Abstract
Background
Evidence is limited on the impact of childhood socioeconomic status (SES), adulthood SES and chronic conditions on risk of incident stroke in later life. We aimed to examine these associations using data from a nationally representative sample of the Health and Retirement Study (HRS).
Methods
Stroke-free participants (n=22,847) aged>50 years in the HRS (1992-2008) were analyzed. Childhood and adulthood SES were assessed using parental and participant’s education attainments. Incident stroke was defined as self-reported first incident stroke.
Results
Of the study sample, 2,298 subjects experienced first incident stroke (10.06%). Cox’s regression models indicate that subjects with low childhood SES had 1.36 times higher risk (95%CI: 1.18-1.57) of first incident stroke than those with high childhood SES. There was an 8% reduction of this association after adjustment for adulthood SES. Adults with diabetes mellitus (DM) had the highest hazard ratio (1.91, 95%CI: 1.63-2.23) for incident stroke, followed by heart disease (1.69, 1.48-1.93), and then hypertension (1.56, 1.40-1.75). Significant interaction effect of childhood SES and DM, and combined effects of SES and chronic conditions on risk of incident stroke were observed.
Conclusions
Both low SES in childhood and adulthood SES predict the risk of stroke. There are significantly combined effects of SES and chronic conditions on the risk of stroke. Improving SES across the lifespan and aggressive control of chronic conditions may play pivotal roles in the prevention of stroke development.
Keywords: Social position, lifespan and stroke
Introduction
Although a declining trend of stroke incidence and mortality has been observed in the United States, since 1930s stroke has continuously been the leading cause of death for Americans (1). In 2010, 137,000 people suffered fatal stroke. It is estimated that Americans would pay nearly $73.7 billion for stroke-related medical costs and disabilities. Several studies have demonstrated that individuals living in low socioeconomic status (SES) in early life have an increased risk of death from cardiovascular disease (CVD), including stroke in their later life (2-4). For example, using nationally representative birth cohort data from Britain, Barker and colleagues showed for the first time that individuals who had low birth weight (an indicator of low SES) are at greater risk of cardiovascular mortality in their adulthood (5,6). Similar findings have been reported from Norway, Sweden, Finland and Japan,(4,7-9) however, few studies have been conducted in the US, and results of those studies are inconsistent (10-13). For example, Gliksman and colleagues used data from the Nurses’ Health Study cohort (1976-1990) and showed that low childhood SES (assessed using father’s occupational status) significantly predicted total risk of coronary heart disease as well as non-fatal myocardial infarction, but did not predict risk of fatal stroke and coronary heart disease (11). A recent report, using earlier released HRS data, examined association of childhood SES and adulthood SES with risk of incident stroke; however, possible cumulative and combined effects of childhood SES, adulthood SES and chronic conditions on risk of stroke need to be further addressed in terms of the disease development and public health impact (10). In the present study, we aimed to add to the narrow body of the literature by using the most recently released data of the Health and Retirement Study (HRS, 1992-2008) to test two novel hypotheses: (1) although low childhood SES significantly predicts the risk of first incident stroke, this association can be influenced by adulthood SES; and (2) there are significant combined effects of childhood SES, adulthood SES and chronic conditions on the risk of stroke.
Materials and Methods
Data
The HRS is a prospective study of individuals of the non-institutionalized population and their spouses or partners in the United States. This study is sponsored primarily by the U.S. National Institute on Aging (NIA). Details of the study are provided elsewhere (14). In brief, the HRS sample is selected using a multi-stage area probability sample design. Enrolment was staggered by birth cohort with enrolments in 1992, 1993 and 1998. The first wave of the study started in 1992 with recruitment of participants born between 1931 and 1941 (i.e., aged 51 to 61 years-old at baseline). Since 1998 the HRS has been merged with four additional cohort studies, including the Study of Asset and Health Dynamics among the Oldest Old (AHEAD: participants born 1890 to 1923 at baseline 1993), Children of the Depression Age (CODA: born 1924 to 1930 at baseline 1998), War Baby (WB: born 1942 to 1947 at baseline 1998), and Early Baby Boomer (EBB: born 1948 to 1953 at baseline 2004). This HRS merger has resulted in a total of five entry cohorts, which surveys a nationally representative sample of Americans over the age of 50 years old (14). The majority of baseline interviews were face-to-face. Biennial follow-up interviews (or proxy interviews for decedent participants) were conducted by telephone or mail through 2008, with wave-to-wave retention rates being around 90%. In the present analysis, we used the combined HRS data (1992-2008) prepared by the RAND Center for the Study of Aging (released in March 2010, and updated in June 2010), because the RAND - HRS Data file is an easy to use longitudinal data set. The RAND – HRS was funded from the NIA and the Social Security Administration (14). A total of 26,856 participants aged ≥51 years are included in the Rand – HRS file. In the present analysis, we excluded 264 cases due to unknown stroke status at enrollment, and 1,471 cases that had prevalent stroke at baseline. Of the remainder (n=25,121) who were stroke-free at baseline, we further excluded 439 cases with missing values of sampling weight assignment, and 1,853 participants with lost to follow-up or a missing value on stroke status. Therefore the final sample size used in the present study is 22,847, with a participation rate of 91% (22,847/25,121). Of them, 17,176 were white, 3,309 were African American (AA), and 2,362 were other racial/ethnic participants. The HRS has been approved by the Institute for Social Research at the University Michigan, and our present analysis using de-identified data from HRS has been approved using the online approval process (14).
Variable measurements
Baseline variables
Demographic characteristics include age, sex, race/ethnicity (White, AA and others), marital status (married or not married) and education attainments (years). We categorized participants into three groups of those with low, middle and high SES according to cutoff-points of those with less than high school (<9 years), high school (9-12 years), and college education levels (≥13 years). Participants’ cigarette smoking status (never, former, or current smokers) and body mass index [weight (kg)/height (m) × height (m)] were included as covariates. We examined three groups of chronic conditions of hypertension, heart disease and diabetes mellitus (DM), because they are well-established risk predictors for stroke (1,15). Chronic conditions were defined based on participants’ self-report of a physician diagnosis of the disease. Participants were asked whether a doctor had ever told them (yes/no) that they had hypertension (or high blood pressure), heart problems (including coronary heart disease, heart attack, congestive heart disease, or the occurrence of heart surgery), and DM (or high blood sugar). The validity of self-reported health conditions has been supported by several studies and confirmed to have a substantial agreement between surveys and medical record reports (16,17).
Participants’ childhood SES is assessed according to parental education attainments, which were measured at the respondent’s first interview. In the analysis, the mother’s and father’s education attainments are recorded separately using 0, 1 and 2 for those with less than high school (i.e., <9 years), high school (9-12 years) and college or higher (≥13 years), respectively. Then, a sum index of childhood SES is created by combining parental education levels (ranged 0 to 4), such that a lower score indicates a lower childhood SES. Three groups of low, middle and high SES were classified with a sum score 0, 1 and ≥2, respectively. The use of parental education attainment as an index of childhood SES, and the use of participants’ education attainments as adult SES, have been demonstrated to be valid measures by several studies (18,19).
Outcome variables
The outcome variables include time in years from the baseline interview to onset (yes or no) of first incident stroke (either a non-fatal or fatal case) or to the last follow-up of those who did not report experiencing a first incident stroke, whichever occurred first.
We ascertained the occurrence of stroke based on the response to the survey question; asked of survey participants (or proxy interviews for decedent participants) at biannual follow-up: “since (last interview date) has a doctor told you that you had a stroke?” In the case of a positive response (i.e., first incident stroke), participants (or proxy interviews for those with first fatal stroke) were then asked for the date (month and year) on which the stroke occurred (14).
Statistical analysis
The characteristics of participants were described using univariate analysis. Correlation between adulthood SES and childhood SES was tested using Spearman’s correlation method. Differences in the first incident stroke rates by demographic, behavioral, body mass index and history of chronic conditions of hypertension, heart disease and DM for White, AA and other racial/ethnic groups were examined using Chi-square test.
To adjust potential confounders, multivariate Cox’s proportional hazards regression models were conducted to examine whether childhood SES, adulthood SES and chronic conditions were significant predictors for risk of first incident stroke. In the analysis, three multivariate models were tested. Model 1 examined the association between childhood SES (low and middle refer to high SES) and risk of first incident stroke with adjustment for age (<60 vs. ≥60), sex (male vs. female), race/ethnicity (white, AA and others) and marital status (yes vs. no). Model 2 examined whether the association between childhood SES and risk of stroke was changed when including adulthood SES as a covariate. Model 3 examined whether childhood SES, adulthood SES and chronic conditions of hypertension, heart disease and diabetes are independent predictors for risk of first incident stroke. The trends of childhood SES and adulthood SES for risk of stroke were tested by recoding low, middle and high SES as a continuous variable. Interactions of childhood SES with adulthood SES, and childhood SES with chronic conditions on the risk of first incident stroke were examined in models 2 and 3.
Sensitivity analyses were conducted as well. In particular, we excluded subjects who had first incident stroke within one-year follow-up from the survey because they might be incident stroke cases at baseline but missed diagnosis by a physician or a health professional. This exclusion can minimize potential misclassification of stroke cases and have a conservative estimate of the associations between risk factors and incident stroke.
Finally, the combined effects of childhood SES and adulthood SES with chronic conditions on the risk of first incident stroke were examined and depicted.
Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC). Because the HRS applied multi-stage sampling design, all data analyses were conducted using SAS procedures for complex sampling surveys to calculate weighted estimates and sampling errors and account for the weighted sample designs (20). In Cox’s models, we used plots of the log (-log) survival curves and Schoenfeld residuals to verify the proportional hazard assumption (21). A two-sided p value ≤0.05 was considered as having statistical significance.
Results
Of the total participants (n=22,847) who were stroke-free at baseline, 75.2% were White, 14.5% were AA and 10.3% were other racial/ethnic groups, with an average age of 61 years old. Prevalence of hypertension, heart disease and diabetes were 36.5%, 12.6% and 8.5%, respectively.
Of the study participants, 2,298 subjects experienced first incident stroke (10.06%). AA had the highest incidence of first stroke (11.14%), followed by white (9.12%), and other racial/ethnic groups (6.22%), within a median follow-up of 9.8 years (interquartile, 4.4 – 15.2 years). Aging and chronic conditions were significantly associated with risk of first incident stroke in all race/ethnic groups. Marriage status was significant, and smoking status and body mass index appeared to have no or a borderline significant association with risk of first incident stroke (Table 1).
Table 1.
First Incident Stroke by Age and Other Factors in White, African American and Other Racial/Ethnic Groups in Health and Retirement Study 1992-2008
| Baseline parameters |
White (n=17,176) Incident stroke |
AA (n=3,309) Incident stroke |
Others (n=2,362) Incident stroke |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % | (SEP)* | p | % | (SEP) | p | % | (SEP) | P | |
| Age, years | |||||||||
| 51–64 | 5.93 | (0.26) | 9.10 | (0.73) | 4.83 | (0.59) | |||
| ≥65 | 15.95 | (0.51) | <0.001 | 17.00 | (1.35) | <0.001 | 11.12 | (1.37) | <0.001 |
| Aged ≥51† | 9.12 | (0.24) | 11.14 | (0.65) | 6.22 | (0.55) | |||
| Gender | |||||||||
| Male | 8.69 | (0.35) | 11.42 | (1.06) | 7.13 | (0.91) | |||
| Female | 9.50 | (0.34) | 0.097 | 10.93 | (0.81) | 0.714 | 5.45 | (0.67) | 0.127 |
| Marital status | |||||||||
| Not married | 10.69 | (0.51) | 11.89 | (0.97) | 7.01 | (1.02) | |||
| Married | 8.58 | (0.27) | <0.001 | 10.43 | (0.87) | 0.261 | 5.85 | (0.65) | 0.326 |
| Smoking status | |||||||||
| Never | 8.58 | (0.36) | 12.13 | (1.02) | 4.92 | (0.70) | |||
| Former smoker | 9.47 | (0.39) | 8.98 | (0.95) | 7.37 | (1.06) | |||
| Current smoker | 9.54 | (0.59) | 0.185 | 12.32 | (1.48) | 0.068 | 7.52 | (1.38) | 0.081 |
| BMI, kg/m2 | |||||||||
| <18.5 | 9.47 | (2.08) | 6.37 | (3.65) | 3.17 | (2.29) | |||
| 18.5-24.9 | 8.74 | (0.44) | 12.77 | (1.75) | 6.63 | (1.31) | |||
| 25-29.9 | 9.25 | (0.35) | 11.65 | (0.98) | 6.74 | (0.83) | |||
| ≥30 | 9.35 | (0.55) | 0.782 | 9.86 | (1.00) | 0.287 | 5.21 | (0.89) | 0.514 |
| Hypertension | |||||||||
| No | 7.04 | (0.28) | 8.24 | (0.91) | 4.66 | (0.64) | |||
| Yes | 12.49 | (0.48) | <0.001 | 13.24 | (0.97) | <0.001 | 10.07 | (1.21) | <0.001 |
| Heart disease | |||||||||
| No | 7.75 | (0.25) | 9.99 | (0.68) | 5.47 | (0.56) | |||
| Yes | 16.08 | (0.83) | <0.001 | 19.06 | (2.41) | <0.001 | 14.62 | (2.83) | <0.001 |
| Diabetes mellitus | |||||||||
| No | 8.63 | (0.25) | 9.56 | (0.68) | 5.12 | (0.54) | |||
| Yes | 13.95 | (1.05) | <0.001 | 18.70 | (2.01) | <0.001 | 12.84 | (2.24) | <0.001 |
SEP: Standard error of proportion.
Test for rates differences across three race/ethnic groups: p<0.001.
Adulthood SES was significantly and positively correlated with childhood SES across all three racial/ethnic groups and in the total combined sample. The corresponding correlation coefficients of childhood SES and adulthood SES were 0.42 (p<0.001), 0.40 (p<0.001), and 0.45 (p<0.001) for white, AA and other racial/ethnic groups, respectively. The overall correlation coefficient between childhood and adulthood SES in the total sample was 0.44 (p<0.001).
Table 2 indicates that after adjustment for demographic variables, subjects with low or middle childhood SES had significantly higher risk of first incident stroke as compared to those with high SES (Model 1). The association remained significant after adjustment for adulthood SES (Model 2), but it resulted in a 4.8% and 8.1% reduction for those with middle and low childhood SES, respectively. Model 3 shows that childhood SES, adulthood SES and chronic conditions were significant predictors for risk of first incident stroke. These associations were independent of multiple covariates. It should be noted that current smoking is a significant predictor of incident stroke in the multivariate analysis (HR=1.59, 95%CI: 1.36-1.85). However, similar to the crude analysis (Table 1), no significant association between BMI and stroke is observed. Model 3 also shows that there was a significant interaction of childhood SES and diabetes on incident stroke (p=0.03). Therefore, we further examined the association of childhood SES and adulthood SES by stratification of diabetes status. The results shows that among those without diabetes, subjects with low childhood SES and low adulthood SES had significantly higher risk of first incident stroke (12.45%) than those with higher childhood and adulthood SES (4.42%). However, patients with diabetes and with low adulthood SES had significantly higher risk of first incident stroke, regardless of childhood SES. The corresponding incidence rates (standard error of proportion) of stroke in patients with diabetes and with low adulthood SES are 17.25% (2.05), 16.34% (7.45) and 27.77% (12.95) among those with low, middle and high childhood SES, respectively.
Table 2.
Hazard Ratios (95%CI) of Childhood SES, Adulthood SES and Chronic Conditions for Risk of Incident Stroke in Health and Retirement Study 1992-2008
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95%CI) | p-value | HR | (95%CI) | p-value | HR | (95%CI) | p-value | |
| Childhood SES | |||||||||
| High | 1 | 1 | 1 | ||||||
| Middle | 1.24 | (1.03 – 1.49) | 0.03 | 1.18 | (0.98 – 1.43) | 0.08 | 1.19 | (0.96 – 1.47) | 0.12 |
| Low | 1.36 | (1.18 – 1.57) | <.0001 | 1.25 | (1.08 – 1.45) | <0.01 | 1.35 | (1.15 – 1.59) | <.001 |
| Test for trend, p-value | <.001 | <.001 | <.001 | ||||||
| Adulthood SES | |||||||||
| High | 1 | 1 | |||||||
| Middle | 1.20 | (1.07 – 1.35) | 0.002 | 1.17 | (1.03 – 1.33) | 0.02 | |||
| Low | 1.37 | (1.17 – 1.59) | <.001 | 1.26 | (1.06 – 1.50) | 0.01 | |||
| Test for trend, p-value | <.001 | <.001 | |||||||
| Smoking status | |||||||||
| Never | 1 | ||||||||
| Former smoker | 1.03 | (0.91 – 1.17) | 0.61 | ||||||
| Current smoker | 1.59 | (1.36 – 1.85) | <.001 | ||||||
| Test for trend, p-value | <0.01 | ||||||||
| Body mass index, kg/m2 | |||||||||
| <18.5 | 1.08 | (0.72 – 1.62) | 0.72 | ||||||
| 18.5-24.9 | 1 | ||||||||
| 25-29.9 | 0.90 | (0.79 – 1.02) | 0.10 | ||||||
| ≥30 | 0.93 | (0.80 – 1.09) | 0.37 | ||||||
| Chronic conditions | |||||||||
| HBP (yes vs. no) | 1.56 | (1.40 – 1.75) | <.001 | ||||||
| HD (yes vs. no) | 1.69 | (1.48 – 1.93) | <.001 | ||||||
| DM (yes vs. no) | 1.91 | (1.63 – 2.23) | <.001 | ||||||
| Interaction effects on stroke | |||||||||
| Childhood SES * Adult SES | 1.61 | (1.09 – 2.38) | 0.017 | 1.22 | (0.75 – 1.99) | 0.41 | |||
| Childhood SES * HBP | 0.96 | (0.75 – 1.23) | 0.74 | ||||||
| Childhood SES * HD | 0.89 | (0.64 – 1.22) | 0.46 | ||||||
| Childhood SES * DM | 1.50 | (1.05 – 2.15) | 0.03 | ||||||
SES: Socioeconomic status. HR: Hazard ratio. HBP: Hypertension, HD: heart disease, DM: Diabetes mellitus.
Model 1: Adjusted for age, race, gender and marital status.
Model 2: Adjusted for age, race, gender, marital status, adulthood SES.
Model 3: Adjusted for all covariates in model 2 plus adult smoking status, body mass index and chronic conditions.
Sensitivity analysis, by excluding those who reported having first incident stroke diagnosed within one year of the follow-up, had similar results to the total sample without this excluding (data not shown). Therefore, we present the results using the total sample in Table 2.
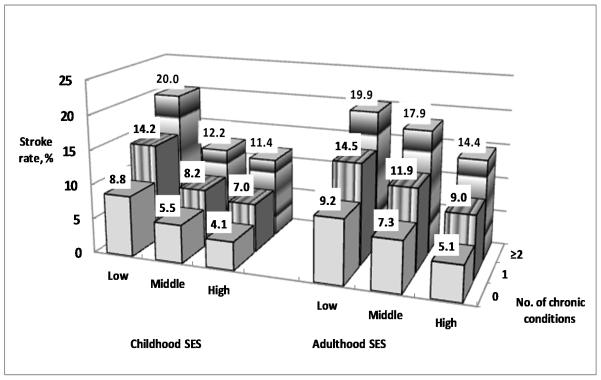
Of three chronic conditions (hypertension, heart disease and diabetes), 32.1% of participants had one chronic condition, and 10.4% had two or more conditions. Figure 1 depicts that subjects with low childhood SES or low adulthood SES and with ≥ two chronic conditions had the highest incident rate of first stroke. Those without a chronic condition and with high SES had the lowest incident rate.
Figure 1.
First incident stroke rates (%) by the combined effects of childhood, adulthood SES and the number of chronic conditions in Health and Retirement Study 1992-2008. Subjects with low childhood SES or low adulthood SES and with ≥ two chronic conditions had the highest incident rate of first stroke. Those without a chronic condition and with high SES had the lowest incident rate.
Discussion
Consistent with other studies suggesting a cause–effective association between low childhood SES and risk of first incident stroke (3,7,19,22-24), the present study provides further evidence to the literature on the cumulative and combined and interaction effects of childhood and adulthood SES and chronic conditions.
The study shows that adulthood SES may modify the association between childhood SES and risk of stroke, and there are significantly combined effects of childhood SES, adulthood SES and chronic conditions on risk of stroke. These results suggest that there are potentially cumulative effects of childhood SES adulthood SES and adult chronic conditions on risk of stroke, because low childhood SES occurs before adulthood SES and before the occurrence of chronic conditions. These findings highlight the importance of control of stroke across the lifespan.
Several studies have observed that smoking and obesity are significant risk factors for stroke. Finding from our present study is consistent with previous studies on the association between smoking and stroke (1). However, we do not observe a significant association between BMI and stroke. A meta-analysis based on findings from 25 studies suggests that overweight and obesity are significantly associated with risk of ischemic stroke, but no evidence for the association with hemorrhagic stroke (25). It is known that the mechanisms of by which BMI as a risk factor for stroke may be different among subtype strokes. We are unable to test in detail on the association between BMI and subtype strokes because information on the classification of subtype strokes is not available in the present study.
Although diabetes poses a serious health problem, very limited data examined a potential interactive effect of diabetes with SES on risk of stroke. Results from the present study suggest that the magnitudes of the associations between childhood SES and risk of stroke are not only modified by adulthood SES, but also are strongly influenced by whether individuals suffer from diabetes. Patients with diabetes and with low adulthood SES shows significantly higher risk of stroke, regardless of childhood SES. This important finding suggests that although low SES in childhood plays an overall negative effect on the risk of stroke in general population, an aggressive control of diabetes for patients with low SES in adulthood may greatly reduce risk of stroke in individuals with diabetes.
In life-course epidemiology studies, several indicators have been used to assess childhood and adulthood SES, including parental education attainments, occupational status and income, or disease status in early life. Each has its own advantages and disadvantages because of their measurable and comparable issues across different cultures, regions and time periods. In the present study, we applied parental education attainments to evaluate SES, due to two advantages: (1) the measures of education level and cutoff-points are consistently used over the course of the HRS, and (2) these measures are relatively stable and comparable than other indicators, as such income and occupational status.
The mechanism by which childhood SES contributes to an increased risk of stroke is still being studied, three possible pathways are postulated. First, the biological pathways may be involved in an intergenerational transmission. In particular, early life experience with physical disadvantages can result in epigenetic modifications, shown to be heritable and consequently able to exert long-term biological influences (6,19). Secondly, psychosocial pathways through which a low SES environment may be transmitted from one generation to the next may exist. The present study has demonstrated that childhood SES is significantly positively correlated with adulthood SES. The combined exposure to a poorer SES at childhood and adulthood may accumulate the risk of stroke across the lifespan.
The present study has several strengths. First, this prospective analysis approach has the advantage of interpreting a causal association between predictors and outcomes. Second, the survey procedures and measures of predictors and outcomes are validated across all waves of the HRS. Third, results from our sensitivity analyses reveal that excluding those who reported having first incident stroke within one-year of the follow-up, produces similar results to the use of the total study sample. This sensitivity analysis enhances the accuracy of the present findings. Fourth, by using data from the largest sample size of Americans aged > 50 years, the present study is able for the first time to explore a significant interactive effect of SES and prevalent DM, and combined effects of SES and chronic conditions on risk of stroke. Beyond previous life-course analysis, these findings address that prevention of stroke is not only for those at early life, but also for those in adulthood. An increased risk of incident stroke in adults with diabetes and with low SES in adulthood, regardless of their SES in childhood, highlights the challenge of further etiological study and control of stroke across the lifespan. Last but not least, although the patterns and epidemiology of stroke in relation to SES and BMI may be different between developed and developing countries in a certain time period of social and environmental transmission, the survey approach of the HRS and findings from the present analysis may provide an overall concept and new insights into setting up surveillance system and monitoring stroke risk across the lifespan in populations living in developing countries (25-28).
Meanwhile, several limitations should be kept in mind when interpreting the results. First, like most longitudinal observational studies, findings from the present study may underestimate the associations of SES across the lifespan and chronic conditions with risk of first incident stroke. For example, we may miss some stroke cases among those who died from other causes but with comorbidity from stroke because these cases were unlikely to be reported as incident stroke. Meanwhile, those who died before age 50, but had high risk of development of stroke or had subclinical stroke were excluded from the study, which may lead to an underestimate of the association between SES and incidence of stroke. Secondly, measures of chronic conditions and incident stroke are based upon respondents’ self-reports of physician’s diagnosis and thus potential recall bias may exist. Thirdly, we are unable to conduct further in-depth analyses of potential mediators, such as serum biomarkers of dyslipidemia, glucose and HbA1c, in relation to risk of stroke and subtype strokes (i.e., ischemic and hemorrhagic), because these measures are not available from the current HRS data.
Despite the aforementioned limitations, findings from the study suggest that both low SES in childhood and adulthood SES predict the risk of stroke. There are significantly combined risk effects of low SES and chronic conditions on stroke. Improving SES across the lifespan and aggressive control of chronic conditions will play pivotal roles in the prevention of stroke development.
Acknowledgments
We acknowledge the support of the Health and Retirement Study (HRS) for providing access to the RAND-HRS data files. The HRS is sponsored by the National Institute on Aging (grant number NIA U01AG009740), and was conducted by the University of Michigan (UM). The RAND – HRS data file was funded from the NIA and the Social Security Administration. The views expressed in this paper are those of the authors and do not necessarily represent the views of the UM. One of the goals of the present study was to establish a frame of reference for setting up a surveillance system and examining stroke risk in Chinese population.
Footnotes
Conflicts of interest: None declared.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Palloni A, Milesi C, White RG, Turner A. Early childhood health, reproduction of economic inequalities and the persistence of health and mortality differentials. Soc.Sci.Med. 2009;68(9):1574–1582. doi: 10.1016/j.socscimed.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braveman P, Barclay C. Health disparities beginning in childhood: a life-course perspective. Pediatrics. 2009;124(Suppl 3):S163–75. doi: 10.1542/peds.2009-1100D. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Liu L. Infant mortality, cancer, and cardiovascular disease mortality: an ecological analysis for 47 prefectures of Japan. Int.J.Cardiol. 2011;149(2):242–243. doi: 10.1016/j.ijcard.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Barker DJ. Human growth and cardiovascular disease. Nestle Nutr.Workshop Ser.Pediatr.Program. 2008;61:21–38. doi: 10.1159/000113163. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ. Fetal nutrition and cardiovascular disease in later life. Br.Med.Bull. 1997;53(1):96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- 7.Claussen B, Davey Smith G, Thelle D. Impact of childhood and adulthood socioeconomic position on cause specific mortality: the Oslo Mortality Study. J.Epidemiol.Community Health. 2003;57(1):40–45. doi: 10.1136/jech.57.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nass CM, Reck K. Clinical challenges: the intersection of diabetes, chronic kidney disease, and cardiovascular disease. Curr.Diab Rep. 2004;4(1):1–2. doi: 10.1007/s11892-004-0001-0. [DOI] [PubMed] [Google Scholar]
- 9.Kuper H, Adami HO, Theorell T, Weiderpass E. The socioeconomic gradient in the incidence of stroke: a prospective study in middle-aged women in Sweden. Stroke. 2007;38(1):27–33. doi: 10.1161/01.STR.0000251805.47370.91. [DOI] [PubMed] [Google Scholar]
- 10.Glymour MM, Avendano M, Haas S, Berkman LF. Lifecourse social conditions and racial disparities in incidence of first stroke. Ann.Epidemiol. 2008;18(12):904–912. doi: 10.1016/j.annepidem.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gliksman MD, Kawachi I, Hunter D, et al. Childhood socioeconomic status and risk of cardiovascular disease in middle aged US women: a prospective study. J.Epidemiol.Community Health. 1995;49(1):10–15. doi: 10.1136/jech.49.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avendano M, Glymour MM. Stroke disparities in older Americans: is wealth a more powerful indicator of risk than income and education? Stroke. 2008;39(5):1533–1540. doi: 10.1161/STROKEAHA.107.490383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen ME. Coronary heart disease from a life-course approach: findings from the health and retirement study, 1998-2004. J.Aging Health. 2010;22(2):219–241. doi: 10.1177/0898264309355981. [DOI] [PubMed] [Google Scholar]
- 14.HRS. Health and Retirement Study [Accessed on June 16, 2010]; Available at: http://hrsonline.isr.umich.edu/
- 15.Yamori Y, Liu L, Mizushima S, Ikeda K, Nara Y, CARDIAC Study Group Male cardiovascular mortality and dietary markers in 25 population samples of 16 countries. J.Hypertens. 2006;24(8):1499–1505. doi: 10.1097/01.hjh.0000239284.12691.2e. [DOI] [PubMed] [Google Scholar]
- 16.Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors?: evidence from the health and retirement study. Stroke. 2009;40(3):873–879. doi: 10.1161/STROKEAHA.108.529479. [DOI] [PubMed] [Google Scholar]
- 17.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am.J.Public Health. 1989;79(11):1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int.J.Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 19.Schreier HM, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain Behav.Immun. 2010;24(8):1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute Inc [Accessed on Dec 18, 2011];Sample Survey Design and Analysis. Available at: http://support.sas.com/rnd/app/da/new/dasurvey.html.
- 21.Tabachnick BG, FL . Using Multivariate Statistics. 5th ed Allyn & Bacon; Boston, MA: 2007. [Google Scholar]
- 22.Bowen ME. Childhood socioeconomic status and racial differences in disability: evidence from the Health and Retirement Study (1998-2006) Soc.Sci.Med. 2009;69(3):433–441. doi: 10.1016/j.socscimed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Naess O, Strand BH, Smith GD. Childhood and adulthood socioeconomic position across 20 causes of death: a prospective cohort study of 800,000 Norwegian men and women. J.Epidemiol.Community Health. 2007;61(11):1004–1009. doi: 10.1136/jech.2006.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J.Epidemiol.Community Health. 2008;62(5):387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 25.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41(5):e418–26. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 26.Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365(9478):2160–2161. doi: 10.1016/S0140-6736(05)66755-4. [DOI] [PubMed] [Google Scholar]
- 27.Mushtaq MU, Gull S, Abdullah HM, Shahid U, Shad MA, Akram J. Prevalence and socioeconomic correlates of overweight and obesity among Pakistani primary school children. BMC Public Health. 2011;11:724. doi: 10.1186/1471-2458-11-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das SK, Banerjee TK, Biswas A, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38(3):906–910. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]