Abstract
In this initial report, we document preoperative outcomes of a behavioral lifestyle intervention delivered to patients prior to bariatric surgery. Participants (N = 240) were 86.7% female, 82.9% white, 52.3% married, and 85.8% had ≥ high school education. Mean BMI was 47.9 ± 6.7 kg/m2 and age was 45.2 ± 11 years. After completing a baseline assessment, patients were randomized to a 6-month, evidence-informed, manualized lifestyle intervention (LIFESTYLE, n = 121) or to preoperative care as usual (USUAL CARE, n = 119). At 6 months, 187 participants remained candidates for bariatric surgery and were included in the analyses. Results indicated that LIFESTYLE participants lost significantly more weight than those receiving USUAL CARE [8.3 ± 7.8 kg vs. 3.3 ± 5.5 kg, F(1,182) = 23.6, p < 0.0001], with an effect size of 0.72. LIFESTYLE patients were more likely to lose at least 5% of initial body weight than those in USUAL CARE (OR = 4.98, p < 0.0001), as were participants who were older (OR = 1.04, p = 0.01 for every year increase in age) or heavier (OR = 1.06, p = 0.02 for each unit increase in BMI). A behavioral lifestyle intervention for severely overweight individuals leads to clinically significant weight loss prior to bariatric surgery. Post-surgery follow-up will allow us to examine the impact of the preoperative intervention on postoperative outcomes.
Introduction
Recent attention has focused on the utility of weight loss immediately prior to bariatric surgery, yet there have been no studies designed to evaluate an evidence-informed, preoperative behavioral lifestyle intervention. Indeed, there are few studies that document the impact of behavioral lifestyle intervention on severely obese individuals who are not planning for bariatric surgery. In a randomized trial, a year-long intensive lifestyle intervention involving diet and initial or delayed physical activity resulted in clinically significant weight loss and favorable changes in cardiometabolic risk factors among adults with class II or III obesity (1). Similarly, in an analysis of results from the intensive behavioral treatment in the Look AHEAD study, individuals with Class II and III obesity and type 2 diabetes had significant weight loss and improvement in caridiovascular disease risk factors (2). These results suggest that intensive behavioral lifestyle intervention can lead to short-term weight loss and health benefits for severely overweight individuals, but it is not known whether lifestyle intervention would yield similar results for patients preparing for bariatric surgery.
There has been debate as to whether patients should be required to participate in a weight loss program immediately prior to bariatric surgery, with concerns raised that insurance-mandated physician supervised diet could delay or impede access to treatment (3). The American society for Metabolic and Bariatric Surgery (ASMBS) has taken the position that the requirement for documentation of prolonged physician supervised weight loss efforts before health insurance carrier approval of bariatric surgery is inappropriate (4). However, the ASMBS notes that individual surgeons and programs should be free to recommend preoperative weight loss according to the specific needs and circumstances of the patient.
Despite the controversy surrounding insurance-mandated physician supervised diets, bariatric surgery programs often recommend preoperative weight loss and lifestyle changes. Clinical reports indicate that preoperative behavioral intervention is well received by patients, with high program satisfaction and perceived usefulness (5), and that that weight loss is safe and achievable in the context of preoperative care (6, 7). Available data also support the benefits of preoperative weight loss, including fewer surgical complications, shorter operative time, less blood loss, and a shorter hospital stay (8). Moreover, a meta-analysis suggests that weight loss is greater 1 year after surgery among patients who had lost weight preoperatively (9).
Nevertheless, we are aware of just one published prospective, randomized trial evaluating the impact of preoperative weight loss (10). Candidates for surgery were randomized to a preoperative weight loss requirement (n = 50) or routine preoperative care without this requirement (n = 50). Patients in the weight loss group were allowed to use any method, but were encouraged to concentrate on diets that had worked well in the past. Of 61 patients who underwent Roux-en-Y gastric bypass, 50 (82%) were followed at 3 months, and 37 (61%) at 6 months after operation. Patients randomized to the weight loss requirement had a shorter operative time and greater weight loss three months after operation than the group receiving routine care, but differences in weight loss were not sustained at 6 months (10). At follow-up one year after operation, there was no difference in outcomes by randomization group, but when patients were divided according to those who did and did not lose at least 5% of initial body weight preoperatively, weight loss at 1 year was greater for those with 5% weight loss (11). However, this was a single study of a relatively small sample required to lose weight by any method, and many important questions remain regarding the optimal approaches to weight loss and behavior change prior to bariatric surgery.
We are currently conducting a randomized, controlled trial to evaluate the benefits of manualized, evidence-informed, 6-month behavioral lifestyle intervention relative to routine care prior to bariatric surgery. In this initial report, we evaluate the impact of the lifestyle intervention on body weight prior to surgery and examine factors associated with preoperative weight loss.
Methods and Procedures
Participants
All patients who were at least 18 years of age and seeking bariatric surgery at a Bariatric Center of Excellence at a large, urban medical center were eligible. Exclusion criteria included: 1) Mental retardation or psychosis; 2) Previously diagnosed genetic obesity syndrome; 3) Participation in a weight management program in the 6 months prior to study enrollment; 4) Uncontrolled psychiatric symptomatology sufficiently severe to require immediate treatment; 5) Pregnant or lactating in the previous 6 months; 6) Taking a medication known to affect body weight in the previous 6 months (e.g., second generation antipsychotics); 8) Any previous weight loss surgery; 9) Medical condition requiring a specialized preoperative regimen (e.g., nonambulatory, on oxygen therapy for chronic obstructive pulmonary disease); and 10) Participation in a conflicting research protocol.
Procedure
All new bariatric surgery patients were asked if they would be willing to be contacted about participation in research. Patients were assured that a decision to hear about research would not obligate them to participate, their medical care would be the same whether or not they agreed to participate, and information provided for research would have no bearing on their candidacy for surgery. The research study was approved by the University of Pittsburgh Institutional Review Board, and all participants provided informed consent.
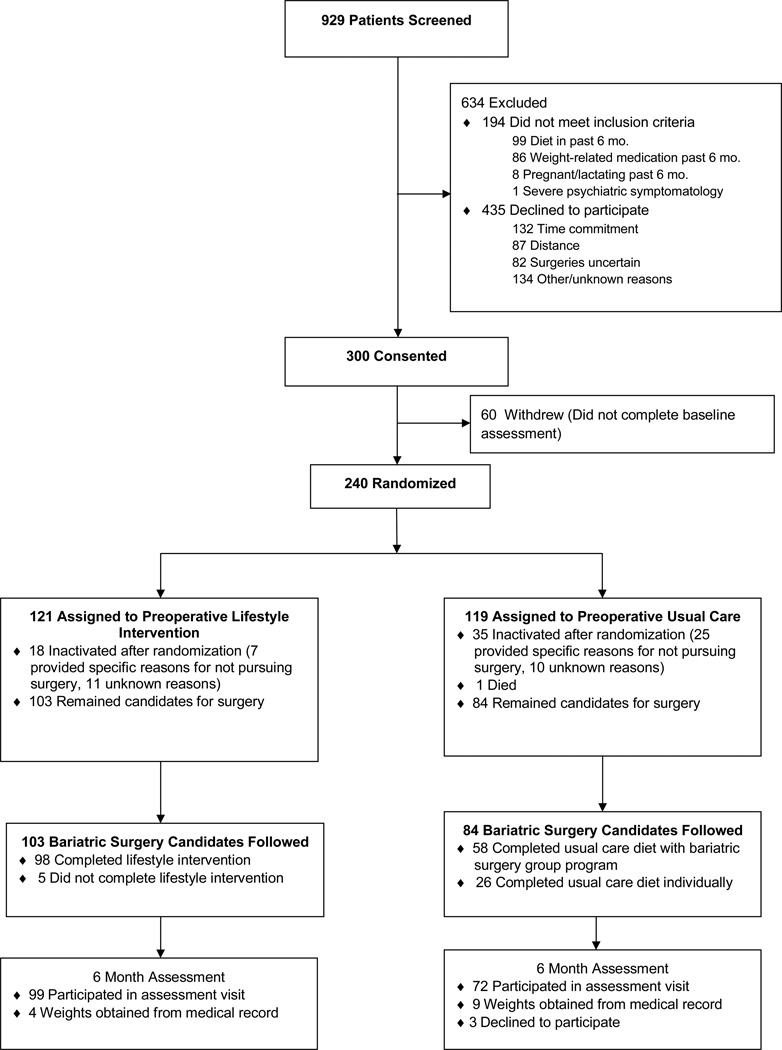
As shown in Figure 1, out of 1,153 patients who agreed to be contacted about participation in research and were screened by telephone, 300 consented to participate. Those who did (n = 240) and did not (n = 60) complete the baseline assessment did not differ significantly in BMI or demographic characteristics. Participants (n = 240) were 86.7% female, 82.9% white, 0.8% Hispanic or Latino, 52.3% married, and 85.8% had education beyond high school education. Mean BMI was 47.9 ± 6.7 kg/m2 and age was 45.2 ±11 years. After completing the baseline assessment, participants were block randomized to behavioral lifestyle intervention (LIFESTYLE, n = 121) or usual preoperative care (USUAL CARE, n = 119), with stratification by BMI. Patients randomized to LIFESTYLE and USUAL CARE did not differ significantly in baseline BMI or demographic characteristics.
Figure 1.
Patient recruitment and flow.
Measures
An investigator-designed questionnaire was used to collect demographic data including sex, age, race/ethnicity, education, employment status, income and marital status. Weight was measured at baseline and 6 months using a digital scale. Height was measured at study entry using a mounted stadiometer. Participants were weighed and height measured in street clothes, without shoes. BMI was calculated as weight in kilograms divided by the square of height in meters.
Patients completed a battery of questionnaires and interviews at each assessment. Baseline measures of depression and eating behavior were included because they may be related to weight loss among bariatric surgery patients (12, 13). The widely used, psychometrically sound Beck Depression Inventory (BDI)(14) self-report questionnaire was used to assess severity of depressive symptoms. A semi-structured clinical interview with good reliability and validity, the overeating section of the Eating Disorder Examination (EDE)(15), was used to document binge eating, defined at least one episode per week with loss of control over the last 3 months, regardless of whether the amount of food consumed was objectively large (≥ 12 objective or subjective bulimic episodes). The EDE has documented validity for assessment of binge eating among patients prior to undergoing bariatric surgery (16). Participants also completed the Eating Behavior Inventory (EBI)(17), a reliable and valid tool used to assess behaviors associated with positive outcomes in lifestyle interventions.
Behavioral lifestyle intervention
Patients randomized to LIFESTYLE participated in an intervention adapted from the behavioral weight management program developed by the University of Pittsburgh Obesity and Nutrition Research Center (ONRC)(18, 19). Adaptations included providing information about how surgery facilitates weight loss, emphasizing the role of self-management, and addressing factors that have been related to postoperative weight control such as eating behaviors and mood. The study intervention was designed to instill realistic expectations regarding preoperative weight loss (5% of initial body weight), or 1 – 2 pounds per week, emphasizing the potential health benefits of lifestyle change pre- and post-surgery.
The objective of the behavioral intervention was to decrease calorie intake through diet and increase energy expenditure through physical activity. Participants were given a goal of 1200–1400 calories per day and instructed to stay within the range while maintaining a balanced diet consistent with nutritional guidelines for bariatric surgery. Participants were prescribed an exercise program based on their choice of activity (e.g., walking or swimming). Strategies for increasing lifestyle activity (e.g., taking the stairs or getting off the bus a stop early) were emphasized. Participants were assisted in self-monitoring and setting small, incremental goals for behavior change. The skills required to make the recommended changes in diet and physical activity were modeled, practiced, and reinforced throughout the intervention.
The lifestyle intervention lasted approximately 6 months (8 weekly face-to-face sessions, followed by 16 weeks of alternating face-to-face sessions and telephone coaching). A combination of face-to-face sessions and telephone coaching was utilized to minimize participant burden and maximize the intensity of counseling. Face-to-face sessions lasted 1 hour, consisting of a weigh-in, review of self-monitoring records, a didactic presentation, and homework. Telephone coaching was shorter in duration (15 – 20 minutes) and included a review of progress, problem solving and goal setting. Interventionists received training in behavioral and surgical management of obesity and regular supervision.
Most health insurance carriers required patients to provide documentation of participation in a 6-month physician supervised diet and activity program before granting approval for surgery. For LIFESTYLE patients, documentation of participation in the study intervention fulfilled this requirement.
Usual care
After randomization, USUAL CARE patients did not have any additional contact with the study staff until the 6 month assessment. As required by health insurance carriers, patients completed a non-standardized, physician-supervised diet and activity program in the context of routine presurgical care. Most patients were seen once a month for 6 months, either in group sessions provided under the auspices of the bariatric surgery program or individually by primary care providers.
Analytic plan
Descriptive statistics were used to summarize demographic characteristics of study participants. Two-sample t tests, Wilcoxon tests and chi-square analyses (or Fisher’s exact tests) were performed for continuous and categorical variables, respectively, to test for differences between study conditions at randomization, and to compare those who were excluded vs. retained on BMI and baseline demographic variables. Statistical significance was set at p ≤ .05, and all tests were two-tailed. All analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC).
Out of 240 patients randomized, 53 were excluded from the study because they were not proceeding to surgery for various reasons such as development of a medical contraindication, an individual decision not to pursue surgery, failure to complete preoperative requirements, or denial of surgery by the insurance carrier. Patients who were excluded (n = 53) did not differ from those who were retained in the study at the 6 month assessment (n = 187) on baseline BMI or demographic characteristics.
To test the hypothesis intervention would have a positive impact on weight loss relative to routine care at 6 months, we fit a longitudinal model using SAS mixed models. We included fixed terms for time (0, 6 months), group (LIFESTYLE, USUAL CARE), and the group by time interaction, as well as initial BMI. Time was treated as a categorical variable, and a random term was included to account for individual variability. Planned contrasts were set to compare conditions in changes from baseline to the 6 months for weight loss. Effect size was calculated based on the t statistic. We computed weight loss and percent weight loss for all participants for whom we collected weight at 6 months (184/187). We then compared conditions in percent weight loss using a linear regression model.
A series of linear regression models was conducted to explore factors that potentially related to weight loss, including age, sex, education, employment status, income, race/ethnicity, marital status, and baseline scores on the BDI, EDE and EBI. We first ran univariate analysis for each variable of interest controlling for group and initial BMI. The final multivariate model was selected using a stepwise method with group and initial BMI forced into the model. Last, following the same modeling strategy, we examined the effects of these factors on the probability that a participant lost at least 5% of initial body weight using logistic regression analysis.
Results
Preoperative weight loss
A mixed model included 187 participants who remained candidates for surgery at the time of the 6 month assessment, and a contrast was set to compare weight change between conditions. There was a significant condition by time interaction: LIFESTYLE participants lost an average of 4.98 kg more weight than USUAL CARE participants from baseline to 6 months [8.3 ± 7.8 kg vs. 3.3 ± 5.5 kg; F(1,182) = 23.6, p < 0.0001]; this translated to an effect size of 0.72. Similar results were observed for LIFESTYLE vs. USUAL CARE, respectively, in a linear regression model for percent weight loss [6.3% ± 5.8% vs. 2.5% ± 4.0%; t(1,182) = 5.01, p < 0.0001].
Factors associated with preoperative weight loss
Results of univariate and multivariate modeling are presented in Table 1. We first ran analysis for the aforementioned predictors, one at a time, controlling for group and initial BMI. The effects of group and BMI were calculated without any other covariates. Group, initial BMI, sex, age and EBI score were significant predictors of weight loss in univariate models; the other covariates, including BDI and EDE, were not significantly associated with weight loss. The final multivariate model, which was constructed based on the stepwise procedure, included intervention condition, initial BMI, sex and age. Consistent with the results from mixed models, there was a significant intervention effect (β = 4.93, t = 5.2, p < 0.0001). Male participants lost an average of 4.83 kg more than females (t = 3.31, p = 0.001). Further, those with larger BMIs lost more weight (β = 0.27, t = 3.6, p = 0.0004), as did older subjects (β = 0.12, t = 2.75, p = 0.01). Results of modeling for percent weight loss yielded a similar pattern of results (data not shown).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | β | P value | β | P value |
| Group (ref: usual care) | 4.94 | <.0001 | 4.93 | <.0001 |
| BMI | .25 | .002 | .27 | .0004 |
| Race (ref: non white) | 2.14 | .13 | - | - |
| Sex (ref: female) | 5.30 | .0004 | 4.83 | .001 |
| Education (ref: ≤ high school) | −.72 | .63 | - | - |
| Employment (ref: not full time) | −.75 | .46 | - | - |
| Income (ref: ≤ $30,000) | .79 | .48 | - | - |
| Married (ref: No) | .22 | .83 | - | - |
| Age | .14 | .002 | .12 | .01 |
| BDI | .08 | .15 | - | - |
| EDE (ref: < 12 binges past 3 mo.) | 1.47 | .22 | - | - |
| EBI | −0.12 | .02 | - | - |
Notes:
BMI = Body Mass Index; BDI = Beck Depression Inventory; EDE = Eating Disorder Examination; EBI = Eating Behavior Inventory.
Positive coefficients correspond to larger weight reduction.
A weight loss of at least 5% of initial body weight was achieved by 53.4% of LIFESTYLE patients and 21.0% of USUAL CARE patients, and logistic models were used to explore factors associated with ≥ 5% weight loss. The final model included group, age, and baseline BMI. Participants in LIFESTYLE were more likely to lose at least 5% initial weight than those in USUAL CARE (OR = 4.98, p < 0.0001). Additionally, those who were older or heavier were more likely to lose at least 5% of their initial weight (OR = 1.04, p = 0.01 for every year increase in age, and OR = 1.06, p = 0.02 for each unit increase in BMI).
Discussion
Results of the present investigation indicate that a 6-month behavioral lifestyle intervention delivered to individuals preparing for bariatric surgery is associated with significantly larger weight losses than those achieved by patients receiving usual care (i.e. an insurance-mandated physician supervised diet) prior to surgery. Average loss of 6.3% of initial body weight among participants randomized to the lifestyle intervention is similar to results from a trial in which patients required to lose weight prior to Roux-en-Y gastric bypass lost 8.2% of body weight (10).
Further, a weight loss of at least 5% of initial body weight was achieved by over half of the lifestyle intervention group at 6 months, an amount of preoperative weight loss that has been associated with shorter operating room times (20) and greater postoperative weight loss one year following Roux-en-Y gastric bypass (11). Furthermore, these results may be compared to data from nonsurgical samples of severely overweight individuals participating in university-based clinical trials of behavioral lifestyle interventions. In a secondary analysis of completers by Goodpaster and colleagues (1), 60 to 80% of participants in the activity interventions lost more than 5% of their baseline body weight at 6 months. Among severely overweight participants receiving the intensive lifestyle intervention in the Look AHEAD trial, 67% achieved a 5% weight loss at 1 year (2). Thus, the percentage of patients achieving 5% weight loss in the present study appears similar to other trails, although more modest.
In our sample (with an average BMI of 48 kg/m2 and age of 45 years), older, heavier patients were more likely to achieve at least 5% preoperative weight loss. However, measures of depression and eating behavior at study entry were not associated with preoperative weight loss. Although findings require replication, they are consistent with the broader literature on behavioral weight control. For example, among a less obese sample of adults at risk for developing type 2 diabetes in the Diabetes Prevention Program (DPP), older participants were particularly successful at meeting their weight loss and physical activity goals, while psychosocial and depression measures were unrelated to goal achievement (21). It has also been well established that heavier patients tend to lose more weight. In the study by Goodpaster and colleagues (1), the heavier participants with class III obesity had a significantly greater percent weight loss than class II obese participants at one year. In secondary analyses from the Look AHEAD study, participants in the intensive lifestyle intervention group with class III obesity had a significantly greater percent weight loss than overweight participants at one year (2).
Strengths of the present study include its randomized, controlled design and evidence-informed, manualized behavioral lifestyle intervention. Nonetheless, it also has limitations. Most notably, the sample of patients who enrolled in the research trial may not be representative of the population of bariatric surgery patients. Indeed, about 3 out of 4 patients screened by telephone for the study were excluded because they were ineligible or not interested. Although initial results are positive, generalizability may be limited, and data on postoperative outcomes are not yet available.
A growing body of evidence suggests that behavioral intervention leads to significant short-term weight loss even among extremely obese individuals, and preoperative weight loss may improve weight loss after bariatric surgery (9–11). Results of the present study suggest that a 6-month, evidence-informed, manualized lifestyle intervention was associated with significantly greater weight loss than routine care prior to surgery. Although weight loss was greater for older, heavier patients, neither binge eating nor depressive symptoms was associated with preoperative weight loss. However, post-surgery follow-up is essential because these factors have been associated with poorer postoperative weight control (12, 13).
In future reports, we will examine whether preoperative behavioral lifestyle intervention has an impact on postoperative outcomes, including fewer complications and greater longer-term weight loss. More research is needed on when to initiate and how to integrate surgical and behavioral interventions in the management of severe obesity. This is particularly true given mounting evidence of a significant minority of bariatric surgery patients who experience poor long-term weight loss (22, 23). Self-regulation of eating and activity are critical for management of severe obesity, and both pre- and postoperative lifestyle interventions (24) will be crucial for optimizing long-term outcomes of bariatric surgery.
Acknowledgement
Research supported by R01DK077102 from the National Institute of Diabetes and Digestive and Kidney Diseases (PI: Melissa A. Kalarchian).
Footnotes
Disclosure: The authors have no conflicts of interest.
REFERENCES
- 1.Goodpaster BH, Delany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304(16):1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unick JL, Beavers D, Jakicic JM, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34(10):2152–2157. doi: 10.2337/dc11-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jamal MK, DeMaria EJ, Johnson JM, et al. Insurance-mandated preoperative dietary counseling does not improve outcome and increases dropout rates in patients considering gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2006;2(2):122–127. doi: 10.1016/j.soard.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Brethauer S. ASMBS Position Statement on Preoperative Supervised Weight Loss Requirements. Surg Obes Relat Dis. 2011;7(3):257–260. doi: 10.1016/j.soard.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Brandenburg D, Kotlowski R. Practice makes perfect? Patient response to a prebariatric surgery behavior modification program. Obes Surg. 2005 Jan;15(1):125–132. doi: 10.1381/0960892052993594. [DOI] [PubMed] [Google Scholar]
- 6.Martin LF, Tan TL, Holmes PA, Becker DA, Horn J, Bixler EO. Can morbidly obese patients safely lose weight preoperatively? Am J Surg. 1995;169(2):245–253. doi: 10.1016/s0002-9610(99)80145-7. [DOI] [PubMed] [Google Scholar]
- 7.Dolfing JG, Wolffenbuttel BH, ten Hoor-Aukema NM, Schweitzer DH. Daily high doses of fluoxetine for weight loss and improvement in lifestyle before bariatric surgery. Obes Surg. 2005;15(8):1185–1191. doi: 10.1381/0960892055002301. [DOI] [PubMed] [Google Scholar]
- 8.Tarnoff M, Kaplan LM, Shikora S. An evidenced-based assessment of preoperative weight loss in bariatric surgery. Obes Surg. 2008;18(9):1059–1061. doi: 10.1007/s11695-008-9603-y. [DOI] [PubMed] [Google Scholar]
- 9.Livhits M, Mercado C, Yermilov I, et al. Does weight loss immediately before bariatric surgery improve outcomes: a systematic review. Surg Obes Relat Dis. 2009;5(6):713–721. doi: 10.1016/j.soard.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Alami RS, Morton JM, Schuster R, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis. 2007;3(2):141–145. doi: 10.1016/j.soard.2006.11.006. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 11.Solomon H, Liu GY, Alami R, Morton J, Curet MJ. Benefits to patients choosing preoperative weight loss in gastric bypass surgery: new results of a randomized trial. J Am Coll Surg. 2009;208(2):241–245. doi: 10.1016/j.jamcollsurg.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Kalarchian MA, Marcus MD, Levine MD, Soulakova JN, Courcoulas AP, Wisinski MS. Relationship of psychiatric disorders to 6-month outcomes after gastric bypass. Surg Obes Relat Dis. 2008;4(4):544–549. doi: 10.1016/j.soard.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM. Loss of control over eating predicts outcomes in bariatric surgery patients: a prospective, 24-month follow-up study. J Clin Psychiatry. 2010;71(2):175–184. doi: 10.4088/JCP.08m04328blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 15.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge eating: Nature, assessment, and treatment. 12th ed. New York: Guilford; 1993. pp. 317–360. [Google Scholar]
- 16.Hsu LK, Mulliken B, McDonagh B, et al. Binge eating disorder in extreme obesity. Int J Obes Relat Metab Disord. 2002 Oct;26(10):1398–1403. doi: 10.1038/sj.ijo.0802081. [DOI] [PubMed] [Google Scholar]
- 17.O'Neil PM, Rieder S. Utility and validity of the eating behavior inventory in clinical obesity research: a review of the literature. Obes Rev. 2005;6(3):209–216. doi: 10.1111/j.1467-789X.2005.00192.x. [DOI] [PubMed] [Google Scholar]
- 18.Kalarchian M, Beagle N, Courcoulas A. Case study: behavioral weight control following bariatric surgery. Bariatric Nursing and Surgical Patient Care. 2007;2:189–192. [Google Scholar]
- 19.Kalarchian MA, Marcus MD. Preoperative Lifestyle Intervention. In: Mitchell JE, de Zwaan M, editors. Psychosocial Assessment and Treatment of Bariatric Surgery Patients. New York: Routledge; 2011. pp. 209–220. [Google Scholar]
- 20.Alvarado R, Alami RS, Hsu G, et al. The impact of preoperative weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15(9):1282–1286. doi: 10.1381/096089205774512429. [DOI] [PubMed] [Google Scholar]
- 21.Wing RR, Hamman RF, Bray GA, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008 Jun;18(6):648–651. doi: 10.1007/s11695-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 23.Elder KA, Wolfe BM. Bariatric surgery: a review of procedures and outcomes. Gastroenterology. 2007 May;132(6):2253–2271. doi: 10.1053/j.gastro.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 24.Kalarchian MA, Marcus MD, Courcoulas AP, Cheng Y, Levine MD, Josbeno D. Optimizing long-term weight control after bariatric surgery: a pilot study. Surg Obes Relat Dis. 2011 doi: 10.1016/j.soard.2011.04.231. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]