Abstract
The objective of this study was to further understand the genetic mechanisms of Vitamin-A-Deficiency (VAD) induced arrest of spermatogonial stem-cell differentiation.
Vitamin A and its derivatives (the retinoids) participate in many physiological processes including vision, cellular differentiation and reproduction. VAD affects spermatogenesis, the subject of our present study. Spermatogenesis is a highly regulated process of differentiation and complex morphologic alterations that leads to the formation of sperm in the seminiferous epithelium. VAD causes early cessation of spermatogenesis, characterized by degeneration of meiotic germ cells, leading to seminiferous tubules containing mostly type A spermatogonia and sertoli cells. These observations led us to the hypothesis that VAD affects not only germ cells but also somatic cells.
To investigate the effects of VAD on spermatogenesis in mice we used adult Balb/C mice fed with Control or VAD diet for an extended period of time (6–28 weeks). We first observed the chronology, then the extent of the effects of VAD on the testes. Using microarray analysis of isolated pure populations of spermatogonia, leydig and sertoli cells from control and VAD 18- and 25-week mice, we examined the effects of VAD on gene expression and identified target genes involved in the arrest of spermatogonial differentiation and spermatogenesis.
Our results provide a more precise definition of the chronology and magnitude of the consequences of VAD on mouse testes than the previously available literature, and highlight direct and indirect (via somatic cells) effects of VAD on germ cell differentiation.
INTRODUCTION
Vitamin-A-Deficiency (VAD) is a serious public health problem in developing countries where dietary intake of vitamin A is low. VAD, a leading cause of preventable blindness in children, increases the risk of disease and death from severe infections. More than 250 million children under 5 years of age suffer from dietary Vitamin-A-Deficiency (1). VAD is mainly a consequence of malnutrition, but may also occur due to inadequate absorption, and hepatic disease (2).
Vitamin A and its derivatives (the retinoids: retinol, retinal, retinoic acid and retinyl esters) play critical roles during embryogenesis as well as in adult tissues (3, 4, 5). They participate in numerous cellular functions including reproduction, development, vision, growth, lipid metabolism, cellular differentiation, proliferation, brain function, and tissue maintenance (6, 7). The biological effects of retinoids are mediated through binding of their active metabolite, retinoic acid (RA), to two families of nuclear receptors: (i) RARs (receptors of all-trans and 9-cis retinoic acid stereoisomers); (ii) RXRs (receptors specific to the 9-cis retinoic acid). Both receptors contain at least three isotypes designated a, b and g, encoded by separate genes (8, 9). These receptors belong to the steroid/thyroid hormone nuclear receptor superfamily and function as ligand-dependent transcription factors binding to Retinoic Acid Response Elements (RAREs) in the promoter of their target genes (10, 11).
Spermatogenesis, a highly regulated process of differentiation and complex morphologic alterations, leads to the formation of sperm in the seminiferous epithelium. In rodent testes, spermatogenesis begins shortly after birth. It encompasses a series of developmental changes, divided into three distinct steps (i.e., spermatogonial mitosis, meiosis of spermatocytes and spermiogenesis of haploid spermatids). These steps are described as a cycle of cellular changes, referred to as stages of the seminiferous epithelial cycle and they occur within defined regions of the epithelium (12). The transition of germ cells from stage to stage and the concomitant change in cellular morphology suggest that germ cell development is mediated by ‘stage-specific’, tightly regulated changes in gene expression. Spermatogenesis is under the control of cell signaling pathways, involving a complex array of hormones and cytokines (13, 14), and requires interaction among sertoli, leydig and germ cells (15, 16, 17). However, the molecular mechanisms regulating spermatogonial stem cell proliferation, differentiation, or dedifferentiation are largely unknown.
The need for vitamin A during normal spermatogenesis has been recognized for decades (18, 19). Retinoic acid nuclear receptors are expressed in testes germ cells, sertoli and leydig cells (20). The membrane receptor for RBP (Retinol Binding Protein), Stra6, is also highly expressed in sertoli cells (21). Vitamin-A-Deficiency induces early cessation of spermatogenesis (22), characterized by degeneration of the meiotic germ cells (23) resulting in seminiferous tubules that contain only sertoli cells, spermatogonia, and some early spermatocytes (24). Dietary vitamin A supplementation and injection of high doses of retinoic acid can correct the VAD-induced loss of mature germ cells in the testes (25). After retinol replacement, the spermatogonia, mainly type A1 that survived VAD treatment, repopulate the regenerated testes. The functional roles of retinoids in spermatogenesis were studied using animal models maintained on a diet deficient in vitamin A and/or vitamin A derivatives. The most extensively studied model is the rat (26), in which time-dependent effects of VAD have been measured. Knockout mice for RARa, the predominant isoform of retinoid nuclear receptor expressed in the testes, have also been studied (27, 18). The induction of Vitamin-A-Deficiency permits investigation of changes in cellular physiology following the first synchronized spermatogenic cycle after vitamin A replacement; that is, when the somatic cells interact with germ cells at a limited number of developmental stages. Therefore, it was used as a simplified model to study spermatogenesis. However, an understanding of the molecular mechanisms and/or consequences of Vitamin-A-Deficiency on germ cells remains elusive. It is not resolved whether RA directly induces spermatogonial differentiation or indirectly via somatic cells.
While most data in the literature concern the effect of VAD in mouse spermatogenesis during development, our study is original in its focus on the effect of Vitamin-A-Deficiency on spermatogenesis in adult mice. We therefore first present results enhancing the description of this animal model by performing a time-point study of the consequences of the VAD diet, not previously documented, to establish a better understanding of the chronology and magnitude. In the second part of our study, we emphasized the effect of VAD in our animal model not only on germ cells but also on somatic cells. Our results confirmed the hypothesis that there is both direct and indirect effects of VAD on gene expression (not only in spermatogonia but also in leydig and sertoli cells).
MATERIALS AND METHODS
Animals
Balb/C male mice were obtained from Charles River Laboratories, Inc. and/or from a colony maintained in NICHD. The animals were housed in groups of 5, maintained on a 12-h light/12-h dark cycle. The animals were allowed to have ad libitum access to food and tap water.
The National Institute of Child and Health and Human Development Animal Care and Use Committee approved the use and care of animals in this study.
Experimental Design
At 60 days of age all male mice were randomly divided into the Control or VAD groups. Both diets, in the form of dry food, were freely available throughout the experiment. The composition of the Vitamin-A-Deficient (AIN-93G vitamin A Free from TestDiet, Richmond, USA) and Control (AIN-93G Growth Purified Diet from TestDiet, Richmond, USA) diets is indicated in Table 1. The Control diet was identical to the VAD plus vitamin A (4000 IU/kg diet).
Table 1. Composition of the diet.
Control and Vitamin-A-Deficient diets were purchased from Test Diet (Richmond, USA) in the form of dry food and stored in sealed bags at 4°C.
| Ingredients | Amount (%) |
|---|---|
| Corn Starch | 39.75 |
| Casein – Vitamin Free | 20.00 |
| Dextrin | 13.20 |
| Sucrose | 10.00 |
| Soybean Oil | 7.00 |
| Powdered Cellulose | 5.00 |
| AIN 93G Mineral Mix a | 3.50 |
| AIN 93G Vitamin Mix (no vitamin A) b | 1.00 |
| L-Cystine | 0.30 |
| Choline Bitartrate | 0.25 |
| t-Butylhydroquinone | 0.0014 |
AIN 93G Mineral Mix consisted of the following: Calcium (0.50%); Phosphorus (0.32%); Potassium (0.36%); Magnesium (0.05%); Sodium (0.12%); Chloride (0.20%); Fluorine (1.0 ppm); Iron (35 ppm); Zinc (35 ppm); Manganese (11 ppm); Copper (6 ppm); Iodine (0.21 ppm); Chromium (1.0 ppm); Molybdenum (0.14 ppm) and Selenium (0.17 ppm).
AIN 93G Vitamin Mix consisted of the following: Vitamin A (0 IU/g); Vitamin D-3 (1 IU/g); Vitamin E (80.7 IU/kg); Vitamin K (as menadione) (0.29 ppm); Thiamin Hydrochloride (6.0 ppm); Riboflavin (6.0 ppm); Niacin (30 ppm); Pantothenic Acid (15 ppm); Folic Acid (2.0 ppm); Pyridoxine (5.8 ppm); Biotin (0.2 ppm); Vitamin B-12 (25 mcg/kg) and Choline Chloride (1250 ppm).
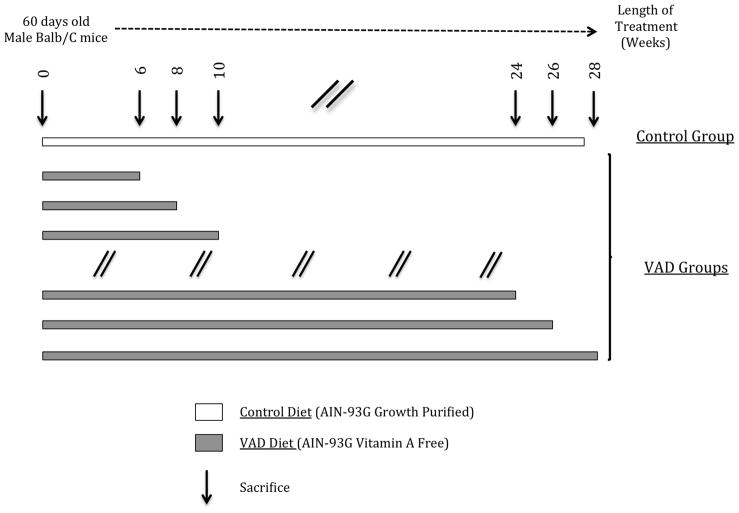
Our experimental design is described in Figure 1. Control and VAD diets started when the mice were 60 days old. The VAD diet was maintained from 6 weeks up to 28 weeks. VAD mice (4–6 mice per group) were sacrificed by decapitation after CO2 asphyxia at 2 weeks intervals throughout this period. Blood, liver and testes of animals were rapidly removed, weighed when applicable, and stored at −80°C for subsequent analysis.
Figure 1.
Experimental Design.
RBP EIA Assay
The Vitamin A status of the mice was determined by measurement of serum RBP levels using the Dual Mouse/Rat RBP4 EIA Kit (Alpo Diagnostics, USA). Following centrifugation of the blood for 15 minutes at 1000xg, serum was stored at −80°C until utilization. The assay was performed according to the manufacturer’s specifications. The absorbance of each sample was determined at 450 nm, and concentrations calculated by interpolation of the regression curve formula. Concentrations were expressed in ng/ml.
Triiodothyronine (T3) Assay
The thyroid hormone status of the mice was determined by measuring serum triiodothyronine levels using the Mouse/Rat Triiodothyronine (T3) ELISA Kit (Calbiotech Inc, USA). Following centrifugation of the blood for 15 minutes at 1000xg, serum was stored at −80°C until utilization. The assay was performed according to the manufacturer’s specification. The absorbance of each sample was determined at 450 nm, and concentrations calculated by interpolation of the standard curve. Concentrations were expressed in ng/ml.
Histology
Testes from Control and VAD groups were fixed for 24h in Bouin’s solution and embedded in paraffin for sectioning at 6 μm. One slide from each animal was stained with Hematoxylin and Eosin (H&E) for light microscopy examination (Paragon Bioservices, Baltimore, USA).
In Situ TUNEL Assay
Terminal transferase mediated DNA nick end-labeling was performed on paraffin sections using a TUNEL assay kit (Roche, USA). The paraffin sections were dewaxed and rehydrated according to standard protocols (washing in xylene, and rehydration through a graded series of ethanol and double distilled water). The samples were incubated with Proteinase K solution (14–22 mg/ml in 10 ml Tris-HCl, pH 7.5) (Roche Diagnostics, Germany) for 25 minutes at room temperature, and rinsed twice in PBS (1X). The sections were then permeabilized for 8 minutes in freshly prepared citrate buffer (0.1% sodium citrate, 0.1% Triton X100) at room temperature, and rinsed twice in PBS. The sections were incubated at 37°C for 1 hour in a dark humid chamber with 50 μl of freshly prepared TUNEL reaction mixture. The slides were thoroughly rinsed in PBS. 50 μl of Converter-POD (peroxidase) was added to each sample and incubated for 30 minutes at 37°C in a humid chamber, and rinsed 3 times in PBS. The slides were incubated with DAB (diaminobenzidine) substrate for 15 minutes at room temperature, rinsed with PBS and counterstained with Hematoxylin, rinsed in distilled water, and mounted. As a negative control, sections were processed though the same procedure, but in the absence of Terminal Transferase enzyme. A positive control was prepared by pretreating the sections with DNase I, which resulted in multiple DNA fragments with 3′-OH ends, where dUTP could be incorporated.
For quantification of apoptotic cells, 20 tubules per sections and 3 sections per animal were observed and the percentage of apoptotic cells calculated.
Quantification of RNA Expression
Total RNA isolation
Mice were killed and both testes were quickly removed and stored at −80°C. Isolation of RNA was performed using the TRIzol® Reagent (Invitrogen, USA), according to the manufacturer’s specification. The quality and the concentration of RNA were determined by measurement of the OD260/OD280 absorbance ratio.
Reverse transcription
cDNA was synthesized using Superscript III reverse transcriptase (Invitrogen, USA), with minor modifications according to the manufacturer’s protocol. Briefly, 1 μg total RNA was incubated at 37°C for 15minutes with 1μl DNAse I (IU/μl) (Invitrogen, USA), and 0.5 μl RNAse Inhibitor (40 U/μl) (Invitrogen, USA). 1 μl of random hexamer primers (Poly-N 6-mer, 1 O.D.) (Eurofins MWG Operon, USA) was added and the mix incubated 10 minutes at 70°C and then placed on ice before addition of reverse transcriptase reaction reagents to a final volume of 20 μl. The reverse transcriptase reaction was performed at 42°C for 60 min.
Real-Time PCR
cDNA quantification by real-time PCR was performed using a 7500 Real Time PCR System (Applied Biosystem, USA). To detect target-gene amplification products, Power SYBR® Green PCR Master Mix (Applied Biosystem, USA) was used according to the manufacturer’s instructions. PCRs were performed on 96-well plates in a final volume of 25 μl containing SYBR® Green PCR Master Mix 1X, 0.5 μM of each primer and cDNA. The amplification conditions were 50°C for 2 min, 95°C for 10 min, followed by forty cycles of denaturation at 95°C for 15 s and annealing and elongation at 60°C for 60s. For each primer used, melting curve analysis showed a single melting peak after amplification, indicating a specific product. Quantification data were analyzed, as previously described (28), using the Applied Biosystem 7300/7500/7500 Fast Real-Time PCR System software. The oligonucleotides primers used are detailed in Table 2. Primers were purchased from Eurofins MWG Operon, USA.
Table 2.
Sequences of primers used for Real-Time PCR
| PCR Primers | Sequence of Forward (F) and Reverse (R) Primers | Reference |
|---|---|---|
| GAPDH | F-5′-gAACATCATCCCTgCATCCA-3′ R-5′-CCAgTgAgCTTCCCgTTCA-3′ |
(29) |
| RBP4 | F-5′-gACAAggCTCgTTTCTCTgg-3′ R-5′-AAAggAggCTACACCC-3′ |
(30) |
| RARα | F-5′-AggCCATCACAACTACCTgC-3′ R-5′-ggAAAgAAgAAggCgTAggg-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| RARβ | F-5′-CAgCTgggTAAATACACCACgAA-3′ R-5′-ggggTATACCTggTACAAATTCTgA-3′ |
(31) |
| RARγ | F-5′-TgCCAgTACTgCAggCTAC-3′ R-5′-TCTgCACTggAgTTCgTggTgTACT-3′ |
(32) |
| RXRα | F-5′-CTTTgACAgggTgCTAACAgAgC-3′ R-5′-ACgCTTCTAgTgACgCATACACC-3′ |
(33) |
| RXRβ | F-5′-CCTCTggACgATCAggTCAT-3′ R-5′-TgTCACgATTTTggACACT-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| RXRγ | F-5′-AggCAggTTTgCCAAgCTTCTg-3′ R-5′-ggAgTgTCTCCAATgAgCTTgA-3′ |
(34) |
| Acrv1 | F-5′-TCCgTggAgAAggAgTATgC-3′ R-5′-CACgggCATCTAgACCTTgT-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| c-kit | F-5′-TCCCAgAAACAggCTgAgTT-3′ R-5′-TTCATgTgATTgCCCAggTA-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| Ccna1 | F-5′-CAgCTATTCTCCTggCTTCg-3′ R-5′-CTgATgCACACTCCTTgACg-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| Cdc25c | F-5′-CCCTCggTgAAgACTCTgAA-3′ R-5′-CCgAgTCgTggAgTTTgTCT-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| Oct4 | F-5′-ACCATgTTTCTgAAgTgCCC-3′ R-5′-TgggAAAggTgTCCCTgTAg-3′ |
Primer-BLAST Primer designing tool (NCBI) |
| Stra8 | F-5′-CgATCTCTCCCACTCCTCCT-3′ R-5′-ggAgTCTggCCTTTTTCTCC-3′ |
Primer-BLAST Primer designing tool (NCBI) |
Leydig Cells Isolation
Control and VAD mouse testes were used for isolation of leydig cells. Decapsulated testes from 2 mice were suspended in RPMI medium (GIBCO Medium 1640, Invitrogen, USA) containing Collagenase (0.6 mg/ml), Hyaluronidase (0.12 mg/ml) and DNAse I (1.25 mg/ml) and incubated at 37°C for 30 minutes in a shaking water bath. The tissues were then allowed to come down the tube and the supernatant (containing interstitial cells/leydig cells) was transferred onto a fresh tube. Total RNA was extracted from the isolated cells using TRIzol ® Reagent (Invitrogen, USA), and cleaned with RNeasy minicolumns (Qiagen, USA). RNA content was determined by measurement of optical density at 260 nm. Only the RNA samples showing an OD260/280 ratio higher than 1.8 were used for microarray hybridization.
Sertoli Cells and Spermatogonia Isolation
The sertoli cells and spermatogonia were isolated by the STATPUT procedure (35) with minor modifications. Decapsulated testes from 2 mice were suspended in RPMI medium (GIBCO Medium 1640, Invitrogen, USA) containing Collagenase, Hyaluronidase and DNAse I (in the concentrations and conditions described above for the leydig cells isolation). The tissues were then allowed to come down the tube and the supernatant (containing interstitial cells) was removed. The pellet was incubated with 3 ml of 0.25% Trypsin/EDTA (GIBCO, Invitrogen, USA), in the presence of DNAse I (0.2 mg/ml) at 37°C for 15 minutes in a shaking water bath. The dispersed cells were washed twice with RPMI medium containing 10% heat inactivated FBS (GIBCO, Invitrogen, USA) to neutralize the protease activity, and filtered through a sterile 0.22 μm nylon to remove any undigested fragments. The cells in suspension were then separated by sedimentation with use of a 2–4% BSA gradient. The cells were allowed to sediment for a standard period of 2.5 h, and fractions of 2-ml volume were collected. The cells of each fraction were examined under a phase contrast microscope, and fractions containing cells of similar size and morphology were pooled and spun down by low-speed centrifugation. Purity of spermatogonia and sertoli cells were estimated and was routinely higher than 90%. Total RNA was extracted from the isolated cells using TRIzol ® Reagent (Invitrogen, USA), and cleaned with RNeasy minicolumns (Qiagen, USA). RNA content was determined by measurement of optical density at 260 nm. Only the RNA samples showing an OD260/280 ratio higher than 1.8 were used for microarray hybridization.
Microarray Processing and Data Analysis
Microarray experiment
Mouse whole genome expression chips U430 2.0 array (Affymetrix, USA) were used in this study. One hundred nanograms of total RNA from spermatogonia were used for each microarray hybridization experiment. We used a two-cycle Target Labeling protocol to obtain sufficient amounts of labeled cRNA target for analysis with arrays. The first-cycle, first-strand and second-strand cDNA synthesis from spermatogonia total RNA was performed, using the Two-cycle cDNA Synthesis Kit (Affymetrix, USA). The unlabeled ribonucleotide mix obtained served as a template in the subsequent in vitro transcription (IVT) reaction, using the MEGAscript® T7 Kit (Ambion, USA). After cleanup of the unlabeled cRNA using the GeneChip® IVT cRNA Cleanup kit (Affymetrix, USA), this cRNA was reverse transcribed in the first-strand cDNA synthesis step of the second-cycle with random primers, using the Two-cycle cDNA Synthesis Kit (Affymetrix, USA). Subsequently, the T7-Oligo (dT) Promoter Primer was used in the second-strand cDNA synthesis to generate double-stranded cDNA template containing T7 promoter sequences. The resulting double-stranded cDNA was then amplified and labeled using a biotinylated nucleotide analog/ribonucleotide mix in the second IVT reaction (GeneChip IVT Labeling Kit, Affymetrix, USA). The labeled cRNA was cleaned and fragmented using the GeneChip Sample Cleanup Module (Affymetrix, USA), and hybridized to GeneChip expression arrays. Labeled cDNA samples were processed according to the manufacturer’s suggestion (GeneChip® Hybridization, Wash and Stain Kit, Affymetrix, USA). Hybridization was carried out at 45°C for 16 h. Each microarray was then subjected to post hybridization washes at room temperature. The chips were scanned in the Affymetrix 7G GeneChip Scanner (Affymetrix, USA).
Microarray data import, normalization, and analysis
Raw expression values in Affymetrix CEL file format (GEO submission no. pending) were generated by GeneChip Operating Software 1.4 (Affymetrix, Santa Clara, CA) and imported into Partek Genomic Suite 6.5 (Partek, St. Charles, MO) for analysis. Cell intensity files from the Affymetrix GeneChip arrays were normalized by the RMA (robust multiarray average) algorithm (36). Potential batch effects were estimated and adjusted using analysis of variance for subsequent calculations. Global gene expression patterns in VAD samples and controls were first resolved and visualized by Principal Component Analysis (PCA). The RMA-normalized data were applied to determine the significant sources of variability in the data with Partek software. PCA was performed using the correlation method, which adjusts the data mean to zero and the SD to 1. All statistical analyses were performed on log2 transformed data, and one-way ANOVA model analysis was performed to identify differentially expressed genes for each sample type. Within this ANOVA model, linear contrasts were used to compare VAD samples from 18 weeks and 25 weeks to the control. To adjust for multiple testing errors, a false discovery rates (FDR) P value adjustment was used. FDR is set to be less than 5% in the analysis to produce a list of significantly differentially expressed genes for each contrast described above. These lists of statistically significant genes were filtered to include only those genes that demonstrated at least a 1.5 fold difference. Unsupervised hierarchical clustering was performed to determine the relative similarities of VAD samples and control. Clustering was performed on log2 values of these means. Interpoint distances were calculated using the coefficient of shape difference based on Euclidean distance. Distance between clusters was determined from the average distance between all pairs of objects in the two different clusters.
Gene network construction
The integrated gene network analysis on the gene set with significant expression changes were generated by Ingenuity Pathways Analysis (Version 6, Ingenuity® Systems, www.ingenuity.com). Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base. The differential gene list generated was overlaid onto a global molecular network developed from information contained in the Ingenuity Pathways Knowledge Base. Networks of these focus genes were then algorithmically generated based on their connectivity. The Functional Analysis identified the biological functions and/or diseases that were most significant to the data set. Fischer’s exact test was used to calculate a p-value determining the probability that each biological function and/or disease assigned to that data set is due to chance alone. A p-value of less than 0.01 was considered significant
Western Blot Analysis
Western blot analysis was performed as described earlier (37) with minor modifications. Testes were homogenized in Tris-HCl 50mM, pH7.5, DTT 2mM, EDTA 2mM, NP40 1%, SDS1%, Glycerol 20% and protein inhibitor cocktail 1%. The homogenized was centrifuged 5 min at 1000xg. A sample of the supernatant was removed for protein assay. Twenty micrograms of protein were loaded per lane in 12% (w/v) SDS-PAGE. Proteins were transferred to 0.2-μm polyvinylidene fluoride membrane and blocked in blocking solution (PBST plus 5% nonfat dried milk) at room temperature for 1 h. Primary antibody (purified mouse monoclonal Anti-vimentin, diluted 1:10000, BD Biosciences, USA)in blocking solution was added, and the membrane was gently agitated at 4°C overnight. After washing with 1× PBST solution, secondary antibody (goat anti-mouse horseradish peroxidase secondary antibody-conjugate, diluted 1:10 000, Bio-Rad, USA) in blocking solution was added, and the mixture was gently shaken at room temperature for 1 h. Immunoreactivity was detected by SuperSignal West Pico chemiluminescent substrate and film exposure.
Immunohistochemistry
Immunohistochemistry was performed as previously described (38). Briefly, formalin-fixed paraffin-embedded tissue arrays were deparafinized in xylenes and hydrated in a gradual series of ethanol. Antigen retrieval was done by heating the slides in citrate buffer at 100 °C. The slides were probed with anti-vimentin antibody (purified mouse monoclonal Anti-vimentin, diluted 1:100, BD Biosciences, USA) overnight at 4°C. Signal was developed using DAB Histochemistry Kit (Invitrogen). Cells were counter stained with hematoxylin.
Statistical Analysis
Control and VAD means were compared and values are given as means and standard errors of the mean (SEM). The statistical significance of differences was calculated by ANOVA followed by an appropriate post-hoc test (Fisher’s LSD test) using Minitab Statistical Software (Minitab, USA). P values < 0.05 were considered to be statistically significant.
RESULTS
Effect of VAD diet on Vitamin A Status
Vitamin A status was determined by measurement of the RBP mouse serum concentrations. Retinol-binding protein (RBP) is a surrogate biochemical marker for retinol to determine vitamin A status (39). Serum retinol concentration reflects vitamin A status, particularly when the body’s reserves of vitamin A are limited, since it is homeostatically controlled and declines when body stores are significantly compromised (40). Serum RBP occurs in a 1:1:1 M complex with retinol and transthyretin (41). Moreover RBP EIA is as reliable in estimating VAD as HPLC retinol (42).
RBP concentrations are shown Figure 2. We did not observe significant variation in serum RBP concentrations between the controls, animals of 6 to 8 months of age, compared to VAD 8- to 16-weeks groups. However we observed significant decreases in RBP concentration in the VAD treated mice at 18 up to 28 weeks as compared to the Control group: VAD 18 weeks, − 28.34% (P≤0.05); VAD 22 weeks, − 39.53% (P≤ 0.05); VAD 24 weeks, − 45.60% (P≤ 0.01); VAD 26 weeks, − 30.76% (P≤ 0.05) and VAD 28 weeks, − 28.36% (P≤ 0.01). Based on the RBP serum concentrations, a decline in vitamin A status of VAD treated-mice was observable after 18 weeks.
Figure 2. RBP immunoassay performed on serum from animals under Control and VAD diets.
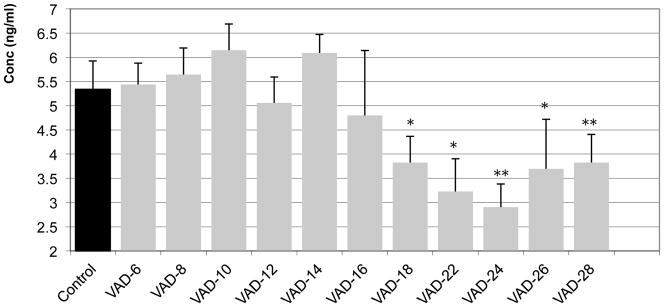
The different groups of VAD diets extend from 6 weeks up to 28 weeks and are compared with the Control Group (6/8 months old) mice. Each column represents the mean of several animals (n=3–12 animals per group).
* Mean value was significantly different in the VAD group compared to the Control group, P ≤ 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control group, P ≤ 0.01 (ANOVA followed by Post-hoc Fisher’s test).
To verify the absence of potential aging effects four groups of animals, aged 60 days, 3 months, 4 months and 6–8 months old, were fed control diet (starting at 60 days old). Serum RBP concentrations in these animals remained unchanged (data not shown).
To further analyze the vitamin A status as a function of duration of the Vitamin-A-Deficient diet, we measured by semi-quantitative RT-PCR the mRNA expression levels of several markers of the retinoid signaling pathway (RBP, RARa, g and RXRa, b, g) in the liver, principal organ of storage of vitamin A (43). Our results (described in Supplemented data 1) document significant decreases in expression of the hepatic retinoid signaling after about 12 weeks of VAD diet.
Effect of VAD diet on adult mouse testis morphology
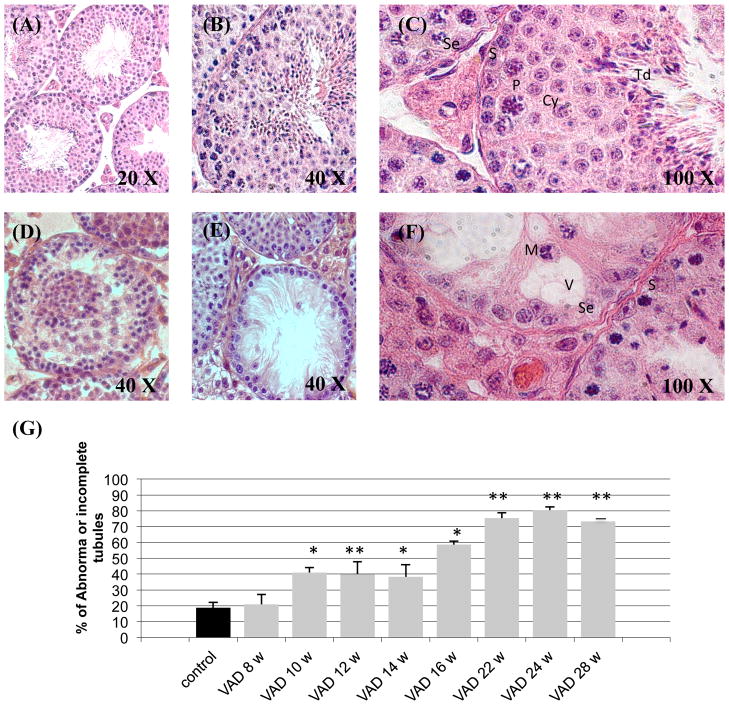
The first aim of our study was to describe, in detail, the temporal effect of Vitamin-A-Deficiency on adult mouse testis. Our histologic examination of testis included Control animals at 6/8 months of age and groups of VAD animals from 8 weeks up to 28 weeks of VAD. Testicular cross sections of the control animals at 6/8 months of age, as described in the literature (44), contained a normal array of germ cells at different developmental stages, namely, mitosis, meiosis, and spermiogenesis (Figure 3(A), (B) and (C)). However, seminiferous tubules from the VAD animals (as early as VAD 10 weeks) exhibited varying degrees of testicular degeneration. Some tubules displayed moderate germ-cell degeneration (Figure 3(D)), while others were more severely affected, containing very few germ cells (Figure 3(E)). In tubules more profoundly affected by the Vitamin-A-Deficiency one could observe sloughing of immature germ cells into the lumen, as well as the presence of large vacuoles and multinucleated bodies (Figure 3(F)). The changes were a function of duration of the VAD diet, as we observed a statistical increase in the number of tubules with a loss of germ cells or evidence of disorganization of their cell-cell interactions (Figure 3(G)). There was a significant increase in the number of abnormal tubules in VAD animals as soon as 10 weeks after the beginning of the diet (VAD 10 w, + 118.43%, P<0.05; VAD 12 w, + 113.11%, P<0.01; VAD 14 w, + 104.21%, P<0.05). This effect of VAD on the morphology of the seminiferous tubules was more severe after 16 weeks of diet (+ 212.57%, P<0.05), and even more dramatic after 22, 24 and 28 weeks of diet (+ 301.01%, + 328.66% and + 290.46%, respectively, P<0.01).
Figure 3. Effect of VAD on mouse testicular morphology as a function of time.
(A) – (F) Photomicrographs of Hematoxylin-stained testicular cross sections from Control (6/8 months old) and VAD animals. The testicular morphology was examined by light microscopy. (A), (B), (C) Sections from a Control (6/8 months old) animal. (D) Represents a section from a VAD 22 weeks mouse; (E) from a VAD 24 weeks mouse and (F) from a VAD 28 weeks mouse. S: Spermatogonia; Se: Sertoli cell; P: Pachytene; Cy: Spermatocyte; Td: Spermatid; V: Vacuole; M: Multinucleated bodies.
(G) For each group testicular cross-sections from several animals (n=3–4) were examined under light microscopy. In each section at least 10 seminiferous tubules were randomly chosen and observed in more details. The number of tubules presenting an abnormal morphology or/and a dramatic loss of germ cell was then calculated as a percentage of the total number of tubules observed for this animal. The observation was realized in such conditions that the observant did not know the group in which each section belongs, until the end of the analysis.
* Mean value was significantly different in the VAD group compared to the Control group, P ≤ 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control group, P ≤ 0.01 (ANOVA followed by Post-hoc Fisher’s test).
Effect of VAD diet on apoptosis in adult mouse testis
Terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay assessed the magnitude of apoptosis during the VAD diet; the results are summarized in Figure 4. Our analysis included Control animals at 6/8 months of age and groups of VAD animals from 8 weeks up to 28 weeks of VAD. Statistically significant increases in apoptotic cells were observed after 10, 12 and 14 weeks of VAD diet +36.26%, +32.26% and + 56.45%, respectively compared to controls. Dramatic increases in the number of tubules containing at least one apoptotic cell occurred after 20 weeks of VAD diet (VAD 20, +127.42%, P<0.01; VAD 22, +135.48%, P<0.01).
Figure 4. Effect of VAD on apoptosis in adult mouse testis, as a function of time.
(A) – (C) Microphotographs of testicular cross sections from Control (6/8 months old) and VAD animals after treatment with the in Situ TUNEL Assay (Roche, USA). The testicular morphology was examined by light microscopy.
The white arrows point in the direction of apoptotic cells.
(A), Section from a Control animal. (B), Section from a VAD 22 weeks mouse. (C), Positive Control staining after DNase treatment of a section from a Control animal.
(D) For each group testicular cross-sections from several animals (n=3–4) were examined under light microscopy. In each section at least 10 seminiferous tubules were randomly chosen and observed in more details. The number of tubules presenting at least one apoptotic cell was then calculated as a percentage of the total number of tubules observed for this animal. The observation was realized in such conditions that the observant did not know the group in which each section belongs, until the end of the analysis.
* Mean value was significantly different in the VAD group compared to the Control group, P ≤ 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control group, P ≤ 0.01 (ANOVA followed by Post-hoc Fisher’s test).
Effect of VAD diet on the expression level of the retinoid signaling pathway in adult mouse testis
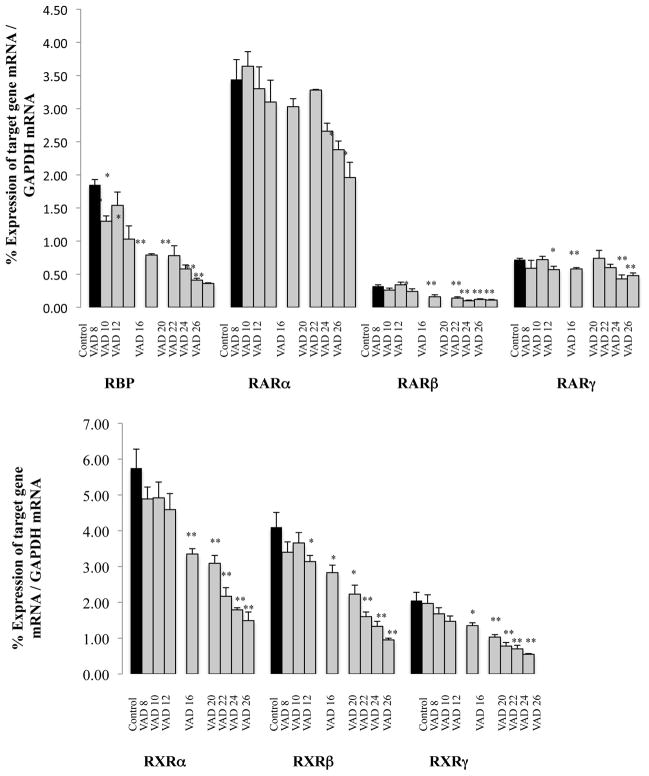
We assessed the retinoid signaling pathway as a function of duration of the Vitamin-A-Deficient diet by measuring the expression levels of several markers of the retinoid signaling pathway (RBP, RARa, b, g and RXRa, b, g) (Figure 5). Immediately after decapitation testes were removed and stored at −80°C. We compared the relative expression of each retinoic acid receptor compared with the other isoforms and noted, as previously described, predominant expression of RARa, RXRa and RXRb (20).
Figure 5. Effect of VAD on the mRNAs expression levels of markers of the retinoids signaling pathway in the testes.
Data bars represent mean ± SEM of triplicate measures performed on 3–5 mice per group.
* Mean value was significantly different in the VAD group compared to the Control group (6/8 months old), P< 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control group (6/8 months old), P< 0.01 (ANOVA followed by Post-hoc Fisher’s test).
The expression level of RBP mRNA in the testis was already significantly decreased after 8 weeks of diet (−30.09%, P<0.01); this change was consistent with the duration of the treatment (VAD 16 weeks, −57.26%, P<0.01; VAD 22 weeks, −68.88%, P<0.01; VAD 26 weeks, −80.60%, P<0.01). Subsequently we examined the expression of the mRNA of RARa, an isoform of the retinoic acid receptor, which seems to have a predominant role in the regulation of spermatogenesis (45, 18). No significant change in expression of this receptor occurred before 24 weeks on VAD diet (VAD 24 weeks, −30.91%; VAD 26 weeks, −43.02%; P<0.05). The testis isoform RARb decreased sooner (VAD 12 weeks, −26.15%, P<0.05; VAD 16, −49.74%, P<0.05; VAD 20, −56.92%, P<0.01; VAD 22, −67.69%, P<0.01; VAD 24, −63.08%, P<0.01 and VAD 26, −66.15%, P<0.01). The isoform RARg demonstrated a lesser decline than RARb: significant but moderate decreases, of mRNA expression in VAD 12, 16 and 22 weeks mice compared with controls (respectively −21.18%, P<0.05; −18.98%, P<0.05 and −15.97%), and more significant decreases for the VAD 24 and 26 weeks animals (−39.82%, P<0.01 and −33.33%, P<0.01). This more dramatic effect of VAD on the expression of RARb compared with the effect on the isoforms a and g is consistent with the published literature. RARb is up regulated in several organs by its own ligand, retinoic acid (46), and contains a RA-responsive element (RARE) in its promoter (47). We also measured VAD consequences on expression of receptors RXRa, b and g. The results of all three isoforms were comparable: the first significant decreases in mRNA expression levels are apparent after 16 weeks of diet, and subsequently as a function of duration a gradual accentuation of these decreases.
To eliminate potential effects of aging we analyzed testes from control animals sacrificed at 60 days, 3 months, 4 months and 6–8 months of age. RT-PCR semi-quantitative analysis did not reveal any age-relative decrease in mRNAs expression (Table 3).
Table 3.
Effect of aging on the expression level of different markers of the retinoids signaling pathway in the testes.
| 60 days old | 3 months old | 4 months old | 6–8 months old | |
|---|---|---|---|---|
| RBP | 1.92 ± 0.12 | 1.94 ± 0.06 | 1.85 ± 0.01 | 1.85 ± 0.05 |
| RARα | 3.80 ± 0.27 | 4.42 ± 0.31 | 4.32 ± 0.62 | 3.45 ± 1.78 |
| RARβ | 0.28 ± 0.01 | 0.34± 0.03 | 0.28 ± 0.04 | 0.32 ± 0.01 |
| RARγ | 0.78 ± 0.10 | 0.59 ± 0.08 | 0.66 ± 0.11 | 0.72 ± 0.07 |
| RXRα | 6.16 ± 0.33 | 6.07 ± 0.44 | 5.72 ± 0.59 | 5.76 ± 0.32 |
| RXRβ | 4.00 ± 0.29 | 4.34 ± 0.38 | 4.55 ± 0.36 | 4.10 ± 0.23 |
| RXRγ | 2.45 ± 0.21 | 2.38 ± 0.07 | 2.19 ± 0.07 | 2.05 ± 0.13 |
Data are expressed as mean ± SEM of measures performed in triplicate from 3–5 animals per group.
Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P ≤ 0.05 (ANOVA followed by Post-hoc Fisher’s test).
Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P ≤ 0.01 (ANOVA followed by Post-hoc Fisher’s test).
Effect of VAD diet on spermatogenesis
To study time-related VAD effects on spermatogenesis, we first examined (Control at 6/8 months of age and VAD 8 to 26 weeks) mRNA expression of specific markers in different ‘normal’ germ cells: specifically Oct4 as a marker for undifferentiated spermatogonia (48), c-kit for differentiated spermatogonia (49), Ccna1 (Cyclin A1) for pachytene spermatocytes (50), Cdc25c (cell division cycle 25 homolog C (S. pombe)) for spermatocytes and round spermatids (51) and Acrv1 (SP10, acrosomal vesicle protein 1) for round spermatids (52). We also used Stra8 (Stimulated by Retinoic Acid Gene 8), both as a marker of spermatogonia and as a known direct target of RA (53).
Immediately after decapitation testes were removed and stored at −80°C. Semi-quantitative RT-PCR demonstrated an increase (+30.29%, though not statistically significant), in expression of Oct4 mRNA in 28-weeks-VAD-diet mice compared to the controls (Figure 6. A), indicative of an increase in undifferentiated spermatogonia. Stra8 mRNA expression (Figure 6. B) was significantly decreased as a consequence of the VAD diet: VAD 12 weeks, −37.23%, P<0.01; VAD 16 weeks, −17.40%, P<0.05; VAD 20 weeks, −34 68%, P<0.05; VAD 22 weeks, −53.86%, P<0.01, VAD 24 weeks, −61.35%, P<0.01 and VAD 26 weeks, −66.59%, P<0.01. We also observed significantly decreased expression of c-kit (Figure 6. C), as a function of length of Vitamin-A-Deficiency (VAD 12, −27.48%, P<0.01; VAD 16, −32.04%, P<0.05; VAD 22, −34.32%, P<0.01; VAD 24, −41.33%, P<0.01 and VAD 26, −43.74%, P<0.01). Our data implicate a decrease in pachytene spermatocytes as highlighted by Cdc25c mRNA expression (Figure 6. D): VAD 12 weeks, −33.89%, P<0.01; VAD 16 weeks, −53.56%, P<0.01; VAD 20 weeks, −41.01%, P<0.01; VAD 22 weeks, −58.73%, P<0.01; VAD 24 weeks, −58.30%, P<0.01 and VAD 26 weeks, −66.61%, P<0.01. Figure 6(E) and (F) identify responses to VAD treatment with dramatic changes in expression of Ccna1 and Acrv1 mRNA: VAD 26 weeks, up to −87.33% compared to controls animals for Ccna1, and decreases up to −74.64% for Acrv1. Cumulatively these results indicate that 20–22 weeks of Vitamin-A-Deficiency induce a decrease in the number of germ cells, and more specifically a decrease in differentiated germ cells (spermatocytes and spermatids). These results are in agreement with previously published data of effects of VAD on spermatogenesis in rats, however, the effects in the mice occur later and are less severe. Indeed studies in rats describe dramatic germ cells loss and tubules containing exclusively sertoli cells and spermatogonia, after 7–12 weeks of Vitamin-A-Deficiency (54, 55).
Figure 6. Effect of VAD on the mRNAs expression levels of markers of different types of germ cells.
(A) Oct4, (B) Stra8, (C) c-kit, (D) Cdc25c, (E) Ccna1 and (F) Acrv1.
Data bars represent mean ± SEM of triplicate measures performed on 3–5 mice per group.
* Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P< 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P< 0.01 (ANOVA followed by Post-hoc Fisher’s test).
To verify the absence of potential aging effects four groups of animals, aged 60 days, 3 months, 4 months and 6–8 months old, were fed control diet (starting at 60 days old) and RT-PCR analysis on the markers described above were performed. No effect of aging was observed (data not shown).
Effect of VAD on spermatogonial transcriptome profiles
Microarray analyses were used to assess the effect of VAD on the transcriptome of pure spermatogonia populations. We contrasted gene profiles in cells from controls (6/8 months old) to mice fed VAD for 18 weeks and 25 weeks. We identified 1900 differentially expressed genes in the 18 week VAD group, and 9987 in the 25 week VAD group (differential expression based on False Discovery Rate at 5% and at least 1.5 fold change: P≤0.00674 and P≤0.0268 respectively). We then carried out a more comprehensive analysis of the control and VAD 25 week spermatogonia. Table 4 identifies potential functional gene clustering and pathways affected by the Vitamin-A-Deficiency.
Table 4.
Gene clusters and pathways primarily affected by the Vitamin-A-Deficiency in spermatogonia
| P Value | |
|---|---|
| 1- Cell cycle – Role of Nek in cell cycle regulation | 8.32 × 10e-7 |
| 2- Reproduction – GnRH signaling | 8.58 × 10e-6 |
| 3- Proteolysis – Role of Parkin in the Ubiquitin-Proteasomal Pathway | 8.72 × 10e-6 |
| 4- Transcription – CREB pathway | 7.31 × 10e-6 |
| 5- Cytoskeleton remodeling – TGF, WNT and cytoskeletal remodeling | 6.25 × 10e-6 |
| 6- Regulation of lipid metabolism – Stimulation of Arachidonic acid production by ACM receptors | 5.32 × 10e-6 |
| 7- Neurodisease – Parkin disorder under Parkinson disease | 5.12 × 10e-6 |
| 8- Cytoskeleton remodelin – Cytoskeleton remodeling | 4.93 × 10e-6 |
| 9- Development – Fit3 signaling | 4.89 × 10e-6 |
| 10- Cell adhesion – Role of tetraspanins in the integrin-mediated cell adhesion | 4.81 × 10e-6 |
| 11- Development – MAG-dependent inhibition of neurite outgrowth | 4.95 × 10e-6 |
| 12- Cytoskeleton remodeling – Reverse signaling by ephrin B | 4.48 × 10e-6 |
Results of microarray analyses performed on spermatogonia population from Control (6/8 months old) and VAD 25 weeks mouse testis.
P values indicate the overall significance of a functional cluster or a canonical pathway, dependent on the number of retinol-regulated genes found in a functional category and the number of the total genes in a functional category in the software package.
Effect of VAD on somatic cells
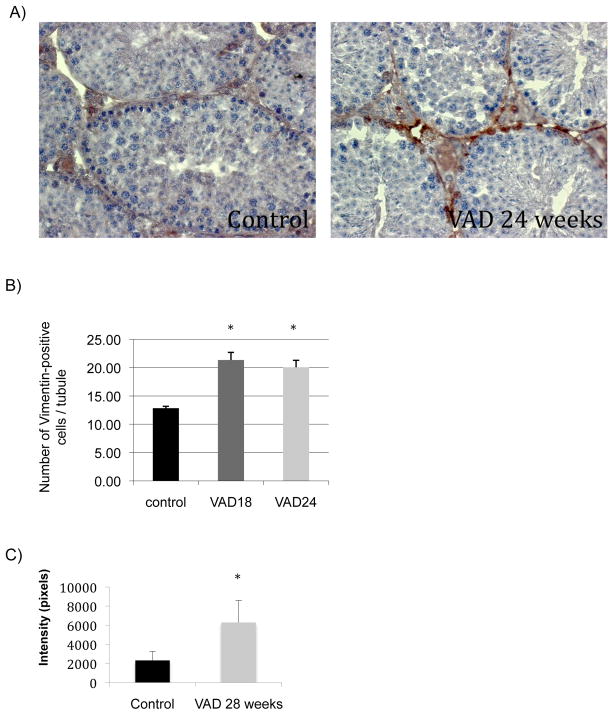
As described earlier we observed several VAD-related alterations in the morphology of the seminiferous tubules. One major difference we observed between Control and VAD groups was an apparent increase of the number of somatic cells, and in particular sertoli cells. To confirm this hypothesis we performed immunohistochemistry staining of Control and VAD mouse testis and western blot analyses using an antibody targeting a known cytoplasmic marker of sertoli cells, vimentin (56). Both techniques pointed out significant increases in expression of vimentin in VAD animals compared to control, supporting our hypothesis of an increase in the number of sertoli cells in these conditions (Figure 7).
Figure 7. Effect of VAD on the expression levels of Vimentin, a marker of Sertoli cells.
(A) Microphotographs of testicular cross sections from Control (6/8 months old) and VAD animals after immunostaining with anti-Vimentin antibody. The sections were examined by light microscopy.
(B) Data bars represent mean ± SEM of results of the immunostaining with anti-Vimentin antibody. For each group testicular cross-sections from several animals (n=5) were examined under light microscopy. In each section at least 10 seminiferous tubules were randomly chosen and observed in more details. The number of Vimentin-positive cells in each tubule was then counted and averaged. The observation was realized in such conditions that the observant did not know the group in which each section belongs, until the end of the analysis.
(C) Data bars represent mean ± SEM of results of the western blot measuring the expression level of Vimentin in Control and VAD 28 week testes.
* Mean value was significantly different in the VAD group compared to the Control group, P< 0.05 (ANOVA followed by Post-hoc Fisher’s test).
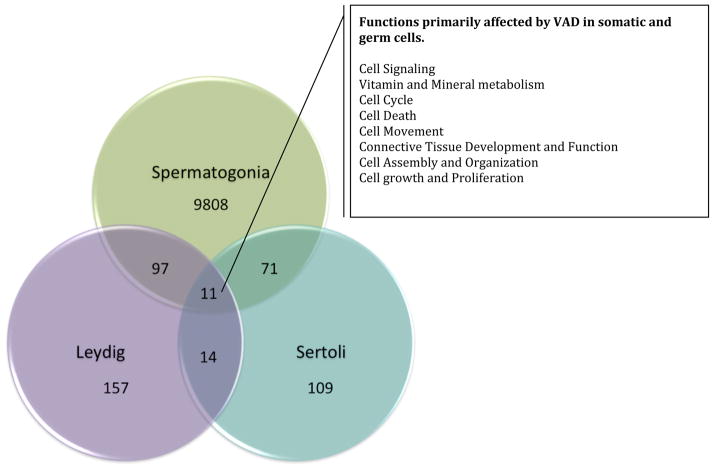
Microarray analyses were also used to assess the effect of VAD on transcriptome profiles in pure sertoli and leydig cells populations. We contrasted gene profiles in cells from controls to mice fed VAD for 28 weeks. We identified 299 differentially expressed genes in the 28 weeks VAD leydig cells group (differential expression based on p<0.05 and at least 1.6 fold change), and 237 genes in the 28 week VAD sertoli cells group (differential expression based on p<0.05 and at least 1.2 fold change)(Table 5). We also identified 11 genes affected in all three population studied (spermatogonia, sertoli and leydig cells), involved in various functions summarized Figure 8.
Table 5.
Gene clusters and pathways primarily affected by the Vitamin-A-Deficiency in Leydig and Sertoli cells
| Gene clusters pathways primarily affected by VAD in Leydig cells | P Value |
|---|---|
| Cellular Growth and Proliferation | 9.92 × 10e-9 |
| Cell Signaling | 9.35 × 10e-5 |
| Cell Cycle | 9.34 × 10e-5 |
| Connective Tissue Development and Function | 9.93 × 10e-3 |
| Cell-To-Cell Signaling and Interaction | 9.52 × 10e-3 |
| Cellular Assembly and Organization | 9.01 × 10e-3 |
| Cell Death | 8.80 × 10e-3 |
| Reproductive System Disease | 8.80 × 10e-3 |
| Reproductive System Development and Function | 8.80 × 10e-3 |
| Gene clusters and pathways primarily affected by VAD in Sertoli cells | P Value |
|---|---|
| Cellular Movement | 6.89 × 10e-11 |
| Reproductive System Disease | 9.08 × 10e-7 |
| Cell Death | 6.17 × 10e-7 |
| Connective Tissue Development and Function | 6.22 × 10e-6 |
| Cellular Growth and Proliferation | 8.13 × 10e-5 |
| Cell Cycle | 7.41 × 10e-5 |
| Cellular Assembly and Organization | 7.56 × 10e-4 |
| Endocrine System Disorders | 7.49 × 10e-4 |
| Cell-To-Cell Signaling and Interaction | 6.67 × 10e-4 |
Results of microarray analyses performed on somatic cell population from Control (6/8 months old) and VAD 28 weeks mouse testis.
P values indicate the overall significance of a functional cluster or a canonical pathway, dependent on the number of retinol-regulated genes found in a functional category and the number of the total genes in a functional category in the software package.
Figure 8.
Venn diagram representing the number of genes affected by VAD in germ cells and somatic cells.
DISCUSSION
In this study, we present a global description of the effect of Vitamin-A-Deficiency on adult spermatogenesis, not only on germ cells but also on the somatic leydig and sertolicells. The initial phase of our investigation defines the temporal sequence and consequences of VAD in mouse testes. We used young adult Balb/C mice at 60 days of age and induced Vitamin-A-Deficiency for a period of 6 to 28 weeks. We identified the onset of the Vitamin-A-Deficiency by measurement of Vitamin-A status, and delineated the earliest measurable effects on the retinoid signaling pathway after 12 weeks of treatment (depressed expression of retinoic acid receptors and retinol binding protein) in liver and testes. Finally, microarray analyses defined effects of VAD on gene expression in germ cells, but also in leydig and sertoli cells, highlighting the importance of somatic cells in the support and maintenance of spermatogenesis.
The data highlight the effect of VAD on germ cell proliferation, differentiation and survival. A significant decrease in differentiated germ cells in Vitamin-A-Deficient animals correlated with increased cellular apoptosis. These results are in concordance with previously published data (57, 26) reporting a VAD-induced “sertoli cell and spermatogonia only” state. However, the VAD-related effect observed in mice in our study is less dramatic than that described in the literature. Indeed, even after 28 weeks of a VAD diet, the mouse testes still have some seminiferous tubules with apparent normal histology. Moreover our RT-PCR results confirmed the presence of spermatocytes and spermatids in these animals, though their number is dramatically reduced. These discrepancies lie first of all in the animal model chosen due to species’ differences in sensitivity to retinoids (as has long been described (58, 59)); and secondly, in various mouse studies, the experimental approach on male mouse offspring used breeding pairs already on a VAD diet, therefore insuring depleted hepatic storage of retinoic acid prior to initiation of the experiment (60, 61). We chose not to follow these protocols so as to negate VAD effects on the development of the testis and, therefore, to isolate and identify the effects on adult spermatogenesis alone.
With our experimental model, we used microarray technology to document the effects of Vitamin-A-Deficiency on gene expression in undifferentiated germ cells (spermatogonia) and somatic leydig and sertoli cells.
We first investigated VAD-induced transcriptome changes that occur in mouse spermatogonia after 18 weeks and 25 weeks of Vitamin-A-Deficiency. The bioinformatic analyses we used identified clusters of genes and/or signaling pathways known to play a role in the regulation of spermatogenesis that might be affected by VAD. Our initial analyses focused on the retinoid signaling pathways and verify its hypoexpression in spermatogonia of the Vitamin-A-Deficient mice. These results confirmed our previous data concerning both the VAD status of these animals and the hypoexpression of the retinoid receptors in the testes. More detailed analyses of the expression profiles of VAD 25 week spermatogonia reveal other pathways that are affected by the Vitamin-A-Deficiency. Consistent with the literature, our results document an effect of VAD expression profiles of gene networks involved in development, proteolysis and lipid metabolism (62). Several publications suggested a role of retinoic acid in the regulation of induction of meiosis during spermatogenesis (63, 64), Wang and Kim, in 1993 (65), demonstrated arrest at the end of S phase of the cell cycle of Vitamin-A-Deficient testes’ germ cells. A more recent study on the temporal profiling of rat transcriptomes in retinol-replenished Vitamin-A-Deficient testes also indicated that retinol may regulate genes involved in cell cycle and associated signaling pathways (62). Our data confirmed these reports and highlight, in particular, the effect of VAD on the Nek (NIMA-related kinase) pathway involved in regulation of mitotic progression (66). Therefore, interruption of germ cell differentiation and arrest of spermatogenesis occurs not only because of RA regulation of induction of meiosis, but also due to an effect on regulation of mitosis.
Multiple studies describe interactions between retinoid and thyroid hormone pathways, in particular interaction with the RXR receptor (67, 68). Depressed expression of thyroid hormone nuclear receptor (TR) has also been reported in the brains of Vitamin-A-Deficient rats (69). The potential effect of thyroid hormone on male reproduction has remained controversial because of the low number of thyroid hormone-binding sites found in the adult organ (70) and the lack of clinical data correlating male sexual function with thyroid disorders. Recent studies, however, have highlighted a relation between neonatal triiodothyronine levels (T3, the active form), sertoli and leydig cells proliferation/maturation and, therefore, testicular development (71, 72). T3 inhibits sertoli cells’ proliferation and stimulates their functional maturation in pre-pubertal rat testes (73, 74). Experimental data indicate expression of TR isoforms in germ cells, not only during neonatal development, but also in adult testes (75). Regulation of sertoli cell number affects the number of spermatogonia and progression of spermatogenesis (76). In our study, we observed a VAD-related 20% decrease in T3 serum concentration (although not statistically significant) (Supplemented data 2). We evaluated the expression profile of the thyroid hormone pathway in our microarray data. In agreement with published literature, we observed deregulation of this signaling pathway in spermatogonia of VAD mice after 18 and 25 weeks of deficient diet. As mentioned above, this hypothyroid status of the VAD mice could therefore lead to abnormal regulation of somatic cell number and maturation stages (71, 72), and our results indicate a VAD-related increased expression of a sertoli cell marker, vimentin, suggesting a deregulation of the number of these cells.
Finally, our results highlight the importance of vitamin-A to cytoskeleton remodeling and cell adhesion. VAD induces disorganization of seminiferous tubules, as shown in our histological data in VAD animals, microarray data from isolated spermatogonia (Table 4), and a dramatic decrease in the expression of connexin-43, a protein involved in the formation of GAP junctions (77)(−57.5% decrease in the mRNA level of VAD 26 weeks testes compared to Control, and −32% decrease in protein level expression between the same groups of animals, data not shown). These data are also in agreement with results obtained in rats by Doyle’s team (62), and the importance of RA regulation of spermatogonial differentiation via both somatic and germ cell compartments of the seminiferous epithelium was recently highlighted (78). A recent study documented disruption of the sertoli cell barrier in RARa−/− mutant mouse testes (79). In total, these results suggest Vitamin-A-Deficiency induces disruption of sertoli/germ cell interfaces leading to inability of the sertoli cells to further sustain the progression of spermatogenesis. Most events of spermatogenesis take place in the environment behind the blood-testis-barrier (BTB), which is created between adjacent sertoli cells near the basement membrane of the seminiferous tubule. The BTB, thus, segregates the seminiferous epithelium into the basal and apical compartment, with spermatogonial renewal taking place in the basal compartment (80). Junctions formed between sertoli cells, and between sertoli and germ cells, continuously undergo restructuring to accommodate the translocation and differentiation of germ cells during spermatogenesis (80, 81). These cell junctions are crucial for coordinating different events of spermatogenesis by sending signals back-and-forth between sertoli and germ cells to regulate spermatogonial cell renewal by mitosis, cell cycle progression, meiosis, spermiogenesis, germ cell movement across the epithelium, spermiation and germ cell apoptosis (82). To explore these factors we performed bioinformatic analyses in the somatic cells to identify VAD-induced transcriptome changes of genes and/or signaling pathways known to play a role in the regulation of spermatogenesis. In sertoli and leydig cells we found the most affected pathways were signaling pathways involved in cellular movement, connective tissue development and function, cellular growth and proliferation, cell death, cell development and reproductive system disease. These results are consistent with an effect of VAD on the integrity of the cell junctions between sertoli cells and germ cells, and the inability of the sertoli and leydig cells to maintain spermatogenesis.
In conclusion we observed the following effects of VAD on adult mouse spermatogenesis: arrest of germ cell differentiation, increased germ cell apoptosis, a general effect on the transcriptome profile of spermatogonia, and a disruption of somatic cell organization and interfaces with germ cells. While the effects on germ cells were sometimes dramatic, our data highlight the importance of VAD-induced effects in somatic cells, and their indirect effects on the progression of spermatogenesis. We suggest Vitamin-A-Deficiency induces deregulation of somatic cell numbers, and a disruption of cell-junctions. As a consequence, sertoli and leydig cells would become unable to regulate and sustain the proliferation and differentiation of the germ cells, and, therefore, contribute to the VAD-induced arrest of spermatogenesis.
Supplementary Material
(A) RBP4, (B) RARa, (C) RARg, (D) RXRa, (E) RXRb and (F) RXRg.
Data bars represent mean ± SEM of triplicate measures performed on 3–5 mice per group.
* Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P< 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P< 0.01 (ANOVA followed by Post-hoc Fisher’s test).
Data bars represent mean ± SEM of triplicate measures performed on 14–15 mice per group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. MDIS working paper #2, updated. WHO; Geneva, Switzerland: 2003. Global prevalence of vitamin A deficiency. [Google Scholar]
- 2.Sethuraman U. Vitamins. Pediat Rev. 2006;27:44–55. doi: 10.1542/pir.27-2-44. [DOI] [PubMed] [Google Scholar]
- 3.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Retinoids in embryonal development. Physiol Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 4.Sporn MB, Roberts AB, Goodman DS. The retinoids: Biology, Chemistry, and Medicine. 2. Raven Press; New York: 1994. [Google Scholar]
- 5.Tang XH, Gudas LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;28(6):345–364. doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- 6.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66(7):606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 7.Guas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226(2):322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amann PM, Eichmüller SB, Schmidt J, Bazhin AV. Regulation of gene expression by retinoids. Curr Med Chem. 2011;18(9):1405–1412. doi: 10.2174/092986711795029618. [DOI] [PubMed] [Google Scholar]
- 9.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 10.Kastner P, Mark M, Ghyselinck N, Krezel W, Dupé V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 11.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 12.Lie PPY, Cheng CY, Mruk DD. Coordinating cellular events during spermatogenesis: a biochemical model. Trends Biochem Sci. 2009;34(7):366–373. doi: 10.1016/j.tibs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker WH. Non-classical actions of testosterone and spermatogenesis. Phil Trans R Soc B. 2010;365:1557–1569. doi: 10.1098/rstb.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng CY, Wong EWP, Yan HHN, Mruk D. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol Cell Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddy EM. Male germ cell gene expression. Recent Prog Horm Res. 2002;57:103–128. doi: 10.1210/rp.57.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Johnson L, Thompson DL, Varner DD. Role of Sertoli cells number and function on regulation of spermatogenesis. Anim Reprod Sci. 2008;105:23–51. doi: 10.1016/j.anireprosci.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30(2):119–132. doi: 10.1210/er.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SS, Wolgemuth DJ. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet Genome Res. 2004;105(2–4):189–202. doi: 10.1159/000078189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell JM, Thompson JN, Pitt GA. Histology of the lesions produced in the reproductive tract of animals fed a diet deficient in vitamin A alcohol but containing vitamin A acid. I. The male rat. J Reprod Fertil. 1963;5:1590167. doi: 10.1530/jrf.0.0050159. [DOI] [PubMed] [Google Scholar]
- 20.Livera G, Rouiller-Fabre V, Pairault C, Levacher C, Habert R. Regulation and perturbation of testicular functions by vitamin A. Reproduction. 2002;124(2):173–180. [PubMed] [Google Scholar]
- 21.Sun H, Kawaguchi R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int Rev Cell Mol Biol. 2011;288:1–41. doi: 10.1016/B978-0-12-386041-5.00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unni E, Rao MR, Ganguly J. Histological and ultrastructural studies on the effect of vitamin A depletion and subsequent repletion with vitamin A on germ cells and Sertoli cells in rat testis. Indian J Exp Biol. 1983;21(4):180–192. [PubMed] [Google Scholar]
- 23.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120(4):956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Palczewski K, Baehr W, Clagett-Dame M. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol Reprod. 2011;84:336–341. doi: 10.1095/biolreprod.110.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Pelt AM, de Rooij DG. Retinoic acid is able to reinitiate spermatogenesis in vitamin A-deficient rats and high replicate doses support the full development of spermatogenic cells. Endocrinology. 1991;128(2):697–704. doi: 10.1210/endo-128-2-697. [DOI] [PubMed] [Google Scholar]
- 26.Wolgemuth DJ, Chung SS. Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor alpha. Soc Reprod Fertil Suppl. 2007;63:11–23. [PMC free article] [PubMed] [Google Scholar]
- 27.Chung SS, Wang X, Wolgemuth DJ. Expression of retinoic acid receptor alpha in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development. 2009;136(12):2091–2100. doi: 10.1242/dev.020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Husson M, Enderlin V, Alfos S, Féart C, Higueret P, Pallet V. Triiodothyronine administration reverses vitamin A deficiency-related hypo-expression of retinoic acid and triidothyronine nuclear receptors and of neurogranin in rat brain. Br J Nutr. 2003;90(1):191–198. doi: 10.1079/bjn2003877. [DOI] [PubMed] [Google Scholar]
- 29.Sabath DE, Broome HE, Prystowsky MB. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91(2):185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 30.Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, Viville S, Mark M. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235(6):1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- 31.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine alpha and beta retinoid acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989;339:714–717. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- 32.Féart C, Pallet V, Boucheron C, Higueret D, Alfos S, Letenneur L, Dartigues JF, Higueret P. Aging affects the retinoic acid and the triiodothyronine nuclear receptor mRNA expression in human peripheral blood mononuclear cells. Eur J Endocrinol. 2005;152(3):449–458. doi: 10.1530/eje.1.01858. [DOI] [PubMed] [Google Scholar]
- 33.Nishizawa H, Manabe N, Morita M, Sugimoto M, Imanishi S, Miyamoto H. Effects of in utero exposure to bisphenol A on expression of RARalpha and RXRalpha mRNAs in murine embryos. J Reprod Dev. 2003;49(6):539–545. doi: 10.1262/jrd.49.539. [DOI] [PubMed] [Google Scholar]
- 34.Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 35.Dym M, Jia MC, Dirami G, Price JM, Rabin SJ, Mocchetti I, Ravindranath N. Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod. 1995;52(1):8–19. doi: 10.1095/biolreprod52.1.8. [DOI] [PubMed] [Google Scholar]
- 36.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, et al. Exploration, normalization, and summaries of high density oligonucleotides array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 37.Pang AL, Peacock S, Johnson W, Bear DH, Rennert OM, Chan WY. Cloning, characterization, and expression analysis of the novel acetyltransferase retrogene Ard1b in the mouse. Biol Reprod. 2009;81(2):302–309. doi: 10.1095/biolreprod.108.073221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Tabatabai ZL, Lee TL, Hatakeyama S, Ohyama C, Chan WY, et al. The Y-encoded TSPY protein: a significant marker potentially plays a role in the pathogenesis of testicular germ cell tumors. Hum Pathol. 2007;38:1470–1481. doi: 10.1016/j.humpath.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gamble MV, Ramakrishnan R, Palafox NA, Briand K, Berglund l, Blaner WS. Retinol binding protein as a surrogate measure for serum retinol: studies in vitamin A-deficient children from the Republic of the Marshall Islands. Am J Clin Nutr. 2001;73:594–601. doi: 10.1093/ajcn/73.3.594. [DOI] [PubMed] [Google Scholar]
- 40.De Pee S, Dary O. Biochemical indicators of Vitamin A Deficiency: serum retinol and serum Retinol Binding Protein. J Nutr; Proceedings of the XX International Vitamin A Consultative Group Meeting; 2002. pp. 2895S–2901S. [DOI] [PubMed] [Google Scholar]
- 41.Soprano DR, Blaner WS. Plasma retinol-binding protein. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. 2. Raven Press; New York, NY: 1994. pp. 257–281. [Google Scholar]
- 42.Hix J, Martinez C, Buchanan I, Morgan J, Milton T, Shankar A. Development of a rapid enzyme immunoassay for the detection of retinol-binding protein. Am J Clin Nutr. 2004;79:93–98. doi: 10.1093/ajcn/79.1.93. [DOI] [PubMed] [Google Scholar]
- 43.Senoo H, Kojima N, Sato M. Vitamin-A-storing cells (stellate cells) Vitam Horm. 2007;75:131–159. doi: 10.1016/S0083-6729(06)75006-3. [DOI] [PubMed] [Google Scholar]
- 44.Doyle TJ, Braun KW, McLean DJ, Wright RW, Griswold MD, Kim KH. Potential functions of retinoic acid receptor A in Sertoli cells and germ cells during spermatogenesis. Ann NY Acad Sci. 2007;1120:114–130. doi: 10.1196/annals.1411.008. [DOI] [PubMed] [Google Scholar]
- 45.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA. 1993;90(15):7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato S, Mano H, Kumazawa T, Yoshizawa Y, Kojima R, Masushige S. Effect of retinoid status on a, b and g retinoic acid receptor mRNA levels in various rat tissues. Biochem J. 1992;286:755–760. doi: 10.1042/bj2860755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann B, Lehmann JM, Zhang X, Hermann T, Husmann M, Graupner G, Pfahl M. A retinoic acid receptor-specific element controls the retinoic acid receptor-b promoter. Mol Endo. 1990;4:1727–1736. doi: 10.1210/mend-4-11-1727. [DOI] [PubMed] [Google Scholar]
- 48.Dym M, He Z, Jiang J, Pant D, Kokkinaki M. Spermatogonial stem cells: unlimited potential. Reprod Fertil Dev. 2009;21(1):15–21. doi: 10.1071/rd08221. [DOI] [PubMed] [Google Scholar]
- 49.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;120(12):5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 50.Ravnik SE, Wolgemuth DJ. Regulation of meiosis during mammalian spermatogenesis: the Atype cyclins and their associated cyclin-dependent kinases are differentially expressed in the germ-cell lineage. Dev Biol. 1999;207(2):408–418. doi: 10.1006/dbio.1998.9156. [DOI] [PubMed] [Google Scholar]
- 51.Wu S, Wolgemuth DJ. The distinct and developmentally regulated patterns of expression of members of the mouse Cdc25 gene family suggest differential functions during gametogenesis. Dev Biol. 1995;170(1):195–206. doi: 10.1006/dbio.1995.1207. [DOI] [PubMed] [Google Scholar]
- 52.Reddi PP, Shore AN, Acharya KK, Herr JC. Transcriptional regulation of spermiogenesis: insights from the study of the gene encoding the acrosomal protein SP-10. J Reprod Immunol. 2002;53(1–2):25–36. doi: 10.1016/s0165-0378(01)00104-8. [DOI] [PubMed] [Google Scholar]
- 53.Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulated sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103(8):2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ismail N, Morales CR, Clermont Y. Role of spermatogonia in the stage-synchronization of the seminiferous epithelium in vitamin-A-deficient rats. Am J Anat. 1990;188:57–63. doi: 10.1002/aja.1001880107. [DOI] [PubMed] [Google Scholar]
- 55.Ismail N, Morales CR. Effects of vitamin A deficiency on the inter-Sertoli cell tight junctions and on the germ cell population. Microsc Res Tech. 1992;20:43–49. doi: 10.1002/jemt.1070200106. [DOI] [PubMed] [Google Scholar]
- 56.Weider K, Bergmann M, Giese S, Guillou F, Failing K, Brehm R. Altered differentiation and clustering of Sertoli cells in transgenic mice showing a Sertoli cell specific knockout of the connexin 43 gene. Differentiation. 2011;82(1):38–49. doi: 10.1016/j.diff.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Morales C, Griswold MD. Retinol-induced stage synchronization in seminiferous tubules of the rat. Endocrinology. 1987;121(1):432–434. doi: 10.1210/endo-121-1-432. [DOI] [PubMed] [Google Scholar]
- 58.Krinsky NI, Mathews-Roth MM, Welankiwar S, Sehgal PK, Lausen NCG, Russel RM. The metabolism of [14C] b-carotene and the presence of other carotenoids in rats and monkeys. J Nutr. 1990;120:81–87. doi: 10.1093/jn/120.1.81. [DOI] [PubMed] [Google Scholar]
- 59.Kishida T, Muto S, Hayashi M, Tsutsui M, Tanaka S, Murakami M, Kuroda J. Strain differences in hepatic cytochrome P450 1A and 3A expression between Sprague-Dawley and Wistar rats. J Toxicol Sci. 2008;33(4):447–457. doi: 10.2131/jts.33.447. [DOI] [PubMed] [Google Scholar]
- 60.McLean DJ, Russel LD, Griswold MD. Biological activity and enrichment of spermatogonial stem cells in Vitamin-A-Deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biol Reprod. 2002;66:1374–1379. doi: 10.1095/biolreprod66.5.1374. [DOI] [PubMed] [Google Scholar]
- 61.Gaemers IC, Sonneveld E, van Pelt AMM, Schrans BH, Themmen AP, van der Saag PT, de Rooij DG. The effect of 9-cis-Retinoic Acid on proliferation and differentiation of A spermatogonia and retinoid receptor gene expression in the Vitamin-A-Deficient mouse testis. Endocrinology. 1998;139:4269–4276. doi: 10.1210/endo.139.10.6272. [DOI] [PubMed] [Google Scholar]
- 62.Doyle TJ, Oudes AJ, Kim KH. Temporal profiling of rat transcriptomes in retinol-replenished vitamin A-Deficient testis. Syst Biol Reprod Med. 2009;55:145–163. doi: 10.3109/19396360902896844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134(19):3401–3411. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- 64.Pellegrini M, Filipponi D, Gori M, Barrios F, Lolicato F, et al. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle. 2008;7(24):3878–3888. doi: 10.4161/cc.7.24.7262. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Kim KH. Vitamin A-Deficient testis germ cells are arrested at the end of S phase of the cell cycle: A molecular study of the origin of synchronous spermatogenesis in regenerated seminiferous tubules. Biol Reprod. 1993;48:1157–1165. doi: 10.1095/biolreprod48.5.1157. [DOI] [PubMed] [Google Scholar]
- 66.O’regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div. 2007;2:25–37. doi: 10.1186/1747-1028-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satyanarayana M, Sarvesh A, Khadeer MA, Ved HS, Soprano DR, et al. Regulation of neuronal thyroid hormone receptor alpha 1 mRNA by hydrocortisone, thyroid hormone and retinoic acid. Dev Neurosci. 1994;16(5–6):255–259. doi: 10.1159/000112117. [DOI] [PubMed] [Google Scholar]
- 68.Jones KE, Yaffe BM, Chin WW. Regulation of thyroid hormone beta-2 mRNA levels by retinoic acid. Mol Cell Endocrinol. 1993;91(1–2):113–118. doi: 10.1016/0303-7207(93)90262-i. [DOI] [PubMed] [Google Scholar]
- 69.Husson M, Enderlin V, Alfos S, Boucheron C, Pallet V, et al. Expression of neurogranin and neuromodulin is affected in the striatum of vitamin A-deprived rats. Brain Res Mol Brain Res. 2004;123(1–2):7–17. doi: 10.1016/j.molbrainres.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 70.Oppenheimer JH, Schwartz HL, Surks MI. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- 71.Rijntjes E, Wientjes AT, Swarts HJM, de Rooij DG, Teerds KJ. Dietary-induced hyperthyroidism marginally affects neonatal testicular development. J Androl. 2008;29:643–653. doi: 10.2164/jandrol.108.005108. [DOI] [PubMed] [Google Scholar]
- 72.Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199:351–365. doi: 10.1677/JOE-08-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cooke PS, Zhao YD, Bunick D. Triiodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment. Biol Reprod. 1994;51:1000–1005. doi: 10.1095/biolreprod51.5.1000. [DOI] [PubMed] [Google Scholar]
- 74.Holsberger DR, Jirawatnotai S, Kiyokawa H, Cooke PS. Thyroid hormone regulates the cell cycle inhibitor p27Kip1 in postnatal murine Sertoli cells. Endocrinology. 2003;144(9):3732–3728. doi: 10.1210/en.2003-0389. [DOI] [PubMed] [Google Scholar]
- 75.Rao JN, Liang JY, Chakraborti P, Feng P. Effect of thyroid hormone on the development and gene expression of hormone receptors in rat testes in vivo. J Endocrinol Invest. 2003;26:435–443. doi: 10.1007/BF03345199. [DOI] [PubMed] [Google Scholar]
- 76.Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- 77.Weider K, Bergmann M, Brehm R. Connexin 43: its regulatory role in testicular junction dynamics and spermatogenesis. Histol Histopathol. 2011;26:1343–1352. doi: 10.14670/HH-26.1343. [DOI] [PubMed] [Google Scholar]
- 78.Gely-Pernot A, Raverdeau M, Célébi C, Dennefeld C, Feret B, Klopfenstein M, et al. Spermatogonia differentiation requires retinoic acid receptor g. Endocrinology. 2012;153 doi: 10.1210/en.2011-1102. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Chung SSW, Choi C, Wang X, Hallock L, Wolgemuth DJ. Aberrant distribution of junctional complex components in retinoic receptor alpha-deficient mice. Microsc Res Tech. 2010;73:583–596. doi: 10.1002/jemt.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev. 2009;20(4):329–338. doi: 10.1016/j.cytogfr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yan HH, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: the biology, regulation, and implication in male contraceptive development. Curr Top Dev Bio. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 82.Cheng CY, Wong EW, Yan HH, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol Cell Endocrinol. 2010;315(1–2):49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) RBP4, (B) RARa, (C) RARg, (D) RXRa, (E) RXRb and (F) RXRg.
Data bars represent mean ± SEM of triplicate measures performed on 3–5 mice per group.
* Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P< 0.05 (ANOVA followed by Post-hoc Fisher’s test).
* * Mean value was significantly different in the VAD group compared to the Control (6/8 months old) group, P< 0.01 (ANOVA followed by Post-hoc Fisher’s test).
Data bars represent mean ± SEM of triplicate measures performed on 14–15 mice per group.