Abstract
An important endeavor involves increasing our understanding of biobehavioral processes underlying different types of obesity. The current study investigated the neural correlates of cognitive control (involving conflict monitoring and response inhibition) in obese individuals with binge eating disorder (BED) as compared to BMI-matched non-BED obese (OB) individuals and lean comparison (LC) participants. Alterations in cognitive control may contribute to differences in behavioral control over eating behaviors in BED and obesity. Participants underwent functional magnetic resonance imaging (fMRI) while completing the Stroop color-word interference task. Relative to the OB and LC groups, activity in the BED group was differentiated by relative hypoactivity in brain areas involved in self-regulation and impulse control. Specifically, the BED group showed diminished activity in the ventromedial prefrontal cortex (vmPFC), inferior frontal gyrus (IFG) and insula during Stroop performance. In addition, dietary restraint scores were negatively correlated with right IFG and vmPFC activation in the BED group, but not in the OB or HC groups. Thus, BED individuals’ diminished ability to recruit impulse-control-related brain regions appears associated with impaired dietary restraint. The observed differences in neural correlates of inhibitory processing in BED relative to OB and LC groups suggest distinct neurobiological contributions to binge eating as a subgroup of obese individuals.
Keywords: binge eating disorder, obesity, inhibition, ventromedial prefrontal cortex, inferior frontal gyrus, restraint, fMRI
Introduction
Approximately one-third of the US population is obese (1) and data suggest that obesity has a complex, heterogeneous etiology (2). Identifying and conceptualizing specific subtypes can lead to more targeted prevention and treatment strategies. Binge eating disorder (BED) is characterized by recurring episodes of binge eating (defined as eating unusually large quantities of food while experiencing a subjective sense of loss of control over the eating) and marked distress, yet without the weight-compensatory behaviors that define bulimia nervosa (3). BED is strongly associated with obesity (4) but also differs from other forms of obesity and eating disorders in behavioral, body-image, psychological, and psychiatric domains (5). Persons with BED consume more calories and feel relatively less full after eating meals relative to non-BED obese individuals. Group differences also extend to non-food stimuli; BED is associated with impairments on executive tasks of cognitive flexibility (6), suggesting more general cognitive or self-regulation difficulties. Consistent with this notion, BED is associated with greater psychiatric comorbidity (7), and binge-eating status, rather than obesity, accounts for many observed differences in psychological (5) and psychiatric domains (4).
One influential yet controversial concept in eating research is the role of dietary restraint, which refers broadly to conscious efforts to control food intake because of weight concerns. Restrained eating, particularly when combined with palatable food and stress, may represent an important factor in the development and maintenance of binge eating (8, 9), although the mechanisms by which this might occur are not well understood. Restraint represents a multidimensional construct, and different subcomponents may have divergent prognostic significance with respect to binge eating and weight (10, 11). Additionally, this construct may vary across different types of disordered eating; for example, in some groups restraint may best reflect dieting intentions and attempts, rather than actual caloric restriction (12). Overall, understanding of how this construct relates to general self-regulatory capacities is limited and to date no study has examined the neural correlates of restraint in BED.
During food cue presentation, differences in prefrontal brain areas are observed in BED, particularly in the ventromedial prefrontal cortex (vmPFC) (13, 14), a region involved in impulse control and decision-making processes, relative to healthy-weight and obese adults (15). Altered processing in this area is consistent with biobehavioral findings in addictive disorders, and contributes to emerging evidence that specific consumption patterns of palatable foods may produce behaviors and brain changes similar to those observed in addiction (8, 9, 16, 17). Individuals characterized by poor impulse control, such as persons with pathological gambling, demonstrate relatively diminished activation of the vmPFC during Stroop performance (18).
In obese individuals, disinhibited behavior inversely relates to vmPFC volume (19). This study, however, did not distinguish between BED and non-BED individuals. Indeed, few neuroimaging studies compare obese BED to obese non-BED groups, thereby making it difficult to differentiate effects related to BED from those attributable to obesity in general. To date, no studies have examined the neurobiological substrates of response inhibition on standard cognitive control tasks (i.e., not food-related) that might distinguish one form of obesity from another, nor how the construct of restraint relates to these general control processes.
The current study sought to investigate similarities and differences in brain areas that underlie cognitive control processes in obese BED individuals, non-BED obese individuals (OB), and a lean comparison (LC) group. Functional magnetic resonance imaging (fMRI) measured neural activity as participants completed the Stroop color-word task, testing the ability to inhibit pre-potent response tendencies. It has been previously shown that Stroop performance (on incongruent relative to congruent trials) recruits prefrontal cortex (PFC), including the dorsolateral PFC, inferior frontal gyrus and the anterior cingulate cortex. Activity in these regions is related to the ability to inhibit pre-potent responses (i.e., reading of words) over less automatic responses (i.e., color naming). In populations characterized by impaired impulse control, such as pathological gambling and bulimia nervosa, Stroop performance is associated with relatively diminished activity in these areas, including within the vmPFC (18, 20). We hypothesized similar prefrontal hypoactivity might also be observed in BED relative to control groups. In bulimia nervosa, another disorder characterized by binge eating, individuals demonstrate diminished recruitment of the left inferior frontal gyrus (IFG) as well as superior and medial temporal areas during self-regulatory processing (21, 22). We therefore hypothesized that individuals with BED may exhibit similar alterations in fronto-temporal networks, relative to non-binge eating groups. We further investigated the neural correlates of inhibitory control with dietary restraint scores. Based on prior reports of disinhibition and vmPFC activity, we hypothesized that dietary restraint scores in the BED group would correlate inversely with vmPFC activity during Stroop performance.
Methods and Procedures
Participants
Thirty-five English-speaking adults aged 19–64 years (mean age: 38.4±12.5, 19 female) participated, where 69% (n=24) were Caucasian, 23% (n=8) were African American, and 9% (n=3) were Asian, Native American, or of other racial heritage; 9% (n=3) identified themselves as Hispanic and 91% (n=32) as non-Hispanic. For all participants, eligibility criteria included no head injury, history of neurological condition or seizure, or medical condition precluding participation in fMRI procedures (e.g. pacemakers, implanted devices). Exclusion criteria included current drug or psychotherapy for psychiatric or medical conditions (e.g. antidepressant therapy, migraine medication, uncontrolled hypertension or diabetes). Participants were also excluded if they met any of the following criteria: pregnant, breast-feeding, color-blind, history of cardiovascular disease, BP>145/90. Structured clinical interviews (SCID; (23)) assessed past and current psychiatric conditions. Across all groups, subjects were excluded for past or current depressive, anxiety or substance use disorder (other than nicotine dependence). In the BED group, participants met proposed DSM-5 criteria for BED. For OB and LC groups, the SCID interview verified no history or current expression of binge eating or other disordered eating behaviors. One BED participant was excluded for structural brain anomalies, leaving a total of 11 individuals in this group. This protocol was approved by the Yale University School of Medicine Human Investigations Committee; all participants provided written informed consent.
Table 1 summarizes demographic information for the 3 study groups. The BED group consisted of 12 treatment-seeking participants from a larger clinical research trial conducted at Yale University School of Medicine. Participation and data collection occurred prior to treatment onset. Body Mass Index (BMI) in this group ranged from 30.0–41.9 (mean BMI: 37.1±3.9). Both obese and lean groups were used as comparisons for the BED group. The obese group consisted of 13 individuals with a BMI ranging from 30.4–42.5 (mean BMI: 34.6±4.1); the lean group consisted of 11 individuals with BMIs ranging from 20.8–24.9 (mean BMI: 23.2±1.1). As expected, the BED and OB groups had significantly higher BMIs than the LC group. The BED and OB groups did not differ on BMI.
Table 1.
Participant demographic and BMI information.
| BED | OB | LC | Test Statistic | |
|---|---|---|---|---|
| n | 11 | 13 | 11 | - |
| Male/Female | 2/9 | 8/5 | 6/5 | χ2(2, 35) = 5.0, p > .05 |
| Age (SD) | 47.6 (12.7)* | 35.4 (9.3) | 32.7 (11.3) | F(2, 32) = 5.7, p < .05 |
| Race White/Black/Other | 9/2/0 | 8/4/1 | 7/2/2 | χ2(4, 35) = 3.1, p > .05 |
| Ethnicity Non-Hispanic/Hispanic | 10/1 | 13/0 | 9/2 | χ2(2, 35) = 2.5, p > .05 |
| BMI (SD) | 37.1 (3.9) | 34.6 (4.1) | 23.2 (1.1)* | F(2, 32) = 53.2, p < .05 |
p< .05
BMI= Body Mass Index
SD= Standard Deviation
All three groups were similar with regard to gender, race and ethnicity (see Table 1). The mean age in the BED group was higher than in the HC and OB groups (who did not differ from each other). To control for potential age effects, age was entered as a covariate in all subsequent analyses.
Measures
Eating Disorder Examination Questionnaire (EDE-Q)
The EDE-Q is a self-report questionnaire that assesses eating disorder features with a focus on the previous 28-day period (24). The EDE-Q assesses the frequency of different forms of overeating, including binge eating, and is comprised of four subscales (Dietary Restraint, Eating Concern, Weight Concern and Shape Concern) and a global severity score. Items are rated on 7-point forced-choice scales, with high scores reflecting greater eating-disorder severity.
FMRI Stroop Task, Data Acquisition, Processing and Analyses
All participants performed an event-related fMRI Stroop color-word interference task and completed two practice runs prior to scanning. During fMRI, participants completed 6 runs of 105 stimuli. Each word stimulus was presented for 1300 msec, with an inter-trial interval of 350 msec. To produce the Stroop effect, incongruent stimuli were presented pseudo-randomly every 13 to 16 congruent stimuli, with seven incongruent events in each run. Behavioral Stroop performance was assessed out-of-scanner during five runs presented immediately following scanning. A microphone recorded verbal responses as reaction times on each trial and errors on incongruent trials were manually recorded by research staff. Due to voice recorder malfunctions, data from four participants (1 BED, 2 OB, and 1 LC) were excluded from analyses. One BED subject had data from one of the five runs discarded, an OB subject had two runs discarded, and one LC subject had four runs discarded.
Images were obtained with a Siemens TIM Trio 3T MRI system. Functional images were aligned with the 8th slice parallel to the plane transecting the anterior and posterior commissures (the AC-PC line). Localizers and functional images were collected using an echo-planar image gradient-echo pulse sequence. The functional images were collected over six runs (repetition time, TR=1500ms, echo time, TE=27ms, flip angle=60°, field of view [FOV]=22cm×22cm, 64x64 matrix, 3.4mm in-plane resolution). Each run consisted of 124 volumes, including an initial rest period of 9 seconds that was removed from analyses.
FMRI data were pre-processed using statistical parametric mapping software (SPM5; Welcome Functional Imaging Laboratory, London UK). All functional and anatomical images were reoriented along the AC-PC line on a subject-by-subject basis. All images were first corrected for varying slice-timing and then realigned to the first slice. These image volumes were used to construct mean functional image volumes, which were then co-registered with each subject's anatomical image, segmented, and normalized to the Montreal Neurological Institute (MNI) standardized space. The images were smoothed with a 6mm full-width-at-half-maximum Gaussian kernel. The image volumes from each run were examined for head motion in excess of one acquisition voxel in translation or 3.5 degrees of rotation. A first-level general linear model (GLM) was used to model the onsets and offset of the congruent and incongruent stimuli using robust regression; motion parameters and high-pass filter parameters were included as additional regressors of no interest. An event-related design modeled the onsets of the congruent and incongruent stimuli using the hemodynamic response function with a time derivative. A temporal high-pass filter of 64sec removed low-frequency signals. Second-level random effects analyses were conducted with Neuroelf analysis package (www.neuroelf.net) using a GLM approach. Between-group differences in Stroop-related activity were assessed with random-effects second level contrasts of incongruent versus congruent trials using t-tests. A Monte-Carlo simulation (e.g. AlphaSim) used a combined voxel-wise and cluster threshold producing a family-wise error (FWE) rate of 5% to correct for multiple comparisons.
Results
Self-report Questionnaires
EDE-Q
The mean global score on the EDE-Q was 1.8 (SD=1.4) across all groups. One-way analysis of variance (ANOVA) revealed a difference between the three groups [F(2,33)=12.0, p<.001]. Comparisons showed that the BED group scored higher [MBED=3.1, SD=1.1] than the OB [MOB=1.6, SD=1.0] and LC [MLC=0.9, SD=1.1] groups, which did not differ from each other [p>.05]. There was a significant between-group difference on the Restraint subscale of the EDE-Q [F(2,33)= 8.4, p=.001]; the BED group scored higher [MBED=2.8, SD=1.3] than the LC group [MLC=0.7, SD=1.1; p<.05], while the obese control group [MOB=1.6, SD=1.3] did not differ from either group (p>.05). A one-way ANOVA examining the Weight Concern subscale revealed a significant difference between the three groups [F(2,33)=9.3, p=.001]. Comparisons showed that the BED group scored higher [MBED=3.3, SD=1.0] than the OB [MOB=1.9, SD=1.0] and LC [MLC=1.1, SD=1.7] groups, which did not differ from each other [p>.05]. On the Eating Concern subscale [F(2,33)=8.8, p=.001], the BED group scored higher [MBED=2.4, SD=1.6] than the OB and LC groups [MOB=0.6, SD=1.1, MLC=0.4, SD=0.9], which did not differ from each other [p>.05]. On the Shape Concern subscale [F(2,33)=8.3, p=.001], the BED group scored higher [MBED=3.7, SD=1.1] than the LC group [MLC=1.3, SD=1.6], while the obese control group [MOB=2.5, SD=1.5] did not differ from either group.
Stroop Behavioral Performance
Collapsed across experimental groups, a paired-samples t-test showed a significant difference in reaction times between congruent and incongruent stimuli collected from out-of-scanner recordings (t=14.90, p<.05). Consistent with the idea of greater interference during incongruent trials, mean(SD) reaction time to congruent stimuli was significantly shorter [612.40(69.52)ms] than the mean reaction time to incongruent stimuli [830.86(105.22)ms].
Multiple one-way ANOVAs examining between-experimental-group differences in reaction times showed no significant between-group differences to incongruent stimuli [F(2,17)=.83, p>.05], or to congruent stimuli [F(2,17)=.79, p>.05], or in the average percent of incongruent stimuli incorrectly identified [F(2,29)=1.72, p>.05], or in the Stroop effect [F(2,17)=.42, p>.05].
Neuroimaging Results – Brain Activation during Stroop Task
Using SPSS, brain activity (percent BOLD signal change) was covaried with subjects’ age for all applicable analyses and was found to have no significant effect on any of the observed group differences.
Main Effects
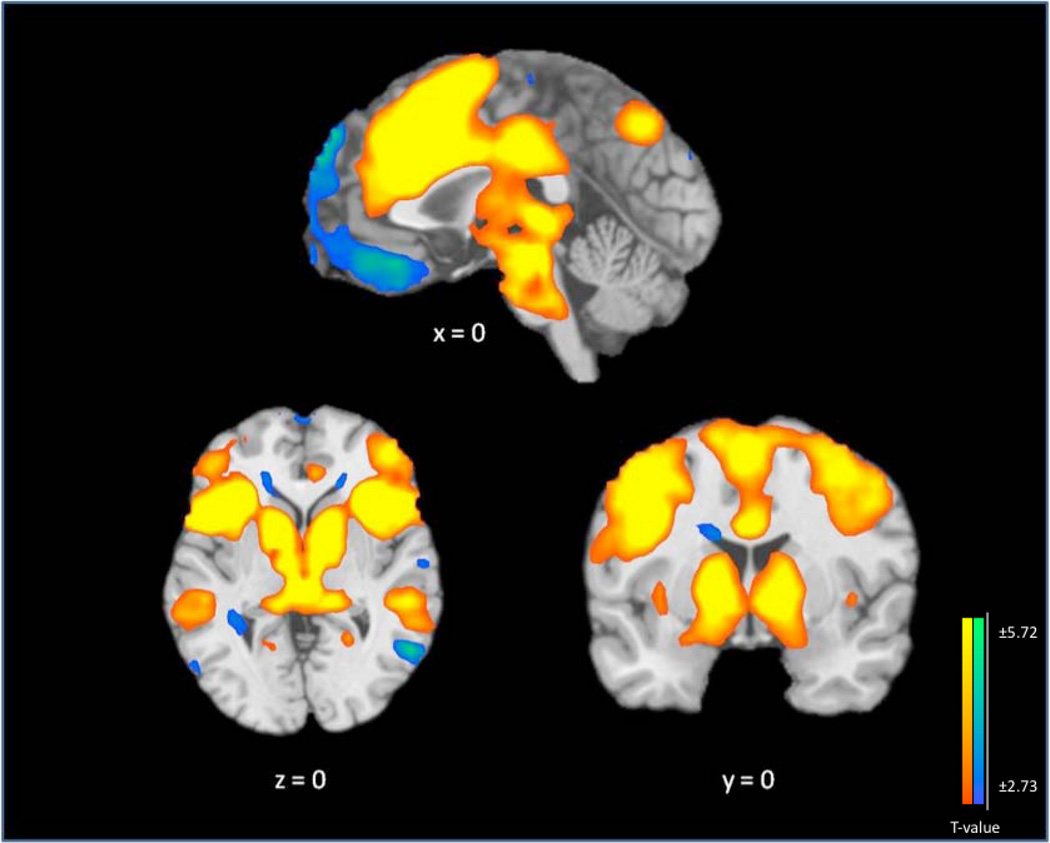
Figure 1 and Table 2 show the main effects of task. Across all three groups, when presented with incongruent stimuli during the fMRI Stroop task, subjects showed an increase in brain activity, relative to the congruent condition, in bilateral regions comprised of the insula and extending anteriorly to the inferior and middle frontal gyri; the midbrain and subcortical structures, including the globus pallidus, red nuclei, caudate, and thalamus; the anterior cingulate, extending dorsally to the cingulate and medial frontal gyri; the fusiform gyri and culmen, extending laterally to the inferior temporal gyri; and the precuneus, extending laterally to the inferior parietal lobules.
Figure 1. Main Effect of Stroop Task Performance.
Change in mean fMRI BOLD signal on Stroop-effect, collapsed across group, contrasting incongruent trials with congruent trials in N=35 participants. The contrast map is thresholded at an uncorrected level of p < 0.01 two-tailed and FWE-corrected at p < 0.05 with a cluster threshold of 28. Blue/green color demonstrates areas of significant differences between incongruent and congruent conditions where relatively less activity occurs in the incongruent condition. Yellow/orange color indicates areas of relative greater activity during the incongruent condition. The right hemisphere of the brain is on the right side. Saggital view is represented at x=0, coronal view at y=0 and axial view at z=0.
Table 2.
Incongruent vs Congruent Effects on the Stroop Task. The main Stroop effect is shown contrasting percent BOLD signal change during incongruent conditions with percent BOLD signal change during congruent conditions, collapsed across all groups.
| MNI Coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| Stroop Main Effect Contrast |
Structure | BA | Left/ Right |
x | y | z | k | T |
| Incongruent> Congruent |
Insula/ Cingulate/ Striatum, Inferior Parietal Lobule/ Striatum/ Midbrain, Thalamus/ Precentral Gyrus/Middle Fontal Gyrus/ Middle Temporal Gyrus/ Middle Temporal Gyrus/ Culmen |
13 | L/R | 36 | 15 | 0 | 24688 | 15.59 |
| Parahippocampal Gyrus/Medial Frontal Gyrus/Posterior Cingulate/Superior Temporal Gyrus/ Inferior Temporal Gyrus/Superior Frontal Gyrus/ Medial Frontal Gyrus |
19 | L | −33 | −42 | −9 | 6013 | −6.84 | |
| Middle Temporal Gyrus | 19 | L | −57 | −72 | 12 | 421 | −6.75 | |
| Cerebellum | - | R | 27 | −87 | −39 | 149 | −4.67 | |
| Cuneus | 19 | R | 6 | −87 | −39 | 407 | −4.29 | |
BA = Brodman’s Area; k = cluster size in voxels
Decreases in brain activity were noted in the left medial orbitofrontal cortex extending dorsally through the ventromedial prefrontal cortex, into the dorsomedial prefrontal cortex, and the superior frontal gyrus. Significant decreases in brain activity were also observed in the left parahippocampal gyrus, right middle temporal gyrus, bilateral superior temporal gyri, bilateral posterior cingulate, bilateral cuneus, and the right tuber. Altogether, these main effect findings are consistent with other fMRI studies of the Stroop-effect (25).
Stroop-Related Between-Group Differences
BED–OB Contrast
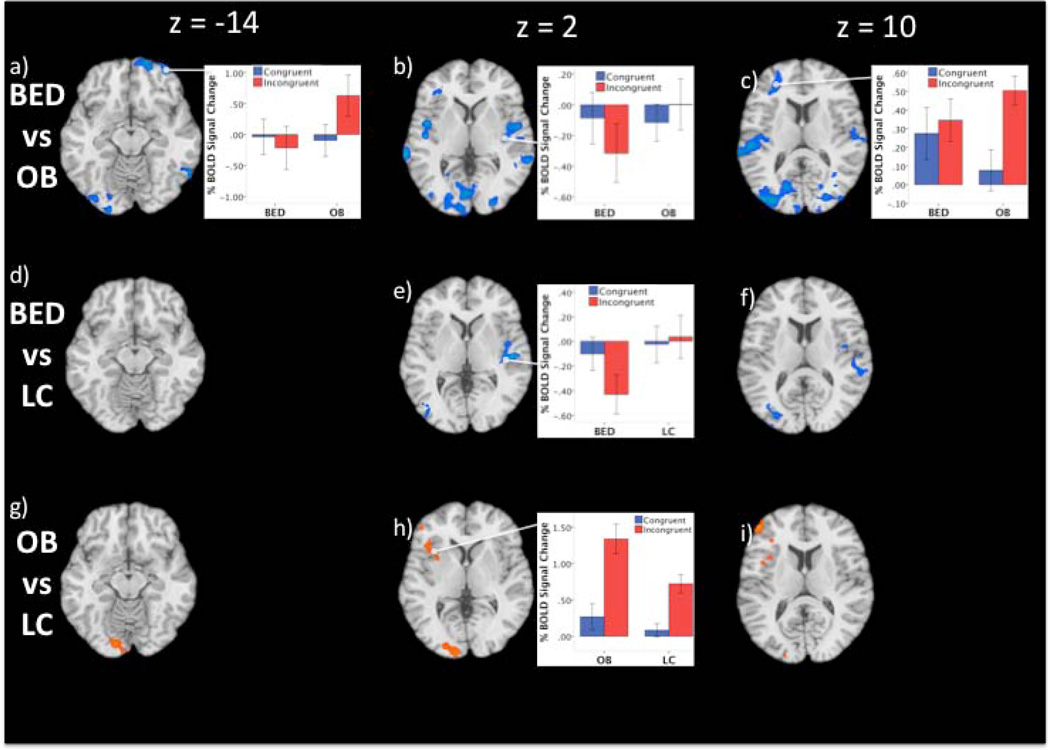
The BED group demonstrated decreased brain activity relative to the OB subjects in the right lateral and anterior/medial orbitofrontal cortex (OFC) extending to vmPFC (Table 3; Figure 2a). These differences were driven by greater activity in the OB group during incongruent trials; the BED group showed activity decreases in both incongruent and congruent conditions. There were also significant differences in the right superior temporal gyrus extending to the insula; both groups demonstrated similar decreases in activity during the congruent condition, but the BED group showed larger decreases during incongruent conditions, whereas the OB group showed little signal changes during incongruent trials (Figure 1b). Group differences were also noted in the left IFG extending to the middle frontal gyrus, where the OB group showed a greater increase during the incongruent trials relative to the congruent ones, whereas the BED group demonstrated similar activity during both the incongruent and the congruent conditions (Figure 1c). Significantly decreased activity was observed in the left middle occipital gyrus extending to the lingual gyrus; while the BED and OB groups demonstrated similar activity decreases during the congruent condition, the BED group demonstrated a greater decrease in the incongruent condition and the OB group showed a greater decrease in the congruent versus the incongruent condition. Significantly decreased activity also occurred in the left superior temporal gyrus extending to the insula; the BED group showed a greater activity decreases during incongruent vs congruent trials, whereas the OB group did not demonstrate between-condition differences. Significant differences were also noted in the middle occipital gyrus extending to the middle temporal gyrus; while both groups showed similar decreases in activity during the congruent condition, the BED group showed a greater decrease during incongruent conditions, whereas the OB group demonstrated slightly increased activity during the incongruent conditions.
Table 3.
Group Differences on the Stroop Task during incongruent vs congruent stimuli presentation
| BED vs OB | |||||||
|---|---|---|---|---|---|---|---|
| Structure | BA | Left/ Right |
x | y | z | k | T |
| Middle/Superior/Orbital Frontal Gyrus | 11 | R | 33 | 57 | −18 | 118 | −5.20 |
| Middle/Inferior Occipital Gyrus/ Lingual Gyrus |
18 | L | −42 | −87 | 9 | 625 | −4.34 |
| Superior Temporal Gyrus/Insula/Precentral Gyrus |
22 | L | −72 | −36 | 6 | 239 | −4.22 |
| Middle Occipital Gyrus | 19 | R | 45 | −87 | 15 | 190 | −3.92 |
| Middle Occipital Gyrus | 42 | R | 60 | −63 | −12 | 206 | −3.87 |
| Superior Temporal Gyrus/Insula | 42 | R | 75 | −18 | 15 | 155 | −3.65 |
| Inferior/Middle Frontal Gyrus | 46 | L | −30 | 36 | 9 | 92 | −3.08 |
| BED vs LC | |||||||
| Structure | BA |
Left/ Right |
x | y | z | k | T |
| Superior Occipital Gyrus/Middle Occipital Gyrus |
19 | L | −33 | −84 | 31 | 197 | −5.41 |
| Superior Temporal Gyrus/Insula | 41 | R | 51 | −23 | 5 | 222 | −4.80 |
| OB vs LC | |||||||
| Structure | BA |
Left/ Right |
x | y | z | k | T |
| Middle/Inferior Frontal Gyrus/Insula | 8 | L | −24 | 12 | 39 | 308 | 4.97 |
| Inferior Frontal Gyrus | 46 | L | −51 | 45 | 6 | 94 | 3.93 |
| Medial Frontal Gyrus | 6 | L | 0 | 15 | 45 | 207 | 3.34 |
| Lingual/ Middle Occipital Gyrus | 18 | L | −12 | −87 | −18 | 139 | 3.22 |
| Cingulate Gyrus | 23 | L | 0 | −27 | 36 | 93 | 2.80 |
BA = Brodman’s Area
k = cluster size in voxels
T = T-value with greatest deviation from 0
Figure 2. Group Differences in Brain Activity during Stroop Task Performance.
Maps show contrasts between the three experimental groups: obese individuals with binge eating disorder (BED; n=11), obese individuals without binge eating disorder (OB; n=13) and lean comparison (LC) participants (n=11) during incongruent vs congruent conditions of the Stroop. The contrast map is thresholded at an uncorrected level of p < 0.05 two-tailed and family-wise-error-corrected at p < 0.05 with a cluster threshold of 90. Orange/yellow color indicates areas of increased activity between groups. Blue areas represent areas of decreased activity between groups. The right hemisphere of the brain is on the right. Images are unmasked.
BED–LC Contrast
BED relative to LC subjects demonstrated significant differences in the right superior temporal gyrus extending to the insula; these differences were driven by activity decreases in the BED group during incongruent versus congruent trials, whereas the LC group showed small increases in activity during both conditions (Figure 1e). Group differences were also noted in the left superior occipital gyrus; BED subjects demonstrated diminished activity during congruent trials but greater decreases during incongruent trials, while the LC group exhibited small decreases during both conditions.
OB–LC Contrast
Stroop-related activity in OB subjects was significantly different than the LC subjects in the left middle frontal gyrus extending to the insula (Table 3; Figure 1h). These differences were driven by relative increased activity during incongruent compared to congruent condition for both groups, with greater activity increases in the OB group. Activity increases were also observed in the bilateral medial frontal gyrus; the OB group demonstrated greater increases during incongruent relative to congruent trials, whereas the LC group showed a small increase during incongruent trials, relative to the OB group, and a small decrease during congruent trials. Groups also differed in the left lingual gyrus extending to the middle occipital gyrus; while groups showed similar decreases here during congruent trials, the OB group showed less of a decrease during incongruent trials, with no between-condition difference in the LC group. The groups also differed in the bilateral cingulate gyrus; while the OB group showed increased activity during incongruent versus congruent trials, little between-condition difference was observed in the LC group.
EDE-Q Restraint Subscale Correlations with Activity during fMRI Stroop Performance BED Group Correlations
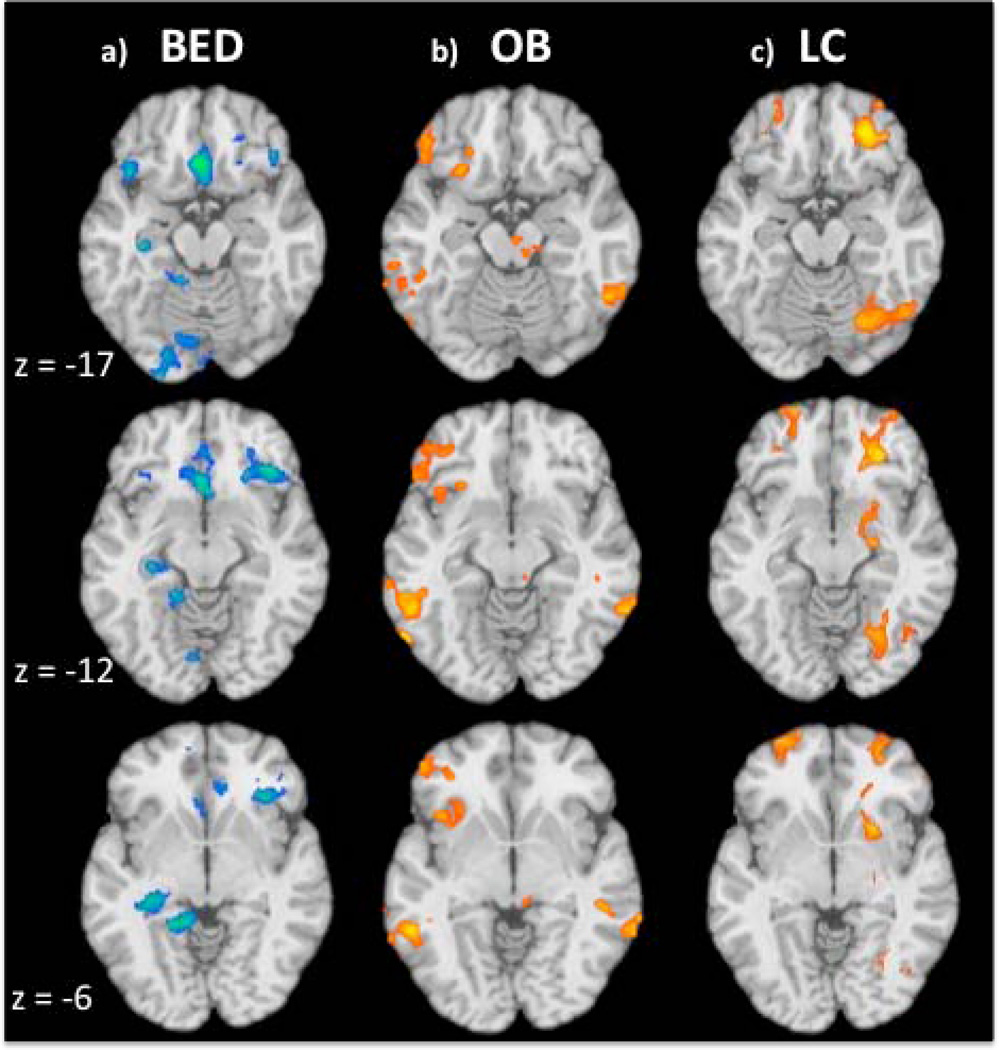
EDE-Q Restraint scores were correlated with BOLD signal change differences during incongruent and congruent conditions in the BED group (Table 4a). Negative correlations were found in the right middle temporal gyrus extending to the IFG and in the left middle temporal gyrus. Negative correlations were also found in the medial OFC including the anterior cingulate and extending to the bilateral vmPFC (Figure 2a). Negative correlations were also observed in the left lingual gyrus, hippocampus and the bilateral superior frontal gyrus and the cerebellum on the right and left sides.
Table 4.
Stroop correlations with EDE-Q-restraint scores in each experimental group
| a) | |||||||
|---|---|---|---|---|---|---|---|
| BED | |||||||
| Structure | BA | Left/ Right |
x | y | z | k | Peak r-value |
| Middle Temporal Gyrus/Inferior Frontal Gyrus/ | 21 | R | 33 | 0 | −39 | 270 | −0.961 |
| Middle/Superior Temporal Gyrus | 21 | L | −51 | 12 | −39 | 207 | −0.952 |
| Anterior Cingulate/vmPFC | 32 | L | −3 | 24 | −15 | 168 | −0.967 |
| Lingual Gyrus | 19 | L | −18 | −45 | −6 | 94 | −0.899 |
| Cerebellum | − | L | −21 | −90 | −30 | 664 | −0.891 |
| Hippocampus | − | L | −30 | −24 | −15 | 96 | −0.882 |
| Superior/Medial Frontal Gyrus | 9 | R | 18 | 60 | 30 | 400 | −0.881 |
| Cerebellum | − | R | 54 | −63 | −39 | 120 | −0.863 |
| b) | |||||||
| OB | |||||||
| Structure | BA |
Left/ Right |
x | y | z | k |
Peak r-value |
| Cuneus | 17 | R | 18 | −78 | 12 | 96 | 0.945 |
| Middle Occipital Gyrus | 19 | L | −60 | −66 | −12 | 204 | 0.889 |
| Superior Temporal Gyrus/Precuneus/Inferior Parietal Lobule/Angular Gyrus |
39 | R | 57 | −57 | 30 | 396 | 0.887 |
| Superior/Inferior Parietal Lobule/Precuneus |
7 | L | −24 | −75 | 54 | 255 | 0.872 |
| Inferior Frontal Gyrus/Insula/ | 11 | L | −24 | 30 | −21 | 405 | 0.854 |
| Inferior Temporal Gyrus | 20 | R | 54 | −54 | −18 | 113 | 0.847 |
| Culmen/Midbrain Substantia Nigra | − | L | −9 | −24 | −36 | 222 | 0.826 |
| Precuneus/Posterior Cingulate | 7 | L | −3 | −36 | 48 | 130 | 0.824 |
| Anterior Cingulate/Superior Frontal Gyrus/Medial Frontal Gyrus |
8 | R | 15 | 21 | 39 | 285 | 0.807 |
| Inferior Frontal Gyrus | 9 | L | −57 | 15 | 24 | 97 | 0.799 |
| Middle Frontal Gyrus | 9 | R | 27 | 21 | 24 | 118 | −0.844 |
| Postcentral Gyrus/Superior/Transverse Temporal Gyrus/Inferior Parietal Lobule |
3 | R | 60 | −6 | 21 | 163 | −0.814 |
| c) | |||||||
| LC | |||||||
| Structure | BA |
Left/ Right |
x | y | z | k |
Peak r-value |
| Inferior/Middle Frontal Gyrus/vmPFC | 47, 11 | R | 27 | 36 | −15 | 200 | 0.951 |
| Inferior/Middle Frontal Gyrus | 46 | R | 48 | 42 | 6 | 103 | 0.901 |
| Middle Frontal/Precentral Gyrus | 6 | R | 24 | 6 | 57 | 117 | 0.893 |
| Inferior/Superior Parietal Lobule/Precuneus | 40 | R | 54 | −30 | 48 | 286 | 0.886 |
| Caudate/Putamen/Ventral Striatum/Thalamus | − | R | 18 | 21 | 3 | 460 | 0.866 |
| Superior Frontal Gyrus | 10 | L | −18 | 72 | 0 | 94 | 0.851 |
| Lingual/Fusiform Gyrus/Declive | 18 | R | 27 | −69 | −15 | 175 | 0.827 |
BA = Brodman’s Area
k = cluster size in voxels
r = Pearson correlation coefficient
vmPFC = ventromedial prefrontal cortex
OFC = orbitofrontal cortex
OB Group Correlations
Regions of significant correlations with EDE-Q Restraint scores in the OB group are listed in Table 4b. Positive correlations (i.e., where greater restraint is related to a greater difference between incongruent and congruent conditions) were found in the right cuneus, left middle occipital gyrus, right superior temporal gyrus, inferior parietal lobule on the right and left sides, right inferior temporal gyrus, culmen extending to the midbrain, left precuneus extending to the posterior cingulate, the anterior cingulate extending to the superior and medial frontal gyrus. Positive correlations also occurred in the left IFG, extending to more medial OFC regions and posteriorly to the insula (Figure 2b).
LC Group Correlations
In the LC group, Restraint scores correlated positively with Stroop-effect-related activity in the right inferior and middle frontal gyrus (including the vmPFC), bilateral caudate extending to the right ventral striatum (Table 4c; Figure 2c) and right middle frontal and precentral gyri, right inferior and superior parietal lobules, superior frontal gyrus and lingual gyrus extending to the declive.
Discussion
This is the first study examining the neural correlates of inhibitory control in obese persons with BED relative to both non-BED obese and lean participants using an fMRI Stroop task. The BED group reported the highest dietary restraint scores, while the OB and the LC group did not statistically differ in their scores. All three groups showed similar performance on the Stroop task, demonstrating longer reaction times to incongruent stimuli compared to congruent ones and committing few errors. Given comparable performance across experimental groups, between-group differences in brain activation can therefore be attributed to alterations in neural substrates underlying cognitive control. Consistent with our hypotheses, the BED group demonstrated diminished activity in frontal regions subserving inhibitory control, including the vmPFC and the IFG. In addition, diminished activity was also noted in the insula, and in superior and middle temporal areas as well as in the middle occipital gyrus. The use of two comparison groups in the current study further demonstrates that the activity differences to incongruent stimuli on the Stroop task appear driven by diminished activation in the BED group, rather than differential activity in the OB or LC groups. In addition, restraint scores were inversely related to vmPFC and IFG activity in the BED group, but not in the OB or the LC groups. Overall, this pattern of negative correlations, together with diminished fronto-temporal activity during a cognitive control task, provide support for distinct differences in inhibitory processing in BED, relative to other forms of obesity. These significant differences in the neurobiological correlates of BED relative to obesity without BED extend the behavioral and psychological empirical literature on the distinctiveness of this diagnostic construct and its validity (5), and provide additional support for the DSM-5 proposal (www.dsm5.org) to make BED a formal diagnosis.
Group Differences during Stroop Performance
Activity differences in the BED group were distinguished by relative hypoactivity in areas involved in self-regulation. Contrasted with the OB group, the BED group showed decreased activity in the left IFG, a brain area implicated in the interaction between cognitive and motivational processes during inhibitory control, including paradigms of attentional set-shifting, task-set shifting and stop-signal inhibition (26). Diminished activity in the IFG may reflect deficits in attentional shifts from congruent to incongruent stimuli in the BED group. Correspondingly, imaging studies of response inhibition in bulimia nervosa, another disorder characterized by binge eating, also report diminished activity during conflict stimuli in the left IFG, as well as the left superior temporal gyrus and the right medial temporal gyrus (21, 22). Our findings are also consistent with lesion and neurodegenerative studies implicating fronto-temporal lesions in the pathogenesis of disordered eating (27, 28). Specifically, imaging studies in patients with compulsive binge eating show diminished volumes in the vmPFC and the right anterior insula - two regions implicated in the BED group in the current study (28). Attenuated recruitment of fronto-temporal circuitry during self-regulatory processing therefore appears as an important distinguishing marker in disorders characterized by binge eating.
Relative to the OB group, the BED group also exhibited diminished activity in the right lateral vmPFC extending towards the IFG. The vmPFC modulates the ability to rapidly adjust prepared responses when reinforcement contingencies change and therefore contributes importantly to inhibitory processes, including reversal learning and attentional conflict tasks such as the Stroop (15, 29). By actively representing the value of an expected outcome, the vmPFC is ascribed an important role in guiding decision-making processes (30), including the choice to consume food (31). In the current study, hypoactivity in the BED group also extended from the vmPFC into more lateral areas of the orbital gyrus, which encompass the secondary gustatory cortex (32). Cognitive factors contribute to the hedonic representation of food in orbitofrontal areas, including biasing the sensory perception of stimuli (33). In the case of BED, altered cognitive processes in OFC/vmPFC areas could exaggerate the signal associated with food reward value and/or diminish inhibitory control. Support for the former idea comes from a study presenting images of high-caloric foods to individuals with BED, bulimia patients, and healthy-weight and overweight controls (14). Relative to the other groups, food-image presentation produced greater recruitment of the vmPFC in the BED group; moreover, scores on the Behavioral Activation Scale positively correlated with vmPFC activity, suggesting related alterations in reinforcement sensitivity. Therefore, alterations in vmPFC activity could distort the perceived palatability of foods and/or override satiety or inhibition signals. Results in the current study provide further support for this latter idea, whereby diminished vmPFC activity during a standard cognitive control task (i.e., in the absence of food cues) suggests more generalized inhibitory signaling impairment in the BED group.
Reduced engagement of self-regulatory mechanisms in BED relative to OB and LC individuals is further suggested by diminished insula recruitment. The insula comprises the primary taste cortex and is involved in the anticipation and consumption of foods, as well as in integrating homeostatic signals (34). Given this latter role, recent studies implicate this area in linking the current bodily state with cognitive and affective processes, thereby influencing decision-making (34). Activity in the anterior insula is related to loss-prediction and, together with ventrolateral prefrontal areas, signals changes in reinforcement contingencies (35). Blunted activity in the BED group suggests potential differences in self-awareness as individuals engage in the Stroop task. In the context of disordered eating, alterations in insula activity may disrupt interoceptive sensitivity to food and satiety cues and underlie dissociative experiences often reported during binges. Altogether, altered recruitment of insula, vmPFC and IFG networks may underlie the sense of loss of control and dissociation and ultimately interfere with individuals’ abilities to balance their experiences of food cravings with their desires for weight loss.
Correlations with Dietary Restraint
The choice to diet is cognitively mediated and involves actively keeping in mind long-term goals (e.g. health, slim body) and disregarding more proximal cues (e.g. hunger signals, food cues). The role of restraint in dieting and eating is poorly understood, possibly because the construct is multifaceted and may include several component processes. These component processes, in turn, may be varyingly expressed in different groups and therefore account for ambiguous findings in studies of restraint (10, 36). The restraint correlational findings in the current study support the idea of self-regulatory impairments and provide further insight into the mechanisms underlying this construct. Restraint scores in the BED group negatively correlated with vmPFC/OFC, IFG and insula activity – areas implicated in inhibitory control and homeostatic signal integration. In contrast, higher restraint scores in the OB group were positively associated with IFG and insula activity. These different correlational patterns suggest that restrained eaters represent a heterogeneous group who may not only employ different weight-regulation strategies (10), but also differ in brain activation relating to their application. Poor communication between cognitive and motivational circuits during inhibitory control may lead an individual to attempt dieting, but to employ strategies less effectively and thereby report greater levels of restraint. This is consistent with the idea that in some individuals, restraint is more closely tied to the intention to diet, rather than actual caloric restriction (12). Indeed, among obese patients with BED, higher eating disorder psychopathology is associated with more frequent dieting attempts (37). Thus, perhaps persons with BED may report greater dietary efforts, but use less successful strategies. It is noteworthy that the OB group demonstrated a similar pattern of positive correlations between dietary restraint and Stroop-related activity in frontal areas, including the IFG and the insula.
Strengths, Limitations and Directions for Future Research
This study is the first to examine the neural correlates of cognitive control in BED relative to two comparison groups. Given that the BED and OB groups were matched on BMI, these findings help distinguish the mechanisms associated with binge eating from those associated with obesity more generally. This study also used the Stroop task, a well-validated assessment of cognitive control. The absence of Stroop behavioral measures during scanning prevents a more precise characterization of neural differences between groups, for example, with respect to parsing out activity related to correct and incorrect trials. Additionally, Stroop performance involves multiple cognitive processes, including attention, conflict monitoring and response inhibition. Future studies should investigate the neural and behavioral correlates underlying these specific dimensions of impulse control in BED. The groups also demonstrated differences in middle occipital regions during the Stroop task; there is evidence that interference effects modulate perceptual processing in visual cortices, with greater conflict associated with reduced cortical signals (38). Future studies could further clarify potential differences in visual processing and how they may relate to aspects of cognitive control in BED. Future studies could also contrast general inhibitory control and food-related inhibitory control in these populations.
Another limitation includes the relatively small numbers of participants within each group, which limits the examination of other potential important influences like gender. Although gender distributions did not differ across the three groups, future studies should examine for potential gender differences. While the BED group included slightly older individuals, age was included as a covariate in all analyses. Finally, racial and ethnic differences exist with respect to demographic characteristics and eating disorder symptoms in treatment-seeking individuals with BED (39); therefore, the predominantly Caucasian BED group in the current study may limit the generalizability to other groups. In addition, the treatment-seeking nature of the current group may distinguish them from a community sample of binge-eaters who are not seeking treatment.
Although still controversial, biobehavioral research increasingly recognizes similarities between BED and addictions and the potential appropriateness of examining BED in an impulse-control-disorder or addiction framework (16, 17). The impaired sense of control during consumption, together with reduced ability to limit the quantity or frequency of use, has drawn parallels to impulse-control and addictive behaviors similarly characterized by diminished control and continued engagement despite negative consequences (2). Neurobiological studies in addicted or impulsive populations also demonstrate diminished ventromedial prefrontal cortical activity during Stroop performance, suggesting that this area may contribute to the cognitive control deficits in these disorders (18, 40). More generally, the current findings indicate that people with BED have difficulty engaging fronto-temporal systems in domains other than feeding, and this tendency may contribute to high rates of co-occurrence between BED and other psychiatric disorders characterized by impaired impulse control (7).
Investigations of biological mechanisms related to the priming effects of specific foods and the manner in which these foods are consumed, constitute an important future direction. Exposure to stressful stimuli, when combined with a restricted diet, can produce an over-consumption of hyper-palatable, energy-dense processed foods (9, 17). Understanding the mechanisms by which these factors combine to produce sensitization or altered endogenous hunger and satiety signals will be important for conceptualizing bingeing as an addictive behavior (16, 17). The nature of different forms of dietary restraint together with intermittent exposure to hyper-palatable food also requires further study in their relation with both self-regulatory behaviors and neurobiological correlates. Longitudinal studies should investigate the link between biobehavioral markers, self-regulatory capacity and the prognostic significance of different types of restraint in specific obesity subtypes.
Conclusions
Identifying neurobiological characteristics distinguishing obesity subtypes is important for understanding mechanisms underlying different types of disordered eating. In the current study, diminished recruitment of frontal systems by individuals with BED supports the idea that BED is characterized by functional disturbances in brain areas implicated in self-control processing. Alterations in fronto-temporal circuits are similarly observed in individuals with bulimia nervosa or neurodegenerative disorders who develop compulsive overeating. Altogether, these results provide evidence for divergent neural substrates of inhibitory control distinguishing BED from other manifestations of obesity.
Figure 3. Correlations between Restraint Scores and Brain Activity during Stroop Task Performance.
Maps show correlations between eating restraint scores on the EDE-Q and activity during Stroop performance in the three experimental groups: a) obese individuals with binge eating disorder (BED; n=11), b) obese individuals without binge eating disorder (OB; n=13) and c) healthy control lean comparison (LC) participants (n=11). The contrast map is thresholded at an uncorrected level of p < 0.05 two-tailed and family-wise-error-corrected at p < 0.05 with a cluster threshold of 90. Orange/yellow color indicates a positive correlation and blue areas represent areas of negative correlations. The right hemisphere of the brain is on the right. Images are unmasked.
Acknowledgements
We gratefully acknowledge Scott Bullock, Jessica Montoya, Naaila Panjwani, Monica Solorzano, Jocelyn Topf, Katie VanBuskirk, Rachel Barnes, and Robin Masheb for their help with the project. Support was provided by the following Grants: National Institutes of Health grants R01-DA019039, P20-DA027844, P50-AA012870, R01-DA020908, R01-AA016599, RL1-AA017539, K12-DA00167, R01 DK073542, PL1-DA024859 and 2K24 DK070052. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
Disclosure
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Psyadon, Ortho-McNeil, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Footnotes
Conflict of Interest
The authors report that they have no financial conflicts of interest with respect to the content of this manuscript.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Davis CA, Levitan RD, Reid C, et al. Dopamine for "wanting" and opioids for "liking": a comparison of obese adults with and without binge eating. Obesity (Silver Spring) 2009;17:1220–1225. doi: 10.1038/oby.2009.52. [DOI] [PubMed] [Google Scholar]
- 3.APA. Diagnostic and statistical manual of mental disorders, 4th edition, Text Revision. Washington, DC: 2004. [Google Scholar]
- 4.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grilo CM, Masheb RM, White MA. Significance of overvaluation of shape/weight in binge-eating disorder: comparative study with overweight and bulimia nervosa. Obesity (Silver Spring) 2010;18:499–504. doi: 10.1038/oby.2009.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchesne M, Mattos P, Appolinario JC, et al. Assessment of executive functions in obese individuals with binge eating disorder. Rev Bras Psiquiatr. 2010;32:381–388. doi: 10.1590/s1516-44462010000400011. [DOI] [PubMed] [Google Scholar]
- 7.Yanovski SZ, Nelson JE, Dubbert BK, Spitzer RL. Association of binge eating disorder and psychiatric comorbidity in obese subjects. Am J Psychiatry. 1993;150:1472–1479. doi: 10.1176/ajp.150.10.1472. [DOI] [PubMed] [Google Scholar]
- 8.Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104:87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathes WF, Brownley KA, Mo X, Bulik CM. The biology of binge eating. Appetite. 2009;52:545–553. doi: 10.1016/j.appet.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westenhoefer J, Pudel V, Maus N. Some restrictions on dietary restraint. Appetite. 1990;14:137–141. doi: 10.1016/0195-6663(90)90014-y. discussion 42-3. [DOI] [PubMed] [Google Scholar]
- 11.Blomquist KK, Grilo CM. Predictive significance of changes in dietary restraint in obese patients with binge eating disorder during treatment. Int J Eat Disord. 2011;44:515–523. doi: 10.1002/eat.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stice E, Fisher M, Lowe MR. Are dietary restraint scales valid measures of acute dietary restriction? Unobtrusive observational data suggest not. Psychol Assess. 2004;16:51–59. doi: 10.1037/1040-3590.16.1.51. [DOI] [PubMed] [Google Scholar]
- 13.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 16.Avena NM, Bocarsly ME, Hoebel BG, Gold MS. Overlaps in the Nosology of Substance Abuse and Overeating: the Translational Implications of "Food Addiction". Curr Drug Abuse Rev. 2011 doi: 10.2174/1874473711104030133. [DOI] [PubMed] [Google Scholar]
- 17.Garber AK, Lustig RH. Is fast food addictive? Curr Drug Abuse Rev. 2011;4:146–162. doi: 10.2174/1874473711104030146. [DOI] [PubMed] [Google Scholar]
- 18.Potenza MN, Leung HC, Blumberg HP, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- 19.Maayan L, Hoogendoorn C, Sweat V, Convit A. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity (Silver Spring) 2011;19:1382–1387. doi: 10.1038/oby.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemps E, Wilsdon A. Preliminary evidence for a role for impulsivity in cognitive disinhibition in bulimia nervosa. J Clin Exp Neuropsychol. 2010;32:515–521. doi: 10.1080/13803390903264122. [DOI] [PubMed] [Google Scholar]
- 21.Marsh R, Horga G, Wang Z, et al. An fMRI Study of Self-Regulatory Control and Conflict Resolution in Adolescents With Bulimia Nervosa. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.11010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh R, Steinglass JE, Gerber AJ, et al. Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Arch Gen Psychiatry. 2009;66:51–63. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.First MB. The DSM series and experience with DSM-IV. Psychopathology. 2002;35:67–71. doi: 10.1159/000065121. [DOI] [PubMed] [Google Scholar]
- 24.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16:363–370. [PubMed] [Google Scholar]
- 25.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 26.Dillon DG, Pizzagalli DA. Inhibition of Action, Thought, and Emotion: A Selective Neurobiological Review. Appl Prev Psychol. 2007;12:99–114. doi: 10.1016/j.appsy.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uher R, Treasure J. Brain lesions and eating disorders. J Neurol Neurosurg Psychiatry. 2005;76:852–857. doi: 10.1136/jnnp.2004.048819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woolley JD, Gorno-Tempini ML, Seeley WW, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology. 2007;69:1424–1433. doi: 10.1212/01.wnl.0000277461.06713.23. [DOI] [PubMed] [Google Scholar]
- 29.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 30.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2010;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolls ET, Yaxley S, Sienkiewicz ZJ. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- 33.Grabenhorst F, Rolls ET. Selective attention to affective value alters how the brain processes taste stimuli. Eur J Neurosci. 2008;27:723–729. doi: 10.1111/j.1460-9568.2008.06033.x. [DOI] [PubMed] [Google Scholar]
- 34.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 35.Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masheb RM, Grilo CM. On the relation of flexible and rigid control of eating to body mass index and overeating in patients with binge eating disorder. Int J Eat Disord. 2002;31:82–91. doi: 10.1002/eat.10001. [DOI] [PubMed] [Google Scholar]
- 37.Roehrig M, Masheb RM, White MA, Grilo CM. Dieting frequency in obese patients with binge eating disorder: behavioral and metabolic correlates. Obesity (Silver Spring) 2009;17:689–697. doi: 10.1038/oby.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- 39.Franko DL, Thompson-Brenner H, Thompson DR, et al. Racial/ethnic differences in adults in randomized clinical trials of binge eating disorder. J Consult Clin Psychol. 2011 doi: 10.1037/a0026700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biol Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]