Abstract
Cytokines generated from macrophages contributes to pathogenesis of inflammation-associated diseases. Here we show that gamma-tocotrienol (γ-TE), a natural vitamin E form, inhibits lipopolysaccharide (LPS)-induced interleukin-6 (IL-6) production without affecting TNFα, IL-10 or cyclooxygenase-2 (COX-2) up-regulation in murine RAW267.4 macrophages. Mechanistic studies indicate that nuclear factor (NF)-κB, but not JNK, p38 or ERK MAP kinases, is important to IL-6 production and γ-TE treatment blocks NF-κB activation. In contrast, COX-2 appears to be regulated by p38 MAPK in RAW cells, but γ-TE has no effect on LPS-stimulated p38 phosphorylation. Despite necessary for IL-6, NF-κB activation by TNFα or other cytokines is not sufficient for IL-6 induction with exception of LPS. CCAAT-enhancer binding protein β (C/EBPβ) appears to be involved in IL-6 formation, because LPS induces C/EBPβ up-regulation, which parallels IL-6 production, and knockdown of C/EBPβ with siRNA results in diminished IL-6. LPS but not individual cytokines is capable of stimulating C/EBPβ and IL-6 in macrophages. Consistent with its dampening effect on IL-6, γ-TE blunts LPS-induced up-regulation of C/EBPβ without affecting C/EBPδ. γ-TE also decreases LPS-stimulated granulocyte-colony stimulating factor (G-CSF), a C/EBPβ target gene. Compared with RAW267.4 cells, γ-TE shows similar or stronger inhibitory effects on LPS-triggered activation of NF-κB, C/EPBβ and C/EBPδ, and more potently suppresses IL-6 and G-CSF in bone marrow-derived macrophages. Our study demonstrates that γ-TE has anti-inflammatory activities by inhibition of NF-κB and C/EBPs activation in macrophages.
Keywords: vitamin E, tocopherol, tocotrienol, inflammation, IL-6, C/EBPβ
INTRODUCTION
Macrophages play important roles in promoting inflammation and inflammation-associated diseases including cancer. During inflammation, in response to endotoxin and cytokines, macrophages release excessive amount of pro-inflammatory mediators including reactive oxygen species, eicosanoids and cytokines such as interleukin 6 (IL-6). IL-6 has been recognized as a key proinflammatory cytokine that contributes to arthritis, cancer and obesity-related promotion of carcinogenesis. Targeting IL-6 by anti-IL-6 has been used clinically to treat anti-TNFα nonresponsive arthritis. Therefore, modulation of IL-6 formation appears to be an attractive strategy for regulating excessive immune response and attenuating inflammation-associated damage.
Vitamin E is a group of lipophilic antioxidants, which include α-, β-, γ-, δ-tocopherol and α-, β-, γ-, δ-tocotrienol. All members of vitamin E family contain a chromanol ring linked with a phytyl side chain, in which tocopherols are saturated and tocotrienols have three double bonds. In addition to antioxidant activities, specific forms of vitamin E have been shown to have anti-inflammatory properties. We have demonstrated that γ-tocopherol (γ-T), δ-tocopherol (δ-T) and γ-tocotrienol (γ-TE) as well as their long-chain metabolites suppress cyclooxygenase- and 5-lipoxygenase-catalyzed proinflammatory eicosanoids. Consistently, γ-T supplementation decreases pro-inflammatory eicosanoids and suppresses eosinophilia in an acute inflammatory and an allergic airway inflammation model.
Besides inhibitory effects on eicosanoids, specific vitamin E forms have been reported to modulate cytokine formation. For instance, γ-tocotrienol (γ-TE) appears to be stronger than its tocophoerol counterparts in inhibition of IL-13-stimulated eotaxin-3 in human lung epithelial cells by up-regulation of PAR-4, which subsequently blocks atypical protein kinase C-mediated STAT6 activation. It has recently been reported that γ-TE inhibits LPS-stimulated IL-6 in murine RAW276.4 macrophages. However, the mechanisms underlying this inhibitory effect have not been identified. In the present study, we investigated the effects and mechanisms of various forms of vitamin E, including α-tocopherol (α-T), γ-T, δ-T and γ-TE, on LPS-stimulated cytokine formation in murine RAW267.4 macrophages. We also extended our study to primary bone marrow derived macrophages (BMDMs) for verifying the observed activities and mechanisms.
MATERIALS AND METHODS
Materials
α-T (99%), γ-T (97–99%) and δ-T (97%) were purchased from Sigma (St Louis, MO). γ-TE (>97%) was a gift from BASF (Germany). Bacterial lipopolysaccharide (LPS; B E. coli 055:B5), recombinant mouse TNFα, IL-1α, IL-1β, IFN-γ and M-CSF were from sigma (St Louis, MO). Primary antibodies against phosphor-IκBα (sc-8404), IκBα (sc-371), C/EBPβ (sc-7962), C/EBPδ (sc-636) and secondary antibodies as well as C/EBPβ siRNA (sc-29862) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Inhibitors of MEK (U0126), p38 MAPK (SB202190), and NF-κB (Parthenolide) were from Calbiochem (La Jolla, CA). JNK inhibitor (SP600125), [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] (MTT), and all other chemicals were from Sigma (St Louis, MO). Cell culture media were from American Type Culture Collection (ATCC) (Manassas, VA).
Cell culture
Murine RAW264.7 macrophages from ATCC were routinely maintained in Dulbecco’s modified eagle medium (DMEM) with 10% fetal bovine serum (FBS). Confluent cells were seeded and allowed to attach overnight at 7 × 105 or 5 × 106 per well in a 24-well or a 6-well plate, respectively. Vitamin E stock solutions were initially made in dimethyl sulfoxide (DMSO) and then diluted in 10mg/mL of fatty acid-free bovine serum albumin (BSA). Cells were incubated in DMEM-1% FBS containing 0.05% DMSO (control) or vitamin E forms for 14 -16 h, and stimulated with LPS (0.1μg/ml) or other stimuli. Cell viability was determined using cellular dehydrogenase/reductase activity by MTT assays
Preparation of bone marrow derived macrophages (BMDMs)
BMDMs from mice was prepared according to a published protocol. The protocol on animal use was approved by the Animal Care and Use Committee at Purdue University and was strictly followed. Briefly, bone marrow was obtained by flushing femur from 7–8 week-old c57BL/6 black mice from Harlan (Indianapolis, IN). Suspension cells were cultured in DMEM containing 10% FBS with penicillin (100U/ml), streptomycin (100μg/ml) and M-CSF (100U/ml). Cells were cultured in 10-cm dish for 5 days, followed by replacing fresh medium for additional two days. Attached cells were harvested by non-enzymatic dissociation solution (Sigma, St Louis, MO) and characterized using flow cytometry, or re-seeded into 24 or 6-well plate for subdequent studies.
Characterization of BMDMs with flow cytometry
Bone marrow derived macrophages were characterized by flow cytometry. Briefly, cells were blocked with mouse immunoglobulin G (Sigma) before stained with phycoerythrin (PE)-conjugated anti-mouse F4/80/EMR1 (#FAB5580P) or PE-conjugated isotype control (#IC006P)(R&D Systems, Minneapolis, MN) for 30 min at room temperature. Cells were then washed and analyzed by Cell Lab Quanta SC-MPL flow cytometer (Beckman Coulter, Brea, CA) with excitation at 488 nm.
Analyses of cytokines
Cytokine accumulation in the media was quantitatively measured using immunoassay kits from R&D Systems (Minneapolis, MN) or eBioscience (San Diego, CA) according to the manufacturers’ instructions. In some experiments, relative levels of selected cytokines and chemokines were determined by proteome profiler mouse cytokine array kit (R&D 0system, Catalog # ARY006, Minneapolis, MN).
Western blot
Cells were lysed in a lysis buffer containing Tris-EDTA, 1% SDS, 1mM DTT, 2mM sodium vanadate and protease inhibitor cocktails (Sigma). Cytosolic and nuclear proteins were extracted using a Pierce Kit (Pierce, Rockford, IL). The resulting solution was heated at 95 °C for 10–15 min. Proteins (25–50 μg) were loaded on 10% pre-cast SDS-PAGE gels. Resolved proteins were transferred onto a PVDF membrane (Millipore, Billerica, MA) and probed by antibodies. Membranes were exposed to chemiluminescent reagent (Perkin Elmer, Waltham, WA) and visualized on a Kodak film. In all the experiments, immunoblotting was first performed with antibodies for proteins of interest. PVDF membranes were then stripped with antibody-stripping solution from EMD Millipore Corporation (Billerica, MA,) and re-immunoblotted with antibodies for internal controls such as β-actin.
Knockdown of C/EBPβ by siRNA transfection
Mouse C/EBPβ siRNA and control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Transfections were performed according to the manufacturer’s instructions with lipofectamine 2000 (Invitrogen, Carlsbad, CA). Briefly, 75% confluent RAW cells were transfected with 10μl of lipofectamine and 10μl of 10μM siRNA in 1ml Opti-MEM® Reduced Serum Medium (CAT#51985-091, Invitrogen) for 6 h. The transfection mixture was replaced with fresh DMEM containing 10% FBS for 16 h. Cells were then stimulated with LPS for 8 h. Media and cells were used for cytokine analysis and western blot, respectively.
Statistical analyses
One-way ANOVA and student T tests were used in statistical analyses.
RESULTS
γ-TE inhibited LPS-stimulated IL-6 but had no effect on up-regulation of TNFα, IL-10 or COX-2 in RAW267.4 macrophages
In confluent RAW264.7 macrophages, LPS treatment led to marked increase of proinflammatory cytokines including IL-6 (4 – 16ng/ml) and TNFα (24 – 33ng/ml) as well as an anti-inflammatory cytokine IL-10 (1 – 12ng/ml). Vitamin E forms differentially inhibited LPS-induced IL-6 and γ-TE was much stronger than tocopherols in this activity (Figure 1A and B). These observations are consistent with a previous study. On the other hand, γ-TE or tocopherols had no significant effects on LPS-stimulated increase of TNFα or IL-10 (Figure 1C and D). Consistent with our previous work, tocopherols or γ-TE did not significantly affect LPS-induced up-regulation of cyclooxygenase-2 (COX-2) (Figure 1E), a key enzyme catalyzing proinflammatory eicosanoids. In all these studies, γ-TE treatment had no obvious impact on cell viability based on cell morphological examination and MTT assays (not shown).
Figure 1. γ-TE inhibited LPS-stimulated IL-6 but had no effect on TNFα, IL-10 or COX-2 in RAW267.4 macrophages.
After preincubated with tocopherols (Panel A) and γ-TE (Panel B, C, D and E) for 14–16 h, RAW 264.7 cells were stimulated by LPS (0.1μg/ml) for 16–18 h. Media were collected to measure IL-6, TNFα and IL-10 and cells were collected to analyze COX-2 expression using immunoblot. αT50, γT10, γT25, γT50, δT10, δT25, δT50, γTE5, γTE10 and γTE20 stand for the corresponding vitamin E at the indicated concentrations (μM), respectively. Relative cytokines are the ratio of cytokine secreted by cells treated with tested compounds to that of solvent controls in the presence of LPS. Ctrl is non-LPS control. Data are shown as mean±S.D. *p<0.05 indicates significant difference between vitamin E-treated and solvent control cells (n >2 per bar).
NF-κB is necessary for IL-6 production and γ-TE inhibited NF-κB activation in RAW 276.4 macrophages
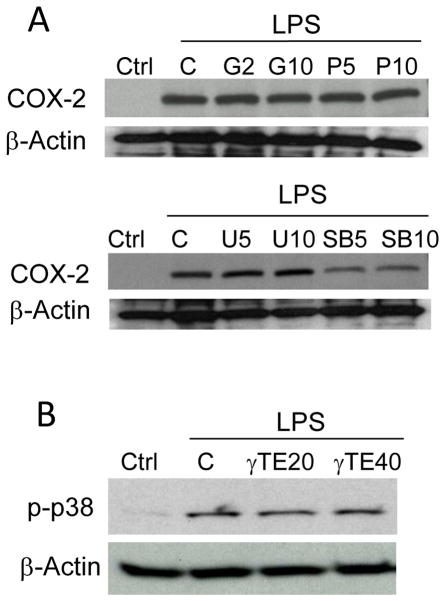
To understand the mechanism underlying the observed effects by γ-TE, we examined potential involvement of several signaling pathways using specific inhibitors. Chemical inhibition of p38 MAPK, but not NF-κB, ERK, protein kinase C (PKC) or JNK, led to significant suppression of LPS-induced COX-2 upregulation (Figure 2A). This suggests that p38 activation plays a key role in COX-2 induction in RAW cells. Interestingly, several previous studies reported that p38 MAPK is important to COX-2 induction in various types of cells. Here we found that γ-TE showed no significant effects on LPS-stimulated p38 activation (Figure 2B), which is consistent with its lack of impact on COX-2 induction in RAW267.4 cells.
Figure 2. COX-2 expression was inhibited by p38 inhibitor and -TE did not affect LPS-induced p38 activation.
Panel A: RAW cells were pre-treated with inhibitors of PKC (G=Go6983, 2 or 10μM), NF-κB (P=Parthenolide, 5 or 10μM), MEK (U=U0126, 10μM) and p38 MAPK (SB=SB202190, 5 or 10μM) for 30 min and then stimulated with 0.1μg/ml LPS for 16 h. Total proteins were analyzed by immunoblot with antibodies for COX-2 or β-actin. Panel B: RAW cells were stimulated with 0.1μg/ml LPS for 10 min after pre-treated with γ-TE (20 or 40μM) for 16 h. Cytosolic proteins were probed by immunoblot.
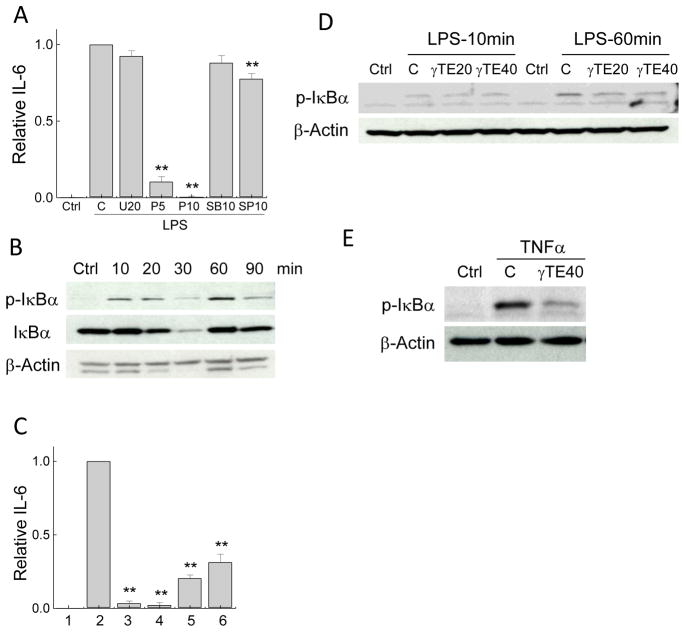
Using the similar approaches, we found that blocking LPS-induced NF-κB activation by parthenolide almost completely abolished IL-6 production, whereas inhibitors of JNK, MEK and p38 MAPK had moderate or no influence on IL-6 (Figure 3A). These results strongly suggest that NF-κB is important for LPS-stimulated IL-6. Interestingly, while LPS caused immediate increase of IκBα phosphorylation, a critical step for NF-κB activation, even stronger κBα phosphorylation was seen after 60-min LPS stimulation (Figure 3B). We reason that the second activation may be caused by cytokine-induced re-stimulation subsequent to the initial Toll-like receptor (TLR)-mediated activation. The importance of the delayed NF-κB activation in IL-6 induction is evident by the observation that addition of parthenolide after LPS treatment resulted in similar suppression of IL-6 to that caused by cells pretreated with parthenolide prior to LPS stimulation (Figure 3C). We found that γ-TE inhibited LPS-stimulated IκBα phosphorylation at the 60-min rather than initial activation (Figure 3D). In addition to LPS, γ-TE also potently inhibited TNFα-triggered phosphorylaion of IκBα (Figure 3E).
Figure 3. NF-κB is necessary for IL-6 expression and γ-TE inhibited NF-κB activation in RAW 276.4 macrophages.
Panel A: RAW 264.7 cells were preincubated with inhibitors for MEK (U=U0126, 20μM), NF-κB (P=Parthenolide, 5 or 10μM), p38 (SB=SB202190, 10μM) and JNK (SP=SP600125, 10μM) for 30 min and stimulated by LPS for 16 h. Relative IL-6 data are the ratio of IL-6 secreted by cells treated with inhibitors to that of solvent controls in the presence of LPS and ctrl is no-LPS control. Panel B: RAW cells were stimulated with 0.1μg/ml LPS for indicated times. Panel C: Bars 1–2 are the relative IL-6 production in ctrl (no LPS) and LPS (16 h)-stimulated cells, respectively. Bar 3 refers to cells pretreated with parthenolide (10μM) for 30 min before LPS treatment. Bars 4–6 are the relative IL-6 secretion when cells were added with parthenolide at 0.5, 1 or 2 h after LPS stimulation. Panel D: RAW cells were pretreated with γ-TE at indicated concentrations (μM) for 14–16 h and then stimulated with 0.1μg/ml LPS for 10 or 60 min. Panel E: Cells were pretreated with γ-TE at 40μM for 14–16 h and then stimulated with TNFα (10ng/ml) for 5min. Data are shown as mean±S.D. *P<0.05 and **P<0.01 are the difference between cells treated with inhibitors and those treated with LPS alone (n >2 per bar). Cytosolic proteins were probed for I Ba phosphorylation by Western blot.
Activation of NF-κB alone is not sufficient to IL-6 production; C/EBP-β plays a role in IL-6 formation and γ-TE inhibited LPS-induced C/EBPβ
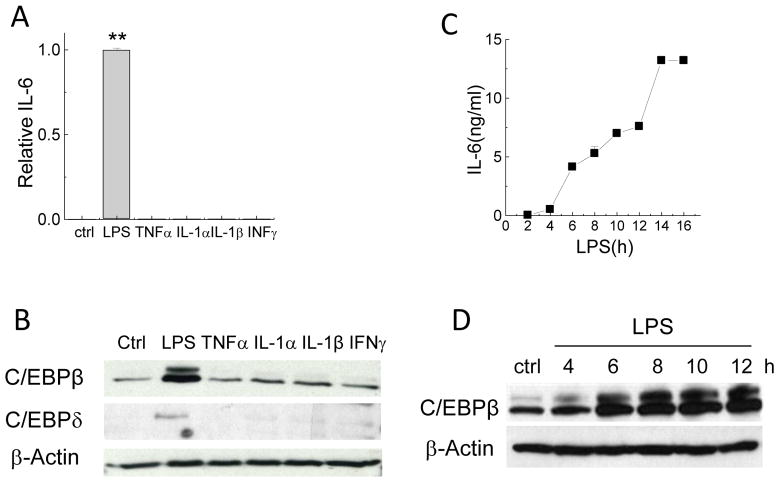
To investigate whether the delayed NF-κB activation was induced by cytokines, we used cytokine array combined with ELISA assays to assess cytokine secretion following LPS stimulation. The results confirmed a rapid surge of TNFα to 1.4 – 1.8ng/mL within 60 min after LPS stimulation (data not shown). Previous studies reported that individual cytokines including TNFα, IL-1α, IL-1β, or IFNγ stimulate IL-6 production in different cell lines. Since γ-TE inhibited TNFα-stimulated NF-κB activation that appears to be essential to IL-6 (Figure 3), we reason that γ-TE may suppress TNFα-induced IL-6. Surprisingly, in RAW264.7 macrophages, LPS but not individual cytokines or a combination of TNFα and IFNγ was capable of stimulating IL-6 (Figure 4A). This observation suggests that despite necessary for IL-6 generation, activation of NF-κB alone is not sufficient for IL-6 induction, which is similarly observed in a previous study in murine fibrosarcoma cells.
Figure 4. LPS but not individual cytokines was capable of inducing IL-6 and LPS-induced C/EBPβ upregulation paralleled IL-6 in a time-dependent manner.
Panels A and B: RAW cells were stimulated with LPS (0.1μg/ml) or TNFα (10ng/ml), IL-1α (10ng/ml), IL-1β (10ng/ml), or INFγ (100ng/ml) for 16 h. Ctrl is no-stimulus control. Media was collected for IL-6 and cells were collected for Western blotting. **P<0.01 is the difference between LPS- and individual cytokine-treated cells. Panels C and D: RAW cells were stimulated with LPS for indicated times (h) and IL-6 (ng/ml) in the media was measured by ELISA. Total proteins from the cells were immunoblotted for C/EBPβ, C/EBPδ or actin. The relative IL-6 is the ratio of IL-6 secreted by cells treated with different stimuli to that of LPS-treated cells. Data are shown as mean±SD (n >2 per bar).
It has been documented that besides NF-κB, other key transcription factors such as CCAAT/enhancer-binding proteins, i.e., C/EBPβ and C/EBPδ, are involved in regulating IL-6. Interestingly, in RAW267.4 macrophages, LPS but not individual cytokines led to enhanced C/EBPβ and C/EBPδ expression (Figure 4B). Furthermore, the upregulation of C/EBPβ by LPS paralleled IL-6 production in a time-dependent manner (Figure 4C and 4D).
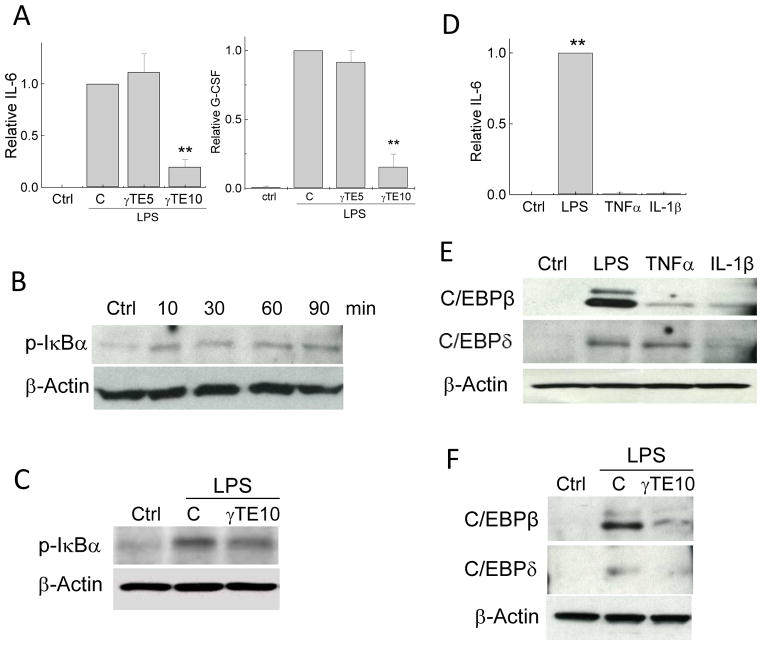
To further verify the role of C/EBPβ in IL-6 production in macrophages, we used siRNA of C/EBPβ to knockdown its expression (Figure 5A). This resulted in dampening secretion of IL-6 (Figure 5B). Importantly, γ-TE attenuated LPS-induced C/EBP β expression in a dose-dependent manner, whereas it had no significant impact on C/EBPδ (Figure 5C). The blunting effect of γ-TE on C/EBPβ together with its inhibition of NF-κB likely account for its suppression of IL-6 production. Furthermore, consistent with its inhibition of C/EBPβ upregulation, γ-TE dose-dependently diminished granulocyte-colony stimulating factor (G-CSF) (Figure 5D), which is known to be regulated by C/EBPβ.
Figure 5. Knockdown of C/EBP-β diminished IL-6 and γ-TE inhibited LPS-induced C/EBPβ and G-CSF.
Panels A and B: RAW cells were transfected with C/EBPβ siRNA and then stimulated with LPS for 8 h. Whole protein was probed for C/EBPβ by Western blot. The relative IL-6 was the ratio of IL-6 secreted by cells transfected with C/EBPβ siRNA to those with control siRNA (ctrl-siRNA) in the presence of LPS. Ctrl is no-LPS control. Panel C: RAW cells were pretreated with γ-TE at indicated concentrations (μM) for 14 h and then stimulated with LPS for 16 h. Total proteins were immunoblotted with anti-C/EBPβ, C/EBPδ or β-actin. Panel D: G-CSF was measured in the media which were used to detect IL-6 in Figure 1. Data are shown as mean±S.D. *P<0.05 and **P < 0.01 were the difference between treated and control cells. n >2 per bar.
γ-TE inhibited LPS-stimulated IL-6 and G-CSF and blocked NF-κB activation and C/EBP-β upregulation in mouse bone marrow-derived macrophages (BMDMs)
We next investigated whether γ-TE is capable of modulating LPS-stimulated IL-6 production and C/EBPβ up-regulation in primary macrophages. Mouse BMDMs were prepared to >95% purity, as indicated by F4/80 positive staining using flow cytometry (data not shown). γ-TE at 10μM did not significantly affect BMDM viability based on morphological examination and MTT assays (data not shown). On the other hand, γ-TE at 20μM decreased BMDM viability after prolonged incubation (data not shown). We therefore used γ-TE at 10μM in all subsequent functional studies.
γ-TE treatment markedly suppressed LPS-stimulated IL-6 by up to 80% in the BMDMs, which was stronger than its inhibitory effect in RAW267.4 macrophages where γTE inhibited IL-6 by 40% (comp. Figure 6A vs. Figure 1). Similarly, γ-TE led to stronger reduction of G-CSF in the BMDMs than in RAW cells (Figure 6A). Unlike observations with RAW267.4 cells where LPS triggered initial (10min) and an even stronger delayed stimulation of IκBα phosphorylation (Figure 3), LPS led to relatively constant increase of IκBα phosphorylation in the BMDMs during 90-min period of post stimulation (Figure 6B). While inhibiting the delayed activation of NF-κB in RAW cells, γ-TE suppressed LPS-stimulated immediate phosphorylation of IκBα in the BMDMs (Figure 6C).
Figure 6. γ-TE inhibited LPS-stimulated activation of NF-κB and C/EBPβ and diminished IL-6 and G-CSF in bone marrow-derived macrophages (BMDMs).
Panel A: BMDMs were pretreated with γ-TE at 5 and 10μM for 8 h and then stimulated with 0.1μg/ml LPS for 16 h. Results of IL-6 and GCF are the ratio of cytokines secreted by cells treated with γ-TE to that of solvent controls in the presence of LPS. Ctrl is no-LPS control. * P<0.01 are the difference between γ-TE treated and solvent control cells. Data are shown as mean±SD (n >2 per bar). Panels B-C: BMDMs were stimulated with LPS for indicated times (B). BMDMs were pretreated with γ-TE at 10μM for 8 h and then stimulated with LPS for 10 min (C). Panels D-E: BMDMs were stimulated with LPS, TNFα or IL-1β for 16 h at dosages indicated in Fig 4. Data analyses were described under Fig 4. Panel F: BMDMs were pretreated with γ-TE at 10μM for 8 h and then stimulated with LPS for 16 h.
Like the results with RAW cells, LPS induced marked up-regulation of IL-6, C/EBPβ and C/EBPδ in the BMDMs (Figure 6D and E). Meanwhile, although TNFα induced upregulation of C/EBPδ, it caused very modest increase of C/EBPβ and IL-6 in the BMDMs (Figure 6E). IL-1β failed to increase IL-6, C/EBPβ or C/EBPδ (Figure 6E). Importantly, consistent with the results in RAW cells, γTE potently inhibited LPS-enhanced C/EBPβ in the BMDMs (Figure 6F). γ-TE also dampened LPS-stimulated upregulation of C/EBPδ (Figure 6F), which was not observed in RAW cells.
DISCUSSION
Our study demonstrates that γ-TE inhibits LPS-stimulated C/EBPβ up-regulation and NF-κB activation, which leads to decreased production of IL-6 and G-CSF in macrophages. Although γ-TE has previously been reported to inhibit IL-6 in RAW267.4 cells, our study provides the molecular mechanism underlying this action. Furthermore, we are the first to demonstrate that γ-TE dampens production of IL-6 and G-CSF and suppresses activation of C/EBPβ and NF-κB in primary bone marrow-derived macrophages, and these inhibitory effects are even stronger than those observed in RAW macrophages. These results support the notion that γ-TE has anti-inflammatory activities, which are in line with a recently published study where tocotrienol-rich supplementation results in lowered IL-6 in LPS-stimulated peripheral blood leukocytes of healthy volunteers.
C/EBPβ, which is also called NF-IL6, is a member of the C/EBP family of leucine zipper transcription factors. C/EBPβ has been demonstrated to play an important role in terminal acquisition of macrophage phenotype and in regulation of several macrophage-specific gene expressions during acute inflammatory responses. Mice with complete knockout of C/EBPβ (−/−) are susceptible to infection by microorganisms and display impaired tumoricidal activities. C/EBPβ has recently been demonstrated to be a critical regulator of the immunosuppressive environment created by growing cancer cells and therefore contributes to tumor metastasis. These studies suggest that modulation of C/EBPβ may have significant effects on immune function and targeting C/EBPβ may be a useful therapy that prevents tumor cells from invading distance tissues, a clinically significant area.
It is well recognized that C/EBPβ plays a role in induction of various cytokines including IL-6, IL-8 and G-CSF. Consistently, we observe that IL-6 secretion parallels C/EBPβ upregulation and IL-6 was partially suppressed by down-regulation of C/EBPβ via siRNA in the RAW cells. The importance of C/EBPβ to IL-6 in the RAW cells and BMDMs is also evident by their correlative increase in response to LPS but not individual cytokines. In contrast, C/EBPδ likely plays a minor role in IL-6 regulation as TNFα-induced up-regulation of C/EBPδ does not correlate with marked induction of IL-6 in the BMDMs (Figure 6). Paradoxically, despite well-accepted involvement in IL-6 production, cells complete lack of C/EBPβ (C/EBPβ −/−) show no significant reduction of various cytokines including IL-6 with exception of G-CSF in macrophages. This may be explained by the possibility that other C/EBP family member(s) such as C/EBPδ compensate for C/EBPβ in certain cytokine regulation in macrophages. To this end, double knockout of C/EBPβ and C/EBPδ but not either alone leads to profound decrease of IL-6. IL-6 production is also partially reduced by C/EBP decoy that inhibits binding of C/EBPs to its cognate DNA sequences. Here we have found that γ-TE treatment partially suppresses LPS-stimulated C/EBPβ up-regulation without affecting C/EBPδ in the RAW cells. On the other hand, γ-TE suppressed both C/EBPβ and C/EBPδ in the BMDMs, where it diminished IL-6 and G-CSF more potently than did in RAW cells.
Besides C/EBPs, γ-TE also blocks NF-κB activation in both RAW cells and BMDMs. Although required for IL-6 expression, NF-κB activation alone appears to be insufficient to IL-6 induction, as activation of NF-κB by cytokines fails to significantly increase IL-6 in macrophages. It is known that IL-6 promoter has single binding sites for C/EBPβ and NF-κB. These two transcription factors have been suggested to synergistically activate IL-6 promoter. Therefore, it is very likely that the dampening effect of γ-TE on IL-6 is a result of dual inhibition of NF-κB and C/EBPβ in macrophages. The inhibitory activities of NF-κB by γ-TE in macrophages are in agreement with a previous study in various cancer cell lines. In particular, Ahn et al. have demonstrated that γ-TE inhibits TNFα-stimulated IκBα phosphorylation by suppressing IκBα kinase (IKK) activity in leukemia KBM-5 cells. γ-TE has also been shown to sensitize pancreatic cancer cells to gemcitabine-induced anticancer effects via suppressing NF-κB. Nevertheless, the molecular mechanisms underlying the inhibition of NF-κB by γ-TE remain to be determined. In contrast to the suppression of C/EBPβ and NF-κB, γ-TE had no effect on p38 activation, which appears to regulate COX-2 expression in RAW cells. Therefore, these data explain the lack of impact of γ-TE on LPS-stimulated COX-2 induction in the present and previous studies.
Our current findings provide additional evidence that γ-TE have anti-inflammatory properties by modulating C/EBPβ and NF-κB as well as their regulated pro-inflammatory cytokines. The concentrations of γ-TE used in the current study are pharmacologically achievable. Since IL-6 and G-CSF are known to contribute to pathogenesis of arthritis and inflammation-promoted carcinogenesis, inhibition of these proinflammatory mediators may contribute to potential prevention and therapy of γ-TE against chronic diseases. Consistent with these mechanistic studies, γ-TE supplementation has recently been shown to inhibit IL-6 in animals and human subjects. Future studies are necessary to further examine in vivo anti-inflammatory effects of specific vitamin E forms as well as their metabolites in different preclinical models related to inflammatory diseases and cancer. The molecular mechanisms underlying inhibition of C/EBPβ and NF-κB should also be investigated.
Acknowledgments
Funding sources: This work was partially supported by grants R21CA133651 and R01AT006882 (QJ)from National Institutes of Health.
ABBREVIATIONS
- α-T, β-T, γ-T, or δ-T
α, β, γ, or δ-tocopherol
- γ-TE
γ-tocotrienol
- C/EBPβ(δ)
CCAAT/enhancer-binding protein beta (delta)
- NF-κB
nuclear factor κ B
- IκBα
NF-κB inhibitor α
- MAPK
mitogen-activated protein kinase
- ERK
extracellular-signal-regulated kinase
- JNK
c-Jun NH(2)-terminal protein Kinases
- G-CSF
granulocyte-colony stimulating factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodrick R, Ruderman EM. Anti-interleukin-6 therapy in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2010;68:211–217. [PubMed] [Google Scholar]
- 6.Bannwarth B, Richez C. Clinical safety of tocilizumab in rheumatoid arthritis. Expert Opin Drug Saf. 2011;10:123–131. doi: 10.1517/14740338.2011.537256. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. The American journal of clinical nutrition. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 8.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. Faseb J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 9.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Molecular aspects of medicine. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunol. 2011;186:1173–1179. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free radical biology & medicine. 2007;43:1176–1188. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Moreland M, Wagner JG, Ames BN, Illek B, Peden DB, Jiang Q. Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. J Nutr Biochem. 2011 Jul 15; doi: 10.1016/j.jnutbio.2011.03.003. [Epub ahead of print]. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ. 2006;13:702–711. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- 17.Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids. 2009;44:787–797. doi: 10.1007/s11745-009-3326-2. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. {gamma}-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14(Unit 14):11. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Dubois RN. Eicosanoids and cancer. Nature reviews. Cancer. 10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, Zamora R, Ford HR. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol. 2006;176:580–588. doi: 10.4049/jimmunol.176.1.580. [DOI] [PubMed] [Google Scholar]
- 23.Dean JL, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. The Journal of biological chemistry. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–186. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 25.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanceau J, Wijdenes J, Revel M, Wietzerbin J. IL-6 and IL-6 receptor modulation by IFN-gamma and tumor necrosis factor-alpha in human monocytic cell line (THP-1). Priming effect of IFN-gamma. J Immunol. 1991;147:2630–2637. [PubMed] [Google Scholar]
- 27.Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990;10:2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asschert JG, Vellenga E, Ruiters MH, de Vries EG. Regulation of spontaneous and TNF/IFN-induced IL-6 expression in two human ovarian-carcinoma cell lines. Int J Cancer. 1999;82:244–249. doi: 10.1002/(sici)1097-0215(19990719)82:2<244::aid-ijc15>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Patestos NP, Haegeman G, Vandevoorde V, Fiers W. Activation of the nuclear factor kappa B is not sufficient for regulation of tumor necrosis factor-induced interleukin-6 gene expression. Biochimie. 1993;75:1007–1018. doi: 10.1016/0300-9084(93)90153-j. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci U S A. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley MN, Zhou L, Smale ST. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Mol Cell Biol. 2003;23:4841–4858. doi: 10.1128/MCB.23.14.4841-4858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su WC, Chou HY, Chang CJ, Lee YM, Chen WH, Huang KH, Lee MY, Lee SC. Differential activation of a C/EBP beta isoform by a novel redox switch may confer the lipopolysaccharide-inducible expression of interleukin-6 gene. J Biol Chem. 2003;278:51150–51158. doi: 10.1074/jbc.M305501200. [DOI] [PubMed] [Google Scholar]
- 34.Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nature immunology. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 36.Mahalingam D, Radhakrishnan AK, Amom Z, Ibrahim N, Nesaretnam K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur J Clin Nutr. 2011;65:63–69. doi: 10.1038/ejcn.2010.184. [DOI] [PubMed] [Google Scholar]
- 37.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 38.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Gorgoni B, Maritano D, Marthyn P, Righi M, Poli V. C/EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mRNAs in macrophages. J Immunol. 2002;168:4055–4062. doi: 10.4049/jimmunol.168.8.4055. [DOI] [PubMed] [Google Scholar]
- 41.Uematsu S, Matsumoto M, Takeda K, Akira S. Lipopolysaccharide-dependent prostaglandin E(2) production is regulated by the glutathione-dependent prostaglandin E(2) synthase gene induced by the Toll-like receptor 4/MyD88/NF-IL6 pathway. J Immunol. 2002;168:5811–5816. doi: 10.4049/jimmunol.168.11.5811. [DOI] [PubMed] [Google Scholar]
- 42.Lu YC, Kim I, Lye E, Shen F, Suzuki N, Suzuki S, Gerondakis S, Akira S, Gaffen SL, Yeh WC, Ohashi PS. Differential role for c-Rel and C/EBPbeta/delta in TLR-mediated induction of proinflammatory cytokines. J Immunol. 2009;182:7212–7221. doi: 10.4049/jimmunol.0802971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rego D, Kumar A, Nilchi L, Wright K, Huang S, Kozlowski M. IL-6 production is positively regulated by two distinct Src homology domain 2-containing tyrosine phosphatase-1 (SHP-1)-dependent CCAAT/enhancer-binding protein beta and NF-kappaB pathways and an SHP-1-independent NF-kappaB pathway in lipopolysaccharide-stimulated bone marrow-derived macrophages. J Immunol. 2011;186:5443–5456. doi: 10.4049/jimmunol.1003551. [DOI] [PubMed] [Google Scholar]
- 44.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 45.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, Koca C, Yadav VR, Tong Z, Gelovani JG, Guha S, Krishnan S, Aggarwal BB. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer research. 2010;70:8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. The Journal of nutrition. 2009;139:884–889. doi: 10.3945/jn.108.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qureshi AA, Reis JC, Papasian CJ, Morrison DC, Qureshi N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids in health and disease. 2010;9:143. doi: 10.1186/1476-511X-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]