten D, Jaffe RB. A radioimmunoassay for dehydroepiandrosterone sulfate in the circulation of rhesus monkeys. Steroids. 1983;42:653–667. doi: 10.1016/0039-128x(83)90129-0. [DOI] [PubMed] [Google Scholar]
- 38.Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- 39.Goncharova ND, Oganyan TE, Taranov AG. Functions of the hypothalamo-hypophyseal-adrenal system in aging in female monkeys. Neurosci Behav Physiol. 2000;30:717–721. doi: 10.1023/a:1026663119704. [DOI] [PubMed] [Google Scholar]
- 40.Parker CR, Jr, Mixon RL, Brissie RM, Grizzle WE. Aging alters zonation in the adrenal cortex of men. J Clin Endocrinol Metab. 1997;82:3898–3901. doi: 10.1210/jcem.82.11.4507. [DOI] [PubMed] [Google Scholar]
- 41.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Brissie RM, Parker CR., Jr Effects of aging on cytochrome b5 expression in the human adrenal gland. J Clin Endocrinol Metab. 2005;90:4357–4361. doi: 10.1210/jc.2005-0017. [DOI] [PubMed] [Google Scholar]
- 42.Sulcova J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocrinol. 1997;154:57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- 43.Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 44.Rose DP, Fern M, Liskowski L, Milbrath JR. Effect of treatment with estrogen conjugates on endogenous plasma steroids. Obstet Gynecol. 1977;49:80–82. [PubMed] [Google Scholar]
- 45.Lobo RA, Goebelsmann U, Brenner PF, Mishell DR., Jr The effects of estrogen on adrenal androgens in oophorectomized women. Am J Obstet Gynecol. 1982;142:471–478. doi: 10.1016/s0002-9378(16)32393-6. [DOI] [PubMed] [Google Scholar]
- 46.Slayden SM, Crabbe L, Bae S, Potter HD, Azziz R, Parker CR., Jr The effect of 17 beta-estradiol on adrenocortical sensitivity, responsiveness, and steroidogenesis in postmenopausal women. J Clin Endocrinol Metab. 1998;83:519–524. doi: 10.1210/jcem.83.2.4562. [DOI] [PubMed] [Google Scholar]
- 47.Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vuga M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause. 2011;18:494–498. doi: 10.1097/gme.0b013e3181fb53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams J, Garcia M, Rochefort H. Estrogenic effects of physiological concentrations of 5-androstene-3 beta, 17 beta-diol and its metabolism in MCF7 human breast cancer cells. Cancer Res. 1981;41:4720–4726. [PubMed] [Google Scholar]
- 49.Cizza G, Ronsaville DS, Kleitz H, Eskandari F, Mistry S, Torvik S, Sonbolian N, Reynolds JC, Blackman MR, Gold PW, Martinez PE. Clinical subtypes of depression are associated with specific metabolic parameters and circadian endocrine profiles in women: the power study. PLoS One. 2012;7:e28912. doi: 10.1371/journal.pone.0028912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan XJ, Dai YB, Wu WF, Kim HJ, Barros RP, Richardson TI, Yaden BC, Warner M, McKinzie DL, Krishnan V, Gustafsson JA. Reduction of dendritic spines and elevation of GABAergic signaling in the brains of mice treated with an estrogen receptor beta ligand. Proc Natl Acad Sci U S A. 2012;109:1708–1712. doi: 10.1073/pnas.1121162109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su IH, Chen Y-C, Hwang W-T, Liu Z, Su T-P, Chen T-J, Barnhart KT, Yang Y-X. Risks and benefits of menopausal hormone therapy in postmenopausal Chinese women. Menopause. 2012 doi: 10.1097/gme.0b013e31824362ff. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honma N, Horii R, Iwase T, Saji S, Younes M, Ito Y, Akiyama F. Clinical importance of androgen receptor in breast cancer patients treated with adjuvant tamoxifen monotherapy. Breast Cancer. 2012 doi: 10.1007/s12282-012-0337-2. [DOI] [PubMed] [Google Scholar]
- 53.Chlebowski RT, Anderson GL, Gass M, Lane DS, Aragaki AK, Kuller LH, Manson JE, Stefanick ML, Ockene J, Sarto GE, Johnson KC, Wactawski-Wende J, Ravdin PM, Schenken R, Hendrix SL, Rajkovic A, Rohan TE, Yasmeen S, Prentice RL. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–1692. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15:603–612. doi: 10.1097/gme.0b013e318174ea4d. [DOI] [PubMed] [Google Scholar]
- 55.Fitzgerald CT, Seif MW, Killick SR, Elstein M. Age related changes in the female reproductive cycle. Br J Obstet Gynaecol. 1994;101:229–233. doi: 10.1111/j.1471-0528.1994.tb13115.x. [DOI] [PubMed] [Google Scholar]


