Abstract
Small molecules that increase the presynaptic function of aminergic cells may provide neuroprotection in Parkinson’s disease as well as treatments for attention deficit hyperactivity disorder (ADHD) and depression. Model genetic organisms such as Drosophila melanogaster may enhance the detection of new drugs via modifier or “enhancer/suppressor” screens, but this technique has not been applied to processes relevant to psychiatry. To identify new aminergic drugs in vivo, we used a mutation in the Drosophila vesicular monoamine transporter (dVMAT) as a sensitized genetic background, and performed a suppressor screen. We fed dVMAT mutant larvae ~1000 known drugs and quantitated rescue (suppression) of an amine-dependent locomotor deficit in the larva. To determine which drugs might specifically potentiate neurotransmitter release, we performed an additional secondary screen for drugs that require presynaptic amine storage to rescue larval locomotion. Using additional larval locomotion and adult fertility assays, we validated that at least one compound previously used clinically as an antineoplastic agent potentiates the presynaptic function of aminergic circuits. We suggest that structurally similar agents might be used to development treatments for Parkinson’s disease, depression and ADHD and that modifier screens in Drosophila provide a new strategy to screen for neuropsychiatric drugs. More generally, our findings demonstrate the power of physiologically based screens for identifying bioactive agents for select neurotransmitter systems.
Keywords: VMAT, Antidepressant, Parkinson’s disease, Neurotransmitter transporter, ADHD
Introduction
Most antidepressants and treatments of ADHD target the same proteins, mandating the development of novel screening strategies. Current treatments for Parkinson’s disease (PD) are also limited and do not slow the underlying neurodegenerative process. The presynaptic proteins required for the exocytotic release of monoamines may serve as novel therapeutic targets for both of these illnesses. These include the release machinery itself, the vesicular monoamine transporter (VMAT), required for transport of all amines into synaptic vesicles, and other proteins that regulate these activities (1). Multiple studies support the potential clinical relevance of these targets. VMAT regulates cytosolic concentrations of dopamine (DA), and the cytosolic pool of DA is neurotoxic (2, 3). Loss of VMAT2 increases DA cell death (4) whereas over-expression of VMAT is neuroprotective (5, 6). Inhibition of VMAT causes a state resembling depression (7, 8), and overexpression of VMAT mimics the effects of psychostimulants (9). To date, drugs that increase the activity or expression of VMAT are not known. More generally, with the exception of the DA precursor L-DOPA, which increases DA storage via increased synthesis, current psychotropic drugs are not able to increase the exocytotic release of biogenic amines via other mechanisms. Amphetamines, by contrast, use alternative mechanisms to release biogenic amines, including efflux through the plasma membrane dopamine transporter, and may be neurotoxic (10).
“Enhancer/suppressor”, or “modifier” screens in Drosophila melanogaster are a powerful method to identify novel genes or drugs in biological pathways of interest (11). To our knowledge, this strategy has not yet been used to identify drugs relevant to psychiatry, and relatively few screens have been reported for models of PD or other neurological diseases. Here we have used the dVMAT larval phenotype as a sensitized genetic background to screen for aminergic drugs and performed additional genetic tests to narrow potential mechanisms. We suggest that this strategy represents a new way to screen for psychotropic drugs in an intact organism without bias toward known drug targets and demonstrate its use to identify a possible new class of aminergic drugs.
Materials and Methods
Drosophila strains and maintenance
All mutations and transgenes have been described previously, including the null dVMAT loss of function mutant dVMATP1 (12), the UAS-DVMAT-A transgene (9) and the tyramine β-hydroxylase (TβH) mutant (13). All lines were outcrossed for five generations into either the wild-type strain Canton S (CS), or w1118 CS10 (w1118 outcrossed 10 times to CS) and maintained at 25°C on standard cornmeal molasses agar media in a 12 hr light dark cycle.
Drug exposure
Initial screens were performed using a library of 1039 drugs (10 mM) solubilized in dimethylsulfoxide (DMS0) (US Drug collection from Microsource Discovery systems, Gaylordsville, CT) and additional chemicals were used for validation assays as indicated (Sigma-Aldrich, St. Louis, MO). All drugs were added with vigorous mixing to molten cornmeal molasses agar media to a final concentration of ≤ 1% DMSO with food coloring (1% v/v, Kroger, Cincinnati, OH) to confirm feeding. Larva homozygous for dVMATP1 (+/−UAS-DVMAT, see text) were use for all assays and differentiated from dVMATP1/CyO siblings based on the observed rate of locomotion.
Larval locomotion assay
Two to three larvae were placed on the food/drug mixture for 30 sec to acclimate and locomotion was scored as the number of 0.4 cm grids crossed over a 2 min period (time 0). After additional incubation on the drug/food mixture for 2 hrs and 24 hrs (23°C) assays were repeated.
Fertility Assay
Three virgin females (0–3 day old) were mated with six CS males (0–3 days old) for three days on standard food then transferred to fresh food containing the indicated drug plus 1% food coloring (Kroger Foods, Cincinatti, OH). Eggs laid over the ensuing 24 hr period were counted.
Results
Weak expression of ‘leaky’ DVMAT generates a functional hypomorph
Octopamine (OA) is required for the initiation and regulation of baseline motor behavior in Drosophila larva (14). OA is thought to play a role in invertebrates similar to that of mammalian noradrenalin but is synthesized via a different enzymatic pathway and contains one rather than two ring oxygens (15). In addition to OA, dopaminergic circuits can influence locomotion under conditions of low OA and are responsible for the effects of several psychostimulants (16). Locomotor rates of wild type larvae are relatively high at baseline making it difficult to detect the effects of exogenously applied amines (Fig. 1a) or known aminergic drugs (data not shown). In contrast, dVMATP1 null larvae show severely reduced baseline larval locomotion (12), and dVMATP1 larvae fed OA show a robust and easily quantified increase in baseline locomotion (Fig. 1b). We reasoned that the dVMAT mutant might also be used as a sensitized genetic background to screen for novel aminergic drugs.
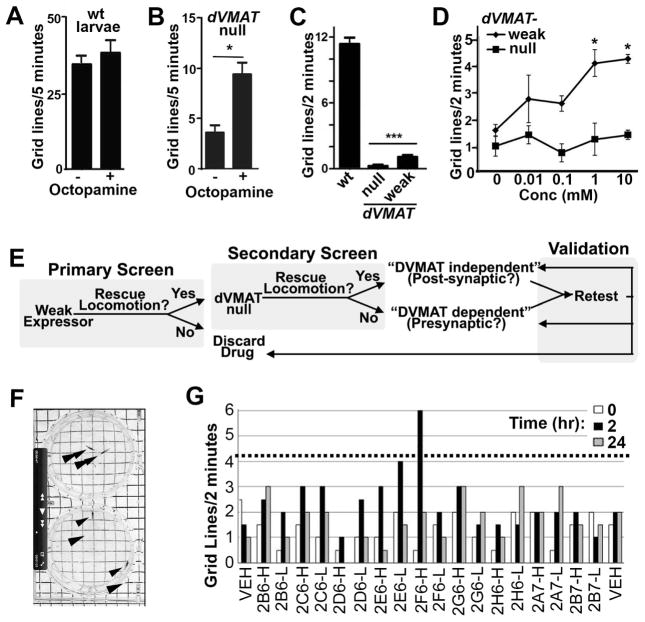
Figure 1. Screen design.
Baseline locomotion is unchanged in wild type larvae (A) but increased in dVMAT mutant larvae fed octopamine (panel B, OA) or vehicle for 2 hrs (1 way ANOVA *p<0.05, n=10 larvae per condition. (C) Locomotion of the dVMAT weak expressor shows a slight increase relative to the dVMAT null mutant but is severely reduced compared to wt larvae (one-way ANOVA p<0.0001, Bonferroni post test, ***p<0.001, mean+/− SEM, n=30–31). (D) Feeding amphetamine increases locomotion in the dVMAT weak expressor but not the null (one-way ANOVA p<0.05, Bonferroni post test *p<0.05, mean +/−SEM, n=8–9 larvae. (E) Screening strategy. In our Primary Screen we assayed drugs for their ability to increase or “rescue” the deficit in locomotion seen in larvae expressing low levels of DVMAT (the dVMAT “Weak Expressor”). In the Secondary Screen, candidate drugs were tested for their ability to increase locomotion in the dVMAT null. Drugs able to rescue locomotion in both the Primary and Secondary screens were designated “DVMAT independent” and therefore potentially acting via post-synaptic mechanisms. Conversely, drugs able to rescue locomotion in the Primary but not the Secondary screen were designated as “DVMAT dependent” and potentially acting via presynaptic mechanisms to increase amine release. During the Validation Phase of the screen, candidates were retested and either confirmed as DVMAT dependent or independent, reclassified or discarded depending on the ability of a second formulation of drug to activate locomotion. (F) A still photo from a videotape of two assay plates. The larva (arrowheads) on the top and bottom plates have moved ~1 grid and 3–4 grids respectively from the center of the plates. (G) Example of primary screen data. Drugs tested at both high (100uM, designated ‘H’) and low (10uM, designated ‘L’) concentrations at 0, 2 and 24 hrs as indicated. Dotted line: 4.2 grids per 2 min, used as cut-off for strong hits (e.g. 2F6-H).
To allow the identification of drugs that require might act presynaptically, we developed a sensitized background that retained some degree of presynaptic function. We crossed into the dVMATP1null background a leaky UAS-DVMAT transgene that expresses low levels of DVMAT in the absence of an additional exogenous Gal4 driver (6). We designate this line (dVMATP1; UAS-DVMAT) the dVMAT “weak expressor”. Locomotion rates of the “weak expressor” are low relative to wt but significantly higher than the dVMAT null (Fig. 1c). To confirm that the dVMAT weak expressor would respond to drugs that act presynaptically, we used amphetamine, which promotes the presynaptic release of monoamines in both mammals (10) and flies (16). Amphetamine feeding caused a dose-dependent increase in locomotion in the dVMAT weak expressor, but not the dVMAT null (Fig. 1d).
Locomotion based primary screen
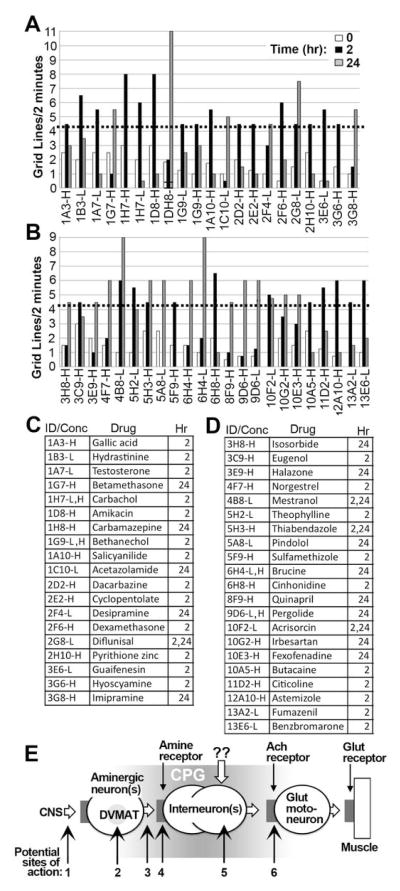
In our primary screen (Fig. 1e) we tested whether any of 1039 drugs (Table S1) would increase locomotion in the dVMAT weak expressor, using two concentrations of each drug (10 and 100 uM). Locomotion was assayed before ingestion (0 hrs) and after 2 and 24 hrs of incubation on food containing each drug. A photo of an assay plate (Fig. 1f) and a representative sample of data from the primary screen is shown (Fig. 1g, See Table S1 for names of all 1039 tested drugs and Fig. S1 for primary screen data). Averaging across all drugs and vehicle controls, the mean+/−SD grid lines traveled over 2 min at 0, 2 and 24 hrs was 1.18+/−0.86, 1.33+/−1.09 and 1.13+/−1.2 respectively (n=2193). We identified 40 “strong hits” as drugs that increased locomotion at either 2 or 24 hr and either 10 or 100 uM >3.5 SD (>4.2 grid lines) above the mean at time 0 (Fig. 2a–d). An additional 76 drugs were scored as “weak hits”: locomotion >2 but < 3.5 SD above the mean (>2.9 and < 4.2 gridlines per 2 min, Table S2).
Figure 2. Primary screen hits.
(A–D) Shown are the 40 of 1039 tested drugs that increased locomotion >3.5 SD above the mean (>4.2 grid lines per 2 min, see dotted line) at either 2 or 24 hr and either the low (L, 10 uM) or high (H, 100uM) concentrations, labeled with the ID number from the Microsource library used for the primary screen. The drugs shown in panels A, B are listed (Table C, D) by their library ID number and their effective concentration (High or Low as in A, B, shown as ID–H or –L in the first column of C and D). The assay times (2 or 24 hrs after administration, or both times) at which the drug yielded a positive locomotor response is listed in the right hand column of C and D. (E) Model of larval locomotor circuit. The larval locomotor circuit remains incompletely defined; we show here a heuristic model of the minimal elements known to be present in the locomotor circuit as an aid for interpretating the results of the screen. Minimal elements of the circuit include (from right to left) the muscle expressing glutamate receptors (Glut receptor) innervated by a glutamatergic motoneuron (Glut motoneuron). Larval motoneurons express Ach receptors that stimulate their activity; they are therefore presumed to be innervated by a cholinergic interneuron but the identity and number of interneurons(s) is not known. Amines including octopamine and under some conditions dopamine are released onto yet to be identified cells (labeled “Interneurons”) to activate the central pattern generator (“CPG”) and thus stimulate locomotion. The cells included in the larval locomotor CPG are not yet clearly defined and the shading of the CPG reflects this ambiguity. It is possible that there are additional non-aminergic inputs to the CPG capable of activating locomotion; these are indicated by “??”. Numbered arrows indicate possible sites of action for drugs including 1) upstream sits in the CNS that activate the aminergic neurons, 2) DVMAT and/or proteins that regulate vesicular storage in the aminergic neurons, 3) the exocytotic release machinery of the aminergic neurons, 4) amine receptors including OA receptors for a activation of baseline locomotion, 5) alternative amine-independent inputs (indicated by “??”), 6) Ach receptors. Targets 1–3 would require presynaptic amine storage and are DVMAT dependent (see text). Targets 4–6 would function independently of DVMAT and presynaptic amine storage.
Secondary screen
The drugs identified in our primary screen could act at several distinct sites in the locomotion circuit, but can be distinguished by their requirement for amine storage and release (Fig. 2e). Those acting presynaptically in aminergic cells or other upstream sites in the CNS will require DVMAT to allow amine storage. These “DVMAT dependent” drugs will stimulate movement in the dVMAT weak expressor but not the null. Conversely “DVMAT independent” drugs that act post-synaptically at the aminergic synapse(s) that stimulate larval locomotion (14, 16) or downstream sites will not require DVMAT for amine storage and release and will stimulate movement in both the DVMAT weak expressor and the null.
Of 40 strong hits from the primary screen, 15 were unable to stimulate movement in the dVMAT null mutant in the secondary screen (Table S3). These drugs were tentatively designated as DVMAT-dependent. In contrast, 11 drugs that strongly stimulated locomotion in the primary screen also strongly stimulated locomotion in the dVMAT null in the secondary screen and were tentatively designated as DVMAT independent (Table S3). A third group of 14 drugs weakly stimulated movement in the dVMAT null in the secondary screen and thus did not clearly fall into either the DVMAT dependent or independent category (Table S3). To facilitate further analysis, we focused on those that were either DVMAT dependent or independent rather than these more ambiguous candidates.
Validation of candidates from primary and secondary screens
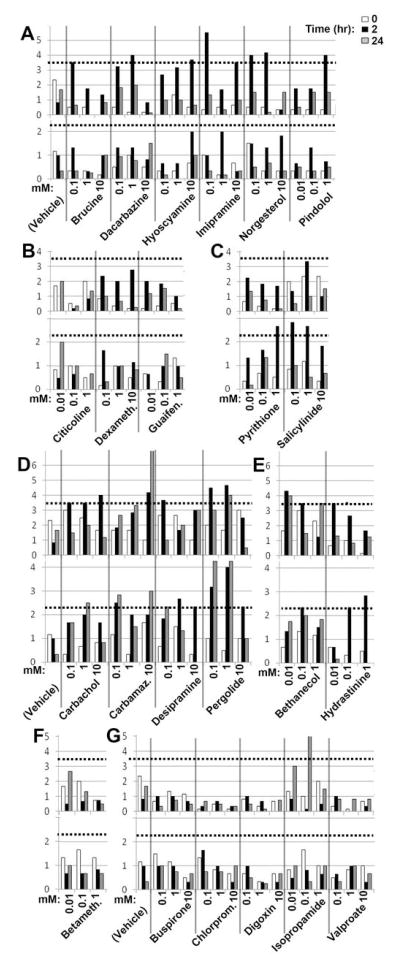
To validate the results of our primary and secondary screens, we obtained from commercial sources 13 of 15 DVMAT dependent drugs (Table S4 lists other drugs that were not available). Six of these were validated to stimulate locomotion in the weak expressor (Fig. 3a, top panel) but not the null (Fig. 3a, bottom panel). An additional class of 2 drugs (Fig. 3c) paradoxically showed increased locomotion in the dVMAT null but not the weak expressor and were not pursued. Four of the drugs tentatively identified as DVMAT independent (and commercially available) were validated as able to stimulate locomotion in both the dVMAT null and the weak expressor (Fig. 3d). In addition, 2 drugs tentatively identified as DVMAT dependent were reclassified as DVMAT independent (Fig. 3e) bringing the total to 6 (Table 1). Retesting a subset of five weak hits from our primary screen (Fig. 3g) identified only one additional DVMAT dependent drug for a total of 7 (Table 1). Since our yield was relatively low, we did not attempt to validate any additional “weak” hits.
Figure 3. Validation assays.
(A–C) Putative DVMAT independent drugs. 6 drugs stimulated locomotion in the weak expressor (A, top panel) but not the null (A, bottom panel) confirming DVMAT dependence. B) 3 failed to stimulate locomotion in either genotype. (C) 2 showed minimal activity in the weak expressor (C, top) but stimulated locomotion in the null (C, bottom, see text). (D–G) Validation of putative DVMAT independent drugs. 4 drugs stimulated locomotion in the weak expressor (D, top) and null (D, bottom) confirming DVMAT independence. Two drugs originally classified as DVMAT dependent stimulated locomotion in both the weak expressor (E, top panel) and the null (E) and were reclassified as DVMAT independent. (F) Betamethasone failed to stimulate locomotion was discarded. (G) Retesting “weak hits”. One drug (isopropamide) activated locomotion in the weak expressor (G, top) but not the null (G) and was classified as DVMAT dependent. Drugs were tested at two concentrations above the minimum that was effective in the primary and secondary screens: 10um, 100um and 1 mM or 100uM, 1mM and 10mM as indicated. Dotted line: cut-off for validated hits (3 SD above the mean at time 0 for all drugs tested in the weak expressor during the validation phase (3.6 grid lines/2 min), or 4 SD above mean at time 0 for all drugs tested in the null during the validation phase (2.2 grid lines/2 min). Abbreviations: Carbamaz., Carbamazepine; Dexameth., Dexamethasone.; Guaifen, Guaifenesin; Betameth., Betamethasone; Chlorprom., Chlorpromazine.
Table 1.
Validated Hits
| Drug | Known Use | Class | |
|---|---|---|---|
| 1 | Desipramine | Antidepressant | VMAT independent |
| 2 | Pergolide | Dopamine receptor agonist | ” |
| 3 | Carbachol | Cholinergic | ” |
| 4 | Carbamazepine | Mood Stabilizer, Antiseizure | ” |
| 5 | *Hydrastinine | Antihemorrhagic | ” |
| 6 | *Bethanechol | Cholinergic | ” |
| 1 | Imipramine | Antidepressant | VMAT dependent |
| 2 | Brucine | Cholinergic | ” |
| 3 | Hyoscyamine | Anticholinergic | ” |
| 4 | Isopropamide iodide | Anticholinergic | ” |
| 5 | Dacarbazine | Chemotherapeutic | ” |
| 6 | Norgesterol | Contraceptive | ” |
| 7 | Pindolol | Beta blocker | ” |
Asterisk indicates drugs reclassified as DVMAT independent during validation.
Several hits identified in our original screen could not be replicated during validation and, as noted above, three others were reclassified. There are several possible reasons why the results of our validation assays were not completely consistent with our initial screens. These include: 1) experimental error resulting from the manual collection of data by different individuals; 2) differences between the formulation, purity and degree of degradation between the drugs used in our initial screens which were from a commercial library, versus those used in the validation assays which were purchased from a separate source. Future screening efforts will employ an automated data collection system (not shown) reducing at least one potential source of varialbility.
Validated hits (Table 1) included several cholinergic compounds, consistent with the known role of acetylcholine in Drosophila locomotion and the cholinergic input to larval motoneurons (17, 18) (See Fig. 2e). Others appeared to represent a range of drugs with varied known uses (Table 1). The mode of action of these drugs in our assay may or may not be similar to the activity for which they are currently used. To address this issue and provide preliminary mechanistic information, we selected for further analysis one of the 7 validated DVMAT dependent drugs and one of the 6 DVMAT independent drugs. We focused on drugs able to give a robust signal at the 2 hr time point during the validation phase rather than those than only stimulated locomotion after 24 hrs since the former would be more likely to act via relatively direct mechanisms as opposed to long-term changes in transcription. Of the DVMAT independent drugs, we focused on pergolide, a known amine receptor agonist in mammals (see below). Of the DVMAT dependent drugs, we focused on dacarbazine, previously used only as an anticancer drug (19) rather than a psychotropic.
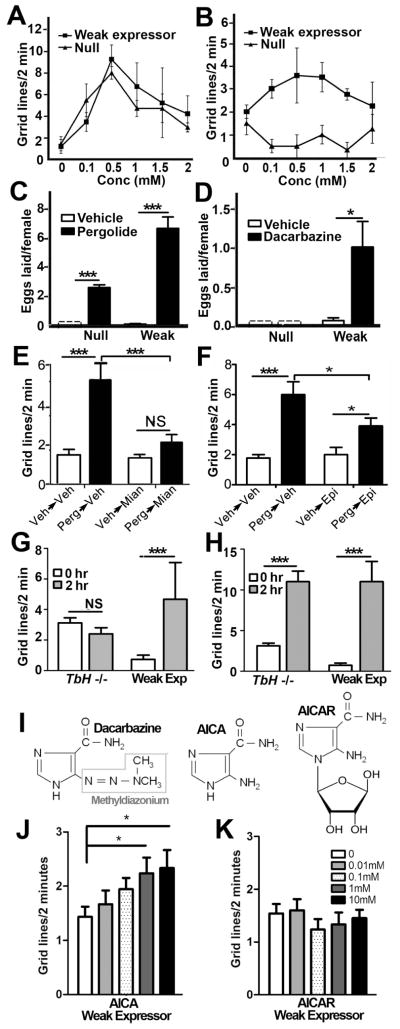
Further tests of pergolide and dacarbazine
Additional dose response experiments show that both dacarbazine (Fig. 4a) and pergolide (Fig. 4b) increase locomotion at concentrations up to 0.5–1mM in the weak expressor. To determine whether pergolide and dacarbazine were generally active as aminergic agents in the fly, we tested their affects on another amine-dependent behavior: egg-laying in the adult. This behavior is controlled predominantly by OA (13, 20–23) although oocyte development may be regulated in part by DA (24). Consistent with its assignment as a DVMAT independent drug in larval locomotion assays, pergolide at 1mM partially rescued egg laying in both the dVMAT null and weak expressors (Fig. 4c). By contrast, 1mM dacarbazine rescued egg-laying in the dVMAT weak expressor but had no effect in the null mutant (Fig. 4d), thus demonstrating that it acts in a DVMAT dependent fashion in at least two independent circuits in larvae and adults.
Figure 4. DVMAT dependent versus independent activities of selected drugs.
(A) Pergolide increased locomotion in the null and weak expressor with a peak effect at 0.5 mM but steadily reduced locomotion at higher concentrations. (B) Dacarbazine increased locomotion in the weak expressor but not the null with a peak effect at 0.5 mM, One-Way ANOVA, ***p<0.001, **0<0.01, *p<0.05. (C) Pergolide at 1mM partially rescued adult egg-laying in both the null and weak expressor. (two-way ANOVA p<0.0001. Bonferroni post test, ***p<0.001). (D) Dacarbazine (1mM) rescued egg-laying in the weak expressor but not the null (two-way ANOVA, Bonferroni post test, *p<0.05). For panels A–D, bars represent mean +/− SEM, n=16–20 larvae per condition. (Dashed lines boxes are a place marker for eggs laid by animals treated with vehicle alone; the actual value of the bar is zero). (E–H) Pergolide function requires OA signaling. (E) Treatment with mianserin. As compared to animals treated with vehicle for 2 hrs followed by vehicle for an additional 2 hrs (Veh=>Veh), animals treated with 1 uM pergolide for 2 hrs followed by vehicle alone (Perg=>Veh) showed an increase in locomotion (For both panels E and F: two-way ANOVA, ***p<0.0001; Bonferroni post test, ***p<0.001, *p<0.05, n=6–12, error bars represent +/− SEM). Larvae fed pergolide then mianserin (0.25 mg/ml, Perg=>Mian) locomote less than larva fed pergolide followed by vehicle (Perg=>Veh). (F) Larvae fed pergolide then epinastine (Perg =>Epi) locomote less than larva fed pergolide then vehicle (Perg =>Veh) but more than controls (Veh=>Veh and Veh=>epinastine) suggesting partial rather than complete inhibition of pergolide’s effects by epinastine. (G, H) Dacarbazine but not pergolide requires presynaptic OA synthesis. (G) Dacarbazine (100 uM for 2 hrs) does not stimulate locomotion in the TbH mutant (HTbH −/−, two-way ANOVA p<0.05, Bonferroni post test, ***p<0.001, n=6. (H) Pergolide (1uM, 2 hrs) stimulates locomotion in the TbH mutant (two-way ANOVA p<0.0001, Bonferroni post test, ***p<0.001). (I) Structures of dacarbazine, 5-amino-4- imimdazole-carboxamide-HCL (AICA) and aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR). (J, K) Dose-dependent increase in locomotion in the dVMAT weak expressor fed AICA (panel J, 2 hrs, two-way ANOVA, p<0.0001. Bonferroni post test, *p<0.05, **p<0.01 as indicated) but not AICAR (panel K) (n=6–15 larvae per condition).
Since the sensitized background used in out primary screen employs a “leaky” UAS-DVMAT transgene that is expressed at low levels in the absence of a cell-specific Gal4 driver, it should allow low levels of amine storage and release from all types of aminergic neurons including those that synthesize DA, 5-HT and OA. However, since baseline larval locomotion and egg-laying are primarily regulated by OA (13, 14, 20, 21) we focused further mechanistic experiments on OA circuits. We first tested the effects of the OA receptor antagonists mianserin and epinastine (25, 26). (Both decrease the locomotion of CS larvae, Fig. S2). dVMAT null larvae were fed 1uM pergolide for 2h, followed by mianserin, epinastine or vehicle alone. Pergolide continued to stimulate locomotion after transfer to food containing vehicle alone (Fig. 4e, f). In contrast, although neither drug completely abolished the effect of pergolide, transfer to food containing mianserin or epinastine reduced the locomotor effects of pergolide by 60% (****p<0.0001 two-way ANOVA; ***p<0.001, Bonferroni post test) and 33% (***p<0.0001, two-way ANOVA; *p<0.05, Bonferroni post test) respectively (Fig. 4e, f, compare first black bars to second black bars). In support of the specificity of mianserin and epinastine for OA circuits, we tested these drugs on tyramine beta hydroxylase (TbH) mutants and found no effect (not shown). The simplest explanation of our results is that pergolide can act in part as an OA receptor agonist in Drosophila, in addition to its activity as a D2 receptor agonist in mammals (27) and that activation of OA receptors by pergolide stimulates larval locomotion.
To determine whether OA synthesis is required for dacarbazine or pergolide to stimulate locomotion, we used the tyramine beta hydroxylase (TbH) mutant, which is unable to convert the precursor tyramine to OA (13). Homozygous TbH mutants show defects in larval locomotor behavior consistent with a requirement for OA (14, 28). Dacarbazine had no effect on locomotion behavior in the TbH null (Fig. 4h) as compared to the dVMAT weak expressor control. In contrast, pergolide (Fig. 4i) increased larval locomotion in both the TbH null and the dVMAT weak expressor. These results further suggest that dacarbazine may act within the OA cell itself to stimulate the amine storage and release, or at a site in the nervous system upstream of the OA cell that regulates baseline locomotion.
A structural analog of dacarbazine increases locomotion in the dVMAT weak expressor but not in the dVMAT null
Dacarbazine is currently used as an antineoplastic agent (29). Toxic effects would limit its use as a psychotropic in mammals, and high doses are toxic to fly larvae (Fig. S3). In mammals, dacarbazine is metabolized to methyldiazonium, which mediates DNA alkylation, plus the non-toxic 5-amino-4-imidazole-carboxamide (AICA) backbone (29) (see Fig. 4j). Unlike dacarbazine, AICA does not show toxicity in the fly at high concentrations (Fig. S3). However, similar to dacarbazine, AICA increases locomotion in a dose-dependent fashion after either 2 hr (Fig. 4k) or 4 hr exposure (Fig. S4) in the dVMAT weak expressor but not the dVMAT null (Figs. S4). One available derivative of AICA is 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR, Fig. 4j) a potential antihyperglycemic agent thought to act in part via stimulation of AMP kinase (30). In contrast to AICA, AICAR did not increase locomotion in the dVMAT weak expressor at 2 hrs or 4 hrs in either the weak expressor or the null (Fig. 4l and S4).
Discussion
We describe here the use of a “modifier” or “enhancer/suppressor” screen for psychotropic drugs in Drosophila melanogaster. Screening for a change in functional output in an intact animal selects for targets that are physiologically relevant, and selects for drugs that are able penetrate a glial barrier to enter the CNS (31). The use of a sensitized background (here the dVMAT mutant) focused our screen on a specific biological process (aminergic signaling) without limiting the range of protein targets. The sensitized genetic background also allowed the detection of pharmacologic activities that are difficult to observe in the wild type. We are not aware of any previous studies using this method to screen for aminergic drugs or any other small molecules relevant to the treatment of psychiatric illness.
Importantly, we have identified drugs that give a desirable functional effect without selecting for a specific protein target. We recognize that additional experiments will be needed to determine the mechanism of action of any drugs identified in this manner. However, we emphasize that because of the unbiased nature of our screen, the potential mechanisms will not be restricted to known drug targets. We therefore propose that this approach will complement other existing methods of drug discovery in which specific protein targets are preselected, and may allow the detection of drugs that act on novel targets unrelated to those already used for the treatments for depression, ADHD and PD.
In preliminary transport experiments, we have not been able to detect a direct effect of dacarbazine on VMAT transport activity (data not shown). Indeed, we are not aware of any psychotropic drugs that can directly increase the physiological activity of a neurotransmitter transporter. Rather we suggest that the drugs we identify are more likely to increase VMAT activity in an indirect fashion. We suggest that the strength of our system, and the use of an in situ aminergic circuit, is that it will allow the detection of drugs that act on a variety of novel targets that act upstream of VMAT, or may otherwise increase amine release via other indirect mechanisms. We further suggest that such targets may not be present in less complex screening platforms, and because they indirectly stimulate neurotransmission, may not be obvious “aminergic” targets for drug development.
Although we used an octopaminergic circuit in our screen, some of the drugs we have identified may be generally active at other types of aminergic nerve terminals, similar to DVMAT and mammalian VMAT2, which are expressed broadly in most if not all aminergic neurons in the CNS (1, 32). Since the molecular mechanisms of monoamine release are conserved across many species, it is also possible that some drugs will be active in mammalian systems. Indeed, assays of peripheral blood in rodents and patients treated with dacarbazine demonstrate an increase in peripheral 5HT metabolites, due to release of 5HT from enterochromaffin cells (33, 34). These observations support the notion that at least some of the drugs we have identified may be active across multiple types of aminergic neurons and have similar effects in mammals.
Since dacarbazine is used as a chemotherapeutic to kill dividing cells (29) it is unlikely to be directly useful as a psychotropic or neuroprotective agent. We find that 5-Aminoimidazole-4-carboxamide (AICA), which lacks an alkylating moiety but is otherwise identical to dacarbazine, mimics its activity in the fly. Another derivative, 5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), which is undergoing trials as an antihyperglycemic agent (30, 35) did not stimulate locomotion. It is likely that additional derivatives will need to be developed if this class of molecules is to be investigated for its potential effects in mammals.
In conclusion, our data support the use of modifier screens in the fly to identify novel aminergic agents. If any of the drugs we have identified or structurally related derivatives are found to increase amine release in mammals they might be used to treat depression or ADHD. Increasing amine storage in vesicles also has the potential to sequester cytotoxic DA metabolites away from their site of action (36). Thus, some identified drugs might provide novel neuroprotective strategies for PD (5, 6, 37). In support of this possibility, preliminary data suggest that a subset of DVMAT-dependent drugs can increase the survival of mammalian dopaminergic neurons in culture (data not shown). More generally, we suggest that modifier screens in Drosophila provide a new way to screen for psychotropic drugs without bias toward previously identified protein targets. Similar screens also might be used to identify novel GABAergic or glutamatergic agents.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health [MH076900], the National Institute of Environmental Health and Safety [ES015747] and NARSAD “The Brain and Behavior Research Foundation” (to D.E.K.), additional funding from an National Institute of Environmental Health and Safety program project grant [ES016732, M.F. Chesselet, PI) and training fellowships from the National Institute of Environmental Health and Safety (to H.O.L. and A.T.). The authors thank Drs. Lori Altshuler and Roland Bainton for helpful suggestions on the manuscript.
Footnotes
Conflict of Interest
The authors declare there are no competing financial interests in relation to the work described.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Chaudhry FA, Boulland JL, Jenstad M, Bredahl MK, Edwards RH. Pharmacology of neurotransmitter transport into secretory vesicles. Handb Exp Pharmacol. 2008;184:77–106. doi: 10.1007/978-3-540-74805-2_4. [DOI] [PubMed] [Google Scholar]
- 2.Hastings TG, Lewis DA, Zigmond MJ. Reactive dopamine metabolites and neurotoxicity: implications for Parkinson’s disease. Adv Exp Med Biol. 1996;387:97–106. doi: 10.1007/978-1-4757-9480-9_13. [DOI] [PubMed] [Google Scholar]
- 3.Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- 4.Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, et al. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sang TK, Chang HY, Lawless GM, Ratnaparkhi A, Mee L, Ackerson LC, et al. A Drosophila model of mutant human parkin-induced toxicity demonstrates selective loss of dopaminergic neurons and dependence on cellular dopamine. J Neurosci. 2007;27:981–992. doi: 10.1523/JNEUROSCI.4810-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawal HO, Chang HY, Terrell AN, Brooks ES, Pulido D, Simon AF, et al. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol Dis. 2010;40:102–112. doi: 10.1016/j.nbd.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freis ED. Mental depression in hypertensive patients treated for long periods with high doses of reserpine. New England Journal of Medicine. 1954;251:1006–1008. doi: 10.1056/NEJM195412162512504. [DOI] [PubMed] [Google Scholar]
- 8.Fukui M, Rodriguiz RM, Zhou J, Jiang SX, Phillips LE, Caron MG, et al. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang H-Y, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, et al. Over-expression of the Drosophila vesicular monoamine transporter increases motor activity and courtship but decreases the behavioral response to cocaine. Molecular Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 10.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 12.Simon AF, Daniels R, Romero-Calderón R, Grygoruk A, Chang HY, Najibi R, et al. Drosophila vesicular monoamine transporter mutants can adapt to reduced or eliminated vesicular stores of dopamine and serotonin. Genetics. 2009;181:525–541. doi: 10.1534/genetics.108.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monastirioti M, Linn CEJ, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. J Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox LE, Soll DR, Wu CF. Coordination and modulation of locomotion pattern generators in Drosophila larvae: effects of altered biogenic amine levels by the tyramine Beta hydroxlyase mutation. J Neurosci. 2006;26:1486–1498. doi: 10.1523/JNEUROSCI.4749-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suo S, Kimura Y, Van Tol HH. Starvation induces cAMP response element-binding protein-dependent gene expression through octopamine-Gq signaling in Caenorhabditis elegans. J Neurosci. 2006;26:10082–10090. doi: 10.1523/JNEUROSCI.0819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, et al. The membrane-raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.82. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorczyca MG, Budnik V, White K, Wu CF. Dual muscarinic and nicotinic action on a motor program in Drosophila. J Neurobiol. 1991 Jun;22(4):391–404. doi: 10.1002/neu.480220407. [DOI] [PubMed] [Google Scholar]
- 18.Rohrbough J, O’Dowd DK, Baines RA, Broadie K. Cellular bases of behavioral plasticity: establishing and modifying synaptic circuits in the Drosophila genetic system. J Neurobiol. 2003;54:254–271. doi: 10.1002/neu.10171. [DOI] [PubMed] [Google Scholar]
- 19.Bonfante V, Santoro A, Viviani S, Valagussa P, Bonadonna G. ABVD in the treatment of Hodgkin’s disease. Semin Oncol. 1992 Apr;19(2 Suppl 5):38–44. discussion 44–35. [PubMed] [Google Scholar]
- 20.Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264:38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Middleton A, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJ. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HG, Seong CS, Kim YC, Davis RL, Han KA. Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster. Dev Biol. 2003;264:179–190. doi: 10.1016/j.ydbio.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Lee HG, Rohila S, Han KA. The octopamine receptor OAMB mediates ovulation via Ca2+/calmodulin-dependent protein kinase II in the Drosophila oviduct epithelium. PLoS One. 2009;4:e4716. doi: 10.1371/journal.pone.0004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willard SS, Koss CM, Cronmiller C. Chronic cocaine exposure in Drosophila: life, cell death and oogenesis. Dev Biol. 2006;296:150–163. doi: 10.1016/j.ydbio.2006.04.448. [DOI] [PubMed] [Google Scholar]
- 25.Unoki S, Matsumoto Y, Mizunami M. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. The European journal of neuroscience. 2005;22:1409–1416. doi: 10.1111/j.1460-9568.2005.04318.x. [DOI] [PubMed] [Google Scholar]
- 26.Ohtani A, Arai Y, Ozoe F, Ohta H, Narusuye K, Huang J, et al. Molecular cloning and heterologous expression of an alpha-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Mol Biol. 2006;15:763–772. doi: 10.1111/j.1365-2583.2006.00676.x. [DOI] [PubMed] [Google Scholar]
- 27.Radad K, Gille G, Rausch WD. Short review on dopamine agonists: insight into clinical and research studies relevant to Parkinson’s disease. Pharmacol Rep. 2005;57:701–712. [PubMed] [Google Scholar]
- 28.Saraswati S, Fox LE, Soll DR, Wu CF. Tyramine and octopamine have opposite effects on the locomotion of Drosophila larvae. J Neurobiol. 2004;58:425–441. doi: 10.1002/neu.10298. [DOI] [PubMed] [Google Scholar]
- 29.Pourahmad J, Amirmostofian M, Kobarfard F, Shahraki J. Biological reactive intermediates that mediate dacarbazine cytotoxicity. Cancer Chemother Pharmacol. 2009;65:89–96. doi: 10.1007/s00280-009-1007-8. [DOI] [PubMed] [Google Scholar]
- 30.Gaidhu MP, Fediuc S, Ceddia RB. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem. 2006;281:25956–25964. doi: 10.1074/jbc.M602992200. [DOI] [PubMed] [Google Scholar]
- 31.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderón R, Chang H-Y, et al. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin and octopamine. J Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- 33.Cubeddu LX, Hoffmann IS, Fuenmayor NT, Malave JJ. Changes in serotonin metabolism in cancer patients: its relationship to nausea and vomiting induced by chemotherapeutic drugs. Br J Cancer. 1992;66:198–203. doi: 10.1038/bjc.1992.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubeddu LX. Serotonin mechanisms in chemotherapy-induced emesis in cancer patients. Oncology. 1996;53 (Suppl 1):18–25. doi: 10.1159/000227636. [DOI] [PubMed] [Google Scholar]
- 35.Boon H, Bosselaar M, Praet SF, Blaak EE, Saris WH, Wagenmakers AJ, et al. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia. 2008;51:1893–1900. doi: 10.1007/s00125-008-1108-7. [DOI] [PubMed] [Google Scholar]
- 36.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.