Abstract
Objective
High-resolution optical coherence tomography (OCT), can be used noninvasively to evaluate vaginal morphologic features, including epithelial thickness, to assess this protective barrier in transmission of sexually transmitted infections and to monitor tissue response to topical medications and hormonal fluctuations. We examined the utility of OCT to measure epithelial thickness noninvasively before and after topical treatment with a drug that causes epithelial thinning.
Study Design
Twelve female sheep were treated with intravaginal placebo (n=4) or nonoxynol-9 (n=8). Vaginal OCT images were obtained before and 24 hours after treatment. Four sheep in the nonoxynol-9 group were also examined on days 3 and 7. Vaginal biopsies were obtained on the last exam day. Epithelial thickness was measured in OCT images and in H&E-stained histological sections from biopsies. Statistical analysis was performed using ANOVA (significance p<0.05).
Results
Baseline OCT epithelial thickness measurements were similar (85±19 μm placebo, 78±20 μm nonoxynol-9; p=0.52). Epithelial thinning was significant after nonoxynol-9 (32±22 μm) compared to placebo (80±15 μm) 24 hours after treatment (p<0.0001). In the four nonoxynol-9-treated sheep followed for 7 days, epithelial thickness returned to baseline by day 3, and increased significantly on day 7. Epithelial thickness measurements from histology were not significantly different than OCT (p=0.98 N-9, p=0.93 HEC).
Conclusion
Drug-induced changes in the epithelium were clearly detectable using OCT imaging. OCT and histology epithelial thickness measurements were similar, validating OCT as a noninvasive method for epithelial thickness measurement, providing an important tool for quantitative and longitudinal monitoring of vaginal epithelial changes.
Keywords: optical coherence tomography, sheep, vagina, nonoxynol-9, noninvasive epithelial thickness measurement
Introduction
The natural protective barrier provided by the vagina is vitally important to women’s health, as evidence indicates that the loss of this barrier has been linked to increased risk of HIV infection.1,2 In addition to this critical role of protection against pathogens, there are implications for drug delivery and mucosal vaccine development, both of which may be affected by changes in the vaginal epithelium due to hormonal fluctuations from the menstrual cycle or hormonal contraceptives.
The epithelial layer is an important component of the protective vaginal barrier, which also includes microflora, vaginal fluid, lamina propria, and immune cells.3 Changes in the integrity of the vaginal epithelium occur under the influence of hormones, inflammation, and infection,4 therefore being able to noninvasively characterize components of the vaginal barrier under various conditions would provide insight into enhancing its protective effect or identifying women at greater risk of infection due to diminished barrier function. A dramatic illustration of the hormonal effects on vaginal epithelium is seen in the macaque, which has large fluctuations in vaginal epithelial thickness during the menstrual cycle, with thicker epithelium in the follicular phase, and thinner epithelium in the luteal phase.3,5 In humans, epithelial thickness variations are also present, however less pronounced, with epithelial thickness variations occurring during the menstrual cycle and with use of hormonal contraceptives.6–9 Both vaginal cell layer6 and epithelial thickness7 decrease in the luteal phase, with decreased epithelial thickness after depomedroxyprogesterone acetate (DMPA) treatment similar to that in the luteal phase.7
Vaginal epithelial thickness may play a role in acquisition of sexually transmitted infections (STI). Genital ulcerative disease, with focal loss of the epithelial barrier, has been linked to an increased risk of HIV.1,2 Macaques are more likely to acquire simian-human immunodeficiency virus (SHIV) in the late luteal phase, and treatment with progestins, mimicking the luteal phase, leads to thinning of the vaginal epithelium and increased infection with simian immunodeficiency virus (SIV).10–12 In contrast, treatment with estrogen, which increases the vaginal epithelial thickness, is protective against SHIV infection.13 Clinical studies have shown a link between increased HIV incidence and the use of DMPA,14,15 a contraceptive that may thin the vaginal epithelium.7,8,16 Nonoxynol-9 (N-9), a spermicide with in vitro action against HIV that was tested for prevention of HIV in clinical trials, actually increased HIV acquisition,17,18 a finding that may be due in part to epithelial disruption and thinning.19 In addition to providing protection from STIs, the vagina is utilized as a route for drug or vaccine delivery; vaginal epithelial integrity and thickness can impact drug delivery and determine whether effects are local or systemic.20–22
Historically, vaginal administration of drugs has been underutilized, but with new drug delivery mechanisms such as advances in intravaginal rings, the numbers of papers and patents related to vaginal drug delivery for local and systemic drug release have recently increased dramatically.20,23 Some current vaginally administered treatments for local or systemic effects include hormone replacement, contraception, infertility treatment, treatment of local infections, and, more recently topical microbicides, medications designed to prevent the acquisition of HIV and other STIs.20,23–26 The vagina is also being explored as a route for vaccine delivery.27 As the number of vaginally administered drugs continues to increase, it is increasingly important to understand drug and vaccine effects on vaginal physiology and microstructure. Drug delivery in the vagina relies on a variety of parameters, including drug properties, vaginal pH, characteristics of vaginal fluid and cervical mucus, and vaginal epithelial thickness.20,22–24 Vaginal epithelial thickness can be affected by local administration of drugs, as seen after treatment with N-9, with epithelial thinning considered a marker of drug toxicity.19 A noninvasive measure of vaginal epithelial thickness could be an important part of drug development to predict uptake of drug as well as drug safety.
Optical coherence tomography (OCT) utilizes a noninvasive probe- based imaging system to achieve resolutions of 15–20 micrometers with depths of up to 1.5 mm, sufficient for visualization of the epithelial layer and underlying lamina propria. Its use has been explored in gynecology to image the lower reproductive tract to evaluate for dysplasia28–30 and to evaluate the epithelial response to drug treatment.19,31,32 These studies emphasized the use of visual-based scoring systems to describe changes in morphology of the epithelium and epithelial-stromal interface. In the toxicity studies, OCT was shown to be more sensitive when compared to standard method of colposcopy for detection of epithelial disruption.19,31,32
In a sheep model characterized as a good model for the human vagina,32–34 we explored the use of OCT to measure vaginal epithelial thickness after the application of vaginal products and noninvasively monitored changes in the vaginal epithelial thickness over time. Whereas prior studies have focused on developing a scoring system for noninvasive assessment of epithelial disruption, the current study focuses on noninvasive longitudinal measurement of epithelial thickness, comparing OCT to histology findings. In this study, we used a compound known to cause epithelial thinning in order to show the ability of OCT to measure drug-induced epithelial thickness changes.
Materials and Methods
All animal studies were approved by the IACUC at the University of Texas Medical Branch in Galveston, Texas. Twelve yearling female sheep were evaluated to determine if epithelial changes were detectable after treatment with either HEC or N-9, to validate OCT epithelial thickness measurement with histology epithelial thickness, and to demonstrate the utility of OCT for longitudinal observations of recovery of the vaginal epithelium after injury. The reproductive tract of the sheep has been used as a model for the evaluation of the vagina and cervix.32–34 Sheep were anesthetized with ketamine/diazepam and isoflurane, intubated, and positioned supine on a V-tilt table. A speculum was placed in the vagina and a colposcope used to visualize the vagina and cervix. The sheep were examined at baseline and after treatment during which OCT images of the vagina were obtained with the Imalux Niris OCT imaging system (Cleveland, OH) as previously described.32 After the baseline examination on day 0, the sheep were treated with a single 5 mL dose of the “universal placebo” hydroxyethyl cellulose gel (HEC, Reprotect, Baltimore, MD)35 (n=4) or 2% N-9 (Gynol-II, Johnson & Johnson, New Brunswick, NJ) (n=8) with the operators masked to treatment group. Twenty-four hours after treatment (day 1), colposcopy and OCT were repeated. In four sheep treated with N-9, examinations were also performed on post-treatment days 3 and 7. On the last day of the study (day 1 for eight sheep; day 7 for four sheep treated with N-9), animals were euthanized and vaginal biopsies obtained at the site of OCT imaging. Biopsies were H&E-stained and digital photographs of histology slides were obtained. Epithelial thickness of twelve OCT images per sheep per exam was measured with Presto 32 software at three points across each image. Epithelial thickness was measured on histology images from four vaginal biopsies per sheep using Image J36 at nine points along the image. Both techniques utilized manual identification of epithelium and then line tools to measure the length of a line spanning from the surface to the epithelial-lamina propria boundary as previously described.16,32 Epithelial thickness measurements were compared using ANOVA with a value of p < 0.05 considered statistically significant. A power calculation was made based on preliminary data in the sheep model. With four sheep per group, the study had 81% power to detect a difference of 40 μm (approximately 3–4 cell layers of stratified squamous epithelial cells) between groups assuming a standard deviation of 16.5 μm.
Results
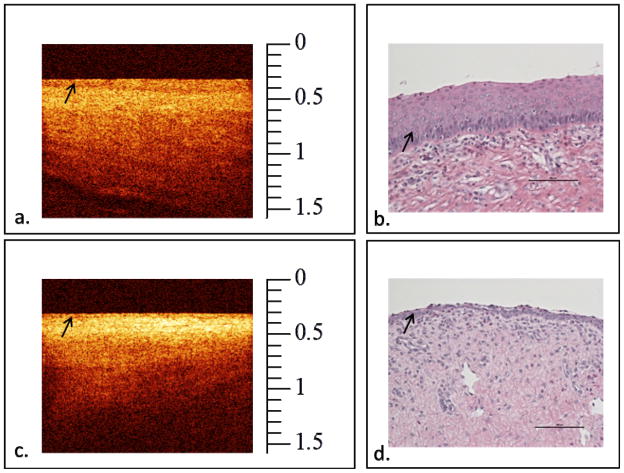
OCT images show clear definition of the vaginal epithelium. Figure 1 shows post-treatment OCT images and corresponding histology images for the placebo and N-9 groups, with obvious thinning of the epithelium detected by OCT and histology in the group treated with N-9. The OCT images are in cross-section similar to standard histology orientation of epithelium with the z-axis reflecting depth into the tissue. The upper dark band represents the glass window of the imaging probe. The next moderately dark band represents the epithelium and the brighter band below the epithelium represents the lamina propria.
Figure 1.
Vaginal OCT images (a, c) and corresponding histology from biopsy (b, d) after treatment with placebo (a, b) or nonoxynol-9 (c, d). The vaginal epithelium (arrows) is clearly defined in the representative OCT image from the placebo treated group (a); however, the epithelium is thinned after treatment with nonoxynol-9 as seen in this representative OCT image (c). The topmost dark band in the OCT images represents the glass window on the OCT probe. Corresponding histology images show similar effects after treatment (OCT scale is in mm, with each horizontal mark = 100 micrometers. Histology magnification 20x objective with bar = 100 micrometers).
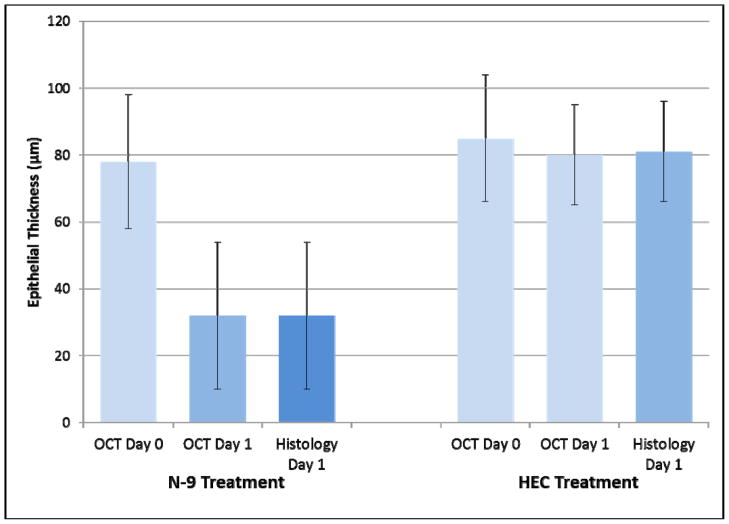
Figure 2 shows that mean vaginal epithelial thickness measured by OCT in the two treatment groups was not significantly different at baseline (P=0.52) (85 ± 19 μm HEC, 78 ± 20 μm N-9), however epithelial thinning measured by OCT was significant in the N-9 group (32 ± 22 μm) when compared to the HEC group (80 ± 15 μm) (P<0.0001) 24 hours after treatment. After treatment, the N-9 group had significantly thinner epithelium than the HEC group when measured by histology as well (P<0.0001). After treatment with either HEC placebo or N-9, mean epithelial thickness measured by OCT was not significantly different than epithelial thickness measured by histology (32 ± 22 μm N-9, 81 ± 15 μm HEC) (P=0.98 and P=0.93, respectively). Effect size calculation using the overall standard deviation of 15.75 μm in this study, showed that there was 80% power to detect a difference of 18 μm between histology and OCT epithelial thickness measurements. This is within the 15–20 μm axial resolution of the OCT imaging system.
Figure 2.
Vaginal epithelial thickness before and after treatment with placebo or nonoxynol-9. Longitudinal measurement with OCT showed that vaginal epithelial thickness decreased after treatment with nonoxynol-9 (*P<0.0001). Vaginal epithelial thickness measured by OCT and histology were similar in each group. (Error bars indicate standard deviation).
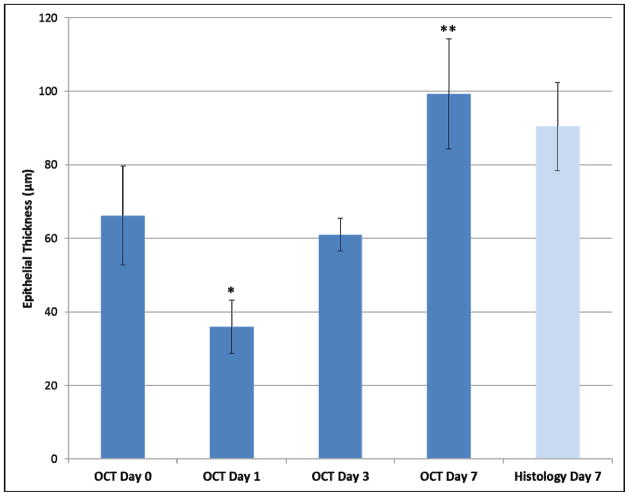
Results from observations in N-9 treated sheep for 7 days after treatment (Figure 3) show that OCT can be used longitudinally to monitor recovery of the vaginal epithelium after injury. After treatment on day 1, vaginal epithelial measurements were thinner than at baseline (36 ± 7 μm Day 1, 66 ± 13 μm Day 0) (p = 0.03). On day 3, the vaginal epithelium recovered in thickness (61 ± 4 μm Day 3) and was similar to that at baseline (p = 0.60). However, on day 7, the vaginal epithelium was thickened at 99 ± 15 μm when compared to baseline (p < 0.001). OCT thickness on day 7 was similar to histology (90 ± 10 μm; p = 0.40).
Figure 3.
The 7 day response of vaginal epithelial thickness after a single treatment with nonoxynol-9 on day 0 following baseline imaging. Vaginal epithelial thickness decreased in response to treatment, returned to baseline on day 3, and was increased on day 7. OCT thickness was similar to histology (histo) thickness on day 7. Error bars indicate standard deviation. (* p = 0.03 compared to baseline Day 0; ** p < 0.001 compared to baseline Day 0).
Comment
Despite the critical role of the vaginal epithelial layer in providing protection against entrance of pathogens or facilitating local delivery of drugs, there is currently no technique that can be used for accurate noninvasive longitudinal measurements of epithelial thickness in multiple locations within the female reproductive tract. The current study demonstates that OCT provides sufficient resolution to noninvasively monitor temporal changes in the thickness of the vaginal epithelium of a large animal model that is similar to humans. Furthermore, comparisons with the accepted standard of histology show that these measurements are an accurate representation of epithelial thickness, further validating OCT as a useful method for noninvasive vaginal epithelial assessment. Of significant interest was the fact that longitudinal measurements demonstrated the dynamic changes in epithelial thickness that can occur over the course of days following an insult with a topical agent. In this study, following initial thinning of the epithelium, the epithelium returned to baseline thickness after 3 days. However an interesting new finding was that the epithelium in these animals continued to thicken until at least one week after treatment. This finding was also present in the investigators’ recent clinical trial in which women had increased vaginal epithelial thickness 7 days after N-9 induced epithelial thinning.19 This epithelial thickening may be part of the normal repair mechanism after epithelial injury. It will be of interest to further evaluate the timecourse of the epithelial repair process with noninvasive imaging in the future.
Because the vagina serves a vital protective barrier function, real-time measures of vaginal thickness may be important to understanding its effect on the transfer of pathogens and drugs across the epithelium, with a thin epithelium more likely to allow passage of both. Since hormonal fluctuations affect vaginal epithelial thickness, understanding changes in the epithelium related to hormonal influence is also likely to be important for predicting drug and pathogen flow across the epithelium during various stages of the menstrual cycle or in the presence of exogenous hormones.
Transmission of sexually transmitted infections has been associated with vaginal epithelial thinning as well. In macaques, hormone-induced thinning of the vaginal epithelium increased the risk for SIV/SHIV infection.10–12 Increased susceptibility to HIV has been shown in women with GUD1,2 and in women treated with DMPA,14,15 presumably in part due to disrupted or thinned epithelium. Concern has been raised that postmenopausal women are at increased risk of acquiring HIV;37,38 vaginal atrophy with decreased epithelial thickness and subsequent susceptiblity to “tearing” may be factors. Wira, et al, have suggested a “window of vulnerability” to infection in women during the luteal phase of the menstrual cycle. During this time, immune response is suppressed, which, along with decreased vaginal epithelial thickness could lead to increased susceptibility to infections.39 Certainly complex interactions of multiple factors, including microflora, vaginal fluid, immune function, and epithelium and lamina propria, are synergistic in protection against infection. Hormonal effects on any of these factors could lead to diminished protection. Vaginal epithelial thickness may also play a role in the acquisition of other STIs. In a mouse model of herpes simplex virus infection, naturally cycling mice are more difficult to infect than are mice treated with DMPA, which thins the epithelium.16,40 Human papilloma virus infection requires access to heparan sulfate proteoglycans on the basement membrane; thinned or disrupted epithelium facilitates such access.41 These findings support the importance of epithelial thickness in the protective barrier function of the vagina.
The vagina is ideally suited for drug delivery, especially for products such as microbicides, hormones for contraception and vaginal atrophy treatment, and more recently for vaccine development.20,24,27 According to Fick’s law of diffusion, epithelial thickness could affect drug delivery across the vaginal epithelium, with the rate of drug diffusion across the vaginal mucosa being inversely proportional to epithelial thickness.22,23 This was seen during topical treatment of vaginal atrophy with conjugated equine estrogens in postmenopausal women. Initial serum estrogen levels were high and then decreased over time, a finding attributed to epithelial thickening after topical estrogen use.21 Katz et al have reported the use of optical imaging to determine the distribution of vaginal gels and are developing mathematical models to predict vaginal drug delivery based many complex factors, including gel and epithelial thickness.42,43 Response to vaccines may be affected by hormonal influence as well, with Bourne et al reporting increased genital herpes vaccine efficacy in ovariectomized mice treated with estradiol.40 During a vaginal immunization study, the phase of the menstrual cycle affected immune response, with women in the mid-follicular phase exhibiting antibodies in cervical secretions and women in the luteal phase lacking them.44 These examples indicate that vaginal epithelial thickness is an important parameter for vaginal drug delivery and possibly for vaginal vaccine administration. The current study shows that drugs can have acute effects on the epithelium with vaginal thinning the day after treatment and that tissue response is ongoing even after discontinuation of the drug, as seen in the group that had increased vaginal epithelial thickness seven days after treatment. As use of the vagina as a route for drug delivery increases, so will the need for developing high resolution imaging methods that can be used to rapidly and noninvasively assess the tissue response after exposure to drugs, both for drug transport and for toxicity evaluations.
Use of OCT could be extended to noninvasivly study the effects of hormonal fluctuations on the epithelium. Macaques treated with two progestogen contraceptives experienced pronounced thinning of the epithelium with return to normal thickness after removal of medication.45 Clinical studies have shown a similar, but less pronounced, effect in women with a decrease in epithelial thickness under progestin-dominant conditions.6,7 On the contrary, other human studies have shown little to no hormonal effects. One study showed similar epithelial thickness during use of contraceptives and during the follicular phase of the menstrual cycle.46 A study examining the use of DMPA47 showed that the epithelium was similar to that during the menstrual cycle. However, in that study, biopsies in DMPA users were obtained 90 days after injection, a time when drug levels would be lowest, and the nonuser control group biopsies were only obtained in the luteal phase. These study design parameters would decrease the likelihood of detecting a difference between groups. The authors recognized a study limitation of the lack of feasibility of taking biopsies at multiple timepoints. In the Mauck study, biopsies were obtained in the follicular and luteal phases and after a single injection of DMPA in the same women. Findings included thinner epithelium in both the luteal phase and after DMPA when compared to the follicular phase.7 In these clinical studies, there were limitations in the numbers of locations for biopsy sampling as well as the timing of biopsies (e.g. phase of menstrual cycle, time after drug administration). The use of OCT is not limited by timing or number of noninvasive “optical” biopsy locations, and therefore would allow further study of these unresolved questions in a noninvasive manner.
In this study, the sheep model afforded the opportunity to follow the timecourse of epithelial thickness following topical drug treatment as well as for obtaining multiple biopsies, which may not be feasible in women using conventional biopsy techniques. While the number of animals used was small, the ease of imaging with OCT allowed for multiple sites to be sampled and followed over multiple timepoints. The results of this study, including quantifying decreased vaginal epithelial thickness after treatment with N-9, then monitoring the subsequent thickening of the epithelial layer after discontinuation of N-9 during tissue repair were similar to findings in the clinical trial except that sheep have a thinner vaginal epithelium than women at baseline and after treatment.19 A limitation of this study is that use of a contact probe may compress tissue and affect tissue thickness measurements. However, the OCT images were obtained by a single experienced operator utilizing the same technique for acquisition of each image using light pressure contact. In addition, the authors have observed that when the vaginal mucosa is compressed, the lamina propria and muscular layers compress first and the epithelial layer remains intact (not published). This is supported by the fact that there was strong agreement between histological findings and OCT measurements.
In summary, the current study has validated the use of OCT with histology in the lower reproductive tract for the evaluation of vaginal epithelial thickness and for noninvasive monitoring of dynamic changes in epithelial thickness that could be induced by topical drug treatment or hormonal influence. Subtle differences of 30–50 micrometer decreases in thickness were detectable, a resolution not currently available with other noninvasive methods. OCT has potential to profoundly enhance research and clinical practice in gynecology by bringing a new tool for longitudinal, high resolution, noninvasive assessment of the morphology and thickness of the vaginal epithelium allowing for multiple “optical biopsies” of multiple sites over time.
Acknowledgments
This study was funded with support from the National Institutes of Health R33AI07606205, R33AI076062-03S1, and U19AI6059801.
The authors would like to thank Brent Bell, B.S. (Cleveland Clinic, Cleveland, OH), and Jinping Yang, M.D. (University of Texas Medical Branch, Galveston, TX), for technical assistance with animal studies and data collection. We gratefully acknowledge support from the National Institutes of Health R33AI07606205, R33AI076062-03S1, and U19AI6059801.
Footnotes
This study was conducted at the University of Texas Medical Branch in Galveston, Texas, USA.
DISCLOSURE: The authors report no Conflict of Interest.
Partial results from this study have been published in poster format at the Microbicides 2010 Conference (M2010), Microbicides: Building Bridges in HIV Prevention, which was held in Pittsburgh, USA on 22–25 May 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen L. VINCENT, Department of Obstetrics and Gynecology, Center for Biomedical Engineering, University of Texas Medical Branch
Gracie VARGAS, Department of Neuroscience, Center for Biomedical Engineering, University of Texas Medical Branch.
Jingna WEI, Center for Biomedical Engineering, University of Texas Medical Branch
Nigel BOURNE, Department of Pediatrics, Sealy Center for Vaccine Development, University of Texas Medical Branch.
Massoud MOTAMEDI, Department of Ophthalmology, Center for Biomedical Engineering, University of Texas Medical Branch.
References
- 1.Dickerson MC, Johnston J, Delea TE, White A, Andrews E. The causal role for genital ulcer disease as a risk factor for transmission of human immunodeficiency virus. An application of the Bradford Hill criteria. Sex Transm Dis. 1996 Sep-Oct;23(5):429–40. doi: 10.1097/00007435-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006 Jan 2;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 3.Veazey RS, Shattock RJ, Klasse PJ, Moore JP. Animal Models for Microbicide Studies. Current HIV Research. 2012;10:79–87. doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaino RJ, Nucci M, Kurman RJ. Diseases of the Vagina. In: Kurman RJ, Ellenson LH, Ronnett BM, editors. Blaustein’s Pathology of the Female Genital Tract. 6. Vol. 2011. New York: Springer; 2011. pp. 105–154. [Google Scholar]
- 5.Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;190:829– 835. doi: 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- 6.Patton DP, Thwin SS, Meier A, Hooton TM, Stapleton AE. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183:967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 7.Mauck CK, Callahan MM, Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;60:1524. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 8.Miller L, Patton DL, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96:431–9. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 9.Bounds W, Szarewski A, David Lowe D, Guillebaud J. Preliminary report of unexpected local reactions to a progestogen releasing contraceptive vaginal ring. Eur J Obstet Gynecol Reprod Biol. 1993;48:123–125. doi: 10.1016/0028-2243(93)90252-8. [DOI] [PubMed] [Google Scholar]
- 10.Vishwanathan SA, Guenthner PC, Lin CY, et al. High Susceptibility to Repeated, Low-Dose, Vaginal SHIV Exposure Late in the Luteal Phase of the Menstrual Cycle of Pigtail Macaques. J Acquir Immune Defic Syndr. 2011;57:261–4. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 11.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–S123. [PubMed] [Google Scholar]
- 12.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance simian immunodeficiency virus vaginal transmission and early viral load. Nature Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Mefford M, Sodorad D, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004;18:1637–1643. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 14.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin HL, Jr, Nyange PM, Richardson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 16.Vincent KL, Bell BA, Johnston RK, et al. Benzalkonium Chloride Causes Colposcopic Changes and Increased Susceptibility to Genital Herpes Infection in Mice. Sex Transm Dis. 2010;37(9):579–84. doi: 10.1097/olq.0b013e3181dac410. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme L, Ramjee G, Alary M, et al. COL-1492 Study Group. Effectiveness of COL-1792, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 18.Hillier SL, Moench T, Shattock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquir Immune Defic Syndr. 2005;39(1):1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- 19.Vincent KL, Stanberry LR, Moench TR, et al. Optical coherence tomography compared with colposcopy for assessment of vaginal epithelial damage: a randomized controlled trial. Obstet Gynecol. 2011;118(6):1354–61. doi: 10.1097/AOG.0b013e318238f563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. J Control Release. 2005;103(2):301–13. doi: 10.1016/j.jconrel.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Carlström K, Karlgren E, Furuhjelm M, Ryd-Kjellén E. Effects of intravaginal oestrogen treatment upon the vaginal absorption of conjugated equine oestrogens. Maturitas. 1982;4:211–283. doi: 10.1016/0378-5122(82)90059-7. [DOI] [PubMed] [Google Scholar]
- 22.Barnhart K, Shalaby W. The Vagina: Physiologic Characteristics Important to Formulators of Microbicides. In: Rencher RF, editor. Vaginal Microbicide Formulations Workshop. Vol. 1998. Philadelphia: Lippincott-Raven Publishers; 1998. pp. 1–15. [Google Scholar]
- 23.Sandri G, Rossi S, Ferrari F, Bonferoni MC, Caramella C. Strategies to Improve Systemic and Local Availability of Drugs Administered via Vaginal Route. In: Touitou E, Barry BW, editors. Enhancement in Drug Delivery 2007. Boca Raton: CRC Press; 2007. pp. 441–470. [Google Scholar]
- 24.Malcolm RK, McCullagh SD, Morrow RJ, Woolfson AD. Vagina and Uterus as Drug-Absorbing Organs. In: Touitou E, Barry BW, editors. Enhancement in Drug Delivery 2007. Boca Raton: CRC Press; 2007. pp. 295–439. [Google Scholar]
- 25.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. CAPRISA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernández-Romero JA, Abraham CJ, Rodriguez A, et al. Zinc Acetate/carrageenan gels exhibit potent activity in vivo against high dose herpes simplex virus 2 vaginal and rectal challenge. Antimicrob Agents Chemother. 2012;56(1):358–68. doi: 10.1128/AAC.05461-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 28.Vincent KL, Levine L, Bell B, Yandell R, Motamedi M. Optical coherence tomography in the evaluation of cervical dysplasia. Gynecol Oncol; Oral presentation at the 34th Annual Meeting of the Society of Gynecologic Oncologists; February 1–4, 2003; New Orleans, LA. 2003. pp. 174–5. [Google Scholar]
- 29.Escobar PF, Rojas-Espaillat L, Tisci S, et al. Optical coherence tomography as a diagnostic aid to visual inspection and colposcopy for preinvasive and invasive cancer of the uterine cervix. Int J Gynecol Cancer. 2006;16:1815–1822. doi: 10.1111/j.1525-1438.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 30.Gallwas JK, Turk L, Stepp H, et al. Optical coherence tomography for the diagnosis of cervical intraepithelial neoplasia. Lasers Surg Med. 2011;43(3):206–12. doi: 10.1002/lsm.21030. [DOI] [PubMed] [Google Scholar]
- 31.Vincent KL, Bell BA, Rosenthal SL, et al. Application of optical coherence tomography for monitoring changes in cervicovaginal epithelial morphology in macaques: potential for assessment of microbicide safety. Sex Transm Dis. 2008;35(3):269–275. doi: 10.1097/OLQ.0b013e31815abad8. [DOI] [PubMed] [Google Scholar]
- 32.Vincent KL, Bourne N, Bell BA, et al. High resolution imaging of epithelial injury in the sheep cervicovaginal tract: a promising model for testing safety of candidate microbicides. Sex Transm Dis. 2009;36(5):312–8. doi: 10.1097/OLQ.0b013e31819496e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moss JA, Malone AM, Smith TJ, et al. Simultaneous Delivery of Tenofovir and Acyclovir via an Intravaginal Ring. Antimicrob Agents Chemother. 2012;56(2):875–82. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta S, Verstraelen H, Peremans K, et al. Vaginal distribution and retention of a multiparticulate drug delivery system, assessed by gamma scintigraphy and magnetic resonance imaging. Int J Pharm. 2012;426(1–2):44–53. doi: 10.1016/j.ijpharm.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Tien D, Schnaare R, Kang F, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 36.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mingjia L, Short R. How oestrogen or progesterone might change a woman’s susceptibility to HIV-1 infection. Aust N Z J Obstet Gynaecol. 2002;42(5):472–475. doi: 10.1111/j.0004-8666.2002.00472.x. [DOI] [PubMed] [Google Scholar]
- 38.Elias C, Heise L. Challenges for the development of female controlled vaginal microbicides. AIDS. 1994;8:1–9. doi: 10.1097/00002030-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–17. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pennock JW, Stegall R, Bell B, et al. Estradiol improves genital herpes vaccine efficacy in mice. Vaccine. 2009 Sep 25;27(42):5830–6. doi: 10.1016/j.vaccine.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010 Jun;118(1 Suppl):S12–7. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henderson MH, Couchman GM, Walmer DK, et al. Optical imaging and analysis of human vaginal coating by drug delivery gels. Contraception. 2007 Feb;75(2):142–51. doi: 10.1016/j.contraception.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz DF, Gao Y, Kang M. Using modeling to help understand vaginal microbicide functionality and create better products. Drug Deliv Transl Res. 2011 Jun;1(3):256–276. doi: 10.1007/s13346-011-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozlowski PA, Williams SB, Lynch RM, et al. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 45.Hild-Petito S, Veazey RS, Larner JM, Reel JR, Blye RP. Effects of two progestin only contraceptives, Depo-Provera, and Norplant II, on the vaginal epithelium of rhesus monkeys. AIDS Res Hum Retroviruses. 1998;14:S125–30. [PubMed] [Google Scholar]
- 46.Eschenbach DA, Patton DL, Meier A, et al. Effects of oral contraceptive pill use on vaginal flora and vaginal epithelium. Contraception. 2000;62:107–112. doi: 10.1016/s0010-7824(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 47.Bahamondes L, Trevisan M, Andrade L, et al. The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera®. Contraception. 2000;62:23–27. doi: 10.1016/s0010-7824(00)00132-3. [DOI] [PubMed] [Google Scholar]