Abstract
Targeting the cellular Ca2+ channels and pumps that underpin parasite Ca2+ homeostasis may realize novel antihelmintic agents. Indeed, the antischistosomal drug praziquantel (PZQ) is a key clinical agent that has been proposed to work in this manner. Heterologous expression data has implicated an action of PZQ on voltage-operated Ca2+ channels, although the relevant in vivo target of this drug has remained undefined over three decades of clinical use. The purpose of this review is to bring new perspective to this issue by discussing the potential utility of free-living planarian flatworms for providing new insight into the mechanism of PZQ action. First, we discuss in vivo functional genetic data from the planarian system that broadly supports the molecular data collected in heterologous systems and the ‘Ca2+ hypothesis’ of PZQ action. On the basis of these similarities we highlight our current knowledge of platyhelminth voltage operated Ca2+ channels, their unique molecular pharmacology and the downstream functional PZQ interactome engaged by dysregulation of Ca2+ influx that has potential to yield novel antischistosomal targets. Overall the broad dataset underscore a common theme of PZQ-evoked disruptions of Ca2+ homeostasis in trematodes, cestodes and turbellarians, and showcase the utility of the planarian model for deriving insight into drug action and targets in parasitic flatworms.
Keywords: Planarians, Schistosomiasis, Platyhelminths, Ca2+ signaling, Voltage-operated Ca2+ channel
1. Introduction
Over a third of the world’s population is estimated to be infected with parasitic worms [1]. As discussed by the authors of this volume, ion channel modifying drugs hold considerable potential for use as antihelmintics with several agents that target ligand-gated ion channels in parasitic worms approved for clinical/veterinary use [2–5]. Voltage-gated ion channels afford similar opportunity for exploitation as druggable targets, given the likely importance of these channels for parasite biology. Our laboratory is particularly interested in the role of platyhelminth voltage-gated Ca2+ channels (Cav channels), given the fundamental role of Ca2+ signals in normal cellular and developmental physiology [6, 7]. Indeed, one important drug proposed to disrupt voltage-operated Ca2+ entry in platyhelminths is praziquantel (PZQ), and the purpose of this review is to provide broad perspective on evidence linking PZQ to changes in Ca2+ homeostasis in both parasitic and free living (planarian) platyhelminths.
PZQ is the key pharmacotherapy used for treating schistosomiasis, as well as cestode infections. It is a crucial treatment: over 200 million people harbor schistosome infections (Schistosoma mansoni, S. japonicum and S. haematobium) and PZQ is the sole therapy available in many areas of endemic disease [8–12]. The associated burden of schistosomiasis manifest through gastrointestinal and liver pathology, anaemia, undernutrition, growth retardation, genitourinary disease (S. haematobium) and increased prevalence of co-morbidities, is arguably second only to HIV/AIDS in impact [9, 13]. The low cost (~$0.07/treatment) and high cure rate with a single dose of PZQ has led to initiatives to increase distribution of the drug [10] but there is a continued anxiety that PZQ-resistant strains of schistosomiasis will emerge [12, 14–17]. In the absence of an effective vaccine/vector control for schistosomiasis, the continued efficacy of PZQ in clearing schistosome infections is critical for reducing the devastating burden of this disease in Africa. Therefore, it remains problematic that despite over three decades of clinical use the target of PZQ still remains ambiguous and synthesized structural derivatives prove less efficacious [18–21]. Resolution of the target and effector mechanisms of PZQ is needed to permit rational design of novel drugs to exploit these same pathways and to discover agents that, unlike PZQ, retain efficacy against all stages of the schistosome life cycle [22–24].
We have attempted to bring new perspective to this longstanding roadblock by studying the action of PZQ in planaria [20, 25], free-living turbellarian flatworms (Figure 1A). Planarians have long been used as a model organism in their own right for studying their remarkable powers of regeneration and rejuvenation [26, 27]. The key point of interest is definition of the properties and behavior of neoblasts, the pluripotent stem cell population that empowers regeneration and normal tissue turnover. Neoblasts are the only mitotically active cells in these organisms [28, 29], and by extension neoblast activity drives progression of the parasitic platyhelminths through the various stages of their life cycle [30]. As an experimental model, planarians are easy to maintain in the laboratory as they are free-living and cultures expand rapidly via asexual reproduction (fission). Protocols to culture these free-living organisms are straightforward, contrasting with the logistical complexity of protocols needed to support the schistosome life cycle. The long standing usage of planarians as an experimental model has spurred development of methods and functional genomic techniques (notably RNA interference, RNAi) to interrogate gene expression and function [31, 32], thereby establishing a deep methodological resource for experimentalists working in this system. The basic regenerative assays are robust, simple to execute, and amenable to pharmacological and RNAi screens [33]. Genomic and transcriptomic data are available, and unsurprisingly, recent analyses demonstrate a high degree of gene conservation and protein sequence homology between planarians and schistosomes [34–39].
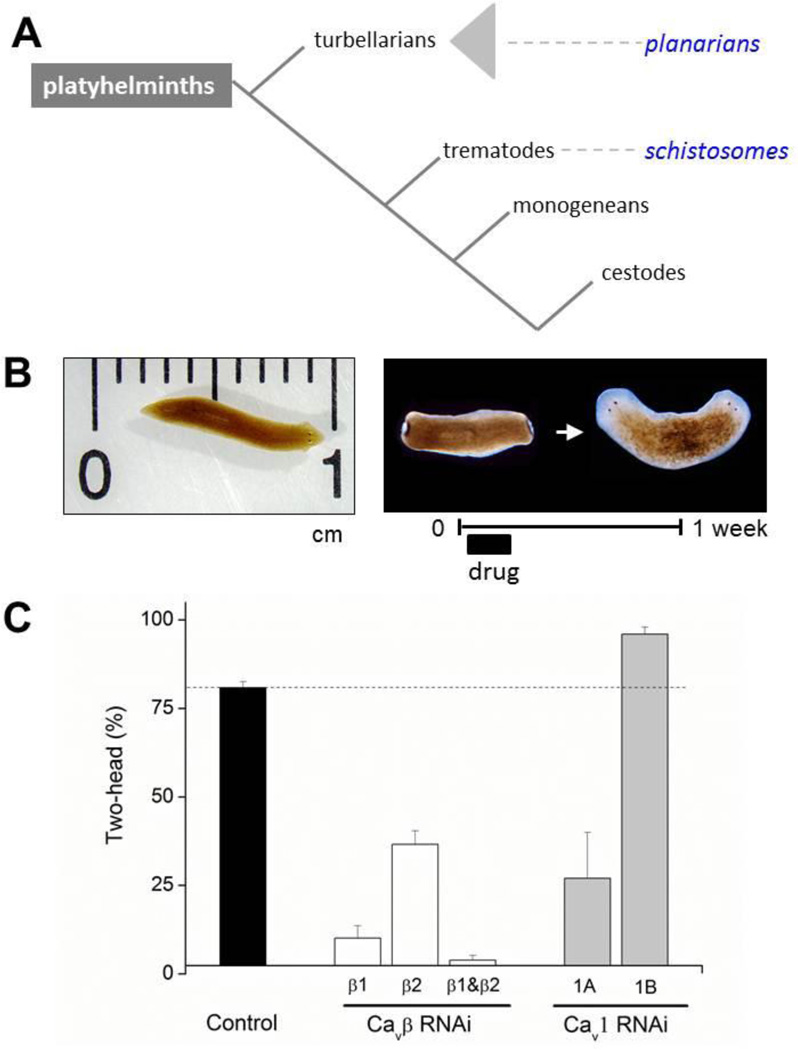
Figure 1. PZQ effects on regeneration of the planarian Dugesia japonica.
(A) Overview of the relationship between free living planarian species to the parasitic platyhelminth groupings. (B) Left, image of intact D. japonica. Right, bipolar trunk fragment regenation after exposure to ±PZQ (70µM, 48hrs). Anterior structures are visually apparent by the presence of the eyespots. This regeneration assay is robust and no polarity defects are observed in the absence of drug (i.e. by surgery alone). (C) Penetrance of PZQ-evoked two-headed regeneration in D. japonica following RNAi knockdown of different Cav complex subunits. Data are summarized from [20, 25].
Here we discuss data concerning the activity of PZQ on free-living planarian flatworms and the potential relevance of these studies for delimiting PZQ targets and effector mechanisms [20, 25]. We believe interrogation of the planarian system can provide new information about the molecular basis of efficacy of existing drugs as well as potentially aid discovery of new agents to mitigate parasitic flatworm infections. That lateral sidesteps between closely related flatworm groupings can provide fresh insight into the workings of this important clinical therapeutic is not in retrospect surprising as we note PZQ was originally introduced as both an anticestodal and antischistocidal agent [40, 41]. Our work on PZQ is but one evidenced example of a theme of using planarians as a ‘parasite model’ to provide information pertinent to the study of schistosomes [42–44].
2. The effects of praziquantel on Ca2+ signaling in parasitic flatworms
First, let us briefly review evidence that supports a link between PZQ and dysregulated Ca2+ homeostasis in parasitic platyhelminths. Early studies on PZQ in schistosomes revealed the drug caused muscle contraction and a sustained paralysis. The PZQ-evoked contraction was dose dependent, rapid (maximum tension < 1 minute) and inhibited by incubation in media with reduced [Ca2+] [45, 46]. Analysis of radioisotopic fluxes revealed the PZQ-evoked muscle contraction was accompanied by a rapid and maintained uptake of 45Ca2+ from external media, an effect that has been widely reproduced [45, 47–49]. Intracellular Ca2+ mobilization was also involved in the sustained contractile response, consistent with a functional relationship between Ca2+ entry and Ca2+-induced Ca2+ release from intracellular Ca2+ stores [46, 50]. Interestingly, certain strains of S. mansoni that show a therapeutic resistance to PZQ treatment also exhibit attenuated responses to PZQ in 45Ca2+ uptake and contractile assays, implying a mechanistic link between these assays and PZQ activity in treating schistosomiasis [49]. PZQ has also been shown to evoke 45Ca2+ uptake in the trematode Opisthorchis viverrini [51], and experiments in cestodes studying the effect of PZQ on Ca2+ flux broadly mirror results reported for schistosomes [52, 53].
In addition to causing paralysis, PZQ disrupts the exterior surface of the parasite. Unlike free living turbellarians, whose surface is covered by a layer of epithelial cells, parasitic platyhelminths possess a unique exterior covering, or tegument, which forms a cellular syncytium and interfaces with the host environment. PZQ results in a rapid (as little as 30 seconds) lesion or ‘blebbing’ of the tegument in many cestodes and trematodes [54–56]. In schistosomes, tegument disruption is also Ca2+ dependent, as PZQ has no effect on the surface of worms incubated in Ca2+ free media [57], and tegument disruption is also attenuated in parasites which show a therapeutic resistance to PZQ [58]. Studies on schistosomes have shown that the Cav1 inhibitor verapamil is also capable of causing tegument lesions, and is lethal in vitro [59]. While at first this seems paradoxical, it is consistent with recent drug screening data (see section 4.2.2) and supports the concept that tegument integrity is highly sensitive to Ca2+ fluxes.
The role of voltage-operated Ca2+ entry in muscle physiology – Cav channels were subsequently shown to mediate neuropeptide and depolarization-evoked contractions in schistomes [60, 61] - suggests a focus on the Cav complex as a possible site of action of PZQ. Such an idea received critical molecular support following the cloning and heterologous expression of schistosome Cavβ subunits [62–64]. The key observation was that co-expression of a specific β subunit (Cavβvar, from either S. mansoni or S. japonicum) with a mammalian Cav2.3 channel resulted in PZQ regulable currents [62]. Specifically peak channel current was increased ~1.5–2-fold in the presence of PZQ, consistent with observations of PZQ activation of Ca2+ entry observed in schistosomes. This property was not exhibited by the other schistosome Cavβ subunit or mammalian Cavβ subunits, unless two serine residues were mutated to mimic residues in Cavβvar [63, 64]. These minimal changes in Cavβ sequence were sufficient to confer PZQ regulation of Cav2.3 currents in Xenopus oocytes [63, 64]. Furthermore, engineering even a single serine replacement into the schistosome Cavβvar was sufficient to abrogate PZQ regulation of Cav currents [63]. Therefore, the importance of this dataset was to reveal that specific, and minimal, changes in Cavβ sequence could impart a PZQ-evoked potentiation of Ca2+ entry currents. These data established a molecular basis for PZQ efficacy at the single amino acid level.
On the basis of this data, the hypothesis was proposed that PZQ acts to disrupt the association Cavβvar and Cavα subunits [62]. The variant serine residues are found in consensus PKC sites in the region of the Cavβ subunit (the β-interaction domain, BID) important for association with the Cavα subunit. Differential phosphorylation would presumably iterate the availability of a PZQ binding site within the broader Cav complex. As expression of the Cavβvar subunit unusually decreased Cav current amplitude, displacement of Cavβvar from Cavα complexes at the cell surface would be expected to relieve this inhibition thereby increasing Ca2+ entry into schistosomes if replicated in situ. This proposal is not in itself unreasonable: several drugs are known to target accessory subunits/modulators of ion channels (discussed in [65]), and the approach of targeting protein-protein interaction interfaces is receiving increasing attention as a therapeutic strategy. Existing examples provide precedent for targeting Cav channels [66–68]. However the key problem for the ‘Cavβ displacement hypothesis’ is simply the lack of supporting structural or biochemical data in the decade since the idea was proposed. Even so, this problem with a single mechanistic hypothesis should not detract from the convincing molecular evidence linking PZQ sensitivity to Cavβ sequence, although further insight is clearly needed.
3. The effects of praziquantel on planarian regeneration
Our research into the biological activity of PZQ in planarians, grew from the serendipitous finding that PZQ miscued the head to tail regenerative polarity of the planarian Dugesia japonica [20]. This result derived from a simple, manual screen in which trunk fragments were excised by amputation of head and tail structures, and incubated in drug-containing solution before the solution was exchanged and the excised fragments left to regenerate for a week (Figure 1B). Surprisingly, we found that PZQ exposure invariably caused regeneration of worms with two heads (‘bipolar’ head), rather than worms with a normal anterior-posterior (AP, head to tail) polarity [20]. In the presence of PZQ, each regenerative blastema yielded head structures (Figure 1B).
Investigation of this unusual property of PZQ revealed that the effect on regenerative polarity was (i) penetrant (at maximal doses of ±PZQ, all regenerating trunk fragments were bipolar); (ii) reflective of a complete duplication of the AP axis, the resulting bipolar worms were viable and able to move and feed; (iii) caused only by an acute exposure to PZQ (a delay of drug exposure after cutting was considerably less effective) and (iv) mimicked by other agents known to impact cellular Ca2+ homeostasis and modulated by media Ca2+ concentration [20]. The opportunity provided by this result was as an unambiguous phenotype that could be used to screen for genes required for mediating this effect in vivo, as the tractability of the planarian system to RNAi at the organismal level allowed interrogation of gene products underpinning the miscued regeneration elicited by PZQ. This is a similar ‘chemical genetic’ logic to that applied in other models to identify drug targets or genes involved in specific biological processes [69, 70]. Therefore, we used the bipolar regenerative phenotype to derive new insight into both the target of PZQ and effectors of this target (e.g. epistatic pathways) in planarians. That two-headed regeneration was phenocopied by known modulators of Ca2+ signaling was especially intriguing, given the previously discussed literature in schistosomes documenting acute effects of PZQ on Ca2+ homeostasis.
3.1 Testing the ‘Ca2+ hypothesis’ of PZQ action by RNAi
Our approach toward functional genetic testing of the ‘Ca2+ hypothesis’ of PZQ action depended on a strategy of stepwise cloning of Cav channel subunits prior to knockdown of these targets by in vivo RNAi. First analyzed were Cavβ subunits: these subunits were more easily cloned than the larger Cavα pore-forming subunits and have merit as target(s) because of their role in supporting Cav channel expression the cell surface [71, 72]. Knockdown of Cavβ subunit provided a route for functional impairment of Ca2+ influx through Cav channels without recourse to a fuller characterization of the pore forming Cavα subunits.
As observed with other platyhelminths studied to date (reviewed in [73]), planarians express two Cavβ subunits, Cavβ1 and the larger Cavβ2 subunit (which lacks the serine residues in the BID domain [20], ~Cavβvar in schistosomes). Both subunits exhibit conservation of Cavβ domains (SH3, HOOK, guanylate kinase-like regions) and residues critical for Cavα interaction that have been well studied in the mammalian Cavβ proteins [72]. In the trunk fragment regeneration assay, knockdown of either of these planarian Cavβ subunits attenuated the ability of PZQ to miscue regeneration (Figure 1C, [20]). Furthermore, in intact worms that were continually exposed to a higher dose of PZQ, individual or combinatorial ablation of the Cavβ subunits conferred resistance to PZQ in lethality assays [20]. Both results suggested that in vivo PZQ efficacy was dependent upon Cavβ function, and by inference the activity of Cav channels.
The next logical step was to characterize the planarian Cavα subunits and ascertain whether similar outcomes occurred following Cavα RNAi. This was important given increasing evidence for roles of Cavβ subunits independent from the core Cav complex [72], and the known promiscuity of ion channel accessory units [65]. Subsequent characterization of these planarian Cavα subunits both confirmed and refined this theme [25]. The ability of PZQ to miscue regeneration could be attenuated by knockdown of a specific HVA Cavα subunit (Cav1A), thereby linking PZQ efficacy to the functionality of a specific Cav complex in vivo. Surprisingly, knockdown of a second Cavα subunit (Cav1B) had the opposite effect, increasing the ability of PZQ to miscue trunk fragment regeneration (Figure 1C, [25]). Therefore, two Cav1 isoforms differentially modulated the ability of PZQ to miscue regeneration, with the effects of PZQ being selectively blocked by Cav1A RNAi. These data suggest that in planarians, PZQ action is mediated via a specific Ca2+ channel complex (Cav1A), although the sensitivity to PZQ can be modulated by the expression levels of other Cav1 isoforms.
The opposing effects of the Cav1 channels seemed inconsistent with a simple model where both channels were directly coupled to muscle depolarization. Indeed examination of the expression patterns of both Cav1A and Cav1B by in situ hybridization revealed a bias toward neuronal expression where Cav1 channels have been shown to function in other systems [74]. The opposing effects of the Cav1 isoforms could then be reconciled to neurons with differing function (excitatory vs inhibitory), or alternatively differential functional coupling within the same cell. An action on the nervous system was also consistent with Ca2+ imaging experiments performed in preperations of dissociated planarian cells, where acute PZQ exposure evoked Ca2+ signals, and the greatest PZQ-evoked 45Ca2+ upotake occurred in a neuronally enriched cell fraction [25]. The effectors of the PZQ-evoked Ca2+ influx were also neuronally derived. PZQ exposure served to decrease transcriptional effects mediated by neuronally derived Hedgehog (Hh) signals [25], which are recently discovered mediators of planarian regenerative outcomes [75, 76]. Consistent with effects of Cav1A RNAi on the bipolar regenerative phenotype, both PZQ-evoked 45Ca2+ uptake in a neuronally enriched cell fraction and PZQ-evoked transcriptional changes in Hh effectors were also attenuated by Cav1A RNAi [25].
So, to summarize, the data studying planarian regeneration are broadly consistent with evidence spanning several decades of research on parasitic platyhelminths, and as such provide in vivo genetic support for the broader ‘Ca2+ hypothesis’ of PZQ action. In both systems, PZQ causes an acute Ca2+ influx, and PZQ efficacy is dependent on Cavβ subunits (despite measuring disparate molecular and organismal level outcomes). Impairment of Cavβ function by mutation, or RNAi, impairs PZQ activity. The planarian data further narrows PZQ efficacy to the expression of a specific Cav1 complex (Cav1A), highlights an action on neuronal signaling and shows that organismal PZQ sensitivity is regulated by Cav channel expression. Obviously, the data do not necessarily assign Cav1A as the direct target for PZQ action as RNAi effects could simply result from epistatic interactions. Such a conclusion could be drawn only following heterologous expression analyses and demonstration of a gain of function of a novel PZQ-evoked Ca2+ influx. The planarian dataset also reminds us that a broader interest in flatworm developmental signaling is not outlandish (PZQ-evoked changes in Hedgehog and Wnt signaling, [25]) given the morphological transitions that are the sine qua non for a parasitic lifecycle.
New contrasts also emerge. For example, both Cavβ subunits regulate PZQ activity in planarians, not just Cavβvar. Knockdown of either planarian Cavβ subunit ablated PZQ-evoked bipolarity [20], suggesting that rather than differences between individual Cavβ subunits being important, it is the differences relating to the in vivo pairings of Cavβ subunits with specific Cavα complexes that is paramount for generating PZQ-sensitive Ca2+ currents. Such an explanation would be consistent with reports of PZQ modulation of Ca2+ entry in organisms lacking Cavβvar [77, 78], and refocus attention on resolving the properties of the pore-forming Cavα complexes.
4. What do we know about the properties of platyhelminth Cavα subunits?
4.1 Cav diversity
Platyhelminths possess a surprisingly diverse repertoire of Cavα subunits. Whereas well characterized invertebrate model systems possess only single representatives of each of the three, conventional Cav channel families (Cav1, Cav2 & Cav3), bioinformatic mining of sequenced platyhelminth genomes reveals the existence of a broader portfolio of Cavα subunits (Table 1). Notably, analysis of the genomes of the three principle species of schistosomes (S. mansoni [79], S. japonicum [80] and S. haematobium [81]) reveals that each species possesses four Cav subunits, comprising two representatives from each of Cav1 and Cav2 classes (Table 1). Best characterized of these are the Cavs from Schistosoma mansoni : SmCav1A (originally named Sm.Cav1), Sm.Cav1B, Sm.Cav2A and Sm.Cav2B, which were first identified over a decade ago [82]. While others have suggested the existence of additional, schistosome Cav subunits from in silico prediction [83], these variants likely represent other classes of four-repeat channels entirely (XP_002575006, a NALNC-like channel [84]) or conform to these known Cav subunits (XP_002571932, Cav2B [83]).
Table 1.
Diversity of Lophotrochozoan Cav channels.
| Cav1 | Cav2 | Cav3 | ||||||
|---|---|---|---|---|---|---|---|---|
| ↓ | ↓ | ↓ | ||||||
| Caenorhabditis elegans | • | • | • | |||||
| Drosophila melanogaster | • | • | • | |||||
| LOPHOTROCHOZOANS | ||||||||
| Capitella capitata | • | • | • | |||||
| Lottia gigantea | • | • | • | |||||
| Lymnaea stagnalis | • | • | • | |||||
| Platyhelminths | ||||||||
| Turbellarians | Dugesia japonica | • | • | • | • | • | ||
| Schmidtea mediterranea | • | • | • | • | • | |||
| Trematodes | Schistosoma mansoni | • | • | • | • | |||
| Schistosoma japonicum | • | • | • | • | ||||
| Schistosoma haematobium | • | • | • | • | ||||
| Clonorchis sinensis | • | • | • | • | ||||
| Cestodes | Taenia solium | • | • | • | • | |||
| Echinococcus multilocularis | • | • | • | • | ||||
| Hymenolepis microstoma | • | • | • | • | ||||
| ↑ | ↑ | ↑ | ↑ | ↑ | ||||
| Cav1A | Cav1B | Cav2A | Cav2B | Cav3 |
Cav channel diversity was examined in classic invertebrate model systems, as well as available lophotrochozoan sequence data. Dots indicate the presence of Cav channels assigned by homology to indicated Cav subunits. Notable outcomes are Cav1 and Cav2 duplication in platyhelminths compared with other lophotrochozoans and the absence of Cav3 channels in parasitic platyhelminths. Sequence identifiers are from NCBI unless otherwise noted: C. elegans (NP_741442.1, NP_741734.1, NP_741848.1), D. melanogaster (Q24270.2, P91645.3, NP_001245544.1), C. capitata (Capca1_51954, Capca1_51958, Capca1_89566)JGI, L. gigantea (Lotgi1_51270, Lotgi1_216445 & Lotgi1_119993, Lotgi1_91235)JGI, L. stagnalis (AAO83838.2, AAO83841.1, AAO83843.2), D. japonica (AEJ87267, AEJ87268, AEJ87269, AEJ87270, AEJ87271), S. mansoni (Smp_020270, Smp_159990,Smp_020170, Smp_004730)GeneDB, S. japonicum (Sjp_0099010, Sjp_0010120 and Sjp_0010110, Sjp_0005280 and Sjp_0073860, Sjp_0016770, Sjp_0096680), S. haematobium (Sha_105781, Sha_200459, Sha_105898, Sha_101457 and Sha_107907)schistodb.net, C. sinensis (GAA55733.1, GAA30063.2, GAA52227.1, GAA56330.1), T. solium (TsM_000783400 and TsM_000783500, TsM_000442900, TsM_000598200, TsM_000175700), Echinococcus multilocularis (EmuJ_000143800.1, EmuJ_000961000.1, EmuJ_000146300.1, EmuJ_000890600.1)GeneDB, Hymenolepis microstoma (HmN_000242200, HmN_000186400, HmN_000427400 and HmN_000427500, HmN_000387900)GeneDB. No identifiers tags are listed for S. mediterranea given the current nature of the genome assembly.
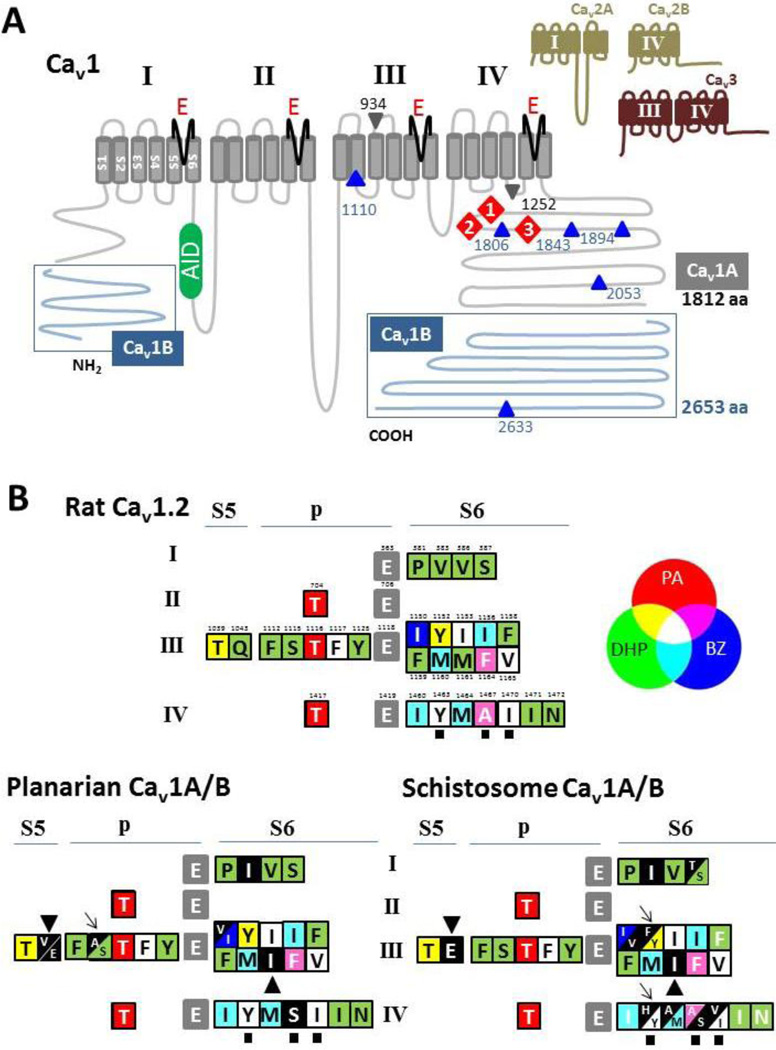
Similarly in planarians, RT.PCR based cDNA cloning identified four high-voltage activated (HVA) subunits in D. japonica, (Dj-Cav1A and Cav1B; Dj-Cav2A and Dj-Cav2B, [25]) that were named as an extension of the original S. mansoni nomenclature (Table 1). These planarian Cav1 channels show ~60% amino acid identity to their schistosome counterparts [25]. Both platyhelminth families conform to the four domain Cav structure with long cytoplasmic loops connecting the first three domains (I and II, II and III) and a shorter loop connecting domains III and IV (Figure 2A). Cav1B is the larger family member in both planarians (longest splice variant, 2689 amino acids) and schistosomes (predicted up to 2570 amino acids), a size approaching the upper range of Cavα subunits characterized to date [85]. Overall identity with mammalian Cav1 proteins is lower (<50%), notwithstanding a clear conversation of this architecture as well as key channel regulatory motifs (Figure 2A). These include: (i) residues important ion permeation and selectivity - notably, the selectivity filter glutamate residues (‘EEEE’ motif), as well as the outer pore tryptophan ring and conserved aspartate residue downstream from the domain II selectivity filter glutamate suggested to be involved in delivering a Ca2+ ion to the selectivity filter [86]; (ii) COOH terminal motifs (EF, PreIQ3, IQ) and transmembrane residues involved in Cav channel regulation/inactivation [87, 88]; and (iii) the region in the domain I–II linker (the alpha-interaction domain, AID), that mediates association with Cavβ subunits.
Figure 2. Molecular architecture of planarian Cav channels.
(A) Schematic representation of planarian Cav1 isoforms (Cav1A & Cav1B). While Cav1B has longer NH2 and COOH terminal regions (blue) than Cav1A, both isoforms share a similar topology (domains I–IV with six transmembrane helices, S1–S6) and contain similar motifs. These include the ‘EEEE’ selectivity filter motif (red), the alpha-interacting domain (AID) in the I–II linker (green) and the cytoplasmic COOH-terminal regions (1, EF; 2, preIQ3; 3, IQ, red diamonds) known to be important for Ca2+ regulation of mammalian Cav1 isoforms. Splice isoforms of both Cav1A (2 variants, grey triangles) and Cav1B (6 variants, blue triangles) have been identified at the indicated residues in Cav1A and Cav1B respectively. Inset, schematic depicting characterized regions of the partially cloned planarian Cavs (Cav2A/2B & Cav3). (B) Overview of residues important for binding Cav ligands and their variation in platyhelminth Cavs. Residues identified in experiments as being important for dihydropyridine (DHP), phenylalkylamine (PA) and benzothiazepine (BZ) binding (summarized in [97, 106–108]) are depicted schematically in terms of their localization (domains I to IV, transmembrane regions S5 & S6, and intervening ‘p’ loop) in rat (numbering of rat Cav1.2, Genbank M67515.1), planarian and schistosome Cav channels. Colouring indicates importance of residue for binding a particular class of ligand, as per additive colour map. For example, if a residue is important for binding all three classes of ligand it is shown in white. Selectivity filter glutamates are shown in grey. Residues that show variation in the platyhelminth Cavs are shown in black, and variation that may be critical for ligand binding is highlighted by different symbols (discussed in text). The ‘YAI’ triad in domain IVS6 is identified by solid squares.
The planarian complement of Cav channels holds further diversity compared with schistosomes owing to the presence of an additional low volateg activated (LVA, Cav3) isoform (Table 1). All five isoforms are also present in Schmidtea mediterranea genome [36] and evidenced at the transcript level by de novo transcriptome sequencing [89]. Although Cav3 was verified only as a partial clone in D. japonica [25], the protein exhibits highest homology to Cav3 channel sequences from other invertebrates, and notably contains aspartate residues in the domain III and IV P loops conforming to the EEDD-type selectivity filter motif diagnostic of LVA channels (compared with the EEEE motif harbored in all HVA Cav channels). These aspartate residues are key features of LVA channels that impart divergent permeation and activation properties compared with HVA channels [90]. Bioinformatic prediction of further sequence of planarian Cav3 (from the S. mediterranea genome) reveal conservation of the selectivity filter glutamate residue together with the highly conserved adjacent aspartate residue in domain II and the glutamate residue in the P-loop of domain I. Therefore, free living planarians possess a fifth Cavα subunit (Cav3) not found in the genomes of parasitic platyhelminths (Table 1).
Profiling available sequencing projects confirms platyhelminths are endowed with unexpected diversity in Cavα subunits compared to ‘classic’ invertebrate model systems (Table 1). This diversity holds clear ramifications for understanding organismal physiology. The lineage specific duplication of HVA channels, absent in other lophotrochozoans is clearly independent from the vertebrate Cav radiation and presents opportunity for unique neofunctionalization of these genes in platyhelminth physiology. This may be particularly important given the lack of Cav3 channels in parasitic platyhelminths and the additional absence of Nav channels in schistosomes [79]. Schistosomes therefore possess a smaller complement of voltage-gated cation influx channels for supporting excitable tissue physiology that exists in the free-living turbellarians. This narrow repertoire of voltage-gated channels, perhaps reflecting the massive gene loss associated with the evolution of parasitism [35, 91], provides opportunity for chemotherapeutic attack and highlights the need for understanding the molecular basis of neuronal and tissue excitability in these pathogens that is clearly divergent from their hosts.
Finally, it is well known that mammalian Cav subunits increase their diversity through alternative splicing and this molecular diversity can impart different functionalities [92–94]. For example, 40 splice variants at a dozen loci have been identified within the human Cav1.2 subunit [95] and specific variants show altered pharmacological, regulatory or electrophysiological signatures [93, 95]. We have identified, but not characterized, splice variants of both Cav1A and Cav1B in planarians (Figure 2A), and suggest the functional repertoire of Cav activity in platyhelminths is likely further expanded by heterogeneity introduced by alternative splicing. In summary, the presence of multiple Cavα genes and associated variants in platyhelminths provides molecular substrate for customization and fine-tuning of responses in the excitable tissues of these organisms.
4.2 Cav properties
What do we know about the molecular pharmacology of platyhelminth Cav channels? The answer is currently little. Practical difficulties in heterologously expressing Cav clones have precluded direct functional insight into the pharmacological profile of these channels. Interpretation of whole animal responses to known Cav modulators is also risky – as the lack of responsiveness to agents established to target mammalian Cavs may simply result from pharmacokinetic considerations (e.g. failure of drug accumulation, xenobiotic defenses) rather than a divergent molecular pharmacology of the channel itself. For example, in C. elegans, nifedipine lacks efficacy against intact worms but is able to antagonize the nematode Cav1 channel (EGL-19) in dissociated specimens [96–98]. Most of our knowledge about flatworm Cav pharmacology therefore depends on three approaches – (i) recordings of endogenous currents and contractile responses in dissociated preparations, (ii) hits to known Cav modulators in pharmacological screens for anithelminthics, and (iii) bioinformatic prediction of pharmacological properties from cloned flatworm Cav sequences. We will briefly discuss these approaches in turn.
4.2.1 Endogenous Cav responses
Cav channel activity can be resolved by studying excitable cells in flatworm musculature and nervous systems. However, electrophysiological recording has not proved trivial. Endogenous currents are generally small (e.g. Imax <100pA in S. mansoni muscle fibres [60, 61] and ~200pA in Dugesia muscle fibres [99]) and prone to rapid rundown within several minutes. Therefore most data derives from studying the contractile response of intact organisms [45, 48, 50] or dissociated muscle fibres [60, 61, 99]. Contractions of isolated S. mansoni, D. japonica and Bdelloura candida muscle fibres require extracellular Ca2+ and can be inhibited by blocking Cav function with known Cav modulators [60, 61]. The major classes of Cav1 ligands are the dihydropyridines (DHPs; antagonists and agonists, e.g. nifedipine and S-(−)-Bay K8644), benzothiazepines (BZs; antagonists, e.g. diltiazem) and phenylalkylamines (PAs; antagonists, e.g. verapamil). Both depolarization and neuropeptide-evoked contractions are inhibited by nicarpidine, as well as verapamil and methoxyverapamil at higher concentrations [60, 61]. Other DHPs, diltiazem, or peptide neurotoxins, fail to inhibit contractile responses [60, 61]. Consistent with this profile, recent data has shown the voltage-operated Ca2+ currents in muscle are reversibly inhibited by verapamil (DHP derivatives were not tested [29]). Intriguingly, this general pharmacological signature parallels the drug profile from the planarian regeneration assay (nicardipine > verapamil, other Cav1 modulators without effect [20]). Less is known about Cav currents in the nervous system: although dissociated neurons from an ectoparasitic turbellarian (B. candida) displayed depolarization-evoked Ca2+ currents that were attenuated by verapamil (~30% decrease, [100]). Therefore, our knowledge about endogenous Cav currents remains quite limited. Key data that is lacking in schistosomes is the resolution of an endogenous PZQ-evoked Ca2+ signal in muscle fibres.
4.2.2 Drug Screens
While interest in flatworm Cav channels has predominantly related to their status as a candidate target for PZQ (Section 3), this relationship underscores the potential of Cavs as antihelminthic targets in their own right. Cav1 modulators have emerged as preliminary ‘hits’ in recent unbiased screens (<2,000 compounds) aimed at discovering new antischistosomals. Verapamil passed an initial phenotypic typing (<3% compounds) against schistosomula but not subsequent screening [101]. Methoxyverapamil and felodipine were also identified as compounds (2 from 30) that delayed miracidial transformation [102]. Nicardipine is also known to be lethal to miracidia [103]. Given the inherent snapshot nature of such unbiased screens (often building from a single compound dosage at a single life cycle stage), it is best to draw only general conclusions. Notably, the pharmacological theme from the tissue assays (section 4.2.1) is extended - BZs and common DHP blockers seem poorly represented, while PAs possess some effectiveness.
4.2.3 Predicted pharmacology of Platyhelminth Cavs
In the absence of a large experimental dataset, what can sequence analysis alone tell us about the pharmacological signature of the platyhelminths Cavs? Sequence comparison between Cav channels with different pharmacological profiles has guided mutagenesis to reveal mutants with impaired or de novo drug sensitivities and thereby identify key residues for ligand binding. Worth bearing in mind is the planarian data showing opposing activities of Cav1A and Cav1B on PZQ efficacy in vivo [25]: agonists at Cav1A would be desired to phenocopy PZQ activity, whereas antagonists of Cav1B should sensitize organisms to PZQ. Therefore, a structural basis for loss of agonist efficacy at Cav1A, and impairment of antagonist potency at Cav1B may help explain why agents widely used to modulate vertebrate Cavs fail to act as schistocidal agents. Given existing Cav1 blockers are widely used cardiovascular therapeutics, this discussion is not meant to infer clinical utility, simply to illuminate why conventional Cav ligands fail to support the ‘Ca2+ hypothesis’ of PZQ action. For example, this analysis may illuminate why PZQ activity, mediated by Cav1A activation, cannot be blocked by conventional Cav1 antagonists or why antagonists at Cav1B are in themselves toxic (note results from Cav blockers in drug screens, section 4.2.2). Auguring from in silico analysis alone is obviously hazardous: often the effects of single amino acid variation can be counterintuitive when analyzed in isolation, and the lack of biophysical characterization of the platyhelminth channels is a problem when drug affinity is closely linked to channel state (e.g. dihydropyridine block is dependent on the voltage sensitivity of mammalian L-type Cavs). Nevertheless, the large body of experimental and modeling studies [104–110] addressing the structural basis of ligand sensing at Cav channels (including studies in invertebrates [97, 111–113]), permits worthwhile speculation on the pharmacological profile of the flatworm Cavs.
All three major classes of Cav ligands (DHPs BZS & PAs) share interactions with critical ligand-sensing residues [97, 105–110], as well as proposed direct interactions with a Ca2+ ion coordinated within the selectivity filer [106–108]. Many of the residues known to interact with these drugs are conserved in the platyhelminth Cavs (for example, 24/28, 12/14 and 13/14 residues experimentally evidenced to regulate DHP, BZ and PA binding in mammalian Cav1.2 channels are identical in the planarian Cav1A channel). However, such analysis is too crude to predict drug effectiveness as single amino acid differences are sufficient to abrogate DHP agonism and antagonism [104]. More detailed scrutinization is required.
First, consider DHP binding. Two groups of residues are important for DHP binding to Cav1 channels – residues that comprise the actual DHP-binding pocket and residues that allosterically impact DHP-binding [96, 114]. Comparison of these residues between rat (Cav1.2), planarian (Dj-Cav1A & Dj-Cav1B) and Schistosoma mansoni (Sm.Cav1A & Sm.Cav1B) Cav clones (Figure 2B) reveals the extent of sequence variation. Some of these changes are conservative and/or exist in the C. elegans channel sequence, which retains sensitivity to many DHPs in vitro [96], so they are likely not key determinants of DHP action. However, other common and unique variants merit discussion. First, are two amino acids in domain III that are variant in both the planarian and schistosome Cav1 channels (Q1043 & M1161 in rat Cav1.2). The M1161 substitution (M to I in the platyhelminth Cav1 channels) was identified as a polymorphism in a C. elegans egl-19 mutant (~Cav1) that conferred resistance to nemadipine-evoked growth defects [97]. This feature may in itself confer low sensitivity to DHP blockade to the platyhelminth Cav1 channels [25]. The M1161 residue in domain IIIS6 is thought to contribute to a hydrophobic pocket which interacts with the ‘portside’ methyl group of DHP ligands [107], and mutagenesis of this residue (to alanine) has been shown to decrease DHP binding affinity (~10-fold [109]) and blockade (~100-fold [97]) in Cav1.2 channels. Second, the highly conserved glutamine in IIIS5 (Q1043 in rat Cav1.2) shows variation (Q/E, or Q/V in Dj-Cav1A). This residue is also unique to DHP modulation (not required for PA or BK binding), and is thought to hydrogen bond with one of the carbonyl oxygen of DHP ligands [107]. Restoration of this residue to glutamine can have variable effects, with evidence for increased, decreased or no effect on DHP blockade depending on the context of the mutation (in isolation, or in concert with other changes [111]) and the channel backbone [115]. Variation at this residue may also impact the potency of DHP agonists (see [111]). The Q/E mutant when introduced into a rat Cav backbone significantly reduced potentiation by (S)-(−)-Bay K8644 suggesting the complete amide side chain of the glutamine residue was needed for full agonist efficacy [115]. Both schistosome Cav1 variants have an asparagine at this location, and this variation may contribute to the lack of potency of (S)-(−)-Bay K8644 in phenocopying PZQ as a schistocidal agent.
Beyond examples of variation common to all platyhelminth Cav1 proteins, Sm.Cav1A is also notable in showing poor conservation of other residues known to be important for DHP binding (only 20/28 identical residues). The additional variation encompasses dual tyrosine residues in the IIIS6 (Y1152 in Cav1.2 vs F1062 in Sm.Cav1A) and IVS6 transmembrane domains (Y1463 vs H1363) which are also considered hydrogen bonding partners with DHP ligands. Indeed, coupled with variation at Q1043 discussed above, it appears Sm.Cav1A shows non-identity across the triad of residues suggested critical for DHP coordination [107]. The variation of S1115 in planarian Cav1A (represented by alanine) also merits comment. S1115 is located three residues proximal to the selectivity filter glutamate residue in the domain III P loop. The S1115A mutational change in rat Cav1.2 reduced the affinity for DHP antagonists (~60-fold reduction in nitrendipine blockade), and also abrogated responsiveness to the DHP agonist S-(−)-Bay K8644 [113]. This alanine is also present in other invertebrate Cav channels that exhibit a blunted pharmacological responsiveness to DHPs [112, 116]. Reverse engineering of the same mutation (A to S) restored sensitivity to nitrendipine and S-(−)-Bay K8644 [112].
Therefore, sequence prospecting provides ample reason to explain the low sensitivity of platyhelminth Cavs to conventional DHP blockade. The DHP that is most effective at retaining antagonistic effects against parasitic [60, 61] and free-living [20] flatworms Cav channels is nicardipine. Is there a structural basis for this observation? Nicardipine possess an ionizable alkylamino group on the 5-position of the pyridine ring and recent studies have suggested that this bulky substituent may interact with unique residues beyond the conventional DHP binding site [117], providing a possible explanation as to why nicardipine can target platyhelminth Cav channels. One recent study brought focus on the role of a residue in IIS6 (A752), which is conserved in the all schistosome and planarian Cav1 channels [114]. Nicardipine therefore may present a structural framework for iteration of DHP-based ligands to identify novel antagonists of platyhelminth Cavs.
What about blockade by the other classes of Cav1 antagonists? Again the majority of residues experimentally implicated in PA and BZ binding appear conserved in the platyhelminth channels (up to 13/14 key residues examined for each class). Again the devil is in the detail, and one possible explanation for the lower sensitivity of these agents is the occurrence of a serine substitution of an important alanine residue in the platyhelminth Cav1B variants (and Dj-Cav1A). Studies of mammalian Cav1 channels, have implicated a triad of residues within the IVS6 transmembrane region (‘YAI triad’) as necessary for high affinity phenylalkylamine blockade through interactions with the amino group proximal methoxylated aromatic ring of many PAs [106, 118]. The alanine to serine substitution in the platyhelminth Cav1 channels correlates with a substitution (A1467S in rat Cav1.2) that has been shown to decrease the affinity of desmethoxyverapamil block by ~11-fold [118]. The importance of the YAI triad also holds for BZ binding, as these residues comprise part of the shared binding site for the two different classes of ligand [119]. Although this alanine residue is conserved in Sm.Cav1A, this particular Cav variant is predicted to harbour substitutions of the two other residues in the critical ‘YAI’ triad (‘HAV’ in Sm.Cav1A, resulting from a Y1463H and a conservative I1470V substitution) as well as another critical tyrosine residue for PA binding in IIIS6. This tyrosine residue in IVS6 (Y1463 in rat Cav1.2 numbering), and the second tyrosine residue in IIIS6 (Y1152 in rat Cav1.2 numbering) are both thought to form hydrogen bonds with the methoxy groups of the two PA aromatic rings [106] are both represented by different residues (Y/F and Y/H) in Sm.Cav1A. These changes would be predicted to decrease PA sensitivity of Sm.Cav1A on the basis of prior mutagenesis studies: the Y1152F substitution in rat Cav1.2 caused an increase (~18-fold) in the concentration of desmethoxyverapamil needed for current blockade [110], and a double mutant (Y1152F, Y1463F) was reported to decrease the potency of desmethoxyverapamil blockade by 100-fold [110]. The Y1463 residue in IVS6 is also important for regulating the potency of diltiazem blockade [120]. Two further residues that regulate BZ (but not PA) block also show variation between the platyhelminth Cavs. First, Sm.Cav1A contains an alanine in domain IVS6 (A1364 in Sm.Cav1A) which is represented by methionine in the other platyhelminth Cavs (and vertebrate Cav1.2). The corresponding mutation M1464A has been shown to decrease sensitivity to diltiazem by ~3-fold [120]. Restoration of this methionine in an invertebrate Cav1 also increased the potency of isradipine (a DHP) blockade [111]. Second, Sm.Cav1B (and Dj-Cav1A) contain a conservative substitution in IIIS6 at position 1150 (I1150V in Cav1.2 numbering): the I1150A mutation has also been shown to decreases BZ sensitivity by ~3-fold [120].
Collectively, these observations suggest a molecular basis for the poor susceptibility of the platyhelminth Cav channels to blockade by the Cav1 blockers widely used in mammalian systems. This divergence explains why conventional Cav1 blockers have provided only equivocal support for the hypothesis that PZQ stimulates L-type Cav channels, and caution against the lack of such evidence being used to argue against an activity of PZQ against schistosome Cavs. Overall, while the divergent pharmacological profiles of the platyhelminth and mammalian Cav1 channels is frustrating for impeding molecular dissection of the action of PZQ and understanding Cav1 physiology in flatworms, there is a silver lining; the realization that divergent pharmacology may ultimately augur selectivity in the activity of novel ligands targeting flatworm Cavs as novel drug leads for treating schistosomiasis.
5. Refractoriness to PZQ action
Molecular insight into the targets/effectors of PZQ action and adaptatory mechanisms will likely come from analysis of situations where PZQ efficacy is decreased. These include de novo mechanisms that emerge in individual strains (‘drug resistance’), natural variation in PZQ potency during the schistosome life cycle [22–24], as well as comparative phylogenetic analysis probing PZQ effectiveness. Some brief comments on these scenarios in the context of the ‘Ca2+ hypothesis’ of PZQ action are worthwhile. While PZQ activity is Cav1 dependant when measuring acute responses (Ca2+ influx, contraction) in cultured flatworms, it is obvious that the anhelminthic effect of PZQ applied to an in vivo infection involves a broader array of influences. These encompass the host immune system [121, 122], as well as the mechanisms controlling PZQ pharmacokinetics [123] and the downstream effectors of Ca2+ influx in the parasite itself. Therefore, it is not difficult to envisage how changes in this broader interactome could impact PZQ efficacy independently from any alteration in target receptor(s) for PZQ. Therefore, in the context of PZQ resistance, a failure to detect changes in specific Cav channel components [124, 125], or a coupling of the Cav complex to effector mechanisms [47] should not detract from the ‘Ca2+ hypothesis’ of PZQ action. PZQ sensitivity could be decreased by changes in pathways both upstream (drug handling) and downstream components (effectors) independent from alterations in the primary drug target, and unraveling such changes is important for identifying novel druggable targets. Identification of such targets will come from unbiased examinations of gene expression in scenarios of natural and acquired refractoriness to PZQ. Indeed, recent microarray profiling studies provide the first chapter of research exploiting such methods [125–127]. Such data are generally supportive of an adaptive organismal Ca2+ toolkit as part of the broader transcriptional adaption to PZQ exposure. Equally, for the free-living planarians it will be important to understand species specific differences in PZQ efficacy. The PZQ-evoked bipolar regeneration of D. japonica, which is a frequently used strain to study drug responsiveness, is not replicated in other planarian species and the molecular basis for this difference is unexplored.
5. Conclusions
The purpose of this review was to discuss the action of PZQ on planarian flatworms and bring new perspective onto in vivo targets relevant to the efficacy of this important therapeutic. The planarian data suggest a focus on specific Cav1 complexes coupling to neuronally derived signaling pathways. Therefore, our data studying the effects of PZQ on planarian regeneration are broadly supportive of the ‘Ca2+ hypothesis’ of PZQ action despite studying a unique organismal output (tissue regeneration) in an amenable model system not widely exploited for antischistosomal drug research. This review has highlighted the divergent molecular pharmacology of platyhelminth Cav channels from their human counterparts, a principle likely shared by downstream Cav effectors and a property that could be exploited by directed drug design in future. Obviously, a variety of different targets for PZQ have been proposed, and our intent was not to discriminate amongst these viable ideas beyond illustrating common principles of PZQ action on Ca2+ signaling in the different flatworm systems. PZQ may have more than one target in vivo, a useful promiscuity that could delay the emergence of drug resistance. Argueably more important than target definition per se is the realization that Cav channels and their downstream effectors represent novel targets for novel antihelminthic drugs.
Highlights.
The antischistosomal action of PZQ may derive from dysregulated Ca2+ homeostasis
New molecular insight supporting this model comes from the planarian model system
PZQ causes Ca2+ influx in a neuronally derived population via a specific Cav complex
The utility of the planarian model for antischistosomal drug research is discussed
Acknowledgements
Work in the laboratory was supported by the NSF (MCB0919933to JSM) and NIH (GM088790 to JSM). JDC was supported by a Stem Cell Biology Training Grant studentship (T32HD060536).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. cited; Available from: http://www.thiswormyworld.org/ [Google Scholar]
- 2.Martin RJ, Robertson AP, Bjorn H. Target sites of anthelmintics. Parasitology. 1997;114(Suppl):S111–S124. [PubMed] [Google Scholar]
- 3.Hu Y, Xiao SH, Aroian RV. The new anthelmintic tribendimidine is an L-type (levamisole and pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl Trop Dis. 2009;3(8):e499. doi: 10.1371/journal.pntd.0000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DD, Siddiqui SZ, Kaji MD, Forrester SG. Pharmacological characterization of the Haemonchus contortus GABA-gated chloride channel, Hco-UNC-49: modulation by macrocyclic lactone anthelmintics and a receptor for piperazine. Vet Parasitol. 2012;185(2–4):201–209. doi: 10.1016/j.vetpar.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, et al. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371(6499):707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 6.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Boulware MJ, Marchant JS. Timing in cellular Ca2+ signalling. Curr Biol. 2008;18(17):R769–R776. doi: 10.1016/j.cub.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caffrey CR. Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol. 2007;11:433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3(9):e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136(13):1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 11.Hotez PJ, Engels D, Fenwick A, Savioli L. Africa is desperate for praziquantel. Lancet. 2010;376(9740):496–498. doi: 10.1016/S0140-6736(10)60879-3. [DOI] [PubMed] [Google Scholar]
- 12.Doenhoff M, Pica-Mattoccia L. Praziquantel for the treatment of schistisomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti Infect Ther. 2006;4(2):199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- 13.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic illness. 2008;4(1):65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 14.Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg. 1995;53:61–62. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 15.Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, et al. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Negl Trop Dis. 2009;3(8):e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster JP, Gower CM, Norton AJ. Evolutionary concepts in predicting and evaluating the impact of mass chemotherapy schistosomiasis control programmes on parasites and their hosts. Evol Appl. 2008;1(1):66–83. doi: 10.1111/j.1752-4571.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ismail M, Bortos S, Metwally A, William S, Farghally A, Tao LF, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 18.Sadhu PS, Kumar SN, Chandrasekharam M, Pica-Mattoccia L, Cioli D, Rao VJ. Synthesis of new praziquantel analogues: potential candidates for the treatment of schistosomiasis. Bioorg Med Chem Lett. 2012;22(2):1103–1106. doi: 10.1016/j.bmcl.2011.11.108. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, William S, Herdtweck E, Botros S, Domling A. MCR synthesis of praziquantel derivatives. Chemical biology & drug design. 2012;79(4):470–477. doi: 10.1111/j.1747-0285.2011.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogi T, Zhang D, Chan JD, Marchant JS. A Novel Biological Activity of Praziquantel Requiring Voltage-Operated Ca2+ Channel β subunits: Subversion of Flatworm Regenerative Polarity. PLoS NTD. 2009;3(6):e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews P, Thomas H, Pohlke R, Seubert J. Praziquantel. Med Res Rev. 1983;3(2):147–200. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- 22.Shaw MK. Schistosoma mansoni: stage-dependent damage after in vivo treatment with praziquantel. Parasitology. 1990;100(Pt 1):65–72. doi: 10.1017/s0031182000060121. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Wang W, Huang YX. New insight into praziquantel against various developmental stages of schistosomes. Parasitol Res. 2011;109(6):1501–1507. doi: 10.1007/s00436-011-2670-3. [DOI] [PubMed] [Google Scholar]
- 24.Pica-Mattoccia L, Cioli D. Sex- and age-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Chan JD, Nogi T, Marchant JS. Opposing roles of voltage-gated Ca2+ channels in neuronal control of stem cell differentiation in vivo. J Neurosci. 2011;31(44):15983–15995. doi: 10.1523/JNEUROSCI.3029-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newmark PA, Sanchez-Alvarado A. Not your father's planarian: a classic model enters the era of functional genomics. Nature Reviews Genetics. 2002;3:210–219. doi: 10.1038/nrg759. [DOI] [PubMed] [Google Scholar]
- 27.Forsthoefel DJ, Newmark PA. Emerging patterns in planarian regeneration. Curr Opin Genet Dev. 2009;19(4):412–420. doi: 10.1016/j.gde.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baguna J. The planarian neoblast: the rambling history of its origin and some current black boxes. Int J Dev Biol. 2012;56(1–3):19–37. doi: 10.1387/ijdb.113463jb. [DOI] [PubMed] [Google Scholar]
- 29.Wagner DE, Wang IE, Reddien PW. Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science. 2011;332(6031):811–816. doi: 10.1126/science.1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brehm K. Echinococcus multilocularis as an experimental model in stem cell research and molecular host-parasite interaction. Parasitology. 2010;137(3):537–555. doi: 10.1017/S0031182009991727. [DOI] [PubMed] [Google Scholar]
- 31.Reddien PW, Bermange AL, Murfitt KJ, Jennings JR, Sánchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Developmental Cell. 2005;8:635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newmark PA, Reddien PW, Cebria F, Sanchez Alvarado A. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc Natl Acad Sci. 2003;100:11861–11865. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan JD, Marchant JS. Pharmacological and functional genetic assays to manipulate regeneration of the planarian Dugesia japonica. Journal of Visualized Experiments. 2011;(54):pii, 3038. doi: 10.3791/3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantarel BL, Korf I, Robb SM, Parra G, Ross E, Moore B, et al. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008;18(1):188–196. doi: 10.1101/gr.6743907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura O, Hirao Y, Tarui H, Agata K. Comparative transcriptome analysis between planarian Dugesia japonica and other platyhelminth species. BMC genomics. 2012;13(1):289. doi: 10.1186/1471-2164-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robb SMC, Ross E, Sanchez Alvarado A. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:D599–D606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandmann T, Vogg MC, Owlarn S, Boutros M, Bartscherer K. The head-regeneration transcriptome of the planarian Schmidtea mediterranea. Genome Biol. 2011;12(8):R76. doi: 10.1186/gb-2011-12-8-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abril JF, Cebria F, Rodriguez-Esteban G, Horn T, Fraguas S, Calvo B, et al. Smed454 dataset: unravelling the transcriptome of Schmidtea mediterranea. BMC genomics. 2010;11:731. doi: 10.1186/1471-2164-11-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blythe MJ, Kao D, Malla S, Rowsell J, Wilson R, Evans D, et al. A dual platform approach to transcript discovery for the planarian Schmidtea mediterranea to establish RNAseq for stem cell and regeneration biology. PLoS ONE. 2010;5(12):e15617. doi: 10.1371/journal.pone.0015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas H, Gönnert R. The efficacy of praziquantel against cestodes in animals. Z Parasitenkd. 1977;52:117–127. doi: 10.1007/BF00389898. [DOI] [PubMed] [Google Scholar]
- 41.Thomas H, Andrews P. Praziquantel - New Cestocide. Pestic Sci. 1977;8(5):556–560. [Google Scholar]
- 42.Collins JJ, Hou X, Romanova EV, Lambrus BG, Miller CM, Saberi A, et al. Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biology. 2010;8(10) doi: 10.1371/journal.pbio.1000509. e10000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamanian M, Kimber MJ, McVeigh P, Carlson SA, Maule AG, Day TA. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC genomics. 2011;12:596. doi: 10.1186/1471-2164-12-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamanian M, Agbedanu PN, Wheeler NJ, McVeigh P, Kimber MJ, Day TA. Novel RNAi-Mediated Approach to G Protein-Coupled Receptor Deorphanization: Proof of Principle and Characterization of a Planarian 5-HT Receptor. PLoS ONE. 2012;7(7):e40787. doi: 10.1371/journal.pone.0040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pax R, Bennett JL, Fetterer R. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Archives Pharmacol. 1978;304:309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- 46.Wolde Mussie E, Vande Waa J, Pax RA, Fetterer R, Bennett JL. Schistosoma mansoni: calcium efflux and effects of calcium-free media on responses of the adult male musculature to praziquantel and other agents inducing contraction. Exp Parasitol. 1982;53(2):270–278. doi: 10.1016/0014-4894(82)90069-8. [DOI] [PubMed] [Google Scholar]
- 47.Pica-Mattoccia L, Orsini T, Basso A, Festucci A, Liberti P, Guidi A, et al. Schistosoma mansoni: Lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Exp Parasitol. 2008;119:332–335. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Fetterer RH, Pax RA, Bennett JL. Praziquantel, potassium and 2,4-dinitrophenol: analysis of their action on the musculature of Schistosoma mansoni. Eur J Pharmacol. 1980;64(1):31–38. doi: 10.1016/0014-2999(80)90366-0. [DOI] [PubMed] [Google Scholar]
- 49.William S, Botros S. Validation of sensitivity to praziquantel using Schistosoma mansoni worm muscle tension and Ca2+-uptake as possible in vitro correlates to in vivo ED50 determination. Int J Parasitol. 2004;34(8):971–977. doi: 10.1016/j.ijpara.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Blair KL, Bennett JL, Pax RA. Praziquantel: physiological evidence for its site(s) of action in magnesium-paralysed Schistosoma mansoni. Parasitology. 1992;104(Pt 1):59–66. doi: 10.1017/s0031182000060807. [DOI] [PubMed] [Google Scholar]
- 51.Ruenwongsa P, Hutadilok N, Yuthavong Y. Stimulation of Ca2+ uptake in the human liver fluke Opisthorchis viverrini by praziquantel. Life Sci. 1983;32(22):2529–2534. doi: 10.1016/0024-3205(83)90234-5. [DOI] [PubMed] [Google Scholar]
- 52.Tayal S, Gupta S, Katiyar JC, Sagar P. Action of praziquantel on calcium transport in Hymenolepis diminuta. Folia Parasitol (Praha) 1988;35(4):329–334. [PubMed] [Google Scholar]
- 53.Prichard RK, Bachmann R, Hutchinson GW, Kohler P. The effect of praziquantel on calcium in Hymenolepis diminuta. Mol Biochem Parasitol. 1982;5(5):297–308. doi: 10.1016/0166-6851(82)90037-8. [DOI] [PubMed] [Google Scholar]
- 54.Becker B, Mehlhorn H, Andrews P, Thomas H. Ultrastructural investigations on the effect of praziquantel on the tegument of five species of cestodes. Z Parasitenkd. 1981;64(3):257–269. doi: 10.1007/BF00927373. [DOI] [PubMed] [Google Scholar]
- 55.Becker B, Mehlhorn H, Andrews P, Thomas H, Eckert J. Light and electron microscopic studies on the effect of praziquantel on Schistosoma mansoni, Dicrocoelium dendriticum and Fasciola hepatica (Trematoda) in vitro. Z Parasitenkd. 1980;63(2):113–128. doi: 10.1007/BF00927527. [DOI] [PubMed] [Google Scholar]
- 56.Bricker CS, Depenbusch JW, Bennett JL, Thompson DP. The Relationship between Tegumental Disruption and Muscle-Contraction in Schistosoma mansoni Exposed to Various Compounds. Zeitschrift Fur Parasitenkunde-Parasitology Research. 1983;69(1):61–71. doi: 10.1007/BF00934011. [DOI] [PubMed] [Google Scholar]
- 57.Xiao SH, Friedman PA, Catto BA, Webster LT., Jr Praziquantel-induced vesicle formation in the tegument of male Schistosoma mansoni is calcium dependent. J Parasitol. 1984;70(1):177–179. [PubMed] [Google Scholar]
- 58.William S, Botros S, Ismail M, Farghally A, Day TA, Bennett JL. Praziquantel-induced tegumental damage in vitro is diminished in schistosomes derived from praziquantel-resistant infections. Parasitology. 2001;122:63–66. doi: 10.1017/s0031182000007137. [DOI] [PubMed] [Google Scholar]
- 59.Senft AW, Gibler WB, Guterman JJ. Influence of Calcium-Perturbing Agents on Schistosomes -Comparison of Effects of Praziquantel and Verapamil on Worm Tegument. J Exp Zool. 1986;239(1):25–36. [Google Scholar]
- 60.Mendonca-Silva DL, Novozhilova E, Cobbett PJR, Silva CLM, Noel F, Totten MIJ, et al. Role of calcium influx through voltage-operated calcium channels and of calcium mobilization in the physiology of Schistosoma mansoni muscle contractions. Parasitology. 2006;133:67–74. doi: 10.1017/S0031182006000023. [DOI] [PubMed] [Google Scholar]
- 61.Novozhilova E, Kimber MJ, Qian H, McVeigh P, Robertson AP, Zamanian M, et al. FMRFamide-Like Peptides (FLPs) Enhance Voltage-Gated Calcium Currents to Elicit Muscle Contraction in the Human Parasite Schistosoma mansoni. PLoS NTD. 2010;4(8):e790. doi: 10.1371/journal.pntd.0000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohn AB, Anderson PAV, Roberts-Misterly JM, Greenberg RM. Schistosome calcium channel β subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem. 2001;40:36873–36876. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 63.Kohn AB, Roberts-Misterly JM, Anderson PAV, Khan N, Greenberg RM. Specific sites in the beta interaction domain of a schistosome Ca2+ channel β subunit are key to its role in sensitivity to the anti-schistosomal drug praziquantel. Parasitology. 2003;127:349–356. doi: 10.1017/s003118200300386x. [DOI] [PubMed] [Google Scholar]
- 64.Kohn AB, Roberts-Misterly JM, Anderson PA, Greenberg RM. Creation by mutagenesis of a mammalian Ca(2+) channel beta subunit that confers praziquantel sensitivity to a mammalian Ca(2+) channel. Int J Parasitol. 2003;33(12):1303–1308. doi: 10.1016/s0020-7519(03)00209-1. [DOI] [PubMed] [Google Scholar]
- 65.Marchant JS, Lin-Moshier Y, Walseth T, Patel S. The molecular basis for Ca2+ signalling by NAADP: two-pore channels in a complex? Messenger. 2012;1(1):63–76. doi: 10.1166/msr.2012.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young K, Lin S, Sun L, Lee E, Modi M, Hellings S, et al. Identification of a calcium channel modulator using a high throughput yeast two-hybrid screen. Nat Biotechnol. 1998;16:946–950. doi: 10.1038/nbt1098-946. [DOI] [PubMed] [Google Scholar]
- 67.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca2+ channel complex. Nat Med. 2011;17(7):822–829. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sieburth D, Ch'ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, et al. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436(7050):510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- 70.Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5(6):967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- 71.Fang K, Colecraft HM. Mechanism of auxiliary beta-subunit-mediated membrane targeting of L-type (Ca(V)1.2) channels. J Physiol. 2011;589(Pt 18):4437–4455. doi: 10.1113/jphysiol.2011.214247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hidalgo P, Neely A. Multiplicity of protein interactions and functions of the voltage-gated calcium channel b-subunit. Cell Calcium. 2007;42:389–396. doi: 10.1016/j.ceca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Jeziorski MC, Greenberg RM. Voltage-gated calcium channel subunits from platyhelminths: potential role in praziquantel action. Int J Parasitol. 2006;36:625–632. doi: 10.1016/j.ijpara.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92(5):2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 75.Rink JC, Gurley KA, Elliott SA, Alvarado AS. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yazawa S, Umesono Y, Hayashi T, Tarui H, Agata K. Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci U S A. 2009;106:22329–22334. doi: 10.1073/pnas.0907464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jim K, Triggle DJ. Actions of Praziquantel and 1-Methyladenine in Guinea-Pig Ileal Longitudinal Muscle. Can J Physiol Pharmacol. 1979;57(12):1460–1462. [Google Scholar]
- 78.Chubb JM, Bennett JL, Akera T, Brody TM. Effects of praziquantel, a new anthelmintic, on electromechanical properties of isolated rat atria. J Pharmacol Exp Ther. 1978;207(2):284–293. [PubMed] [Google Scholar]
- 79.Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, et al. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–360. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Consortium TSjGSaFA. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–352. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, et al. Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012;44(2):221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 82.Kohn AB, Lea JM, Roberts-Misterly JM, Anderson PAV, Greenberg RM. Structure of three high voltage-activated calcium channel α1 subunits from Schistosoma mansoni. Parasitology. 2001;123:489–497. doi: 10.1017/s0031182001008691. [DOI] [PubMed] [Google Scholar]
- 83.Prole DL, Taylor CW. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE. 2011;6(10):e26218. doi: 10.1371/journal.pone.0026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu TZ, Feng ZP. A sodium leak current regulates pacemaker activity of adult central pattern generator neurons in Lymnaea stagnalis. PLoS ONE. 2011;6(4):e18745. doi: 10.1371/journal.pone.0018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Senatore A, Spafford JD. Transient and big are key features of an invertebrate T-type channel (LCav3) from the central nervous system of Lymnaea stagnalis. J Biol Chem. 2010;285(10):7447–7458. doi: 10.1074/jbc.M109.090753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tikhonov DB, Zhorov BS. Possible roles of exceptionally conserved residues around the selectivity filters of sodium and calcium channels. J Biol Chem. 2011;286(4):2998–3006. doi: 10.1074/jbc.M110.175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi C, Soldatov NM. Molecular determinants of voltage-dependent slow inactivation of the Ca2+ channel. J Biol Chem. 2002;277(9):6813–6821. doi: 10.1074/jbc.M110524200. [DOI] [PubMed] [Google Scholar]
- 88.Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, et al. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102(23):8089–8096. doi: 10.1073/pnas.0502506102. discussion 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adamidi C, Wang Y, Gruen D, Mastrobuoni G, You X, Tolle D, et al. De novo assembly and validation of planaria transcriptome by massive parallel sequencing and shotgun proteomics. Genome Res. 2011;21(7):1193–1200. doi: 10.1101/gr.113779.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Talavera K, Staes M, Janssens A, Klugbauer N, Droogmans G, Hofmann F, et al. Aspartate residues of the Glu-Glu-Asp-Asp (EEDD) pore locus control selectivity and permeation of the T-type Ca(2+) channel alpha(1G) J Biol Chem. 2001;276(49):45628–45635. doi: 10.1074/jbc.M103047200. [DOI] [PubMed] [Google Scholar]
- 91.Olson PD, Zarowiecki M, Kiss F, Brehm K. Cestode genomics - progress and prospects for advancing basic and applied aspects of flatworm biology. Parasite Immunol. 2012;34(2–3):130–150. doi: 10.1111/j.1365-3024.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 92.Ernst WL, Noebels JL. Expanded alternative splice isoform profiling of the mouse Cav3.1/alpha1G T-type calcium channel. BMC Mol Biol. 2009;10:53. doi: 10.1186/1471-2199-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res. 2005;68(2):197–203. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 94.Andrade A, Denome S, Jiang YQ, Marangoudakis S, Lipscombe D. Opioid inhibition of N-type Ca2+ channels and spinal analgesia couple to alternative splicing. Nat Neurosci. 2010;13(10):1249–1256. doi: 10.1038/nn.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, et al. Transcript scanning reveals novel and extensive splice variations in human l-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem. 2004;279(43):44335–44343. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- 96.Kwok TCY, Ricker N, Fraser R, Chan AW, Burns A, Stanley EF, et al. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- 97.Kwok TCY, Hui K, Kostelecki W, Ricker N, Selman G, Feng ZP, et al. A genetic screen for dihydropyridine (DHP)-resistant worms reveals new residues required for DHP-blockage of mammalian calcium channels. PLoS Genetics. 2008;4(5) doi: 10.1371/journal.pgen.1000067. e10000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee RY, Lobel L, Hengartner M, Horvitz HR, Avery L. Mutations in the alpha1 subunit of an L-type voltage-activated Ca2+ channel cause myotonia in Caenorhabditis elegans. EMBO J. 1997;16(20):6066–6076. doi: 10.1093/emboj/16.20.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cobbett P, Day TA. Functional voltage-gated Ca2+ channels in muscle fibers of the platyhelminth Dugesia tigrina. Comp Biochem Physiol. 2003;(134):593–605. doi: 10.1016/s1095-6433(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 100.Blair KL, Anderson PAV. Properties of Voltage-Activated Ionic Currents in Cells from the Brains of the Triclad Flatworm Bdelloura candida. J Exp Biol. 1993;185:267–286. [Google Scholar]
- 101.Abdulla MH, Ruelas DS, Wolff B, Snedecor J, Lim KC, Xu F, et al. Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl Trop Dis. 2009;3(7):e478. doi: 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taft AS, Norante FA, Yoshino TP. The identification of inhibitors of Schistosoma mansoni miracidial transformation by incorporating a medium-throughput small-molecule screen. Exp Parasitol. 2010;125(2):84–94. doi: 10.1016/j.exppara.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawamoto F, Shozawa A, Kumada N, Kojima K. Possible roles of cAMP and Ca2+ in the regulation of miracidial transformation in Schistosoma mansoni. Parasitol Res. 1989;75(5):368–374. doi: 10.1007/BF00931132. [DOI] [PubMed] [Google Scholar]
- 104.Ito H, Klugbauer N, Hofmann F. Transfer of the high affinity dihydropyridine sensitivity from L-type To non-L-type calcium channel. Mol Pharmacol. 1997;52(4):735–740. doi: 10.1124/mol.52.4.735. [DOI] [PubMed] [Google Scholar]
- 105.Striessnig J, Hoda J-C, Wappl E, Koschak A. The Molecular Basis of Ca2+ Antagonist Drug Action-Recent Developments. In: Zamponi GW, editor. Voltage-Gated Calcium Channels. Kluwer Academic/Plenum Publishers; 2005. pp. 262–280. [Google Scholar]
- 106.Cheng RC, Tikhonov DB, Zhorov BS. Structural model for phenylalkylamine binding to L-type calcium channels. J Biol Chem. 2009;284(41):28332–28342. doi: 10.1074/jbc.M109.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tikhonov DB, Zhorov BS. Structural model for dihydropyridine binding to L-type calcium channels. J Biol Chem. 2009;284(28):19006–19017. doi: 10.1074/jbc.M109.011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tikhonov DB, Zhorov BS. Molecular modeling of benzothiazepine binding in the L-type calcium channel. J Biol Chem. 2008;283(25):17594–17604. doi: 10.1074/jbc.M800141200. [DOI] [PubMed] [Google Scholar]
- 109.Peterson BZ, Johnson BD, Hockerman GH, Acheson M, Scheuer T, Catterall WA. Analysis of the dihydropyridine receptor site of L-type calcium channels by alanine-scanning mutagenesis. J Biol Chem. 1997;272(30):18752–18758. doi: 10.1074/jbc.272.30.18752. [DOI] [PubMed] [Google Scholar]
- 110.Hockerman GH, Johnson BD, Abbott MR, Scheuer T, Catterall WA. Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels in transmembrane segment IIIS6 and the pore region of the alpha1 subunit. J Biol Chem. 1997;272(30):18759–18765. doi: 10.1074/jbc.272.30.18759. [DOI] [PubMed] [Google Scholar]
- 111.Senatore A, Boone A, Lam S, Dawson TF, Zhorov B, Spafford JD. Mapping of dihydropyridine binding residues in a less sensitive invertebrate L-type calcium channel (LCav1) Channels. 2011;5(2):173–187. doi: 10.4161/chan.5.2.15141. [DOI] [PubMed] [Google Scholar]
- 112.Izumi-Nakaseko H, Yamaguchi S, Ohtsuka Y, Ebihara T, Adachi-Akahane S, Okamura Y. DHP-insensitive L-type-like Ca channel of ascidian acquires sensitivity to DHP with single amino acid change in domain III P-region. FEBS Lett. 2003;549(1–3):67–71. doi: 10.1016/s0014-5793(03)00772-5. [DOI] [PubMed] [Google Scholar]
- 113.Yamaguchi S, Okamura Y, Nagao T, Adachi-Akahane S. Serine residue in the IIIS5-S6 linker of the L-type Ca2+ channel alpha 1C subunit is the critical determinant of the action of dihydropyridine Ca2+ channel agonists. J Biol Chem. 2000;275(52):41504–41511. doi: 10.1074/jbc.M007165200. [DOI] [PubMed] [Google Scholar]
- 114.Hui K, Kwok TC, Kostelecki W, Leen J, Roy PJ, Feng ZP. Differential sensitivities of Cav1.2 IIS5-S6 mutants to 1,4-dihydropyridine analogs. Eur J Pharmacol. 2009;602(2–3):255–261. doi: 10.1016/j.ejphar.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 115.Wappl E, Mitterdorfer J, Glossmann H, Striessnig J. Mechanism of dihydropyridine interaction with critical binding residues of L-type Ca2+ channel alpha 1 subunits. J Biol Chem. 2001;276(16):12730–12735. doi: 10.1074/jbc.M010164200. [DOI] [PubMed] [Google Scholar]
- 116.Jeziorski MC, Greenberg RM, Clark KS, Anderson PA. Cloning and functional expression of a voltage-gated calcium channel alpha1 subunit from jellyfish. J Biol Chem. 1998;273(35):22792–22799. doi: 10.1074/jbc.273.35.22792. [DOI] [PubMed] [Google Scholar]
- 117.Lin M, Aladejebi O, Hockerman GH. Distinct properties of amlodipine and nicardipine block of the voltage-dependent Ca2+ channels Cav1.2 and Cav2.1 and the mutant channels Cav1.2/Dihydropyridine insensitive and Cav2.1/Dihydropyridine sensitive. Eur J Pharmacol. 2011;670(1):105–113. doi: 10.1016/j.ejphar.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hockerman GH, Johnson BD, Scheuer T, Catterall WA. Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels. J Biol Chem. 1995;270(38):22119–22122. doi: 10.1074/jbc.270.38.22119. [DOI] [PubMed] [Google Scholar]
- 119.Kasinathan RS, Goronga T, Messerli SM, Webb TR, Greenberg RM. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB J. 2010;24(1):128–135. doi: 10.1096/fj.09-137091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hockerman GH, Dilmac N, Scheuer T, Catterall WA. Molecular determinants of diltiazem block in domains IIIS6 and IVS6 of L-type Ca(2+) channels. Mol Pharmacol. 2000;58(6):1264–1270. doi: 10.1124/mol.58.6.1264. [DOI] [PubMed] [Google Scholar]
- 121.Brindley PJ, Sher A. Immunological involvement in the efficacy of praziquantel. Exp Parasitol. 1990;71(2):245–248. doi: 10.1016/0014-4894(90)90028-b. [DOI] [PubMed] [Google Scholar]
- 122.Doenhoff MJ, Sabah AA, Fletcher C, Webbe G, Bain J. Evidence for an immune-dependent action of praziquantel on Schistosoma mansoni in mice. Trans R Soc Trop Med Hyg. 1987;81(6):947–951. doi: 10.1016/0035-9203(87)90360-9. [DOI] [PubMed] [Google Scholar]
- 123.Messerli SM, Kasinathan RS, Morgan W, Spranger S, Greenberg RM. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol Biochem Parasitol. 2009;167(1):54–59. doi: 10.1016/j.molbiopara.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valle C, Troiana AR, Festucci A, Pica-Mattoccia L, Liberti P, Wolstenholme A, et al. Sequence and level of endogenous expression of calcium channel β subunits in Schistosoma mansoni displaying different susceptibilities to praziquantel. Mol Biochem Parasitol. 2003;130(2):111–115. doi: 10.1016/s0166-6851(03)00171-3. [DOI] [PubMed] [Google Scholar]
- 125.Aragon AD, Imani RA, Blackburn VR, Cupit PM, Sandra DM, Goronga T, et al. Towards an understanding of the mechanism of action of praziquantel. Mol Biochem Parasitol. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jolly ER, Chin CS, Miller S, Bahgat MM, Lim KC, DeRisi J, et al. Gene expression patterns during adaptation of a helminth parasite to different environmental niches. Genome Biol. 2007;8(4):R65. doi: 10.1186/gb-2007-8-4-r65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hines-Kay J, Cupit PM, Sanchez MC, Rosenberg GH, Hanelt B, Cunningham C. Transcriptional analysis of Schistosoma mansoni treated with praziquantel in vitro. Mol Biochem Parasitol. 2012 doi: 10.1016/j.molbiopara.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]