Abstract
The cytokine IL-1 is critical to the pathogenesis of a variety of human conditions and diseases. Unlike most other cytokines, IL-1 is counterbalanced by two endogenous inhibitors. The functional significance of IL-1 receptor antagonist (IL-1RA) is well documented due to the clinical utilization of the recombinant human IL-1RA analog, anakinra. In contrast, much less is known about the type 2 IL-1 receptor (IL-1R2), which acts as a decoy receptor for IL-1. While IL-1R2 is structurally similar to the type 1 IL-1 receptor (IL-1R1) responsible for IL-1 signal transduction, its truncated cytoplasmic domain and lack of Toll-IL-1 receptor (TIR) region renders IL-1R2 incapable of transmembrane signaling. IL-1R2 competes with IL-1R1 for ligands and for the IL-1R1 co-receptor, IL-1 receptor accessory protein (IL-1RAP). Additionally, IL-1R2 exists in both a membrane bound and soluble form (sIL-1R2) that has biological properties similar to both a decoy receptor and a binding protein. Thus far, IL-1R2 has been implicated in arthritis, endometriosis, organ transplantation, sepsis/sickness behavior, diabetes, atherosclerosis, autoimmune inner ear disease (AIED), Alzheimer’s disease and ulcerative colitis. In this review, we will detail the functional properties of IL-1R2 and examine its role in human disease.
Keywords: IL-1R2, IL1R2, IL-1 receptor type II, IL-1 decoy receptor, CD121b, IL-1RB, type II IL-1 receptor
1. Introduction
The IL-1 system includes an interesting array of at least 21 distinct molecules encompassing receptors, co-receptors, ligands, and endogenous antagonists (Dinarello, 2009). IL-1α and IL-1β (collectively referred to as IL-1) serve as soluble and principally extracellular activators of the IL-1 system, whereas IL-1 receptor antagonist (IL-1RA) is a competitive inhibitor that prevents IL-1α and IL-1β from interacting with the IL-1 receptor 1 (IL-1R1). IL-1R1, in turn, associates with IL-1 receptor accessory protein (IL-1RAP) to create a transmembrane signaling complex that initiates IL-1-dependent intracellular signaling (Korherr et al., 1997). Somewhat unique to the IL-1 system is the existence of two distinct types of IL-1Rs, namely IL-1R1 and the type 2 IL-1 receptor (IL-1R2). IL-1R1, as noted above, is responsible for IL-1 signal transduction. IL-1R2 serves as an endogenous inhibitor of IL-1 signaling (Fig. 1). IL-1R2 was first characterized by McMahon et al. in 1991 (McMahan et al., 1991) and is considered the prototypical decoy receptor. More recently, analogous decoy receptors have been identified for IL-18 and TNF (Mantovani et al., 2001). The purpose of these non-signaling receptors is still somewhat unclear, but functionally, they serve as important negative regulators.
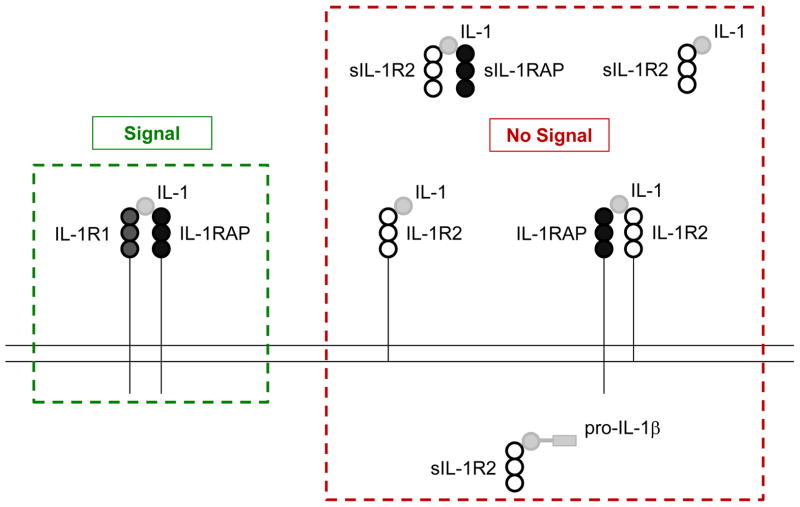
Figure 1. How IL-1R2 can block IL-1 signaling.
Functional IL-1 signaling requires IL-1R1 and IL-1-dependent recruitment of IL-1RAP. Membrane bound IL-1R2 serves as a negative regulator of IL-1 by competing with IL-1R1 for IL-1 and by complexing with IL-1RAP which prevents IL-1RAP from heterodimerizing with IL-1R1. IL-1 can also interact with sIL-1R2 and the sIL-1R2 + sIL-1RAP complex. The sIL-1R2 + sIL-1RAP complex has a greater affinity for IL-1 than sIL-lR2 or IL-1R2, alone. Finally, the processing of pro-IL-1β to mature IL-1β by caspase-1 can be prevented by the binding of pro-IL-1β to intracellular sIL-1R2. Abbreviations: IL-1 receptor type 1 (IL-1R1), IL-1 receptor type 2 (IL-1R2), soluble IL-1R2 (sIL-1R2), IL-1 receptor accessory protein (IL-1RAP).
The IL-1 pathway regulates inflammation, angiogenesis, hematopoiesis and cognition (Rachal Pugh et al., 2001, Shieh et al., 1991). As the first interleukin to be characterized, IL-1 was initially termed endogenous pyrogen for its ability to produce fever in animals and humans. Subsequent work demonstrated the importance of IL-1 to a variety of sickness behaviors, including anorexia, lethargy, locomotion and social exploration (Dantzer, 2001). Confirming the mechanistic importance of IL-1 to sickness were experiments demonstrating that IL-1 antagonism mitigates LPS-dependent reductions in social exploration and food-motivated behavior in mice (Laye et al., 2000, Bluthe et al., 1992, Kent et al., 1992). Overall, much of what is known about IL-1 bioaction is derived from work using IL-1 and IL-1R1 knock out (KO) mice or administered IL-1RA. Given the near absence of IL-1R2 mouse models and IL-1R2 recombinant/synthetic molecules/analogues, the functional role of IL-1R2 is often extrapolated from work with IL-1RA. IL-1R2, however, is quite unique and is likely much more than a redundancy within the system of endogenous IL-1 antagonists.
2. IL-1R2 gene
In humans, the IL-1R2 gene (IL1R2) is located on the long arm of chromosome 2 at band 2q12. In mice, IL1R2 is found in the centromere proximal position of chromosome 1 (Copeland et al., 1991). In both humans and mice, the genes for IL-1R2 and IL-1R1 are adjacent (Dale and Nicklin, 1999, Sims et al., 1995) with the IL-1R2 and IL-1R1 demonstrating similar transmembrane regions but only a 28% homology in their extracellular domains. The IL-1R2 cDNA and amino acid sequences are similar across species. Examination of bovine IL-1R2 cDNA yielded a sequence homology of 79%, 69% and 69% when compared to human, mouse and rat, respectively (Yu et al., 1997). The amino acid sequence of bovine IL-1R2 is 71% identical with human, 58% identical with mouse and 59% identical with rat.
3. IL-1R2 message
IL-1R2 mRNA, in vivo, is up-regulated following middle cerebral artery occlusion, (Wang et al., 2000), acute hypoxia (Johnson et al., 2007) and LPS administration (Herman et al., 2010, Gabellec et al., 1996). In vitro, AtT-20 cells treated with IL-1β or TNF-α can increase the number of IL-1R2 gene transcripts within 3 hrs (Bristulf and Bartfai, 1995). Depending on the stimulus, time of peak expression of IL-1R2 mRNA varies from 2 h following acute hypoxia (Johnson et al., 2007) or LPS administration (Herman et al., 2010) to 12 h post cerebral artery occlusion (Wang et al., 2000). The half-life of IL-1R2 transcripts is 110 min in primary murine dendritic cells treated with LPS (Zeisel et al., 2011). Given the rapidity of IL-1R2 mRNA up-regulation and its relatively short half-life, IL-1R2 appears to be an early response gene. Support for this contention is in the identification of an NF-κB binding site within the IL-1R2 promoter region (Yan et al., 2008).
4. IL-1R2 protein
IL-1R2, in humans and non-human primates, is a protein comprised of 398 amino acids. In mice and rats, it is slightly longer at 410 and 416 amino acids, respectively. As a decoy receptor, IL-1R2 cannot signal. This is due to its lack of an intracellular TIR domain, a conserved region shared by IL-1R1 and the Toll-like receptors (TLRs) as part of the IL-1/TLR superfamily (Dunne and O’Neill, 2003, Xu et al., 2000). Interestingly, Heguy et al. constructed a functional receptor by combining the extracellular and transmembrane domains of IL-1R2 with the intra-cytoplasmic domain of IL-1R1 (Heguy et al., 1993). With three immunoglobulin-like extracellular domains and a single helical transmembrane domain, IL-1R2 is structurally similar to IL-1R1. IL-1R2, however, lacks approximately 200 cytoplasmic amino acids critical to the TIR (Slack et al., 2000). Thus, with only a 29 amino acid cytoplasmic region, IL-1R2 is a 68 kDa glycoprotein in comparison to IL-1R1 which is 80 kDa (Sims et al., 1988).
The affinity of IL-1R2 for IL-1β is 10−10 M, while its affinity for IL-1α is 100 times less at 10−8 M. IL-1R1 binds IL-1α at 10−10 M, but binds IL-1β at 10−9 M (McMahan et al., 1991). In addition, the affinity of IL-1R1 for IL-1RA is similar to its affinity for IL-1α and IL-1β (Symons et al., 1995). In contrast, IL-1R2 binds IL-1RA approximately 100 times less effectively than IL-1R1 (McMahan et al., 1991). These differences in affinity suggest that IL-1R2 and IL-1RA may have different biologic roles.
The IL-1R2 receptor exists in both membrane bound and soluble forms (sIL-1R2). Generation of sIL-1R2 appears to occur via two mechanisms. First, alternative splicing has been shown to generate sIL-1R2 in patients with autoimmune inner ear disease (AIED) (Vambutas et al., 2009). How alternatively spliced sIL-1R2 is secreted is currently not known. Second, matrix metalloproteinases can cleave full-length membrane bound IL-1R2, shedding the extracellular domain as a 45–47 kDa sIL-1R2 (Orlando et al., 1997). This IL-1R2 ectodomain liberation appears to require aminopeptidase regulator of TNFR1 shedding (ARTS-1) (Cui et al., 2003). Interestingly, IL-1R2 shedding can occur slowly (hrs) in response to IL-4, IL-13 and glucocorticoids (Colotta et al., 1996, Colotta et al., 1994) and rapidly (mins) in response to N-formyl-methionine-leucine-phenylalanine, LPS, TNF, reactive oxygen species and phorbol esters (Penton-Rol et al., 1999, Orlando et al., 1997, Sambo et al., 1996, Colotta et al., 1995).
Like IL-1R2, sIL-1R2 binds circulating IL-1. The IL-1β/sIL-1R2 dissociation rate is very low, and from a physiological perspective, the IL-1β/sIL-1R2 interaction has been deemed essentially irreversible (Arend et al., 1994). This makes it unlikely that sIL-1R2 acts as an IL-1 carrier or a protein that protects IL-1 from degradation. Interestingly, sIL-1R2 can sequester pro-IL-1β, interfering with the ability of caspase-1 to enzymatically generate mature IL-1β from its pro-form (Symons et al., 1995).
5. IL-1R2 expression
IL-1R2 is natively found on neutrophils, B-cells, monocytes and macrophages (Colotta et al., 1996, McMahan et al., 1991). It can also be induced in keratinocytes and endothelial cells (Lukiw et al., 1999, Groves et al., 1995, McMahan et al., 1991). Monocytes at rest possess 1.3 × 103 receptors/cell and after 24 hrs of IL-13 treatment express 3.5 × 103 receptors/cell. In comparison, 12.0 × 103 sIL-1Rs/cell were elaborated into the media in the same time period (Colotta et al., 1996). IL-1R1 is expressed by almost all cell types, at least at low levels (Dower et al., 1985). This suggests that IL-1R2 protects specific cell types from IL-1. sIL-1R2, on the other hand, is ubiquitously present in the plasma of healthy individuals (Giri et al., 1994) at a relatively high concentration when compared to serum IL-1β and IL-1RA. In healthy women, serum concentrations of sIL-1R2 are nearly two-fold higher than IL-1RA (570.5 ± 79.1 pg/mL vs 11,328.9 ± 384.9 pg/mL) (Chun et al., 2012) while serum IL-1β is essentially undetectable in healthy human subjects (0.3 ± 0.5 pg/mL) (Hasdai et al., 1996). It remains unclear why the basal expression of IL-1R2 exceeds that of basal IL-1. It is also unknown if any or all of the IL-1R2s are occupied by IL-1 in the healthy state.
6. IL-1R2 function
Monoclonal antibody blocking studies show that when IL-1 is prevented from interacting with IL-1R2, IL-1 bioaction in neutrophils, lymphocytes and monocytes is not inhibited (Sims et al., 1993, Colotta et al., 1993). Blocking the IL-1/IL-1R1 interaction in neutrophils and monocytes does prevent IL-1-induced production of IL-6, IL-8 and TNF-α (Sims et al., 1993). As recently reviewed by Weber et al, the initial step in IL-1 signaling is IL-1 binding to IL-1R1 with subsequent IL-1R1 heterodimerization with IL-1RAP (Greenfeder et al., 1995). The IL-1R1/IL-1RAP complex then scaffolds a functional signaling complex comprised of myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinases (IRAK) and TNF-receptor associated factor 6 (TRAF-6) resulting in the activation of the NF-κB and mitogen-activated protein kinases (MAPK) (Weber et al., 2010). IL-1R2 serves as a negative regulator of IL-1 signaling by competing with IL-1R1 for IL-1 and by complexing with IL-1RAP once it binds IL-1, thereby sequestering both the ligand and the accessory protein required for signal transduction (Malinowsky et al., 1998, Lang et al., 1998). Additionally, sIL-1R2 can bind IL-1 and bind soluble IL-1RAP (sIL-1RAP) (Smith et al., 2003), which is a product of IL-1RAP alternative splicing (Jensen et al., 2000). The interaction of sIL-1R2 with sIL-1RAP increases the affinity of sIL-1R2 for IL-1 by over 100-fold without impacting affinity for IL-1RA. Current evidence, however, does not support an interaction between IL-1, sIL-1R2 and full-length IL-1RAP (Smith et al., 2003).
7. IL-1R2 in disease
IL-1-mediated inflammation contributes to the pathology of many diseases including rheumatoid arthritis, adult-onset Still’s disease, type 2 diabetes, gout, systolic heart failure and pustular psoriasis (Dinarello et al., 2012). Therefore, inhibition of IL-1 signaling is considered a major therapeutic target. Experimentally, transfection or overexpression of IL-1R2 has been used to create anti-inflammatory profiles in animal models of collagen induced arthritis (Bessis et al., 2000), IL-1-induced inflammation (Rauschmayr et al., 1997) and cardiac allograft surgery (Simeoni et al., 2007). In vitro, the expression IL-1R2 has been suppressed by pro-inflammatory agents like LPS (Penton-Rol et al., 1999) and interferon-γ (INF-γ) (Chang et al., 2009). Increased IL-1R2 expression has been induced by immunosuppressive and anti-inflammatory agents such as dexamethasone (Re et al., 1994), prostaglandins (Spriggs et al., 1992), glucocorticoids, IL-4 (Colotta et al., 1993), IL-13 (Colotta et al., 1994), IL-27 (Kalliolias et al., 2010) and aspirin (Daun et al., 1999). In transgenic mice over-expressing IL-1R2 in the epidermis, phorbol ester-induced epidermal and dermal inflammation is blunted. Engineered to over-express IL-1R2 on keratinocytes, these mice are protected from IL-1-induced acute cutaneous vascular leakage. Interestingly, the anti-IL-1 effect observed was predominantly local, and induced IL-1R2 over-expression did not inhibit immune responses when mice were challenged systemically with IL-1 (Rauschmayr et al., 1997).
Given that glucocorticoids increase the expression of IL-1R2 and sIL-1R2, the hypothalamic-pituitary-adrenal (HPA) axis may be critical to their up-regulation. In mice, LPS administration is associated with increased circulating levels of corticosterone and adrenocorticotropic hormone (ACTH). Similarly, intravenous injection of IL-1β induces hypothalamic production of corticotropin releasing factor (CRF) and ACTH (Rivier, 1994). In contrast, administration of anti-IL-1 antibodies decreases endotoxin-mediated ACTH production (Rivier et al, 1989). Recently, Ohmori et al. found that psychologically stressed PhD students had increased gene expression of IL-1R2 in blood cells (Ohmori et al, 2005), indicating a link between the HPA axis and IL-1R2. Although the direct effect of the HPA axis on IL-1R2 and vice versa is not known, it is reasonable to hypothesize that IL-1R2 expression is triggered by the HPA axis resulting in a diminution of IL-1 signaling in the brain.
Finally, evidence for the importance of IL-1 in human disease pathogenesis is provided by the use of recombinant IL-1RA (anakinra) in the treatment of rheumatoid arthritis (Dinarello, 2011, Dinarello, 2009). Table 1 highlights the role of IL-1R2 in specific diseases and conditions where its function is best elucidated.
Table 1.
IL-1R2 as a therapy
| Disease/Condition | Rational | Method | Result | Reference |
|---|---|---|---|---|
| Arthritis | IL-1-induced inflammation implicated in pathology of RA, increased sIL-1R2 is correlated with less severe RA | Gene therapy using hsIL-1R2 transfected cells injected s.c. in the back of CIA mice |
|
Bessis et al., 2000 |
| sIL-1R2 injected i.v. into rabbit RA model |
|
Dawson et al., 1999 | ||
|
| ||||
| Endometriosis | Reduce chronic inflammation induced by up- regulated IL-1 and loss of sIL-1R2 | IL-1R2 cDNA transfected into endometriotic cells with IL-1β stimulation in culture |
|
Akoum et al., 2007 Khoufache et al., 2012 |
| Nude mice implanted with endometrial tissue and administered i.p. hsIL1-R2 |
|
|||
|
| ||||
| Organ Transplantation | Prevent increased IL-1-mediated myocardial reperfusion injury | Gene therapy using IL-1R2-Ig fusion protein to mitigate allograft rejection in rat heart transplantation model |
|
Simeoni et al., 2007 |
|
| ||||
| Sickness | IL-1RA i.c.v. abrogates IL-1β-induced sickness behaviors | Block brain IL-1R2 with i.c.v.anti-IL-1R2 antibody (2 ng/mouse) prior to IL-1β administration in mice |
|
Cremona et al., 1998 |
Rheumatoid Arthritis (RA), Human soluble IL-1R2 (hsIL-1R2), subcutaneous (s.c.), collagen-induced arthritis (CIA), intravenous (i.v.), intracerebroventricular (i.c.v.), myeloperoxidase (MPO), prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), Monocyte chemotactic protein-1 (MCP1), matrix metalloproteinase-2 (MMP2), matrix metalloproteinase-9 (MMP9), tissue inhibitor of metalloproteinases 1 (TIMP1), tissue inhibitor of metalloproteinases 2 (TIMP2), tumor necrosisfactor-α (TNF-α), transforming growth factor-β (TGF-β)
The first dedicated comprehensive review of IL-1R2 in the biomedical literature.
7.1 Arthritis
IL-1 is implicated in the pathogenesis of rheumatoid arthritis (RA). Interestingly, increased concentrations of sIL-1R2 are found in the synovial fluid (Arend et al., 1994) and plasma of individuals with RA (Jouvenne et al., 1998). Levels of sIL-1R2 negatively correlate with severity of disease, implicating sIL-1R2 as a natural antagonist of IL-1-driven joint destruction. In contrast, plasma levels of IL-1RA correlate positively with disease progression, suggesting that IL-1RA is a marker of acute inflammation tied to episodic disease exacerbation (Jouvenne et al., 1998). Due to the ability of IL-1R2 to block IL-1 driven joint destruction, IL-1R2 may be a better therapeutic for RA than IL-1RA. Advantages of IL-1R2 over IL-1RA as a therapeutic is its longer half-life (reduced treatment intervals) and its ability to sequester IL-1RAP and sIL-1RAP. What is not yet clear is the volume of distribution of IL-1R2 in comparison to IL-1RA. However, given its size and avidity for IL-1RAP, it may have use as a joint space injectable, which would reduce complications associated with systemic anti-IL-1 therapy.
Support for IL-1R2 as an anti-arthritic is seen in animal models. In the mouse collage-induced arthritis (CIA) model, recombinant IL-1R2 delivered via implanted human keratinocytes engineered to overexpress human sIL-1R2 (hsIL-1R2) reduced joint concentrations of IL-6 and myeloperoxidase, and also mitigated the histologic and clinical presentation of arthritis (Bessis et al., 2000). In a rabbit model of RA, intravenous administration of sIL-1R2 substantially reduced joint swelling and erosion (Dawson et al., 1999).
7.2 Endometriosis
Endometriosis is a pathologic process in which endometrial epithelium manifests outside the internal uterine environment (Gazvani and Templeton, 2002). Peritoneal fluid from women with endometriosis demonstrates elevated levels of IL-1, and it is thought that IL-1-mediated inflammation is a key component in the manifestation and/or progression of endometriosis (Kondera-Anasz et al., 2005, Taketani et al., 1992). Early stage endometriosis is associated with lower concentrations of serum sIL-1R2 (Kharfi and Akoum, 2002) with values averaging 10,008.3 ± 273.5 pg/mL versus 11,328.9 ± 384.9 pg/mL for healthy fertile women (Chun et al., 2012). In addition, extra-uterine endometrial tissue has reduced IL-1R2 (Akoum et al., 2001) and sIL-1R2 protein expression when compared to its intra-uterine counterpart (Akoum et al., 2007). Interestingly, menstruation is a robust localized inflammatory process characterized by tissue necrosis and acute inflammation. While evidence of behavioral change related to neuroimmune activation is suggested by some menstruation-associated sickness-like symptoms, frank sickness behavior is remarkably absent given the pronounced neutrophilic infiltrates. Therefore, containment of the inflammatory response and communication of its presence to the brain must be highly regulated. Whether or not IL-1R2 is essential to this process is unknown. However, given the significant symptomology of endometriosis (especially pain) in comparison to menstruation, it is likely that locally generated IL-1R2 is important to preventing IL-1-associated sickness symptoms from manifesting.
In endometriosis, IL-1β appears to drive vascular endothelial growth factor (VEGF) and monocyte chemotactic protein 1 (MCP1) secretion. Transfection of cultured endometriotic cells to enhance IL-1R2 generation reduces VEGF and MCP1 production by these cells (Akoum et al., 2007). Further evidence that IL-1R2 can dampen the progression of endometriosis is seen in nude mice implanted with human endometrial tissue. In these studies, hsIL1-R2 administered intraperitonially curtailed number, volume and dissemination of endometrial implants, as well as suppressed expression of various adhesion, angiogenesis, tissue remodeling and cell survival factors (Khoufache et al., 2012). It is not known whether pain and/or sickness behaviors associated with endometriosis are impacted by exogenously administered IL-1R2.
7.3 Organ transplantation
Because IL-1 is a central mediator of myocardial reperfusion injury and a key contributor to immune responses leading to acute graft rejection, neutralizing IL-1 via endogenous IL-1 antagonists may be prudent. Gene therapy to specifically up-regulate IL-1R2 reduced allograft rejection and prolonged graft survival in a rat model of heart transplantation (Simeoni et al., 2007). In these studies, donor heart was transfected with an adenoviral driven vector, AdIL-1R2-Ig, constructed to increase expression of sIL-1R2 immediately prior to transplantation. Transfected hearts had a reduction in infiltrating macrophages and CD4+ T cells as well as fewer TNF-α and TGF-β gene transcripts (Simeoni et al., 2007). As seen in RA and endometriosis, localized expression and/or delivery of IL-1R2 may be especially beneficial.
7.4 Sepsis/sickness
Individuals critically ill with sepsis or operative trauma have significant elevations in IL-1RA and sIL-1R2. Increased sIL-1R2 in such cases is mainly associated with more dire inflammation (Pruitt et al., 1995). For example, acute meningococcal infections raise plasma IL-1RA and sIL-1R2, but the highest sIL-1R2 concentrations are found in those with endotoxemia, complement-activation and shock. In contrast to the serum concentration of IL-1RA, which rapidly wanes during recovery, sIL-1R2 continues to increase (van Deuren et al., 1997). A confounding factor is that administration of dexamethasone to patients with bacterial meningitis (so as to reduce neuroinflammation and cerebral edema) (Hoffman and Weber, 2009), may also induce production of IL-1R2 (Vambutas et al., 2009). Direct relevance of IL-1R2 to sickness has been demonstrated in animals, but to a very limited extent. In IL-1 treated mice, blockade of brain IL-1R2 with a neutralizing antibody increases IL-1-induced sickness behavior (Cremona et al., 1998). In these studies, mice administered i.c.v IL-1β+ i.c.v anti-IL-1R2 antibody ate significantly less than mice receiving i.c.v. IL-1β+ IgG (Cremona et al., 1998). Unfortunately, this is a rare example of how IL-1R2 impacts the brain and behavior.
7.5 Diabetes
Type 2 diabetes (T2D) is a disease driven by inflammation (Donath and Shoelson, 2011). In a leptin resistant mouse model of T2D (db/db mice) (Chen et al., 1996), db/db mice have impaired up-regulation of IL-1RA and IL-1R2 in response to both LPS and IL-1β. These mice also display prolonged LPS- and IL-1β-induced sickness behaviors (O’Connor et al., 2005). In response to acute hypoxia, db/db mice are delayed in recovery, and this delay is coupled to a failure of db/db mice to up-regulate the endogenous inhibitors of IL-1β (Johnson et al., 2007). For IL-1RA, this is likely due to the importance of leptin in driving IL-1RA gene expression (Maedler et al., 2004). Why IL-1R2 gene expression is impacted in the db/db mouse remains unclear, but it could be due to the role of leptin in fostering M2 macrophage activation (Kredel et al., 2012), due to M2 macrophages being a key source of IL-1R2 (Mantovani et al., 2009). As would be predicted, high-fat diet (HFD) fed mice have dysregulated expression of IL-1R2. In this model of diabesity, IL-1R2 gene transcripts are reduced in the brain. Interestingly, fasting increased IL-1R2 gene transcripts in the hypothalamus of lean mice, but not in the hypothalamus of HFD-fed mice (Lavin et al., 2011). These findings support leptin as a regulator of IL-1R2 gene transcripts and suggest that diabesity-associated leptin resistance may impair its expression. Due to its presence in the hypothalamus, IL-1R2 may also be important to appetite regulation.
7.6 Artherosclerosis
IL-1 induced inflammation appears to contribute to the atherosclerotic process by increasing leukocyte adhesion and transmigration and enhancing foam cell and fatty streak formation (von der Thusen et al., 2003, Elhage et al., 1998). Interestingly, macrophages from hyperlipidemic patients have decreased IL-1R2 mRNA and protein expression, as does a macrophage cell line treated in vitro with lipoproteins. Taken together, these findings indicate that IL-1-dependent inflammation is relatively unchecked during atheroma formation (Pou et al., 2011). Currently, no research has examined if exogenous IL-1R2 can mitigate atherosclerosis, but Tedui et al. has shown that administration of IL-1RA to ApoE KO mice decreases lesion size (Tedui et al., 2010).
7.7 AIED
AIED is hypothesized to be a systemic autoimmune disease where unique sequestered cochlear antigens cause abnormal responses in peripheral blood mononuclear cells (PCMCs) (Vambutas et al., 2009). In terms of IL-1R2, autologous perilymph from individuals with AIED does not evoke expression of membrane associated IL-1R2 when incubated with the same individuals peripheral blood mononuclear cells (PBMCs). sIL-1R2 up-regulation, however, is seen. Glucocorticoid-responsive IL-1R2 is variably impacted by AIED. Therefore, IL-1R2 protein expression in PBMCs from patients with AIED may be able to predict whether an individual will be responsive to steroid therapy (Vambutas et al., 2009).
7.8 Alzheimer’s disease
Elevated levels of sIL-1R2 are found in the cerebrospinal fluid of individuals with Alzheimer’s disease (Garlind et al., 1999). Since Alzheimer’s disease is characterized by chronic glial inflammation (Tuppo and Arias, 2005), increased sIL-1R2 may be a marker of disease progression. Whether dementing illness-associated increases in expression of endogenous antagonists to IL-1 represent compensatory anti-inflammation or have a causative role in memory loss, is not currently known. Given the sensitivity of memory to changes in IL-1/IL-1RA balance, it would not be surprising if increased CNS IL-1R2 in Alzheimer’s disease was not only a response to neurodegeneration, but also a negative affecter of memory.
7.9 Ulcerative Colitis
A genome-wide candidate gene study suggested a causative role for IL-1R2 in the pathogenesis of ulcerative colitis (Anderson et al., 2011). How this supports the hypothesis that ulcerative colitis is caused by a dysregulated mucosal immune response against commensal gut flora (Xavier and Podolsky, 2007) needs significant further study.
10. Conclusions
Since its discovery in 1991 (McMahan et al., 1991), IL-1R2 has been characterized as a decoy receptor responsible for capturing IL-1 and reducing IL-1 bioavailability. Given that it can disrupt IL-1R1/IL-1RAP heterodimerization, and that its soluble form can function like a binding protein, the biology of IL-1R2 is varied and complex. Since sIL-1R2 can interact with sIL-1RAP, it is also possible that sIL-1R2 could foster improved IL-1 bioaction because sIL-1RAP may interfere with the ability of IL-1R1 to heterodimerize with membrane-associated IL-1RAP (Smith et al., 2003). In essence, sIL-1RAP may be a functional homologue of sIL-1R2. Additional work is needed to determine if such competing properties exist and are relevant to the IL-1 system.
Although anakinra is a valuable therapeutic tool, it has a short in vivo half-life which necessitates daily injection (Kaiser et al., 2012). Furthermore, anakinra has an affinity for IL-1R1 similar to that of IL-1. Thus, a 100–1,000-fold excess of anakinra relative to IL-1 is recommended for efficient blockade of IL-1 signaling (Gabay et al., 2010). IL-1R2 is an attractive candidate as a therapeutic because of its higher affinity for IL-1β and lower affinity for IL-1RA when compared to IL-1R1. Recently, the soluble IL-1 receptor rilonacept was introduced and is FDA approved for cryopyrin-associated periodic syndromes that include familial cold autoinflammatory syndrome and Muckle-Wells Syndrome (Regeneron Pharmaceuticals, Inc, 2008). With similar decoy properties as sIL-1R2, it is a fusion protein containing the extracellular domains of IL-1R1 and IL-1RAP coupled to the Fc region of human IgG1. This IL-1R1 analogue can bind IL-1β and IL-1α with high affinity and has a higher affinity for IL-1β than IL-1RA (Dinarello et al., 2012, Stahl et al., 2009, Economides et al., 2003). Adding to its therapeutic appeal is it’s once per wk dosing schedule (Regeneron Pharmaceuticals, Inc, 2008).
Finally, KO animal models have contributed significantly to the understanding of numerous inflammatory bioactives. An IL-1R2 KO mouse has been developed (Taconic, Germantown, NY), but almost no information is available on the phenotype of these mice. Given the potential for significant regional differences in IL-1R2 expression within the whole body and within individual organs, tissue-specific IL-1R2 KO mice are especially important to furthering the understanding of IL-1R2 bioaction.
Acknowledgments
Support: This research was supported by the National Institutes of Health (DK064862, NS058525 and AA019357 to GGF).
Abbreviations
- IL-1R
IL-1 receptor
- IL-1R1
IL-1 receptor type 1
- IL-1R2
IL-1 receptor type 2
- IL-1RA
IL-1 receptor antagonist
- AA
amino acid
- TIR
Toll-IL-I receptor
- WT
wild type
- PMN
polymorphonuclear leukocyte
- mIL-1R2
membrane bound IL-1R2
- sIL-1R2
soluble IL-1R2
- IL-1RAP
IL-1 receptor accessory protein
- PMA
phorbol 12-myristate 13-acetate
- RA
rheumatoid arthritis
- CIA
collagen induced arthritis
- LPS
lipopolysaccharide
- KO
knock out
- FCH
familial combined hyperlipidemia
- HFD
high Fat Diet
- LFD
low Fat Diet
- AIED
autoimmune inner ear disease
- PBMC
peripheral blood mononuclear cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akoum A, Jolicoeur C, Kharfi A, Aube M. Decreased expression of the decoy interleukin-1 receptor type II in human endometriosis. Am J Pathol. 2001;158:481–489. doi: 10.1016/S0002-9440(10)63990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoum A, Lawson C, Herrmann-Lavoie C, Maheux R. Imbalance in the expression of the activating type I and the inhibitory type II interleukin 1 receptors in endometriosis. Hum Reprod. 2007;22:1464–1473. doi: 10.1093/humrep/dem021. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagace C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panes J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcalyst [package insert] Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2008. [Google Scholar]
- Arend WP, Malyak M, Smith MF, Jr, Whisenand TD, Slack JL, Sims JE, Giri JG, Dower SK. Binding of IL-1 alpha, IL-1 beta, and IL-1 receptor antagonist by soluble IL-1 receptors and levels of soluble IL-1 receptors in synovial fluids. J Immunol. 1994;153:4766–4774. [PubMed] [Google Scholar]
- Bessis N, Guery L, Mantovani A, Vecchi A, Sims JE, Fradelizi D, Boissier MC. The type II decoy receptor of IL-1 inhibits murine collagen-induced arthritis. Eur J Immunol. 2000;30:867–875. doi: 10.1002/1521-4141(200003)30:3<867::AID-IMMU867>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Dantzer R, Kelley KW. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- Bristulf J, Bartfai T. Interleukin-1 beta and tumour necrosis factor-alpha stimulate the mRNA expression of interleukin-1 receptors in mouse anterior pituitary AtT-20 cells. Neurosci Lett. 1995;187:53–56. doi: 10.1016/0304-3940(95)11336-u. [DOI] [PubMed] [Google Scholar]
- Chang SY, Su PF, Lee TC. Ectopic expression of interleukin-1 receptor type II enhances cell migration through activation of the pre-interleukin 1alpha pathway. Cytokine. 2009;45:32–38. doi: 10.1016/j.cyto.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chun S, Kim H, Ku SY, Suh CS, Kim SH, Kim JG. The Association Between Endometriosis and Polymorphisms in the Interleukin-1 Family Genes in Korean Women. Am J Reprod Immunol. 2012;68:154–163. doi: 10.1111/j.1600-0897.2012.01136.x. [DOI] [PubMed] [Google Scholar]
- Colotta F, Orlando S, Fadlon EJ, Sozzani S, Matteucci C, Mantovani A. Chemoattractants induce rapid release of the interleukin 1 type II decoy receptor in human polymorphonuclear cells. J Exp Med. 1995;181:2181–2186. doi: 10.1084/jem.181.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Polentarutti N, Minty A, Caput D, Ferrara P, Mantovani A. Interleukin-13 induces expression and release of interleukin-1 decoy receptor in human polymorphonuclear cells. J Biol Chem. 1994;269:12403–12406. [PubMed] [Google Scholar]
- Colotta F, Saccani S, Giri JG, Dower SK, Sims JE, Introna M, Mantovani A. Regulated expression and release of the IL-1 decoy receptor in human mononuclear phagocytes. J Immunol. 1996;156:2534–2541. [PubMed] [Google Scholar]
- Copeland NG, Silan CM, Kingsley DM, Jenkins NA, Cannizzaro LA, Croce CM, Huebner K, Sims JE. Chromosomal location of murine and human IL-1 receptor genes. Genomics. 1991;9:44–50. doi: 10.1016/0888-7543(91)90219-5. [DOI] [PubMed] [Google Scholar]
- Cremona S, Laye S, Dantzer R, Parnet P. Blockade of brain type II interleukin-1 receptors potentiates IL1beta-induced anorexia in mice. Neurosci Lett. 1998;246:101–104. doi: 10.1016/s0304-3940(98)00238-9. [DOI] [PubMed] [Google Scholar]
- Cui X, Rouhani FN, Hawari F, Levine SJ. Shedding of the type II IL-1 decoy receptor requires a multifunctional aminopeptidase, aminopeptidase regulator of TNF receptor type 1 shedding. J Immunol. 2003;171:6814–6819. doi: 10.4049/jimmunol.171.12.6814. [DOI] [PubMed] [Google Scholar]
- Dale M, Nicklin MJ. Interleukin-1 receptor cluster: gene organization of IL1R2, IL1R1, IL1RL2 (IL-1Rrp2), IL1RL1 (T1/ST2), and IL18R1 (IL-1Rrp) on human chromosome 2q. Genomics. 1999;57:177–179. doi: 10.1006/geno.1999.5767. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Daun JM, Ball RW, Burger HR, Cannon JG. Aspirin-induced increases in soluble IL-1 receptor type II concentrations in vitro and in vivo. J Leukoc Biol. 1999;65:863–866. doi: 10.1002/jlb.65.6.863. [DOI] [PubMed] [Google Scholar]
- Dawson J, Engelhardt P, Kastelic T, Cheneval D, MacKenzie A, Ramage P. Effects of soluble interleukin-1 type II receptor on rabbit antigen-induced arthritis: clinical, biochemical and histological assessment. Rheumatology (Oxford) 1999;38:401–406. doi: 10.1093/rheumatology/38.5.401. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Blocking interleukin-1beta in acute and chronic autoinflammatory diseases. J Intern Med. 2011;269:16–28. doi: 10.1111/j.1365-2796.2010.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Dower SK, Kronheim SR, March CJ, Conlon PJ, Hopp TP, Gillis S, Urdal DL. Detection and characterization of high affinity plasma membrane receptors for human interleukin 1. J Exp Med. 1985;162:501–515. doi: 10.1084/jem.162.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, Pyles EA, Xu X, Daly TJ, Young MR, Fandl JP, Lee F, Carver S, McNay J, Bailey K, Ramakanth S, Hutabarat R, Huang TT, Radziejewski C, Yancopoulos GD, Stahl N. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med. 2003;9:47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, Bayard F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Gabellec MM, Griffais R, Fillion G, Haour F. Interleukin-1 receptors type I and type II in the mouse brain: kinetics of mRNA expressions after peripheral administration of bacterial lipopolysaccharide. J Neuroimmunol. 1996;66:65–70. doi: 10.1016/0165-5728(96)00021-5. [DOI] [PubMed] [Google Scholar]
- Garlind A, Brauner A, Hojeberg B, Basun H, Schultzberg M. Soluble interleukin-1 receptor type II levels are elevated in cerebrospinal fluid in Alzheimer’s disease patients. Brain Res. 1999;826:112–116. doi: 10.1016/s0006-8993(99)01092-6. [DOI] [PubMed] [Google Scholar]
- Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117–126. doi: 10.1016/s0020-7292(01)00577-x. [DOI] [PubMed] [Google Scholar]
- Giri JG, Wells J, Dower SK, McCall CE, Guzman RN, Slack J, Bird TA, Shanebeck K, Grabstein KH, Sims JE. Elevated levels of shed type II IL-1 receptor in sepsis. Potential role for type II receptor in regulation of IL-1 responses. J Immunol. 1994;153:5802–5809. [PubMed] [Google Scholar]
- Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- Groves RW, Giri J, Sims J, Dower SK, Kupper TS. Inducible expression of type 2 IL-1 receptors by cultured human keratinocytes. Implications for IL-1-mediated processes in epidermis. J Immunol. 1995;154:4065–4072. [PubMed] [Google Scholar]
- Hasdai D, Scheinowitz M, Leibovitz E, Sclarovsky S, Eldar M, Barak V. Increased serum concentrations of interleukin-1 beta in patients with coronary artery disease. Heart. 1996;76:24–28. doi: 10.1136/hrt.76.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heguy A, Baldari CT, Censini S, Ghiara P, Telford JL. A chimeric type II/type I interleukin-1 receptor can mediate interleukin-1 induction of gene expression in T cells. J Biol Chem. 1993;268:10490–10494. [PubMed] [Google Scholar]
- Herman AP, Misztal T, Herman A, Tomaszewska-Zaremba D. Expression of interleukin (IL)-1β and IL-1 receptors genes in the hypothalamus of anoestrous ewes after lipopolysaccharide treatment. Reprod Domest Anim. 2010;45:e426–e433. doi: 10.1111/j.1439-0531.2010.01595.x. [DOI] [PubMed] [Google Scholar]
- Hoffman O, Weber RJ. Pathophysiology and treatment of bacterial meningiti. Ther Adv Neurol Disord. 2009;2:1–7. doi: 10.1177/1756285609337975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LE, Muzio M, Mantovani A, Whitehead AS. IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol. 2000;164:5277–5286. doi: 10.4049/jimmunol.164.10.5277. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenne P, Vannier E, Dinarello CA, Miossec P. Elevated levels of soluble interleukin-1 receptor type II and interleukin-1 receptor antagonist in patients with chronic arthritis: correlations with markers of inflammation and joint destruction. Arthritis Rheum. 1998;41:1083–1089. doi: 10.1002/1529-0131(199806)41:6<1083::AID-ART15>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Kaiser C, Knight A, Nordstrom D, Pettersson T, Fransson J, Florin-Robertsson E, Pilstrom B. Injection-site reactions upon Kineret (anakinra) administration: experiences and explanations. Rheumatol Int. 2012;32:295–299. doi: 10.1007/s00296-011-2096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF-alpha and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J Immunol. 2010;185:7047–7056. doi: 10.4049/jimmunol.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharfi A, Akoum A. Soluble interleukin-1 receptor type II blocks monocyte chemotactic protein-1 secretion by U937 cells in response to peripheral blood serum of women with endometriosis. Fertil Steril. 2002;78:836–842. doi: 10.1016/s0015-0282(02)03335-6. [DOI] [PubMed] [Google Scholar]
- Khoufache K, Kibangou Bondza P, Harir N, Daris M, Leboeuf M, Mailloux J, Lemyre M, Foster W, Akoum A. Soluble Human Interleukin 1 Receptor Type 2 Inhibits Ectopic Endometrial Tissue Implantation and Growth: Identification of a Novel Potential Target for Endometriosis Treatment. Am J Pathol. 2012 doi: 10.1016/j.ajpath.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A, Jonca M. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;123:198–203. doi: 10.1016/j.ejogrb.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Korherr C, Hofmeister R, Wesche H, Falk W. A critical role for interleukin-1 receptor accessory protein in interleukin-1 signaling. Eur J Immunol. 1997;27:262–267. doi: 10.1002/eji.1830270139. [DOI] [PubMed] [Google Scholar]
- Kredel LI, Batra A, Stroh T, Kuhl AA, Zeitz M, Erben U, Siegmund B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut. 2012 doi: 10.1136/gutjnl-2011-301424. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lang D, Knop J, Wesche H, Raffetseder U, Kurrle R, Boraschi D, Martin MU. The type II IL-1 receptor interacts with the IL-1 receptor accessory protein: a novel mechanism of regulation of IL-1 responsiveness. J Immunol. 1998;161:6871–6877. [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG. Fasting induces an anti-inflammatory effect on the neuroimmune system which a high-fat diet prevents. Obesity. 2011;19:1586–94. doi: 10.1038/oby.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laye S, Gheusi G, Cremona S, Combe C, Kelley K, Dantzer R, Parnet P. Endogenous brain IL-1 mediates LPS-induced anorexia and hypothalamic cytokine expression. Am J Physiol Regul Integr Comp Physiol. 2000;279:R93–8. doi: 10.1152/ajpregu.2000.279.1.R93. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Martinez J, Pelaez RP, Bazan NG. The interleukin-1 type 2 receptor gene displays immediate early gene responsiveness in glucocorticoid-stimulated human epidermal keratinocytes. J Biol Chem. 1999;274:8630–8638. doi: 10.1074/jbc.274.13.8630. [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowsky D, Lundkvist J, Laye S, Bartfai T. Interleukin-1 receptor accessory protein interacts with the type II interleukin-1 receptor. FEBS Lett. 1998;429:299–302. doi: 10.1016/s0014-5793(98)00467-0. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328–336. doi: 10.1016/s1471-4906(01)01941-x. [DOI] [PubMed] [Google Scholar]
- McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, Grubin CE, Wignall JM, Jenkins NA, Brannan CI. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Satpathy A, Hartman ME, Horvath EM, Kelley KW, Dantzer R, Johnson RW, Freund GG. IL-1beta-mediated innate immunity is amplified in the db/db mouse model of type 2 diabetes. J Immunol. 2005;174:4991–4997. doi: 10.4049/jimmunol.174.8.4991. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Morita K, Saito T, Ohta M, Ueno S, Rokutan KJ. Assessment of human stress and depression by DNA microarray analysis. Med Invest. 2005;52(Suppl):266–71. doi: 10.2152/jmi.52.266. [DOI] [PubMed] [Google Scholar]
- Orlando S, Matteucci C, Fadlon EJ, Buurman WA, Bardella MT, Colotta F, Introna M, Mantovani A. TNF-alpha, unlike other pro- and anti-inflammatory cytokines, induces rapid release of the IL-1 type II decoy receptor in human myelomonocytic cells. J Immunol. 1997;158:3861–3868. [PubMed] [Google Scholar]
- Orlando S, Sironi M, Bianchi G, Drummond AH, Boraschi D, Yabes D, Mantovani A. Role of metalloproteases in the release of the IL-1 type II decoy receptor. J Biol Chem. 1997;272:31764–31769. doi: 10.1074/jbc.272.50.31764. [DOI] [PubMed] [Google Scholar]
- Penton-Rol G, Orlando S, Polentarutti N, Bernasconi S, Muzio M, Introna M, Mantovani A. Bacterial lipopolysaccharide causes rapid shedding, followed by inhibition of mRNA expression, of the IL-1 type II receptor, with concomitant up-regulation of the type I receptor and induction of incompletely spliced transcripts. J Immunol. 1999;162:2931–2938. [PubMed] [Google Scholar]
- Pou J, Martínez-González J, Rebollo A, Rodríguez C, Rodríguez-Calvo R, Martín-Fuentes P, Cenarro A, Civeira F, Laguna JC, Alegret M. Type II interleukin-1 receptor expression is reduced in monocytes/macrophages and atherosclerotic lesions. Biochim Biophys Acta. 2011;1811:556–63. doi: 10.1016/j.bbalip.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Pruitt JH, Copeland EM, 3rd, Moldawer LL. Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock. 1995;3:235–251. doi: 10.1097/00024382-199504000-00001. [DOI] [PubMed] [Google Scholar]
- Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1beta. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Rauschmayr T, Groves RW, Kupper TS. Keratinocyte expression of the type 2 interleukin 1 receptor mediates local and specific inhibition of interleukin 1-mediated inflammation. Proc Natl Acad Sci U S A. 1997;94:5814–5819. doi: 10.1073/pnas.94.11.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re F, Muzio M, De Rossi M, Polentarutti N, Giri JG, Mantovani A, Colotta F. The type II “receptor” as a decoy target for interleukin 1 in polymorphonuclear leukocytes: characterization of induction by dexamethasone and ligand binding properties of the released decoy receptor. J Exp Med. 1994;179:739–743. doi: 10.1084/jem.179.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. Stimulatory effect of interleukin-1 on the hypothalamic-pituitary-adrenal axis of the rat: influence of age, gender and circulating sex steroids. J Endocrinol. 1994;140:365–372. doi: 10.1677/joe.0.1400365. [DOI] [PubMed] [Google Scholar]
- Rivier C, Chizzonite R, Vale W. In the mouse, the activation of the hypothalamic-pituitary-adrenal axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1. Endocrinology. 1989;125:2800–2805. doi: 10.1210/endo-125-6-2800. [DOI] [PubMed] [Google Scholar]
- Sambo P, Fadlon EJ, Sironi M, Matteucci C, Introna M, Mantovani A, Colotta F. Reactive oxygen intermediates cause rapid release of the interleukin-1 decoy receptor from human myelomonocytic cells. Blood. 1996;87:1682–1686. [PubMed] [Google Scholar]
- Shieh JH, Peterson RH, Moore MA. IL-1 modulation of cytokine receptors on bone marrow cells. In vitro and in vivo studies. J Immunol. 1991;147 [PubMed] [Google Scholar]
- Simeoni E, Dudler J, Fleury S, Li J, Pagnotta M, Pascual M, von Segesser LK, Vassalli G. Gene transfer of a soluble IL-1 type 2 receptor-Ig fusion protein improves cardiac allograft survival in rats. Eur J Cardiothorac Surg. 2007;31:222–228. doi: 10.1016/j.ejcts.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Sims JE, March CJ, Cosman D, Widmer MB, MacDonald HR, McMahan CJ, Grubin CE, Wignall JM, Jackson JL, Call SM. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988;241:585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Sims JE, Gayle MA, Slack JL, Alderson MR, Bird TA, Giri JG, Colotta F, Re F, Mantovani A, Shanebeck K. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci U S A. 1993;90:6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JE, Painter SL, Gow IR. Genomic organization ot the type I and type II IL-1 receptors. Cytokine. 1995;7:483–490. doi: 10.1006/cyto.1995.0066. [DOI] [PubMed] [Google Scholar]
- Slack JL, Schooley K, Bonnert TP, Mitcham JL, Qwarnstrom EE, Sims JE, Dower SK. Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J Biol Chem. 2000;275:4670–4678. doi: 10.1074/jbc.275.7.4670. [DOI] [PubMed] [Google Scholar]
- Smith DE, Hanna R, Della F, Moore H, Chen H, Farese AM, MacVittie TJ, Virca GD, Sims JE. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity. 2003;18:87–96. doi: 10.1016/s1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- Spriggs MK, Nevens PJ, Grabstein K, Dower SK, Cosman D, Armitage RJ, McMahan CJ, Sims JE. Molecular characterization of the interleukin-1 receptor (IL-1R) on monocytes and polymorphonuclear cells. Cytokine. 1992;4 doi: 10.1016/1043-4666(92)90042-p. [DOI] [PubMed] [Google Scholar]
- Stahl N, Radin A, Mellis S. Rilonacept-CAPS and Beyond. Ann NY Acad Sci. 2009:1182. doi: 10.1111/j.1749-6632.2009.05074.x. [DOI] [PubMed] [Google Scholar]
- Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 1995;92:1714–1718. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani Y, Kuo TM, Mizuno M. Comparison of cytokine levels and embryo toxicity in peritoneal fluid in infertile women with untreated or treated endometriosis. Am J Obstet Gynecol. 1992;167:265–270. doi: 10.1016/s0002-9378(11)91672-x. [DOI] [PubMed] [Google Scholar]
- Tedui A, Ait-Oufella H, Mallat Z. Cytokines and Atherosclerosis. In: George SJ, Johnson J, editors. Atherosclerosis. Wiley-Blackwell; Strauss GmbH: 2010. pp. 63–84. [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Vambutas A, DeVoti J, Goldofsky E, Gordon M, Lesser M, Bonagura V. Alternate splicing of interleukin-1 receptor type II (IL1R2) in vitro correlates with clinical glucocorticoid responsiveness in patients with AIED. PLoS One. 2009;4:e5293. doi: 10.1371/journal.pone.0005293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deuren M, van der Ven-Jongekrijg J, Vannier E, van Dalen R, Pesman G, Bartelink AK, Dinarello CA, van der Meer JW. The pattern of interleukin-1beta (IL-1beta) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood. 1997;90:1101–1108. [PubMed] [Google Scholar]
- von der Thusen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- Wang X, Romanic AM, Yue TL, Feuerstein GZ, Ohlstein EH. Expression of interleukin-1beta, interleukin-1 receptor, and interleukin-1 receptor antagonist mRNA in rat carotid artery after balloon angioplasty. Biochem Biophys Res Commun. 2000;271:138–143. doi: 10.1006/bbrc.2000.2588. [DOI] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) Pathway. Sci Signal. 2010;3 doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, Tong L. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- Yan B, Chen G, Saigal K, Yang X, Jensen ST, Van Waes C, Stoeckert CJ, Chen Z. Systems biology-defined NF-kappaB regulons, interacting signal pathways and networks are implicated in the malignant phenotype of head and neck cancer cell lines differing in p53 status. Genome Biol. 2008;9:R53. doi: 10.1186/gb-2008-9-3-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PW, Chen HT, Czuprynski CJ, Schuler LA. Molecular characterization of the bovine type II IL-1 receptor. Cytokine. 1997;9:1–8. doi: 10.1006/cyto.1996.0129. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Kostler WJ, Molotski N, Tsai JM, Krauthgamer R, Jacob-Hirsch J, Rechavi G, Soen Y, Jung S, Yarden Y, Domany E. Coupled pre-mRNA and mRNA dynamics unveil operational strategies underlying transcriptional responses to stimuli. Mol Syst Biol. 2011;7:529. doi: 10.1038/msb.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]