Abstract
Introduction
Both the state of pregnancy as well as disruption of vaginal flora and immune mediators may increase the risk of human immunodeficiency virus (HIV)-1 acquisition.. The objective of this study was to define immune changes in lower genital and systemic immunity associated with normal pregnancy.
Methods
Prospective cohort enrolled low risk pregnant and non-pregnant women ages 18 to 35. Pregnant women at < 14 weeks and non-pregnant women in follicular phase of the menstrual cycle were included. Cervical and vaginal fluid was collected. Concentrations of immune mediators were measured using ELISA-based methods or multiplex immunoassay. Samples were inoculated onto various culture media allowing for growth of Lactobacillus spp, G. vaginalis, E.coli, Enterococcus spp, anaerobic gramnegative rods, Candida, S. aureus, Ureaplasma spp, and Mycoplasma hominis. Concentrations of immune mediators and vaginal colonization frequencies were compared between the pregnant and non-pregnant groups.
Results
Genital tract concentration of IL-1β was higher during pregnancy compared to non-pregnant participants. Serum CRP concentrations were higher in all trimesters of pregnancy. Concentrations of secretory leukocyte protease inhibitor didn’t differ between groups. Lactobacillus was more commonly isolated from vaginal cultures of non-pregnant participants (100% vs. 70.2%, p=0.02). Identification of Candida, G. vaginalis, M. hominis and S. aureus was common and not different between groups. Ureaplasma spp was isolated from over 60% pregnant participants.
Conclusions
The pro-inflammatory cytokine, IL-1β, as well as the systemic marker of inflammation, CRP, are increased during pregnancy. The impact of these pro-inflammatory changes during pregnancy deserves further study.
Keywords: Pregnancy, genital flora, vaginal immunity, inflammation, cytokines
INTRODUCTION
Women account for half of all people living with HIV-1 globally1. The vast majority of incident HIV worldwide is caused by heterosexual intercourse, and the female lower genital tract is the primary site of acquisition2. As a result of infection in women, there are now nearly 2 million children living with HIV, the vast majority of these perinatally infected3. A large, longitudinal study following over 10,000 women in Rakai, Uganda found that women were at significantly increased risk of HIV acquisition during pregnancy compared to non-pregnant women4. The biological reasons for the increased risk of HIV acquisition during pregnancy have not been elucidated. It has been suggested that mucosal immunity in the genital tract is compromised during pregnancy. Wira et al have suggested that this risk may be related to hormonal changes as they reported ovulation to be a time of vulnerability5. Concentrations or expression of certain antimicrobial peptides, cytokines, and chemokines have been shown to be altered under certain conditions in pregnancy, such as bacterial vaginosis6, trichomoniasis7, or premature rupture of membranes8.
The immune function of the female lower genital tract is a complex interplay of host factors that serve to protect against infection and disruption of the normal flora. The lower tract requires cytokines, chemokines, antimicrobial peptides, and genital flora to recognize threats and react to maintain homeostasis. Disruption in several individual components of lower genital tract immunity has been associated with HIV acquisition. Unfortunately these studies lack a consensus on what concentration is considered abnormal and which mediators should be measured. There is also a paucity of data attempting to characterize the cytokine environment in pregnant women without genital tract infections. One study comparing pregnant and non-pregnant women with symptomatic bacterial vaginosis (BV) reported higher levels of Interleukin (IL)-1β, IL-6, and IL-8 among pregnant women compared to non-pregnant women9. There is relatively little known regarding the normal state of lower genital tract immunity in pregnant women at different trimesters of pregnancy.
Seen as a major component of local vaginal immunity, normal vaginal flora is thought to impede the bacterial overgrowth of virulent exogenous bacteria. Changes in the normal vaginal flora can cause detrimental effects to a normal pregnancy. For example, BV, characterized by the shift in the microflora from one characterized by a predominance of Lactobacillus to a complex microflora with a predominance of G. vaginalis and obligately anaerobic bacteria, has been linked to an increased risk of HIV acquisition and transmission10. Our objective was to systematically characterize the local and systemic immune milieu of normal pregnancy and the normal microbial flora in pregnancy compared to the non-pregnant state.
METHODS
Study population
This prospective cohort study recruited pregnant and non-pregnant women at the various clinics serving Women & Infants Hospital in Providence, RI from 2007 to 2010. Inclusion criteria included: 1) pregnancy documented by urine HCG, serum HCG, or ultrasound with low risk pregnancy status and a gestational age less than 14 weeks’, 2) healthy non-pregnant women age 18–35 years, not planning pregnancy within the next year, 3) willingness to avoid the use of intravaginal products and willingness to use condoms during sexual intercourse 48 hours prior to each exam. Exclusion criteria included acute systemic illness, chronic illness (hypertension, pre-existing diabetes mellitus, autoimmune disease, history of thromboembolic disease), use of systemic steroids in past three months, immunization in past one month, active alcohol, drug, or tobacco use, immunocompromised state, known active infection, symptomatic vaginal discharge, current urinary tract infection, antibiotic use within one month of enrollment, prior preterm birth (<36 weeks’), current or planned cerclage, history of pre-eclampsia prior to 36 weeks’ gestation, and multiple gestation. The Women & Infants Hospital Institutional Review Board approved this study. Written informed consent was obtained from each participant before enrollment. Women considered low risk after their first prenatal appointments were recruited by study staff to participate. Non-pregnant women were recruited from physician offices as well as through advertisements on local college campuses.
Study visit procedures
A total of four study visits were planned, one enrollment visit and three follow-up visits. In pregnant patients, visits occurred at less than 14 weeks’, 14 to 28 weeks’, after 28 weeks’ gestation, and again at the postpartum visit, approximately 4–6 weeks after delivery. In non-pregnant patients, study appointments were scheduled at approximately 12 week intervals in order to mimic the time duration between visits for the pregnant participants, excluding menses. In all study participants, each visit was standardized to include a brief questionnaire about recent exposures such as use of antibiotics or unprotected intercourse. A pelvic examination with collection of cervical and vaginal swabs, wet mount, vaginal pH, and a single blood sample were then conducted.
A Dacron swab was used for collection of cervical and vaginal fluid as previously described9, 11–14. All cervical swabs were placed in 100 µL phosphate buffered saline (PBS) and stored at −70°C until the assays were performed. Concentrations of interleukin-1β (IL-1), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), Interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), Granulocyte macrophage colony-stimulating factor (GM-CSF) and Macrophage inflammatory protein-1α (MIP-1α) were measured using the Luminex® multiplex bead assay via a previously validated method15. Serum concentrations of these mediators were consistently too low to measure and were discontinued during the course of the study.
Vaginal samples were used for the measurement of the antimicrobial peptide secretory leukocyte protease inhibitor (SLPI). Specimens were collected from the posterior fornix using a Dacron swab and stored at −70°C. The concentration of SLPI was determined using the commercially available Quantikine Human SLPI Immunoassay kits (R&D Systems Inc, Minneapolis, MN) per manufacturer’s instructions. C-reactive protein (CRP) was measured from serum samples using a highly sensitive assay. Serum samples collected from each participant during each visit were frozen and stored at −70°C. The CRP assay employed a simple sandwich ELISA adapted from that of Erhardt et al16.
Specimens for vaginal cultures were collected via swab placed in Port-a-cul transport gel (Becton-Dickinson, Sparks, MD) and transported via overnight shipping to the reference laboratory in Pittsburgh, PA within 24 hours of collection for the following organisms: M. hominis, Ureaplasma spp, G.vaginalis, Lactobacillus spp, S. aureus, E. coli, Enterococcus spp, Candida spp and anaerobic gram negative rods, both pigmented and non-pigmented. The swabs were removed from the transport gel and inoculated onto Columbia agar supplemented with 5% sheep blood, two plates of human bilayer Tween (HBT) agar, Rogosa agar, A-8 agar, Ureaplasma broth, and one plate of pre-reduced laked blood kanamycin agar. The Columbia agar, one set of HBT agar plates, the A-8 agar plate, and the Ureaplasma broth were incubated at 36°C in 5–7% CO2 for a minimum of 48 hours. The remaining plates were incubated within an anaerobic glove box at 36°C for a minimum of 5 days. Biochemical tests were used to identify the Enterococcus spp, E.coli, and GBS. Lactobacilli spp were tested for production of H2O2 using a qualitative assay. Anaerobic gram-negative rods were identified by their characteristic colony morphology on selective media and Gram stain morphology.
Statistical analysis
Statistical power was calculated based on previously reported SLPI concentrations in pregnancy7. We performed a sample size calculation using a two group repeated measures design. The present study with 47 pregnant and 16 non-pregnant patients was able to detect an 85% difference in SLPI concentrations at the enrollment visit with 80% power. The study lacked sufficient statistical power to assess differences in adverse pregnancy outcomes. Cytokines, chemokines, and SLPI concentrations were not normally distributed. Therefore, comparisons were made between study arms using Wilcoxon rank-sum test. Adjustment for variables such as white race and overweight (BMI>=25) was performed by the Van Elteren test. Organism growth was compared between groups by the Chi-square test or the Cochran-Mantel-Haenszel test (adjusted analysis). Statistical analysis was performed using SAS 9.2 (SAS Institute, Cary, NC). We conducted two-sided hypothesis tests with a p value less than 0.05 considered statistically significant. To account for multiple testing, we adjusted p-values by the Benjamini-Hochberg method with a false discovery rate of 0.0517. This method controls the proportion of type I errors among all significant hypothesis tests at 5%. The sample size did not afford adequate power to make meaningful comparisons between individual microflora and mediators.
RESULTS
A total of 47 pregnant and 16 non-pregnant women were enrolled. The mean age and BMI for both groups were similar (Table 1). More women identified themselves as Hispanic in the pregnant cohort compared to the non-pregnant participants, 44.6% versus 18.8% respectively. Within the pregnant group, 66% of participants were married or had a partner compared to 37.6% in the non-pregnant group. Medicaid recipients were more frequently represented within the pregnant group (43.5%) than the non-pregnant group (12.5%).
Table 1.
Demographic characteristics for all enrolled participants
| Characteristic | Pregnant (n=47) | Non-pregnant (n=16) |
|---|---|---|
| Age, median (range) | 24.7 (18–35) | 25.6 (18–34) |
| BMI, median (range) | 25 (16.2–52.4) | 24.6 (19.9–39.8) |
| Race, n (%) | ||
| Caucasian | 17 (36.2) | 9 (56.3) |
| Hispanic | 21 (44.7) | 3 (18.8) |
| Black | 4 (8.5) | 0 |
| Asian | 2 (4.3) | 1 (6.3) |
| More than 1 race | 2 (4.3) | 1 (6.3) |
| Other | 2 (4.3) | 2 (12.5) |
| Employment, n (%) | ||
| Unemployed | 13 (27.7) | 2 (12.5) |
| Full or part-time | 32 (68.1) | 14 (87.6) |
| Education, n (%) | ||
| None | 1 (2.1) | 0 |
| Junior high school | 5 (10.6) | 1 (6.3) |
| High school/GED | 11 (23.4) | 3 (18.8) |
| Some College/ graduate | 30 (63.8) | 12 (75.1) |
| Marital status, n (%) | ||
| Single | 16 (34.0) | 10 (62.5) |
| Married/Partnered | 31 (66.0) | 6 (37.6) |
| Insurance, n (%) | ||
| Uninsured | 7 (15.2) | 3 (18.8) |
| Medicaid | 20 (43.5) | 2 (12.5) |
| Private | 17 (37.0) | 11 (68.8) |
| Other | 2 (4.4) | 0 |
BMI = Body mass index, GED = General educational development
The median concentrations of the genital tract immune mediators and serum CRP at each visit are illustrated in Table 2. The majority of mediators including IL-4, IL-6, IL-10, SLPI, GM-CSF, IFN-γ, TNF-α and MIP-1α did not differ between the pregnant and the non-pregnant women. IL-1β increased during pregnancy, a difference most pronounced during the first trimester (median 497 g/mL vs. 40 g/mL, p = 0.008). Non-pregnant women were also more likely to have undetectable levels of this cytokine, 7% vs. 25%. A similar effect was seen with serum levels of C-reactive protein (CRP). Concentrations of this marker of systemic inflammation were elevated in pregnancy, a trend that can be seen across trimesters. The serum concentration of CRP then trended downward in the postpartum visit suggesting this change is pregnancy-related. An analysis excluding women who delivered preterm did not significantly alter these findings. Figure 1 graphically illustrates the difference between pregnant and non-pregnant groups for all study visits for both IL-1β and CRP. A Freidman’s test revealed that IL-1 β concentrations did not vary over time in the non-pregnant group, p=0.94.
Table 2.
Median immune marker concentration in each study arm
| Immune marker |
Pregnant (n=47) Median (range) |
Non-pregnant (n=16) Median (range) |
Adjusted p value |
|---|---|---|---|
| Il-1 β | |||
| First visit | 497 (8.2–58192) | 40 (8.2–2032) | 0.008 |
| Second visit | 254 (8.2–15109) | 189 (8.2–1983) | 0.4 |
| Third visit | 200 (8.2–4639) | 165.5 (8.2–836) | 0.05 |
| Fourth visit | 401 (63–3415) | 91 (8.2–467) | 0.04 |
| IL-6 | |||
| First visit | 505.5 (9.6–5246) | 332.5 (6.9–885) | 0.07 |
| Second visit | 515.5 (9.6–8383) | 676 (307–2205) | 0.4 |
| Third visit | 376.5 (9.6–4883) | 729.5 (9.6–2107) | 0.8 |
| Fourth visit | 574.5 (255–3655) | 643 (179–1068) | 0.4 |
| IFN-γ | |||
| First visit | 112 (2.0–743) | 6.9 (2.0–381) | 0.1 |
| Second visit | 71 (2.0–1038) | 104 (6.9–730) | 0.6 |
| Third visit | 13 (2.0–807) | 131 (6.9–388) | 0.2 |
| Fourth visit | 171 (6.9–1036) | 76 (6.9–288) | 0.6 |
| IL-10 | |||
| First visit | 6.9 (6.9–537) | 6.9 (6.9–291) | 0.3 |
| Second visit | 6.9 (6.9–563) | 6.9 (6.9–486) | 0.6 |
| Third visit | 6.9 (6.9–543) | 99.5 (6.9–375) | 0.7 |
| Fourth visit | 243 (6.9–3228) | 226 (6.9–291) | 0.4 |
| GM-CSF | |||
| First visit | 20.0 (6.9–1834) | 6.9 (6.9–224) | 0.08 |
| Second visit | 6.9 (6.9–5869) | 6.9 (6.9–3615) | 0.9 |
| Third visit | 6.9 (6.9–1337) | 32.5 (6.9–628) | 0.5 |
| Fourth visit | 274 (6.9–1799) | 57 (6.9–388) | 0.2 |
| TNF-α | |||
| First visit | 6.9 (6.9–630) | 6.9 (6.9–91) | 0.07 |
| Second visit | 6.9 (6.9–931) | 6.9 (6.9–499) | 0.8 |
| Third visit | 6.9 (6.9–580) | 6.9 (6.9–489) | 0.9 |
| Fourth visit | 153 (6.9–499) | 39 (6.9–254) | 0.1 |
| MIP-1-α | |||
| First visit | 439 (6.9–1661) | 12 912–1003) | 0.1 |
| Second visit | 285 (12–1566) | 378 (12–1580) | 0.8 |
| Third visit | 160 (12–1688) | 373 (12–1451) | 0.7 |
| Fourth visit | 838 (12–3088) | 12 (12–734) | 0.03 |
| IL-4 | |||
| First visit | 8.2 (8.2–829) | 8.2 (8.2–379) | 0.1 |
| Second visit | 8.2 (8.2–960) | 8.2 (8.2–736) | 0.6 |
| Third visit | 8.2 (6.9 –744) | 8.2 (8.2–538) | 0.2 |
| Fourth visit | 262 (8.2–770) | 8.2 (8.2–402) | 0.3 |
| SLPI | |||
| First visit | 525525 (8.2–6008198) | 437310 (11760–6856532) | 0.08 |
| Second visit | 500851 (850.8–8085927) | 488458 (70936–2741354) | 0.9 |
| Third visit | 317831 (44542–6591398) | 1042767 (155370–11924538) | 0.07 |
| Fourth visit | 350403 (21442–1970467) | 756734 (99390–1006787) | 0.08 |
| CRP | |||
| First visit | 6.0 (0.5–27.4) | 0.9 (0.1–9.8) | 0.005 |
| Second visit | 5.1 (0.9–27.8) | 0.8 (0.1–15.4) | <0.0001 |
| Third visit | 4.6 (0.4–15.0) | 0.7 (0.1–8.7) | 0.03 |
| Fourth visit | 2.7 (0.2–2545) | 0.4 (0.1–2.8) | 0.1 |
All values presented as pg/mL reflect genital concentrations, except for serum CRP, presented as mg/L.
IL –interleukin, IFN – interferon, GM-CSF – granulocyte-macrophage colony stimulating factor, TNF – Tumor necrosis factor, MIP – macrophage inflammatory protein, SLPI – Secretory leukocyte protease inhibitor, CRP – C-reactive protein.
p values adjusted for race and body mass index
Figure 1. Scatterplot of vaginal concentration of IL-1β and serum CRP in pregnant and non-pregnant women.
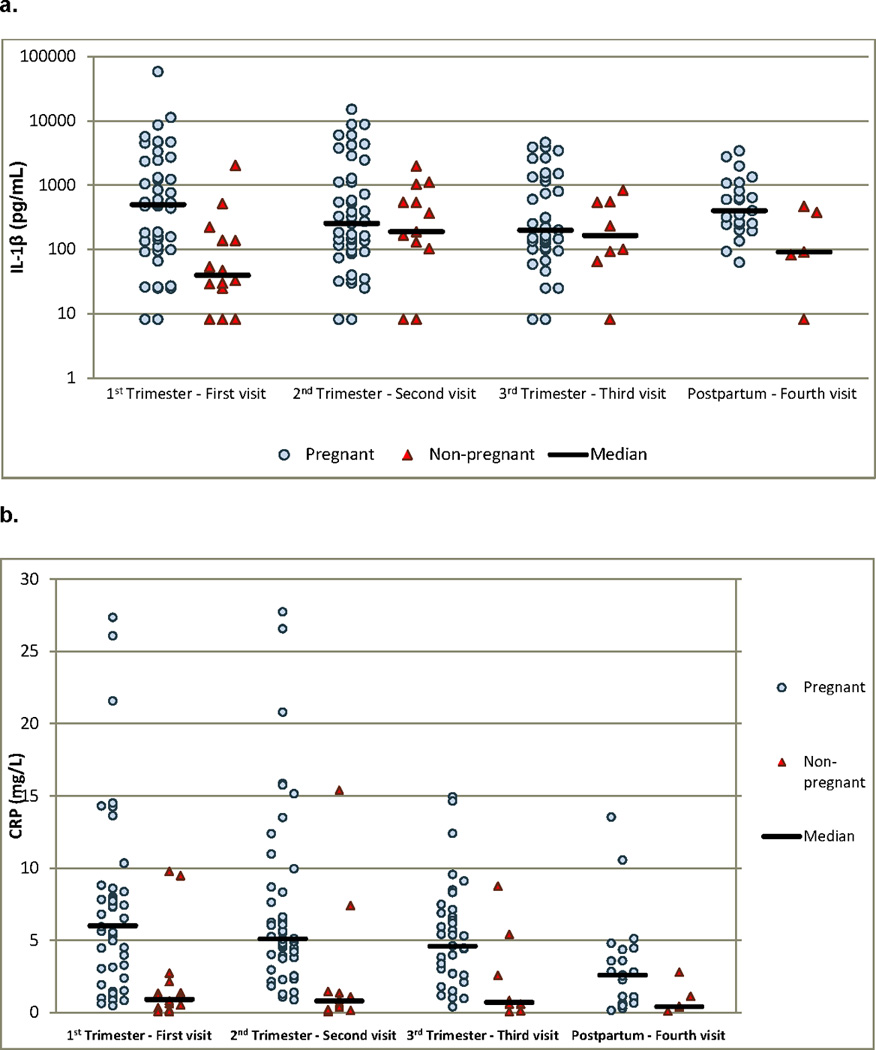
a. Levels of IL-1β reported in pg/nl. The Y-axis has been transformed to logarithmic scale in order to allow better comparison among groups. A statistically significant difference was noted between groups during the first trimester and the postpartum period (p = 0.008 and p = 0.04, respectively).
b. Serum CRP levels reported in ng/L. A statistically significant difference was noted between groups during the first, second and third trimester (p = 0.005, p < 0.0001 and p = 0.03, respectively).
The frequency of cultures that yielded positive results for each group is displayed in Table 3. Hydrogen peroxide-producing strains of Lactobacillus was less frequently isolated from first trimester pregnant women compared to non-pregnant (71.2% vs. 100%, p = 0.02). During the second and third trimester, there was no difference in colonization by these lactobacilli between groups. Among women seen postpartum, colonization by hydrogen-peroxide producing strains of lactobacilli dropped precipitously to 17.7%, while all (100%) of the non-pregnant participants remained colonized over the same period of time (p=0.01). Only one patient (2.7%) in the pregnant cohort met criteria for diagnosis of bacterial vaginosis during the third trimester by Amsel criteria18. Across time, the average pH between groups did not differ with an overall median of 4.4 in all visits.
Table 3.
Organisms isolated during each visit
| Organism | Pregnant (n=47) % |
Non-pregnant (n=16) % |
p value* |
|---|---|---|---|
| Lactobacillus H2O2 (+) | |||
| First visit | 70.2 | 100 | 0.02 |
| Second visit | 75.6 | 93.3 | 0.3 |
| Third visit | 75.0 | 87.5 | 0.9 |
| Fourth visit | 16.7 | 100 | 0.01 |
| Lactobacillus H2O2 (−) | |||
| First visit | 57.4 | 68.7 | 0.9 |
| Second visit | 71.1 | 93.3 | 0.3 |
| Third visit | 72.2 | 87.5 | 0.4 |
| Fourth visit | 75.0 | 80 | 0.9 |
| E. coli | |||
| First visit | 2.1 | 6.3 | 0.8 |
| Second visit | 2.2 | 0 | 0.8 |
| Third visit | 5.6 | 12.5 | 0.0005 |
| Fourth visit | 8.3 | 20 | 0.9 |
| Candida | |||
| First visit | 19.2 | 0 | 0.07 |
| Second visit | 20 | 0 | 0.05 |
| Third visit | 25 | 12.5 | 0.4 |
| Fourth visit | 4.2 | 0 | -- |
| G. vaginalis | |||
| First visit | 44.7 | 31.3 | 0.6 |
| Second visit | 42.2 | 13.3 | 0.06 |
| Third visit | 38.9 | 12.5 | 0.5 |
| Fourth visit | 50 | 20 | 0.8 |
| S. aureus | |||
| First visit | 8.5 | 0 | 0.3 |
| Second visit | 2.2 | 0 | 0.5 |
| Third visit | 0 | 0 | -- |
| Fourth visit | 4.2 | 0 | -- |
| Enterococcus spp. | |||
| First visit | 12.8 | 31.3 | 0.07 |
| Second visit | 17.8 | 20 | 0.9 |
| Third visit | 25 | 12.5 | 0.6 |
| Fourth visit | 12.5 | 40 | 0.2 |
| AGNR Pigmented | |||
| First visit | 17.4 | 0 | 0.1 |
| Second visit | 8.9 | 13.3 | 0.6 |
| Third visit | 11.1 | 0 | 1 |
| Fourth visit | 45.8 | 20 | 0.4 |
| AGNR Non-pigmented | |||
| First visit | 40.4 | 0 | 0.004 |
| Second visit | 24.4 | 20 | 0.9 |
| Third visit | 33.3 | 12.5 | 0.4 |
| Fourth visit | 66.7 | 0 | 0.02 |
| Ureaplasma spp. | |||
| First visit | 66 | 31.3 | 0.05 |
| Second visit | 65.9 | 26.7 | 0.03 |
| Third visit | 61.8 | 14.3 | 0.2 |
| Fourth visit | 34.8 | 0 | 0.3 |
| M. hominis | |||
| First visit | 19.2 | 0 | 0.09 |
| Second visit | 15.6 | 0 | 0.1 |
| Third visit | 20 | 0 | 0.1 |
| Fourth visit | 20.8 | 25 | 0.2 |
H2O2- hydrogen peroxide producing
AGNR - Anaerobic Gram-Negative Rods.
p value adjusted for race and body mass index
There was significantly greater colonization of E. coli in the non-pregnant participants when compared to pregnant women in the third trimester, 12.5% versus 5% respectively p=0.0005. Ureaplasma spp was highly prevalent with a positive culture in over 60% of pregnant patients throughout trimesters, and was isolated more frequently during the second trimester as compared to the non-pregnant state, 65.9% versus 26.7% (p=0.03).
We examined pregnancy outcomes in the context of alterations in immunity (Table 4). In this low-risk cohort, the median gestational age at delivery was 39.0 weeks (33.0–41.0 weeks) and the average birth weight was 3255 grams (2070–4640 grams). The preterm birth rate was lower than the national average, at 8.9%. Gestational age at birth as well as adverse maternal and neonatal outcomes were evaluated according to the microorganism isolated during each trimester. Culture of various organisms was not associated with gestational age at birth, maternal, or neonatal adverse outcomes. Cervical cytokine concentrations and serum CRP levels in the highest quartile for each mediator were not associated with adverse perinatal/maternal outcomes.
Table 4.
Pregnancy outcomes among pregnant women (n=45*)
| Outcomes | Median (range) |
|---|---|
| GA at delivery (weeks) | 39.0 (33.0–41.0) |
| Preterm delivery <37 weeks | 4/45 (8.9%) |
| Birth weight (g) | 3255 (2070–4640) |
| Apgar-5 minute | 9 (8–9) |
| Adverse neonatal outcomes | 6/45 (13.3%) |
| Weight <1500g | 0 |
| APGAR-5 minute <7 | 0 |
| IUFD | 0 |
| IUGR | 2 |
| NICU admission | 4 |
| Delivery complications | 4/45 (8.9%) |
| Oligohydramnios | 0 |
| PPROM | 1 |
| Chorioamnionitis | 1 |
| PTL | 2 |
One patient had a miscarriage at 12 weeks and one participant declined further participation resulting in 45 birth outcomes.
GA – gestational age, IUFD – intrauterine fetal demise, IUGR – intrauterine growth restriction, NICU- neonatal intensive care unit, PPROM – preterm premature rupture of membranes, PTL – preterm labor, g-grams
DISCUSSION
In this study, we found that cervical IL-1β, a pro-inflammatory cytokine, and serum CRP, a marker of systemic inflammation, are increased in normal pregnancy compared to non-pregnant women. Pregnancy-mediated changes to vaginal and systemic immunity and to vaginal flora have been incompletely elucidated. However, a previous study documented an increase in cervical cytokines in pregnant compared to non-pregnant women with BV9. Conflicting results as well as differing techniques and clinical outcomes have made interpretation of these data difficult. Establishing normal parameters and determining physiologic changes attributable to pregnancy allows for a valid comparison when assessing inflammation as it relates to risk of HIV acquisition.
Concentration of cytokines within the vagina or amniotic cavity has been the subject of much research. Our results indicate that pregnancy is associated with fairly few local and systemic changes in immunity. Vaginal concentrations of IL-1β, a pro-inflammatory cytokine, are increased in pregnant women across each trimester. These results confirm the findings of Kutteh et al, who followed 36 women throughout pregnancy and found increasing concentrations of IL-1β19. They differ, though, from the findings of Walter et al, in a recently published study comparing immunomodulatory factors in the cervicovaginal lavage (CVL) of 23 pregnant to 25 non-pregnant women20. These authors did not find a difference in IL-1β concentrations but did find suppression of macrophage-derived chemokine (CCL22) in the CVL of pregnant women. Our findings support those of other investigators as well. We detected a statistically significant decline in IL-6 levels during the first trimester, a trend also reported by others. Donders et al found that pregnant women were less likely to have detectable levels of IL-6 and IL-8 and showed a decreasing trend in concentration especially during the second trimester21. In the study by Beigi et al, reporting increased concentrations of IL-6 in pregnant women, the women were enrolled at a median gestational age of 13 weeks’; therefore changes in this cytokine over the three trimesters were not evaluated9. Our findings differ from results reported by other authors with respect to concentration of IL-10, a major anti-inflammatory cytokine. Cervical concentrations of this mediator within our study population did not differ between pregnant and non-pregnant participants. Others have reported a reduction in concentrations of IL-10 during the first trimester. Our study differs in site of sample collection. The study showing a decrease in IL-10 was from serum and may reflect systemic changes rather than those of the lower genital tract22. We were unable to reliably measure serum cytokine concentrations in this low-risk cohort and therefore only report serum CRP concentrations here. Concentrations of the pro-inflammatory cytokines IFN-γ and anti-inflammatory IL-4 have also been reported to decrease during the second trimester22, a pattern that was not observed in our study cohort.
Concentrations of SLPI were not different between the groups in this study. This is in contrast to the findings of Draper et al who reported a 10-fold increase in this peptide during pregnancy7. Helmig et al also demonstrated that SLPI is increased in cervical mucus of pregnant women23. One of the major reasons explaining this difference in SLPI levels could be that our study cohort only included women that were considered very low risk; this is in stark contrast to cohorts used by others that included both symptomatic patients and patients diagnosed with sexually transmitted infections.
We found a statistically significant increase in serum highly sensitive C-reactive protein concentration during pregnancy. This increase tended to be higher during the first trimester and decrease as pregnancy progressed. Multiple studies have been conducted measuring this analyte during pregnancy with the use of different detection techniques and cut-off values. Some authors have reported an increase in concentration of CRP with labor, delivery and the immediate postpartum period; although the variability of the values used as abnormal makes generalization difficult 24, 25. Various authors have attempted to associate serum concentrations of this acute phase reactant with adverse pregnancy outcomes. To date, studies have yielded conflicting results 26, 27. The usefulness of this inflammatory marker during pregnancy and the ability to predict adverse outcomes remains to be determined. In our study, we used samples collected from low-risk patients that lacked confounding variables that could alter results such as labor, and controlled for variables that may alter the values such as race and BMI. A more sensitive assay was also used to process our samples, another factor adding to the validity of our results.
The effect that pregnancy exerts on vaginal flora is not clearly understood. We have previously reported that perturbations in vaginal flora among low risk pregnant women do not cause a consistent impact on local vaginal immunity 28. The definition of normal flora also varies throughout the literature. Abnormal vaginal flora, despite known associations, is functionally a poor predictor of adverse pregnancy outcomes especially in low-risk patients since most women with abnormal flora will actually go on to have normal pregnancy outcomes 29. Lactobacillus spp are considered a normal component of the vaginal flora and these bacteria are thought to inhibit the growth of pathogenic bacteria. The pregnant group were less frequently colonized by hydrogen peroxide-producing lactobacilli compared to non-pregnant women, and they experienced a decrease in the detection of lactobacilli in the postpartum period. The women in the study group did not have a clinical diagnosis of BV per Amsel’s criteria but Gram stains for Nugent score were not performed. Therefore, it is possible that there could have been asymptomatic BV which did not meet the clinical criteria for diagnosis in the pregnant women. In this study, pregnant women had more complex flora than did non-pregnant women. Our findings differ from those of Aagaard et al. in that while their group found a difference in the vaginal flora among pregnant compared to non-pregnant women using 16S rRNA gene sequencing, the women in that cohort actually had less diverse flora during pregnancy. This study did not use Nugent score and therefore it is possible that there could have been higher rates of asymptomatic BV in the non-pregnant group30. Beigi et al reported that pregnant and non-pregnant women experiencing BV had very similar microflora9.
The frequency of G. vaginalis was similar in pregnant and non-pregnant women. G. vaginalis is found in 100% of women with the diagnosis of BV, but can also be found among women without this condition. Koumans et al showed that the diagnosis of BV is not more common in pregnant patients compared to non-pregnant women31. Despite the association of G. vaginalis with BV, it is also highly prevalent among normal women as demonstrated in our results. Our study was not designed to detect differences across the menstrual cycle, and the effect of various stages of the cycle cannot be examined with these data.
The isolation of Ureaplasma spp. in over 60% of our pregnant participants has important implications. Although various studies have implicated vaginal colonization of Ureaplasma spp. with adverse pregnancy outcomes 32, 33, our results indicate that this microbe is part of the normal vaginal flora in pregnant patients. M. hominis was also more commonly isolated in specimens from pregnant patients, again indicating a possibility that this microbe is not pathogenic when found in the vagina during pregnancy. In a study involving over 900 women, Lee et al. also demonstrated that positive vaginal cultures for M. hominis was not a risk for preterm birth34.
This study is among the first to simultaneously characterize local and systemic immunity in the setting of known vaginal culture data among low risk pregnant women. Despite the small sample size, we compared various aspects of vaginal immunity among pregnant and non-pregnant participants at once thus allowing for a better understanding of the vaginal milieu during pregnancy. Our results merit validation with larger study populations as well as consideration in studies assessing risk of HIV acquisition and vaginal microbicide development.
Acknowledgments
Financial Support: K12HD050108 Brown University Women’s Health Reproductive Health Research Career Development Program, K23HD062340-01 (Anderson), and K24AI066884 (Cu-Uvin)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflict of interest.
Presented at the 38th Annual Meeting of the Infectious Diseases Society for Obstetrics and Gynecology, Chicago, IL.
REFERENCES
- 1.UNAIDS. Report on the Global Aids Epidemic. UNAIDS; 2010. p. 10. [Google Scholar]
- 2.UNAIDS. World AIDS Day Report. (UNAIDS) Joint United Nations Programme on HIV/AIDS. 2011 copyright. [Google Scholar]
- 3.UNAIDS. AIDS Epidemic Update, 2008. 2012;Vol [Google Scholar]
- 4.Gray RH, Li X, Kigozi G, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 5.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008 Oct 1;22(15):1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stock SJ, Duthie L, Tremaine T, Calder AA, Kelly RW, Riley SC. Elafin (SKALP/Trappin-2/proteinase inhibitor-3) is produced by the cervix in pregnancy and cervicovaginal levels are diminished in bacterial vaginosis. Reprod Sci. 2009 Dec;16(12):1125–1134. doi: 10.1177/1933719109341998. [DOI] [PubMed] [Google Scholar]
- 7.Draper DL, Landers DV, Krohn MA, Hillier SL, Wiesenfeld HC, Heine RP. Levels of vaginal secretory leukocyte protease inhibitor are decreased in women with lower reproductive tract infections. Am J Obstet Gynecol. 2000 Nov;183(5):1243–1248. doi: 10.1067/mob.2000.107383. [DOI] [PubMed] [Google Scholar]
- 8.Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004 Oct;191(4):1331–1338. doi: 10.1016/j.ajog.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Beigi RH, Yudin MH, Cosentino L, Meyn LA, Hillier SL. Cytokines, pregnancy, and bacterial vaginosis: comparison of levels of cervical cytokines in pregnant and nonpregnant women with bacterial vaginosis. J Infect Dis. 2007 Nov 1;196(9):1355–1360. doi: 10.1086/521628. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial Vaginosis Associated with Increased Risk of Female-to-Male HIV-1 Transmission: A Prospective Cohort Analysis among African Couples. Plos Med. Jun;9(6) doi: 10.1371/journal.pmed.1001251. e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherpes TL, Marrazzo JM, Cosentino LA, Meyn LA, Murray PJ, Hillier SL. Hormonal contraceptive use modulates the local inflammatory response to bacterial vaginosis. Sex Transm Infect. 2008;84:57–61. doi: 10.1136/sti.2007.026625. [DOI] [PubMed] [Google Scholar]
- 12.Simhan HN, Caritis SN, Hillier SL, Krohn MA. Cervical anti-inflammatory cytokine concentrations among first-trimester pregnant smokers. Am J Obstet Gynecol. 2005;193:1999–2003. doi: 10.1016/j.ajog.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Simhan HN, Caritis SN, Krohn MA, Martinez de Tejada B, Landers DV, Hillier SL. Decreased cervical proinflammatory cytokines permit subsequent upper genital tract infection during pregnancy. Am J Obstet Gynecol. 2003;189:560–567. doi: 10.1067/s0002-9378(03)00518-0. [DOI] [PubMed] [Google Scholar]
- 14.Yudin MH, Landers DV, Meyn L, Hillier SL. Clinical and cervical cytokine response to treatment with oral or vaginal metronidazole for bacterial vaginosis during pregnancy: a randomized trial. Obstet Gynecol. 2003;102:527–534. doi: 10.1016/s0029-7844(03)00566-0. [DOI] [PubMed] [Google Scholar]
- 15.de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–3132. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 18.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 19.Kutteh WH, Franklin RD. Quantification of immunoglobulins and cytokines in human cervical mucus during each trimester of pregnancy. Am J Obstet Gynecol. 2001;184:865–872. doi: 10.1067/mob.2001.113853. discussion 872–74. [DOI] [PubMed] [Google Scholar]
- 20.Walter J, Fraga L, Orin MJ, et al. Immunomodulatory factors in cervicovaginal secretions from pregnant and non-pregnant women: a cross-sectional study. BMC Infect Dis. 11:263. doi: 10.1186/1471-2334-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donders GG, Vereecken A, Bosmans E, Spitz B. Vaginal cytokines in normal pregnancy. Am J Obstet Gynecol. 2003;189:1433–1438. doi: 10.1067/s0002-9378(03)00653-7. [DOI] [PubMed] [Google Scholar]
- 22.Shimaoka Y, Hidaka Y, Tada H, et al. Changes in cytokine production during and after normal pregnancy. Am J Reprod Immunol. 2000;44:143–147. doi: 10.1111/j.8755-8920.2000.440303.x. [DOI] [PubMed] [Google Scholar]
- 23.Helmig R, Uldbjerg N, Ohlsson K. Secretory leukocyte protease inhibitor in the cervical mucus and in the fetal membranes. Eur J Obstet Gynecol Reprod Biol. 1995;59:95–101. doi: 10.1016/0028-2243(94)02023-8. [DOI] [PubMed] [Google Scholar]
- 24.Keski-Nisula L, Kirkinen P, Ollikainen M, Saarikoski S. C-reactive protein in uncomplicated parturients delivered by cesarean section. Acta Obstet Gynecol Scand. 1997;76:862–867. doi: 10.3109/00016349709024366. [DOI] [PubMed] [Google Scholar]
- 25.De Meeus JB, Pourrat O, Gombert J, Magnin G. C-reactive protein levels at the onset of labour and at day 3 post-partum in normal pregnancy. Clin Exp Obstet Gynecol. 1998;25:9–11. [PubMed] [Google Scholar]
- 26.Trochez-Martinez RD, Smith P, Lamont RF. Use of C-reactive protein as a predictor of chorioamnionitis in preterm prelabour rupture of membranes: a systematic review. BJOG. 2007;114:796–801. doi: 10.1111/j.1471-0528.2007.01385.x. [DOI] [PubMed] [Google Scholar]
- 27.van de Laar R, van der Ham DP, Oei SG, Willekes C, Weiner CP, Mol BW. Accuracy of C-reactive protein determination in predicting chorioamnionitis and neonatal infection in pregnant women with premature rupture of membranes: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2009;147:124–129. doi: 10.1016/j.ejogrb.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Anderson BL, Cu-Uvin S, Raker CA, Fitzsimmons C, Hillier SL. Subtle perturbations of genital microflora alter mucosal immunity among low-risk pregnant women. Acta Obstet Gynecol Scand. 90:510–515. doi: 10.1111/j.1600-0412.2011.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gondo F, da Silva MG, Polettini J, et al. Vaginal flora alterations and clinical symptoms in low-risk pregnant women. Gynecol Obstet Invest. 71:158–162. doi: 10.1159/000316051. [DOI] [PubMed] [Google Scholar]
- 30.Aagaard K, Riehle K, Ma J, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 7:e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 32.Kacerovsky M, Boudys L. [Preterm premature rupture of membranes and Ureaplasma urealyticum] Ceska Gynekol. 2008;73:154–159. [PubMed] [Google Scholar]
- 33.Kacerovsky M, Pavlovsky M, Tosner J. Preterm premature rupture of the membranes and genital mycoplasmas. Acta Medica (Hradec Kralove) 2009;52:117–120. doi: 10.14712/18059694.2016.115. [DOI] [PubMed] [Google Scholar]
- 34.Lee SE, Romero R, Kim EC, Yoon BH. A high Nugent score but not a positive culture for genital mycoplasmas is a risk factor for spontaneous preterm birth. J Matern Fetal Neonatal Med. 2009;22:212–217. doi: 10.1080/14767050802616994. [DOI] [PMC free article] [PubMed] [Google Scholar]


