Abstract
Objectives
We previously showed that acute alcohol intoxication (AAI) reduces lymphatic myogenic constriction in response to step increases in luminal pressure. Because of the known role of Ca2+ in smooth muscle contractile responses, we investigated how alcohol impacts cyclic Ca2+ and whether changes in RhoA/ROCK mediated Ca2+ sensitivity underlie the alcohol-induced reduction of myogenic responsiveness.
Methods
AAI was produced by intragastric administration of 30% alcohol in rats. Mesenteric lymphatics were cannulated and loaded with Fura-2 AM to [Ca2+]i for 30 min after AAI. Active GTP-bound RhoA levels were determined by ELISA. To determine ROCK's ability to restore myogenic responsiveness following AAI, isolated lymphatics were transfected with constitutively active ca-ROCK protein.
Results
Lymphatics from alcohol-treated rats displayed significantly larger Ca2+ transients. Also, step increases in luminal pressure caused a gradual rise in the basal [Ca2+]i between transients that was greater in lymphatics submitted to AAI, compared to vehicle control. RhoA-GTP was significantly reduced in lymphatics from the AAI group, compared to vehicle control. Transfection with ca-ROCK protein restored the myogenic response of lymphatic vessels isolated from AAI animals.
Conclusions
The data strongly suggest that the alcohol-induced inhibition of mesenteric lymphatic myogenic constriction is mediated by reduced RhoA/ROCK-mediated Ca2+ sensitivity.
Keywords: calcium, lymphatic smooth muscle, myogenic tone, ROCK, ethanol
INTRODUCTION
The microcirculation and lymphatics of the gut maintain the tightly controlled environment required for optimal gastrointestinal (GI) function and health. Secondary to the gut barrier formed by enterocytes, the portal circulation, liver, lymph nodes and mesenteric lymphatics regulate the entry of toxins, toxicants, and pathogens into the central circulation. Systemic inflammation caused by traumatic injury or chronic disease can compromise blood flow to the gut, leading to increased gut permeability that correlates with extent of the injury [1, 2, 3]. Mesenteric lymphatics serve as conduits for both antigens and lymphocytes to the mesenteric lymph nodes [4], which form the border between mucosal immunity and the remainder of immune system [5]. Mesenteric lymphatics also serve as the primary route for non-bacterial, tissue injury GI-derived factors, which can contribute to progression to multiple organ injury and dysfunction [6, 7]. Considering the multifunctional role of lymphatics of the gut, understanding how insults from disease or injury impact lymphatic function is key for advancing our knowledge of immunity in the digestive tract.
Our previous work has shown that acute alcohol intoxication (AAI) in rats modifies the contractile cycle of isolated mesenteric collecting lymphatic vessels. This is characterized by reduced contraction frequency, a larger stroke volume, and a significant loss of myogenic constriction in response to step increases in luminal pressure. Combined, these modifications appear to enhance the ability of lymphatics to transport lymph during the alcohol-intoxicated state [8]. Both clinical and pre-clinical studies have demonstrated that alcohol significantly increases lymph flow [9, 10]. AAI is associated with a high risk and incidence of traumatic injuries, complicating management and worsening patient outcomes [11]. We speculate that increased lymphatic flow in alcohol-intoxicated trauma victims could lead to an overwhelming amount of inflammatory, potentially toxic factors and/or pathogens entering the systemic circulation following traumatic injury or infection; enhancing the risk for tissue injury, including liver, lung, and brain [6, 7]. Thus, understanding the contractile regulatory mechanisms disrupted by alcohol intoxication is of clinical relevance.
Lymphatic pumping is mediated by the smooth muscle layer present on collecting lymphatics. Contraction of smooth muscle is dependent upon [Ca2+]i, and the force generated is dependent on the sensitivity of the contractile elements to Ca2+ [12]. Smooth muscle [Ca2+]i is in turn determined by membrane potential and binding of extracellular stimuli such as hormones and neurotransmitters to their specific receptors, which in turn determine the influx and efflux of Ca2+ across the sarcolemma, sarcoplasmic reticulum membrane, and membranes of other internal stores. Binding of Ca2+ to calmodulin leads to activation of the myosin light chain kinase (MLCK), leading to phosphorylation of regulatory myosin light chains (MLC) and actin-myosin-mediated contraction. Signaling events that regulate myosin light chain phosphatase (MLCP) can also modulate Ca2+ sensitivity; defined as the overall responsiveness of the molecular contractile mechanism, to a given [Ca2+]i [13, 14, 15]. The small GTPase RhoA and its downstream effector Rho-kinase (ROCK) increase Ca2+ sensitivity by ROCK-mediated phosphorylation of the MLCP regulatory/targeting subunit, MYPT-1. MLCP activity is stimulated when phosphorylated by either ROCK or PKC and inhibited by the kinase CPI-17 [16, 17].
We, and others, have shown that rapid, transient increases in [Ca2+]i precede each phasic contraction in isolated lymphatics [18, 19, 20]. In addition, we have shown that step increases in luminal pressure cause a gradual rise in the basal [Ca2+]i between transients, which may contribute to myogenic contraction [18]. In the current study, we investigated whether the aforementioned alcohol intoxication-induced modulation of the lymphatic contractile cycle is associated with changes in cyclic mobilization of Ca2+. In addition, we also examined whether alcohol intoxication may affect the RhoA/ROCK pathway, which may act as part of the Ca2+-sensitizing mechanism to enhance lymphatic vessel tone [21].
METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center and were performed in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011). Male Sprague-Dawley rats (270-350 g body wt) were housed in a controlled temperature (22 °C) and controlled illumination (12:12 h light dark cycle) environment. After arrival, the rats were submitted to a one-week acclimation period and were provided standard rat chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan) and water ad libitum.
Gastric catheter placement and alcohol administration protocol
AAI was produced as previously described [8, 22]. Briefly, rats were anesthetized with ketamine and xylazine (90 and 9 mg/kg, respectively). A sterile catheter was aseptically placed into the antrum of the stomach, routed subcutaneously through a trocar, and exteriorized at the nape of the neck. After cannula placement, animals were returned to individual cages and allowed 48 hours to recover from surgery, with food and water provided ad libitum.
Following a two day recovery period, conscious and unrestrained animals received an intragastric bolus of 30% ethyl alcohol (2.5 g/kg) via the gastric catheter. Intragastric administration of alcohol at this dose typically produces a blood alcohol level of 200-300 mg/dl within 30 min. of administration [8]. A time-matched control group received isovolumic administration of vehicle (water).
Collecting lymphatic isolation and pressure step protocol
The responsiveness of lymphatic vessels to step changes in pressure was studied as previously described [8]. Briefly, 30 min. after alcohol or vehicle (water) was administered, the rats were anesthetized with ketamine and xylazine (90 and 9 mg/kg, respectively and the small intestine and mesentery were exteriorized and excised, and placed in ice-cold albumin physiological salt solution (APSS). Rats were immediately euthanized after removal of the mesentery by overdose with ketamine/xylazine. In each experiment, a section of mesentery was pinned in a dissection chamber containing ice-cold APSS, and a collecting lymphatic vessel was carefully dissected from surrounding adipose and connective tissue with the aid of a stereomicroscope. The isolated lymphatic was transferred to an isolated vessel chamber (Living Systems Instrumentation, Burlington VT) and was mounted onto two resistance-matched glass micropipettes. Isolated lymphatic vessels with only one valve were used to ensure optimal pressure control in the entire segment [23]. The overall time from removal of mesentery and lymphatic isolation and cannulation was 30-40 min. The chamber was transferred to an inverted microscope (Nikon Eclipse TE2000U with Plan Fluor 10×/0.3 objective, DIC L/N1, ∞/0.17, WD 16) and connected to a heating unit (Living Systems) at 37 °C. Rapid time-lapse image sets were acquired using the image acquisition software (Nikon Elements AR software). The pressure protocol for myogenic response was previously described by us [8].
Ratiometric measurement of [Ca2+]i
Intraluminal pressure was initially set at 2 cm H2O during 45-60 min for equilibration and development of spontaneous lymphatic contractions. After this period, the isolated lymphatic smooth muscle was loaded with a Ca2+-sensing dye Fura 2-acetoxymethyl ester (AM) (Molecular Probes, Eugene, OR). The bath was exchanged to an APSS solution containing Fura-2 AM (2 μM) and pluronic acid (0.2 % wt/vol) for 30 minutes at 37 °C. Fura-2 AM was applied to the abluminal side of the vessel to restrict loading to the smooth muscle layer [24, 25]. After 30 minutes the bath was changed back to APSS solution without Fura-2 AM. Following an additional 20 min. equilibration period, ratiometric Fura-2 measurements were collected in isolated lymphatics by illuminating at alternating wavelengths of 340 and 380 nm via a dichroic mirror (400 nm; Chroma Technology Corp. 400DCLP) for durations of 50 ms each. The epifluorescent light was collected through a wide band emission filter (510 nm, 80 nm band width; Chroma Technology Corp. D510/80m) and acquired by a Photometrics HQ2 camera. We collected 2 minutes of data at which included 30 s at a baseline intraluminal pressure of 2 cm H2O, a 60 s step increase to 4, 6, 8, 10 or 12 cm H2O, and 30 s after a return to 2 cm H2O.
A region of interest (ROI) that included the entire lymphatic vessel and surrounding area was selected to analyze the average [Ca2+]i, so that the vessel was fully tracked during relaxation and contraction. An increase in the 340/380 ratio indicates an increase in [Ca2+]i [18].
RhoA G-LISA luminescence assay
The active GTP-bound pool of RhoA was measured using the RhoA G-LISA™ luminescence assay (Cytoskeleton, Inc., Denver, CO) according to the manufacturer's instructions in lymphatics isolated from a separate group of animals treated with alcohol or vehicle (water) for 30 min. From each rat, 3 to 4 mesenteric lymphatic branches were isolated and immediately placed in 90 μl of ice-cold tissue lysis buffer. The mixtures were sonicated and centrifuged, and protein concentrations were measured using Precision Red reagent (Cytoskeleton, Inc.) at 600 nm of absorbance reading to equilibrate samples. The amount of active GTP-bound RhoA in the equilibrated samples was then determined by ELISA. For both the protein assay and ELISA, data was collected on a Tecan Infinite M200 plate reader (Tecan, Switzerland).
Protein Transfection
Lymphatics were transfected with constitutively active ca-ROCK protein (Millipore) as previously described for isolated venules [26], with some modifications. The ca-ROCK protein was mixed with TransIT-LT1 polyamine transfection reagent (Mirus, Madison, WI). While the lymphatic was held at a luminal pressure of 2 cm H2O, the transfection mixed was added to the isolated lymphatic bath at a final concentration of 2 μg/ml for ca-ROCK and 10 μl/ml for TransIT-LT1. This concentration was selected after a preliminary study showing that it could effectively increase lymphatic tone (data not shown). The transfection mix was added to the abluminal side of the vessels to minimize access to the inner, endothelial layer. The addition of TransIT-LT1 alone at a final concentration of 10 μl/ml served as a transfection control. Before transfection, a pressure protocol was applied for myogenic tonic measurement. The protocol was repeated 10 min and 30 min after transfection with ca-ROCK in mesenteric lymphatics isolated from rats treated with either vehicle (water) or alcohol.
Statistical Data Analysis
For [Ca2+]i measurement data, representative tracings of the 340/380 ratio over time are shown. Basal [Ca2+]i is represented by the 340/380 ratio during diastole (F0). Magnitudes of Ca2+ transients were calculated as the difference between the peak minus the preceding basal 340/380 ratio (Fpeak - F0). Also, the change in basal [Ca2+]i following pressure steps is represented as (F̄/F̄0) where F̄ represents the average basal 340/380 ratio (between transients) after the step increase in pressure and F̄0 is the average basal 340/380 ratio prior to the pressure step. Summarized data are presented as mean ± SE and the N indicated. Student t-tests or two-way ANOVA followed by Bonferroni t-tests were used where appropriate. Statistical significance was accepted at P<0.05.
RESULTS
Impact of alcohol intoxication on calcium transients during step pressure increase
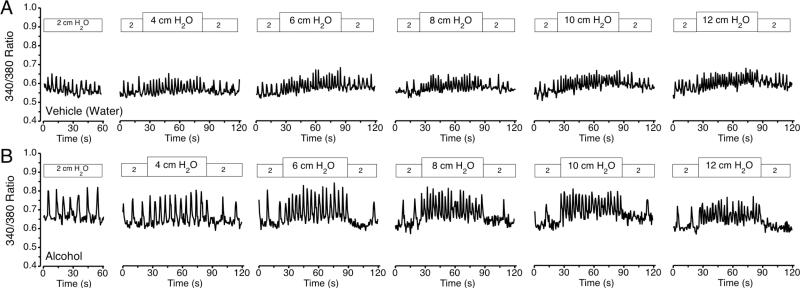
A representative set of tracings of the 340/380 ratio, indicative of cytosolic [Ca2+] over time from an isolated mesenteric collecting lymphatic is shown in Fig. 1. The isolated lymphatic was initially perfused at a baseline luminal pressure of 2 cm H2O and then subjected to step increases to 4, 6, 8, 10 and 12 cm H2O, each followed by a step return to baseline. In response to upward pressure step increases, the frequency of Ca2+ transients increased, and their magnitude remained stable (Fig. 1A). In addition, immediately after step increases in pressure to 6, 8, 10, and 12 cm H2O, the basal [Ca2+]i between transients rose gradually over time. When the pressure was stepped back down to 2 cm H2O, the frequency of transients decreased (and in some cases briefly ceased), and the basal [Ca2+]i between transients gradually decreased. A representative set of tracings of the 340/380 ratio over time of a mesenteric lymphatic isolated from an AAI rat is shown in Fig. 1B.
Figure 1.
Representative tracing of the 340/380 ratio, indicative of [Ca2+]i vs. time in isolated mesenteric collecting lymphatics, from the vehicle control (A) and alcohol-intoxication (B) groups at luminal pressure of 2, 4, 6, 8, 10 and 12 cm H2O.
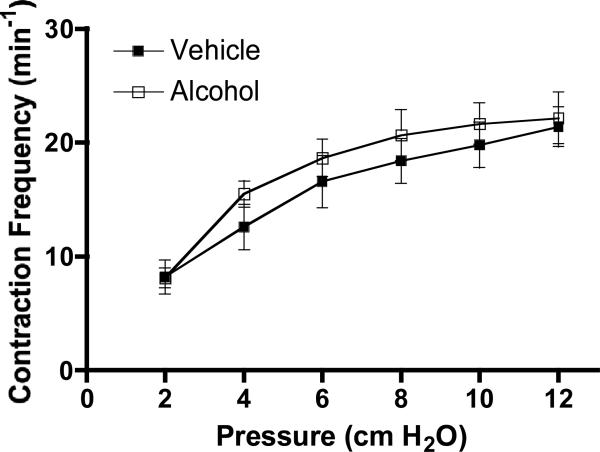
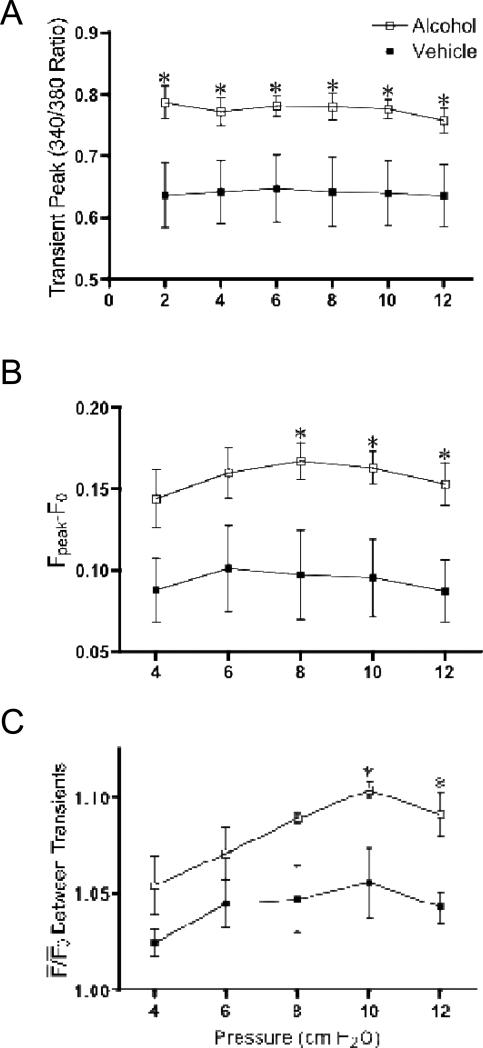
A summary of the frequency of Ca2+ transients in vehicle-treated and AAI rats is shown in Fig. 2. The frequency of Ca2+ transients increased similarly in lymphatic vessels of both experimental groups when pressure step elevations were imposed. Within each group, the mean peak 340/380 ratio did not change with pressure steps. However, at all of the luminal pressures studied, the mean peak 340/380 ratio was significantly higher in lymphatics isolated from AAI rats than vehicle-treated rats (Fig. 3A). In addition, the mean magnitude of the Ca2+ transients (Fpeak - F0), was significantly higher in the AAI group vs. vehicle control after upward pressure steps to 8, 10, and 12 cm H2O (Fig. 3B). The time to peak was not significantly different between the two groups (1015 ± 99 ms for vehicle control and 969 ± 68 ms for AAI, P=0.71). The mean times to half decay were also not significant between the two groups (582 ± 6 ms for vehicle control and 644 ± 6 ms for AAI, P=0.46).
Figure 2.
The change in the frequency of Ca2+ transients, which matches changes in contraction frequency (CF), was not significantly different from acute alcohol intoxicated rats at each pressure studied. For vehicle, N=5 rats; for alcohol N=6 rats studied.
Figure 3.
Acute alcohol intoxication increases the magnitude of Ca2+ transients and the basal [Ca2+]i between transients in response to step elevations in luminal pressure in mesenteric lymphatic vessels. A. Mean peak of Ca2+ transients, B. Magnitude of Ca2+ transients (Fpeak – F0), and C. Basal [Ca2+]i between transients at different luminal pressures, of isolated mesenteric lymphatics from control and acute alcohol intoxicated rats. *P<0.05, vehicle (N=5 rats) vs. alcohol (N=6 rats).
Mean change in basal [Ca2+] between transients in response to step increases in pressure
Lymphatics isolated from both experimental groups showed a rise in the basal 340/380 ratio after each pressure step increase. However, in contrast to our prediction, this rise was significantly greater in lymphatics obtained from AAI rats than in vehicle controls at upward pressure steps to 10 and 12 cm H2O (Fig. 3C).
Impact of alcohol intoxication on calcium sensitivity of lymphatic contractile mechanisms
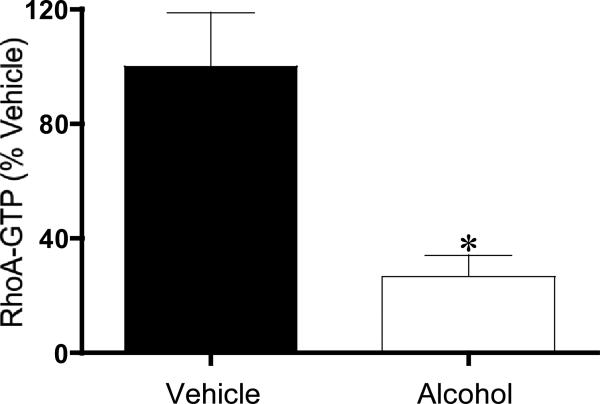
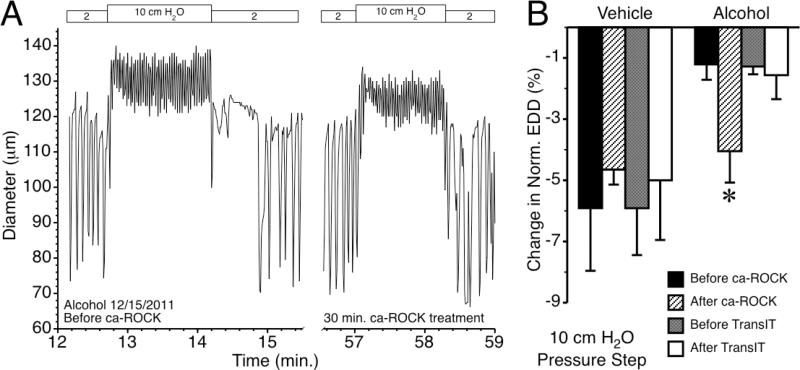
Ca2+ sensitization in lymphatics is partly mediated by RhoA/ROCK [21]. RhoA is a switch protein that when bound to GTP, activates its downstream mediator ROCK. RhoA-GTP was significantly reduced in lymphatics from the AAI rats compared with vehicle controls (Fig. 4). We tested the hypothesis that increasing ROCK activity would rescue the alcohol-induced suppression in lymphatic myogenic constriction (Fig. 5). An example tracing of a lymphatic diameter isolated from an alcohol-treated rat is shown in Fig. 5A. The left panel is the response to a step increase in intraluminal pressure from 2 to 10 cm H2O, before this alcohol treated lymphatic vessel was transfected with constitutively active ca-ROCK protein. The right panel shows the same lymphatic vessel's response to the same pressure step protocol after transfection with ca-ROCK protein for 30 min. Fig. 5B shows the average lymphatic myogenic constriction, represented by the normalized decrease in lymphatic end diastolic diameter, from rats that received intragastric water (vehicle control) prior to isolation, and from AAI rats before and after ca-ROCK transfection. Our results show that constitutively active ca-ROCK protein significantly enhanced lymphatic myogenic constriction of lymphatics from AAI rats, restoring the mean constriction to a level similar to that observed in lymphatics from vehicle-treated controls after the initial stretching of the vessel caused by the upward pressure step. Transfection of isolated lymphatics from vehicle-treated control rats with ca-ROCK did not significantly change the myogenic response. We also tested whether the transfection reagent used to deliver ca-ROCK, TransIT-LT1, affected myogenic constriction. TransIT-LT1 alone did not impact the changes in normalized end diastolic diameter following an upward pressure step from 2 to 10 cm H2O on both groups, vehicle control and AAI.
Figure 4.
Acute alcohol intoxication significantly reduces RhoA-GTP levels in mesenteric lymphatic vessels. *P<0.05, N=3 rats for both the vehicle and alcohol groups.
Figure 5.
Transfection of lymphatics with constitutively active ca-ROCK protein restores myogenic constriction in response to pressure. A. Diameter over time for a single, isolated, rat collecting lymphatic from an alcohol-treated rat, before and after transfection with 2 μg/ml ca-ROCK protein. Applied luminal pressures are shown on the top. B. Mean lymphatic myogenic constriction, expressed as the change in normalized end diastolic diameter (EDD) immediately following the upward pressure step from 2 to 10 cm H2O in lymphatic vessels. The lymphatics were isolated from rats treated with either vehicle (water) or alcohol. The change in normalized EDD was assessed before and after treatment with either 2 μg/ml ca-ROCK diluted in TransIT-LT1 (added at 10 μl/ml; N=4 each for the vehicle and alcohol groups) or TransIT-LT1 alone (10 μl/ml; N=3 for the vehicle and alcohol groups). *P<0.05, Alcohol vs. Alcohol + ca-ROCK.
DISCUSSION
We examined Ca2+ transient dynamics and a potential Ca2+ sensitizing mechanism in lymphatic vessels isolated from alcohol-intoxicated rats. Our results provide evidence that AAI modifies lymphatic contractions by altering both cyclic Ca2+ release and Ca2+ sensitivity. We observed that upward steps in luminal pressure caused a gradual increase in the “basal” [Ca2+]i between transients that were greater in lymphatics from alcohol-intoxicated rats than vehicle controls. Thus, the alcohol-induced loss of lymphatic myogenic constriction in response to pressure [8] cannot be explained by a decrease in [Ca+2]i. Rather, our results suggest that alcohol-induced inhibition of lymphatic myogenic constriction might be due to inhibition of a Ca2+-sensitizing mechanism. RhoAGTP (active RhoA) was significantly reduced in lymphatics from the AAI group compared with vehicle controls, and experimental addition of ca-ROCK essentially rescued the loss of lymphatic myogenic constriction produced by alcohol. Combined, our data suggest that the alcohol-induced reduction of lymphatic myogenic constriction is due to inhibition of a RhoA/ROCK-mediated Ca2+-sensitizing mechanism.
Findings from these studies are in agreement with our previous work showing that each isolated lymphatic phasic contraction is preceded by a transient rise in [Ca2+]i, highlighting the central role of Ca2+ in the pacemaking mechanism. In addition, the frequency of Ca2+ transients is sensitive to changes in luminal pressure [18]. Modulation of Ca2+ release from intracellular stores has been suggested to be involved in the pressure-induced activation of lymphatic contraction [27]. The pacemaking in lymphatic vessels is not generated by a single current but relies on complex interactions between multiple ion currents [28]. Evidence suggests that cyclic depolarization arises in mesenteric lymphatic smooth muscle through spontaneous or agonist-induced release of Ca2+ from IP3-sensitive Ca2+ stores, which activates Ca2+-activated chloride channels (ClCa) [29]. Lymphatic vessels isolated from alcohol-intoxicated animals showed an increase in the magnitude of Ca2+ transients, which could potentially underlie the trend for elevated contraction amplitude observed in our studies [8]. We also determined the time to peak and time to half decay and found these were not altered by alcohol. It should be noted that the time to peak was much higher, and the half time for decay slightly higher than typical times observed in single cells [30, 31]. This is most likely because we determined the 340/380 ratio in a relatively large area, so that the speed at which the calcium transient was transmitted along the length of the vessel strongly influenced these parameters. The mechanism for the alcohol-induced increase in Ca2+ transient magnitude might be due to enhanced release of Ca2 from internal stores, or sarcolemmal entry as well as to changes in sarcoplasmic reticulum reuptake [32]. In addition, alcohol could also directly impact the oscillating mechanism responsible for transient Ca2+ release. Another possibility is that the resulting decrease in RhoA/ROCK-mediated tonic contractility might produce a feedback response to enhance cyclic release of Ca2+. These questions represent a future area of investigation.
In the current study, the contraction frequency was not significantly different between alcohol and control. We previously showed that lymphatic vessels from AAI rats present a decreased contraction frequency compared to controls [8]. The lack of a significant difference in this study compared to our previous work may be due to modifications in our experimental protocol such as the Fura-2 loading, which in addition to the exposure to the dye itself also increased the time from mesentery excision to measurement performance by about one hour. In addition, image acquisition for this protocol involved exposure to a more intense excitation light source in the UV range that could negatively impact contractions. To minimize negative effects we collected image sets over relatively short periods of time, and used the shortest exposure time available with our equipment.
The myogenic response in vessels reflects an improved excitation-contraction coupling, resulting from membrane depolarization and increased Ca2+ conductance [33]. We previously showed that acute alcohol intoxication causes a significant loss of lymphatic myogenic constriction in response to upward luminal pressure steps [8]. We also recently demonstrated in isolated mesenteric lymphatic vessels that the upward steps in luminal pressure elicit a gradual rise in basal [Ca2+]i after increased luminal pressure [18]. Based on these findings we expected that the alcohol-induced loss of stretch-induced lymphatic myogenic constriction would be due to decreased [Ca2+]i. However, we observed the opposite. Alcohol intoxication increased the magnitude of phasic Ca2+ mobilizations in lymphatic smooth muscle, and the gradual rise in basal [Ca2+]i persisted, suggesting that alcohol-induced inhibition of lymphatic myogenic constriction is not due to an impaired Ca2+ release or entry into the cytoplasm. These findings led us to investigate if a Ca2+ sensitizing mechanism was affected.
Ca2+ sensitivity could be modulated, resulting in increased force generated at any given [Ca2+]i. In small arteries, studies have shown that myogenic response is suppressed by inhibition of either PKC or ROCK [34, 35, 36, 37, 38]. Thus, both ROCK- and/or PKC-dependent mechanisms of Ca2+ sensitization contribute to the arterial myogenic response. Additional reports in the literature also suggest that the ROCK pathway may be involved in maintaining lymphatic vessel pump activity and tone [21]. In addition, indirect evidence suggests that the Ca2+-sensitizing CPI-17 may also improve lymphatic tone [39]. The results from this study suggest that RhoA/ROCK signaling contributes to Ca2+-sensitivity in mesenteric collecting lymphatics. Furthermore, our results suggest that alcohol reduces myogenic constriction in lymphatic vessels due to reduction of RhoA/ROCK-mediated Ca2+ sensitivity, in spite of an increase in [Ca2+]i.
While our data suggest that a certain basal ROCK activity is required for lymphatic myogenic constriction, we did not test whether luminal pressure causes RhoA or ROCK activation. The RhoA G-LISA assay requires relatively large amounts of protein to detect changes in RhoA-GTP. Thus, a methodological drawback was that we required several long vessels to perform the assay, and could not measure RhoA-GTP in single, pressurized lymphatic vessels. This also presented the limitation that endothelial vs. smooth muscle RhoA could not be differentiated. Our functional studies with ca-ROCK also did not answer the question as to whether luminal pressure increases can activate the RhoA/ROCK pathway, because ca-ROCK lacks a regulatory domain and cannot be controlled by intracellular signals like endogenous ROCK. This may explain in part why ca-ROCK did not enhance myogenic responsiveness of lymphatics from vehicle-treated rats, which presumably have intact RhoA-ROCK signaling. It may also be possible that substrates may preferentially bind to endogenous activated ROCK, which would explain why ca-ROCK did not enhance myogenic constriction in lymphatics from vehicle-treated rats. New strategies will need to be developed to address this question, and may include transfection with full-length, wild-type ROCK, using pharmacological strategies to inhibit ROCK, or developing more sensitive methods to study ROCK activity, enabling detection in individual lymphatic vessels.
Another potential limitation is that we cannot exclude the possibility that some loading of the endothelium with either Fura-2 or ca-ROCK may have occurred. To minimize this possibility, we applied both Fura-2 AM and the ca-ROCK transfection mix only to the exterior, abluminal side of the isolated lymphatic vessels to restrict access to the endothelium [24, 25]. Also, although our experiments did not involve imposed flow, there is the possibility that alcohol may impact endothelial signaling to cause relaxation [40, 41]. Future experiments focusing on potential roles of the endothelium, including those in which the endothelium is removed will help resolve this issue.
Taken together the results from our studies and reports in the literature indicate that acute alcohol intoxication uncouples the close association between phasic and tonic contraction in lymphatics. This is reflected in the increased magnitude of phasic Ca2+ mobilization and attenuated lymphatic myogenic constriction mediated by impaired Ca2+-sensitizing mechanisms. Future studies, aimed at examining how alcohol affects the stretch-induced increases in cytosolic Ca2+ concentrations and on myosin light chain phosphatase (MLCP) activity will be very important to developing a detailed understanding of the complex mechanisms underlying alcohol's effects on lymphatic function.
PERSPECTIVE.
The lymphatics of the gastrointestinal tract have a high rate of lymph formation, accounting for two-thirds of the lymph formed in the body, and also serve as the primary route for gut-derived tissue injury factors that contribute to progression of tissue dysfunction and multiple organ injury. Alcohol intoxication increases lymph flow in the gut, which can potentially increase transport of toxins or injury factors to remote areas of the body, complicating injuries and worsening outcomes for trauma victims. The current study shows that the changes in the lymphatic contraction pattern caused by alcohol are due in part to the combination of altered cyclic release of Ca2+ from internal stores and reduced sensitivity of contractile proteins due to inhibition of RhoA.
Acknowledgments
FUNDING
This work was supported by NIH Grants R21AA020049, T32AA007577-12 and F32AA021049.
LIST OF ABBREVIATIONS
- Ca2+
Calcium
- [Ca2+]i
Cytosolic calcium
- RhoA
Ras homolog gene family, member A
- ROCK
Rho-kinase
- GTP
Guanosine triphosphate
- Ca-ROCK
Constitutively active ROCK protein
- GI
Gastrointestinal
- MLC
Myosin light chains
- MLCK
Myosin light chain kinase
- MLCP
Myosin light chain phosphatase
- MYPT1
myosin phosphatase target subunit 1
- CPI-17
C-kinase potentiated Protein phosphatase-1 Inhibitor
- PKC
Protein kinase C
- APSS
Albumin physiological salt solution
- Fura-2 AM
Fura 2 acetoxymethyl ester (AM)
- ROI
Region of interest
- IP3
Inositol trisphosphate
- ClCa
Calcium activated chloride channel
- CF
Contraction frequency
REFERENCES
- 1.Faries PL, Simon RJ, Martella AT, et al. Intestinal permeability correlates with severity of injury in trauma patients. J Trauma. 1998;44:1031–5. doi: 10.1097/00005373-199806000-00016. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 2.Langkamp-Henken B, Donovan TB, Pate LM, et al. Increased intestinal permeability following blunt and penetrating trauma. Crit Care Med. 1995;23:660–4. doi: 10.1097/00003246-199504000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Senthil M, Brown M, Xu DZ, et al. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958–65. doi: 10.1097/01.ta.0000215500.00018.47. discussion 65-7. [DOI] [PubMed] [Google Scholar]
- 4.Wu TF, MacNaughton WK, von der Weid PY. Lymphatic vessel contractile activity and intestinal inflammation. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):107–10. doi: 10.1590/s0074-02762005000900018. [DOI] [PubMed] [Google Scholar]
- 5.Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med. 2006;203:497–500. doi: 10.1084/jem.20060227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwin A. Deitch D-ZXaQL. Gut lymph hypotesis of early shock and trauma-induced multiple organ dysfunction syndrome: A new look at gut origin sepsis. Journal of Organ Dysfunction. 2006;2:10. [Google Scholar]
- 7.Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 1207(Suppl 1):E103–11. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 8.Souza-Smith FM, Kurtz KM, Molina PE, et al. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation. 17:514–24. doi: 10.1111/j.1549-8719.2010.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baraona E, Lieber CS. Intestinal lymph formation and fat absorption: stimulation by acute ethanol administration and inhibition by chronic ethanol administration and inhibition by chronic ethanol feeding. Gastroenterology. 1975;68:495–502. [PubMed] [Google Scholar]
- 10.Bartos V, Brzek V. Effect of acute ethanol administration on the thoracic duct lymph flow in man. Lymphology. 1978;11:54–6. [PubMed] [Google Scholar]
- 11.Hadfield RJ, Mercer M, Parr MJ. Alcohol and drug abuse in trauma. Resuscitation. 2001;48:25–36. doi: 10.1016/s0300-9572(00)00315-4. [DOI] [PubMed] [Google Scholar]
- 12.Fay FS, Shlevin HH, Granger WC, Jr., et al. Aequorin luminescence during activation of single isolated smooth muscle cells. Nature. 1979;280:506–8. doi: 10.1038/280506a0. [DOI] [PubMed] [Google Scholar]
- 13.Karaki H, Ozaki H, Hori M, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- 14.Ratz PH, Berg KM, Urban NH, et al. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288:C769–83. doi: 10.1152/ajpcell.00529.2004. [DOI] [PubMed] [Google Scholar]
- 15.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 16.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–85. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitazawa T, Eto M, Woodsome TP, et al. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 18.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of Cytosolic Ca2+ in Isolated Contractile Lymphatics. J Vis Exp. 2011;58:e3438. doi: 10.3791/3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H2573–7. doi: 10.1152/ajpheart.00002.2003. [DOI] [PubMed] [Google Scholar]
- 20.Zawieja DCKE, Pullin J. Dynamics of the Microlymphatic System. Prog Appl Microcirc Basel, Karger. 1999;23:33–41. [Google Scholar]
- 21.Hosaka K, Mizuno R, Ohhashi T. Rho-Rho kinase pathway is involved in the regulation of myogenic tone and pump activity in isolated lymph vessels. Am J Physiol Heart Circ Physiol. 2003;284:H2015–25. doi: 10.1152/ajpheart.00763.2002. [DOI] [PubMed] [Google Scholar]
- 22.Phelan H, Stahls P, Hunt J, et al. Impact of alcohol intoxication on hemodynamic, metabolic, and cytokine responses to hemorrhagic shock. J Trauma. 2002;52:675–82. doi: 10.1097/00005373-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Davis MJ, Davis AM, Ku CW, et al. Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol. 2009;296:H293–302. doi: 10.1152/ajpheart.01040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falcone JC, Kuo L, Meininger GA. Endothelial cell calcium increases during flow-induced dilation in isolated arterioles. Am J Physiol. 1993;264:H653–9. doi: 10.1152/ajpheart.1993.264.2.H653. [DOI] [PubMed] [Google Scholar]
- 25.Hill MA, Zou H, Davis MJ, et al. Transient increases in diameter and [Ca(2+)](i) are not obligatory for myogenic constriction. Am J Physiol Heart Circ Physiol. 2000;278:H345–52. doi: 10.1152/ajpheart.2000.278.2.H345. [DOI] [PubMed] [Google Scholar]
- 26.Breslin JW, Sun H, Xu W, et al. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol. 2006;290:H741–50. doi: 10.1152/ajpheart.00238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atchison DJ, Rodela H, Johnston MG. Intracellular calcium stores modulation in lymph vessels depends on wall stretch. Can J Physiol Pharmacol. 1998;76:367–72. [PubMed] [Google Scholar]
- 28.Toland HM, McCloskey KD, Thornbury KD, et al. Ca(2+)-activated Cl(-) current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol. 2000;279:C1327–35. doi: 10.1152/ajpcell.2000.279.5.C1327. [DOI] [PubMed] [Google Scholar]
- 29.von der Weid PY, Rahman M, Imtiaz MS, et al. Spontaneous transient depolarizations in lymphatic vessels of the guinea pig mesentery: pharmacology and implication for spontaneous contractility. Am J Physiol Heart Circ Physiol. 2008;295:H1989–2000. doi: 10.1152/ajpheart.00007.2008. [DOI] [PubMed] [Google Scholar]
- 30.Sarin V, Muthuchamy M, Heaps CL. Ca(2)(+) sensitization of cardiac myofilament proteins contributes to exercise training-enhanced myocardial function in a porcine model of chronic occlusion. Am J Physiol Heart Circ Physiol. 301:H1579–87. doi: 10.1152/ajpheart.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganitkevich V, Isenberg G. Stimulation-induced potentiation of T-type Ca2+ channel currents in myocytes from guinea-pig coronary artery. J Physiol. 1991;443:703–25. doi: 10.1113/jphysiol.1991.sp018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haorah J, Knipe B, Gorantla S, et al. Alcohol-induced blood-brain barrier dysfunction is mediated via inositol 1,4,5-triphosphate receptor (IP3R)-gated intracellular calcium release. J Neurochem. 2007;100:324–36. doi: 10.1111/j.1471-4159.2006.04245.x. [DOI] [PubMed] [Google Scholar]
- 33.Uchida E, Bohr DF. Myogenic tone in isolated perfused resistance vessels from rats. Am J Physiol. 1969;216:1343–50. doi: 10.1152/ajplegacy.1969.216.6.1343. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RP, El-Yazbi AF, Takeya K, et al. Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J Physiol. 2009;587:2537–53. doi: 10.1113/jphysiol.2008.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schubert R, Kalentchuk VU, Krien U. Rho kinase inhibition partly weakens myogenic reactivity in rat small arteries by changing calcium sensitivity. Am J Physiol Heart Circ Physiol. 2002;283:H2288–95. doi: 10.1152/ajpheart.00549.2002. [DOI] [PubMed] [Google Scholar]
- 36.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 37.VanBavel E, van der Meulen ET, Spaan JA. Role of Rho-associated protein kinase in tone and calcium sensitivity of cannulated rat mesenteric small arteries. Exp Physiol. 2001;86:585–92. doi: 10.1113/eph8602217. [DOI] [PubMed] [Google Scholar]
- 38.Yeon DS, Kim JS, Ahn DS, et al. Role of protein kinase C- or RhoA-induced Ca(2+) sensitization in stretch-induced myogenic tone. Cardiovasc Res. 2002;53:431–8. doi: 10.1016/s0008-6363(01)00496-5. [DOI] [PubMed] [Google Scholar]
- 39.Dougherty PJ, Davis MJ, Zawieja DC, et al. Calcium sensitivity and cooperativity of permeabilized rat mesenteric lymphatics. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1524–32. doi: 10.1152/ajpregu.00888.2007. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–6. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 41.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–37. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]