Abstract
Galectins (Gal) exert many activities, including regulation of inflammation and adipogenesis. We evaluated modulation of Gal-1, 3, 9 and 12 in visceral (VAT) and subcutaneous (SAT) adipose tissue in diet induced obesity (DIO) and in ob/ob mice. Both age and diet modulated expression of galectins, with DIO mice having higher serum Gal-1 and Gal-3 versus lean mice after 13–17 weeks of high-fat diet. In DIO mice there was a progressive increase in expression of Gal-1 and Gal-9 in SAT, whereas Gal-3 increased in both VAT and SAT. Expression of Gal-12 declined over time in VAT of DIO mice, similar to adiponectin. Obesity lead to increased production of Gal-1 in adipocytes, whereas the increased Gal-3 and Gal-9 of obesity mostly derived from the stromovascular fraction. Expression of Gal-12 was restricted to adipocytes. There was increased production of Gal-3 and Gal-9, but not Gal-1, in CD11c− and CD11c+ macrophages from VAT of DIO versus lean mice. Expression of Gal-1, 3, 9 and 12 in VAT and SAT of ob/ob mice followed a trend comparable to DIO mice. Rosiglitazone reduced serum Gal-1, but not Gal-3 and modulated expression of Gal-3 in VAT and Gal-9 and Gal-12 in SAT of DIO mice. High-fat feeding lead to increased adiposity in Gal-1 KO versus WT mice, with loss of correlation between leptin and adiposity and no alterations in glucose and insulin levels. Thus, obesity leads to differential modulation of Gal-1, 3, 9 and 12 in VAT and SAT, with Gal-1 acting as a modulator of adiposity.
Introduction
Obesity is the primary risk factor for development of Type 2 Diabetes (T2D) and significantly increases risk of other chronic conditions, such as cardiovascular disease and several types of cancer (1). Obesity is characterized by chronic inflammation stemming from an expanded adipose tissue, particularly adipose tissue located in the intra-abdominal compartment (visceral adipose tissue, VAT). Whereas expansion of VAT is highly correlated with co-morbidities, a selective increase of subcutaneous adipose tissue (SAT) does not have major metabolic consequences (2). Although the exact mechanisms underlying the differential role of VAT versus SAT in disease risk have yet to be identified, data indicate a higher inflammatory potential of VAT compared to SAT in terms of increased production of pro-inflammatory mediators and reduced expression of the protective adipokine adiponectin (3). In particular, accumulation of pro-inflammatory CD11c+ macrophages in VAT of obese subjects has been causally linked to development of insulin resistance (4)
Galectins (Gal) are a family of 15 structurally related proteins that bind N-acetyllactosamine, a common disaccharidefound on many N- or O-linked glycans, but they can also specifically interact with other ligands (5). Members of the galectin family exert a variety of activities, including regulation of immune and inflammatory responses, apoptosis, tumor development and progression, neural degeneration, atherosclerosis, diabetes, wound repair and adipogenesis (6). Because of their known involvement in regulating inflammation, insulin resistance and/or adipogenesis, we selected four members of the Gal family, Gal-1, Gal-3, Gal-9 and Gal-12, for the current study.
Gal-3 is the best studied member of the family in the context of inflammation, obesity and T2D. Overall, Gal-3 exerts pro-inflammatory activities and its production by leukocytes is increased during inflammatory responses (5). Gal-3 KO mice exhibit decreased inflammation in a variety of models (5), although they develop accelerated atherosclerosis and kidney damage when fed an atherogenic diet (7, 8). In humans, serum Gal-3 levels correlate with body mass index and are elevated in T2D patients (9, 10). In contrast with the pro-inflammatory effects of Gal-3, Gal-1 exerts immunosuppressive, anti-inflammatory and pro-resolving effects in several settings (11). Administration of Gal-1 protects NOD mice from diabetes by acting on T cells (12), whereas Gal-1 KO mice exhibit increased inflammatory responses in several experimental models, even though phase-specific effects of Gal-1 have been reported (11, 13). Gal-1 is expressed in SAT in overweight/obese subjects (14), but its expression has not been studied in VAT or compared in lean versus obese humans or animals. Gal-9 also exerts anti-inflammatory and anti-microbial activities and induces maturation of dendritic cells, in part by binding to the T cell immunoglobulin and mucin domain-3 (15). Ablation or neutralization of Gal-9 leads to enhanced inflammatory responses in various experimental settings, whereas administration of exogenous Gal-9 is protective against development of diabetes in NOD mice (5, 16). Data on production, regulation and role of Gal-9 in adipose tissue and in obesity are not currently available. Gal-12 is predominantly expressed by adipocytes, where it localizes to lipid droplets (17), and is required for adipocyte differentiation in vitro, but also induces adipocyte apoptosis (18, 19). Deficiency of Gal-12 in mice results in increased lipolysis, reduced adiposity and amelioration of insulin resistance (17). Expression of Gal-12 is increased in both VAT and SAT by calorie restriction in monkeys (18), while treatment with several insulin resistance-inducing hormones, including TNFα, downregulates Gal-12 expression in 3T3-L1 cells in vitro (20).
The above-mentioned evidence supports a potentially important role for Gal-1, 3, 9 and 12 in the regulation of adipogenesis, insulin resistance and the inflammatory response therein. However, systematic analyses on the expression and production of these members of the galectin family in obesity are lacking. In the current report, we investigated modulation of expression of Gal-1, 3, 9 and 12 in epididymal VAT and in SAT in a murine model of diet-induced obesity (DIO) as well as in genetic obesity (leptin-deficient ob/ob mice). Because prior evidence indicates that PPARγ agonists modulate expression of some galectins (18, 21), we also investigated the effect of the thiazolidinedione rosiglitazone (Rosi) on adipose tissue expression of Gal-1, 3, 9 and 12. Finally, we used Gal-1 KO mice to begin investigating the role of this galectin in modulating the response to DIO in mice as a complement to studies evaluating the role of Gal-3 and Gal-12 in metabolic disease and obesity (7, 8, 19, 22, 23).
Materials and Methods
Animals
Animal studies were approved by the Animal Care and Use Committee of the University of Illinois at Chicago. For induction of DIO, male C57BL6 mice were fed a high-fat diet (HFD, 60 Kcal% fat, 7% Kcal/fructose, from Research Diets, New Brunswick, NJ) ad libitum for various lengths of time beginning at 4 weeks of age, while control groups received standard chow diet for the same period. For experiments evaluating the effect of Rosi, mice were fed chow or HFD for 13 weeks. During the last 4 weeks of feeding, half of the animals were switched to a low fat diet (LFD, 10% Kcal% fat, 7% Kcal/fructose) or HFD containing 0.01% Rosi, for a total experimental time of 17 weeks. Leptin-deficient obese ob/ob mice and their lean littermates fed a standard chow diet were examined at 7–8 weeks of age. For experiments evaluating the effect of Gal-1 deficiency, male WT and Gal-1 KO C57BL6 mice were fed chow or HFD for 13 weeks. All mice were from The Jackson Laboratories (Bar Harbor, ME). Body composition was evaluated by DXA.
Separation of adipocytes from the stromo-vascular fraction (SVF)
Collagenase digestion was used to separate the SVF from adipocytes of epididymal VAT and SAT obtained from lean and DIO mice, as previously described (24). After digestion and washing, the two fractions were immediately frozen for subsequent mRNA extraction or quantification of Gal-1 and Gal- 3 levels by ELISA. Part of the SVF was used for flow cytometry analysis as described below.
Flow cytometry
Cells from the SVF were resuspended in PBS/BSA prior to surface staining with FITC-conjugated anti-F4/80 antibodies (eBioscience, San Diego, CA) and APC-conjugated anti-CD11c antibodies (BD Biosciences, San Jose’, CA). For intracellular staining, cells were fixed and permeabilized using reagents from eBioscience and subsequently stained with PE-conjugated anti-Gal-3 or anti-Gal-9 or with PE-conjugated rat IgG as a negative control (eBioscience). For detection of intracellular Gal-1, after fixing and permeabilization cells were incubated with a rat-anti-mouse Gal-1 antibody or IgG, followed by a PE-conjugated donkey-anti-rat antibody, both from R&D Systems (Minneapolis, MN). Samples were analyzed on a C6 Accuri cytometer (BD Biosciences).
Splenocyte cultures
Single-cell suspensions of splenocytes were obtained by pressing the spleen through a 100 μm mesh strainer. After washing, cells were resuspended at 2×106 in RPMI containing penicillin and streptomycin and cultured overnight in 24-well plates. Ten μl of Triton-X were subsequently added to the cultures to lyse cells for evaluation of Gal-1 and Gal-3 total production
Measurement of circulating and tissue-associated mediators
Levels of leptin, Gal-1 and Gal-3 were measured in serum, homogenates of the adipocytes and SVF and/or splenocyte cultures using ELISA kits from R&D Systems. Blood glucose was measured using a glucometer and serum insulin by ELISA (Alpco, Salem, NH) in mice fasted for 4 hours.
RNA expression analysis
Total RNA was isolated from epididymal VAT and SAT using Trizol and reverse transcribed. Gene expression of Gal-1, Gal-3, Gal-9, Gal-12, CD68, CCL2, and adiponectin was assessed by real-time RT-PCR using the TaqMan system and primers from Applied Biosystems (Foster City, CA). Relative expression was calculated using the ΔΔCT method after normalizing for expression of the geometric mean of GAPDH, actin and S18.
Statistical analysis
Data are expressed as mean +/− SEM. Statistical significance of differences were evaluated by ANOVA, Kruskal-Wallis test or Mann-Whitney, as appropriate. Statistical analyses were performed with SPSS Statistics version 17.0 software.
Results
Effect of HFD on circulating levels of Gal-1 and Gal-3
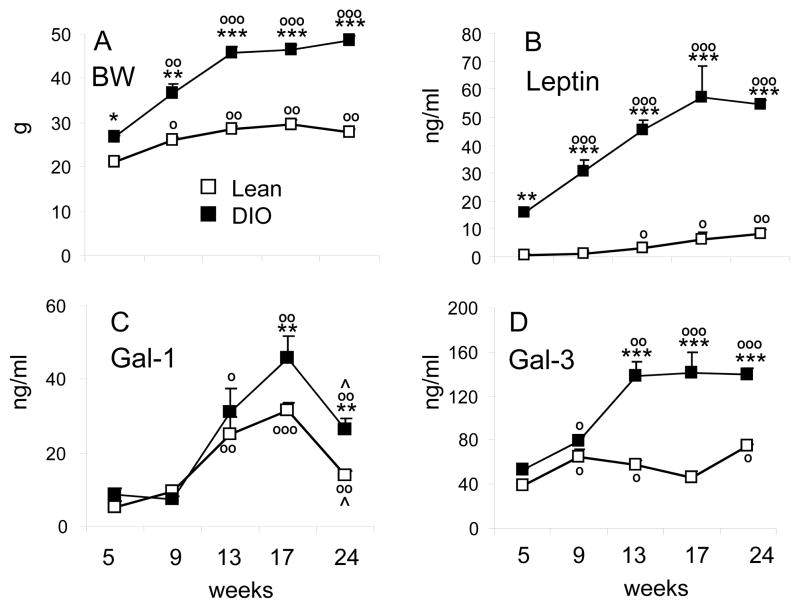
As expected, we observed a progressive elevation in body weight (Fig. 1A) and serum leptin (Fig. 1B) in both lean and DIO mice, with significantly higher values in the latter group at each time point. A significant increase in serum Gal-1 levels with time was observed in both lean and DIO mice, with Gal-1 levels significantly higher in DIO compared to lean mice at the 13, 17 and 24 weeks time points (Fig. 1C). Serum Gal-1 peaked at 17 weeks, and by 24 weeks declined in both lean and DIO mice. A minor, although significant, increase in circulating Gal-3 levels was observed with age in lean groups (Fig. 1D). In contrast, serum Gal-3 levels in DIO mice sharply increased between week 9 and 13 and then reached a plateau (Fig. 1D). Thus both age and, more markedly, DIO were associated with regulation of circulating Gal-1 and Gal-3 levels.
Figure 1. Time course of HFD-induced changes in body weight, serum leptin, Gal-1 and Gal-3.
Mice were fed regular chow (Lean, open squares) or HFD (DIO, black squares) for 5, 9, 13, 17 or 24 weeks. Body weight (A), serum leptin (B), Gal-1 (C) and Gal-3 (D) were measured at the indicated time points. Data are mean +/−SEM of 5–10 mice per group. *p<0.05, **p<0.01, ***p<0.001 vs lean at matching age; op<0.05, oop<0.01, ooop<0.001 vs respective 5 weeks; ^p<0.05 versus respective 17 weeks.
Effect of HFD on mRNA expression of galectins in VAT and SAT
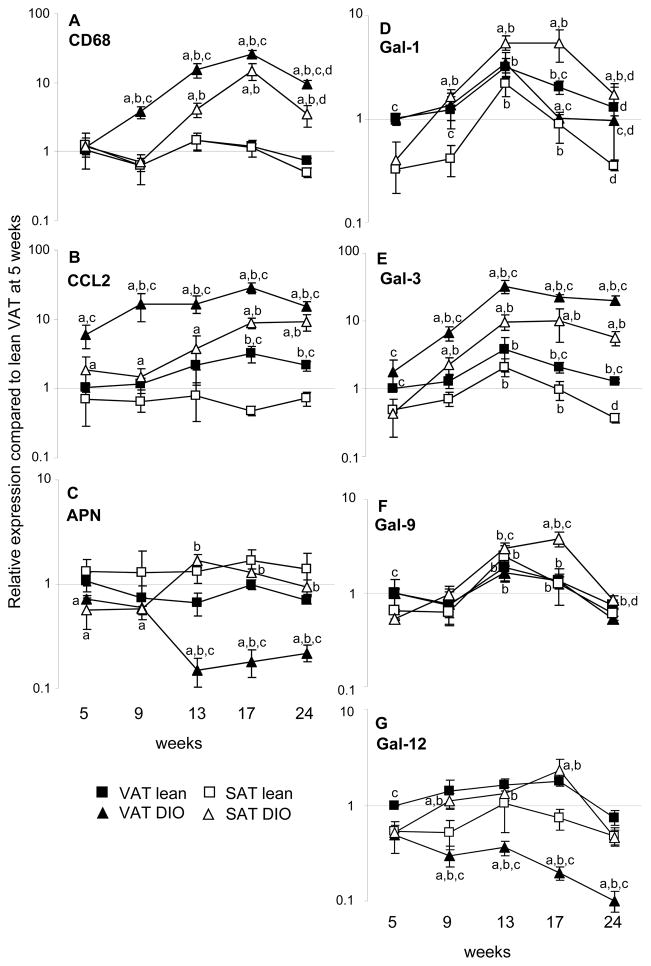
Feeding a HFD lead to a significant and progressive infiltration by macrophages and development of inflammation in VAT and, at the later time points, in SAT, as quantified by expression of CD68 and CCL2 (Fig 2A–B). In contrast, expression of adiponectin declined in VAT of DIO mice between 5 and 9 weeks to 20% of the level observed at 5 weeks (Fig. 2C). In contrast, a 2–4-fold increase in adiponectin expression was observed in SAT of DIO mice beginning at 13 weeks. Expression of adiponectin did not significantly change with time in adipose tissue of lean mice,
Figure 2. Time course of HFD-induced changes in expression of galectin mRNA in VAT and SAT.
Mice were fed regular chow (Lean, squares) or HFD (DIO, triangles) for 5, 9, 13, 17 or 24 weeks. Expression of CD68 (A), CCL2 (B), Adiponectin (APN) (C), Gal-1 (D), Gal-3 (E), Gal-9 (F), and Gal-12 (G) was evaluated by qRT-PCR in VAT (black symbols) and SAT (open symbols). Data are reported as gene expression relative to Lean VAT at 5 weeks using the ΔΔct method. Data are mean +/−SEM of 5–10 mice per group. ap<0.01 vs respective tissue in lean mice at same time point; bp<0.01 vs respective tissue at 5 weeks;, cp<0.01 vs respective SAT at same time point;; dp<0.01 vs respective tissue at 13–17 weeks.
At the 5-week time point, expression of Gal-1 was significantly higher in VAT compared to SAT in both lean and DIO mice (Fig. 2D). Feeding a HFD lead to a higher increase of Gal-1 expression in SAT compared to VAT, with levels peaking at 13–17 weeks and declining at 24 weeks. In DIO mice, peak levels of Gal-1 in SAT were 14-fold higher compared with levels observed at 5 weeks, whereas only a 3-fold increase was observed in VAT (Fig. 2D). A 7-fold increase in Gal-1 expression was also observed in SAT of lean mice at 13 weeks, with a return to baseline by 24 weeks. Expression of Gal-1 in lean VAT followed the same kinetics and magnitude change as observed in DIO mice. In lean mice, expression of Gal-1 remained higher in VAT compared to SAT throughout the whole time course. In contrast, HFD induced a switch in the relative expression of Gal-1 in the two adipose compartments, with SAT of DIO mice expressing significantly higher levels compared to VAT at each time point past 5 weeks.
Expression of Gal-3 was significantly higher in VAT compared to SAT of both lean and DIO mice at 5 weeks (Fig. 2E). Gal-3 progressively increased with time of HFD, resulting in significantly higher levels of Gal-3 in both VAT and SAT of DIO mice at each time point past 5 weeks (Fig. 2E). Expression of Gal-3 reached a plateau at 13–24 weeks in adipose tissue of DIO mice, with levels that were 20–30-times higher in VAT and 5–10-times higher in SAT compared to the respective levels at 5 weeks. At each time point, VAT expressed significantly higher levels of Gal-3 mRNA compared to SAT (Fig. 2E). A blunted elevation of Gal-3 mRNA was also observed with time in lean mice, reaching a 3-fold increase in both VAT and SAT at 13 weeks compared with 5 weeks and returning to baseline thereafter. Expression of Gal-3 was significantly higher in both SAT (5–10 folds) ad VAT (10–25-folds) of DIO mice compared with the respective time points and tissues of lean mice beginning at week 9 (Fig. 2E).
Similar to Gal-1 and Gal-3, expression of Gal-9 was also higher in VAT compared to SAT at 5 weeks in both lean and DIO mice (Fig. 2F). The highest induction of Gal-9 was observed in SAT of DIO mice, with a 6–8-fold increase at 13–17 weeks compared with levels at 5 weeks and expression still significantly elevated at 24 weeks (1.6-fold). In lean mice, there was a 4-fold increase in Gal-9 levels at 13 weeks compared to 5 weeks, with a gradual return to baseline by 24 weeks. A much less sustained modulation of Gal-9 was observed in VAT, with levels peaking at 13–17 weeks and maximal elevation reaching 1.8 folds compared to 5 weeks in both lean and DIO mice.
Expression of Gal-12, which is mostly produced by adipocytes ((18–20) and Fig. 3D), followed a trend similar to that of adiponectin (Fig. 2C). In fact, a progressive and significant decline in mRNA expression of Gal-12 was observed in VAT of DIO mice (Fig. 2G). The lowest expression of Gal-12 was observed at 24 weeks in DIO VAT, at levels that were 80% lower compared to those at 5 weeks and 95% lower than Gal-12 levels observed in lean VAT at the same time point. In contrast with the decline in VAT, expression of Gal-12 in DIO SAT was significantly elevated at 9, 13 and 17 weeks compared to 5 weeks, reaching a peak at 13 weeks with a 4-fold increase and returning to baseline at 24 weeks. As a result of differential regulation in VAT versus SAT of DIO mice, significantly higher levels of Gal-12 were present in SAT compared to VAT of DIO mice past the 5 week time point. In contrast, in lean mice expression of Gal-12 did not significantly change with time and was higher in VAT compared to SAT at each time point.
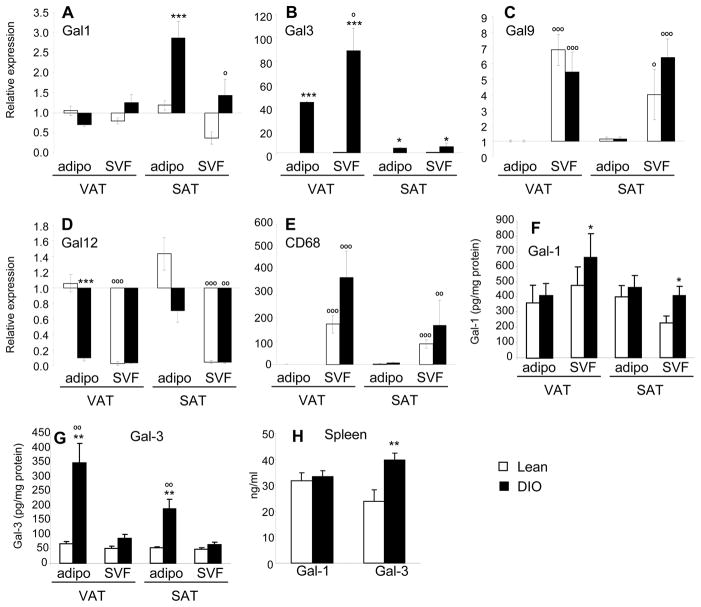
Figure 3. Expression of galectins in SVF versus adipocytes of lean and DIO mice.
Mice were fed regular chow (Lean, open columns) or HFD (DIO, black columns) for 13 weeks. Relative mRNA expression of Gal-1 (A), Gal-3 (B), Gal-9 (C), Gal-12 (D) and CD68 (E) in SVF and adipocytes in VAT and SAT. Data are reported as gene expression in SVF relative to the adipocyte fraction of lean mice in the same tissue using the ΔΔct method. Levels of Gal-1 (F) and Gal-3 (G) protein in homogenates of SVF and adipocytes obtained from VAT and SAT. Panel E: Levels of Gal-1 and Gal-3 protein in splenocyte cultures. Data are mean +/−SEM of 4–5 mice per group.*p<0.05, **p<0.01, ***p<0.001 vs respective lean. op<0.05, oo p<0.01, ooo p<0.001 vs respective adipocyte fraction.
Characterization of galectin-producing cells in VAT and SAT of lean and DIO mice
The relative expression of galectin mRNA and protein levels in adipocytes versus the SVF was evaluated after 13 weeks of chow or HFD. Gal-1 mRNA was expressed at comparable levels in adipocytes and SVF derived from VAT of lean or DIO mice. In SAT, adipocytes from DIO mice expressed the highest level of Gal-1 (Fig. 3A). At the protein level, Gal-1 was detectable in both the SVF and the adipocyte fraction in VAT and SAT, with DIO mice having significantly higher levels of Gal-1 protein in adipocytes compared to lean mice, while no significant differences between lean and DIO mice were observed in the SVF (Fig. 3F). Moreover, no significant differences in production of Gal-1 were detected in cultures of splenocytes obtained from lean and DIO mice (Fig. 3H)
Both adipocytes and SVF expressed significantly higher levels of Gal-3 mRNA in VAT of DIO compared to lean mice, with DIO SVF expressing the highest levels (Fig. 3B). The same pattern was observed in SAT, albeit with less marked difference between lean and DIO mice. Significantly higher levels of Gal-3 protein were detectable in the SVF of DIO compared to lean mice in both VAT and SAT, with no significant difference in adipocytes (Fig. 3G). In agreement with data obtained from the SVF, significantly higher levels of Gal-3 were produced by splenocyte cultures of DIO compared to lean mice (Fig. 3H).
Gal-9 was predominantly expressed in the SVF in both VAT and SAT of lean and DIO mice, with no significant differences between the two groups, while, as expected (18–20), Gal-12 was predominantly expressed by adipocytes in both tissues and groups (Fig. 3C–D). However, Gal-12 expression in VAT adipocytes from DIO mice was significantly reduced compared with levels observed in the adipocyte fraction of lean mice, in agreement with data presented in Fig. 2G.
Expression of the macrophage marker CD68 was exclusively present in the SVF in both VAT and SAT (Fig. 3E), confirming adequate separation between the SVF and adipocytes and lack of cross-contamination of the adipocyte fraction with lipid-laden macrophages.
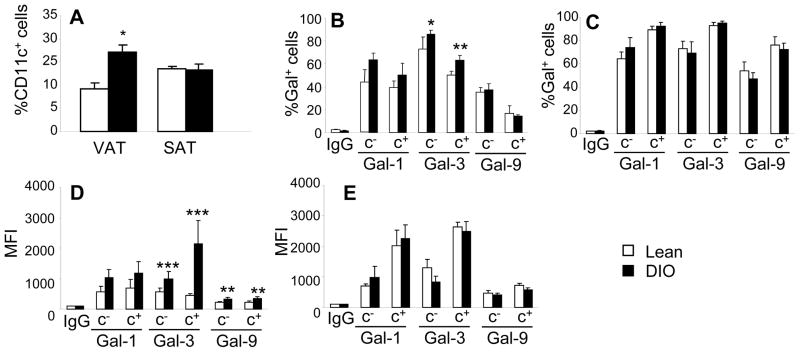
The SVF was analyzed by flow cytometry using the gating strategy shown in Fig. S1A. Because Gal-12 was only detectable in the adipocyte fraction ((17) and Fig. 3D), we did not evaluate its production in cells of the SVF. Feeding a HFD lead to a significantly higher percentage of pro-inflammatory F4/80+/CD11c+ cells in VAT of DIO mice compared to VAT of lean mice, whereas no significant differences in percentages of F4/80+/CD11c+ cells were observed between lean and DIO mice in SAT (Fig. 4A). A significantly higher percentage of Gal-3+ cells was present in both the F4/80+/CD11c− and F4/80+/CD11c+ SVF populations in VAT of DIO mice compared to lean animals (Fig. 4B), whereas no significant differences between lean and DIO mice were observed in the percentage of cells expressing intracellular Gal-1 or Gal-9 (Fig. 4B). Furthermore, no significant differences in the percentage of cells expressing Gal-1, Gal-3 or Gal-9 was observed in SAT of lean versus DIO mice in either cell population (Fig. 4C). The median fluorescence intensity (MFI) for intracellular Gal-3 and Gal-9 was significantly higher in both F4/80+/CD11c− and F4/80+/CD11c+ cells in VAT of DIO mice compared to lean mice, with F4/80+/CD11c+ of DIO mice having the highest expression of Gal-3 (Fig. 4E). In contrast, no significant differences in MFI were observed for Gal-1 in VAT and for any Gal in SAT (Fig. 4F). Representative plots from each tissue and group are shown in Fig. S1B.
Figure 4. Characterization of galectin-producing cells in VAT and SAT of lean and DIO mice.
Mice were fed regular chow (Lean, open columns) or HFD (DIO, black columns) for 13 weeks. Panel A: Percentage of CD11c+ cells in the F4/80+ population of VAT and SAT in lean and DIO mice. Panels B–C: Percentage of Gal+ cells in the F4/80+/CD11c− (c−) and F4/80+/CD11c+ (c+ ) population of VAT (B) and SAT (C). Panel D–E: Median Fluorescence Intensity (MFI) of Gal+ cells in the F4/80+/CD11c− (c−) and F4/80+/CD11c+ (c+ ) population of VAT (D) and SAT (E). DIO. Data are expressed as fold-increase in MFI versus respective IgG isotype. Data are mean +/−SEM of 8 mice per group.*p<0.05, **p<0.01, ***p<0.001 vs respective lean.
Effect of genetic obesity on serum levels and adipose tissue expression of galectins
To clarify whether modulation of galectin production in DIO mice was due to feeding a HFD or to obesity per se, serum levels of Gal-1 and Gal-3 as well as mRNA expression of each galectin were evaluated in genetically obese ob/ob mice fed normal chow. As expected, body weight of ob/ob mice was significantly higher compared with that of their lean WT littermates (Fig. 5A). The concentration of Gal-3 in serum of ob/ob mice was approximately double compared to that of WT mice (Fig. 5C), whereas circulating levels of Gal-1 trended towards higher levels in ob/ob mice without reaching statistical significance (Fig. 5B). The pattern of adipose tissue inflammation in ob/ob mice was comparable to that observed in DIO mice (Fig. 5D). With the exception of Gal-9, modulation of expression of the other galectins in SAT and VAT of ob/ob mice was comparable to results obtained in the DIO model. In fact, a significant upregulation of Gal-1 was observed in SAT, but not VAT, of ob/ob mice compared to lean animals, with SAT expressing significantly higher Gal-1 levels that VAT in the obese group (Fig. 5E). In contrast, Gal-3 expression was significantly upregulated in both SAT and VAT of ob/ob mice compared to WT mice (Fig. 5F). No significant differences between ob/ob and WT or between SAT and VAT were observed for expression of Gal-9 (Fig. 5G), whereas significantly lower expression of Gal-12 was present in VAT, but not SAT, of ob/ob compared to WT mice (Fig. 5H)
Figure 5. Galectins in genetically obese ob/ob mice.
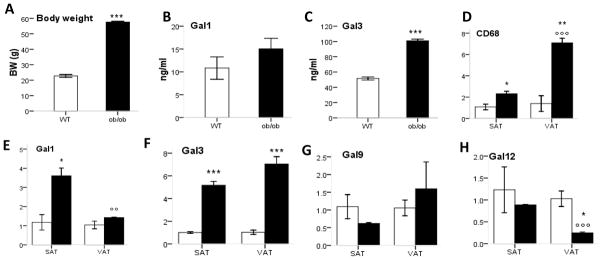
Lean WT (open bars) and obese ob/ob (black bars) mice fed regular chow were studied at 8 weeks of age. Body weight is shown in Panel A. Serum levels of Gal-1 (B) and Gal-3 (C) were measured by ELISA. Expression of CD68 (D), Gal-1 (E), Gal-3 (F), Gal-9 (G) and Gal-12 (H) was evaluated by qRT-PCR in VAT and SAT. Data in panels D–H are reported as gene expression relative to the WT group using the ΔΔct method. Data are mean +/− SEM of 5 mice per group. *p<0.05, **p< 0.01,***p< 0.001 ob/ob versus WT; oo p< 0.01, ooo p< 0.01 VAT versus SAT in respective strain.
Effect of Rosiglitazone on galectins in lean and DIO mice
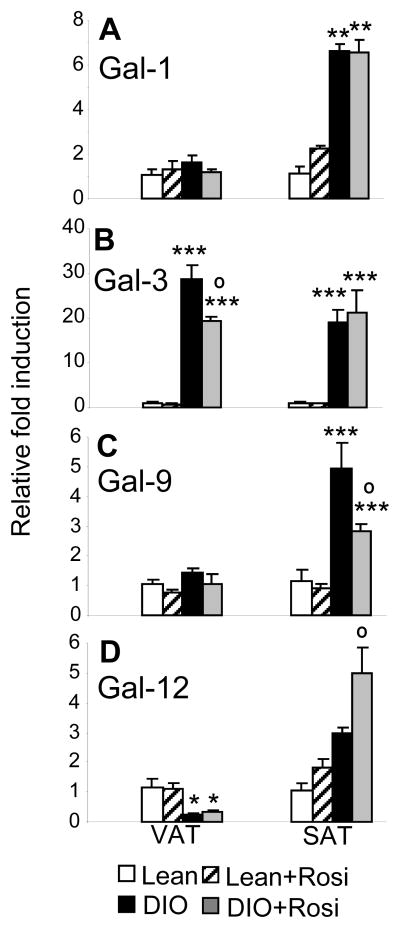
Administration of the insulin-sensitizing PPARγ activator Rosi lead to a significant increase adiposity and serum levels of adiponectin, without changes in circulating leptin levels, in both lean and DIO groups (Table 1), as expected (25). Circulating levels of Gal-1 were significantly reduced by Rosi in the DIO group, without significant effects in lean mice (Table 1). However, Rosi did not alter the upregulation of Gal-1 observed in SAT of DIO mice compared to lean mice and did not affect Gal-1 mRNA expression in VAT of either lean or DIO mice (Fig. 6A).
Table 1.
Effect of Rosiglitazone on serum adiponectin, Gal-1 and Gal-3 levels in leanand DIO mice
| Lean | Lean + Rosi | DIO | DIO + Rosi | |
|---|---|---|---|---|
| % fat mass | 15.12 +/− 0.20 | 21.44 +/− 1.91 o | 50.82 +/− 0.89*** | 55.80 +/− 2.36*** o |
| Leptin (ng/ml) | 2.03 +/− 0.21 | 2.27 +/− 0.46 | 48.45 +/− 7.82*** | 37.26 +/− 8.51*** |
| Adiponectin (μg/ml) | 7.21 +/− 0.76 | 10.99 +/− 0.31 o | 7.40 +/− 1.13 | 11.13 +/− 0.30 o |
| Gal-1 (ng/ml) | 31.4 +/− 2.1 | 30.8 +/− 4.7 | 45.8 +/− 5.8* | 31.0 +/− 2.2 |
| Gal-3 (ng/ml) | 46.1 +/− 5.6 | 62.3 +/− 8.2 | 141.5 +/− 18.4*** | 151.9 +/- 21.5*** |
Mice were fed regular chow (Lean) or HFD (DIO) for 17 weeks, with or without Rosi added to the diet during the last 4 weeks. Body composition was evaluated by DXA. Serum was obtained for measurement of leptin, adiponectin, Gal-1 and Gal-3 by ELISA.
p<0.05 versus each other group;
p< 0.001 versus lean and lean+Rosi;
p< 0.05 versus respective lean or DIO without Rosi.
Figure 6. Effect of rosiglitazone on expression of galectin mRNA in lean and DIO mice.
Mice were fed regular chow (Lean) or HFD (DIO) for 17 weeks, with or without Rosi in the diet during the last 4 weeks. Expression of Gal-1 (A), Gal-3 (B), Gal-9 (C) and Gal-12 (D) was evaluated in VAT and SAT of Lean (open bars), Lean+Rosi (hatched bars), DIO (black bars) and DIO+Rosi (grey bars) by qPCR. Data are expressed as fold increase over Lean mice in either VAT or SAT using the ΔΔct method. Data are mean +/− SEM of 10 mice per group. *p<0.05, **p< 0.01,***p< 0.001 versus respective lean group; op<0.05 versus respective DIO without Rosi.
Administration of Rosi did not significantly affect circulating levels of Gal-3 (Table 1), but lead to a significant reduction in Gal-3 expression in VAT of DIO mice, without effects in SAT or in lean mice (Fig. 6B). Administration of Rosi also significantly reduced expression of Gal-9 (Fig. 6C) and increased expression of Gal-12 (Fig. 6D) in SAT of DIO mice.
Gal-1 KO mice
To begin exploring the functional role of Gal-1 in modulating obesity, we compared lean and DIO WT mice to lean and DIO Gal-1 KO mice. As indicated in Table 2, DIO Gal-1 KO mice developed significantly higher adiposity compared to DIO WT mice, with no significant changes in body weight. However, the increased adiposity of DIO Gal-1 KO mice was not associated with the expected elevation in circulating leptin levels or with increased adipocyte size (Table 2 and Figure S2). In fact, the strong positive correlation between fat mass and circulating leptin levels observed in lean and DIO WT mice was lost in both lean and DIO WT Gal-1 KO mice (Fig. S3). Despite the elevated adipose mass of Gal-1 KO mice, fasting glucose and insulin levels, as well as circulating levels of adiponectin, were not significantly altered compared to WT mice (Table 2). Thus, increased adiposity in DIO Gal-1 KO mice was not associated with further worsening of glucose control induced by HFD.
Table 2.
Phenotype of lean and DIO Gal-1 KO mice
| WT Lean | Gal-1 KO Lean | WT DIO | Gal-1 KO DIO | |
|---|---|---|---|---|
| BW (g) | 28.0 +/− 1.0 | 27.6 +/− 0.1 | 46.3 +/− 1.0*** | 45.0 +/− 1.1*** |
| % fat mass | 8.2 +/− 2.4 | 13.1 +/− 2.5 | 40.9 +/− 2.3*** | 53.4 +/− 3.9*** oo |
| VAT adipocyte size (μm) | 136.7+/− 21.2 | 115.3 +/− 8.4 | 347.3+/− 10.7*** | 374.9 +/− 17.6*** |
| SAT adipocyte size (μm) | 93.5 +/− 18.7 | 93.6 +/− 26.5 | 244.6 +/− 33.7*** | 274.8 +/− 31.7*** |
| Leptin (ng/ml) | 1.75 +/− 0.28 | 0.96 +/− 0.52 | 57.1 +/− 20.47*** | 33.90 +/− 2.08*** |
| Adiponectin (μg/ml) | 4.91 +/− 0.42 | 5.18 +/− 0.19 | 4.65 +/− 0.56 | 4.74 +/− 0.46 |
| Glucose (mg/dl) | 159.2 +/− 16.3 | 157.6 +/− 12.2 | 229.6 +/− 13.4** | 201.2 +/− 11.0** |
| Insulin (ng/ml) | 1.06 +/− 0.21 | 0.74 +/− 0.08 | 1.76 +/− 0.15* | 2.49 +/− 0.51*** |
WT and Gal-1 KO mice were fed regular chow (Lean) or HFD (DIO) for 13 weeks. Blood was obtained for measurement of leptin, adiponectin, glucose and insulin. Fat mass was evaluated by DXA. Mean size of adipocytes in VAT and SAT was evaluated using ImageJ. Data are mean +/− SEM of 5 mice per group.
p<0.05,
p<0.05,
p< 0.001 versus respective lean;
p< 0.01 versus WT DIO.
Discussion
In the present report we demonstrate that both DIO and genetic obesity in mice are associated with modulation of expression and production of Gal-1, 3, 9 and 12 in VAT and SAT, suggesting these four members of the galectin family participate in regulating local inflammatory processes, adipose tissue remodeling, metabolism and adipogenesis during obesity. The pattern of expression in VAT versus SAT, timing of regulation, cellular source as well as regulation by the PPARγ agonist Rosi varied for each galectin, in agreement with the different putative roles described for these molecules in other settings. The experiment using Gal-1 KO mice indicates that this galectin actively participates in modulating adipose tissue mass in mice, with opposite effects on adipose mass compared to Gal-12 deficiency (17).
Gal-1
Expression of Gal-1 selectively increased in SAT of obese mice, with a much less sustained induction in VAT. This pattern of regulation is in agreement with the anti-inflammatory effects of Gal-1 and the observation that SAT has a lower inflammatory potential than VAT (2, 11). Previous reports indicated that several cell types, including leukocytes and adipocytes, can produce Gal-1 (26). Accordingly, our data demonstrate that both adipocytes and SVF produced Gal-1 in VAT, whereas adipocytes were the predominant cell type expressing Gal-1 in SAT. Importantly, the increase in Gal-1 production induced by obesity was limited to adipocytes.
The presence of significantly higher serum Gal-1 levels in DIO compared to lean mice at 17 and 24 weeks is in agreement with the significantly elevated circulating Gal-1 levels reported in overweight T2D patients compared to lean healthy controls (27). The significant decline in circulating levels and adipose tissue expression of Gal-1 between 17 and 24 weeks corresponds to a time of major remodeling of the architecture and pattern of inflammatory response in adipose tissue in obese mice (28). However, a decline in Gal-1 expression at 24 weeks was also observed in lean animals, in agreement with previous data showing a 50% reduction of Gal-1 expression in the colonic mucosa in 3-month-old versus 1-month-old mice (29). Epigenetic mechanisms may explain age-related regulation of Gal-1 production, that has been suggested to influence susceptibility to inflammatory stimuli (29), although exact mechanisms and pathogenetic implications, particularly in obesity, remain to be investigated.
Experiments performed in Gal-1 KO mice indicate that Gal-1 deficiency leads to increased adiposity in DIO mice. Gal-1 has been proposed as an early marker of adipocyte differentiation and to be actively involved in adipose tissue development (14, 26), with potential mechanisms including the ability of Gal-1 to act as a pro-apoptotic, pro-angiogenesis and anti-fibrotic mediator (30, 31). Our data also indicate that Gal-1 deficiency leads to loss of correlation between fat mass and circulating leptin and that the increased adiposity of Gal-1 KO mice is not associated with augmented adipocyte size. These features are reminiscent of the effects of PPARγ activators, including Rosi. Interestingly, Rosi significantly suppressed circulating levels of Gal-1, potentially implicating modulation of this galectin in the effects of TZDs. However, Gal-1 deficiency did not result in alterations of circulating adiponectin levels. More detailed studies are necessary to confirm these findings and identify the mechanisms by which Gal-1 affects production of leptin and adipose tissue development.
Gal-3
At variance with Gal-1, expression of Gal-3 progressively increased with DIO in both VAT and SAT, with VAT having relatively higher expression, in agreement with previous data (10, 11). Results indicate that upregulation of Gal-3 in obesity predominantly occurred in the SVF, with proinflammatory CD11c+ macrophages of DIO VAT producing the highest amounts of Gal-3, in agreement with a previous study (24). Circulating levels of Gal-3, as well as its production by splenocytes, were markedly increased in obesity, as reported in humans (9, 10). Data obtained in ob/ob mice paralleled the results of the DIO model, indicating that regulation of Gal-3 during obesity is leptin-independent. Moreover, circulating levels of Gal-3 mirrored the pattern of induction in VAT and SAT in both DIO and ob/ob mice, suggesting that adipose tissue is a major contributor to systemic levels of Gal-3 during obesity. However, our data show that Rosi reduced Gal-3 expression in VAT of DIO mice, but did not significantly affect circulating levels. These data are in agreement with the previously reported inhibitory effect of Rosi on mucosal induction of Gal-3 in a model of allergic rhinitis (21), and suggest that Rosi differentially regulates production and/or release of galectins by different tissues and cell types.
Overall, the pattern of modulation of Gal-3 in obesity was consistent with its role as a pro-inflammatory mediator with a potential etiological relevance. However, some caution is needed since published data point to a complex role of Gal-3 in modulating the deleterious consequences of atherogenic diets and in development of fatty liver in mice, indicating the need for further studies aimed at better characterizing the specific role played by Gal-3 in adipose tissue during obesity (7, 8, 22, 23, 32, 33).
Gal-9
Obesity lead to a significant increase of Gal-9 mRNA expression selectively in SAT of DIO mice. However, no elevation of Gal-9 expression was observed in SAT of ob/ob mice compared to lean controls, suggesting either that leptin is necessary for upregulation of Gal-9 in SAT or that the increased expression observed in DIO mice was the result of HFD rather than obesity. This possibility is supported by data indicating that dietary components modulate production of Gal-9 in the intestine of mice (34).
In agreement with data demonstrating production of Gal-9 by macrophages (35), we found that the SVF predominantly expressed Gal-9 and that obesity lead to a significant increase in Gal-9 production by both CD11c− and CD11c+ macrophages in VAT. Substantial evidence indicates an important role for Gal-9 in dampening inflammation by modulating the interaction of macrophages with T lymphocytes via Tim3 (15). The selective upregulation of Gal-9 in SAT of DIO mice is consistent with its anti-inflammatory effects, although more data are necessary to better understand its specific roles and mechanisms of regulation. The need for further studies is also underscored by the observation that Rosi prevented elevation of Gal-9 mRNA expression in SAT of DIO mice, at variance with previous data indicating lack of inhibition by PPARγ agonists on expression of Gal-9 by endothelial cells in vitro (36). Similar to Gal-1, Gal-9 expression also declined at 24 weeks in both lean and DIO mice. Data on age-related changes in Gal-9 expression are not currently available, indicating the need for further studies to elucidate this response.
Gal-12
Expression of Gal-12 was exclusively restricted to adipocytes, in agreement with previous reports (17, 19). A progressive decrease of Gal-12 expression was observed in VAT of DIO mice, with Gal-12 also being significantly lower in VAT of ob/ob mice compared to their lean counterpart. The pattern of regulation of Gal-12 was comparable to that of adiponectin. However, Gal-12 and adiponectin are likely to play opposite roles in modulating obesity, since Gal-12 deficiency is associated with reduced adiposity and increased insulin sensitivity (17), whereas adiponectin is protective against development of insulin resistance (37). Thus, strategies leading to upregulation of Gal-12 production may be helpful in obesity. Treatment with insulin resistance-inducing hormones, including TNFα, downregulates Gal-12 expression in 3T3-L1 cells in vitro, whereas caloric restriction and PPARγ agonists increase adipose tissue expression of Gal-12 (18, 20). Our data demonstrating increased expression of Gal-12 in DIO mice in response to Rosi are in agreement with these previous results, although we could only demonstrate a significant impact of Rosi in SAT, but not VAT.
Conclusions
We report a thorough description of the pattern of expression of selected members of the galectin family and detail their differential modulation in adipose tissue during obesity. Whereas Gal-1, 3 and 9 likely participate in the response to excessive caloric intake by either increasing (Gal-3) or downregulating (Gal-1 and Gal-9) inflammation and modulate adipose tissue remodeling (11), expression of Gal-12 by adipocytes is more directly related to lipolysis and glucose metabolism (17). Data obtained in Gal-1 KO mice reinforce the concept that members of the galectin family are active participants in modulation of adipose tissue development in obesity. These data can inform galectin-based strategies to counteract the pathological processes occurring during initiation and progression of obesity and its associated morbidities.
Supplementary Material
Acknowledgments
This work was supported by NIH grant DK083328 to GF.
References
- 1.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–16. doi: 10.1016/j.amjmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Rutkowski JM, Davis KE, Scherer PE. Mechanisms of obesity and related pathologies: the macro-and microcirculation of adipose tissue. FEBS J. 2009;276:5738–5746. doi: 10.1111/j.1742-4658.2009.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norling LV, Perretti M, Cooper D. Endogenous galectins and the control of the host inflammatory response. J Endocrinol. 2009;201:169–184. doi: 10.1677/JOE-08-0512. [DOI] [PubMed] [Google Scholar]
- 6.Yang R-Y, Rabinovich GA, Liu F-T. Galectins: structure, function and therapeutic potential. Expert Reviews Mol Med. 2008;10:1–24. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 7.Iacobini C, Menini S, Oddi G, Ricci C, Amadio L, Pricci F, Olivieri A, et al. Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: evidence for a protective role of galectin-3 as an AGE receptor. FASEB J. 2004;18:1773–1775. doi: 10.1096/fj.04-2031fje. [DOI] [PubMed] [Google Scholar]
- 8.Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Cordone S, Taurino M, et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice. Role of lipoxidation via receptor-mediated mechanisms. Arterioscler Thromb Vasc Biol. 2009;29:831–836. doi: 10.1161/ATVBAHA.109.186791. [DOI] [PubMed] [Google Scholar]
- 9.de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, et al. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2011 doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 10.Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, Schnitzbauer A, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010;95:1404–1411. doi: 10.1210/jc.2009-1619. [DOI] [PubMed] [Google Scholar]
- 11.Cooper D, Ilarregui JM, Pesoa SA, Croci DO, Perretti M, Rabinovich GA. Multiple functional targets of the immunoregulatory activity of galectin-1: Control of immune cell trafficking, dendritic cell physiology, and T-cell fate. Methods Enzymol. 2010;480:199–244. doi: 10.1016/S0076-6879(10)80011-4. [DOI] [PubMed] [Google Scholar]
- 12.Perone MJ, Bertera S, Shufesky WJ, Divitto SJ, Montecalvo A, Mathers AR, Larregina AT, et al. Suppression of autoimmune diabetes by soluble galectin-1. J Immunol. 2009;182:2641–2653. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal AJ, Sampaio AL, Maione F, Greco KV, Niki T, Hirashima M, Perretti M, et al. Endogenous galectin-1 and acute inflammation: emerging notion of a galectin-9 pro-resolving effect. Am J Pathol. 2011;178:1201–1209. doi: 10.1016/j.ajpath.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed M, Neville MJ, Edelmann MJ, Kessler BM, Karpe F. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity. 2009;18:27–34. doi: 10.1038/oby.2009.208. [DOI] [PubMed] [Google Scholar]
- 15.Pan H-F, Zhang N, Li W-X, Tao J-H, Ye D-Q. TIM-3 as a new therapeutic target in systemic lupus erythematosus. Mol Biol Rep. 2009;37:395–398. doi: 10.1007/s11033-009-9833-7. [DOI] [PubMed] [Google Scholar]
- 16.Chou F-C, Shieh S-J, Sytwu H-K. Attenuation of TH1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur J Immunol. 2009;39:2403–2411. doi: 10.1002/eji.200839177. [DOI] [PubMed] [Google Scholar]
- 17.Yang RY, Yu L, Graham JL, Hsu DK, Lloyd KC, Havel PJ, Liu FT. Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice. Proc Natl Acad Sci U S A. 2011;108:18696–18701. doi: 10.1073/pnas.1109065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotta K, Funahashi T, Matsukawa y, Takahashi M, Nishizawa H, Kishida K, Matsuda M, et al. Galectin-12, an adipose-expressed galectin-like molecule possessing apoptosis-inducing activity. J Biol Chem. 2001;276:34089–34097. doi: 10.1074/jbc.M105097200. [DOI] [PubMed] [Google Scholar]
- 19.Yang R-Y, Hsu DK, Yu L, Chen H-Y, Liu F-T. Galectin-12 is required for adipogenic signaling and adipocyte differentiation. J Biol Chem. 2004;279:29761–29766. doi: 10.1074/jbc.M401303200. [DOI] [PubMed] [Google Scholar]
- 20.Fasshauer M, Klein J, Lossner U, Paschke R. Negative regulation of adipose-expressed galectin-12 by isoproterenol, tumor necrosis factor a, insulin and dexamethasone. Eur J Endocrinol. 2002;147:553–559. doi: 10.1530/eje.0.1470553. [DOI] [PubMed] [Google Scholar]
- 21.Han JL, Ding RY, Zhao L, Ren Z, Jiang XJ. Rosiglitazone attenuates allergic inflammation and inhibits expression of galectin-3 in a mouse model of allergic rhinitis. J Int Med Res. 2008;36:830–836. doi: 10.1177/147323000803600426. [DOI] [PubMed] [Google Scholar]
- 22.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, et al. Galectin-3 ablation protects mice from diet-induced NASH: A major scavenging role for galectin-3 in liver. J Hepatol. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi Y, Tsuneyama K, Nomoto K, Fujimoto M, Salunga TL, Nakajima T, Miwa S, et al. Nonalcoholic steatohepatitis and hepatocellularcarcinoma in galectin-3 knockout mice. Hepatol Res. 2008;38:1241–1251. doi: 10.1111/j.1872-034X.2008.00395.x. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Lu M, Nguyen MT, Bae EJ, Chapman J, Feng D, Hawkins M, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aprahamian T, Bonegio RG, Richez C, Yasuda K, Chiang LK, Sato K, Walsh K, et al. The peroxisome proliferator-activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J Immunol. 2009;182:340–346. doi: 10.4049/jimmunol.182.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Mariman E, Keijer J, Bouwman F, Noben JP, Robben J, Renes J. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell Mol Life Sci. 2004;61:2405–2417. doi: 10.1007/s00018-004-4256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Feng Q, Chen Y, Zuo J, Gupta N, Chang Y, Fang F. Proteomics-based identification of differentially-expressed proteins including galectin-1 in the blood plasma of type 2 diabetic patients. J Proteome Res. 2009;8:1255–1262. doi: 10.1021/pr800850a. [DOI] [PubMed] [Google Scholar]
- 28.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 29.Kellermayer R, Balasa A, Zhang W, Lee S, Mirza S, Chakravarty A, Szigeti R, et al. Epigenetic maturation in colonic mucosa continues beyond infancy in mice. Hum Mol Genet. 2010;19:2168–2176. doi: 10.1093/hmg/ddq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T cell immunity: past, present and future. Clin Immunol. 2012;142:107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okano K, Tsuruta Y, Yamashita T, Takano M, Echida Y, Nitta K. Suppression of renal fibrosis by galectin-1 in high glucose-treated renal epithelial cells. Exp Cell Res. 2010;316:3282–3291. doi: 10.1016/j.yexcr.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Iacobini C, Menini S, Ricci C, Scipioni A, Sansoni V, Mazzitelli G, Cordone S, et al. Advanced lipoxidation end-products mediate lipid-induced glomerular injury: role of receptor-mediated mechanisms. J Pathol. 2009;218:360–369. doi: 10.1002/path.2536. [DOI] [PubMed] [Google Scholar]
- 33.Iacobini C, Oddi G, Menini S, Amadio L, Ricci C, Di Pippo C, Sorcini M, et al. Development of age-dependent glomerular lesions in galectin-3/AGE-receptor-3 knockout mice. Am J Physiol Renal Physiol. 2005;289:F611–621. doi: 10.1152/ajprenal.00435.2004. [DOI] [PubMed] [Google Scholar]
- 34.de Kivit S, Saeland E, Kraneveld AD, van de Kant HJ, Schouten B, van Esch BC, Knol J, et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. 2012 doi: 10.1111/j.1398-9995.2011.02771.x. [DOI] [PubMed] [Google Scholar]
- 35.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, et al. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J Exp Med. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imaizumi T, Kumagai M, Nishi N, Hirashima M, Hatakeyama M, Tamo W, Yoshida H, et al. 15-deoxy-delta(12,14)-prostaglandin J2 inhibits IFN-gamma-induced galectin-9 expression in cultured human umbilical vein endothelial cells. Int Arch Allergy Immunol. 2003;131:57–61. doi: 10.1159/000070436. [DOI] [PubMed] [Google Scholar]
- 37.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.