Abstract
The sexually dimorphic medial preoptic nucleus (POM) in Japanese quail has for many years been the focus of intensive investigations into its role in reproductive behaviour. The present paper delineates a sequence of descending pathways that finally reach sacral levels of the spinal cord housing motor neurons innervating cloacal muscles involved in reproductive behaviour. We first retrogradely labeled the motor neurons innervating the large cloacal sphincter muscle (mSC) that forms part of the foam gland complex (Seiwert and Adkins-Regan, 1998, Brain Behav Evol 52:61–80) and then putative premotor nuclei in the brainstem, one of which was nucleus retroambigualis (RAm) in the caudal medulla. Anterograde tracing from RAm defined a bulbospinal pathway, terminations of which overlapped the distribution of mSC motor neurons and their extensive dorsally directed dendrites. Descending input to RAm arose from an extensive dorsomedial nucleus of the intercollicular complex (DM-ICo), electrical stimulation of which drove vocalizations. POM neurons were retrogradely labeled by injections of tracer into DM-ICo, but POM projections largely surrounded DM, rather than penetrated it. Thus, although a POM projection to ICo was shown, a POM projection to DM must be inferred. Nevertheless, the sequence of projections in the male quail from POM to cloacal motor neurons strongly resembles that in rats, cats and monkeys for the control of reproductive behaviour, as largely defined by Holstege and co-workers (e.g., Holstege et al., 1997, Neuroscience 80: 587–598).
Keywords: Foam gland, cloacal motor neurons, bulbospinal projections, nucleus retroambigualis, intercollicular nucleus, DM, medial preoptic nucleus
In mammals considerable experimental effort has been expended to define the neural pathways that mediate control of reproductive behaviour (Pfaff et al., 1994). In females one major objective has been to characterize the nuclei and define the pathways that control the species typical, estrogen-dependent mating posture signifying sexual receptivity, known in rats and cats as lordosis. Although there are differing accounts of the origins of the bulbospinal projections to spinal motor neurons involved in lordosis (cf Pfaff et al, 1994; VanderHorst and Holstege, 1995; Holstege et al., 1997), a strong case can be made for the view that the relevant synaptically connected nuclei are, from rostral to caudal, the ventromedial nucleus of the hypothalamus (VMN), the periaqueductal gray (PAG) of the midbrain, nucleus retroambiguus (NRA) in the caudal medulla, and its bulbospinal projections to spinal motor neurons innervating a host of striated muscles of the trunk and limbs involved in the production of the lordosis posture (Ogawa et al., 1991; Flanagan-Cato, 2011; VanderHorst and Holstege, 1995; 1996; 1997a; VanderHorst et al., 2000a;. 2000b). In the present context it is appropriate to emphasize that an early designation of NRA as a respiratory premotor nucleus – because of its projections upon spinal motor neurons innervating expiratory muscles – is misleading, in the sense that its spinal projections are by no means confined thereto. Pathways for the control or modulation of male reproductive behaviour, such as mounting and possibly penile erection, have also implicated a PAG-NRA-lumbosacral spinal cord sequence of connections (Holstege et al., 1997; VanderHorst and Holstege 1997b). With respect to hypothalamic control, however, it is generally recognised that it is the medial preoptic area that is critical for male sexual behaviour, rather than VMN (Hull et al., 2002).
In birds comparatively little is known about the neural pathways that control reproductive behaviour, in particular those caudal to the hypothalamus. In female birds in breeding condition, sexual receptivity in many species is indicated by an estrogen-dependent posture called the copulation solicitation display (CSD), which closely resembles lordosis in mammals. The supraspinal control of this posture is, however, unknown except for the fact that estradiol benzoate stimulates this behaviour in ring doves (Streptopelia risoria) when stereotaxically implanted in VMN (Gibson and Cheng, 1979). In male birds, as in male mammals, the medial preoptic nucleus (POM), plays a major role in reproductive behaviour, evidenced in particular in the Japanese quail (Coturnix japonica), which has become the model species of choice for the investigation of the neuroendocrine control of reproductive behaviour in birds (Panzica et al., 1996; Balthazart et al., 2009; Ball and Balthazart, 2009; 2010).
Further understanding of how POM might mediate control of reproductive behaviour in birds entails the identification of neural pathways that link the nucleus with final common path motor neurons, specific groups of which lie in the sacral spinal cord and innervate the various cloacal muscles involved in appetitive and/or consummatory aspects of reproductive behaviour, as well as in other behaviours such as voiding (Seiwert and Adkins-Regan, 1989). By far the largest of these cloacal muscles, both in terms of absolute size and number of motor neurons innervating it, is the circular, sphincter cloacae muscle (mSC; Seiwert and Adkins-Regan, 1998). In male Japanese quail mSC is hypertrophied on the dorsal wall of the cloaca, where its three muscular layers overlie and interdigitate with units of the proctodeal gland, which continuously secretes a clear, colorless fluid. The muscle and gland are together known as the foam gland complex (Klemm et al., 1973; King, 1981a), which in adult birds typically reaches the size of a walnut. Visible, vigorous contractions of the foam gland are stimulated simply by the male’s view of the female as well as during copulation (Seiwert and Adkins-Regan, 1998). These contractions reflect the sexual motivation of the male (Ball and Balthazart, 2010). They are inhibited by electrolytic lesions of POM and controlled by testicular secretions (suppressed by castration and restored by exogenous testosterone given systemically or directly implanted in POM) (Balthazart et al., 1998, Riters et al., 1998). By these contractions the muscles of the gland are thought to whip the gland’s secretions into a meringue-like foam that is transferred to the female’s cloaca during copulation and is also deposited during voiding (McFarland et al., 1968; Ikeda and Taji, 1954; Seiwert and Adkins-Regan, 1989). Females do not normally possess a hypertrophied foam gland, although the same muscles of the dorsal cloaca are present as in males and the number of motor neurons innervating these muscles is similar to that in males (Seiwert, 1994). A foam gland complex up to 60% of the size of the male’s can be induced in the female by testosterone treatment over a two-week period (Adkins and Adler, 1972; Schumacher and Balthazart, 1983). The motor neurons innervating mSC and three other cloacal muscles are reported to be intermixed in the lateral motor column of lamina IX of synsacral segments 7 to 10 (Seiwert and Adkins-Regan, 1998). They constitute, therefore, a principal target of descending pathways mediating control of cloacal movements characteristic of reproductive and other vital behaviours in this species.
Since POM does not project to the spinal cord, there has to be one or more synapses between it and cloacal motor neurons. One of these synapses is thought to lie in the central gray matter (GCt) surrounding the cerebral aqueduct of the midbrain, to which POM projects (Berk and Butler, 1981; Balthazart et al., 1994; Absil et al., 2001; Carere et al., 2007). GCt has specifically been suggested to be the medial component of the laterally extensive intercollicular nucleus (ICo; Kingsbury et al., 2011), which is likely homologous with the periaqueductal gray (PAG) of mammals. In male starlings Riters and Alger (2004) indeed showed, using anterograde tracers, that POM projects to both ICo and GCt, and also suggested that this projection included DM, a distinct dorsomedial subnucleus within the ICo complex. In both songbirds and non-songbirds, electrical stimulation of DM drives vocalizations and accompanying respiratory patterns that can resemble the call of the species, even in surgically anesthetised birds (e.g., Brown, 1965; Potash, 1970; Phillips and Peek, 1975; Seller, 1981; Wild et al., 1997). This is explained by the fact that DM projects upon a suite of vocal-respiratory brainstem nuclei that includes the vocal motor nucleus (n. tracheosyringealis, or XIIts) and nucleus retroambigualis (RAm; Wild et al., 1997). RAm projects upon lower thoracic and upper lumbar motor neurons that innervate expiratory muscles, and also projects upon XIIts, a projection thought to underlie the coordination of expiration and vocalization in the production of species-typical calls or songs (Wild, 1993; 1994; Reinke and Wild, 1997; 1998; Sturdy et al., 2003; Kubke et al. 2005). Although the resemblance of the projections of DM and RAm in birds to those of the lateral PAG and NRA in mammals has been noted in the context of vocalization and respiration (Wild et al., 1997; Wild et al., 2009), it has not hitherto been asked whether RAm also has projections to the sacral spinal cord that, like those of NRA, may be involved in reproductive behaviour.
How ICo might mediate control of reproductive behaviour in birds is unknown, for the direct or indirect connections of ICo with cloacal motor neurons have not been defined. Some ICo neurons have been shown to project as far as the upper cervical spinal cord in pigeons, ducks and geese, but they do not appear to reach as far as lumbosacral levels (Cabot et al., 1982; Webster and Steeves, 1988). In the present study, we investigated (1) whether RAm projects to sacral spinal levels housing cloacal motor neurons, (2) whether RAm neurons receive projections directly from either POM and/or DM-ICo, and (3) whether POM projects upon DM-ICo.
MATERIALS AND METHODS
Fifty-three male Japanese quail (Coturnix japonica) were used in the present study. All anesthetic and surgical procedures were in agreement with the relevant Belgian laws regarding the Protection and Welfare of Animals and the Protection of Experimental Animals, and all protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Anesthetic regime
Males, weighing 200–250 gms, were anesthetised by an injection in the breast muscle of 0.1 ml of ketamine (100 mg/ml) and 0.2 ml of xylazine (Rompun 2%, Bayer), which produced surgical anesthesia within 5–10 minutes and lasted for up to 3.5 hours.
Surgical regime for recording, stimulation and injection of tracers in the brain
The bird’s head was held in a Kopf stereotaxic frame with pigeon beak and ear bars such that the beak was angled down at 45 degrees with respect to the horizontal (Karten and Hodos, 1967). The skin was reflected from the midline of the skull and the dura exposed by drilling through the bone at chosen positions. Although a stereotaxic atlas exists for the Japanese quail (Baylé et al., 1974), it lacks detail for the brainstem and, furthermore, caudal brainstem nuclei such as RAm could not be targeted by recording electrodes or injection pipettes using vertical penetrations through the most caudal cerebellum, because this region was covered by large blood sinuses. Therefore, a sagittal plate of the atlas (L0.5) was used to calculate an angle from which caudal brainstem nuclei could be targeted by angling the electrode or pipette 38 degrees backwards through a more rostral cerebellar lobule clear of blood sinuses (~3.8 mm caudal to inter-aural zero; but note that this does not correspond to the coordinates in the Baylé et al. quail atlas). Electrode and pipette movements in the ‘z’ direction were controlled by a Kopf Micropositioner, Model 2650. Target coordinates were worked out by trial and error, guided by recordings of single and multiunit activity using either tungsten microelectodes (Frederick Haer and Company, Bowdoinham, NM; 3–5 MΩ) or glass micropipettes (internal diameter ~10 microns) filled with one of a number of neural tracers dissolved in phosphate buffered saline (PBS), pH 7.6. Signals were band-passed filtered between 300–5000 Hz, amplified x10k (A-M Systems, Model 1800) and monitored auditorily. Spike trains were recorded on computer using an ADI MacLab 8/30 and Scope software. Since RAm was a prime target, rhythmical multiunit activity in phase with observed expiration (Wild, 1993) was the principal guide to the site of tracer injection in this case. Such activity was recorded ~ 0.8 mm lateral to the midline and ~ 5.5 mm along the angled trajectory.
Electrical stimulation of 3–6 volts supplied by a Grass model S88 stimulator was used to evoke vocalizations (crowing) from the midbrain. Two-second trains of 0.2 ms square wave pulses at 200 pps were supplied every 4 seconds to a tungsten microelectrode advanced though the overlying optic tectum and medial to the inferior colliculus, known in birds as MLd. Once robust crowing had been evoked the tungsten microelectrode was withdrawn and replaced with a glass micropipette filled with tracer, which was then lowered through the same brain entry point to the same depth and an injection was made using either air pressure of iontophoresis. Approximate coordinates for these injections were: A5.0, L2.2, D4.6 mm.
Injection of tracers in the spinal cord
A laminectomy was performed at predetermined levels of the sacral spinal column and unilateral air pressure injections of tracer were made into one or two segments of the cord (between SS7and SS10) via a glass micropipette (internal diameter ~ 20 microns) inserted through the dorsal horn and dorsolateral funiculus into more ventrolateral regions.
Injection of tracers in cloacal muscles
The anesthetized bird was placed on its back and the legs taped to the substrate such that the large foam gland was exposed. The thin skin of the gland was cut and separated from the underlying sphincter cloacae muscle. Several (3–5) injections of tracer were then made via a glass micropipette using air pressure into different parts of the muscle, usually on one side only. In one case as much as possible of the exposed muscle on one side was multiply injected to maximize the number of retrogradely labeled motor neurons. In two other cases the much smaller transverse cloacal muscle (mTC) received a single injection of tracer.
Injected tracers
In most cases different fluorescent cholera toxin B-chain (CTB) tracers were used, either singly or in paired combinations in the same bird: CTB Alexafluor 594, 555 or 488 (Molecular Probes; 1.0% in 0.1 M PBS, pH 7.6). These tracers (e.g., a green and a red), or in some cases Fast Blue (Polysciences; Philadelphia, PA; 2% aqueous), which fluoresces in the UV range, were injected using a WPI pneumatic PicoPump (Model PV 800) that supplied timed pulses of air to the injection pipette via a 0.5 M length of tubing. Biotinylated dextran amine (BDA, Invitrogen, Eugene, OR; 10K MW, 10% in PBS) was also used in some cases, in combination with a second injection of unconjugated CTB (List Biological Laboratories, Campbell, CA; 1% in PBS) at another site. BDA and CTB were injected either iontophoretically using a Stoelting high voltage current source (2–4 μA, positive current) or via air pressure.
Following completion of each experiment, the bird was recovered in a warmed environment, usually overnight, and allowed to survive 3–5 days, after which it was deeply anesthetised with an intramuscular injection of an overdose of ketamine/xylazine and perfused through the heart with 300 ml normal saline followed by 500 ml 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, pH 7.4. Brains and spinal cords were post-fixed for 24 hours and then equilibrated in 30% sucrose buffer for 24–48 hrs.
Histology
Brains were blocked in the transverse stereotaxic plane and 40-micron thick sections were cut on a freezing, sliding microtome. Spinal cords were sectioned at 40 microns either in the longitudinal or transverse planes, from the middle of the lumbosacral enlargement through levels caudal to the caudal limit of mSC motor neurons (SS 10: Seiwert and Adkins-Reagan, 1998). Longitudinal spinal cord sections were collected from dorsal to ventral, while transverse sections were collected from caudal to rostral. All sections were collected serially in PBS; if intended to be analysed using fluorescence microscopy, they were mounted serially on Superfrost slides (Fisher), air dried, and coverslipped using either 9:1 glycerol-PBS or a hard-setting fluorescent mounting medium (VectaShield, Vector Laboratories, Burlingame, CA).
Immunohistochemistry
Sections requiring immunohistochemistry were first treated for 20 min with 50% methanol in PBS containing 1% hydrogen peroxide and washed 3×10 min in PBS. They were then incubated for 1 hr in streptavidin-horseradish peroxidase (HRP) conjugate (Invitrogen), 1:1,000 in 0.4% PBS-Triton X-100, washed 3×10 min in PBS, and treated with 0.025% 3,3′-diamino-benzidine (DAB) in PBS containing 0.015% cobalt chloride to produce a black reaction product. If the brain had also received an injection of CTB, the sections were thoroughly washed in PBS and then incubated overnight in a goat anti-CTB antibody (List Laboratories, Campbell, CA) at 1:33,000 final dilution in 0.4% PBS-Triton X-100 and 25 μl/ml normal rabbit serum. The CTB antibody was raised against purified choleragenoid and does not result in labeling following preabsorption of the antibody with excess concentration of choleragenoid (Stocker et al., 2006), and no labeling is seen in material in which a CTB injection has not been performed (Kubke et al., 2004). Sections were then incubated for 1 hr in a biotinylated rabbit anti-goat secondary antibody (Sigma-Aldrich, St Louis, MO) 1:300 in PBS-Triton X-100, washed 3×10 min in PBS, and incubated for a further hour in streptavidin-HRP at 1:1,000 in PBS. CTB was visualized using the DAB mixture without cobalt chloride, which yielded a brown reaction product. Sections were mounted on Superfrost slides, dehydrated in a graded alcohol series and coverslipped with Eukitt (Fluka).
Section analysis
Sections were viewed in brightfield or fluorescence using a Leica DMRB FL.100 microscope, photographed with a Leica DFC480 digital camera and images stored on a Macintosh computer running Leica FireCam 3.4.1 software. Fluorescent images were exported to PhotoShop where they were adjusted for brightness and contrast, and red/green composite images were constructed using the ‘difference’ module. Some sections were drawn using an Olympus microscope equipped with a drawing tube and redrawn and labeled using Canvas 9.04 (ACD Systems, Victoria, BC, Canada).
RESULTS
Cloacal motor neurons
Injections of CTB, Fast Blue, or CTB Alexafluor 488, 594 or 555 were made into the mSC of 10 males. Nine of these injections were subtotal but were distributed throughout different parts of the muscle unilaterally. In one of the males an attempt was made to inject as much of mSC as possible on one side and two others received an injection of one fluorescent tracer into mSC and another fluorescent tracer into m. transverse cloacae (mTC) on the same side.
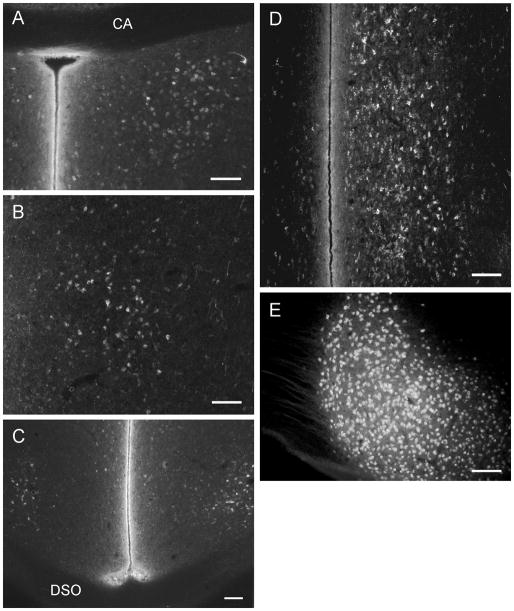
Longitudinal sections of the spinal cord in different cases showed that all labeled neuronal cell bodies were located in the lateral motor column of the ventral horn between SS segments 7 and 10 (Fig. 1A), confirming the results of Seiwert and Adkins-Regan (1998). Transverse sections from cases with subtotal mSC injections showed more clearly that the labeled motor neurons occupied primarily the ventral part of the lateral motor column of the ventral horn (Fig. 1B). The great majority of mSC labeled motor neurons were ipsilateral to the side of injection, although a few were found contralaterally, possibly due to spread of tracer across the midline of the muscle. Labeled mTC motor neurons were located slightly more dorsal than mSC motor neurons, but the full extent of labeling from the much smaller mTC was not determined. The case receiving a near-total injection of mSC on one side showed that labeled motor neuron cell bodies were densely packed and occupied most of the lateral motor column (Fig. 1C).
Figure 1.
Photomicrographs of retrogradely labeled motor neurons in the ventral horn (vh) of the sacral spinal cord following injections of CTB 488 (A, longitudinal section) or CTB 555 (B, C, transverse sections) into the ipsilateral foam gland. In A multiple injections were made into the gland, in B only a single injection in the gland was made, while in C as much of the gland as possible on one side was injected. Note in A the medial extension of dendrites at this ventral level of the cord. In contrast, note in B and C the very extensive dorsally directed dendrites that extend into the white matter and towards the medial aspect of the lateral funiculus (lf) and neck of the dorsal horn (dh). The asterisk denotes marginal, not dorsal root ganglion cells. Scale bar = 200 μm.
In both longitudinal and tranverse sections the ramification of motor neuron dendrites was revealed to be very extensive indeed. In longitudinal sections it appeared that the majority of dendrites were directed medially as far as the midline, but transverse sections revealed this to be a false impression created by cutting though dorsally directed dendrites orthogonal to their long axis. In fact, the majority of the dendrites were directed dorsally into the white matter and through the lateral aspect of the grey matter to reach as far as the medial part of the lateral funiculus and the base of the dorsal horn (Fig. 1C).
Location of bulbospinal neurons
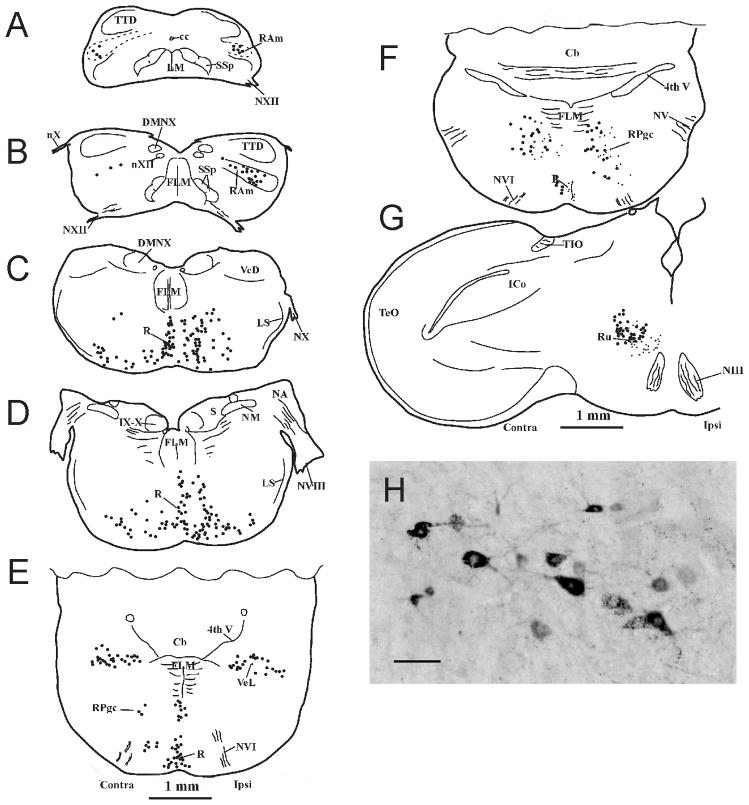
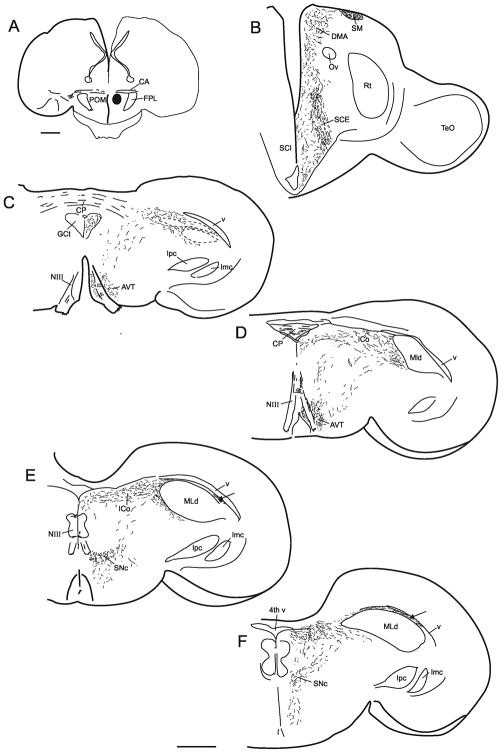
Injections of either unconjugated or fluorescent CTB were made into the sacral spinal cord at the level of SS8–9 in 7 males. The general pattern of retrograde labeling of supraspinal structures, in terms of which nuclei were labeled, was similar across these cases, although the number of neurons labeled in these nuclei in each case varied considerably, presumably depending on the exact location of the injections and the degree of tracer uptake. An example of the mediolateral location and rostrocaudal distribution of this retrograde labeling is shown in Figure 2. All but one of the nuclei was labeled bilaterally, with an ipsilateral predominance. From caudal to rostral, the most caudal labeled neurons were located between dorsal and ventral horns of the spino-medullary junctional region. Slightly more rostrally labeled neurons of heterogeneous size were scattered along a dorsomedial-to-ventrolateral diagonal throughout the dorsal central region of the caudal medulla. Together these two caudal regions correspond to caudal parts of RAm (Wild et al., 2009). More rostrally in the medulla many neurons in the raphe were labeled, accompanied by numerous labeled neurons in the centromedial and ventromedial medulla, with fewer labeled neurons extending into ventrolateral regions. In the pons labeling of the raphe continued, but labeling of adjacent ventromedial regions was largely absent. The large neurons of the lateral vestibular nucleus were distinctly labeled, as were the gigantocellular neurons of the reticular formation. At midbrain levels the contralateral red nucleus was labeled, but no labeled neurons were observed in any part of the intercollicular nucleus. Apart from a few labeled neurons in the caudomedial hypothalamus in some cases, the only other labeled nucleus, and the most rostrally located, was the paraventricular nucleus (PVN, not shown). Labeling in this nucleus extended as far rostral as the level of the anterior commissure.
Figure 2.
A–G. A caudorostral series of schematic transverse sections through the brainstem of the quail showing the location of neurons (1 dot = 1 neuron) retrogradely labeled by an injection of CTB into the sacral spinal cord. Those on the right of each section are ipsilateral to the injection. Note in G that the absence of labeled neurons in ICo also pertains to the ipsilateral side. In H are shown photomicrographically some of the labeled neurons in RAm (Scale bar = 100 μm).
Bulbospinal projections
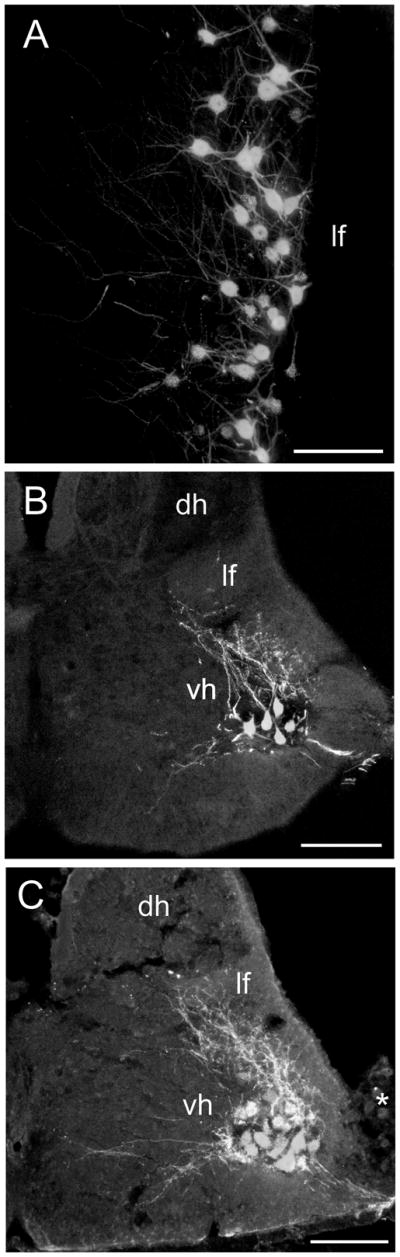
It was beyond the scope of this study to inject systematically all the brainstem nuclei that were retrogradely labeled by injections in the sacral spinal cord. Here we concentrate on RAm (Fig. 3A) because, as mentioned in the Introduction, the putative homologous nucleus in mammals (NRA) has been shown by Holstege and co-workers to project to regions of the spinal cord ventral horn housing motor neurons purported to be involved in reproductive behaviour in both male and female cats and rats (Holstege et al., 1997; VanderHorst and Holstege 1995; 1996; 1997; 2000a; 2000b). Injections of fluorescent CTBs were aimed at RAm in 8 quail, following the recording of respiratory related unit activity in the caudal medulla (Fig. 3B) (see Methods and Materials). All but one quail also received another injection of CTB of a different colour into mSC, ipsilaterally. Of the 8 injections aimed at RAm, 2 did not produce any projection to the spinal cord, presumably because their injections were off target, 1 being centered dorsal to RAm and 1 ventral to RAm. One of the remaining 6 cases had a partial hit of RAm and produced sparse projections to the spinal cord, but in the other 5 cases the injections were well centered on RAm and produced abundant anterograde labeling throughout the sacral spinal cord, always with the same axonal trajectory and pattern of termination. In 2 of these cases labeling was also assessed in upper lumbar regions and found to be present in the lateral motor column of the ventral horn, as previously reported for RAm projections to expiratory motor neurons at these levels (Wild, 1993; 1994). In the one case not receiving a second injection of CTB into mSC, with the cord being sectioned longitudinally, labeled fibers were observed in the more medial part of the lateral funiculus and, further ventrally, putative terminations were present throughout the lateral motor column, frequently appearing in proximity to the ghosts of unlabeled motor neurons (Fig. 4A). In cases that also received an injection into mSC (e.g., of CTB 488), cloacal motor neurons and their extensively projecting dendrites were retrogradely labeled, to the extent that their cell bodies were greatly oversaturated photographically (This could not be substantially corrected in Photoshop without also reducing the visibility of the distal dendrites. Because most of the putative terminations appeared to be in relation to dendrites rather than cell bodies, we considered it more important to be able to see the dendrites). Labeled descending axons from the RAm injection (e.g., of CTB 555) occupied the medial part of the lateral funiculus ipsilateral to the RAm injection and labeled fibers and presumptive terminations completely overlapped the distribution of retrogradely labeled cloacal motor neurons within the ventral horn (Figs. 4B). In transverse sections labeled fibers could be seen to approach the labeled cell bodies and their proximal dendrites from the medial part of the lateral funiculus and frequently appeared to terminate in their vicinity (Fig. 4C, D). Labeled fibers were not observed in the contralateral lateral funiculus at sacral levels, but labeled fibers and putative terminations were almost as common in the contralateral lateral motor column at these levels as they were ipsilaterally. This suggests that descending RAm axons crossed the midline approximately at the level of termination, although unequivocal visual proof of this was not obtained.
Figure 3.
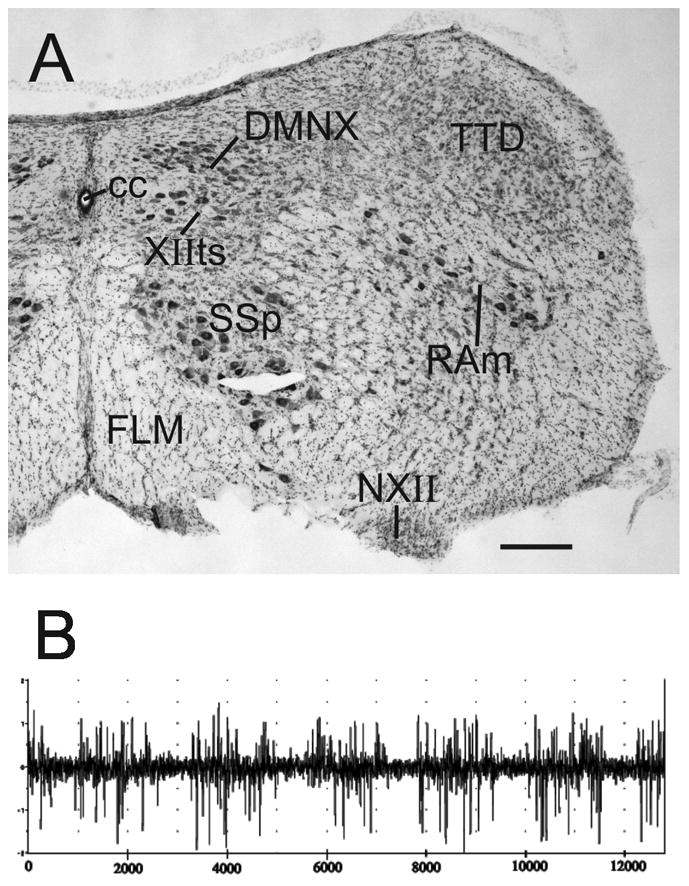
A. Nissl counterstained right transverse hemisection through the caudal medulla showing the diagonal distribution of RAm neurons. Scale bar = 200 μm. B. An extracellular multiunit recording of respiratory-related activity in RAm. Although the phase of respiration was not measured, it was clear from visual inspection of the anesthetised bird that the rhythmical bursts of discharges over the 12 seconds corresponded to expiration.
Figure 4.
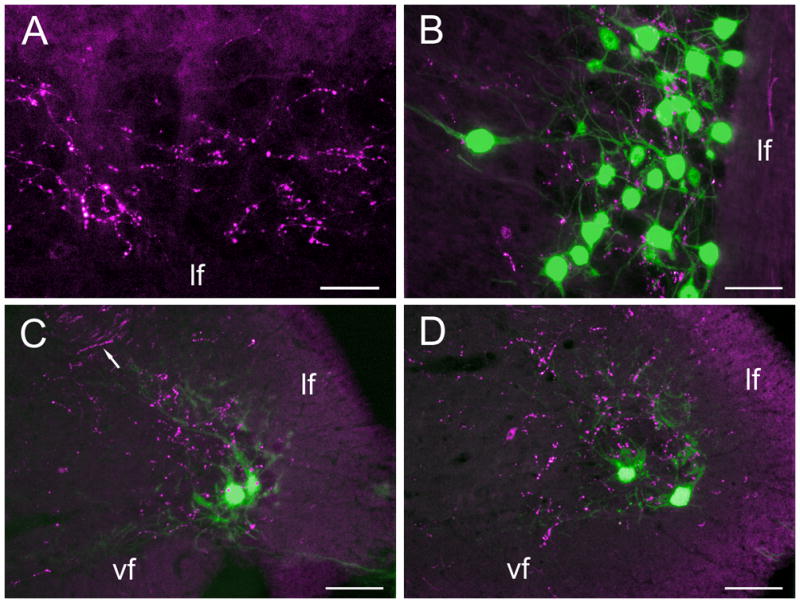
Photomicrographs of anterogradely labeled fibers and putative terminations (red) and retrogradely labeled neurons (green) in the right half of the sacral spinal cord following an injection of CTB 555 into RAm and of CTB 488 into the ipsilateral foam gland in the same animal. A and B are longitudinal sections, C and D are transverse sections. A is turned 90 degrees clockwise and shows only labeled fibers and putative terminations (the foam gland was not injected) throughout the ventral horn adjacent to the lateral funiculus (lf). B shows both fibers and cell bodies with their extensive medially directed dendrites at this ventral level of the cord. In C dorsally directed green dendrites can be seen approximating red axons in the medial part of the lateral funiculus (arrow). vl: ventral funiculus. Green cell bodies are inadvertently oversaturated photographically, but a reduction of their saturation also reduced the visibility of their distal dendrites – see text. Scale bars = 100 μm.
Retrograde labeling from RAm injections
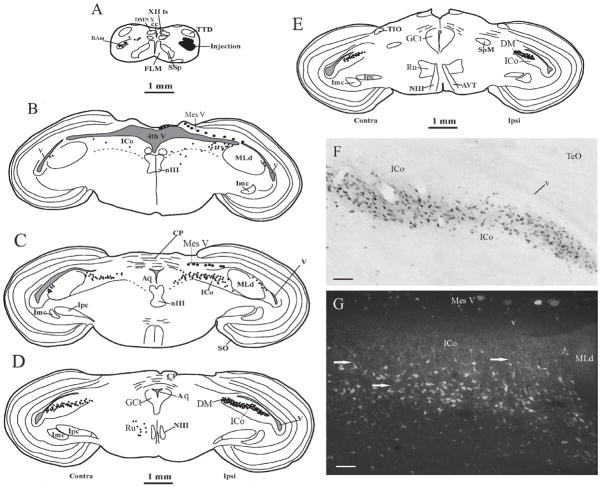
Each of the RAm injections that produced anterograde labeling in the spinal cord also produced abundant retrograde labeling of specific brainstem nuclei, with a pattern similar to that produced by RAm injections in other avian species (Wild et al., 1997). The major difference in the quail, however, was in the unparalleled extent and density of retrograde labeling of a mediolaterally extensive band of neurons within the midbrain intercollicular complex (ICo), predominantly ipsilaterally (Fig. 5). (In other avian species the nucleus within ICo that projects upon RAm (and XIIts) has been called DM (see Wild et al., 1997) because of its dorsomedial position with respect to the central nucleus of the inferior colliculus (MLd). In the present study we chose to adopt the same name (DM) for the midbrain nucleus that was retrogradely labeled from RAm injections, even though, in the quail, the nucleus extends much more medially and rostrally than in any other avian species thus far examined – see also Discussion). Most caudally, numerous labeled neurons were scattered throughout ICo medial to MLd, with a few extending medially into the central gray (GCt) and a small cluster between the lateral border of MLd and the ventricle (Fig. 5 B and C). Proceeding rostrally throughout more rostral levels of MLd, the labeled neurons clustered more and more densely and approximated the labeled neurons lateral to MLd until they formed a continuous, obliquely oriented band (Fig. 5F). This band then extended rostrally from the rostral pole of MLd as far as the diencephalic-mesencephalic border (Figs. 5D, E). A distinct feature of many of the DM neurons, particularly caudally, was a dorsally directed process into the overlying ICo (Fig. 5G). Some retrogradely labeled neurons were also observed in the more lateral parts of POM in two of the RAm injection cases, but we consider that this putative projection requires substantial further verification retrogradely and anterogradely before it can be accepted; it is therefore not dealt with further here (see figure 11).
Figure 5.
B–E. Schematic representations of the location of ICo and DM cell bodies (1 dot = 1 cell body) retrogradely labeled by an injection of CTB centered on RAm (shown in black in A). Note that the majority of labeled neurons in DM are ipsilateral to the injection, and in B and C are split into a numerous medial group and a small group lateral to MLd. Rostral to MLd (D and E) DM cells form a continuous band that extends as far as the caudal border of the diencephalon. Large dots represent labeled neurons of the mesencephalic nucleus of the trigeminal nerve (Mes V) inadvertently labeled by dorsal spread from the RAm injection (see Wild and Krützfeldt, 2012). F and G are photomicrographs of labeled DM neurons, those in F forming a continuous band rostral to MLd and those more caudally in G showing dorsally directed processes (arrows) into ICo. Scale bars = 100 μm.
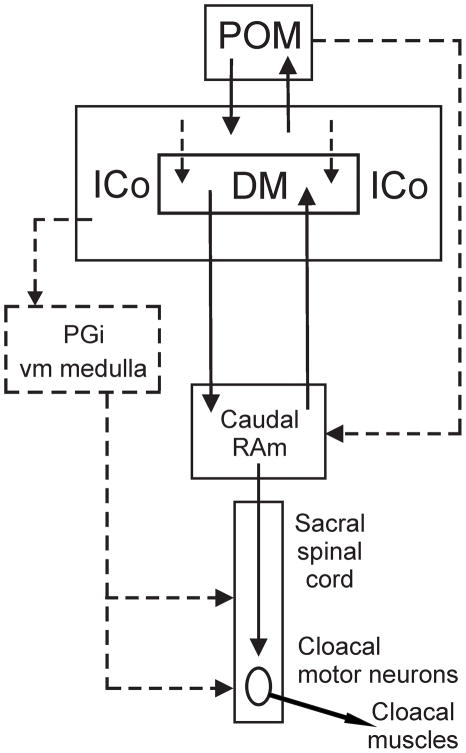
Figure 11.
Schematic depiction of the projections defined in the present study (solid lines). Note that DM is shown as a specific subnucleus within ICo, although caudally DM neurons are scattered within ICo rather than being tightly clustered. Dashed lines indicate possible projections that require further definition.
Anterograde projections resulting from injections in DM-ICo
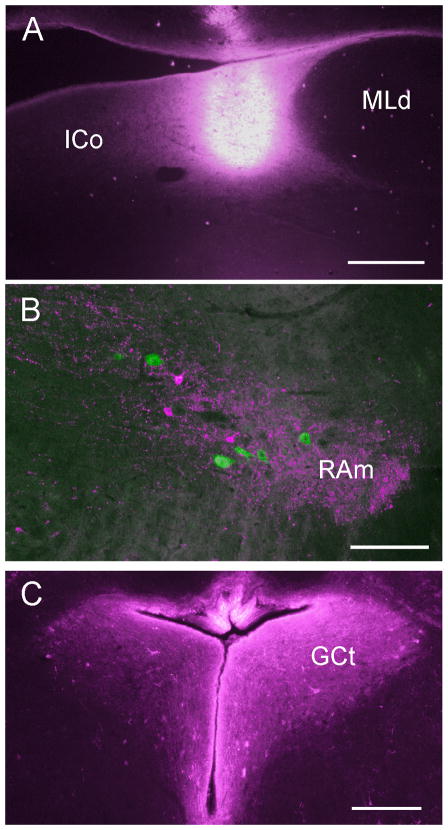
Because of the narrow dorsoventral extent of DM, injections aimed at this structure also included parts of the surrounding ICo, with a variable degree of involvement of the overlying ventricular lining and tectum. Injections centered on DM were made possible in 4 males by first using electrical stimulation to evoke vocalizations from DM (see Materials and Methods). Air pressure injections of CTB 555 were then made at depths at which robust crowing and other less intense vocalizations had been evoked. A curious feature of this stimulation was that vocalizations continued for as much as 30 seconds after the stimulus was turned off. Figure 6A shows an example of an injection located medial to MLd, centered on DM and largely confined within the dorsal and ventral boundaries of ICo. This and other injections at different loci in DM-ICo produced anterograde labeling in all the more caudal brainstem nuclei previously shown to receive projections from DM in other avian species, including XIIts and RAm (Wild et al., 1997). Figure 6B shows a terminal field in RAm in one of two cases in which an injection of CTB 488 had also been made into the sacral spinal cord on the same side. Each injection into DM-ICo also produced anterograde and some retrograde labeling throughout other parts of ICo, including GCt, which at rostral levels is isolated from the rest of ICo by the posterior commissure (Fig. 6C). Diffuse projections from the injections also reached the ipsilateral ventral pons and upper medulla (not shown – see Discussion and figure 11).
Figure 6.
Photomicrographs showing, in A, an injection of CTB 555 in ICo medial to MLd; in B, a terminal field (red) in RAm resulting from the injection shown in A and retrogradely labeled neurons (green) resulting from an injection of CTB 484 in the sacral spinal cord in the same case. Note the presence of a few red retrogradely labeled cells within the terminal field, indicating reciprocity of the ICo-RAm projections (see Wild et al., 2009). C: Predominantly ipsilateral terminations in the central gray (GCt) resulting from the injection in A. Scale bars = 200μm.
Retrograde labeling resulting from injections in DM-ICo
Injections of CTB 555 in DM-ICo retrogradely labeled neurons in lower brainstem respiratory-vocal nuclei, including RAm, as seen in other avian species (Wild et al., 1997). They also labeled neurons in POM, in an unidentified hypothalamic nucleus ventral and caudal to POM, and in the lateral hypothalamus bilaterally (Fig. 7A–C). Dense retrograde labeling was also found more caudally in the hypothalamus, including the ventromedial nucleus (VMN, Fig. 7D), but by far the densest labeling was produced throughout the medial and ventral arcopallium, ipsilaterally (Fig. 7E), with more scattered labeling in the hyperpallium apicale (HA, not shown).
Figure 7.
Photomicrographs of neurons retrogradely labeled by an injection in ICo, such as is shown in figure 6A. Those in A are in POM, those in B are located ventral to POM at about the same rostrocaudal level, those in C are in the lateral hypothalamus bilaterally, those in D are in the more caudal hypothalamus, including VMN, and those in E are in the ventromedial arcopallium. Scale bars = 100μm.
Projections of POM to DM and ICo, etc
To determine the specificity of DM and ICo hypothalamic afferents, injections of BDA, CTB or fluorescent CTB were made in POM in 20 cases. For unknown reasons, iontophoretic injections of BDA in POM proved less than satisfactory, in that solid deposits of tracer could not be made, despite variation in pipette tip size, current strength, etc. Figure 8 traces the projections throughout the ICo and other nuclei resulting from one of several cases of iontophoretic BDA injections in POM that labeled only a variable number of single neurons at the injection site. This injection was paired with a pressure injection of CTB centered on POM on the opposite side. Labeled fibers, many with varicosities typical of BDA labeling, could be traced ventrally, dorsally and caudally from the injection site, predominantly ipsilaterally. Ventrally, numerous fibers descended through the stratum cellulare externum (SCE), while more scattered fibers and terminations were present in the stratum cellulare internum (Fig. 8B). More caudally terminations were apparent in the tuberal nucleus. Dorsally fibers ascended through the medial diencephalon, providing terminations to the dorsal anterior and dorsal posterior thalamic nuclei (DMA and DMP) and specifically to the nucleus of the stria medullaris (Fig. 8B). Further caudally fibers and presumptive terminations extended throughout the rostrocaudal and mediolateral extent ICo, including GCt. They also extended laterally between the lateral border of MLd and the overlying ventricle to terminate in the same place where retrogradely labeled cells were found following injections in RAm (see above). Labeled fibers also headed ventromedially from ICo to form diffuse terminal fields, first in the ventral area of Tsai (AVT), mostly lateral to the exiting third nerve, but also medial to it (Fig. 8C, D), and then in the substantia nigra pars compacta (SNc) (Fig. 8E, F).
Figure 8.
A–F: Schematic hemisections depicting the projections (short fine lines and dots) resulting from a BDA injection in POM, shown as black in A and photomicrographically in figure 9. Arrows in E and F point to fibres and terminations lateral to MLd. The two areas enclosed by dashed lines in C receive little or no labeling. The more dorsal area is DM, the more ventral one receives input from the arcopallium – see figure 9. Scale bars = 1 mm.
Injections of CTB that were centered on POM replicated this pattern of labeling, but showed more clearly that there were two separate regions within rostrolateral ICo that received little or no projection from hypothalamic injections, one lying dorsal to the other (Fig. 9). They also indicated that connections between POM and ICo, GCT, SNc and AVT were reciprocal, by showing retrogradely labeled cells scattered throughout these nuclei, largely with the exception of the two regions that did not receive anterograde projections.
Figure 9.
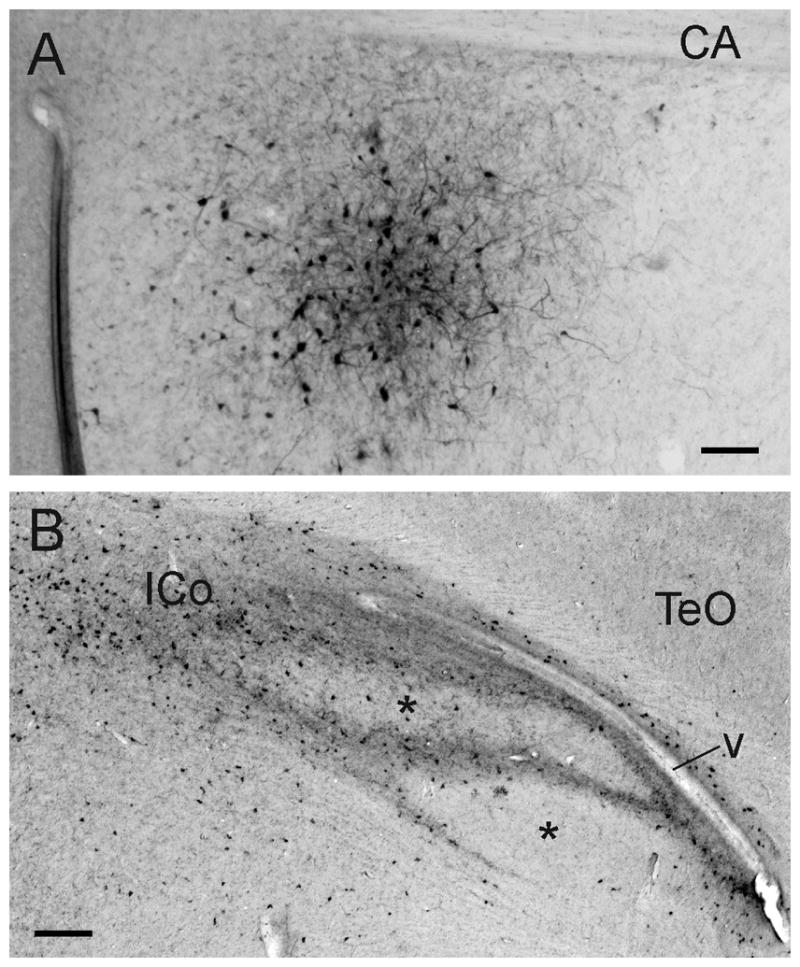
A: Photomicrograph of an iontophoretic BDA injection in POM (just ventral to the anterior commissure, CA) that gave rise to the projections depicted in figure 8. B: anterograde and retrograde labeling in ICo following an injection of CTB centered on POM. Note the two relatively unlabeled areas (asterisks), one dorsal to the other. The dorsal one is DM, the ventral one an area receiving projections from the ventromedial arcopallium (see text and figures 8 and 10E). Scale bars = 100 μm.
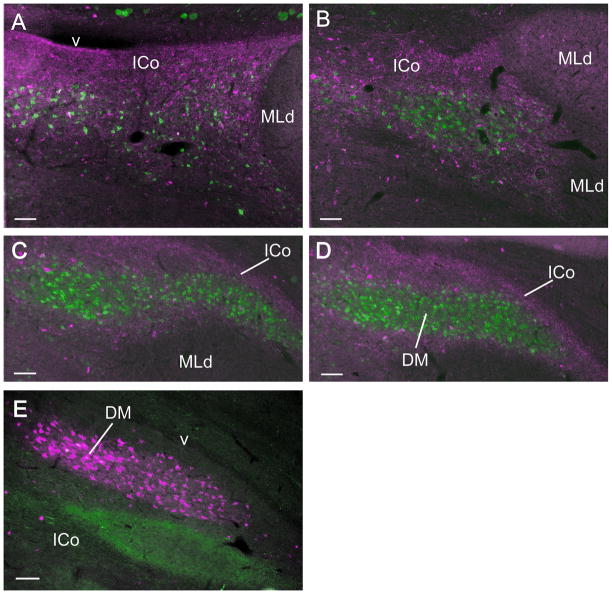
In 2 cases a large injection of CTB 488 centered on POM was combined with an injection of CTB 555 in RAm. Figure 10 shows that, at more caudal levels of ICo, medial to MLd, cells retrogradely labeled from the RAm injection were scattered throughout the diffuse terminal field resulting from the POM injection (Fig. 10A). In contrast, more rostrally in ICo, where the retrogradely labeled cells began to be more densely packed, medial to the hilus of MLd, the POM fibers and terminations tended to surround the whole labeled nucleus rather than individual labeled cells (Fig. 10B). Progressively more rostrally still, where the retrogradely labeled cells were densely packed to form the mediolaterally extensive band, the anterograde labeling from the POM injection surrounded the nucleus but penetrated it only very sparsely (Fig. 10C, D). As discussed above, the results from these two double label cases were consistent with the observations of the lack of anterograde labeling in the more dorsal of the two rostrolateral parts of ICo following CTB injections in POM. The more ventral of these two rostrolateral ICo regions was labeled anterogradely by injections in the arcopallium – as shown in another 2 cases in which injections of CTB 488 in the arcopallium were combined with an injection of CTB 555 in RAm. As in pigeons (Wild et al., 1997), arcopallial efferents did not appear to target DM; in the quail they formed an oval or spindle shaped terminal field in ICo, ventral to DM and rostral to MLd (Fig. 10E).
Figure 10.
A–D: Anterograde (red) and retrograde (green) labeling in ICo and DM following an injection of CTB 555 into POM and an injection of CTB 484 into RAm in the same case. Note how in A the retrogradely labeled cells are interspersed among the anterogradely labeled fibers and terminations, whereas in B–D they form the tightly clustered DM nucleus that does not admit the anterogradely labeled fibers, but is surrounded by them. E: DM (red) retrogradely labeled from an injection of CTB 555 in RAm, dorsal to and separate from a terminal field (green) in ICo resulting from an injection of CTB 484 into the medial arcopallium. Scale bar = 100 μm.
DISCUSSION
In this, the first account of neural pathways possibly mediating control of reproductive behaviour in birds, we have defined a series of projections originating in the hypothalamus, synapsing in DM-ICo and RAm, and terminating at the spinal motor neurons innervating the largest known cloacal muscle of any avian species (mSC). This muscle and its role in reproductive behaviour and voiding was described and analysed by Seiwert and Adkins-Regan (1998) and their findings set the scene for the present investigation. With regard to the anatomical aspects of their work at the level of the spinal cord, we would differ from their conclusions in only one respect, which is that the extensive dendrites of mSC motor neurons, as for the dendrites of many lower limb motor neurons in the lumbosacral spinal cord of the chick (Okado et al., 1990), are primarily directed dorsally rather than rostrally or medially. This could be important in the light of our further observations of the position of bulbospinal axons in the medial part of the lateral funiculus, to which the mSC motor neurons extended their dorsally directed dendrites. At this distance from mSC cell bodies -where their dendrites were narrower and their fluorescent intensity low – the likelihood of synaptic contacts between the bulbospinal axons and cloacal motor neuron dendrites could not realistically be assessed, but from this position bulbospinal axons approached and apparently terminated in the vicinity of mSC motor neurons in the lateral motor column of the ventral horn. Electron microscopy will be required to determine whether synaptic contacts are made on the distal dendrites of motor neurons and/or more proximally, nearer the cell body. Regardless, the overlap of bulbospinal projections and the distribution of mSC motor neurons and their dendrites was strikingly clear.
Origin of bulbospinal projections to the sacral spinal cord
Although several potential origins of bulbospinal projections to the sacral spinal cord were identified retrogradely in the present study, only RAm’s spinal projections were systematically investigated anterogradely. Some of the reasoning contributing to this strategy is documented below.
The rubrospinal pathway is known in pigeons to descend through the dorsolateral funiculus, to project to all levels of the spinal cord, and to terminate in the base of the dorsal horn and intermediate regions, rather than the ventral horn. It was therefore thought unlikely to be a prime candidate for specific control of reproductive behaviour (Wild et al., 1979).
PVN neurons were also retrogradely labeled from sacral spinal cord injections in the present study. As in mammals, PVN in birds is a premotor nucleus in the autonomic nervous system. Its spinal projections descend in the dorsolateral funiculus and terminate in the column of Terni, the avian equivalent of the intermediolateral cell column of mammals, but located medially in the spinal cord cross section rather than in the lateral grey horn (Terni, 1923; Cabot et al., 1982; Webster and Steeves, 1991). Preganglionic sympathetic neurons in the column of Terni at lumbosacral levels, and especially preganglionic neurons in the sacral parasympathetic nucleus, may be involved in the modulation of copulatory reflexes, the afferent limb of which is the pudendal nerve that innervates the reproductive tract (Ohmori et al., 1987). Future studies might also wish to determine whether PVN in quail projects upon mSC motor neurons, in analogous fashion to PVN’s projection upon Onuf’s nucleus in cats (Holstege and Tan, 1987).
Lateral vestibular (VeL) neurons were also specifically labeled in the present study. Projections of VeL in mammals terminate in the medial and ventromedial parts of the intermediate zone and polysynaptically influence motor neurons innervating extensor muscles (review in Kuypers, 1981); hence their implication in the trunk extensor component of lordosis in rats (Pfaff et al., 1994). In birds, as in mammals, vestibular projections at lumbar levels occupy the ventral funiculus, but the location of their spinal terminations is unknown (Cabot et al., 1982; Wold, 1978; Webster and Steeves, 1991).
A raphe-spinal system with a wide axonal distribution throughout the dorsolateral, lateral and ventrolateral funiculi has been demonstrated in pigeons, with lamina IX motor neurons being one of its targets (Cabot et al., 1982). However, the location of this system’s descending axons in pigeons is quite different from that observed in the medial part of the lateral funiculus of the sacral spinal cord of quail following RAm injections in the present study. Future studies, however, will need to investigate the possible presence of raphe-spinal projections to cloacal motor neurons.
The spinal projections and terminations of neurons making up the various pontine and medullary reticular nuclei of birds are also largely unknown, but it is possible that some of these may target the ventral horn of sacral segments and hence be involved in reproductive behaviour in some way. For instance, although a paragigantocellular nucleus (PGi) (as distinct from nucleus paragigantocellularis lateralis (PGL)) is not indicated in either the pigeon (Karten and Hodos, 1967) or chicken (Puelles et al., 2007) brain atlases, neurons in a similar ventral position to PGi at the level of the rostral medulla are depicted as the origin of projections to at least the lumbosacral enlargement in ducks (Webster and Steeves, 1988) and chickens (see figure 9 in Hassouna et al., 2001). Also, many neurons in a similar position were retrogradely labeled from sacral spinal cord injections in the present study, and diffuse projections to this region resulted from injections in DM/ICo. Thus these ventral regions of the upper medulla in quail, which may or may not correspond to, or include, a PGi-like nucleus, are deserving of investigation as to their specific spinal targets in regard to reproductive behavior. In rats, PGi is reported to project upon bulbospongiosus motor neurons in Onuf’s nucleus and to be involved in genital reflexes, although whether these projections are mono- or multisynaptic is not clear (cf Murphy and Hoffman, 2001, and Tang et al., 1999). In any case, the PGi projections in mammals exert an inhibitory effect on male genital reflexes, with disinhibition as a result of PGi lesions revealing a marked increase in excitation of unspecified origin (Yells et al., 1992; Liu et al., 1999). Notwithstanding these considerations, it should be remembered (a) that most avian species, including quail, do not possess an intromittent organ, and (b) the evolutionary relationship of the avian phallus to the mammalian penis is unclear (King 1981b), so avian motor neurons equivalent to those innervating the bulbospongiosus muscle in mammals may not be present in birds. Nevertheless, foam gland motor neurons in quail could be equivalent to those in Onuf’s nucleus that innervate the external anal sphincter, interestingly also called the sphincter cloacae in female rhesus monkeys because of the relatively poor differentiation of pelvic floor muscles in this species (Hartman and Straus, 1961). Projections to motor neurons in Onuf’s nucleus that innervate the sphincter cloacae in female rhesus monkeys receive distinct projections from NRA (VanderHorst and Holstege, 2000b), as mSC motor neurons appear to do from RAm in quail. Since mSC in quail also has an established role in reproductive behaviour, as well as voiding (Seiwert and Adkins-Regan, 1998), it would be interesting to know whether mSC motor neurons are anatomically and/or physiologically differentiated on this basis.
RAm-sacral spinal cord projection
There was a strong comparative incentive for us to concentrate our investigations on RAm. Firstly, as mentioned previously, Holstege and co-workers have shown in cats, rats and monkeys that NRA is more than a respiratory premotor nucleus. In addition to projections to spinal motor neurons innervating muscles concerned with intrathoracic pressure, as in expiration, it also has distinct, targeted projections to many motor neuron groups at lumbosacral levels involved in species-specific reproductive postures, such as lordosis, and, especially in males, to bulbospongiosus motor neurons (VanderHorst and Holstege, 1995, 1996; 1997; Holstege et al., 1997). Secondly, Wild has proposed that in birds the homolog of the mammalian NRA is RAm (Wild, 1993; Wild et al., 2009). This proposal was made on the basis of its similar position to NRA in the caudal medulla, its projections upon expiratory motor neurons (Wild, 1993; 1994), its afferent projections from a Köllicker-Fuse-like nucleus in the pons (Wild et al., 2009) and a nucleus in the midbrain (DM) that is clearly involved in the production of emotional vocalizations and species-specific calls (cf Holstege, 1989; Wild et al., 1997). It was therefore natural to ask whether RAm, like NRA, also had projections to the sacral cord, which we have not only been able to answer in the affirmative, but have also found that these projections terminate specifically in the vicinity of motor neurons innervating the large muscle of the foam gland (mSC), previously demonstrated to be involved in reproductive behaviour and voiding (Seiwert and Adkins-Regan, 1998). Most of the putative terminations appeared to be in relation to dendrites rather than cell bodies, as found by VanderHorst and Holstege (1997b) for NRA projections to Onuf’s motor neurons in male cats and by VanderHorst et al. (2000b) for NRA projections to pelvic floor motor neurons in female monkeys. In the quail it is probable, however, that the RAm projections also target other cloacal motor neurons which, according to Seiwert and Adkins-Regan (1998), are intermixed in the sacral ventral horn. One of these groups of motor neurons is that innervating m. transversus cloacae (mTC), which in pigeons has been shown to act as a respiratory muscle, moving the vent and cloacae cranially during each expiration and thereby helping to compress the caudal air sacs (Baumel et al., 1990).
Since RAm in quail appears to be multifunctional, as shown for NRA in several mammals, it is also natural to ask whether the organization of the nucleus reflects this in some way. The small size of RAm in most birds will render this question difficult to answer physiologically, but in the present study it was nevertheless clear that the RAm neurons retrogradely labeled from the sacral spinal cord were located most caudally in the nucleus, extending into the uppermost cervical spinal cord; and they were also heterogeneous in size and morphology. In a detailed physiological study of NRA neurons in estrus cats, Boers et al. (2005) did not find a somatotopic organization of cells projecting to different levels of the cord, but they did identify a novel population of NRA neurons projecting to lower lumbar and sacral levels that had no or very little respiratory drive and had low conduction velocities. The authors suggested that these non-expiratory bulbospinal NRA units could be involved in mating behaviours. We suggest that birds, too, might possess a special group of RAm neurons concerned with mating behaviour. Such neurons would likely have been included in our RAm injections, despite the fact that we used expiratory-related unit activity as our guide for those injections.
DM and its connections
Injections of CTB 488 or 555 centered on RAm in the present study retrogradely labeled a very extensive midbrain nucleus, which we have here called DM. Traditionally the designation ‘DM’ has been used to refer to a much smaller nucleus dorsomedial to MLd that projects upon the vocal motor nucleus (nXIIts) in songbirds and pigeons (Nottebohm et al., 1976; Gurney, 1981). Wild et al. (1997), however, showed that DM also projects on RAm, thereby helping to account for the coordinated respiratory-vocal activity involved in vocalization. In the present study, our RAm injections did not include XIIts, so DM in quail could be even larger than shown in figure 5. Even as defined here, however, it is considerably more extensive rostrocaudally than the DM defined by estrogen receptor immunohistochemistry and α2-adrenergic receptor autoradiography in a study investigating functional heterogeneity within the ICo complex of quail (Ball et al., 1989). These authors placed DM within caudal levels of the ICo complex and found more rostral parts of ICo were characterized by the presence of cholinergic muscarinic receptors. However, the more rostral part of ICo defined by Ball et al. (1989) is clearly not coincident with the more rostral part of DM defined in the present study, because the latter extends rostral to MLd, whereas the part of ICo defined by presence of cholinergic muscarinic receptors lies ventral to MLd (see fig. 1C of Ball et al., 1989).
These considerations not only confirm that ICo is functionally heterogeneous, as would be expected on the basis of its proposed homology with PAG in mammals (Kingsbury et al., 2011), but also suggest that DM is itself functionally heterogeneous, perhaps in ways not yet fully recognized. Puelles et al. (2007), for instance, have given the name ‘midbrain vocal area’ to a region in the chick midbrain that strongly resembles the distribution of neurons retrogradely labeled from RAm injections in the present study, but it is not really known whether this area is totally vocal. Previously it was assumed that DM’s downstream projections upon XIIts and RAm had a common function of organizing and/or coordinating vocal-respiratory output for the purpose of making species-specific vocalizations (Wild et al., 1997; Phillips and Peek, 1975). Shaw (2000), however, showed that electrical stimulation of DM during crowing in freely moving quail not only stopped crowing but also stopped the head bobbing movements that accompany crowing in this species. The control of postural (head and neck) responses is thought to be mediated by paramedian regions of the pons (Dubbeldam and den Boer-Visser, 2002), from which neurons in subventricular regions of the optic lobe have been retrogradely labeled in pigeons (Reiner and Karten, 1982), but whether these neurons are ICo neurons or DM neurons, or both is unclear. The present results now suggest that some part of DM may be involved in the control of reproductive behaviour, via its projections to those parts of RAm projecting to the sacral spinal cord. This particular projection could be involved with the maintenance of cloacal sphincteric control during the highly elevated air sac pressures characteristic of crowing. Alternatively, or as well, the DM projection to caudal RAm could be more directly involved in reproductive behaviour, via RAm’s projection to mSC motor neurons. One argument for vocalization and reproductive behaviour being under separate DM control is that, although vocalizations frequently occur during mating in many species, crowing in male quail, while serving to attract a female (Goodson and Adkins-Regan 1997), does not usually accompany the consummatory mating sequence of grasping the neck of the female with the beak, mounting, and cloacal contact (Balthazart, personal observations). However, this negative correlation is possibly explained by the fact that crowing – which is executed with an open beak – is incompatible with grasping, which is executed with a closed beak.
The finding of estrogen receptors in the region of DM (Ball et al., 1989) may be regarded as supporting an involvement with reproductive behavior, but until estrogen receptor immunohistochemistry is combined with retrograde labelling of DM neurons, it cannot be concluded that DM neurons, as opposed to ICo neurons, actually possesses estrogen receptors. The distribution of androgen receptors in male quail brain (Balthazart et al., 1992) appears to correspond more to the region of ICo ventral and rostral to MLd rather than to DM as defined in the present study. Furthermore, until double retrograde labeling experiments are carried out from separate DM target nuclei in the same bird, it is difficult to know whether the projections arise from separate or the same DM neurons. In summary, it is presently unclear whether or to what extent DM is topographically organized with respect to its downstream targets.
Most of the DM neurons retrogradely labeled from RAm injections were densely packed into an obliquely oriented band. The proximity of some DM neurons to each other could suggest the presence of gap junctions as a means of inter-cellular communication in this nucleus, a suggestion supported by the fact that injections in DM-ICo in the present study spread throughout most of the nucleus. Thus, DM may play a significant role as an integrator and/or organizer of inputs from a variety of sources. At first sight, it appeared that the hypothalamus was not one of these sources, because most of DM was not penetrated to any great extent by hypothalamic afferents, except caudally where DM neurons were scattered within the ICo complex. Similarly in the study of DM connectivity in pigeons and zebra finches (Wild et al., 1997), injections of biotinylated dextran amine confined to DM did not retrogradely label any hypothalamic neurons, a negative finding thought curious at the time. DM neurons, therefore, may receive their hypothalamic input by extending their dendrites into the surrounding ICo field (but see below). Nor does DM appear to receive direct projections from the arcopallium, again a finding confirming those of Wild et al. (1997) in pigeons. There are, however, major arcopallial inputs to ICo in pigeons and doves (Zeier and Karten, 1971; Cheng et al., 1987), but in the present study in quail these were concentrated in an oval shaped region ventral to rostral levels of DM. These results further confirm the fact that non-songbirds, such as quail and pigeons, do not appear to have any arcopallial-dorsal midbrain connection resembling the RA-DM connection in oscine songbirds that is usually depicted as part of the song control system (Nottebohm et al., 1976; Wild, 1993); a connection that nevertheless remains enigmatic from the functional point of view.
As noted previously (Wild et al., 1997), the DM projections to RAm appear to be directly analogous to those from lateral PAG to NRA in mammals that have been implicated in the control of intrathoracic and intra-abdominal pressure, a control required for vocalization, expiration and reproductive behaviour, among other behaviours (Holstege, 1989; Gerrits and Holstege, 1996; Holstege et al., 1997; VanderHorst and Holstege, 1996; VanderHorst et al., 2000a). Indeed, these studies were a major motivator of the present work.
POM-midbrain pathway
The sexually dimorphic medial preoptic nucleus in quail extends from the level of the anterior commissure, to which it is subjacent, through more rostral levels until it lies in a paramedian position dorsal to the optic chiasm at the level of the diverging septomesencephalic tract. A lesion study of POM in male quail found that more caudal parts of the nucleus were primarily concerned with consummatory sexual behaviour while more rostral levels were primarily concerned with appetitive sexual behaviour (Balthazart et al., 1998; Balthazart and Ball, 2007). Anterograde projections from these two regions of POM using DiI implants into different parts of the nucleus did not reveal differential projections (Balthazart et al., 1994), but this warrants closer examination in vivo and using other tracers. A reciprocal connection of POM with the medial part of ICo (GCt) was shown by Balthazart et al. (1994), but very sparse anterograde labeling of ICo was observed. Berk and Butler (1981) in pigeons also found relatively sparse labeling of ICo as a result of injections of tritiated amino acids into POM. In the present study BDA fibers and terminations were observed throughout much of ICo and GCt, even though the injections in POM were far from ideal (see Methods and Materials), and massive projections to these regions were seen following injections of either CTB or fluorescent CTB that were centered on POM, but with spread to underlying parts of the hypothalamus. Even so, it was clear that there were specific regions within ICo that did not receive hypothalamic projections, regions that were subsequently observed to house DM neurons in those cases in which an injection of fluorescent CTB of another colour had also been made in RAm. Similar findings of hypothalamic projections to ICo that appear to have avoided DM were shown by Berk (1987; see his figure 3B) in pigeons.
As mentioned in the section above dealing with DM, the hypothalamic projections only sparsely penetrated the major part of DM consisting of a dense band of closely clustered neurons. We did observe that some DM neurons extended their dendrites into the overlying ICo, but we do not know whether DM neurons normally receive POM input in this way. DM may receive POM inputs indirectly, via ICo neurons and possibly via neurons located in the most medial aspects of ICo, that is, in GCt (see Absil et al., 2001; Carere et al., 2007), but anterograde tracing experiments will be required to confirm this. Injections centered on DM, but with involvement of the surrounding ICo, did retrogradely label POM (and other hypothalamic neurons, including VMN), but the conclusion that POM projects directly to DM, as well as to ICo, must remain tentative. Furthermore, although there is ample evidence that the medial preoptic area projects to PAG in mammals (Veening et al., 1991), as POM does to GCt in quail (Absil et al., 2001; Carere et al., 2007), we are not aware of any published evidence in mammals that shows that POM projects specifically upon PAG neurons that, in turn, project upon NRA neurons having sacral spinal cord targets. Despite these uncertainties, there seems to be a substantial degree of similarity between the projections as outlined here in quail to those defined in several mammalian species by Holstege and others for the control of reproductive behaviour. Clearly, much more work is required in quail, especially in females, to render this similarity anything more than superficial. As with the neural control of respiration, however, evolution seems to have ensured that, despite the very different peripheral structures and mechanisms involved in reproductive behaviour in birds and mammals, they appear to be controlled by descending pathways that are fundamentally similar in their pattern of organization.
Acknowledgments
Grant Sponsor: National Institute of Mental Health; Grant Number: MH50388;
Grant Sponsor: Belgian FRFC; Grant number: 2.4537.09;
Grant sponsor: European Union, Marie Curie Fellowship to J. M. Wild; Grant Number: 272178 (RPINBIRDS).
We are extremely grateful to Professor Vincent Seutin for providing space and facilities suitable for electrophysiological recording.
ABBREIVIATIONS
- Aq
Cerebral aqueduct
- AVT
Area ventralis Tsai
- CA
Commissura anterior
- Cb
Cerebellum
- cc
Canalis centralis
- CP
Commissura posterior
- dh
Cornua dorsalis
- DM
Dosomedial nucleus of the intercollicular complex
- DMA
Nucleus dorsmedialis anterior thalami
- DMP
Nucleus dorsmedialis posterior thalami
- DMNX
Nucleus motorius dorsalis nervi vagi
- DSO
Decussatio supraoptica dorsalis
- FLM
Fasciculus longitudinalis medialis
- FPL
Fasciculus prosencephali lateralis
- GCt
Substantia grisea centralis
- ICo
Nucleus intercollicularis
- Imc
Nucleus isthmi, pars magnocellularis
- Ipc
Nucleus isthmi, pars parvocellularis
- lf
Funiculus lateralis
- LS
Lemniscus spinalis
- MesV
Nucleus mesencephalicus nervi trigemini
- MLd
Nucleus mesencephalicus lateralis pars dorsalis
- mSC
Musculus cloacae sphinctericus
- mTC
Musculus cloacae transversus
- nIII
Nucleus nervi oculomotorii
- NV
Nervi trigemini
- NVI
Nervi abducens
- NVIII
Nervus octavus
- nIX-X
Nucleus nervi glossopharyngei et nucleus motorius dorsalis nervi vagi
- NXII
Nervi hypoglossi
- nXII
Nucleus nervi hypoglossi
- nXIIts
Nucleus nervi hypoglossi, pars tracheosyingealis
- NRA
Nucleus retroambiguus
- Ov
Nucleus ovoidalis
- PAG
Periaqueductal gray
- PGi
Nucleus paragigantocellularis
- PGL
Nucleus paragigantocellularislateralis
- POM
Nucleus preopticus medialis
- PVN
Nucleus periventricularis magnocellularis
- R
Nucleus raphe
- RA
Nucleus robustus arcopallialis
- RAm
Nucleus retroambigualis
- Rt
Nucleus rotundus
- RPgc
Nucleus reticularis pontis caudalis, pars gigantocellularis
- Ru
Nucleus ruber
- S
Nucleus solitarius
- SCE
Stratum cellulare externum
- SCI
Stratum cellulare internum
- SM
Nucleus stria medullaris
- SNc
Substantia nigra pars compacta
- SO
Stratum opticum
- SpM
Nucleus spiriformis medialis
- SSp
Nucleus supraspinalis
- TeO
Tectum opticum
- TIO
Tractus isthmo-opticus
- TTD
Nucleus et tractus descendens nervi trigemini
- v
ventriculus
- VeD
Nucleus vestibularis descendens
- VeL
Nucleus vestibularis lateralis
- vf
Funiculus ventralis
- vh
Cornua ventralis
- VMN
Nucleus ventromedialis hypothalami
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
Author contributions:
The experiments were conceived by JMW and JB. JMW carried out all the experiments and wrote the paper, with input from JB. JMW made the figures, assisted by JB.
Contributor Information
J Martin Wild, Department of Anatomy with Radiology, Faculty of Medical and Health Science, University of Auckland, Auckland, New Zealand.
Jacques Balthazart, Research Group in Behavioural Neuroendocrinology, GIGA Neurosciences, University of Liege, Liege, Belgium.
LITERATURE CITED
- Absil P, Riters LV, Balthazart J. Preoptic aromatase cells project to the mesencephalic central gray in the male Japanese quail (Coturnix japonica) Horm Behav. 2001;40:369–383. doi: 10.1006/hbeh.2001.1702. [DOI] [PubMed] [Google Scholar]
- Adkins EK, Adler NT. Hormonal control of behaviour in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. doi: 10.1037/h0033315. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. How useful is the appetitive and consummatory distinction for our understanding of the neuroendocrine control of male sexual behaviour in birds? Horm Behav. 2008;53:307–311. doi: 10.1016/j.yhbeh.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Neuroendocrine regulation of reproductive behaviour in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Vol. 2. San Diego: Academic Press; 2009. pp. 855–895. [Google Scholar]
- Ball GF, Balthazart J. Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR Journal. 2010;51:310–325. doi: 10.1093/ilar.51.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Foidart A, Balthazart J. A dorsomedial subdivision within the nucleus intercollicularis identified in the Japanese quail (Coturnix corturnix japonica) by means of α2-adrenergic receptor autoradiography and estrogen receptor immunohistochemistry. Cell Tiss Res. 1989;257:123–128. doi: 10.1007/BF00221641. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Dupiereux V, Aste N, Viglietti-Panzica C, Baresse M, Panzica GC. Afferent and efferent connections of the sexually dimorphic medial preoptic nucleus of the male quail revealed by in vitro transport of DiI. Cell Tiss Res. 1994;276:455–475. doi: 10.1007/BF00343944. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Gérard M, Appeltants D, Ball GF. Appetitive and consummatory male sexual behaviour in Japanese quail are differentially regulated by subregions of the preoptic medial nucleus. J Neurosci. 1998;18:6512–6527. doi: 10.1523/JNEUROSCI.18-16-06512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Arnold AP, Adkins-Regan E. Sexual differentiation of brain and behavior in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. 2. Vol. 3. San Diego: Academic Press; 2009. pp. 1745–1787. [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviours. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumel JJ, Wilson JA, Bergren DR. The ventilatory movements of the avian pelvis and tail: function of the muscles of the tail region of the pigeon (Columba livia) J Exp Biol. 1990;151:263–277. doi: 10.1242/jeb.151.1.263. [DOI] [PubMed] [Google Scholar]
- Baylé JD, Ramade F, Oliver J. Stereotaxic topography of the brain of the quail. J Physiol (Paris) 1974;68:219–241. [PubMed] [Google Scholar]
- Berk ML. Projections of the lateral hypothalamus and bed nucleus of the stria terminalis to the dorsal vagal complex in the pigeon. J Comp Neurol. 1987;260:140–156. doi: 10.1002/cne.902600111. [DOI] [PubMed] [Google Scholar]
- Berk ML, Butler AB. Efferent projections of the medial preoptic nucleus and medial hypothalamus in the pigeon. J Comp Neurol. 1981;203:379–399. doi: 10.1002/cne.902030305. [DOI] [PubMed] [Google Scholar]
- Boers J, Ford TW, Holstege G, Kirkwood PA. Functional heterogeneity among neurons in the nucleus retroambiguus with lumbosacral projections in female cats. J Neurophysiol. 2005;94(4):2617–2629. doi: 10.1152/jn.00370.2005. [DOI] [PubMed] [Google Scholar]
- Brown JL. Vocalization evoked from the optic lobe of a songbird. Science. 1965;149:1002–1003. doi: 10.1126/science.149.3687.1002. [DOI] [PubMed] [Google Scholar]
- Carere C, Ball GF, Balthazart J. Sex differences in projections from preoptic area aromatase cells to the periaqueductal gray in Japanese quail. J Comp Neurol. 2007;500:894–907. doi: 10.1002/cne.21210. [DOI] [PubMed] [Google Scholar]
- Cabot JB, Reiner A, Bogan N. Avian bulbospinal pathways: anterograde and retrograde studies of cells of origin, funicular trajectories and laminar terminations. Prog Brain Res. 1982;57:79–108. doi: 10.1016/S0079-6123(08)64125-4. [DOI] [PubMed] [Google Scholar]
- Cheng M-F, Akesson TR, de Lanerolle NC. Retrograde HRP demonstration of afferent projections to the midbrain and nest calls in the ring dove. Brain Res Bull. 1987;18:45–48. doi: 10.1016/0361-9230(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Dubbeldam JL, den Boer-Visser AM. The central mesencephalic grey in birds: nucleus intercollicularis and substantia grisea centralis. Brain Res Bull. 2002;57:349–352. doi: 10.1016/s0361-9230(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Flanagan-Cato LM. Sex differences in the neural circuit that mediates female sexual receptivity. Front Neuroendocrinol. 2011;32:124–135. doi: 10.1016/j.yfrne.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: A wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol. 1996;373:173–185. doi: 10.1002/(SICI)1096-9861(19960916)373:2<173::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gibson MJ, Cheng MF. Neural mediation of estrogen-dependent courtship behavior in female ring doves. J Comp Physiol Psychol. 1979;93:855–867. [Google Scholar]
- Goodson JL, Adkins-Regan E. Playback of crows of male Japanese quail elicits female phonotaxis. Condor. 1997;99:990–993. [Google Scholar]
- Gurney ME. Hormonal control of cell form and number in the zebra finch song system. J Neurosci. 1981;1:658–673. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CG, Straus WL. The Anatomy of the Rhesus Monkey (Macaca Mulatta) New York: Hafner Publishing Co; 1961. [Google Scholar]
- Hassouna E, Yamamoto M, Imagawa T, Uehara M. Distribution of reticulospinal neurons in the chicken by retrograde transport of WGA-HRP. Tissue and Cell. 2001;33:141–147. doi: 10.1054/tice.2000.0154. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Holstege G, Tan J. Supraspinal control of motoneurons innervating the striated muscles of the pelvic floor including urethral and anal sphincters in the cat. Brain. 1987;110:1323–1344. doi: 10.1093/brain/110.5.1323. [DOI] [PubMed] [Google Scholar]
- Holstege G, Kerstens L, Moes MC, VanderHorst VGJM. Evidence for a periaqueductal gray-nucleus retroambiguus-spinal cord pathway in the rat. Neuroscience. 1997;80:587–598. doi: 10.1016/s0306-4522(97)00061-4. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male sexual behaviour. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain & Behav. San Diego, CA: Academic press; 2002. pp. 3–137. [Google Scholar]
- Ikeda K, Taji K. On the foamy ejaculate of Japanese quail (Coturnix coturnix japonica, T. et S.) Sci Rep Matsyama Agr Coll. 1954;3:1–4. [Google Scholar]
- Karten HJ, Hodos W. A Stereotaxic Atlas of the Brain of the Pigeon (Columba livia) Johns Hopkins Press; Baltimore: 1967. [Google Scholar]
- King AS. Cloaca. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 2. London: Academic Press; 1981a. pp. 63–105. [Google Scholar]
- King AS. Phallus. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 2. London: Academic Press; 1981b. pp. 107–147. [Google Scholar]
- Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS One. 2011;6(6):e20720. doi: 10.1371/journal.pone.0020720.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RD, Knight CE, Stein S. Gross and microscopic morphology of the glandula proctodealis (foam gland) of Coturnix c. japonica (Aves) J Morphol. 1973;141:171–184. doi: 10.1002/jmor.1051410205. [DOI] [PubMed] [Google Scholar]
- Kubke MF, Yazaki-Sugiyama Y, Mooney R, Wild JM. Physiology of neuronal subtypes in the respiratory-vocal integration nucleus retroambigualis of the male zebra finch. J Neurophysiol. 2005;94:2379 – 2390. doi: 10.1152/jn.00257.2005. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Handbook of Physiology, Section 1: The Nervous System, Vol. II, Motor Control, Part 1. Vol. 13. American Physiological Society; Bethesda, MD: 1981. Anatomy of the descending pathways; pp. 597–666. [Google Scholar]
- Liu YC, Sachs BD. Erectile function in male rats after lesions in the lateral paragigantocellular nucleus. Neurosci Lett. 1999;262:203–206. doi: 10.1016/s0304-3940(99)00070-1. [DOI] [PubMed] [Google Scholar]
- McFarland LZ, Warner RL, Wilson WO, Mather FB. The cloacal gland complex of the Japanese quail. Experientia. 1968;24:941–943. doi: 10.1007/BF02138670. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behaviour. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Kow L-M, McCarthy MM, Pfaff DW, Schwartz-Giblin S. Midbrain PAG control of female reproductive behavior: In vitro electrophysiological characterization of actions of lordosis-relevant substances. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. Plenum Press; New York: 1991. pp. 211–235. [Google Scholar]
- Ohmori Y, Watanabe T, Fujioka T. Projections of visceral and somatic primary afferents to the sacral spinal cord of the domestic fowl revealed by transganglionic transport of horseradish peroxidase. Neurosc Lett. 1987;74:175–179. doi: 10.1016/0304-3940(87)90145-5. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. ch 36. Raven Press, Ltd; New York: 1994. pp. 107–220. [Google Scholar]
- Phillips RE, Peek FW. Brain organization and neuromuscular control of vocalization in birds. In: Caryl P, Wright PG, Vowles DM, editors. Neural and endocrine aspects of behaviour in birds. Elsevier Scientific Publishing Co; Amsterdam: 1975. pp. 243–274. [Google Scholar]
- Potash LM. Vocalizations elicited by electrical brain stimulation in Coturnix coturnix japonica. Behav. 1970;36:149–167. doi: 10.1163/156853970x00286. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martinez-de-la-Torre Paxinos G, Watson C, Martinez S. An Atlas featuring Subdivisions and Mammalian Homologies. Academic Press; New York: 2007. The Chick Brain in Stereotaxic Coordinates. [Google Scholar]
- Reiner A, Karten HJ. Laminar distribution of the cells of origin of the descending tectofugal pathways in the pigeon (Columba livia) J Comp Neurol. 1982;204:165–187. doi: 10.1002/cne.902040206. [DOI] [PubMed] [Google Scholar]
- Reinke H, Wild JM. Distribution and connections of inspiratory premotor neurons in the brainstem of the pigeon (Columba livia) J Comp Neurol. 1997;379:347–362. [PubMed] [Google Scholar]
- Reinke H, Wild JM. Identification and connections of inspiratory premotor neurons in songbirds and budgerigar. J Comp Neurol. 1998;391:147–163. [PubMed] [Google Scholar]
- Riters LV, Absil P, Balthazart J. Effects of brain testosterone implants on appetitive and consummatory components of male sexual behavior in Japanese quail. Brain Res Bull. 1998;47:69–79. doi: 10.1016/s0361-9230(98)00064-1. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behaviour and morphology in male and female Japanese quail. Physiol Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. [DOI] [PubMed] [Google Scholar]
- Seiwert CM. PhD dissertation. Cornell University; Ithaca, New York: 1994. The Neuromuscular System Controlling Foam Production in Japanese Quail: An Investigation of Structure and Function. [Google Scholar]
- Seiwert CM, Adkins-Regan E. The foam production system of the male Japanese quail: characterization of structure and function. Brain Behav Evol. 1998;52:61–80. doi: 10.1159/000006553. [DOI] [PubMed] [Google Scholar]
- Seller TJ. Midbrain vocalization centers in birds. Trends Neurosci. 1981;12:301–303. [Google Scholar]
- Shaw BK. Involvement of a midbrain vocal nucleus in the production of both acoustic and postural components of crowing behavior in Japanese quail. J Comp Physiol A. 2000;186:747–757. doi: 10.1007/s003590000128. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. J Neurosci. 2003;23:1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Giullano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999;414:167–192. [PubMed] [Google Scholar]
- Terni T. Ricerche anatomiche sul sistema nervosa autonoma degli Ucceli. Arch Ital Anat Embryol. 1923;20:433–510. [Google Scholar]
- VanderHorst VGJM, Holstege G. Caudal medullary pathways to lumbosacral motoneuronal cell groups in the cat; evidence for direct projections possibly representing the final common pathway for lordosis. J Comp Neurol. 1995;359:457–475. doi: 10.1002/cne.903590308. [DOI] [PubMed] [Google Scholar]
- VanderHorst VJGM, Holstege G. A concept for the final common pathway for vocalization and lordosis behavior in the cat. Prog Brain Res. 1996;107:327–341. doi: 10.1016/s0079-6123(08)61874-9. [DOI] [PubMed] [Google Scholar]
- VanderHorst VGJM, Holstege G. Estrogen induces axonal outgrowth in the nucleus retroambiguus–lumbosacral motoneuronal pathway in the adult female cat. J Neurosci. 1997a;17:1122–1136. doi: 10.1523/JNEUROSCI.17-03-01122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderHorst VGJM, Holstege G. Nucleus retroambiguus projections to lumbosacral motoneuronal cell groups in the male cat. J Comp Neurol. 1997b;382:77–88. doi: 10.1002/(sici)1096-9861(19970526)382:1<77::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Terasawa E, Ralston HJ, 3rd, Holstege G. Monosynaptic projections from the lateral periaqueductal gray to the nucleus retroambiguus in the rhesus monkey: implications for vocalization and reproductive behavior. J Comp Neurol. 2000a;424:251–268. doi: 10.1002/1096-9861(20000821)424:2<251::aid-cne5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Terasawa E, Ralston HJ, 3rd, Holstege G. Mono-synaptic projections from the nucleus retroambiguus to motoneurons supplying the abdominal wall, axial, hindlimb, and pelvic floor muscles in the female rhesus monkey. J Comp Neurol. 2000b;424:233–250. doi: 10.1002/1096-9861(20000821)424:2<233::aid-cne4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Veening J, Buma P, Ter Horst GJ, Roeling TAP, Luiten PGM, Nieuwenhuys R. Hypothalamic projections to the PAG in the rat: topographical, immuno-electronmicroscopical and functional aspects. In: Depaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. New York, London: Plenum Press; 1991. pp. 387–415. [Google Scholar]
- Webster DMS, Steeves JD. Origins of brainstem spinal projections in the duck and goose. J Comp Neurol. 1988;273:573–583. doi: 10.1002/cne.902730411. [DOI] [PubMed] [Google Scholar]
- Webster DMS, Steeves JD. Funicular organization of avian brainstem-spinal projections. J Comp Neurol. 1991;312:467–476. doi: 10.1002/cne.903120312. [DOI] [PubMed] [Google Scholar]
- Wild JM. The avian nucleus retromabigualis: a nucleus for breathing, singing and calling. Brain Res. 1993;606:319–324. doi: 10.1016/0006-8993(93)91001-9. [DOI] [PubMed] [Google Scholar]
- Wild JM. The auditory-vocal-respiratory axis in birds. Brain Behav Evol. 1994;44:192–209. doi: 10.1159/000113577. [DOI] [PubMed] [Google Scholar]
- Wild JM, Cabot JB, Cohen DH, Karten HJ. Origin, course, and terminations of the rubrospinal tract in the pigeon (Columba livia) J Comp Neurol. 1979;187:639–654. doi: 10.1002/cne.901870402. [DOI] [PubMed] [Google Scholar]
- Wild JM, Li D, Eagleton C. Projections of the dorsomedial nucleus of the intercollicular complex (DM) in pigeon (Columba livia) and zebra finch (Taeniopygia guttata) J Comp Neurol. 1997;377:392–413. doi: 10.1002/(sici)1096-9861(19970120)377:3<392::aid-cne7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wild JM, Kubke MF, Mooney R. Avian nucleus retroambigualis: cell types and projections to other respiratory-vocal nuclei in the brain of the zebra finch (Taeniopygia guttata) J Comp Neurol. 2009;512:768–783. doi: 10.1002/cne.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Krützfeldt NOE. Trigeminal and telencephalic projections to jaw and other upper vocal tract premotor neurons in songbirds: sensorimotor circuitry for beak movements during singing. J Comp Neurol. 2012;520:590–605. doi: 10.1002/cne.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold JE. The vestibular nuclei in the domestic hen (Gallus domesticus) IV. The projection of the spinal cord. Brain Behav Evol. 1978;15:41–62. doi: 10.1159/000123771. [DOI] [PubMed] [Google Scholar]
- Yells DP, Hendricks SE, Prendergast MA. Lesions of the nucleus paragigantocellularis: effects on mating behaviour in male rats. Brain Res. 1992;596:73–79. doi: 10.1016/0006-8993(92)91534-l. [DOI] [PubMed] [Google Scholar]
- Zeier H, Karten HJ. The archistriatum of the pigeon: organization of afferent and afferent connections. Brain Res. 1971;31:313–326. doi: 10.1016/0006-8993(71)90185-5. [DOI] [PubMed] [Google Scholar]