Abstract
Background
Black women tend to be diagnosed with breast cancer at a more advanced stage than whites and subsequently experience elevated breast cancer mortality. We sought to determine whether there are racial differences in tumor natural history that contribute to these disparities.
Methods
We used the University of Wisconsin Breast Cancer Simulation Model, a validated member of the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network, to evaluate the contribution of racial differences in tumor natural history to observed disparities in breast cancer incidence. We fit eight natural history parameters in race-specific models by calibrating to the observed race- and stage-specific 1975–2000 U.S. incidence rates, while accounting for known racial variation in population structure, underlying risk of breast cancer, screening mammography utilization, and mortality from other causes.
Results
The best fit models indicated that a number of natural history parameters must vary between blacks and whites to reproduce the observed stage-specific incidence patterns. The mean of the tumor growth rate parameter was 63.6% higher for blacks than whites (0.18, SE 0.04 vs. 0.11, SE 0.02). The fraction of tumors considered highly aggressive based on their tendency to metastasize at a small size was 2.2 times greater among blacks than whites (0.41, SE 0.009 vs. 0.019, SE 0.008).
Conclusion
Based on our simulation model, breast tumors in blacks grow faster and are more likely to metastasize earlier than tumors in whites. These differences suggest that targeted prevention and detection strategies that go beyond equalizing access to mammography may be needed to eliminate breast cancer disparities.
Keywords: Breast Cancer Natural History, black women, white women, Simulation Model, Racial Disparities
Introduction
Disparities in breast cancer incidence and mortality between black and white women in the U.S. have long been a public health concern. Compared to whites, blacks have had higher breast cancer mortality rates despite lower incidence rates for almost thirty years [1,2]. The disparity in mortality continues to grow; in 2008, the breast cancer mortality rate for blacks was 40% higher than that for whites (31 vs. 22 per 100,000 women, respectively) [3].
The mortality disparity appears to stem primarily from the tendency for breast cancer in black women to be diagnosed at a more advanced stage (Fig. 1) and have more aggressive features [2,4–15]. These differences could potentially be due to racial variation in the natural history of the disease (e.g., growth rates), screening mammography utilization, and/or screening performance. Historically, blacks have trailed behind whites in utilization of screening mammography [16,17,12]. However, a number of studies have demonstrated that screening utilization has been comparable over the past fifteen years [2,18], and yet the difference in stage at diagnosis persists [2,8,4,6,7,9,12,15,14]. The sensitivity of screening mammography does not appear to be worse in blacks than among whites [19]. Additionally, several studies have observed that blacks are diagnosed with breast cancer at a later stage and are more likely to have tumors with poorer prognosis than whites independent of health care access or mammography [20]. These findings suggest that variation in the tumor natural history may be driving racial disparities in breast cancer outcomes [13,21–23].
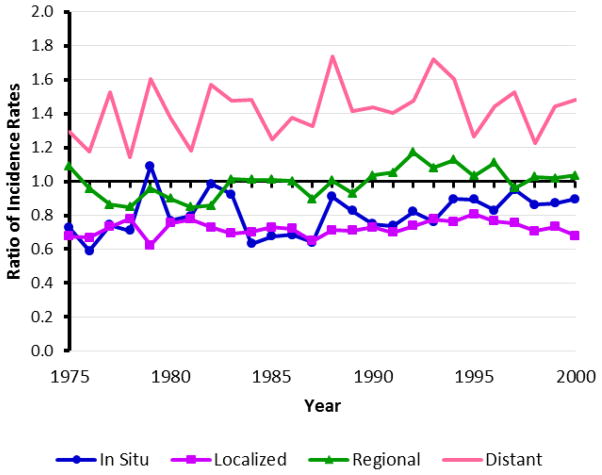
Fig. 1.
Ratio of U.S. observed age-adjusted incidence rates for blacks versus whites for each breast cancer stage. U.S. observed stage-specific incidence rates for women over 20 years of age were obtained from SEER and age-adjusted to the U.S. standard population in the year 2000.
The objective of this study was to evaluate whether racial differences in the natural history of breast cancer could explain the observed stage-specific incidence patterns among black and white women in the United States. We used the University of Wisconsin Breast Cancer Simulation Model (UWBCS) [24], a validated simulation model of the epidemiology of breast cancer, which was previously developed for the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET). The detailed natural history component of the UWBCS allows an evaluation of the role of racial variation of tumor growth and aggressiveness in breast cancer disparities within a framework that also considers racial variation in screening utilization and other important factors.
Methods
Overview
We modified the original UWBCS [24] to create race-specific models for blacks and whites. The original model structure was retained in each case, but race-specific inputs were developed for key components such as mammography utilization. The best fit natural history parameters were estimated separately for blacks and whites by calibrating to the observed 1975–2000 race-specific incidence data from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute.
UWBCS Model Overview
The UWBCS is a discrete-event, stochastic simulation model of breast cancer epidemiology in the U.S. female population and has previously been described in detail [24]. The model was designed to match age- and stage-specific breast cancer incidence rates and age-specific mortality rates in the U.S. female population between 1975 and 2000. It has been used to address a variety of questions including those related to the natural history of breast cancer in the U.S. female population [24], the relative contributions of breast cancer screening and treatment improvements to the reduction in breast cancer mortality observed in the 1990’s [25], the cost-effectiveness of different modes of screening mammography [26,27], and the comparative effectiveness of different screening strategies [21].
The original UWBCS model is not race-specific and is populated by about 3 million women, divided into birth cohorts to mimic the adult female population of the U.S. between 1975 and 2000. Individual women in the simulation experience four interacting stochastic processes which are modeled over time: the natural history of breast cancer from occult onset to breast cancer death or cure, breast cancer detection by screening mammography or clinical detection, the dissemination and effectiveness of breast cancer treatments over time, and death from non–breast cancer causes.
Breast cancer is modeled as a progressive disease. The natural history component of the UWBCS model assumes that invasive breast cancer originates from the progression of carcinoma in situ. Since the biologic onset of tumors is unobservable at first, its rate in the model is approximated using breast cancer incidence in the absence of screening at a later time point by utilizing an age-period-cohort (APC) model developed by Holford [28]. Over time, tumors progress in size and spread probabilistically to lymph nodes. Tumors grow according to a Gompertz-type function with a growth rate that follows a gamma distribution [29]. At the time of diagnosis, tumors are classified according to SEER historical stages (in situ, local, regional, or distant extent of disease). The heterogeneity in tumors in the population is approximated with four types of tumors: tumors with limited malignant potential (LMP), regular tumors, aggressive and highly aggressive tumors. Tumors with LMP are tumors in in situ or early localized stages of breast cancer that will never pose a lethal threat to the host women. Aggressive and highly aggressive tumors are tumors classified as regional or distant at a very small size of the focal primary tumor (particularly, at onset in the model). Depending on the effectiveness of treatments received in the model, uncontrolled growth and spread of invasive breast tumor may lead to death from breast cancer.
A statistical model described elsewhere [30,31] is used to describe mammography use and dissemination over time for 5-year birth cohorts of U.S. women. Screening initiation is assigned on the basis of birth year, and screening frequency (annual, biennial, or irregular) varies by birth year and age. Tumors can be detected by screening mammography or other means, and detection probabilities depend on the tumor size. Detected breast cancers are treated according to the year-specific prevalent clinical practice for a tumor in the given stage in a woman of a given age group [32]. Treatment effectiveness depends on the tumor stage at diagnosis and improves over time according to historical improvements.
The model parameters of the original model were calibrated to breast cancer incidence rates as reported by the SEER Program of the National Cancer Institute, hence the model results apply to the U.S. population.
Race-Specific Modifications of Model’s Fixed Input Parameters
We modified four fixed inputs of the original UWBCS model to make them race-specific: the population age-structure, mortality from non-breast cancer causes, breast cancer incidence in the absence of screening, and mammography dissemination over time.
The race-specific population age-structure was estimated from Census data [33]. We estimated the cohort-specific mortality from non-breast cancer causes for each race using death rates reported in Berkeley Mortality Database for years 1968–1992 and the National Center for Health Statistics (Centers for Disease Control and Prevention) for years 1900–1967 and 1993–2000 [34,35].
To estimate the background incidence of breast cancer in the absence of screening between 1975 and 2000 for white and black women separately, we adjusted the age-period-cohort model by Holford [28] for each race by utilizing SEER-reported race-, sex- and age-specific breast cancer incidence rates for years 1973 to 1981 when use of screening mammography was negligible [36]. We assumed that the age-specific ratio of breast cancer incidence in black versus white women would have remained constant between 1982 and 2000 if screening mammography had not been introduced.
As described in Cronin et al. (2009) [37], we used race-specific screening data from the Breast Cancer Surveillance Consortium (BCSC) regarding age at screening initiation and screening patterns to generate our race-specific mammography dissemination parameters.
Model Calibration and Race-Specific Input Parameters Fitted During Calibration
Eight parameters of the original UWBCS model which govern the tumor natural history properties were modified to allow race-specific values. These included:
LMP Fraction: The proportion of tumors with limited malignant potential.
LMP Dwell Time: The maximum sojourn time for LMP tumors, in years; after this time an undiagnosed tumor with limited malignant potential is no longer detectable by screening.
Onset Proportion: The ratio of age-specific biologic onset rate of tumors to age-specific incidence rate in the absence of screening. This parameter is used to estimate the unobservable biologic onset rates of tumors necessary to produce the observed incidence rates in the following years as dictated by the age-period-cohort (APC) model described elsewhere [28].
APC Lag: The number of years between biologic onset of tumors and their clinical surfacing in the absence of screening produced by the APC model [28]. This is a model-specific parameter used to represent the time between a tumor’s biologic onset in the UWBCS and its possible discovery via self or clinical detection at a later time point in the APC model.
Mean Growth: The mean of the gamma distribution used in the Gompertz-type function that models tumor growth rate [29].
Variance Growth: The variance of the gamma distribution that is used in the Gompertz-type function that models tumor growth rate.
Percent Aggressive: The fraction of tumors classified as regional at a very small size of the focal primary tumor, that is, 2 mm in diameter. This parameter represents aggressive tumors that advance to regional cancer stage quickly and is included to avoid depleting the reservoir of tumors to be discovered at the regional stage under all reasonable screening regimens [24].
Percent Highly aggressive: analogous to the Percent Aggressive parameter, this represents the fraction of tumors classified as distant stage at a very small size of the focal primary tumor (2 mm in diameter).
For model calibration, we determined biologically plausible ranges for each natural history input parameter, partitioned those ranges (Table 1), and ran the model for all possible parameter combinations which totaled to 378,000 simulations for each race. We then determined the combinations of parameters for which the stage-specific age-adjusted incidence rates among women over 20 years of age matched the U.S. observed rates as reported by SEER most closely.
Table 1.
Breast Cancer Natural History Input Parameters Used in UWBCS Model Calibration [24]
| Parameter Name | Best fit in the original combined race UWBCS model | Sampled Parameter Values |
|---|---|---|
| LMP Fraction | 0.42 | 0.00, 0.10, 0.20, 0.30, 0.40, 0.50, 0.60 |
| LMP Dwell Time | 2 | 1, 2, 3 |
| Onset Proportion | 0.90 | 0.80, 0.85, 0.90, 0.95, 1.00 |
| APC Lag | 3 | 1, 3, 5, 7 |
| Mean Growth | 0.12 | 0.01, 0.05, 0.10, 0.15, 0.20 |
| Variance Growth | 0.012 | 0.006, 0.010, 0.040, 0.070, 0.100 |
| Percent Aggressive | 0.01 | 0.00, 0.01, 0.02, 0.03, 0.04, 0.05 |
| Percent Highly Aggressive | 0.02 | 0.00, 0.01, 0.02, 0.03, 0.04, 0.05 |
Abbreviations: UWBCS, University of Wisconsin Breast Cancer Simulation; LMP, limited malignant potential; APC, Age-Period-Cohort
Like in the original UWBCS model, to enforce the general trend in the incidence curves, we determined envelopes around the U.S. observed age-adjusted incidence rates of breast cancer for each of the four breast cancer stages in 1975–2000 [24]. We then evaluated the fit of the age-adjusted incidence rate curves produced by the model relative to the envelopes. A score between 0 and 104 was assigned to each curve based on the number of points it fell outside of the envelopes for all four stages of breast cancer in 1975–2000. The score of 0 implied that the produced incidence curves never fell outside of the envelopes; the score of 104 meant that they were completely outside of the envelopes, that is, in each year between 1975 and 2000 for all four breast cancer stages. As in the original UWBCS model calibration procedure [24], we considered the input vector to be acceptable if the incidence curves score of its output did not exceed 10; such input vectors we ranked for further analysis. The ranking was based on the probability of the age-specific breast cancer incidence in the corresponding output to match the observed incidence as reported in SEER for each year and stage of breast cancer; the higher the probability, the higher the rank. To calculate this probability, we assumed that the age- and stage-specific breast cancer incidence in each given year followed a Poisson distribution with parameter equal to the expected breast cancer incidence given the model. For each race, the set of input vectors within the top 100 ranks and an envelope score no greater than 10 formed a joint posterior distribution of acceptable model parameters.
Results
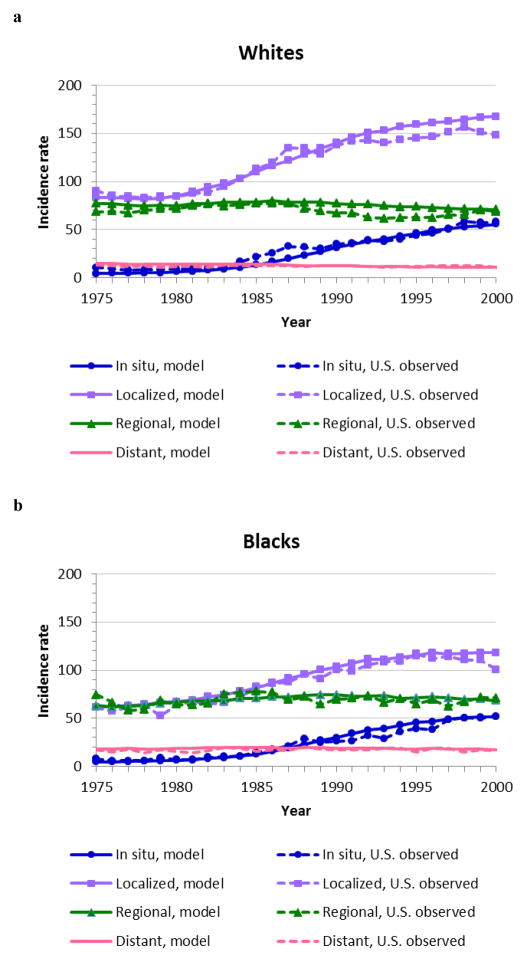
We found 69 acceptable natural history parameter vectors for black and 48 for white women. The mean stage-specific incidence curves produced by these acceptable parameter values are shown in Fig. 2 for each race. Both race-specific models demonstrated excellent matching to the observed stage-specific U.S incidence based on SEER data.
Fig. 2.
Model fit for the average of the joint posterior distribution of acceptable model input parameters: (a) for white women (W), (b) for black women (B). Incidence rates are given per 100,000 women over 20 years of age and age-adjusted to the U.S. standard population in the year 2000.
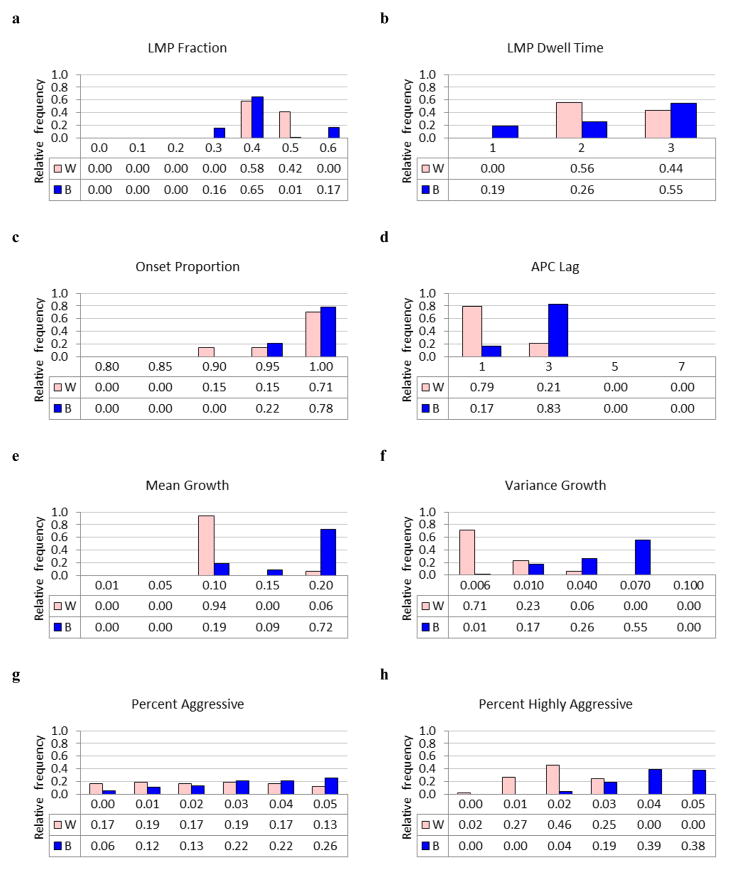
The posterior distributions of acceptable model input parameters are shown in Fig. 3 and their summary statistics (mean and standard error) are displayed in Table 2. LMP Fraction, LMP Dwell Time and Onset Proportion were similar for white and black women, although posterior uncertainty was greater for blacks. APC Lag was longer for blacks (2.65, SE 0.76) than whites (1.42, SE 0.82).
Fig. 3.
Joint posterior distribution of acceptable model parameters for black and white women. (a) LMP Fraction; (b) LMP Dwell Time; (c) Onset Proportion; (d) APC Lag; (e) Mean Growth; (f) Variance Growth; (g) Percent Aggressive; (h) Percent Highly aggressive. Horizontal axes represent parameter values.
Table 2.
Summary Statistics for Calibrated Input Parameters
| Parameter | Whites | Blacks | Posterior Distribution of the Difference, (Whites-Blacks)* | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SE | Mean | SE | Mean | 95% Credible Interval | |
| LMP Fraction | 0.44 | 0.05 | 0.42 | 0.09 | 0.02 | [−0.20, 0.20] |
| LMP Dwell Time | 2.44 | 0.50 | 2.36 | 0.79 | 0.05 | [−1.00, 2.00] |
| Onset Proportion | 0.98 | 0.04 | 0.99 | 0.02 | −0.01 | [−0.10, 0.05] |
| APC Lag | 1.42 | 0.82 | 2.65 | 0.76 | −1.21 | [−2.00, 2.00] |
| Mean Growth | 0.11 | 0.02 | 0.18 | 0.04 | −0.07 | [−0.10, 0.00] |
| Variance Growth | 0.009 | 0.008 | 0.051 | 0.024 | −0.041 | [−0.064, 0.000] |
| Percent Aggressive | 0.024 | 0.017 | 0.032 | 0.015 | −0.008 | [−0.050, 0.030] |
| Percent Highly Aggressive | 0.019 | 0.008 | 0.041 | 0.009 | −0.022 | [−0.040, 0.000] |
Based on the random sample (N=1,000) from the posterior distribution of the difference in the parameters, Whites – Blacks.
Abbreviations: LMP, limited malignant potential; APC, Age-Period-Cohort; SE, standard error
A number of aspects of tumor aggressiveness differed among blacks and whites. Compared to tumors among whites, tumors among blacks tended to grow at a faster rate (Mean Growth), had higher variation in their growth rates (Variance Growth), and were more likely to have spread to regional (Percent Aggressive) and distant (Percent Highly aggressive) sites while still at a small tumor size. The mean of the posterior distribution of the parameter governing tumor growth rate, Mean Growth, was 63.6% higher for blacks than whites (0.18, SE 0.04 vs. 0.11, SE 0.02, respectively), and the mean of the posterior distribution of the other tumor growth parameter, Variance Growth, was almost five-fold higher for blacks than whites (0.051, SE 0.024 vs. 0.009, SE 0.008). The fraction of tumors considered aggressive and highly aggressive based on their tendency to metastasize at a small size, Percent Aggressive and Percent Highly aggressive, was 33% and 2.2 times greater for black (0.032, SE 0.015 and 0.041, SE 0.009, respectively) than for white women (0.024, SE 0.017 and 0.019, SE 0.008, respectively).
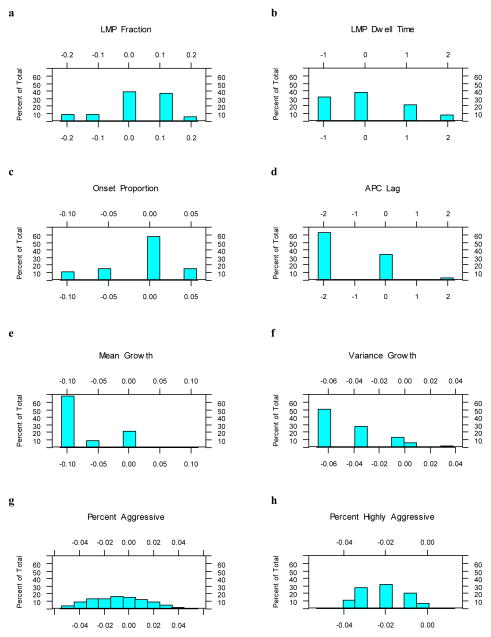
However, since the differences between the separate posterior distributions from the two race models may reflect differences in the associated sampling distributions rather than substantive differences between the models, we also examined the posterior distributions for the differences in parameters between the two race models. To this end, for each parameter from each race model, we generated random samples of size 1000 from the corresponding posterior distributions and calculated pairwise differences between the two samples, whites minus blacks. The posterior distribution for the difference in parameters between the race models are shown in Fig. 4; their means and 95% credible intervals (CI) are presented in Table 2. The 95% credible intervals were formed by the 2.5th and 97.5th percentiles of these posterior distributions [38].
Fig. 4.
Histogram (percent of total) of the posterior distribution for the differences in parameters between the two race models (whites – blacks). (a) LMP Fraction; (b) LMP Dwell Time; (c) Onset Proportion; (d) APC Lag; (e) Mean Growth; (f) Variance Growth; (g) Percent Aggressive; (h) Percent Highly aggressive. Horizontal axes represent the difference in parameter values, whites minus blacks.
The 95 % credible intervals for the mean differences between the posterior distributions for Mean Growth (Mean = −0.07, 95% CI −0.10, 0.00), Variance Growth (Mean = −0.041, 95% CI −0.064, 0.000), and Percent Highly Aggressive (Mean = −0.022, 95% CI −0.040, 0.000) support lower values of the parameter for whites than for blacks. The other credible intervals support no difference between the parameters for whites and blacks.
Discussion
We used a previously validated discrete-event stochastic simulation model of breast cancer epidemiology to evaluate differences in the natural history of breast cancer between black and white women. Model parameters were estimated for the two racial/ancestry groups while accounting for known differences in mammography utilization, and the resulting “best” models closely resembled the observed breast cancer rates as published by the SEER Program [36]. The best fit parameter estimates of our model indicated that the parameter governing tumor growth rates for blacks was almost two-fold higher than for whites, and a higher fraction of tumors in blacks had spread to a regional or distant stage at a very small size (one-third and over two-fold, respectively). The posterior distribution for the difference in parameters between the two race models also supported higher tumor growth rates in blacks and higher percent of highly aggressive tumors in blacks than in whites. This implies that, based on the model, tumors in blacks grow faster and tend to spread to advanced stages of breast cancer at a smaller size of the focal primary tumor compared to cancers among whites. These differences in natural history by race appear to drive the tendency for a more advanced stage distribution among black women.
One important consequence of these results is that with any given interval between two subsequent mammograms, our model suggests that breast cancer in blacks tends to progress to a more advanced stage than in whites. Thus, even with the same rate of adherence to breast cancer screening guidelines, we would expect a more advanced stage at diagnosis in black women. These findings are consistent with observational studies noting that blacks are diagnosed with breast cancer at a more advanced stage and are more likely to present with tumors of a more aggressive phenotype than whites [11,6–8,12,39], even with similar access to screening mammography [2,9,10]. Late stage at diagnosis is associated with poorer prognosis, and consequently higher breast cancer mortality in black women. Recently a group of investigators used two different simulation models to examine the potential impact of variation in natural history, mammography utilization, and adjuvant treatment on race-specific trends in breast cancer mortality [40]. They concluded that the majority of the disparities in breast cancer mortality between black and white women in the U.S. is attributable to differences in the natural history of the disease, while the differences in breast cancer screening and adjuvant treatment are weaker contributors [40]. Combined with our results regarding disparities in stage at diagnosis, these modeling studies provide consistent evidence that variation in tumor natural history by race is needed to explain the observed race-specific trends in both incidence and mortality.
Variation in tumor natural history could be due to differences in risk factor distributions by race, which in turn influence tumor biology. Blacks are more likely than whites to be diagnosed with hormone receptor negative breast cancers [2,4,6,7,9–11,15,41] which have limited adjuvant treatment options. This variation in tumor subtypes could be due to differences in genetic predisposition or differences in the prevalence of risk factors such as postmenopausal hormone therapy, reproductive factors, or body mass index which may have differential influence on risk of tumor subtypes [42,9,43,11,13,44,45]. Specifically, the rapid increase in obesity rates observed in the U.S. over the period of time considered in the study disproportionately affected black women [46]. Obesity has shown to affect mammography utilization and effectiveness [47,48], which, in turn, may lead to more advanced stage at diagnosis and worse outcomes. However, the direct effect of obesity on the natural history of breast cancer remains unclear. In particular, while the version of the model used for this study did not account for individual risk factors, we are currently working on extending the model to account for individual risk factors including body mass index, breast density, postmenopausal hormone use, and family history of breast cancer. It is also possible that differences in tumor biology between black and white breast cancers are mostly or even completely due to different tumor subtype distributions [45]. In particular, a recent international study concluded that among women in West Africa, the founder population of most African-Americans, the proportion of tumors associated with poorer prognosis is much higher; specifically, hormone receptor-negative breast tumors were found to be predominant and the majority of breast tumors triple-negative [45]. The authors also noted that breast cancer subtypes are determined by both environmental exposures and genetic background. More research is needed to better understand differences in the tumor subtype distribution according to race and ethnicity, whether tumor subtypes are inherently different with respect to biology and tumor aggressiveness, and whether there are residual differences in tumor biology within a given tumor subtype according to race/ethnicity.
Differences in breast cancer treatment by race may also contribute to the observed disparities in breast cancer mortality between black and white women. Though some studies reported similar breast cancer treatment utilization for black and white women [49], others noted the existence of race-specific differences. In particular, black women were reported to receive suboptimal treatment for breast cancer, largely due to differential access to care [7] and being less likely to be treated at high-quality hospitals [50]. Blacks were more likely than whites to receive no surgery [51], less likely to receive appropriate surgery [6] and follow-up radiation therapy after undergoing breast conserving surgery [52,53], experienced greater delays between breast cancer diagnosis and treatment initiation [17,54,55], were about twice as likely to experience underuse of appropriate adjuvant therapy [56], and were more likely to terminate treatment prematurely and miss appointments [57,58]. Furthermore, obesity which is more prevalent in black women compared to white [46] may also decrease treatment efficacy [59]. Due to higher prevalence of obesity and other comorbidities, black breast cancer patients may receive lower adjuvant chemotherapy dose proportion and dose intensity [60,61]. However, we did not incorporate these factors into our model, largely due to the absence of appropriate high-quality data. A recent study using two other CISNET breast cancer simulation models who examined treatment-related parameters by race was not able to match breast cancer mortality, particularly in blacks, and concluded that the differences in mortality by race remained largely unexplained (38–46%) [40]. More research is needed that explores differences in breast cancer treatment by race as a contributor to disparities in breast cancer mortality.
The limitations of our study are driven by the modeling assumptions and accuracy of the data inputs and model calibration targets. Consequently, our study has the same limitations due to model assumptions as the non-race-specific UWBCS model [24]. The required inputs to the model had to be estimated from the available data, which often required manipulation to conform to the desired format (e.g., five- or ten-year aggregated or single-year data). The model inputs also relied on race-specific data over many years, and the precision of these data for blacks was less robust than for whites due to smaller sample sizes, changes in methods for collecting race-specific data over time, and variation in how individuals report their race. Self-reported race information reflects race as a complex social construct rather than as an objective assessment of genetic predisposition such as could be provided by ancestral informative markers (AIM). One recent study suggested that self-reported race more than percent African ancestry as measured by AIMs is related to breast cancer tumor characteristics such as estrogen receptor status, stage at diagnosis and grade [62]. Social factors that, combined with inherited risk, influence whether women report their race as “white” or “black” are difficult to quantify. However, since we used the largest and highest quality data sources available, including SEER and the Breast Cancer Screening Consortium, we believe our race-specific data inputs were the best possible estimates.
Our sampling approach was constrained by the computational intensity of the project. Approximately 5 minutes were required to perform a single model run for a given parameter combination on a stand-alone computer. While we were unable to fully explore the parameter space in gaps between the constant step sizes and may have not adequately covered the parameter space for three parameters for blacks, namely, percent highly aggressive tumors, mean growth, and LMP dwell time, we used high throughput computing resources to sample over 378,000 input parameter combinations within the biologically plausible parameter space for each race. This detailed sampling provides a low likelihood that more optimal parameter value combinations were missed.
In conclusion, we used a simulation model to characterize potential differences in the natural history of breast cancer between black and white women in the United States that contribute to the observed disparities in breast cancer mortality. Our findings indicate that, on average, breast tumors in black women grow faster and are more often highly aggressive than those in whites. These factors drive the more advanced stage at diagnosis in black compared with white women which, in turn, is associated with higher breast cancer mortality. These results suggest that targeted breast cancer prevention and screening strategies that are sensitive to differences in tumor natural history may be needed to eliminate racial disparities in breast cancer outcomes in the United States.
Acknowledgments
Financial Support
This work is supported by grants R03CA130727, R01CA088211, and U01CA152958 from the National Cancer Institute. The modeling of screening dissemination used as an input was partially supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, U01CA70040, HHSN261201100031C). A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/
Footnotes
Disclosure of Potential Conflict of Interests
Dr. Alagoz served as a consultant for GE Health Care between June 2012 and July 2012.
References
- 1.Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56(3):168–183. doi: 10.3322/canjclin.56.3.168. 56/3/168 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Ghafoor A, Jemal A, Cokkinides V, Cardinez C, Murray T, Samuels A, Thun MJ. Cancer statistics for African Americans. CA Cancer J Clin. 2002;52 (6):326–341. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. [Accessed November 24, 2011]. based on November 2010 SEER data submission, posted to the SEER web site. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 4.Miller BA, Hankey BF, Thomas TL. Impact of sociodemographic factors, hormone receptor status, and tumor grade on ethnic differences in tumor stage and size for breast cancer in US women. American journal of epidemiology. 2002;155 (6):534–545. doi: 10.1093/aje/155.6.534. [DOI] [PubMed] [Google Scholar]
- 5.Vastag B. Breast cancer racial gap examined: no easy answers to explain disparities in survival. JAMA : the journal of the American Medical Association. 2003;290(14):1838–1842. doi: 10.1001/jama.290.14.1838. [DOI] [PubMed] [Google Scholar]
- 6.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of internal medicine. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. ioi10945 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 8.Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M. Trends in breast cancer by race and ethnicity. CA Cancer J Clin. 2003;53 (6):342–355. doi: 10.3322/canjclin.53.6.342. [DOI] [PubMed] [Google Scholar]
- 9.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10(1):1–14. doi: 10.1634/theoncologist.10-1-1. 10/1/1 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97(6):439–448. doi: 10.1093/jnci/dji064. 97/6/439 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Amend K, Hicks D, Ambrosone CB. Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res. 2006;66(17):8327–8330. doi: 10.1158/0008-5472.CAN-06-1927. 66/17/8327 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Smith-Bindman R, Miglioretti DL, Lurie N, Abraham L, Barbash RB, Strzelczyk J, Dignan M, Barlow WE, Beasley CM, Kerlikowske K. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Ann Intern Med. 2006;144(8):541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. 144/8/541 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA : the journal of the American Medical Association. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. 295/21/2492 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Vicini F, Jones P, Rivers A, Wallace M, Mitchell C, Kestin L, Jaiyesimi I, Dekhne N, Martinez A. Differences in disease presentation, management techniques, treatment outcome, and toxicities in African-American women with early stage breast cancer treated with breast-conserving therapy. Cancer. 2010;116(14):3485–3492. doi: 10.1002/cncr.25088. [DOI] [PubMed] [Google Scholar]
- 15.DeSantis C, Jemal A, Ward E. Disparities in breast cancer prognostic factors by race, insurance status, and education. Cancer causes & control : CCC. 2010;21(9):1445–1450. doi: 10.1007/s10552-010-9572-z. [DOI] [PubMed] [Google Scholar]
- 16.Jazieh AR, Buncher CR. Racial and age-related disparities in obtaining screening mammography: results of a statewide database. South Med J. 2002;95 (10):1145–1148. [PubMed] [Google Scholar]
- 17.Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Medical care. 2005;43(2):141–148. doi: 10.1097/00005650-200502000-00007. 00005650-200502000-00007 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Peek ME, Han JH. Disparities in screening mammography. Current status, interventions and implications. J Gen Intern Med. 2004;19(2):184–194. doi: 10.1111/j.1525-1497.2004.30254.x. 30254 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yankaskas BC, Gill KS. Diagnostic mammography performance and race: outcomes in Black and White women. Cancer. 2005;104(12):2671–2681. doi: 10.1002/cncr.21550. [DOI] [PubMed] [Google Scholar]
- 20.Wojcik BE, Spinks MK, Optenberg SA. Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer. 1998;82(7):1310–1318. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1310::AID-CNCR14>3.0.CO;2-9. [pii] [DOI] [PubMed] [Google Scholar]
- 21.Mandelblatt JS, Cronin KA, Bailey S, Berry DA, de Koning HJ, Draisma G, Huang H, Lee SJ, Munsell M, Plevritis SK, Ravdin P, Schechter CB, Sigal B, Stoto MA, Stout NK, van Ravesteyn NT, Venier J, Zelen M, Feuer EJ. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med. 2009;151(10):738–747. doi: 10.1059/0003-4819-151-10-200911170-00010. 151/10/738 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID, Jatoi I. Racial disparities in breast cancer outcome: insights into host-tumor interactions. Cancer. 2007;110(9):1880–1888. doi: 10.1002/cncr.22998. [DOI] [PubMed] [Google Scholar]
- 23.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(9):1342–1349. doi: 10.1200/JCO.2005.03.3472. 24/9/1342 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Fryback DG, Stout NK, Rosenberg MA, Trentham-Dietz A, Kuruchittham V, Remington PL. The Wisconsin Breast Cancer Epidemiology Simulation Model. J Natl Cancer Inst Monogr. 2006;(36):37–47. doi: 10.1093/jncimonographs/lgj007. 2006/36/37 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. 353/17/1784 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–782. doi: 10.1093/jnci/djj210. 98/11/774 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Tosteson AN, Stout NK, Fryback DG, Acharyya S, Herman BA, Hannah LG, Pisano ED. Cost-effectiveness of digital mammography breast cancer screening. Ann Intern Med. 2008;148(1):1–10. doi: 10.7326/0003-4819-148-1-200801010-00002. 148/1/1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holford TR, Cronin KA, Mariotto AB, Feuer EJ. Changing patterns in breast cancer incidence trends. J Natl Cancer Inst Monogr. 2006;(36):19–25. doi: 10.1093/jncimonographs/lgj016. 2006/36/19 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Spratt JS, Spratt JA. Growth Rates. In: Donegan WL, editor. Cancer of the Breast. 5. Saunders; Philadelphia (PA): 2002. pp. 443–476. [Google Scholar]
- 30.Cronin KA, Yu B, Krapcho M, Miglioretti DL, Fay MP, Izmirlian G, Ballard-Barbash R, Geller BM, Feuer EJ. Modeling the dissemination of mammography in the United States. Cancer causes & control : CCC. 2005;16(6):701–712. doi: 10.1007/s10552-005-0693-8. [DOI] [PubMed] [Google Scholar]
- 31.Cronin KA, Mariotto AB, Clarke LD, Feuer EJ. Additional common inputs for analyzing impact of adjuvant therapy and mammography on U.S. mortality. J Natl Cancer Inst Monogr. 2006;(36):26–29. doi: 10.1093/jncimonographs/lgj005. [DOI] [PubMed] [Google Scholar]
- 32.Mariotto AB, Feuer EJ, Harlan LC, Abrams J. Dissemination of adjuvant multiagent chemotherapy and tamoxifen for breast cancer in the United States using estrogen receptor information: 1975–1999. J Natl Cancer Inst Monogr. 2006;(36):7–15. doi: 10.1093/jncimonographs/lgj003. [DOI] [PubMed] [Google Scholar]
- 33.United States Census Bureau Population Estimates. 2008 http://www.census.gov/popest/archives/
- 34.Berkeley Mortality Database Data for the United States. 2008 http://www.demog.berkeley.edu/~bmd/states.html.
- 35.Centers for Disease Control and Prevention National Center for Health Statistics. 2008 http://www.cdc.gov/nchs/
- 36.Software: Surveillance Research Program, National Cancer Institute SEER*Stat software version 6.6.2. Data: Surveillance, Epidemiology, and End Results (SEER) Program. Available from: www.seer.cancer.gov/seerstat. Available from: www.seer.cancer.gov. SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2009 Sub. 1973–2007<Single Ages to 85+, Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2007 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission.
- 37.Cronin KA, Miglioretti DL, Krapcho M, Yu B, Geller BM, Carney PA, Onega T, Feuer EJ, Breen N, Ballard-Barbash R. Bias associated with self-report of prior screening mammography. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1699–1705. doi: 10.1158/1055-9965.EPI-09-0020. 18/6/1699 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wasserman LA. Springer Texts in Statistics. 1. Springer; The United States of America: 2004. All of Statistics: A Conscise Course in Statistical Inference. [Google Scholar]
- 39.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, Schwartz GF, Park PK, Rosenberg AL, Brill K, Mitchell EP. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 40.van Ravesteyn NT, Schechter CB, Near AM, Heijnsdijk EA, Stoto MA, Draisma G, de Koning HJ, Mandelblatt JS. Race-specific impact of natural history, mammography screening, and adjuvant treatment on breast cancer mortality rates in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(1):112–122. doi: 10.1158/1055-9965.EPI-10-0944. 1055-9965.EPI-10-0944 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joslyn SA. Hormone receptors in breast cancer: racial differences in distribution and survival. Breast Cancer Res Treat. 2002;73 (1):45–59. doi: 10.1023/a:1015220420400. [DOI] [PubMed] [Google Scholar]
- 42.McCullough ML, Feigelson HS, Diver WR, Patel AV, Thun MJ, Calle EE. Risk factors for fatal breast cancer in African-American women and White women in a large US prospective cohort. American journal of epidemiology. 2005;162(8):734–742. doi: 10.1093/aje/kwi278. kwi278 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Hall IJ, Moorman PG, Millikan RC, Newman B. Comparative analysis of breast cancer risk factors among African-American women and White women. American journal of epidemiology. 2005;161(1):40–51. doi: 10.1093/aje/kwh331. 161/1/40 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Fregene A, Newman LA. Breast cancer in sub-Saharan Africa: how does it relate to breast cancer in African-American women? Cancer. 2005;103(8):1540–1550. doi: 10.1002/cncr.20978. [DOI] [PubMed] [Google Scholar]
- 45.Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, Zhang B, Grushko T, Zhang C, Oluwasola O, Malaka D, Malami S, Odetunde A, Adeoye AO, Iyare F, Falusi A, Perou CM, Olopade OI. Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(27):4515–4521. doi: 10.1200/JCO.2008.19.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA : the journal of the American Medical Association. 2002;288 (14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 47.Cohen SS, Palmieri RT, Nyante SJ, Koralek DO, Kim S, Bradshaw P, Olshan AF. Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer. 2008;112(9):1892–1904. doi: 10.1002/cncr.23408. [DOI] [PubMed] [Google Scholar]
- 48.Elmore JG, Carney PA, Abraham LA, Barlow WE, Egger JR, Fosse JS, Cutter GR, Hendrick RE, D’Orsi CJ, Paliwal P, Taplin SH. The association between obesity and screening mammography accuracy. Archives of internal medicine. 2004;164(10):1140–1147. doi: 10.1001/archinte.164.10.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Worthington J, Waterbor JW, Funkhouser E, Falkson C, Cofield S, Fouad M. Receipt of standard breast cancer treatment by African American and White women. Int J Med Sci. 2008;5 (4):181–188. doi: 10.7150/ijms.5.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Medical care. 2009;47(7):765–773. doi: 10.1097/MLR.0b013e31819e1fe7. [DOI] [PubMed] [Google Scholar]
- 51.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112(1):171–180. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94 (5):334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 53.Joslyn SA. Racial differences in treatment and survival from early-stage breast carcinoma. Cancer. 2002;95(8):1759–1766. doi: 10.1002/cncr.10827. [DOI] [PubMed] [Google Scholar]
- 54.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(27):4135–4141. doi: 10.1200/JCO.2009.27.2427. JCO.2009.27.2427 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Archives of internal medicine. 2006;166(20):2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 56.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. 24/9/1357 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Hershman D, McBride R, Jacobson JS, Lamerato L, Roberts K, Grann VR, Neugut AI. Racial disparities in treatment and survival among women with early-stage breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(27):6639–6646. doi: 10.1200/JCO.2005.12.633. 23/27/6639 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Hershman DL, Unger JM, Barlow WE, Hutchins LF, Martino S, Osborne CK, Livingston RB, Albain KS. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(13):2157–2162. doi: 10.1200/JCO.2008.19.1163. JCO.2008.19.1163 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Archives of internal medicine. 2005;165(11):1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 60.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 61.Griggs JJ, Culakova E, Sorbero ME, van Ryn M, Poniewierski MS, Wolff DA, Crawford J, Dale DC, Lyman GH. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(3):277–284. doi: 10.1200/JCO.2006.08.3063. JCO.2006.08.3063 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Reding KW, Carlson CS, Kahsai O, Chen CC, McDavid A, Doody DR, Chen C, Ornelas I, Lowe K, Bernstein L, Weiss L, McDonald JA, Simon MS, Strom B, Marchbanks PA, Burkman R, Spirtas R, Liff JM, Malone KE. Examination of ancestral informative markers and self-reported race with tumor characteristics of breast cancer among Black and White women. Breast Cancer Res Treat. 2012;134(2):801–809. doi: 10.1007/s10549-012-2099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]