Introduction
Although improvements in breast cancer treatment continue to be made allowing a majority of women to survive many years after diagnosis, prevention of this disease is still of outmost importance. Towards this goal women identified to be at increased breast cancer risk based on the Gail Score have been offered tamoxifen treatment and more recently raloxifene for prevention. However, these pharmaceutical agents have undesirable side effects and many women refuse treatment or discontinue it. Also other factors not evaluated in the Gail Score for example obesity or adult weight gain also increase risk. This has led to recommendations for women to lose weight if obese or to prevent weight gain to decrease their risk.
Although human studies are limited as to actual documentation that weight loss or prevention of weight gain will reduce breast cancer risk [1] in animal models calorie restriction consistently prevents the development of mammary tumors. In most cases chronic calorie restriction (CCR) protocols have been used whereby the same degree of calorie restriction is used on a daily basis. This approach has been consistently reported to prevent or delay the development of spontaneous and carcinogen-induced mammary tumors [2-8]. Interestingly, the consumption of high-fat diets during CCR protocols did not alter the protective effect of this intervention [9-12]. In contrast ad libitum consumption of high fat diets shorted latency and/or increased the incidence of spontaneous and carcinogen-induced mammary tumors [13]. Further, we have reported that transgenic MMTV-TGF-α mice fed a moderately high-fat diet (32.5% fat calories) that gained weight and became obese, i.e., obesity-prone mice had a shortened tumor latency compared to mice fed a low fat diet [14]. Interestingly, mice fed the same high fat diet that did not gain weight, i.e., obesity-resistant, had shorter mammary tumor latency than did low-fat mice. Thus, the impact of dietary fat content on mammary tumor development may be dependent upon whether ad libitum or calorie restriction is utilized.
Although CCR is the most widely implemented protocol for mammary tumor prevention, a few investigators have evaluated the impact of intermittent calorie restriction (ICR) on mammary tumor development. For example, fasting/refeeding regimens have been reported to reduce mammary tumor incidence in comparison to ad libitum feeding [15-17]. However, in one case it appeared that fasted/refed C3H/Ou mice fed a high fat diet developed more spontaneous mammary tumors compared to a fasted/refed group fed a low fat diet [16]. Further, two different ICR protocols implemented in rats fed high fat diets did not provide protective against the development of carcinogen-induced mammary tumors [18,19].
More recently we have reported that multiple cycles of three weeks of 50% caloric restriction followed by three weeks of refeeding reduced the incidence of mammary tumors in two transgenic mouse strains, MMTV-TGF-α [20-22] and MMTV-neu [23], to a greater extent than did the same degree of calorie restriction implemented by CCR. The diets used were based on AIN-93M formulations providing ~9% fat calories to ad libitum-fed mice. Here, we used the ICR protocol to determine how a higher fat intake affected this protective effect of ICR. We also evaluated the influence of consumption of a higher fat diet on serum IGF-1 and IGFBP-3 as previously MMTV-TGF-α mice with mammary tumors had higher terminal IGF-1 and lower IGFBP-3 serum levels than did mice without mammary tumors regardless of dietary intervention and the elevated IGF-I levels were present prior to tumor detection. [22].
Another aspect of our investigation was to measure the serum adipokines, leptin and adiponectin, which are synthesized and secreted from adipose tissue and have been implicated in breast cancer development [24-26]. For example, in in vitro experiments addition of leptin enhanced proliferation of human breast cancer cell lines [27-31]; while in contrast, adiponectin reduced breast cancer cell proliferation [32-35]. Further findings from two human studies [36,37] and from our in vitro study which evaluated the impact of different adiponectin:leptin ratios on human breast cancer cell proliferation [38] suggested that the adiponectin:leptin ratio may be more important in determining how these two proteins affect mammary tumor development than either alone. In support, in both longitudinal and cross-sectional studies we have shown that the ICR protocol was associated with an elevated adiponectin:leptin ratio in relationship to reduced tumor incidence of MMTV-TGF-α mice [39,40].
We report here that even when mice were fed a high-fat diet during refeeding that the ICR protocol still was more protective than CCR in this model of hormone-responsive breast cancer.
Material and Methods
Animals and Study Design
MMTV-TGF-α female mice that overexpress human TGF-α were produced at the Hormel Institute (Austin, MN, USA) and genotyped as previously described [20]. At 8 weeks of age mice were housed individually and provided ad libitum access to water and powdered AIN-93M diet. At 10 weeks of age mice were assigned to AL, CCR or ICR groups. Mice in the AL group (N=45) had free access to a 22.7% fat by calories diet. For the ICR protocol during each 3 week period of restriction ICR mice (N=45) were provided a diet 19% fat by calories and fed at 50% of the consumption level of AL mice and in each 3 week refeeding period were provided with a 33.6% fat by calories diet at 100% of food intake of age-matched AL mice. CCR mice (N=45) were given a 29.6% fat by calories diet formulated to match the calorie/nutrient intake of ICR mice for each 6-week cycle when given at 75% of age-matched ad-libitum consumption. Formula and nutrient information of all research diets are presented in Table 1. The goal was that ICR and CCR mice consumed the same absolute amounts of fat and other nutrients except for carbohydrate in comparison to the AL mice.
Table 1.
Formula and Nutrient Information of Research Diets.
| Group | Ad libitum | Chronic Restricted |
Intermittent (restriction) |
Intermittent (refeeding) |
|---|---|---|---|---|
| Diet* | TD 06531 | TD 06530 | TD 98213 | TD 03438 |
| Casein | 151.2 | 201.0 | 280.0 | 162.4 |
| L-Cystine | 2.0 | 2.6 | 3.6 | 2.1 |
| Corn Starch | 397.38 | 323.874 | 322.8 | 324.269 |
| Maltodextrin | 155.0 | 133.0 | 98.0 | 150.0 |
| Sucrose | 100.0 | 87.0 | 61.0 | 100.0 |
| Soybean Oil | 97.3 | 130.0 | 80.0 | 154.5 |
| Cellulose | 45.0 | 53.0 | 59.584 | 50.0 |
| Mineral Mix, AIN-93M-MX (94049) |
37.8 | 50.4 | 70.0 | 40.6 |
| Vitamin Mix, AIN-93-VX (94047) |
10.8 | 14.4 | 20.0 | 11.6 |
| Choline Bitartrate | 3.5 | 4.7 | 5.0 | 4.5 |
| TBHQ, antioxidant | 0.02 | 0.026 | 0.016 | 0.031 |
| Nutrient information (% of kcal) | ||||
| Protein | 13.7 | 17.7 | 30 | 13.7 |
| Carbohydrate | 63.6 | 52.7 | 51 | 52.7 |
| Fat | 22.7 | 29.6 | 19 | 33.6 |
| Kcal/g | 3.9 | 4.0 | 3.8 | 4.2 |
All diets were made by Harlan Teklad.
Food intakes were determined daily and body weights weekly at which time mice were palpated for mammary tumors. Once detected mammary tumor growth was monitored with calipers. All mice were euthanized by CO2 overdose at predetermined endpoints of either 79 (end of restriction) or 82 (end of refeeding) weeks of age or when mammary tumor size exceeded 20 mm in length or the mouse lost more then 25% of its body weight. When results are presented for ICR mice during restriction periods they are further classified as ICR-Restricted and during refeeding periods as ICR-Refed.
Tissue Sample collection and Histopathological analysis
At sacrifice, fat pads (mammary, retroperitoneal and parametrial), livers, mammary tumors and any abnormally appearing organs or tissues were removed and weighed. A sample of each was placed in 10% neutral buffered formalin. The remaining tissues were stored at −70°C. Left mammary fad pads, mammary tissue, mammary tumors and samples that appeared abnormal were sent to the Department of Pathology and Laboratory Medicine of the Mayo Foundation (Rochester, MN, USA) for histopathological analyses to determine malignancy and/or disease status.
Serum Measurements
Over the course of the study (10 weeks = baseline and cycles 5, 8, and 11) blood samples were obtained from three cohorts corresponding to the first, second and third weeks of the restriction and refeeding periods of ICR mice. For each mouse two samples were obtained per cycle such that for cohort one a sample was obtained corresponding to the first week of restriction and the first week of refeeding (week 1 and 4 of the cycles 5, 8, 11), for cohort two after two weeks of restriction and two weeks of refeeding (week 2 and 4 of the cycles 5, 8, 11) and cohort three after three weeks of restriction and three weeks of refeeding (week 3 and 6 of the cycles 5, 8, 11). Also terminal blood samples were collected from all mice at death. Blood samples were obtained under anesthesia from the orbital sinus. Serum samples were stored at −20°C. IGF-1 was measured using mouse/rat IGF-1 ELISA (DSL-10-29200, Diagnostic Systems Laboratory, Webster, TX) kits. Serum IGFBP-3 levels were determined using a commercial human IGFBP-3 ELISA kit (DSL-10-6600, Diagnostic Systems Laboratory, Webster, TX). Leptin was measured using the mouse Leptin ELISA kit (EZML-82K, Linco Research, St. Charles, MO). Serum adiponectin levels were determined using a mouse Adiponectin ELISA kit (EZML-60K, Linco).
Statistical Analysis
Results are presented as mean ± standard error of the mean (SEM). Serum data were analyzed by ANOVA followed by Neuman-Keuls test or t-test. Number of mammary tumors per mouse, mammary tumor incidence and grade were analyzed by the Chi-squared and two-group log-rank test. Kaplan-Meier analysis was used for evaluation of time of tumor detection. Graph Pad Prism version 4 was used for statistical analysis.
Results
Food Intake
As expected based from the experimental design cumulative energy intakes were lower for CCR (6038 ± 11 kcal/mouse, 22% reduction) and ICR (6131 ± 11 kcal/mouse, (21% reduction) mice, respectively compared to AL mice (ANOVA P<0.0001, ICR and CCR P< 0.001 versus AL). The total calorie intake of the AL mice was 7766 ± 99 kcal/mouse. However, cumulative fat intakes were similar, 194 ± 2.g, 196. ± 0.4 g and 195 ± 0.4 g for AL, CCR and ICR groups respectively.
Body and Fat Pad Weights
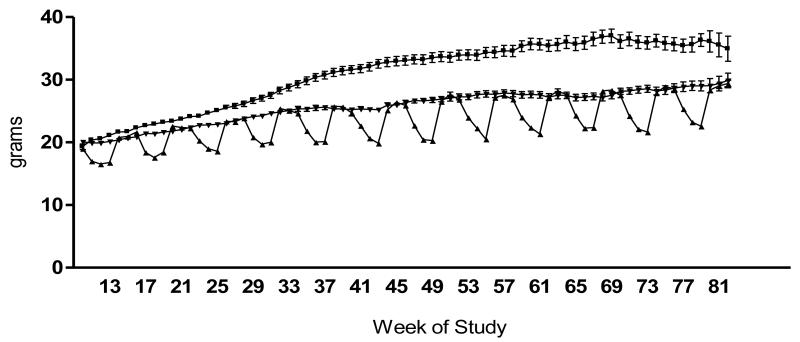
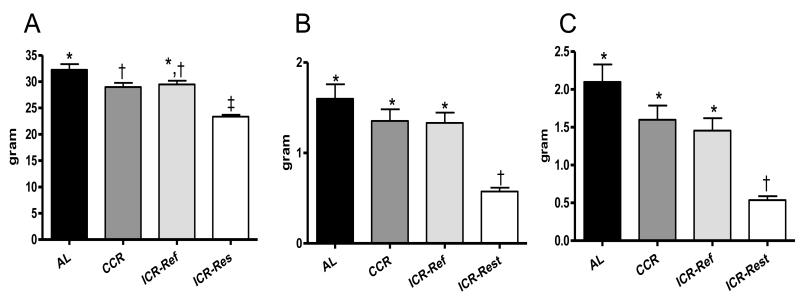
Body weights over the course of the study are presented in Figure 1. The body weight curve for AL mice was significantly higher than for CCR and ICR mice (P<0.001). ICR mice exhibited a pattern of weight loss during caloric restriction periods followed by rapid weight regain during the first week of refeeding. Terminal body and fat pad weights are presented in Figure 2. At euthanasia ICR-Restricted mice had body and fat pad weights significantly less than AL (P<0.001), CCR (P<0.001) and ICR-Refed (P<0.01) mice. In addition, AL mice had significantly heavier body weights than CCR (P<0.05) mice, while CCR and ICR-Refed mice had similar body weights (Figure 2A). There were no significant differences in mammary (Figure 2B) and internal (Figure 2C) fat pad weights among AL, CCR and ICR-Refed mice which were all significantly heavier than those of the ICR-Restricted mice.
Fig 1.
Body weight curves of TGF-α female mice over the course of the experiment. Ad libitum-Fed (AL) (■) n=12-45 depending on age; Intermittent Calorie Restricted (ICR) (▼) n=45; Chronic Calorie Restricted (CCR) (▲) n=19-45 depending on age. ANOVA P < 0.0001, All dietary groups are significantly different from each other (P<0.001).
Fig 2.
Final Body and Fat Pad Weights of TGF-α Female Mice. A, B and C, AL (n = 42), CCR (n = 44), ICR-Refed (n = 22) and ICR-Restricted (n = 23) mice. A. Terminal Body Weight. Bars represent means of Terminal Body Weights. ANOVA P < 0.05. B. Mammary Fad Pad Weight (combined right and left mammary fat pads. Bars represent means of Mammary Fat Pad Weights. ANOVA P> 0.01. C. Internal Fat Pad Weight (combined parametrial and retroperitoneal fat pads). Bars represent means of Internal Fat Pad Weights. ANOVA P < 0.05. In A, B and C ICR-Refed mice were euthanized during a refeeding period and ICR-Restricted mice were euthanized during a restriction period. Columns with different superscripts are significantly different from each other.
Pathology
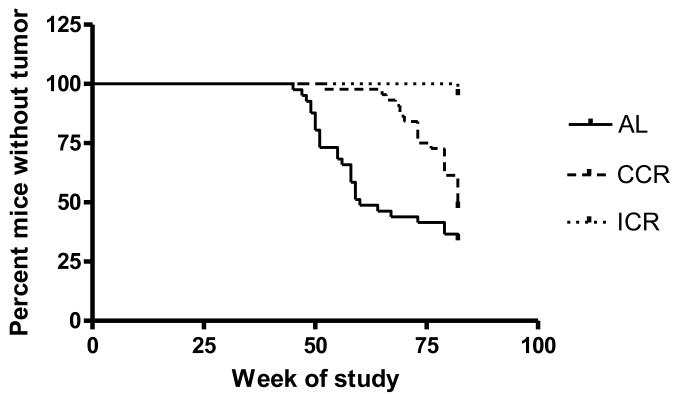
Four mice died from unknown illnesses (three AL and one CCR) prior to the age when mammary tumors usually develop, i.e., 39 weeks of age, and were removed from further consideration. Tumor incidence of 66.7% for AL mice (28/42) was the highest, followed by 52.3% for CCR mice (23/44), and 4.4% for ICR mice (2/45) was the lowest (Table 2). All groups were significantly different from each other. Tumors become palpable at age 45 weeks in AL mice, 52 weeks in CCR mice. Two mammary tumors found in ICR mice were not palpable and only detected following necropsy at 82 weeks of age with one tumor visual and the second identified as a focus of adenocarcinoma in the mammary fat pad by the pathologist. Kaplan-Meier analysis revealed a significant difference among AL, CCR and ICR groups in time of tumor detection (P<0.0001, Figure 3). It should be noted that the due to the limited tumors obtained for the ICR mice number of tumors per mouse and tumor weight were not included in statistical analysis. Tumor-bearing AL mice developed between 1 to 5 mammary tumors per mouse; while CCR mice had 1 to 3 (P<0.0001). Interestingly CCR mice had significantly higher mammary tumor weight than did AL mice.
Table 2.
Characteristic of Mammary Tumors from Ad libitum-fed (AL), Chronic Calorie Restricted (CCR) and Intermittent Calorie Restricted (ICR) TGF-α female mice
| AL (42)# | CCR (44) | ICR (45) | |
|---|---|---|---|
| Age of MT detection (weeks) | 57.5±1.9a | 74.5±1.6b | 82.0±0.0b |
| % of mice in group with MT (number with MT/total mice) |
66.7% (28/42)c | 52.3% (23/44)d | 4.4% (2/45)e |
| MTs weight (g) | 0.42±0.07* | 0.75±0.24 | 0.051 |
| Number of MTs/mouse | 1.54±0.19 1-5* |
1.70±0.16 1-3 |
1.00±0.00 1 |
| Total number of MTs (%) | 40 | 39 | 2 |
| Low grade and a carcinoma in situ | 0 (0%) | 2 (5 %) | 0 (0%) |
| Grade 2 | 28 (70%) | 23 (59%) | 2 (100%) |
| Grade 3 | 10 (25 %) | 12 (31%) | 0 (0%) |
| Grade 4 | 2 (5 %) | 2 (5 %) | 0 (0%) |
number in parentheses is final n value.
Values are mean ± SE
Values within a row with different superscript letters are significantly different by Chi-square analysis. P<0.01
Values within a row with different superscript letters are significantly different by Chi-square analysis: P<0.0001
Values within a row with different superscript star are significantly different by t-test (only AL and CCR, but not ICR –Only one mouse with weighed tumor).
Fig 3.
Proportion of mice without palpable mammary tumor over the course of the experiment. Vertical plots represent number of new mice with palpable tumor in weekly basis. Kaplan-Meier analysis, P < 0.0001, χ2=40.9. Age of tumor detection is significantly different among all dietary groups.
A summary of mammary tumor histopathology is presented in Table 2. A total of 40 tumors were identified in 28 AL mice, 39 tumors in 23 CCR mice, and two tumors in two ICR mice. The majority of the mammary tumors regardless of dietary group were classified as grade 2 and 3 adenocarcinomas with others classified as either Grade 4 or low-grade (adenocarcinomas/carcinomas in situ). In eight mice additional benign or malignant growths were noted. One AL, two ICR and three CCR mice had myeloproliferative disorder. There were two AL mice with ovarian tumors (one a low grade adenocarcinoma and other was benign). In addition, non-malignant pathology of ovaries, hemorrhagic corpus luteus cystd were detected in one AL, one CCR and two ICR mice.
Terminal IGF-1 and IGFBP-3 Serum Levels
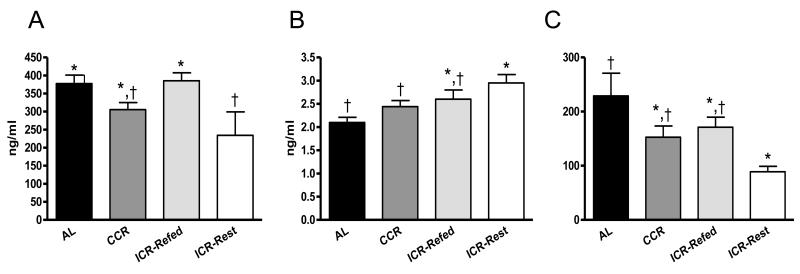
At euthanasia ICR-Restricted mice had serum IGF-1 levels significantly lower than AL (P<0.01) and ICR-Refed (P<0.05) mice (Figure 4A). There were no significant differences in terminal IGF-1 concentrations among AL, CCR and ICR-Refed mice or between CCR and ICR-Restricted mice. ICR-Restricted mice had significantly higher IGFBP-3 serum level than did AL (P<0.01) and CCR (P<0.05) mice, while there was no statistical difference (P>0.05) for terminal IGFBP-3 among the AL, CCR, ICR-Refed mice or between ICR-Restricted and ICR-Refed mice (Figure 4B). ICR-Restricted mice also had a serum IGF-1:IGFBP-3 ratio (Figure 4C) significantly lower than AL (P<0.001) mice. There were no significant differences for the IGF-1:IGFBP-3 ratio between ICR-Refed, CCR and AL mice and also between ICR-Refed, CCR and ICR-Restricted mice.
Fig 4.
Terminal IGF-1 and IGFBP-3 Serum Level and Ratio of IGF1:IGFBP-3 of TGF-α Female Mice. A, Terminal IGF-1 serum levels. Bars represent means of IGF-1 concentrations. ANOVA P < 0.05. AL (n = 37), CCR (n = 38), ICR-Refed (n = 22) and ICR-Restricted (n = 23) mice. B, Terminal IGFBP-3 serum levels. Bars represent means of IGFBP-3 concentrations. ANOVA P < 0.01. AL (n = 37), CCR (n = 38), ICR-Refed (n = 22) ICR-Restricted (n = 23) mice. C, Ratio of IGF-1 to IGFBP-3 levels. Bars represent means of IGF-1:IGFBP-3 Ratio. ANOVA P < 0.01. AL (n = 37), CCR (n = 38), ICR-Refed (n = 22) and ICR-Restricted (n = 23) mice. In A, B and C ICR-Refed mice were euthanized during a refeeding period and ICR-Restricted mice were euthanized during a restriction period. Columns with different superscripts are significantly different from each other.
Correlations between IGF-1, IGFBP-3, IGF-1:IGFBP-3 ratio and body and fat pad weight measurements were done (not shown). In general, serum IGF-1 concentrations of all mice positively correlated with terminal body weight (P=0.0495, r=0.1798, number of XY pairs – 120, but not with mammary and internal fad pad weights (not shown). There were no correlations of weight parameters with either terminal IGFBP-3 concentration or the IGF-1:IGFBP-3 ratio. Interestingly, when all mice that developed mammary tumors were considered together there was no statistical difference in terminal IGF-1 or IGFBP-3 between tumor-free and tumor-bearing mice, however mice with mammary tumors had significantly higher IGF-1:IGFBP-3 than mice without mammary tumors (t-test: P = 0.0133).
Longitudinal IGF-1 Serum Levels
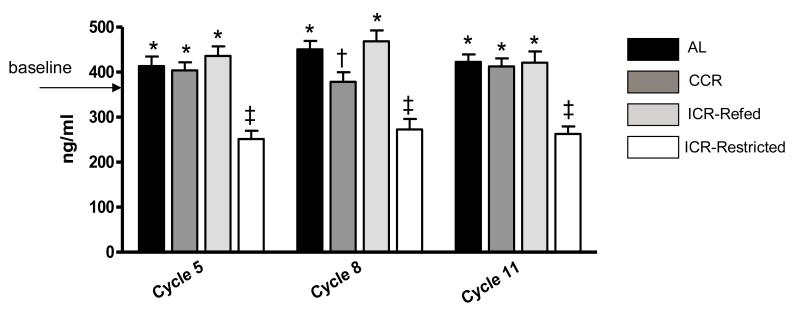
Samples for prospective serum IGF-1 evaluation were obtained when mice were enrolled (baseline, 10 weeks old) and during cycles 5 (34-39 weeks old), 8 (52-57 weeks old), and 11 (72-75 weeks old) as described in the methods section. Baseline IGF-1 concentration was 379.26±17.64 ng/ml (Figure 5). For AL and CCR mice there were little differences in values obtained over the six week periods and these results were combined. For the ICR mice values were combined for three weeks of restriction and three weeks of refeeding. As shown in Figure 5, while IGF-1 serum concentration for CCR mice were maintained at baseline level with increasing age, AL mice had slightly increased IGF-1 reaching significance in Cycle 8 (P<0.01). ICR-Restricted mice consistently had significantly lower IGF-1 levels compared with both their refeeding (P<0.001) and baseline (P<0.001) measurements. The most dramatic change in IGF-1 concentrations between restriction and refeeding periods for ICR mice was in Cycle 8 when the IGF-1 value was statistically higher in refeeding (P<0.05) and lower in restriction (P<0.001) in comparison with the baseline level. Over the course of the study there were no significant differences in IGF-1 serum levels between mice that eventually developed tumors compared to those that did not in any groups (data not shown).
Fig 5.
Serum IGF-1 Levels for Ad libitum-Fed (AL), Chronic Calorie Restricted (CCR) and Intermittent Restricted (ICR) TGF-α Mice (combined with and without mammary tumor) over the Course of the Study. Bars represent means of IGF-1 concentrations. Cycle 5: ANOVA P<0.001. AL n = 36, CCR n= 36, ICR-Refed n=18 and ICR-Restricted n=18. Cycle 8: ANOVA P<0.05. AL n = 36, CCR n= 36, ICR-Refed n=18 and ICR-Restricted n=18. Cycle 11: ANOVA P<0.001. AL n = 32, CCR n= 36, ICR-Refed n=18 and ICR-Restricted n=18.
Terminal Adiponectin and Leptin Serum Levels
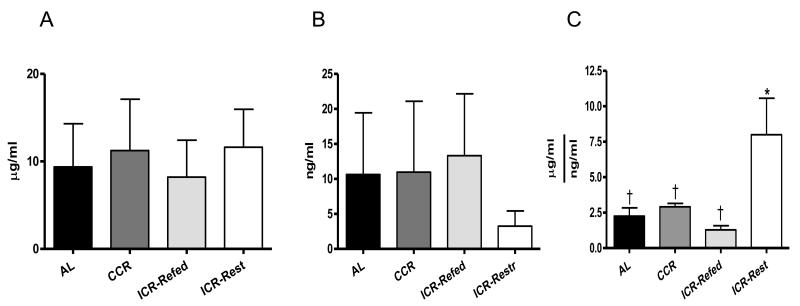
At euthanasia serum adiponectin levels were similar among the groups (Figure 6A). Serum leptin was reduced in the ICR-Restricted mice by 75% compared to the other three groups but did not reach statistical significance, probably due to the small sample size (Figure 6B). At euthanasia ICR-Restricted mice had serum adiponectin:leptin ratio significantly higher than CCR (P<0.01), ICR-Refed (P<0.01) and AL (P<0.01) mice (Figure 6C). There were no significant differences in the adiponectin:leptin ratio between ICR-Refed, CCR and AL mice.
Fig 6.
Terminal Adiponectin, Leptin Serum Level and Ratio of Adiponectin:Leptin of TGF-α Female Mice. A. Terminal Adiponectin serum levels. Bars represent means of Adiponectin concentrations. ANOVA P > 0.05, AL (n = 23), CCR (n = 18), ICR-Refed (n = 22) and ICR-Restricted (n = 23) mice. B. Terminal Leptin serum levels. Bars represent means of Leptin concentrations. ANOVA P> 0.05, AL (n = 17), CCR (n = 18), ICR-Refed (n = 9) and ICR-Restricted (n = 7) mice. C. Terminal Adiponectin:Leptin Ratio. Bars represent means of Adiponectin:Leptin Ratio. ANOVA P < 0.001, AL (n = 17), CCR (n = 17), ICR-Refed (n = 9) and ICR-Restricted (n = 7) mice. In A, B and C ICR-Refed mice were euthanized during a refeeding period and ICR-Restricted mice were euthanized during a restriction period. Columns with different superscripts are significantly different from each other.
We also examined the correlations between adiponectin, leptin, adiponectin:leptin ratio and body and fat pad weights (not shown). Terminal leptin serum concentration of all mice positively correlated with final body weights (P<0.0001. r=0.67, number of XY pairs −51), mammary fat pad weights (P<0.0001. r=0.73, number of XY pairs −50) and internal fat pad weights (P<0.0001. r=0.67, number of XY pairs −50). In contrast, terminal serum adiponectin concentration and adiponectin:leptin ratio were not correlated with body weights, mammary and internal fad pad weights. We also did not find significant differences in adiponectin, leptin or adiponectin:leptin ratio serum levels between AL, CCR and ICR tumor-bearing and tumor-free mice (data not shown).
Longitudinal Adipokines Serum Levels
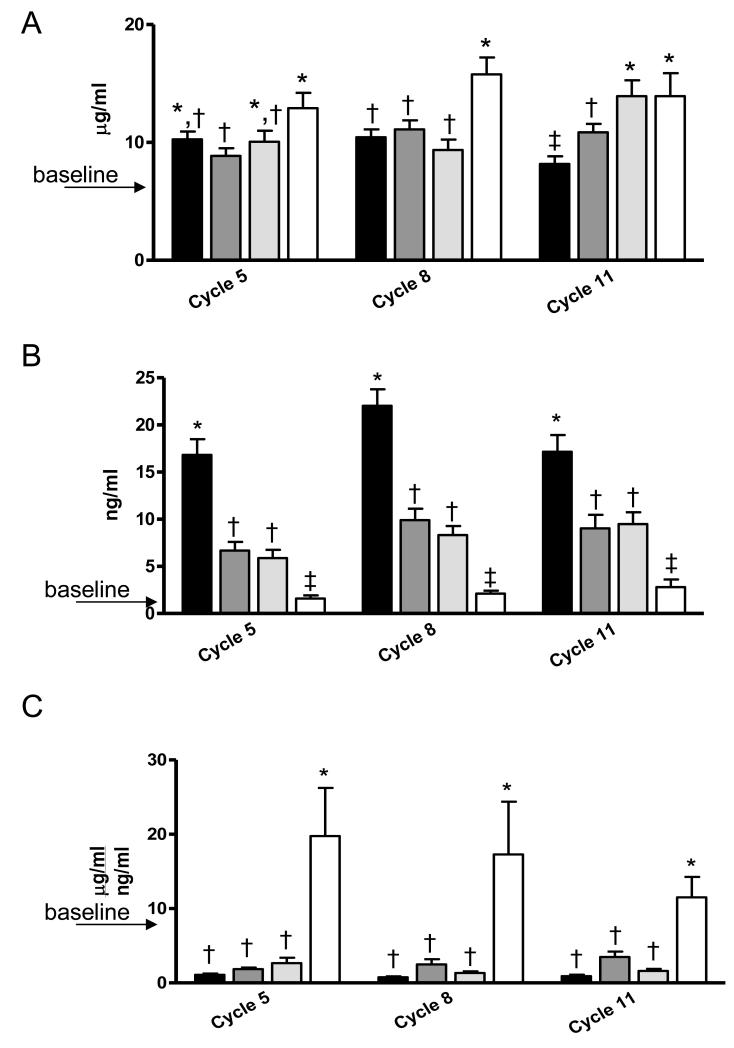
One of our goals was to evaluate serum adipokine concentrations over the course of the study. Baseline adiponectin concentration was 5.90 ± 0.32 μg/ml (Figure 7A). Serum adiponectin increased over time in comparison to baseline for all dietary groups (P<0.001) except AL mice in Cycle 11 (P<0.05). Interestingly, whereas CCR mice reached a significantly higher adiponectin level than did AL mice in Cycle 11 (P<0.05), ICR-Restricted mice already had statistically higher adiponectin level than AL mice in Cycle 8 (P<0.01) which was maintained thereafter (P<0.05). Further, while ICR-Restricted mice had significantly higher adiponectin serum concentration in comparison to ICR-Refed mice only in Cycle 5 (P<0.01) it is important to note that ICR-Restricted mice had significantly higher adiponectin serum levels across the study in comparison to CCR mice (P<0.01 for Cycles 5 and 8 and P<0.05 for Cycle 11).
Fig 7.
Serum Adiponectin, Leptin Levels and Ratio of Adiponectin:Leptin Levels for Ad libitum-Fed (AL), Chronic Calorie Restricted (CCR) and Intermittent Restricted (ICR) TGF-α Mice (combined with and without mammary tumor) over the Course of the Study.
A, Serum Adiponectin Levels for AL, CCR, ICR-Refed and ICR-Restricted mice during cycles 5, 8, and 11. Bars represent means of Adiponectin concentrations. Cycle 5: ANOVA P<0.05. AL n = 36, CCR n= 36, ICR-Refed n=17 and ICR-Restricted n=17. Cycle 8: ANOVA P<0.05. AL n = 36, CCR n= 36, ICR-Refed n=17 and ICR-Restricted n=17. Cycle 11: ANOVA P<0.01. AL n = 32, CCR n= 36, ICR-Refed n=17 and ICR-Restricted n=16.
B, Serum Leptin Levels for AL, CCR and ICR mice during cycles 5, 8, and 11. Bars represent means of Leptin concentrations. Cycle 5: ANOVA P<0.01. AL n = 36, CCR n= 34, ICR-Refed n=17 and ICR-Restricted n=15. Cycle 8: ANOVA P<0.01. AL n = 36, CCR n= 35, ICR-Refed n=17 and ICR-Restricted n=16. Cycle 11: ANOVA P<0.01. AL n = 32, CCR n= 35, ICR-Refed n=17 and ICR-Restricted n=17.
C, Serum Adiponectin:Leptin Ratio for AL, CCR and ICR mice during cycles 5, 8, and 11.Bars represent means of Adiponectin:Leptin Ratio. Cycle 5: ANOVA P<0.01. AL n = 36, CCR n= 34, ICR-Refed n=17 and ICR-Restricted n=15. Cycle 8: ANOVA P<0.01. AL n = 36, CCR n= 35, ICR-Refed n=17 and ICR-Restricted n=16. Cycle 11: ANOVA P<0.01. AL n = 32, CCR n= 35, ICR-Refed n=17 and ICR-Restricted n=15.
In A, B and C ICR-Refed mice were euthanized during a refeeding period and ICR-Restricted mice were euthanized during a restriction period. Columns with different superscripts are significantly different from each other.
Baseline level of leptin at 10 weeks of age was 0.65 ± 0.10 ng/ml (Figure 7B). Serum leptin concentrations increased for AL, CCR and ICR-Refed mice over the study in comparison to baseline (P<0.01 in Cycle 5 and P<0.001 in Cycles 8 and 11). However, in contrast to adiponectin, in each cycle ICR-Restricted mice had leptin levels similar to the baseline level. Over the course of the study AL mice had the highest serum concentrations (P<0.001 versus all groups in all cycles. This was followed by CCR and ICR-Refed mice with similar leptin values. The ICR-Restricted mice had the lowest leptin serum levels in every cycle.
We also calculated adiponectin:leptin ratio over the course of the study (Figure 7C). As can be seen AL, CCR and ICR-Refed mice had reduced values in all cycles while there was elevated in ICR-Restricted mice compared to the baseline levels. Within each cycle values were similar for AL, CCR and ICR-Refed mice and statistically lower than those calculated for ICR-Restricted mice. Over the course of the study there were no significant differences in adiponectin and leptin serum levels or in adiponectin:leptin ratio values between AL, CCR and ICR tumor-bearing and tumor-free mice (data not shown).
Discussion
Here we report that when dietary fat content is increased intermittent calorie restriction still resulted in far superior protection in the prevention of mammary tumor development than did the same degree of calorie restriction implemented in a chronic fashion. These results complement earlier findings that consistently indicated the ICR reduced mammary tumor incidence and extended latency in MMTV-TGF-α mice fed a low-fat diet in comparison to mice fed the same reduced calories (and other nutrients) chronically. [20-22,40].
A protective effect of fasting/refeeding on spontaneous mammary tumor development has also been reported although comparison to chronic calorie restriction was not done [16,17,41,42]. In one case mice fed a high fat diet appeared to develop more spontaneous mammary tumors than did those fed a low fat diet but overall the degree of protection was still very significant compared to the ad libitum fed mice [16]. In contrast, it appeared that there was not a protective effect of intermittent restriction protocols using high fat diets introduced near the time of carcinogen administration on the development of this type of mammary tumors [18,19]. However, when the ICR intervention was started several months after carcinogen administration mammary tumor incidence was reduced by 50% compared to ad libitum fed rats [43]. In light of other studies reporting that fasting/refeeding at the time of carcinogen administration may enhance tumorigenesis this suggests that the results of the two former studies were due to timing of the intervention rather than the consumption of a high fat diet per se. We have also reported that MMTV-neu mice that develop estrogen receptor negative mammary tumors had reduced incidence following intermittent calorie restriction compared to both ad libitum and CCR mice [23].
Although limited in number there are additional reports of that ICR provides protection in other malignancies. For example, ICR implemented in the TRAMP mouse model of prostate cancer in a two week restriction/two week refeeding protocol from 7-50 weeks of age resulted in delayed prostate tumor detection and death, while there was little effect of CCR in comparison to ad libitum-fed TRAMP mice [44,45]. In mice with xenografts resulting from inoculation of a human prostate cancer cell line subjected to several different ICR regimens survival was improved by either one or two days of fasting per week with controlled refeeding for the remainder of the week [46]. In several different lymphoma models ICR reduced disease incidence even when the intervention was initiated in older animals [47,48].
There has also been interest in the impact of weight fluctuation and how it might affect breast cancer development. In a retrospective study of postmenopausal women with a history of weight cycling no association of weight cycling with breast cancer development was found [49]. However, several studies reported that women diagnosed with anorexia nervosa who likely experienced periods of weight loss and regain were at reduced risk for breast cancer [50,51].
Results of an intervention study which compared the effects of CCR versus ICR in overweight women identified at high risk for breast cancer have recently been published [53]. The overall calorie restriction was 25% with weight reduction similar between the groups as were most of the measurements made. Interestingly, when the authors combined the fasting insulin and glucose values to calculate the insulin resistance index using the HOMA (homeostatic model assessment), they found that it was reduced to a greater extent compared to starting values in the ICR versus CCR group. This indicates that the ICR group had a reduction in insulin resistance compared to the CCR group. A slight decrease in the leptin:adiponetin ratio was also found. A follow-up study by these researchers (M. Harvie personal communication) compared three types of calorie restriction diets in a similar population of women in a four month protocol. Women were assigned to either a restricted low carbohydrate diet two days a week, an ad libitum low carbohydrate diet two days a week or a standard daily restricted Mediterranean diet seven days a week. With respect to weight loss and HOMA measurements the two ICR diets were superior to the CCR approach. Although these studies do not include breast cancer as an outcome they clearly demonstrate the feasibility of implementing this approach in humans.
Epidimiological [56-58] and rodent [59,60] studies have shown that elevated levels of IGF-1 are associated with increased risk of breast/mammary cancer. Further, energy-restricted diets significantly reduce levels of circulating IGF-1 in rodents [21,54,55]. Previously we found that CCR [20,21] and ICR-Restricted [21] transgenic MMTV-TGF-α mice had reduced terminal IGF-1 levels compared with ad libitum-fed mice and lower serum IGF-1 levels were associated with the prevention of mammary tumors after long term CCR [20,21]. In a more recent study, terminal IGF-1 serum levels of ICR mice were significantly lower after both three weeks of restriction and three weeks of refeeding compared to AL and CCR groups and mice with mammary tumors had higher terminal IGF-1 and lower IGFBP-3 levels than mice without tumors regardless of whether they were ad libitum-fed or calorie restricted [22]. In the present study there was less impact on terminal IGF-I levels as a result of reduced calorie intake. However, IGFBP-3 levels tended to be higher which was reflected by a reduction in the IGF-I:IGFBP-3 ratio. We also did not find significant differences in IGF-1 serum levels between AL, CCR, and ICR mice with or without mammary tumors, although we again found that mice that developed mammary tumors had significantly higher IGF-1:IGFBP-3 ratio than mice without tumors. These results may at least in part be due to the higher dietary fat content and this issue remains to be investigated in more detail.
We have investigated the adipokines, leptin and adiponectin because of their potential roles in tumorigenesis. As cited in the Introduction in vitro experiments indicate that leptin enhances breast cancer cell proliferation while adiponectin reduces it. In women serum leptin has not been consistently found to be elevated in those with postmenopausal breast cancer as reviewed in [61] However, adiponectin has been reported to be reduced in women with breast cancer compared to those without [37,62,63] and in several studies the ratio of adiponectin:leptin was reduced in women with breast cancer [35,36]. We have published in vitro studies indicating the importance of this ratio in cell proliferation of both breast and prostate cancer cell lines [38,64]. Further, in both a prospective and a cross-sectional study we reported that periods of 50% calorie restriction resulted in a high adiponectin:leptin ratio which is not observed with 25% calorie reduction [39,40]. In those studies a low-fat diet was used. Here, we show that this relationship is also observed in association with a higher fat intake. Thus, we consistently find an increase in the adiponectin:leptin ratio associated with an intervention that reduced mammary tumor incidence. Interestingly women on an ICR protocol for 6 months had a reduced leptin:adiponectin ratio or if calculated differently an increased adiponectin:leptin ratio compared to women fed the same reduced calorie intake in a chronic fashion [53].
Conclusions
In summary, we demonstrate that even when mice were fed a high-fat diet during refeeding, the ICR protocol was still more protective than CCR with respect to the prevention of mammary tumors. While a higher fat consumption did not eliminate the protective effect of CCR, it did result in an increased tumor burden and the protection was not as robust as seen in our earlier studies using a low-fat diet. The two modes of calorie restriction had different influences on serum IGF-1 and adipokine levels and on their ratios. While values of IGF-1, IGFBP-3 and IGF-1:IGFBP-3 ratio of CCR mice were similar with those obtained for AL mice, IGF-1 levels of ICR mice during restriction periods were consistently lower than during refeeding and in comparison to CCR and AL levels. ICR-restricted mice also had higher terminal IGFBP-3 and lower IGF-1:IGFBP-3 ratio in comparison with AL mice. Mice in AL, CCR and ICR-refed groups had raised adiponectin and leptin values in comparison to baseline concentrations and AL mice had significantly higher leptin serum levels over the study period in comparison to all dietary groups. The significantly reduced tumor incidence in ICR mice was associated with an elevated terminal serum adiponectin:leptin. In addition when mice from all dietary groups which developed mammary tumors were considered together they had significantly higher IGF-1:IGFBP-3 than mice without tumors. These findings support considering ways to implement dietary intervention that may alter the course of breast tumor development in at risk women.
Acknowledgements
This research was supported by NIH-CA 101858 and The Hormel Foundation.
Grant Support: NIH-CA 101858 and The Hormel Foundation
List of abbreviations
- ICR
intermittent calorie restriction
- CCR
chronic caloric restriction
- AL
ad libitum
- IGF-1
insulin like binding factor-1
- IGFBP-3
insulin like growth factor binding protein-3
- MMTV-TGF-α
mouse mammary tumor virus transforming growth factor-α
- MT
mammary tumor
Footnotes
Competing interests: none
Authors’ contributions:
Dr. Olga Rogozina oversaw mouse breeding and day to day undertaking of the animal care including body weight and food intake measurements as well as tumor monitoring. She conducted data analyses and was involved in data interpretation and manuscript preparation.
Mr. Katai Nkhata and Ms. Emily Nagle performed analysis and calculations of serum samples.
Dr. Joseph Grande analyzed all slide samples for determination of mammary tumor status as well has any other histology.
Dr. Margot Cleary conceived the experimental design, procured funding and was involved in data analysis, interpretation and participated in manuscript preparation.
Ethical Standards
All procedures with mice were performed under the guidelines and with approval of the University of Minnesota Institutional Animal Care and Use Committee. The University of Minnesota is an AAALAC accredited institution.
Conflict of interest: we declare that we do not have a financial relationship with the organizations that sponsored the research.
Reference List
- 1.Harvie M, Howell A. Energy balance adiposty and breast cancer - energy restriction strategies for breast cancer prevention. Obes Rev. 2006;7:33–47. doi: 10.1111/j.1467-789X.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW. Caloric restriction and 7,12- dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989;49:4130–4134. [PubMed] [Google Scholar]
- 3.Klurfeld DM, Welch CB, Davis MJ, Kritchevsky D. Determination of degree of energy restriction necessary to reduce DMBA-induced mammary tumorigenesis in rats during the promotion phase. J Nutr. 1989;119:286–291. doi: 10.1093/jn/119.2.286. [DOI] [PubMed] [Google Scholar]
- 4.Klurfeld DM, Lloyd LM, Welch CB, et al. Reduction of enhanced mammary carcinogenesis in LA/N-cp (corpulent) rats by energy restriction. Proc Soc Exp Biol Med. 1991;196:381–384. doi: 10.3181/00379727-196-43202. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes G, Chandrasekar B, Troyer DA, et al. Dietary lipids and calorie restriction effect mammary tumor incidence and gene expression in mouse mammary tumor virus/v-Ha-ras transgenic mice. Proc Natl Acad Sci USA. 1995;92:6494–6498. doi: 10.1073/pnas.92.14.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Haegele AD, Thompson HJ. Effect of caloric restriction on pre-malignant and malignant stages of mammary carcinogenesis. Carcinogenesis. 1997;18:1007–1012. doi: 10.1093/carcin/18.5.1007. [DOI] [PubMed] [Google Scholar]
- 7.Dirx MJM, Zeegers MPA, Dagnelie PC, et al. Energy restriction and the risk of spontaneous mammary 8 tumors in mice: a meta-analysis. Int J Cancer. 2003;106:766–770. doi: 10.1002/ijc.11277. [DOI] [PubMed] [Google Scholar]
- 8.Bunk B, Zhu P, Klinga K, et al. Influence of reducing luxury calories in the treatment of experimental mammary carcinoma. Brit J Cancer. 1992;65:845–851. doi: 10.1038/bjc.1992.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beth M, Berger MR, Aksoy M, Schmahl D. Comparison between the effects of dietary fat level and calorie intake on methlynitrosourea-induced mammary carcinogenesis in female SD rats. Int J Cancer. 1987;39:737–744. doi: 10.1002/ijc.2910390614. [DOI] [PubMed] [Google Scholar]
- 10.Klurfeld DM, Welch CB, Lloyd LM, Kritchevsky D. Inhibition of DMBA-induced mammary tumorigenesis by caloric restriction in rats fed high-fat diets. Int J Cancer. 1989;43:922–925. doi: 10.1002/ijc.2910430532. [DOI] [PubMed] [Google Scholar]
- 11.Welsch CW, House JL, Herr BL, et al. Enhancement of mammary carcinogenesis by high levels of dietary fat: a phenomenon dependent on ad libitum feeding. J Natl Cancer Inst. 1990;82:1615–1620. doi: 10.1093/jnci/82.20.1615. [DOI] [PubMed] [Google Scholar]
- 12.Boissonneault GA, Elson CE, Pariza MW. Net energy effects of dietary fat on chemically induced mammary carcinogenesis in F344 rats. J Natl Cancer Inst. 1986;76:335–338. [PubMed] [Google Scholar]
- 13.Welsch CW. Relationship between dietary fat and experimental mammary tumorigenesis: a review and critique. Cancer Res. 1992;52(Suppl.):2040s–2048s. [PubMed] [Google Scholar]
- 14.Cleary MP, Grande JP, Maihle NJ. Effect of a high fat diet on body weight and mammary tumor latency in MMTV-TGF- mice. Int J Obesity. 2004;28:956–962. doi: 10.1038/sj.ijo.0802664. [DOI] [PubMed] [Google Scholar]
- 15.Engelman RW, Day NK, Good RA. Calorie intake during mammary development influences cancer risk: lasting inhibition of C3H/HeOu mammary tumorigenesis by peripubertal calorie restriction. Cancer Res. 1994;54:5724–5730. [PubMed] [Google Scholar]
- 16.Chen R-F, Good RA, Engelman RW, et al. Suppression of mouse mammary tumor proviral DNA and protooncogene expression: association with nutritional regulation of mammary tumor development. Proc Natl Acad Sci USA. 1990;87:2385–2389. doi: 10.1073/pnas.87.7.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson AJ, Hoelzel F. Apparent prolongation of the life span of rats by intermittent fasting. J Nutr. 1946;31:363–375. doi: 10.1093/jn/31.3.363. [DOI] [PubMed] [Google Scholar]
- 18.Harris SR, Brix AE, Broderson JR, Bunce OR. Chronic energy restriction versus energy cycling and mammary tumor promotion. Proc Soc Exp Biol Med. 1995;209:231–236. doi: 10.3181/00379727-209-43897. [DOI] [PubMed] [Google Scholar]
- 19.Tagliaferro AR, Ronan AM, Meeker LD, et al. Cyclic food restriction alters substrate utilization and abolishes protection from mammary carcinogenesis in female rats. J Nutr. 1996;126:1398–1405. doi: 10.1093/jn/126.5.1398. [DOI] [PubMed] [Google Scholar]
- 20.Cleary MP, Jacobson MK, Phillips FC, et al. Weight-cycling decreases incidence and increases latency of mammary tumor development to a greater extent than does chronic restriction in mouse mammary tumor virus-transforming growth factor- female mice. Cancer Epidemiol Biomarkers Prev. 2002;11:836–843. [PubMed] [Google Scholar]
- 21.Cleary MP, Hu X, Grossmann ME, et al. Prevention of mammary tumorigenesis by intermittent caloric restriction, does caloric intake during refeeding modulate the response? Exp Biol Med. 2007;232:70–80. [PubMed] [Google Scholar]
- 22.Rogozina OP, Bonorden MJL, Grande JP, Cleary MP. Serum insulin like growth factor-I and mammary 9 tumor development in ad libitum-fed, chronic calorie-restricted and intermittent calorie-restricted MMTV-TGF- mice. Cancer Prev Res. 2009;2:712–719. doi: 10.1158/1940-6207.CAPR-09-0028. [DOI] [PubMed] [Google Scholar]
- 23.Pape-Ansorge KA, Grande JP, Christensen TA, et al. Effect of moderate caloric restriction and/or weight- cycling on mammary tumor incidence and latency in MMTV-neu female mice. Nutr Cancer. 2002;44:161–168. doi: 10.1207/S15327914NC4402_07. [DOI] [PubMed] [Google Scholar]
- 24.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 25.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocrine-Related Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 26.Barb D, Pazaitou-Panayiotou K, Mantzoros CS. Adiponectin: a link between obesity and cancer. Expert Opin Investig Drugs. 2006;15:917–933. doi: 10.1517/13543784.15.8.917. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Juneja SC, Maihle NJ, Cleary MP. Leptin- a growth factor for normal and malignant breast cells and normal mammary gland development. J Natl Cancer Inst. 2002;94:1704–1711. doi: 10.1093/jnci/94.22.1704. [DOI] [PubMed] [Google Scholar]
- 28.Laud K, Gourdou I, Pessemesse L, et al. Identification of leptin receptors in human breast cancer: functional activity in the T47-D breast cancer cell line. Mol Cell Endocrinol. 2002;188:219–226. doi: 10.1016/s0303-7207(01)00678-5. [DOI] [PubMed] [Google Scholar]
- 29.Dieudonne M-N, Machinal-Quelin F, Serazin-Leroy V, et al. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem Biophys Res Comm. 2002;293:622–628. doi: 10.1016/S0006-291X(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 30.Ray A, Nkhata KJ, Cleary MP. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. 2007;30:1499–1509. [PubMed] [Google Scholar]
- 31.Garofalo C, Sisci D, Surmacz E. Leptin interferes with the effects of the antiestrogen ICI 182.780 in MCF-7 breast cancer cell lines. Clin Cancer Res. 2004;10:6466–6475. doi: 10.1158/1078-0432.CCR-04-0203. [DOI] [PubMed] [Google Scholar]
- 32.Dieudonne M-N, Bussiere M, Dos Santos E, et al. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Comm. 2006;345:271–279. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 33.Grossmann ME, Nkhata KJ, Mizuno NK, et al. Effects of adiponectin on breast cancer cell growth and signaling. Brit J Cancer. 2008;98:370–378. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahata C, Miyoshi Y, Irahara N, et al. Demonstration of Adiponectin Receptors 1 and 2 mRNA expression in human breast cancer cells. Cancer Lett. 2007;250:229–236. doi: 10.1016/j.canlet.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Körner A, Pazaitou-Panayiotou K, Kelesidis T, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J Clin Endocrinol Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 36.Chen D-C, Chung Y-F, Yeh Y-T, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 37.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 38.Grossmann ME, Ray A, Dogan S, et al. Balance of adiponectin and leptin in relationship to breast cancer 0 cell growth. Cell Res. 2008;18:1154–1156. doi: 10.1038/cr.2008.293. [DOI] [PubMed] [Google Scholar]
- 39.Rogozina OP, Bonorden MJL, Seppanen C, et al. Effect of chronic and intermittent calorie restriction on serum adiponectin and leptin and mammary tumorigenesis. Cancer Prev Res. 2011;4:568–581. doi: 10.1158/1940-6207.CAPR-10-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dogan S, Rogozina OP, Loshkin A, et al. Effects of chronic vs intermittent calorie restriction on mammary tumor incidence and serum adiponecin and leptin levels in MMTV-TGF- mice at different ages. Oncology Letters. 2010;1:167–176. doi: 10.3892/ol_00000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shankaraiah K, Halberg F, Yunis E, Watson LM. Alternate-day feeding alters the circadian system, reduces breast cancer incidence and prolongs life. In: Halberg F, Reale L, Tarquini B, editors. Proceedings of II International Symposium on Chronobiologic Approach to Social Medicine; Rome. Instituto Itaniano di Medicina Sociale; 1984. pp. 633–48. [Google Scholar]
- 42.Shao R, Dao ML, Day NK, Good RA. Dietary manupulation of mamamry tumor development in adult C3H/Bi mice. Proc Soc Exp Biol Med. 1990;193:313–317. doi: 10.3181/00379727-193-43041. [DOI] [PubMed] [Google Scholar]
- 43.Buison AM, Pellizzon MA, Brogan KE, et al. Weight cycling did not increase tumor incidence in high fat- fed rats treated weith a low-dose 7,12-dimethylbenzyl(1)anthracene. Nutr Res. 2005;25:1097–1108. [Google Scholar]
- 44.Bonorden MJL, Rogozina OP, Kluczny CM, et al. Intermittent caloric restriction delays tumor detection and increases survival in TRAMP mice. Nutr Cancer. 2009;61:265–275. doi: 10.1080/01635580802419798. [DOI] [PubMed] [Google Scholar]
- 45.Bonorden MJL, Rogozina OP, Kluczny CM, et al. Cross-sectional analyses of intermittent versus chronic caloric restriction in the TRAMP mouse. The Prostate. 2009;69:317–326. doi: 10.1002/pros.20878. [DOI] [PubMed] [Google Scholar]
- 46.Buschemeyer WC, III, Klink JC, Mavropoulos JC, et al. Effect of intermittent fasting with or without caloric restriction on prostate cancer growth and survival in SCID mice. Prostate. 2010;70:1037–1043. doi: 10.1002/pros.21136. [DOI] [PubMed] [Google Scholar]
- 47.Descamps O, Riondel J, Ducros V, Rousell A-M. Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mech Age Dev. 2005;126:1185–1191. doi: 10.1016/j.mad.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 49.Trentham-Dietz A, Newcomb PA, Egan KM, et al. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes Control. 2000;11:533–542. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 50.Mellemkjaer L, Emborg C, Gridley G, et al. Anorexia nervosa and cancer risk. Cancer Causes Control. 2001;12:173–177. doi: 10.1023/a:1008974414116. [DOI] [PubMed] [Google Scholar]
- 51.Michels KB, Ekbom A. Caloric restriction and incidence of breast cancer. JAMA. 2004;291:1226–1230. doi: 10.1001/jama.291.10.1226. [DOI] [PubMed] [Google Scholar]
- 52.Christou NV, Leiberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on 1 weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obesity. 2011;35:714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunn SE, Kari FW, French JE, et al. Dietary restriction reduces insulin-like growth-like factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 55.Powolny AA, Wang S, Carlton PS, et al. Interrelationships between dietary restriction, the IGF-I axiz, and expression of vascular endothelial growth factor by prostate adenocarcinomas in rats. Mol Carcinog. 2008;47:458–465. doi: 10.1002/mc.20403. [DOI] [PubMed] [Google Scholar]
- 56.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 57.Peyrat JP, Bonneterre J, Hecquet B, et al. Plasma insulin-like growth factor-1 (IGF-1) concentrations in human breast cancer. Eur J Cancer. 1993;29A:492–497. doi: 10.1016/s0959-8049(05)80137-6. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Jin F, Shu X-O, et al. Insulin-like growth factors and breast cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2002;11:705–712. [PubMed] [Google Scholar]
- 59.de Ostrovich KK, Lambertz I, Colgy JKL, et al. Paracrine overexpression of insulin-like growth factor-1 enhances mammary tumorigenesis in vivo. Am J Pathol. 2008;173:824–834. doi: 10.2353/ajpath.2008.071005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hadsell DL, Bonnette SG. IGF and insulin action in the mammary gland: lessons from transgenic and knockout models. J Mammary Gland Biol & Neo. 2000;5:19–30. doi: 10.1023/a:1009559014703. [DOI] [PubMed] [Google Scholar]
- 61.Cleary MP, Ray A, Rogozina OP, et al. Targeting the adiponectin-leptin ratio for prevention of postmenopausal breast cancer. Frontiers in Bioscience. 2009 Jun 1;S1:329–357. doi: 10.2741/S30. [DOI] [PubMed] [Google Scholar]
- 62.Petridou E, Mantzoros C, Dessypris N, et al. plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–997. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 63.Tworoger SS, Eliassen AH, Kelesidis T, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–1516. doi: 10.1210/jc.2006-1975. [DOI] [PubMed] [Google Scholar]
- 64.Nkhata KJ, Ray A, Schuster TF, et al. Effects of adiponectin and leptin co-treatment on human breast cancer cell growth. Oncol Rep. 2009;21:1611–1619. doi: 10.3892/or_00000395. [DOI] [PubMed] [Google Scholar]