SUMMARY
Aims and methods
To evaluate the clinical value of 18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET in relation to tumour localization and the patient’s genetic status in a large series of pheochromocytoma/paraganglioma (PHEO/PGL) patients and to discuss in detail false-negative results.
A retrospective study of PGL patients who were investigated with 18F-FDOPA PET or PET/CT imaging in two academic endocrine tumour centers was conducted (La Timone University Hospital, Marseilles, France and National Institutes of Health (NIH), Bethesda, MD, USA).
Results
One hundred sixteen patients (39.7% harboring germline mutations in known disease susceptibility genes) were evaluated for a total of 195 PHEO/PGL foci. 18F-FDOPA PET correctly detected 179 lesions (91.8%) in 107 patients (92.2%).
Lesion-based sensitivities for parasympathetic PGLs (head, neck, or anterior/middle thoracic ones), PHEOs, and extra-adrenal sympathetic (abdominal or posterior thoracic) PGLs were 98.2% [96.5% for Timone and 100% for NIH], 93.9% [93.8% and 93.9%], and 70.3% [47.1% and 90%], respectively (P<0.001).
Sympathetic (adrenal and extra-adrenal) SDHx-related PGLs were at a higher risk for negative 18F-FDOPA PET than non-SDHx-related PGLs (14/24 vs 0/62, respectively, p<0.001). By contrast, the risk of negative 18F-FDOPA PET was lower for parasympathetic PGLs regardless of the genetic background (1/90 in SDHx vs 1/19 in non-SDHx tumours, p= 0.32).
18F-FDOPA PET failed to detect 2 head and neck PGLs (HNPGL), likely due to their small size, while most missed sympathetic PGL were larger and may have exhibited a specific 18F-FDOPA-negative imaging phenotype. 18F-FDG PET detected all the missed sympathetic lesions.
Conclusions
18F-FDOPA PET appears to be a very sensitive functional imaging tool for HNPGL regardless of the genetic status of the tumours. Patients with false-negative tumours on 18F-FDOPA PET should be tested for SDHx mutations.
Keywords: Positron emission tomography, 18F-fluorodihydroxyphenylalanine, paraganglioma, radiopharmaceuticals, genetics
INTRODUCTION
Paragangliomas (PGL) are members of a family of neuroendocrine neoplasms that develop in the adrenal medulla (also called pheochromocytomas (PHEO)), in the extra-adrenal chromaffin cells that persist postnatally in the pre-aortic region or within sympathetic ganglia or from paraganglionic chemoreceptor cells wich are concentrated in the head and neck. They are intimately associated with the autonomic nervous system.
A high percentage (up to 35%) of PGLs are hereditary, which can lead to multifocal disease. Molecular genetic research has resulted in the identification of 10 susceptibility genes so far for tumours of the paraganglial system. Mutations in any of the succinate dehydrogenase subunit genes (collectively SDHx) are each associated with a distinct PGL syndrome, with a high percentage of extra-adrenal locations 1, 2. SDHB mutations are associated with more aggressive tumour behavior and higher rates of malignancy 3–5. Imaging investigations in these patients need to be more exhaustive and genetically specific for more effective and personalized management in the future.
To achieve this goal, positron emission tomography (PET) imaging has gained an increasing role in PGL imaging, paralleled by great efforts to develop new tracers. There is growing evidence that suggests a link between imaging phenotypes and certain genetic mutations. For example, it has been shown that SDHB-related tumours are better detected by 18F-fluorodeoxyglucose (18F-FDG) PET than with other radiopharmaceuticals 6–9. However, limited data are available in other genotypes and in non-metastatic PGL/PHEO.
The present study aims to evaluate the clinical value of 18F-fluorodihydroxyphenylalanine (18F-FDOPA) PET in relation to tumour localization and genetic background in a large series of non-metastatic PHEO/PGL and to discuss in detail the false-negative results.
MATERIAL AND METHODS
Patients
18F-FDOPA PET studies performed for PGL imaging in two academic endocrine tumour centers were reviewed. Only patients who fulfilled the following criteria were included and their images were reinterpreted:
At least one PHEO/PGL at the time of PET study.
Absence of multiple endocrine neoplasia type 2 (MEN2) or neurofibromatosis type. MEN2 and NF1-related PHEO were also excluded because they raise specific problems for clinicians, and many do not need functional imaging.
Genetic screening for SDHB, SDHC, SDHD and von Hippel-Lindau (VHL).
Absence of a previous history of metastases and absence of presumed metastases on the complete imaging work-up.
Genetic testing
The SDHB, SDHC, SDHD and VHL genes were amplified using exon flanking from DNA isolated from the blood samples of all patients with PHEO/PGL. Genetic testing for TMEM127, SDHA, and SDHAF2 gene mutations was not performed.
18F-FDOPA PET scanning
At La Timone University Hospital, 18F-FDOPA was used in the setting of marketing authorization. 18F-FDOPA was supplied by Iason GmbH, Graz, Austria, and quality control was performed according to European pharmacopeia by the manufacturer. At Timone, 18F-FDOPA was extemporaneously neutralized by the radiopharmacist using a bicarbonate buffer kit supplied by the manufacturer, and the pH was checked in order to ensure that the solution was kept between 4.0 and 5.0. Patients fasted for a minimum of 3 hours before 18F-FDOPA injection (IASOdopa®, 4 MBq/kg) without carbidopa pre-treatment. The PET emission scan started approximately 60 minutes after 18F-FDOPA injection. Three-dimensional images were acquired using a GE Discovery ST PET/computed tomography (CT) hybrid scanner (General Electrics Medical System).
CT was performed first from at least the skull base to the upper thighs. Immediately after the CT, PET images were acquired in 3-dimensional mode, at 3 min per table position. PET images were reconstructed iteratively (OSEM algorithm) on a 256 × 256 matrix using CT data for attenuation correction. Co-registered images were displayed on a workstation (Xeleris; GE Healthcare) with 3D representation as well as axial, coronal, and sagittal slices.
At the National Institutes of Health, 18F-FDOPA is an investigational agent approved by the FDA. It is produced using a standard procedure which has been previously described 10. 18F-FDOPA was used as an experimental tracer after approval by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health (protocol 00-CH-0093). All patients provided written informed consent. Patients fasted for a minimum of 4 hours before 18F-FDOPA injection (444 MBq). The tracer injection was preceded by oral administration of 100 mg of carbidopa, 1 h before tracer injection. The PET emission scan started approximately 30 minutes after 18F-FDOPA injection.
For 9 patients, images were acquired using an mCT PET/CT hybrid scanner (Biograph-128 mCT PET/CT scanner, Siemens Medical Solutions). CT was performed first from at least the skull base to the upper thighs, followed by PET imaging (3-dimensional mode) using an acquisition time of 5 min per table position. PET images were reconstructed iteratively (OSEM algorithm) on a 256 × 256 matrix using CT data for attenuation correction.
For 53 patients, an Advance scanner (GE Healthcare) was used with a rod source for attenuation correction. 5-min emission images in 2-dimensional mode were obtained from at least the skull base to the mid-thighs. PET images were reconstructed on a 256 × 256 matrix using an iterative algorithm. Analysis was performed on attenuation-corrected images.
Analysis of data
At each participating institution, the 18F-FDOPA PET scans were reviewed by one experienced nuclear medicine physician who was blinded to the reports of other functional and anatomic imaging studies. 18F-FDOPA uptake was considered pathologic if there was nonphysiological extra-adrenal focal uptake, asymmetric adrenal uptake with a concordant enlarged gland, or adrenal uptake more intense than the liver 11. Lesions were classified into sympathetic and parasympathetic by location, based on knowledge of embryology and development. Specifically, foci in the head and neck and anterior and middle mediastinum were considered to be derived from parasympathetic PGLs, whereas those in the adrenal gland, posterior mediastinum, and abdomen were considered to be derived from sympathetic PGLs 12. 18F-FDOPA results were then compared to on-site reports of concomitant thoracoabdominal CT and CT and/or MRI of the neck (all patients), 123I-MIBG (Timone) or 18F-fluorodopamine PET/CT (NIH), 18F-FDG PET/CT (103 cases, 44 out of 54 at Timone and 59 out of 62 at the NIH) when available. 18F-FDOPA PET scans with FN issues were also reviewed in detail in an unblinded fashion.
Standard of truth
Pathological analysis of the tumour was considered the standard of truth for the diagnosis of a PGL. In cases where no surgical resection was performed, the diagnosis of PGL was made if lesions were located in a classical anatomic site for PGL and after confirmation with a second imaging procedure.
Statistics
The sensitivity of 18F-FDOPA PET was calculated for patients and for individual lesions. The sensitivity for tumours at different locations was calculated and compared using Fisher’s exact test. We did not perform a quantitative analysis using SUV and relate these values to the different genetic change because three different scanners were used (two PET/CT and one PET) with different radiopharmaceuticals and imaging protocols.
P values of less than 0.05 were taken to be statistically significant. All statistical analyses were performed using SPSS 17.0 software.
RESULTS
Patients
We identified 116 patients (54 Timone/62 NIH) (62 females, 54 males, ages 14 to 83) who underwent 18F-FDOPA PET (63 PET/CT and 53 PET) from 2007 to 2012 and fulfilled the inclusion criteria. Forty-six (39.7%) patients harbored germline mutations, specifically SDHB (20), SDHC (5), SDHD (17), VHL (3), and MAX (MYC associated factor X) (1). PHEO/PGLs were distributed as follows: 100 HNPGL, 9 parasympathetic thoracic, 49 adrenal, 34 extra-adrenal abdominal, and 3 sympathetic thoracic (Table 1). The total number of lesions was 195 (105 NIH/90 Timone) with 114 SDHx-related PGLs (90 parasympathetic/24 sympathetic) and 81 non-SDHx-related PGLs (19 parasympathetic/62 sympathetic). This was confirmed histologically at 138/195 sites. Thirty patients harbored more than 1 lesion (13 Timone/17 NIH).
Table 1.
Lesion-based sensitivity of 18F-FDOPA PET across different tumour locations in relation to genotype.
| A Parasympathetic (HNPGL and parasympathetic thoracic) | B Adrenal | C Sympathetic Extra-adrenal (Abdominal and sympathetic thoracic) | All locations | |
|---|---|---|---|---|
| Number of PGL/PHEO | 109 [57/52] | 49 [16/33] | 37 [17/20] | 195 [90/105] |
| Per-lesion sensitivity | 107 (98.2%) [96.5%/100%] | 46 (93.9%) [93.8%/93.9%] | 26 (70.3%) [47.1%/90%] | 179 (91.8%) [86.7%/96.2%] |
| Genotype of 18F-FDOPA negative tumours | 1 SDHD, 1 sporadic | 3 SDHD | 7 SDHD 4 SDHB |
1 sporadic [1/0] 11 SDHD [8/3] 4 SDHB [3/1] |
Results of the two series are detailed ([Timone/NIH]) just below the pooled results.
The SDHD-related vagal PGL was millimetric and located just below another highly avid PGL of the vagus nerve.
The sporadic carotid body tumour was mainly fibrotic, with a minimal residual volume of neuroendocrine tissue.
18F-FDOPA PET was more sensitive in parasympathetic PGL than in sympathetic PGL. A vs B vs C: P<0.001, A vs B: P=0.173, A vs C: P<0.001, B vs C: P=0.006, A vs B+C: P<0.001 (Fisher’s exact test).
Overall results
The patient-based sensitivity was 92.2% (107/116), which was not different between the two involved institutions: Timone (patient-based analysis: 88.9%; 95% CI [77.4–95.8%]) and NIH (95.2%; 95% CI [86.5–99.0%]; p=0.300). However, the lesion-based sensitivity was 91.8% (175/195), and it was significantly higher at NIH compared to Timone (96.2% vs 86.7%, p=0.019), related to differences in sensitivities in group C only.
18F-FDOPA PET and PET/CT had a higher diagnostic value for the detection of parasympathetic PGLs than sympathetic PGLs (pooled results, P<0.001) (Table 1).
We identified 9 patients with false-negative 18F-FDOPA PET (for a total of 16 lesions) (Table 2). Sympathetic (adrenal and extra-adrenal) SDHx-related PGLs were at a higher risk for negative 18F-FDOPA PET than non-SDHx PGLs (14/24 vs 0/62, respectively, p<0.001). By contrast, the risk of negative 18F-FDOPA PET was lower for parasympathetic PGLs regardless of the genetic background (1/90 in SDHx vs 1/19 in non-SDHx tumours, p= 0.32). At La Timone University Hospital, all but two parasympathetic foci were detected by 18F-FDOPA PET/CT, including sporadic and inherited cases. Two HNPGLs (in 2 patients) were missed. Every HNPGL detected by 18F-FDOPA exhibited excellent tumour-to-background ratios. One small jugular focus in an SDHB patient remained occult on both multiphasic CT, MRI, and angioMRI. 18F-FDOPA PET/CT detected all sporadic PHEO/PGL of sympathetic origin (adrenal, sympathetic thoracic, and extra-adrenal abdominal), but missed 10 extra-adrenal abdominal sympathetic tumours in 5 SDHx mutation carriers.
Table 2.
Clinical characteristics of patients with false-negative 18F-FDOPA PHEO/PGL.
| Patient | Institution | Genetic status | Total number of lesions | Number of FDOPA+ lesions | Lesions missed by 18F-FDOPA PET | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Tumour location | Origin | Size (mm) | 18F-FDG PET | Gold standard | |||||
| 1 | Timone | Sporadic | 1 | 0 | Carotid body | Parasympathetic | 30 | nd | Surgery |
| 2 (fig 1) | Timone | SDHD | 8 | 4 | Adrenal | Sympathetic | 9 | + | Surgery |
| Abdo aortocaval | Sympathetic | 26 | + | Surgery | |||||
| Abdo lateral caval | Sympathetic | 17 | + | Surgery | |||||
| Abdo iliac bifucation | Sympathetic | 6 | + | Surgery | |||||
| 3 | Timone | SDHD | 8 | 2 | Abdo pre-aortic | Sympathetic | 21 | + | Surgery |
| Abdo lateral aortic | Sympathetic | 18 | + | Surgery | |||||
| 4 (fig 2) | Timone | SDHD | 7 | 5 | Cervical vagal | Parasympathetic | 5 | − | Imaging |
| Abdo lateral aortic | Sympathetic | 14 | + | Imaging | |||||
| 5 (fig 3) | Timone | SDHB | 2 | 0 | Abdo lateral-aortic | Sympathetic | 28 | + | Surgery |
| Abdo pre-aortic (Zuckerkandl) | Sympathetic | 13 | + | Surgery | |||||
| 6 | Timone | SDHB | 1 | 0 | Abdo lateral aortic | Sympathetic | 45 | + | Imaging |
| 7 (fig 4) | NIH | SDHD | 4 | 2 | Adrenal | Sympathetic | 27 | + | Surgery |
| Adrenal | Sympathetic | 34 | + | Surgery | |||||
| 8 (fig 5) | NIH | SDHD | 10 | 9 | Post mediastinum | Sympathetic | 30 | + | Imaging |
| 9 | NIH | SDHB | 1 | 0 | Abdo lateral aortic | Sympathetic | 15 | + | Surgery |
Abbreviations: nd: not done; Abdo: abdominal.
At the National Institutes of Health, 18F-FDOPA PET/CT and PET detected all PGLs of parasympathetic origin (HNPGL and thoracic) with excellent tumour-to-background ratios. 18F-FDOPA PET missed two adrenal PHEOs in one SDHD patient. One sympathetic thoracic (paravertebral) lesion in an SDHD mutation carrier did not show any significant 18F-FDOPA uptake, and one extra-adrenal abdominal PGL was missed in an SDHB mutation carrier. All PHEO/PGLs in non-SDHx patients had significant 18F-FDOPA uptake.
False-negative cases
La Timone University Hospital results
At La Timone University Hospital, a false-negative sporadic carotid body tumour was found to be primarily fibrotic with minimal residual neuroendocrine tissue, as revealed by a small area of positivity on synaptophysin immunostaining (patient #1). The second missed HNPGL was a 5 mm SDHD-related vagal PGL (patient #4). 18F-FDG PET/CT also failed to detect this lesion.
Among patients with adrenal PHEO and/or extra-adrenal abdominal PGL, false-negative 18F-FDOPA PET/CT scans only occurred in SDHx patients (3 unrelated SDHD and 2 unrelated SDHB cases). All had diameters greater than or equal to 6 mm and were detected by 18F-FDG PET/CT. 18F-FDOPA PET/CT failed to identify 4 abdominal PGLs in the first case (patient #2, Figure 1), 2 in the second case (patient #3) and 1 in the latter case (patient #4, Figure 2). Two patients with SDHB mutations also had false-negative lesions with 18F-FDOPA. A 17-year-old male (patient #5) had two 18F-FDOPA-negative extra-adrenal abdominal PGLs (Figure 3). The other SDHB patient (patient #6), a 14-year-old male, had a large extra-adrenal PGL located in the right renal hilum that was completely negative for 18F-FDOPA.
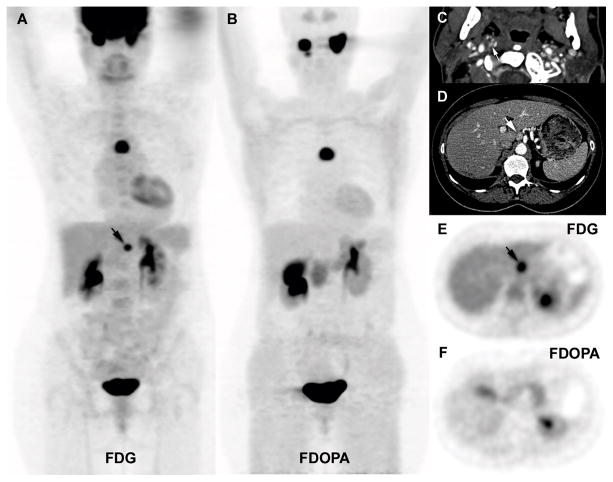
Figure 1.
Multicentric SDHD-related PGL syndrome (2 HNPGL, 2 PHEO, and 4 extra-adrenal PGL). A. Fused axial 18F-FDG PET/CT images centered over the tumours (6 positive tumours, arrows). B. Matching axial 18F-FDOPA PET/CT images show 2 positive tumours (arrows). Missed tumours on 18F-FDOPA PET/CT were located as follows: 9 mm left PHEO, 26 mm interaortocaval, 17 mm lateral caval, 6 mm iliac bifurcation.
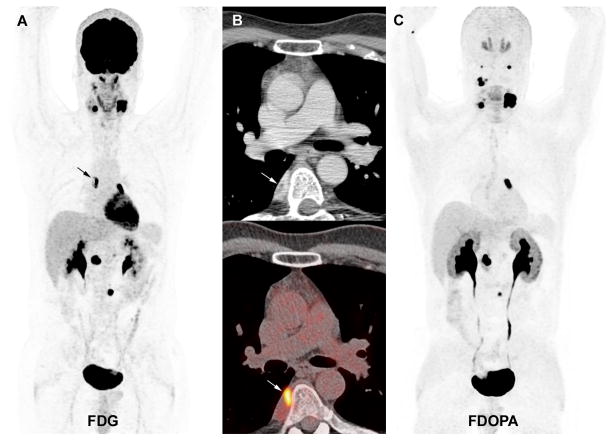
Figure 2.
Multicentric SDHD-related PGL syndrome (5 HNPGL, 1 thoracic and 1 extra-adrenal PGL). A. 18F-FDG PET (maximal intensity projection (MIP)). B. 18F-FDOPA PET (MIP). C. Axial contrast-enhanced CT showing a 5 mm cervical PGL of the vagus nerve missed by 18F-FDOPA PET/CT (arrow). D. Axial contrast-enhanced CT image centered on a 14 mm abdominal extra-adrenal PGL located lateral to the celiac trunk (arrow). E. Axial 18F-FDG PET image centered over the positive tumour (arrow). F. Axial 18F-FDOPA PET image centered over the false-negative abdominal extra-adrenal PGL.
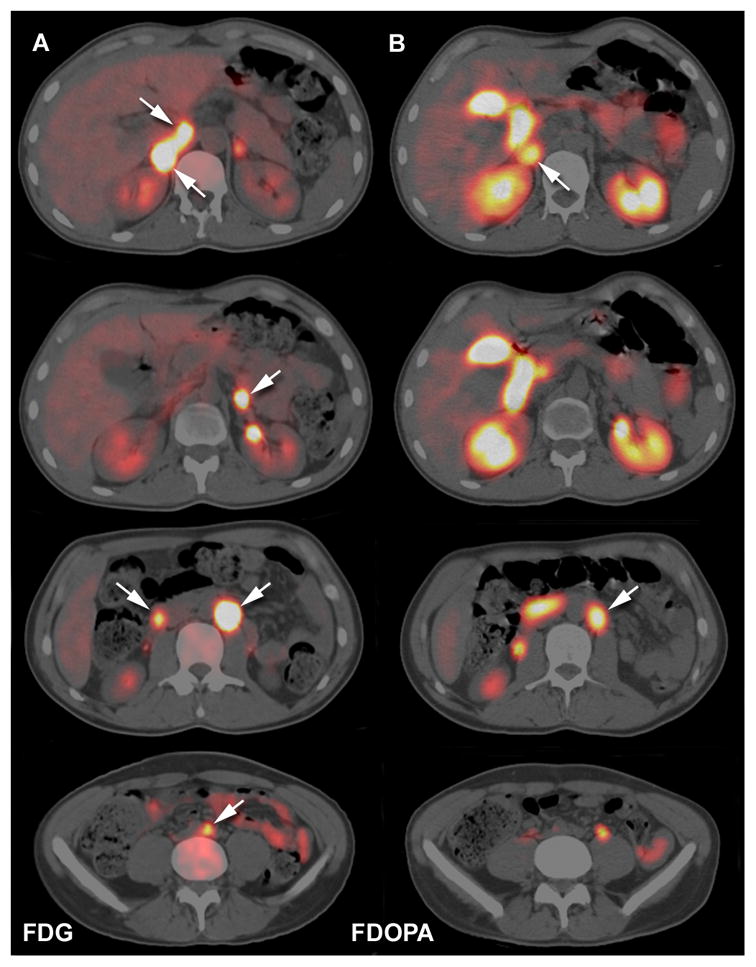
Figure 3.
Multicentric SDHB-related PGL (2 extra-adrenal PGL, 1 adrenal PHEO). A. Enhanced coronal and axial CT slices at the level of the tumours (reconstruction in the lower left image). B. Fused axial 18F-FDG PET/CT images centered over the tumours (2 positive extra adrenal tumours 28 and 13 mm in diameter, arrows). C. Fused axial 18F-FDOPA PET/CT images centered over the tumours. 18F-FDOPA-negative tumour sites were lateral aortic (at the level of the superior mesenteric artery) and preaortic (PGL derived from the organ of Zuckerkandl). False-positive uptake was seen in an enlarged left adrenal gland (top row). The adrenal gland weighed 8 g (normal 5 to 6 g) and showed cortical hyperplasia, but the adrenal medulla was normal.
NIH results
At the NIH, the 2 missed adrenal PHEO occurred in an SDHD mutation carrier (patient #7). In this patient, the concomitant parasympathetic lesions (1 HNPGL and 1 cardiac PGL) were 18F-FDOPA positive (Figure 4). In another SDHD patient (patient #8), a sympathetic thoracic lesion (paravertebral) was missed by 18F-FDOPA PET/CT while the other lesions were clearly identified (Figure 5). Finally, in an SDHB mutation carrier, 18F-FDOPA PET missed a solitary retroperitoneal (para-aortic) sympathetic PGL (patient #9). In all cases, the missed lesions had high 18F-FDG uptake and were also detected by 18F-FDA PET.
Figure 4.
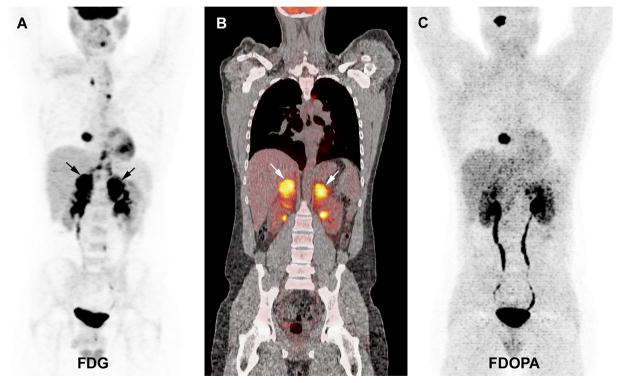
Multicentric SDHD-related PGL syndrome (1 HNPGL, 1 cardiac, 2 adrenal PHEO). A. 18F-FDG PET (MIP image) showing positive cardiac PGL and bilateral PHEO (arrows). Non-specific uptake in the mediastinum corresponds to brown fat. The HNPGL is not visible on this projection. B. Coronal fused 18F-FDG PET/CT image centered over the PHEO (2 positive tumours, arrows). C. 18F-FDOPA PET (MIP image) showing positive HNPGL and cardiac PGL, negative bilateral PHEO.
Figure 5.
Multicentric SDHD-related PGL syndrome (multiple HNPGL, 1 parasympathetic thoracic PGL, 1 sympathetic thoracic (cardiac) PGL, and 2 sympathetic retroperitoneal PGL). A. 18F-FDG PET/CT (MIP image) shows multiple foci, including uptake in a paravertebral sympathetic legion (arrow). B. Axial CT and fused 18F-FDG PET/CT images centered over the sympathetic thoracic PGL (arrows). C. 18F-FDOPA PET (MIP image) showing positive parasympathetic and extra-adrenal sympathetic PGL, but negative sympathetic thoracic PGL.
The results are summarized in Table 2.
DISCUSSION
The present study describes the sensitivity of 18F-FDOPA PET in a large cohort of non-metastatic PGLs/PHEOs with and without associated genetic mutations.
We excluded metastatic patients from the analysis, because our aim was focused on the evaluation of the relationship between primary tumour origin/genotype and 18F-FDOPA uptake. Inclusion of patients with metastatic disease would have contributed to a significant increase in the number of SDHB-related PHEOs or PGLs and chromaffin-derived (sympathetic) lesions. Furthermore, these patients are known to be best evaluated by 18F-FDG PET 9.
The patient and lesion-based sensitivities were 92.2% and 91.8% respectively, which are consistent with most previous studies 13, 14.
18F-FDOPA PET failed to detect 16/195 PGLs/PHEOs. False-negative results in molecular imaging should be considered as important as positive findings since they may have important implications for the pathophysiology, prognosis, and treatment of patients with these tumours. In a recent meta-analysis performed on 11 studies (275 patients), the pooled sensitivity and specificity of a per lesion-based analysis of 18F-FDOPA PET(CT) in PHEO/PGL were 79% (95% CI 76–81%) and 95% (95% CI 84–99%), respectively 13. Possible sources of false-negative results have been listed (i.e., small lesions, tumour location near organs with high physiological uptake, dedifferentiation) but have not been sufficiently examined, particularly with regard to the tumour location and the patient’s genetic status.
In our series, as in others, HNPGLs (SDHx-related as well as their sporadic counterparts) exhibit a high avidity for 18F-FDOPA 15–20. We confirmed that 18F-FDOPA PET and PET/CT are highly sensitive (98.2%) for the detection of HNPGLs, regardless of the genetic status of the tumours. Both HNPGLs missed by 18F-FDOPA PET/CT in our series were very small. One was 30 mm but was almost completely fibrotic, with only a tiny area of residual neuroendocrine tissue; the second was only 5 mm in size and located just below another highly avid lesion. These false-negative findings are attributed to the limits of resolution of the BGO-based GE Discovery ST PET/CT used and partial volume effect that profoundly decreases the visibility of tiny lesions. By contrast, the missed extra-adrenal abdominal PGL were larger and may have exhibited a specific 18F-FDOPA negative imaging phenotype. We found a significantly higher number of missed tumours at Timone (12/90) compared to the NIH (4/105), which was related to a higher rate of missed lesions in group C at Timone. Since this group was proportionally equivalent at both participating institutions (19%) and 18F-FDOPA PET sensitivities were high or similar in other groups between institutions, the lower sensitivity observed at Timone in group C was most likely due to chance (4 lesions were missed in a single patient).
One of the most interesting results of the study is that the sensitivity was lower in cases of both SDHD (patient-based 12/17, 70.6%) and SDHB mutations (17/20, 85%); therefore, we suggest that patients with 18F-FDOPA-negative PHEO/PGL should undergo testing for SDHx mutations. Interestingly, all but one of the false-negative results observed in SDHB or SDHD patients were of sympathetic (chromaffin) origin. In a prospective study by Fottner et al., 2 SDHx-related extra-adrenal abdominal sympathetic PGLs were not detected with 18F-FDOPA PET, in spite of the centimeter size of the lesions and positivity of 123I-MIBG scintigraphy 18. This imperfect sensitivity of 18F-FDOPA PET for detecting extra-adrenal abdominal tumours has also been noted by Fiebrich et al. 17. Taken together, these data indicate that SDHx deficiency might impact tumour biology and subsequently the cellular avidity for 18F-FDOPA.
Hypothetically, several models have been invoked to explain the discordance between high 18F-FDG uptake and low avidity for specific tracers in SDHB-related metastatic tumours (i.e., activation of the hypoxia signalling pathway, dedifferentiation, proliferation). The present study demonstrates in a large series that we can broaden the concept of a molecular imaging pattern of SDHx-related tumours to non metastatic PGLs. Interestingly, it appears that genotype alone does not determine imaging phenotype, since different tumours in the same patient often exhibit different imaging patterns. In our series, results of the detection of HNPGLs using 18F-FDOPA PET or PET/CT suggest uptake in these tumours is not affected by genetic status. This is not true for PHEOs/PGLs in other locations, where 18F-FDOPA positivity appears to be affected rather by genetic background and tumour origin (sympathetic vs parasympathetic) than biochemical phenotypes.
Interestingly, we found relatively high sensitivities of 18F-FDOPA PET/CT in sympathetic non-metastatic SDHB-related PGLs consistent with previously published studies 8, 14. By contrast, 18F-FDOPA PET/CT was found to be less sensitive for sympathetic metastatic SDHB-related PGLs in which tumour de-differentiation and the loss of the L-amino acid transporter system could lead to a decline in 18F-FDOPA uptake8.
18F-FDG PET findings were only detailed in lesions missed by 18F-FDOPA PET, since this was the major endpoint of the study.
It is well known that chromaffin cells derive from pluripotent neural crest (NC) progenitors. It has been shown that NC pluripotent progenitors might be found in different sites until late in development, and even in postnatal and adult life 21. Some of them are able to self-renew and may be the origin of several tumour types. The discordance between high 18F-FDG/low 18F-FDOPA uptake pattern might be related to a certain degree of functional dedifferentiation of some tumours regarding Amine Precursor Uptake and Decarboxylation (APUD phenotype) that may occur in the same patient. Other explanations might involve epigenetic modifications and/or somatic mutations that may regulate key proteins involved in tracer uptake. It has been shown that inhibition of succinate dehydrogenase alters epigenetic processes 22.
We acknowledge several limitations to our study: retrospective, two institutions with different patient populations, different PET cameras, different radiopharmaceuticals and imaging protocols. We also have to admit that the high proportion of HNPGLs in our series is probably related to a certain bias due to long-standing collaboration with ENT physicians. However, it is the largest study showing extensive data related to primary tumours, and the patient-based sensitivity was not statistically different between the two participating institutions. Furthermore, differences in acquistion timing (30 vs 60 min) or patient premedication (carbidopa or not) may only slightly influence PET sensitivity, since PHEO/PGL usually exhibit intense and prolonged uptake values 23, 24.
In conclusion, 18F-FDOPA PET appears to be a very sensitive functional imaging tool for HNPGL regardless of the genetic status of the tumours. Patients with false-negative primary tumours on 18F-FDOPA PET should be tested for SDHx mutations. Some unanswered questions arise from lesions missed by functional imaging and head-to-head comparison of genetic/molecular findings and imaging phenotypes might be of particular interest 25.
Acknowledgments
The authors would like to thank Victoria Martucci for her technical assistance in the preparation of this manuscript. This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Development and the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
References
- 1.Timmers HJ, Taieb D, Pacak K. Current and future anatomical and functional imaging approaches to pheochromocytoma and paraganglioma. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2012;44:367–372. doi: 10.1055/s-0031-1299712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taieb D, Neumann H, Rubello D, Al-Nahhas A, Guillet B, Hindie E. Modern nuclear imaging for paragangliomas: beyond SPECT. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:264–274. doi: 10.2967/jnumed.111.098152. [DOI] [PubMed] [Google Scholar]
- 3.Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, Khau Van Kien P, Corvol P, Plouin PF, Jeunemaitre X. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer research. 2003;63:5615–5621. [PubMed] [Google Scholar]
- 4.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, Bertagna X, Schlumberger M, Jeunemaitre X, Gimenez-Roqueplo AP, Plouin PF. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. The Journal of clinical endocrinology and metabolism. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan AM, Trivedi H, Adams KT, Kebebew E, Pacak K, Hughes MS. Comparison of clinical and imaging features in succinate dehydrogenase-positive versus sporadic paragangliomas. Surgery. 2011;150:1186–1193. doi: 10.1016/j.surg.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, Pacak K. Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. Journal of the National Cancer Institute. 2012;104:700–708. doi: 10.1093/jnci/djs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonte JS, Robles JF, Chen CC, Reynolds J, Whatley M, Ling A, Mercado-Asis LB, Adams KT, Martucci V, Fojo T, Pacak K. False-negative (1)(2)(3)I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocrine-related cancer. 2012;19:83–93. doi: 10.1530/ERC-11-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-Fluoro-L-DOPA, 18F-Fluoro-Deoxyglucose, and 18F-Fluorodopamine PET and 123I-MIBG Scintigraphy in the Localization of Pheochromocytoma and Paraganglioma. Journal of Clinical Endocrinology and Metabolism. 2009;94:4757–4767. doi: 10.1210/jc.2009-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. Journal of Clinical Oncology. 2007;25:2262–2269. doi: 10.1200/JCO.2006.09.6297. [DOI] [PubMed] [Google Scholar]
- 10.Luxen A, Perlmutter M, Bida GT, Van Moffaert G, Cook JS, Satyamurthy N, Phelps ME, Barrio JR. Remote, semiautomated production of 6-[18F]fluoro-L-dopa for human studies with PET. Int J Rad Appl Instrum A. 1990;41:275–281. doi: 10.1016/0883-2889(90)90191-i. [DOI] [PubMed] [Google Scholar]
- 11.Taieb D, Timmers HJ, Hindie E, Guillet BA, Neumann HP, Walz MK, Opocher G, de Herder WW, Boedeker CC, de Krijger RR, Chiti A, Al-Nahhas A, Pacak K, Rubello D. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging. 2012;39:1977–1995. doi: 10.1007/s00259-012-2215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS, Kang EY, Kim KA, Lee NJ. Extraadrenal paragangliomas of the body: imaging features. American Journal of Roentgenology. 2006;187:492–504. doi: 10.2214/AJR.05.0370. [DOI] [PubMed] [Google Scholar]
- 13.Treglia G, Cocciolillo F, de Waure C, Di Nardo F, Gualano MR, Castaldi P, Rufini V, Giordano A. Diagnostic performance of 18F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis. European journal of nuclear medicine and molecular imaging. 2012;39:1144–1153. doi: 10.1007/s00259-012-2087-y. [DOI] [PubMed] [Google Scholar]
- 14.Rischke HC, Benz MR, Wild D, Mix M, Dumont RA, Campbell D, Seufert J, Wiech T, Rossler J, Weber WA, Neumann HP. Correlation of the Genotype of Paragangliomas and Pheochromocytomas with Their Metabolic Phenotype on 3,4-Dihydroxy-6-18F-Fluoro-L-Phenylalanin PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:1352–1358. doi: 10.2967/jnumed.111.101303. [DOI] [PubMed] [Google Scholar]
- 15.Hoegerle S, Ghanem N, Altehoefer C, Schipper J, Brink I, Moser E, Neumann HP. 18F-DOPA positron emission tomography for the detection of glomus tumours. European journal of nuclear medicine and molecular imaging. 2003;30:689–694. doi: 10.1007/s00259-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 16.Taieb D, Tessonnier L, Sebag F, Niccoli-Sire P, Morange I, Colavolpe C, De Micco C, Barlier A, Palazzo FF, Henry JF, Mundler O. The role of 18F-FDOPA and 18F-FDG-PET in the management of malignant and multifocal phaeochromocytomas. Clinical endocrinology. 2008;69:580–586. doi: 10.1111/j.1365-2265.2008.03257.x. [DOI] [PubMed] [Google Scholar]
- 17.Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van der Wal JE, Sluiter WJ, de Vries EG, Links TP. 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess. The Journal of clinical endocrinology and metabolism. 2009;94:3922–3930. doi: 10.1210/jc.2009-1054. [DOI] [PubMed] [Google Scholar]
- 18.Fottner C, Helisch A, Anlauf M, Rossmann H, Musholt TJ, Kreft A, Schadmand-Fischer S, Bartenstein P, Lackner KJ, Kloppel G, Schreckenberger M, Weber MM. 6-18F-fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to 123I-metaiodobenzyl-guanidine scintigraphy in the detection of extraadrenal and hereditary pheochromocytomas and paragangliomas: correlation with vesicular monoamine transporter expression. Journal of Clinical Endocrinology and Metabolism. 2010;95:2800–2810. doi: 10.1210/jc.2009-2352. [DOI] [PubMed] [Google Scholar]
- 19.Charrier N, Deveze A, Fakhry N, Sebag F, Morange I, Gaborit B, Barlier A, Carmona E, De Micco C, Garcia S, Mancini J, Palazzo FF, Lavieille J, Zanaret M, Henry JF, Mundler O, Taieb D. Comparison of [111In]pentetreotide-SPECT and [18F]FDOPA-PET in the localization of extra-adrenal paragangliomas: The case for a patient-tailored use of nuclear imaging modalities. Clinical endocrinology. 2011;74:21–29. doi: 10.1111/j.1365-2265.2010.03893.x. [DOI] [PubMed] [Google Scholar]
- 20.King KS, Chen CC, Alexopoulos DK, Whatley MA, Reynolds JC, Patronas N, Ling A, Adams KT, Xekouki P, Lando H, Stratakis CA, Pacak K. Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-D-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. The Journal of clinical endocrinology and metabolism. 2011;96:2779–2785. doi: 10.1210/jc.2011-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35:657–669. doi: 10.1016/s0896-6273(02)00827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cervera AM, Bayley JP, Devilee P, McCreath KJ. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Molecular cancer. 2009;8:89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hentschel M, Rottenburger C, Boedeker CC, Neumann HP, Brink I. Is there an optimal scan time for 6-[F-18]fluoro-L-DOPA PET in pheochromocytomas and paragangliomas? Clinical nuclear medicine. 2012;37:e24–29. doi: 10.1097/RLU.0b013e318238f550. [DOI] [PubMed] [Google Scholar]
- 24.Timmers HJ, Hadi M, Carrasquillo JA, Chen CC, Martiniova L, Whatley M, Ling A, Eisenhofer G, Adams KT, Pacak K. The effects of carbidopa on uptake of 6-18F-Fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:1599–1606. doi: 10.2967/jnumed.107.042721. [DOI] [PubMed] [Google Scholar]
- 25.Sandgren J, Andersson R, Rada-Iglesias A, Enroth S, Akerstrom G, Dumanski JP, Komorowski J, Westin G, Wadelius C. Integrative epigenomic and genomic analysis of malignant pheochromocytoma. Experimental & molecular medicine. 2010;42:484–502. doi: 10.3858/emm.2010.42.7.050. [DOI] [PMC free article] [PubMed] [Google Scholar]