Abstract
The current study evaluated processes underlying two common symptoms (i.e., state regulation problems and deficits in auditory processing) associated with a diagnosis of autism spectrum disorders. Although these symptoms have been treated in the literature as unrelated, when informed by the Polyvagal Theory, these symptoms may be viewed as the predictable consequences of depressed neural regulation of an integrated social engagement system, in which there is down regulation of neural influences to the heart (i.e., via the vagus) and to the middle ear muscles (i.e., via the facial and trigeminal cranial nerves). Respiratory sinus arrhythmia (RSA) and heart period were monitored to evaluate state regulation during a baseline and two auditory processing tasks (i.e., the SCAN tests for Filtered Words and Competing Words), which were used to evaluate auditory processing performance. Children with a diagnosis of autism spectrum disorders (ASD) were contrasted with aged matched typically developing children. The current study identified three features that distinguished the ASD group from a group of typical developing children: 1) baseline RSA, 2) direction of RSA reactivity, and 3) auditory processing performance. In the ASD group, the pattern of change in RSA during the attention demanding SCAN tests moderated the relation between performance on the Competing Words test and IQ. In addition, in a subset of ASD participants, auditory processing performance improved and RSA increased following an intervention designed to improve auditory processing.
Keywords: respiratory sinus arrhythmia, heart rate variability, autism, auditory processing, Polyvagal Theory
1.0 Introduction
Difficulties in state regulation and deficits in auditory processing are prevalent symptoms is autism spectrum disorders (ASD), although neither are a criterion for diagnosis. Problems in state regulation may be expressed as atypical social and emotional behaviors (e.g., Bachevalier & Loveland, 2005), low thresholds to be reactive, tantrums, difficulties in sustaining attention, and sleep disorders. The auditory processing deficits may be experienced as language and speech delays, difficulties in extracting human voice from background sounds, hyperacusis, or as a general compromise in social communication skills (Dissanayake & Sigman, 2001; Frith & Baron-Cohen, 1987; Hayes & Gordon, 1977; Klin, 1992; Lockyer & Rutter, 1969).
The mechanisms mediating state regulation and auditory processing are assumed to represent disparate response systems. From an empirical perspective state regulation is manifested in observable behaviors, while auditory processing is manifested in expressive and receptive language skills. These problems are not unique to individuals diagnosed with autism, and similar symptoms have been observed in other clinical disorders including fragile X syndrome, attention deficit disorder, post-traumatic stress disorder, and anxiety disorders.
In typically developing children both domains exhibit a developmental trajectory. During the first few years of life language and listening skills improve and are paralleled by improved state regulation. The improved state regulation is often observed as an increased ability to sustain attention, while actively inhibiting responses to distracters. During aging, the reverse is observed and there are increases in problems associated with both domains.
Since deficits in state regulation and auditory processing lack diagnostic specificity, researchers who study the neurobiological and biobehavioral features of a specific clinical diagnosis, such as autism, have not focused on these domains. Rather, research in clinical neuroscience has targeted investigations of potential biomarkers (e.g., genetic, neurochemical) unique to a diagnostic category. This strategy assumes the possibility that a biomarker or set of biomarkers could be identified, which would provide needed information to reconceptualize the diagnosis and treatment of autism. However, research need not be focused on specificity of neurobiological features and can be directed at understanding the neural mechanisms leading to compromised behaviors common to several diagnostic categories. Thus, in spite of this lack of diagnostic specificity, difficulties in state regulation and auditory processing are prevalent, and degrade quality of life by interfering with an ability to participate in social interactions and educational opportunities in the home, clinic, and classroom. This focus on observable behavior and measureable neurobiological processes, independent of clinical diagnosis, is consistent with the NIMH Strategic Plan for Research Domain Criteria.
State regulation and auditory processing are dependent on response systems that are studied by different scientific disciplines, which have little interaction and virtually no common language. For example, the study of psychiatric disorders (i.e., diagnoses), behavioral problems (e.g., state regulation and tantrums), psychological difficulties (e.g., emotional instability), auditory processing disorders (e.g., difficulties understanding verbal instructions), and cognitive deficits (e.g., language delays) represent research domains investigated by separate disciplines. These distinctions contribute to the current models of inquiry applied in clinical neuroscience, which rely on separate disciplines to categorize, investigate, treat, and explain the neurobiological mechanisms of clinical disorders.
Since deficits in state regulation and auditory processing frequently are observed in the same individual, do they share common neural mechanisms? If these deficits have a common neural substrate, will an understanding of this “common” neural mechanism provide insights into the management of symptoms frequently observed in individuals with autism? The above questions may require a new conceptualization of the phenotypic features of autism, especially if features, such as deficits in state regulation and auditory processing, are shared with other clinical disorders.
The Polyvagal Theory (Porges, 1995, 1998, 2001, 2003, 2007) proposes a strategy that applies evolution as an organizing principle to understand the link between state regulation and auditory processing. According to the Polyvagal Theory, the well-documented phylogenetic shift in neural regulation of the autonomic nervous system provided mammals with a neural circuit that promoted social interactions in safe contexts by supporting calm physiological states and an ability to process relatively soft vocalizations in a frequency band distinct from the lower frequencies associated with predators (see Porges & Lewis, 2009). This “mammalian” circuit functions as the neural substrate for an integrated social engagement system. Many of the behavioral attributes of autism appear to be convergent with a compromise in this hypothetical social engagement system.
The phylogenetic origin of the social engagement system is intertwined with the evolutionary changes in vertebrate autonomic nervous systems. As the muscles of the face and head emerged as social engagement structures, a new component of the autonomic nervous system (i.e., a myelinated vagus) evolved that was regulated by the nucleus ambiguus, a medullary nucleus ventral to the dorsal motor nucleus of the vagus. This convergence of neural mechanisms regulating heart and face provides the neural substrate for an integrated social engagement system with synergistic facial and visceral components. The product of this evolution is a system that co-opted a variety of structures and processes to support social behavior including such disparate processes as ingestion and state regulation, and integrated these processes with social engagement behaviors involving facial expressivity, head gesture, listening, and vocalizations (see Table 1).
Table 1.
Muscles of the face and neck and their respective methods of innervation, functions, and features of clinical presentation.
| Striated muscles | Cranial Nerve | Functions | Clinical features |
|---|---|---|---|
| Muscles of mastication | V | Ingestion | Ingestive disorders |
| Middle ear muscles | V, VII | Listening | Hyperacusis, auditory processing deficits, language delays |
| Facial muscles | VII | Facial expression | Poor gazea, flat affect |
| Laryngeal and pharyngeal muscles | IX,X | Vocalization, prosody | Difficulties in coordinating sucking, swallowing, breathing, and vocalizations; lack vocal prosody |
| Neck muscles | XI | Head gestures | Lack of head gestures |
The regulation of eyelid opening, via the levator palpebrae superioris, is regulated by cranial nerve III (oculomotor). The oculomotor nerve does not contain special visceral efferent pathways and the levator palpebrae superioris is a smooth muscle. However, a branch of the facial nerve regulates the obicularis oculi, a striated muscle, which opposes the levator palpebrae superioris muscle in regulating the opening of the eyelids. Hemi-paralysis of the facial nerve (i.e., Bell’s palsy) results in dropping of the eyelids and a hyperacusis due to dampened neural tone to the stapedius, a middle ear muscle.
Special visceral efferent pathways originating in brainstem nuclei travel through several cranial nerves to regulate the striated muscles of the face and head (Parent & Carpenter, 1996). These “brain-face” circuits form, via a synergistic “collaboration” with the myelinated vagus, form the neural substrate of the social engagement system (see Porges, 1998, 2001, 2003). The social engagement system is controlled from higher brain circuits and via feedback from the periphery that regulate brainstem nuclei (i.e., lower motor neurons) to control eyelid opening (e.g., looking), facial muscles (e.g., emotional expression), middle ear muscles (e.g., extracting human voice from background noise), muscles of mastication (e.g., ingestion), laryngeal and pharyngeal muscles (e.g., prosody and intonation), and head turning muscles (e.g., social gesture and orientation). Collectively, these muscles function both as determinants of engagement with the social environment and as filters that limit social stimuli. The neural pathways involved in raising the eyelids also tense the stapedius muscle in the middle ear, which functionally dampens the transmission of low frequency background sounds and facilitates the ability to hear the acoustic frequencies associated with human speech. Thus, the neural mechanisms for making eye contact are shared with those needed to listen to human voice. As a cluster, deficits in features of the social engagement system (see Table 1) such as difficulties in gaze, extraction of human voice from background sounds, facial expression, head gesture, and dampened vocal prosody are common features of ASD.
Based on the Polyvagal Theory, it would be hypothesized that the deficits in behavioral and psychological features of the social engagement system would be paralleled by reduced vagal influences to the heart via the myelinated vagus and measured by the amplitude of respiratory sinus arrhythmia (RSA). According to the Polyvagal Theory, dampened vagal regulation of the heart via the myelinated vagus is an adaptive response strategy to support mobilization (i.e., fight-flight behaviors) in dangerous environments. Since the Polyvagal Theory articulates a hierarchy of neural circuits, the metabolic resources necessary for fight-flight behaviors are not efficiently available unless there is a retraction of the vagal brake (i.e., the calming influence of the myelinated vagus on the sympathetic nervous system enables social engagement behaviors to spontaneously occur). Thus, understanding the neural mechanisms defining the social engagement system provides a plausible model to explain why both auditory processing and state regulation difficulties are prevalent in ASD.
In the current study we focus on documenting the covariation of two processes, vagal regulation of the heart and auditory processing skills, theoretically linked by the Polyvagal Theory. Consistent with a psychophysiological perspective, the experimental design provides an opportunity to evaluate this covariation by contrasting typically developing children and adolescents with ASD participants. Since the prevalence of deficits in state regulation and poor auditory processing skills is high in ASD, the experimental design provides an opportunity to evaluate the covariation of these processes through different analytic strategies. Thus, hypotheses regarding the covariation between RSA and auditory processing are tested via: 1) group contrasts, 2) individual differences, and 3) the response of ASD participants to an intervention designed to improve auditory processing.
2.0 Method
2.1 Participants
All participants, with and without a diagnosis of Autism Spectrum Disorders (ASD), were recruited from the Chicago area and tested in protocols approved by the University of Illinois at Chicago Institutional Review Board. Potential participants were excluded if they were taking medications or had a medical condition that might influence autonomic function. The participants ranged in age between 6 and 21 years. The control and ASD groups did not differ in age (see Table 2).
Table 2.
Means and SDs for baseline measures and age at assessment in complete ASD and control populations.
| ASD (n = 78) | Control (n = 68) | |
|---|---|---|
|
|
||
| Baseline RSA (ln(ms2)) | 6.36 ± 1.38 | 7.79 ± 1.07 |
| Baseline heart period (ms) | 680.4 ± 110.0 | 861.5 ± 162.8 |
| Age in months | 156.5 ± 46.6 | 164.2 ± 52.0 |
2.1.1 Autism Spectrum Disorder (ASD)
Seventy-eight participants (8 female) between the ages of six and 21 years were recruited from the Easter Seals Metropolitan Chicago Therapeutic School and Center for Autism Research, local support groups for parents of children with autism, local chapters of autism advocacy groups, clinician referrals, or through self-referrals. Participant screening criteria included a clinical diagnosis of ASD and a parent or guardian who spoke fluent English.
Cognitive assessments were obtained on sixty-one ASD participants. If the participant’s school or clinical records contained documentation that intelligence testing was conducted by a licensed practitioner, then these values were used an assessment of cognitive function. If intelligence scores were not available or if it was not possible to confirm that the test was administered by a licensed practitioner, the Kaufman Brief Intelligence Test (KBIT, Kaufman & Kaufman, 1990) was administered to assess cognitive ability. The mean IQ composite score was 79.54 (SD = 25.97).
2.1.2 Control Participants
Sixty-eight typically developing participants (9 female) between the ages of six and 21 years were recruited via internet, newspaper, and magazine advertisements. Potential participants were excluded if they had a psychiatric diagnosis, were taking medications or had a medical condition that might influence autonomic function. The Kaufman Brief Intelligence Test (K-BIT, Kaufman & Kaufman, 1990) was used to assess cognitive ability in sixty-six of the control participants. The mean IQ composite score was 95.18 (SD = 14.84).
2.2 Measures
2.2.1 Auditory Processing
Auditory processing was evaluated with the Filtered Words (FW) and the Competing Words (CW) subtests from the SCAN Test for Auditory Processing Disorder (Keith, 1986, 2000). Two forms of the SCAN were administered, SCAN-A for participants 12 years of age and older and SCAN-C for participants younger than 12 years of age. The FW subtest assesses the ability to decipher human speech from background sounds (i.e., a component of receptive language skills). The CW subtest is a dichotic listening task, which identifies developmentally delayed or damaged central auditory pathways. Analyses were conducted on age-normed standard scores derived from linear z-scores transformed to a standard score scale with a mean of 10 and a standard deviation of three.
2.2.2 Heart Rate and RSA
Electrocardiogram (ECG) was assessed with either a Biopac MP150 physiological acquisition system (Biopac Systems, Inc., Santa Barbara, CA), an EZ-IBI interbeat interval monitor (UFI, Morro Bay, CA), or a Biolog ambulatory heart rate monitor (UFI, Morro Bay, CA). Each monitoring system sampled ECG at 1 KHz with minimal artifact. Self-adhesive ECG electrodes were placed in a three-lead configuration on the participant’s chest. The ECG and heart period data from the monitoring devices were visually inspected and edited offline with CardioEdit software (Brain-Body Center, University of Illinois at Chicago). Editing consisted of integer arithmetic (i.e., dividing intervals between heart beats when detections of R-wave from the ECG were missed or adding intervals when spuriously invalid detections occurred). RSA was calculated with CardioBatch software (Brain-Body Center, University of Illinois at Chicago) consistent with the procedures developed by Porges (1985). The Porges method quantifies the amplitude of RSA with age-specific parameters that are sensitive to the maturational shifts in the frequency of spontaneous breathing. The method includes the following steps: 1) R-R intervals are timed to the nearest ms to produce a time series of sequential heart periods; 2) sequential heart periods are resampled into 250 ms intervals to produce time-based data; 3) the time-based series is detrended by a 51-point cubic moving polynomial (Porges & Bohrer, 1990) that is stepped through the data to create a smoothed template and the template is subtracted from the original time-based series to generate a detrended residual series; 4) the detrended time series is bandpassed to extract the variance in the heart period pattern associated with spontaneous breathing (0.12–1.0 Hz to account for the wide range of ages in the study cohort); and 5) the natural logarithm of the variance of the bandpassed time series is calculated as the measure of the amplitude of RSA (Riniolo & Porges, 1997).
2.3 Protocol
2.3.1 Study 1
Assessment of RSA was conducted in each participant in both the ASD and control groups during a two-minute baseline period and during the SCAN challenge. The ASD participants varied in functional level and several were not able to complete the intelligence and auditory processing tasks. Analyses were conducted to maximize the number of participants. This approach maximized sample sizes when contrasting heart period and RSA during baseline. Smaller sample sizes were available for contrasts involving cognitive function (i.e., intelligence tests) and auditory processing (i.e., SCAN subtests). Group contrasts evaluating RSA and heart period baselines were similar with maximized and reduced sample sizes.
2.3.2 Study 2
Approximately one week after baseline testing, a subset (n=33) of the participants with ASD were assessed following an intervention, the Listening Project Protocol. The intervention was designed to exercise the middle ear muscles (i.e., stapidius, tensor tympani) to functionally improve auditory processing by enhancing the transmission of frequencies associated with human voice. The intervention presented acoustic stimulation, which was generated by dynamically filtering human vocal music to amplify the features of vocal prosody. The intervention consisted of five daily sessions of approximately one hour during which the participant passively listened to the acoustic stimulation through headphones in a quiet room, while researchers provided social support to insure that the participants remained calm. The frequency bands were temporally modulated within each session and the band of frequencies that were modulated progressively increased across the five sessions.
Theoretically, the changing frequency bands were presented to trigger feedback to increase the neural regulation of middle ear structures to dampen the perception of background low frequency sounds and to potentiate the extraction of human voice. Borg and Counter (1989) described a role of the middle ear muscles in facilitating the extraction of human speech by dampening the transmission of low frequency noise from the external environment to the inner ear. The Borg and Counter model explains why auditory hypersensitivity is a symptom of Bell’s palsy, a condition characterized by a lateralized paralysis of the facial nerve including the pathway regulating the stapedius muscle in the middle ear.
Borg and Counter (1986) provide a scientific basis to investigate whether improvements in auditory processing would occur if neural regulation of the middle ear muscles were rehabilitated through the exercises embedded in the Listening Project Protocol. By contrasting pre and post intervention measures of the SCAN tests and RSA, the functional enhancement of neural regulation of the middle ear muscles and the vagal regulation of the heart may be evaluated. The extrapolation from improving auditory processing to improving state regulation via improved vagal regulation of the heart is based on the theoretical model elaborated in Porges and Lewis (2009) and linked to the social engagement system described in the Polyvagal Theory (Porges, 2011).
3.0 Results
3.1 Study 1
The ASD (n = 78) participants had significantly lower RSA, F(1, 144) = 48.6, p < 001, and shorter heart period, F(1, 144) = 63.4, p < .001, at baseline than typically developing participants (n = 68). The descriptive data are provided in Table 2. Since the study evaluated individuals across a broad age range, the relation between age in months and the cardiac parameters were evaluated. Consistent with the well-documented relation between the slowing of heart rate and the increase in body size associated with maturation, longer heart period (i.e., slower heart rate) at baseline was significantly correlated with increased age across all participants, r(142) = 0.46, p < .001, within the ASD group, r(76) = 0.44, p < .001, and within the control group, r(66) = 0.53, p < .001. RSA was not correlated to age in either population. RSA was significantly correlated with heart period across all participants, r(142) = 0.67, p < .001, within the ASD group, r(76) = .69, p < .001, and within the control group, r(66) = 0.46, p < .001.
Similar to the analyses described above (see Table 3), when the groups were redefined to include only participants who had both IQ and autonomic measures, group differences in heart period and RSA were of similar magnitude. The control group had significantly higher IQ scores than the ASD group, F(1,125) = 17.69, p < .001. Descriptive statistics for the redefined sample are provided in Table 3. In addition, correlations of similar magnitude were observed between heart period and age and between RSA and heart period within groups and in the combined cohort. Similar to the larger cohort, the redefined groups had significantly different heart period and RSA during baseline. IQ was not correlated with heart period or RSA in either the ASD or control group. However, there was a trend towards a positive relation within the ASD group between higher IQ being associated with larger values for baseline RSA, r(59) = 0.230, p < .074.
Table 3.
Means and SDs for IQ, baseline measures, and age at assessment in ASD and control populations after selecting participants with IQ scores.
| ASD (n = 61) | Control (n = 66) | |
|---|---|---|
|
|
||
| IQ | 79.5 ± 26.0 | 95.2 ± 14.8 |
| Baseline RSA (ln(ms2)) | 6.33 ± 1.22 | 7.82 ± 1.01 |
| Baseline heart period (ms) | 683.6 ± 111.0 | 866.0 ± 161.2 |
| Age in months | 155.4 ± 49.7 | 166.2 ± 51.5 |
The sample was further restricted to contain only ASD (n = 32) and control (n = 49) participants, who had measures of heart period and RSA while participating in the SCAN tests for auditory processing. Repeated measures analyses of variance identified a significant group x condition interaction for both heart period and RSA (see Table 4). As illustrated in Figure 1, the ASD group not only had significantly lower RSA and shorter heart periods (i.e., faster heart rate), but increased RSA during the SCAN.
Table 4.
Statistical analysis of difference in physiological measures from baseline to SCAN task via repeated-measures ANOVA.
| Condition | Group | Group x Condition | |
|---|---|---|---|
|
|
|||
| RSA (ln(ms2)) | F(1, 79) = 11.6, p < 0.01 | F(1, 79) = 64.2, p < 0.001 | F(1, 79) = 10.0, p < 0.01 |
| Heart Period (ms) | F(1, 79) = 18.3, p < 0.001 | F(1, 79) = 41.0, p < 0.001 | F(1, 79) = 24.6, p < 0.01 |
Figure 1.
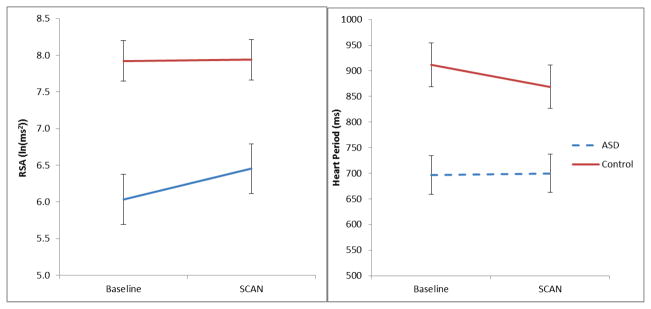
RSA (respiratory sinus arrhythmia) and HP (heart period) during baseline and SCAN task conditions. Error bars represent +/− two standard errors of the mean. ASD, N = 32. Control, N = 49.
There were group differences in performance on the two subscales of the SCAN test for auditory processing. Since it was not possible to monitor autonomic measures on all the ASD participants who successfully completed the SCAN, analyses on the SCAN scales were conducted on a larger sample. The ASD group (n = 41) performed significantly poorer than the control group (n = 49) on FW, F (1, 88) = 56.0, p < .001, and CW, F (1, 88) = 14.9, p < .001, subtests. Descriptive statistics are provided in Table 5. Across group assignment, there was a significant correlation between IQ and performance on the FW subtest, r(81) = 0.57, p < .001, and CW subtest, r(81) = 0.42, p < .001. However, only within the ASD group was IQ correlated with performance on the CW subtest, r(32) = .66, p < .001. There were no significant correlations between performance and heart period and between performance and RSA.
Table 5.
Means and SDs for SCAN subtest standard scores in ASD and control populations.
| ASD (n = 41) | Control (n = 49) | |
|---|---|---|
|
|
||
| Filtered Words Standard Score | 2.90 ± 2.37 | 7.16 ± 2.93 |
| Competing Words Standard Score | 4.10 ± 3.00 | 7.00 ± 3.96 |
Correlations are reported in Table 6 between SCAN performance and the changing physiological state from baseline to SCAN testing. For the FW subtest, greater decreases in RSA and heart period were significantly correlated with better performance across groups. Within groups there were similar relationships for changes in heart period and performance. Only in the control group was suppression of RSA correlated with performance on the FW subtest. Changes in either RSA or heart period within each group were not significantly related to performance on the CW subtest. However, across groups there was a significant correlation between change in heart period and performance on the CW subtest.
Table 6.
Correlations between change (SCAN minus baseline scores) in physiological measures and SCAN subtest performance .
| ASD (n =37) | Control (n = 49) | All Participants (N = 86) | |
|---|---|---|---|
|
|
|||
| Change in RSA/FW | r = −0.227 | r = −0.388* | r = −0.419** |
|
|
|||
| Change in HP/FW | r = −0.403* | r = −0.544** | r = −0.633** |
|
|
|||
| Change in RSA/CW | r = 0.120 | r = −0.089 | r = −0.137 |
|
|
|||
| Change in HP/CW | r = −0.047 | r = −0.081 | r = −0.239* |
FW = Filtered Words; CW = Competing Words; HP = heart period;
= p < 0.05;
= p <0.001
Correlations (see Table 7) were calculated between performance on each of the SCAN subtests and IQ. Across both groups there were significant correlations between IQ and performance on each of the subscales. However, only within the ASD group were these correlations significant.
Table 7.
Correlations between IQ and SCAN subtest performance.
| ASD (n = 34) | Control (n = 49) | All participants (N =83) | |
|---|---|---|---|
|
|
|||
| IQ/ Filtered Words | r = 0.483, p < .05 | r = 0.179, p = .22 | r = 0.568, p <.001 |
| IQ/ Competing Words | r = 0.656, p <.001 | r = 0.081, p = .58 | r = 0.417, p <.001 |
Since suppression of RSA has been used as an index of sustained attention and mental effort (e.g., Porges, 1992), we tested the hypothesis that the reported correlation between IQ and performance would be moderated by the pattern of change in RSA from baseline to the SCAN task. Moderation analysis is a methodology that statistically defines the relationship between two variables as a function of a third variable (Kraemer, Wilson, Fairburn, & Agras, 2002). In this study we evaluated whether the relation between IQ and performance on the SCAN subscales was related to the degree that RSA either decreased or increased while performing the SCAN.
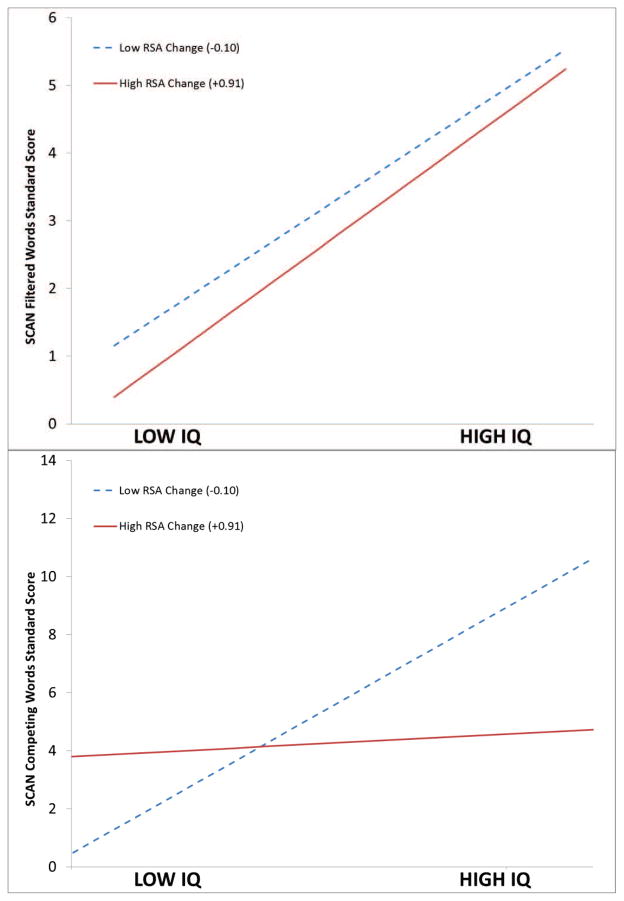
As illustrated in the upper panel of Figure 2, the parallel lines indicate a non-significant interaction (i.e., no moderation) between change in RSA and the relation between IQ and performance on the FW test. In contrast, the lower panel of Figure 2 illustrates the significant moderation effect of change in RSA on the correlation between IQ and performance on the CW subscale. When RSA decreases during the SCAN condition, IQ and performance on the CW task are positively correlated. Note that when RSA increases, IQ is not correlated with CW performance. The moderation effect is first confirmed by a significant effect for the interaction between the independent variable (IQ) and the proposed moderator (change in RSA) in the regression model. Simple slopes plots illustrate the observed relationship between IQ and the SCAN scores for individuals with RSA change scores at +/− 1 SD of the observed values. As illustrated in Table 8, inclusion of the interaction term, between change in RSA and IQ, explains an additional 10% of the total variance in CW performance but only 0.02% of the total variance in FW performance.
Figure 2.
Simple slopes plot illustrating the interaction between IQ and RSA change, from baseline to SCAN, in the prediction of the SCAN standard scores. Filtered Words p < .935; Competing Words p < .001.
Table 8.
Coefficient of determination (R2) and significance of SCAN subtest performance predicted by the interaction between IQ scores and change in RSA from baseline to SCAN.
| R2 | Increase in R2 due to interaction | F (3, 26) | P | |
|---|---|---|---|---|
|
|
||||
| Filtered Words | 0.259 | 0.0002 | 0.0068 | 0.935 |
| Competing Words | 0.565 | 0.0977 | 11.265 | 0.0001 |
| Predicting CW performance | |||
|---|---|---|---|
|
| |||
| R2 | F | P | |
| IQ | 0.47 | 24.30 | <0.0001 |
| Interaction (IQ x ΔRSA) | 0.10 | 5.84 | 0.0230 |
| Total model | 0.57 | 11.27 | 0.0001 |
3.2 Study 2
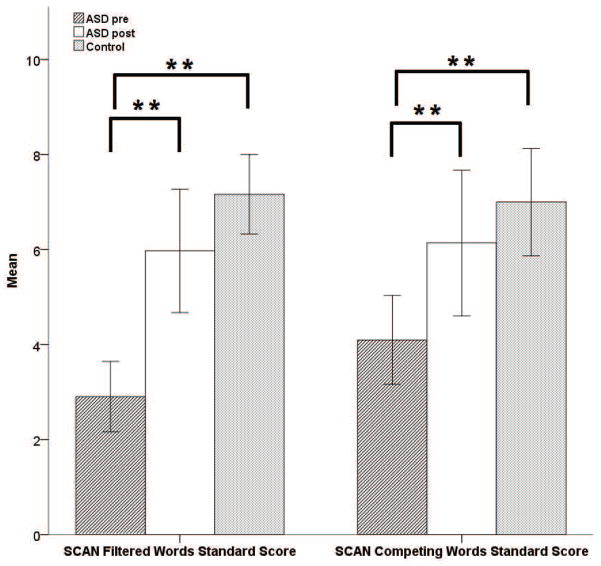
A subset of the ASD participants participated in an intervention designed to improve auditory processing by stimulating middle ear muscle function. In addition, based on the assumption of an integrated social engagement system postulated in the Polyvagal Theory, it was hypothesized that the intervention would increase the vagal influence to the heart through brainstem mechanisms involved in regulating both middle ear muscles and the heart. Means and standard deviations for the Pre/Post intervention assessments are reported in Table 9. Significant increases in baseline RSA, F (1, 28) = 6.45, p < .05, and significant improvements in FW, F (1, 35) = 22.87, p < .001, and CW, F (1, 35) = 15.37, p < .001, subscales of the SCAN were observed. As illustrated in Figure 3, the significant post-intervention improvements by the ASD group on the SCAN subscales were no longer significantly different that the performance levels of the control group.
Table 9.
Means and SDs for baseline measures and SCAN subtest percentile scores in ASD population pre- and post-intervention. N = 35.
| Variable | Pre-intervention | Post- intervention | F(1, 34) | p | |
|---|---|---|---|---|---|
|
|
|||||
| Baseline RSA | 5.98 ± 0.86 | 6.40 ± 0.91 | 8.32 | 0.007 | |
| Baseline heart period | 691.5 ± 104.2 | 707.7 ± 97.5 | 1.20 | 0.281 | |
| Filtered Words Standard Score | 2.92 ± 2.42 | 5.77 ± 3.76 | 20.74 | <0.001 | |
| Competing Words Standard Score | 3.94 ± 2.61 | 5.80 ± 4.20 | 13.67 | 0.001 | |
Figure 3.
SCAN standard scores for ASD participants before and after intervention compared to performance of control participants. Error bars represent +/− two standard errors of the mean. ** = p < .01.
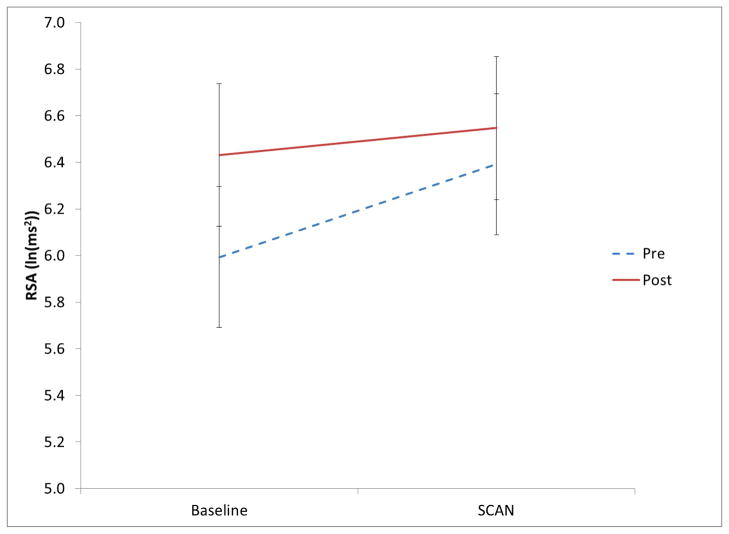
To evaluate the influence of the intervention on autonomic reactivity, analyses of variance were conducted with pre/post intervention and condition (baseline, SCAN) as repeated measures. As illustrated in Figure 4, there was a significant condition by pre/post intervention interaction, F (1, 32) = 6.35, p < .05. Post intervention RSA was higher and the increase in RSA during the SCAN task was dampened. The intervention did not influence the heart period response pattern. Post-intervention there were no relations between individual differences in the change in RSA or heart period from baseline to SCAN and performance on the auditory processing tasks.
Figure 4.
RSA during baseline and SCAN pre- and post-intervention (error bars are +/− two standard errors of the mean).
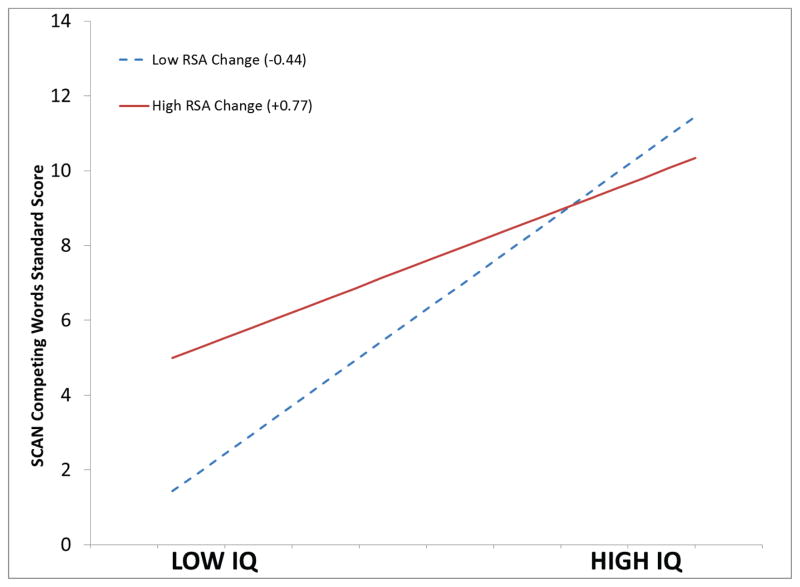
In addition, paralleling the post-intervention dampened RSA reactivity, the moderation of change in RSA on the correlation between IQ and CW observed prior to the invention was no longer significant (see Table 10). During the pre-intervention (see Study 1 above), change in RSA from baseline to SCAN explained 10% of the variance relating CW performance to IQ. As illustrated in Figure 4, following the intervention, this moderating influence was reduced to 2% of the variance. Post-intervention performance on the CW task retained a significant correlation with IQ, r(33) = 0.450, p < .01, although it was no longer moderated by changes in RSA (see Figure 5).
Table 10.
Comparison of moderation analyses pre- and post-intervention: Evaluating the relation between IQ and SCAN Competing Words standard scores, with change in RSA from baseline to SCAN condition as a moderator. F and p-values represent the significance of R2 increase due to the interaction between IQ scores and change in RSA.
| R2 | Increase in R2 due to interaction | F(3, 26)a | p | |
|---|---|---|---|---|
|
|
||||
| Pre-intervention | 0.565 | 0.0977 | 11.27 | 0.0001 |
| Post-intervention | 0.2612 | 0.0237 | 0.868 | 0.3598 |
29 participants were evaluated in both conditions.
Figure 5.
Simple slopes plot illustrating the interaction between IQ and RSA change, from baseline to SCAN, in the prediction of the SCAN standard scores post-intervention. p < .36.
4.0 Discussion
The group contrasts identified three features that distinguish the ASD group from a group of typical developing children: 1) baseline RSA, 2) RSA reactivity, and 3) auditory processing performance. The interpretation of the interrelationships among these variables is dependent on the Polyvagal Theory and the functional model of an integrated social engagement system derived from the theory. The neurobiological substrate of the social engagement system provides the basis for a developmentally expanding repertoire of social communication strategies, which reflect the emergent properties of a uniquely mammalian link between the source nuclei in the brainstem regulating both the heart via a myelinated vagal efferent pathway to the sinoatrial node and the striated muscles of the face and head via special visceral efferent pathways exiting five cranial nerves (V, VII, IX, X, XI).
When the social engagement system is compromised, emergent behavioral, psychological, and autonomic features converge with several features prevalent in ASD. Consistent with this model, not only do features of the social engagement system become windows of assessment, but due to the integrated nature of the system, these features also become portals for possible intervention. Thus, there is the optimistic possibility that the entire integrated social engagement system can be “rehabilitated” via an intervention designed to exercise a specific portal. This optimistic scenario is supported by the outcome of the intervention delivered, which improved in auditory processing, increased RSA, and normalized RSA reactivity.
4.1 Baseline RSA and heart period
Baseline RSA and heart period were significantly lower in the ASD group. Vagal regulation, via the myelinated vagus and measured by the amplitude of RSA, provides a critical neural pathway to regulate behavior state by dampening sympathetic-adrenal reactivity. Thus, the poor state regulation observed in ASD may be a behavioral manifestation of a chronic depression of the functional influence of the myelinated vagus. These findings, consistent with recently published studies with children diagnosed with autism (Bal et al., 2010; Patriquin, Scarpa, Friedman, & Porges, 2011; Van Hecke et al., 2009) support this hypothesis. The studies report findings consistent with a dampened social engagement system: 1) the amplitude of RSA is lower in ASD samples relative to age-matched typically developing children (Bal et al., 2010; Ming, Julu, Brimacombe, Connor, & Daniels, 2005; Van Hecke et al., 2009), and 2) within samples of children with ASD higher amplitude RSA is associated with better social behavior (Bal et al., 2010; Patriquin et al., 2011; Van Hecke et al., 2009), and better receptive language abilities (Patriquin et al., 2011).
Although RSA and heart period reflect vagal influences, RSA was uncoupled from the maturational influences observed in heart period. Thus, although heart period was correlated with age, RSA, which is more sensitive to cardiac vagal tone than heart period (Porges, 2007; Lewis, Furman, McCool & Porges, 2012), was unrelated to age in both ASD and control groups.
The observed age related changes in heart period are due to influences, other than vagal, that change with maturation. Heart period is a composite variable that is not only influenced by neural tone via both the parasympathetic and sympathetic branches of the autonomic nervous systems, but is, in part, determined by the size of the heart. The size of the heart imposes limits on the temporal parameters of electrical potentials arising from the myocardium, which are manifested in the ECG. The maturational increase in body size is paralleled by a decrease in heart rate, reflecting the expanded time necessary to complete a heartbeat. The relation between body size and heart rate is a well-documented allometric law in mammals (Meijler, 1985).
4.2 Baseline RSA and function
RSA has been proposed to be an indicator of function in ASD (Porges, 2005). This relation is supported in the literature with higher RSA related to more competent social behavior (Bal et al., 2010) and receptive language (Patriquin et al., 2011). In the current study, the relation between RSA and function in ASD was investigated by calculating correlations between RSA and IQ and between RSA and performance on the auditory processing tests. Consistent with the association of positive functional features with higher levels of RSA, the ASD group had significantly lower scores on RSA and the measures of function (i.e., IQ, FW, CW). However, within the ASD group there was only a marginal trend between RSA and IQ and there were no significant correlations between RSA and auditory processing performance. This inconsistency between the current findings and the literature might be due to the range of function that characterized the ASD group. In the current study the distribution of IQ scores differed from the studies cited above. The IQ scores were less variable than the participants described in the Patriquin et al. (2011) study and substantially lower than the ASD participants described in the Bal et al. (2010) and van Hecke et al. (2009) studies.
4.3 IQ and auditory processing
Similar to the findings with IQ, the ASD group performed significantly poorer on both auditory processing tasks. Across groups, IQ was correlated with performance on each of the SCAN subscales. Within the ASD group, but not in the control group, IQ was significantly correlated with performance on the CW subtest. This covariation between performance on a dichotic listening task and mental function in the ASD group is consistent with studies that have demonstrated a relation between dichotic listening skills and cognitive function (e.g., Hugdahl et al., 2009).
4.4 RSA increases degrade performance on tasks requiring sustained attention
In the current study we explored the response pattern of RSA and heart period, while participants were performing an auditory processing assessment to determine whether ASD children exhibit a reduction of the vagal brake, which is associated with mental effort in typically developing samples (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996). Suppression of RSA during attention demanding tasks has frequently been used as a psychophysiological index of mental effort and sustained attention (e.g., Vicente, Thornton, & Moray, 1987; Porges, 1992). Several studies have reported dampened RSA reactivity to social and cognitive challenges in children with atypical development including social phobia (Schmitz, Kramer, Tuschen-Caffier, Heinrichs, & Blechert, 2011), fragile X syndrome (Heilman, Harden, Zageris, Berry-Kravis, & Porges, 2011), selective mutism (Heilman et al., 2012), sleep problems (El-Sheikh & Buckhalt, 2004), poor maternal-child relationships (Calkins, Graziano, Berdan, Keane, & Degnan, 2008) , increased risk for obesity (Graziano, Calkins, Keane, & O’Brien, 2011), and lower peer status (Graziano, Keane, & Calkins, 2007).
The evolutionary roots of this response may be linked to the adaptive function of withdrawal of the vagal inhibition on the sinoatrial node and the parallel release of inhibition on sympathetic influences on the heart. The withdrawal of vagal inhibition promotes opportunities to increase cardiac output to support mobilization without requiring sympathetic excitation. If sympathetic excitation is required to support mobilization, then the sympathetic influences will not be opposed by vagal inhibition and will be efficiently manifested in cardiac output. Thus, the transitory withdrawal of the vagal brake during tasks of sustained attention may represent an adaptive precautionary vigilance response preparing the individual to mobilize if a novel person, object, or event would become threatening (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996).
The pattern of autonomic reactivity during the SCAN task was investigated to explore the possibility that poor auditory processing observed in the ASD group was, in part, determined by the autonomic reaction during the task. As illustrated in Figure 1 there was a significant group by condition interaction. During the SCAN tests, the ASD group increased RSA, while the control group did not exhibit any change.
The SCAN condition can be viewed as an attention task, since SCAN subtests require the participants to direct their attention to the auditory signals. Within a context of a sustained attention task, the small RSA changes in the control group might reflect either a bias not to suppress RSA due to minimal attentional demands or the central tendency of a distribution of individuals who are either suppressing or not suppressing.
In contrast to the typical group, the ASD group responded with an atypical increase in RSA. An increase in RSA during an attention demanding task may be incompatible with efficient processing of sensory stimuli and may functionally dampen input and negatively impact on performance. Consistent with this speculation, Dale, O’Hara, Schein, and Porges (2011) reported that only infants with behavioral state regulation problems increased RSA in response to a cognitive task. Moreover, when infants with these “regulatory disorders” were studied longitudinally, features of state regulation problems were an early marker for developmental vulnerabilities including ASD (see Degangi, Breinbauer, Doussard-Roosevelt, Porges, & Greenspan, 2000).
The plausibility that increases in RSA negatively impact on the processing of sensory stimuli may be evaluated by investigating the covariation between individual differences in auditory processing and direction and amplitude of RSA responses from baseline to SCAN. Across both groups there was a significant correlation between change in RSA and performance on the FW task and a trend on the CW task.
Consistent with the above hypothesis, increases in RSA from baseline were related to poorer performance. Within group effects were less robust, perhaps due to the reduced range of within group performance. Poorer performance on the Filtered Words task was related to increases in RSA only in the control group. Consistent with the above findings relating changes in RSA and auditory processing, Lewis, et al. (2011) demonstrated a covariation between the transfer function of the middle ear structures and changes in RSA. They reported that greater decreases in RSA, while attending to a number in noise task, were related to enhanced absorption of middle ear structures in the mid-frequency range (i.e., between 2000 Hz and 4000 Hz) associated with the second and third formants. Functionally, when there is more neural tone to the middle ear muscles, the eardrum absorbs more acoustic energy in the mid-frequency range and reflects more acoustic energy in the low frequencies. The mid-frequency range characterizes the acoustic features conveying information needed to distinguish words in human speech. The low frequency range characterizes background noises. This strategy improves the accurate processing of human speech. If RSA increased, the middle ear “transfer function” dampened the absorption of acoustic frequencies necessary to distinguish features of vocalizations required to accurately discriminate among specific words. Performance paralleled the middle ear transfer function with greater absorption in the mid-frequency range being related to better performance on a number in noise task.
Lewis et al. (2011) demonstrated that individual differences in the middle ear transfer function are related to changes in RSA (i.e., greater middle ear muscle tension is associated with greater decreases in RSA during auditory processing tasks). These findings suggest that the covariation of these two components of the social engagement system synergistically reflect “states” that either interfere with or promote an efficient processing of human speech.
4.5 RSA reactivity moderates the relation between IQ and Competing Words (CW) performance in ASD
As noted above, the direction that RSA changes during the SCAN condition is related to the transfer function of the middle ear structures and functionally may influence auditory processing and moderate the reported relationship between IQ and CW performance. Thus, hypothetically the linear mapping of IQ on CW performance would be strongest in participants who decrease or do not change RSA and weakest in participants who increase RSA. The moderation analyses clearly support this hypothesis and demonstrate the significant interaction of change in RSA on the correlation between of IQ and CW performance. As illustrated in Figure 2, only when RSA does not increase is there a significant relation between IQ and performance on the CW test.
4.6 The Listening Project Protocol: An intervention designed to exercise the middle ear structures
Based on the above findings, the impact of an intervention, designed to exercise the middle ear muscles, on RSA and auditory processing performance was evaluated. The intervention was predicated on the documented neuroanatomical basis of an integrated social engagement system. This model assumes that neural exercises, which would improve the function of a feature of the system, would (due to common brainstem regulatory mechanisms) have positive influences on other features of the system. Thus, exercising the middle ear muscles to change the transfer function of the middle ear would not only improve auditory processing performance, but also would improve vagal regulation of the heart. The changes following the intervention support these speculations. RSA significantly increased, auditory processing significantly improved, the pattern of RSA reactivity during the SCAN condition was no longer characterized by RSA increases, and the correlation between IQ and CW performance was no longer moderated by the pattern of RSA reactivity. Consistent with the interpretation that the Listening Project Protocol functionally improves neural regulation of the middle ear muscles, preliminary testing of a new device that measures the middle ear transfer function (Lewis & Porges, patent pending), has provided examples illustrating that the intervention normalizes the transfer function of middle ear structures.
Although the intervention resulted in positive effects with documented improvements in both RSA and auditory processing, the findings need to be cautiously interpreted. The data are preliminary and do not represent a randomized clinical trial. There was no control group that received alternative acoustic stimulation or headphones without music. The protocol required a pre and post testing and the effects of time without an intervention were not evaluated. Neither were the long-term effects of the intervention monitored. Even with these limitations, the findings related to auditory processing are encouraging, since it had been previously assumed that the decrements in auditory processing skills frequently observed in ASD were permanent and due to atypical neural circuitry in the central nervous system. The findings suggest that for many individuals with ASD, the deficits in auditory processing might be due to a deficit in neural regulation of peripheral structures (i.e., middle ear muscles) that is paralleled by a deficit in the vagal regulation of the heart.
The data from the intervention support a model of an integrated social engagement system. The intervention can be conceptualized as a “neural exercise” that challenges the middle ear muscles with computer modulated vocalizations. Functionally, the intervention improved auditory processing performance, increased RSA, and normalized RSA reactivity. Although middle ear muscle tone was not assessed in this study, subsequent preliminary research with a new measurement strategy suggests that the transfer function of the middle ear structures can be modified by listening to stimuli similar to those used in the intervention. Moreover, preliminary research has documented that the transfer function of the middle ear structures is related to both RSA reactivity during sustained attention and auditory processing (see Lewis et al., 2011).
4.7 Summary
The current study evaluated two common symptoms (i.e., state regulation problems and deficits in auditory processing) associated with a diagnosis of ASD, which have been treated in the literature as unrelated. However, when these symptoms are informed by the Polyvagal Theory, they may be viewed as the predictable consequences of a depressed social engagement system.
Features of the social engagement system may serve as a clinical checklist converging on many of the deficits observed of ASD. The convergence between the features of the hypothetical social engagement system and clinical symptoms is evident from the description of the structures and functions of the system described in Table 1. Thus, a systematic evaluation of the system’s functions would provide definable and measureable neural pathways for several clinical features observed in ASD.
Although individuals with ASD have a compromised social engagement system, a compromised social engagement system does not define autism. Rather than having diagnostic specificity, the social engagement system appears to be blunted in many psychiatric disorders other than ASD. Moreover, context and physiological state appear to provide opportunities for the social engagement system either to function or to shut down. In general, states that promote defense and mobilization reduce access to the system; states that promote calm physiological associated with safety provide opportunities to engage and to “exercise” the system. Understanding the covariation and potential interactions of symptoms associated with an integrated social engagement system may inform clinicians and lead to improved treatment models and management strategies.
Autistic children (ASD) had depressed RSA and auditory processing performance.
RSA reactivity moderated the relation between dichotic listening and IQ in ASD.
RSA and auditory processing are theoretically linked to a social engagement system.
An intervention was tested with ASD that stimulated the social engagement system.
Auditory processing improved and RSA increased in ASD following intervention.
Acknowledgments
Support for the research described in this manuscript was provided, in part, by grants from the National Institute of Mental Health (MH060625), Unicorn Children’s Foundation, Cure Autism Now Foundation, Nancy Lurie Marks Family Foundation, and Autism Speaks. The contents of this manuscript are solely the responsibility of the authors and do not represent the official views of NIH or other funding agencies. We would like to thank the staff and students at Easter Seals Therapeutic School and Center for Autism Research for their support and participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachevalier J, Loveland K. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Vaughan-Van Hecke A, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disabilities. 2010;40:358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Borg E, Counter SA. The middle-ear muscles. Scientific American. 1989;26:74–80. doi: 10.1038/scientificamerican0889-74. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Berdan LE, Keane SP, Degnan KA. Predicting cardiac vagal regulation in early childhood from maternal-child relationship quality during toddlerhood. Developmental Psychobiology. 2008;50:751–766. doi: 10.1002/dev.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LP, O’Hara EA, Schein R, Porges SW. Nine-month RSA regulation and regulatory disorders predict 54-month behavior problems. Infant Mental Health Journal. 2011;32:473–486. doi: 10.1002/imhj.20306. [DOI] [PubMed] [Google Scholar]
- Degangi GA, Breinbauer C, Doussard-Roosevelt J, Porges SW, Greenspan S. Prediction of childhood problems at three years in children experiencing disorders of regulation during infancy. Infant Mental Health Journal. 2000;21:156–175. [Google Scholar]
- Dissanayake C, Sigman M. Attachment and emotional responsiveness in children with autism. International Review of Research in Mental Retardation. 2001;23:239–266. [Google Scholar]
- El-Sheikh M, Buckhalt JA. Vagal regulation and emotional intensity predict children’s sleep problems. Developmental Psychobiology. 2005;46:307–317. doi: 10.1002/dev.20066. [DOI] [PubMed] [Google Scholar]
- Frith U, Baron-Cohen S. Perception in autistic children. In: Cohen DJ, Donnellan A, Paul R, editors. Handbook of Autism and Pervasive Developmental Disorders. New York, NY: John Wiley & Sons; 1987. pp. 85–102. [Google Scholar]
- Graziano PA, Keane SP, Calkins SD. Cardiac vagal regulation and early peer status. Child Development. 2007:78. doi: 10.1111/j.1467-8624.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- Graziano PA, Calkins SD, Keane SP, O’Brien M. Cardiovascular regulaton profile predicts developmental trajectory of BMI and pediatric obesity. Obesity. 2011;19:1818–1825. doi: 10.1038/oby.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes R, Gordon A. Auditory abnormalities in autistic children. Lancet. 1977;2:767. doi: 10.1016/s0140-6736(77)90278-1. [DOI] [PubMed] [Google Scholar]
- Heilman KJ, Harden ER, Zageris DM, Berry-Kravis E, Porges SW. Autonomic regulation in fragile x syndrome. Developmental Psychobiology. 2011;53:785–795. doi: 10.1002/dev.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KJ, Connolly SD, Padilla WO, Wrzosek MI, Graczyk PA, Porges SW. Sluggish vagal brake reactivity to physical exercise challenge in children with selective mutism. Development and Psychopathology. 2012;24:241–250. doi: 10.1017/S0954579411000800. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Westerhausen R, Alho K, Medvedev S, Laine M, Hamalainen H. Attention and cognitive control: unfolding the dichotic listening story. Scandinavian Journal of Psychology. 2009;50:1–22. doi: 10.1111/j.1467-9450.2008.00676.x. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test Manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Keith RW. SCAN: A screening test for auditory processing disorders. San Antonio, TX: The Psychological Corporation; 1986. [Google Scholar]
- Keith RW. SCAN-C: Test for auditory processing disorders in children-revised. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Klin A. Listening preferences in regard to speech in four children with developmental disabilities. Journal of Child Psychology and Psychiatry. 1992;33:763–769. doi: 10.1111/j.1469-7610.1992.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Stanfill S, Davila M, Macellaio M, Zageris D, Coleman D, Aylward S, McCue K, Heilman K, Porges SW. Neural regulation of sensory gating in the auditory periphery: Relationship with listening and heart rate dynamics. Society for Psychophysiological Research; Boston, Massachusetts: 2011. [Google Scholar]
- Lewis GF, Furman SA, McCool MF, Porges SW. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biological Psychology. 2012;89:349–364. doi: 10.1016/j.biopsycho.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer L, Rutter M. A five to fifteen year follow-up study of infantile psychosis: III. Psychological aspects. British Journal of Psychiatry. 1969;115:865–882. doi: 10.1192/bjp.115.525.865. [DOI] [PubMed] [Google Scholar]
- Meijler FL. Atrioventricular conduction versus heart size from mouse to whale. Journal of the American College of Cardiology. 1985;5:363–365. doi: 10.1016/s0735-1097(85)80060-7. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain Development. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Parent A, Carpenter MB. Carpenter’s Human Neuroanatomy. 9. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, Porges SW. Respiratory sinus arrhythmia: A marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology. 2011 doi: 10.1002/dev.21002. [DOI] [PubMed] [Google Scholar]
- Porges SW. Respiratory sinus arrhythmia: An index of vagal tone. In: Orlebeke JF, Mulder G, Van Dornen LJP, editors. Psychophysiology of Cardiovascular Control: Models, Methods, and Data. New York: Plenum; 1985. pp. 437–450. [Google Scholar]
- Porges SW. Autonomic regulation and attention. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults. Hillsdale, N.J: Lawrence Erlbaum Associates; 1992. pp. 201–223. [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32:301–18. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Love: An emergent property of the mammalian autonomic nervous system. Psychoneuroendocrinology. 1998;23(8):837–861. doi: 10.1016/s0306-4530(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–46. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. Social engagement and attachment: a phylogenetic perspective. Annals of New York Academy of Sciences. 2003;1008:31–47. doi: 10.1196/annals.1301.004. [DOI] [PubMed] [Google Scholar]
- Porges SW. The vagus: A mediator of behavioral and visceral features associated with autism. In: Bauman ML, Kemper TL, editors. The Neurobiology of Autism. Baltimore: Johns Hopkins University Press; 2005. pp. 65–78. [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-regulation. New York: WW Norton; 2011. [Google Scholar]
- Porges SW, Bohrer RE. Analyses of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. New York: Cambridge University Press; 1990. pp. 708–753. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal "brake" predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porges SW, Lewis GF. The polyvagal hypothesis: Common mechanisms mediating autonomic regulation, vocalizations, and listening. In: Brudzynski SM, editor. Handbook of Mammalian Vocalizations: An Integrative Neuroscience Approach. Amsterdam: Academic Press; 2009. pp. 255–264. [Google Scholar]
- Riniolo TC, Porges SW. Inferential and descriptive influences on measures of respiratory sinus arrhythmia: Sampling rate, R-wave trigger accuracy, and variance estimates. Psychophysiology. 1997;34:613–621. doi: 10.1111/j.1469-8986.1997.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Kramer M, Tuschen-Caffier B, Heinrichs N, Blechert J. Restricted autonomic flexibility in children with social phobia. Journal of child psychology and psychiatry. 2011;52:1203–1211. doi: 10.1111/j.1469-7610.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- Van Hecke AV, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Denver J, Bazhenova O, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development. 2009;80:1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Vicente KJ, Thornton DC, Moray N. Spectral analysis of sinus arrhythmia: A measure of mental effort. Human Factors: The Journal of the Human Factors and Ergonomics Society. 1987;29:171–182. doi: 10.1177/001872088702900205. [DOI] [PubMed] [Google Scholar]