Abstract
PLA2G7 gene product is a secreted enzyme whose activity is associated with coronary heart disease (CHD). The goal of our study is to investigate the contribution of PLA2G7 promoter DNA methylation to the risk of CHD. Using the bisulphite pyrosequencing technology, PLA2G7 methylation was measured among 36 CHD cases and 36 well-matched controls. Our results indicated that there was a significant association between PLA2G7 methylation and CHD (adjusted P = 0.025). Significant gender-specific correlation was observed between age and PLA2G7 methylation (males: adjusted r = −0.365, adjusted P = 0.037; females: adjusted r = 0.373, adjusted P = 0.035). A breakdown analysis by gender showed that PLA2G7 methylation was significantly associated with CHD in females (adjusted P = 0.003) but not in males. A further two-way ANOVA analysis showed there was a significant interaction between gender and status of CHD for PLA2G7 methylation (gender*CHD: P = 6.04E−7). Moreover, PLA2G7 methylation is associated with the levels of total cholesterols (TC, r = 0.462, P = 0.009), triglyceride (TG, r = 0.414, P = 0.02) and Apolipoprotein B (ApoB, r = 0.396, P = 0.028) in females but not in males (adjusted P>0.4). Receiver operating characteristic (ROC) curves showed that PLA2G7 methylation could predict the risk of CHD in females (area under curve (AUC) = 0.912, P = 2.40E−5). Our results suggest that PLA2G7 methylation changes with aging in a gender-specific pattern. The correlation between PLA2G7 methylation and CHD risk in females is independent of other parameters including age, smoking, diabetes and hypertension. PLA2G7 methylation might exert its effects on the risk of CHD by regulating the levels of TC, TG, and ApoB in females. The gender disparities in the PLA2G7 methylation may play a role in the molecular mechanisms underlying the pathophysiology of CHD.
Introduction
DNA methylation often occurs in a CpG dinucleotide context and promoter DNA methylation can regulate the expression level of gene. Vertebrate CpG islands (CGIs) are short interspersed CpG-rich DNA sequences predominantly nonmethylated in or near approximately 40% of promoters of mammalian genes [1]. CGI hypermethylation of gene promoter usually silences gene expression [2]. Aberrant DNA methylation has extensively studied for the pathogenesis of multiple cancers including colorectal cancer [3], lung cancer [4], and leukemia [5]. However, only a few studies [6]–[10] have indicated an involvement of DNA promoter methylation in the susceptibility of coronary heart disease (CHD) that is the top killer in the world.
Gender disparities exist in the incidence, clinical presentation, diagnosis, and the surgical treatment of CHD [11]. For example, there are significantly more men die of CHD than women each year [11], [12]; Men suffered more from CHD and showed significantly more often chest pain localized on the right side of the chest [13]; Women were treated less intensively in the acute phase of acute coronary syndrome (ASC), while men were more often referred for coronary angiography [14]–[18]. Gender-related studies in CHD have identified a handful of biomarkers to clarify the differences between women and men [19]. It has been reported that by regulating expression of target gene, hormone-induced DNA methylation may increase or reduce the risk of complex diseases such as CHD [20]. Therefore, the gender difference in the epidemiological studies is likely attributable to the epigenetic modifications [21]–[23] such as DNA methylation.
PLA2G7 gene product is a secreted enzyme whose activity is associated with CHD [24], [25]. Circulating lipoprotein-associated phospholipase A2 (Lp-PLA2) may indicate the inflammation level which plays a key role in the development of CHD [26], [27]. Lp-PLA2 is expressed abundantly in the necrotic core of coronary lesions [28], and once in the arterial wall facilitates hydrolysis of phospholipids [29]. PLA2G7 gene expression and its expression quantitative locus were associated with CHD [30], [31]. In light of the previous findings, we hypothesized that promoter DNA methylation of PLA2G7 gene in peripheral blood might contribute to the risk of CHD. Thus, the goal of this study was to assess whether PLA2G7 gene promoter DNA methylation is associated with the risk of CHD and whether the association, if exists, is gender-specific.
Materials and Methods
Sample and Clinical Data
A total of 36 CHD cases and 36 age- and sex-matched controls were collected from the patients in the Ningbo Lihuili Hospital. The details of the inclusion criteria were presented in our previous publication [32]. All the individuals were Han Chinese originated from Ningbo city in the Eastern China. Blood samples were collected in 3.2% citrate sodium-treated tubes and then stored at −80°C. The study protocol was approved by the Ethical Committee of Ningbo Lihuili Hospital, and the informed written consent was obtained from all the subjects.
Biochemical Analyses
Human genomic DNA was prepared from peripheral blood samples using the nucleic acid extraction analyzer (Lab-Aid 820, Xiamen City, China). DNA concentrations were determined by the ultramicro nucleic acid ultraviolet tester (NANODROP 1000, Wilmington, USA). Plasma levels of TG, TC, high density lipoprotein (HDL), and low density lipoprotein (LDL) were measured using an enzymatic end point assay [33]. The ApoA, ApoB and ApoE levels were measured by the transmission turbidimetric method [34]. The plasma Lp(a) concentrations were determined by a sandwich enzyme-linked immunosorbent assay method [Macra-Lp(a), SDI, Newark, Delaware]. The concentrations of ALT, AST, ALP and GGT in plasma were measured by the IFCC reference measurement systems [35]–[37]. The ALB level was worked through the Bromocresol green method [38]. All the tests applied the standard procedures recommended by the manufacturers. DNA methylation was measured by the pyrosequencing technology which combines sodium bisulfite DNA conversion chemistry (EpiTech Bisulfite Kits; Qiagen), polymerase chain reaction (PCR) amplification (Pyromark PCR Kit; Qiagen) and sequencing by synthesis assay (Pyromark Gold Q24 Reagents; Qiagen) of the CGI region on PLA2G7 gene promoter. PCR primers were designed by PyroMark Assay Design software. Sequences of the PCR primers were shown in Table S1.
Statistical Analyses
Using the SPSS package (version 16.0), a series of statistical analyses were performed to investigate the association of the promoter DNA methylation of PLA2G7 gene with previous history of CHD and various biochemical factors. All the P values were adjusted for the history of smoking, diabetes and hypertension. A two-tailed p-value<0.05 was considered to be significant.
Results
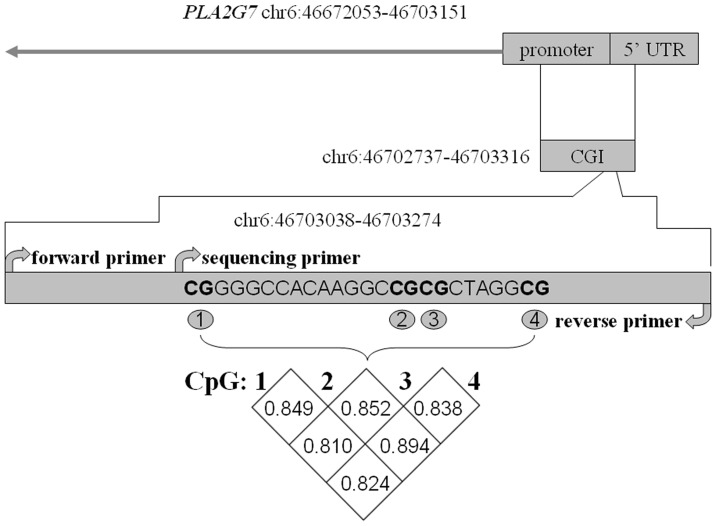
As shown in Figure 1, the bisulphate pyrosequencing assay was carried on a fragment (hg19, chr6:46702737–46703316) in the promoter region of PLA2G7 gene. This fragment contained 4 CG sites that could be measured to evaluate the methylation levels of PLA2G7 gene promoter. Significant correlation of the DNA methylation levels was found among these 4 CpGs (Figure 1, r >0.8, P<0.0001). No gender difference was observed for the promoter DNA methylation levels of PLA2G7 gene (Table 1, adjusted P = 0.226). There was a significant higher promoter DNA methylation of PLA2G7 gene in the CHD cases than in the non-CHD controls (Table 2, adjusted P = 0.025).
Figure 1. Significant correlation among the four CpGs in PLA2G7 gene promoter.
Table 1. Characteristics of subjects according to gendera.
| Characteristics | Men (n = 36)Mean±s.e. | Women (n = 36)Mean±s.e. | P value |
| Age | 62.0±5.4 | 62.2±5.4 | 0.896 |
| TG (mmol/L) | 2.39±0.82 | 2.75±0.80 | 0.054 |
| TC (mmol/L) | 4.19±0.85 | 4.64±0.84 | 0.060 |
| HDL (mmol/L) | 1.08±0.20 | 1.20±0.25 | 0.173 |
| LDL (mmol/L) | 1.59±0.90 | 1.54±0.95 | 0.444 |
| ApoA I (g/L) | 0.88±0.14 | 0.91±0.13 | 0.789 |
| ApoB (g/L) | 0.63±0.15 | 0.67±0.16 | 0.226 |
| ApoE (g/L) | 4.2±1.2 | 6.8±8.3 | 0.005d |
| Lp(a) (g/L) | 0.27±0.36 | 0.31±0.33 | 0.298c |
| hs-CRP (mg/L) | 3.2±3.4 | 3.5±3.5b | 0.664c |
| ALB (g/L) | 42.2±4.1 | 41.4±3.2 | 0.126 |
| GLB (g/L) | 22.6±3.7 | 25.4±3.6 | 0.112 |
| A/G | 1.9±0.3 | 1.7±0.3 | 0.033 |
| ALT (IU/L) | 24±14 | 23±15 | 0.502 |
| AST (IU/L) | 21±7 | 22±8 | 0.808 |
| ALP (IU/L) | 60±15 | 70±18 | 0.130 |
| GGT(IU/L) | 29±20 | 25±25 | 0.929c |
| mean PLA2G7 methylation (%) | 5.08±2.65 | 5.15±3.29 | 0.226 |
P values were adjusted for the history of smoking, diabetes and hypertension.
An outlier due to the clerical error was eliminated.
Log-transformation was used.
Nonparametric rank test was applied.
Table 2. Characteristics of subjects from cases and controlsa.
| Characteristics | Cases (n = 36)Mean±s.e. | Controls (n = 36)Mean±s.e. | P value |
| Age | 62.5±5.5 | 61.7±5.2 | 0.802 |
| Gender (M/F) | 18/18 | 18/18 | 1.000 |
| TG (mmol/L) | 2.66±0.86 | 2.48±0.80 | 0.554 |
| TC (mmol/L) | 4.49±0.90 | 4.34±0.86 | 0.591 |
| HDL (mmol/L) | 1.15±0.24 | 1.13±0.22 | 0.747 |
| LDL (mmol/L) | 1.51±0.98 | 1.62±0.86 | 0.700c |
| ApoA I (g/L) | 0.90±0.11 | 0.90±0.16 | 0.845 |
| ApoB (g/L) | 0.66±0.17 | 0.63±0.15 | 0.686 |
| ApoE (g/L) | 6.5±8.4 | 4.5±1.2 | 0.910d |
| Lp (a) (g/L) | 0.32±0.38 | 0.27±0.31 | 0.325c |
| hs-CRP (mg/L) | 3.5±3.5 | 3.1±3.5b | 0.257d |
| ALB (g/L) | 41.6±3.8 | 42.0±3.6 | 0.487 |
| GLB (g/L) | 24.2±3.7 | 23.8±4.1 | 0.942 |
| A/G | 1.8±0.3 | 1.8±0.3 | 0.697 |
| ALT (IU/L) | 26±17 | 21±11 | 0.168 |
| AST (IU/L) | 23±9 | 21±6 | 0.398 |
| ALP (IU/L) | 65±19 | 65±16 | 0.912 |
| GGT (IU/L) | 28±26 | 27±20 | 0.721c |
| mean PLA2G7 methylation (%) | 6.41±2.62 | 4.98±3.06 | 0.025 |
P values were adjusted for the history of smoking, diabetes and hypertension.
An outlier due to the clerical error was eliminated.
Log-transformation was used.
Nonparametric rank test was applied.
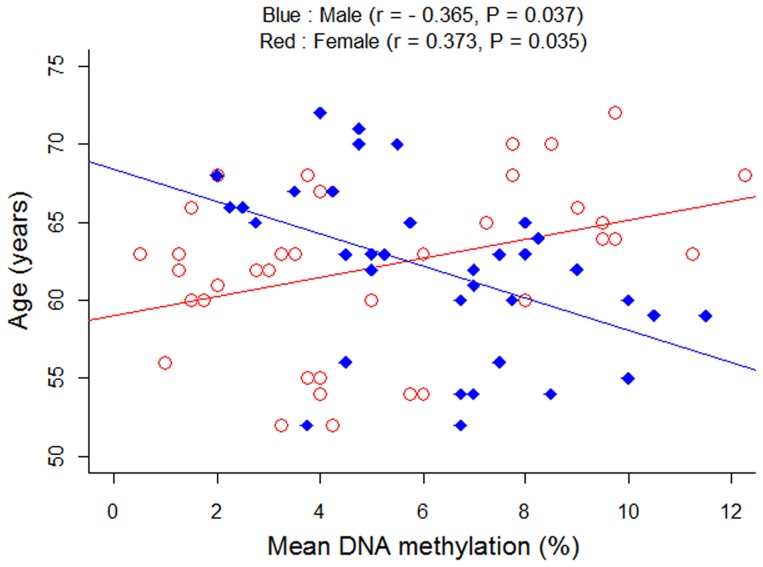
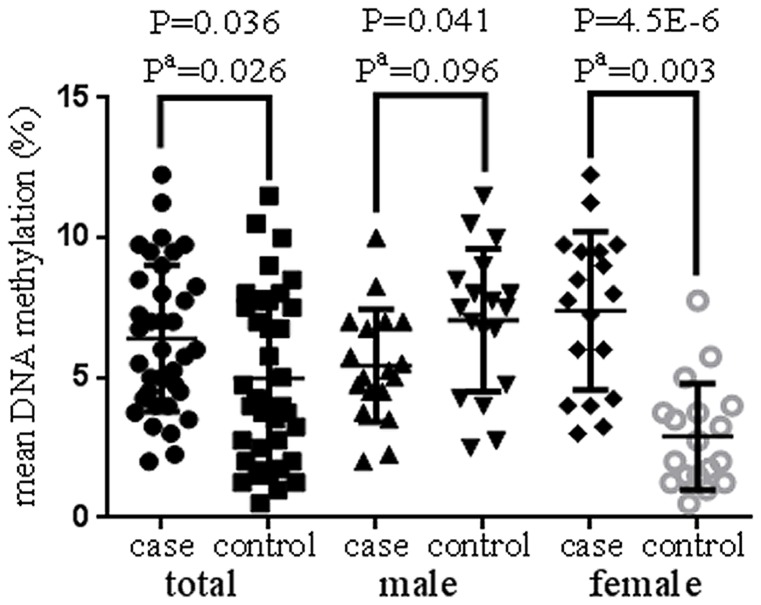
It was intriguing that we observed a gender-specific pattern of the correlation between age and PLA2G7 gene methylation (Figure 2, males: adjusted r = −0.365, adjusted P = 0.037; females: adjusted r = 0.373, adjusted P = 0.035). A further breakdown analysis by gender found that the significant association only existed in the female subgroup (Figure 3, females: uncorrected P = 4.5E−6, adjusted P = 0.003; males: uncorrected P = 0.041, adjusted P = 0.096). To note, the adjusted P values were corrected by age, the history of smoking, diabetes and hypertension. A further two-way ANOVA analysis also showed there was a significant interaction between gender and status of CHD for the methylation level of PLA2G7 gene (gender*CHD: P = 6.04E−7).
Figure 2. Correlation between PLA2G7 methylation and agea.
a) r values and P values were adjusted for the history of smoking, diabetes and hypertension.
Figure 3. Comparison of PLA2G7 methylation levels between cases and controls.
a) P values were adjusted for age, the history of smoking, diabetes and hypertension.
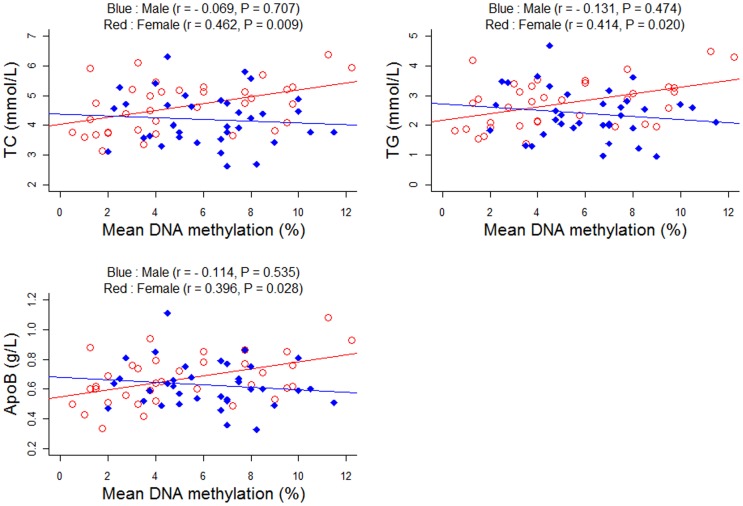
As shown in Table 1 and 2, a total of 16 phenotypes were involved in the present study. Among these phenotypes, only the ratio of albumin to globulin (A/G) showed a significant difference between men and women (Table 1, adjusted P = 0.033). No significant association was observed between A/G phenotype and CHD risk in either the female subgroup (P = 0.293) or the male subgroup (P = 0.716). And there is no correlation between the PLA2G7 methylation and A/G phenotypes in either the female subgroup (plot 3b, r = −0.062, P = 0.739) or the male subgroup (r = −0.050, P = 0.786). No significant association of the rest phenotypes with the risk of CHD was found (Table 2). Interestingly, a significant female-specific association was found between PLA2G7 promoter DNA methylation and phenotypes including TC, TG and ApoB (Figure 4, adjusted P<0.03 in females; adjusted P>0.4 in males).
Figure 4. Pearson correlation between PLA2G7 DNA methylation and TC, TG, ApoBa.
a) r values and P values were adjusted for age, the history of smoking, diabetes and hypertension.
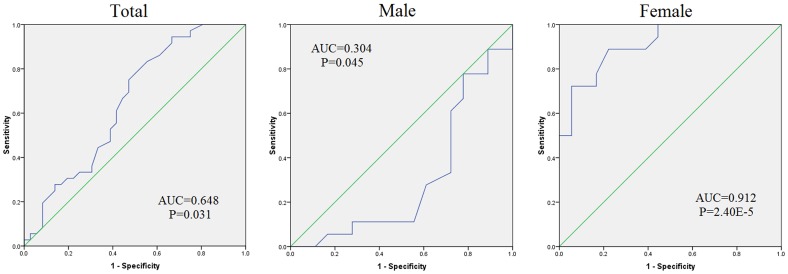
As shown in Figure 5, the receiver operating characteristic (ROC) analyses of curves showed that PLA2G7 promoter methylation could predict the risk of CHD in the total samples (Figure 5, area under curve (AUC) = 0.648, P = 0.031). The subgrouped analyses showed a female-dependent effect of PLA2G7 methylation in the prediction of CHD (Figure 5, males: AUC = 0.304, P = 0.045; females: AUC = 0.912, P = 2.40E−5).
Figure 5. ROC curves of PLA2G7 in total, male and female samples.
Discussion
CHD cases were observed to have significantly higher genomic DNA methylation in peripheral lymphocytes in comparison to non-CHD controls [39]. In addition, candidate epigenetics analysis showed that altered ABCA1 promoter DNA methylation was associated with the risk of CHD [10]. In the present study, we had recruited 36 cases and 36 gender- and age-matched controls to test the association between DNA methylation of PLA2G7 gene and the risk of CHD. We found that PLA2G7 methylation changes with aging in a gender-specific pattern, which accords with the previous observation that the pattern of DNA methylation changes individually over time [40].
PLA2G7 is the coding gene for Lp-PLA2 whose abnormal activity can cause high risk of CHD [30] and may serve as a diagnostic marker for CHD [27]. However, controversies remained in the association between PLA2G7 variants and the risk of CHD [27], [30], [31], [41], [42]. The present study demonstrated that PLA2G7 methylation changes with aging differently in two genders. Its female-specific contribution to the risk of CHD provides epigenetic clues to explain the inconsistency in the epidemiological studies.
Epidemiologic evidence from observational studies and randomized clinical trials identified high risk factors of CHD among older men and women [43]. Among the conventional risk factors, diabetes mellitus and hyperlipidemia was shown to impact more on women while smoking was found to affect more on men [44]. To test the difference between male cases and male controls, the unadjusted P value reached the significant level (P<0.05, Figure 3). However, the significance disappeared after corrected by age, the history of smoking, diabetes and hypertension (P = 0.096). So we speculate that environmental factors such as aging, smoking, diabetes and hypertension may play their roles in the risk of CHD through affecting PLA2G7 methylation.
Gender difference exists in both the age of onset [45] and the long-term prognosis of CHD patients [46]. Estrogen regulates the enzymatic activity of PLA2G7 protein and thus causes significant changes in the metabolism of cholesterols and apolipoproteins [47]–[49]. In the present study, we observed a gender-dependent correlation between age and PLA2G7 methylation, and an interaction between PLA2G7 methylation and gender. TC, TG, and ApoB are known as important risk factors of CHD [50], [51]. And these findings suggest that PLA2G7 methylation may exert its effects on the risk of CHD by regulating the levels of TC, TG, and ApoB in females.
PLA2G7 methylation was shown to be controlled by the interactive effect of CHD status and gender. Female cases had a higher PLA2G7 methylation level than female controls while it was the opposite in males, although the latter comparison was not significant after correction with confounding factors such as smoking and status of diabetes and hypertension. The trend of correlation between PLA2G7 methylation and age was clearly opposite in the two genders. The ROC curves showed a much higher accuracy of PLA2G7 methylation to predict CHD in females than in males.
Although there is a paucity of evidence showing a gender-dimorphism of methylation-mediated PLAG7 expression in CHD, we observe a possible connection with APOE gene that may help explain our results. As shown in the Figure S1, there is an APOE-dependent correlation between PLA2G7 expression and aging in mice (F = 12.42, P = 0.0074) [52]. Moreover, a female-specific interaction was found between APOE4 genotype and the metabolism of HDL [53], which is an important protective factor in cardiovascular diseases including CHD. Another piece of evidence has shown a significant association between APOE expression and aging [54]. All the above evidence about APOE gene might give a plausible explanation for the sex-dimorphism of the association of PLA2G7 promoter methylation with aging in CHD, although cautions need to be taken without direct supportive evidence. Future investigation of APOE interaction with PLA2G7 is needed to confirm this speculation.
There were some limitations in our study. Firstly, the sample size in our study is relatively small. Future investigation with more samples needs to be performed to confirm our findings. Secondly, only a fragment of the CGI was selected to stand for the whole promoter of PLA2G7. Thirdly, PLA2G7 methylation was measured in the whole peripheral blood which contained the DNA from a mixture of lymphocytes, granulocytes and other cell types. Fourthly, although we tried our best to control the confounding factors that may affect the methylation level of PLA2G7, there existed a possibility of an unknown factor that might confound the alteration of PLA2G7 methylation. Fifth, we didn’t explore the mechanism why PLA2G7 methylation correlated with TG, TC and ApoB in females. The exact interactions among them remained to be explained in the future work. Sixth, some P values in our study will not retain significance after being corrected by the number of tests. Therefore, we can’t exclude a chance of random positive findings among these results. Finally, the ages of female samples in our study range from 52 to 72. The mean age of natural menopause was 48.72±3.51 for Chinese female residents in city according to an epidemiological report in China [55]. As shown in Figure S2, the significance of association is mainly contributed by the eldly females. Thus, our results might be not feasible in the younger population.
In summary, we revealed PLA2G7 methylation as a gender-dependent marker of aging and its female-specific association with the risk of CHD and biochemistry factors such as TC, TG, and ApoB. These findings could establish a molecular link between aging and the risk of CHD and thus contribute to a better understanding of the molecular mechanisms underlying the pathophysiology of CHD. The above clues may help improve the current clinical diagnosis and treatment of CHD.
Supporting Information
Comparison of PLA2G7 methylation levels between cases and controls in different age groups in femalesa. a) P values were adjusted for age, the history of smoking, diabetes and hypertension.
(TIF)
Correlation between PLA2G7 methylation and agea.
(TIF)
Primer information of PLA2G7 methylation assay.
(DOC)
Funding Statement
The research was supported by the grants from National Natural Science Foundation of China (31100919), K. C. Wong Magna Fund in Ningbo University, Advanced Key Scientific and Technological Programs of Ningbo (2011C51001), Science and Technology Innovation team of Ningbo (2011B82015), Ningbo natural science foundation (2011A610036, 2011A610052), Zhejiang provincial natural science foundation (LY12H16002), and Ningbo social development research projects (2012C50032). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Fatemi M, Pao MM, Jeong S, Gal-Yam EN, Egger G, et al. (2005) Footprinting of mammalian promoters: use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res 33: e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deaton AM, Bird A (2011) CpG islands and the regulation of transcription. Genes Dev 25: 1010–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim MS, Lee J, Sidransky D (2010) DNA methylation markers in colorectal cancer. Cancer Metastasis Rev 29: 181–206. [DOI] [PubMed] [Google Scholar]
- 4. Kerr KM, Galler JS, Hagen JA, Laird PW, Laird-Offringa IA (2007) The role of DNA methylation in the development and progression of lung adenocarcinoma. Dis Markers 23: 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Akalin A, Garrett-Bakelman FE, Kormaksson M, Busuttil J, Zhang L, et al. (2012) Base-pair resolution DNA methylation sequencing reveals profoundly divergent epigenetic landscapes in acute myeloid leukemia. PLoS Genet 8: e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, et al. (1999) Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res 43: 985–991. [DOI] [PubMed] [Google Scholar]
- 7. Turunen MP, Aavik E, Yla-Herttuala S (2009) Epigenetics and atherosclerosis. Biochim Biophys Acta 1790: 886–891. [DOI] [PubMed] [Google Scholar]
- 8. Bressler J, Shimmin LC, Boerwinkle E, Hixson JE (2011) Global DNA methylation and risk of subclinical atherosclerosis in young adults: the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Atherosclerosis 219: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friso S, Lotto V, Choi SW, Girelli D, Pinotti M, et al. (2012) Promoter methylation in coagulation F7 gene influences plasma FVII concentrations and relates to coronary artery disease. J Med Genet 49: 192–199. [DOI] [PubMed] [Google Scholar]
- 10. Guay SP, Brisson D, Munger J, Lamarche B, Gaudet D, et al. (2012) ABCA1 gene promoter DNA methylation is associated with HDL particle profile and coronary artery disease in familial hypercholesterolemia. Epigenetics 7: 464–472. [DOI] [PubMed] [Google Scholar]
- 11. Lawton JS (2011) Sex and gender differences in coronary artery disease. Semin Thorac Cardiovasc Surg 23: 126–130. [DOI] [PubMed] [Google Scholar]
- 12.Anderson J, Kessenich CR (2001) Women and coronary heart disease. Nurse Pract 26: 12, 18, 21–13 passim; quiz 32–13. [DOI] [PubMed]
- 13. Bosner S, Haasenritter J, Hani MA, Keller H, Sonnichsen AC, et al. (2009) Gender differences in presentation and diagnosis of chest pain in primary care. BMC Fam Pract 10: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stone PH, Thompson B, Anderson HV, Kronenberg MW, Gibson RS, et al. (1996) Influence of race, sex, and age on management of unstable angina and non-Q-wave myocardial infarction: The TIMI III registry. JAMA 275: 1104–1112. [PubMed] [Google Scholar]
- 15. Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, et al. (1998) Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Arch Intern Med 158: 981–988. [DOI] [PubMed] [Google Scholar]
- 16. Roger VL, Farkouh ME, Weston SA, Reeder GS, Jacobsen SJ, et al. (2000) Sex differences in evaluation and outcome of unstable angina. JAMA 283: 646–652. [DOI] [PubMed] [Google Scholar]
- 17. Gan SC, Beaver SK, Houck PM, MacLehose RF, Lawson HW, et al. (2000) Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med 343: 8–15. [DOI] [PubMed] [Google Scholar]
- 18. Mahon NG, McKenna CJ, Codd MB, O’Rorke C, McCann HA, et al. (2000) Gender differences in the management and outcome of acute myocardial infarction in unselected patients in the thrombolytic era. Am J Cardiol 85: 921–926. [DOI] [PubMed] [Google Scholar]
- 19. Sbarouni E, Georgiadou P, Voudris V (2011) Gender-specific differences in biomarkers responses to acute coronary syndromes and revascularization procedures. Biomarkers 16: 457–465. [DOI] [PubMed] [Google Scholar]
- 20. Kaminsky Z, Wang SC, Petronis A (2006) Complex disease, gender and epigenetics. Ann Med 38: 530–544. [DOI] [PubMed] [Google Scholar]
- 21.McDonald KL, Rapkins RW, Olivier J, Zhao L, Nozue K, et al.. (2012) The T genotype of the MGMT C>T (rs16906252) enhancer single-nucleotide polymorphism (SNP) is associated with promoter methylation and longer survival in glioblastoma patients. Eur J Cancer. [DOI] [PubMed]
- 22. Docherty SJ, Davis OS, Haworth CM, Plomin R, D’Souza U, et al. (2012) A genetic association study of DNA methylation levels in the DRD4 gene region finds associations with nearby SNP. Behav Brain Funct 8: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, et al. (2012) FTO genotype is associated with phenotypic variability of body mass index. Nature 490: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sertic J, Skoric B, Lovric J, Bozina T, Reiner Z (2010) [Does Lp-PLA2 determination help predict atherosclerosis and cardiocerebrovascular disease?]. Acta Med Croatica 64: 237–245. [PubMed] [Google Scholar]
- 25. Vittos O, Toana B, Vittos A, Moldoveanu E (2012) Lipoprotein-associated phospholipase A2 (Lp-PLA2): a review of its role and significance as a cardiovascular biomarker. Biomarkers 17: 289–302. [DOI] [PubMed] [Google Scholar]
- 26. Goncalves I, Edsfeldt A, Ko NY, Grufman H, Berg K, et al. (2012) Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler Thromb Vasc Biol 32: 1505–1512. [DOI] [PubMed] [Google Scholar]
- 27. Ferguson JF, Hinkle CC, Mehta NN, Bagheri R, Derohannessian SL, et al. (2012) Translational studies of lipoprotein-associated phospholipase A(2) in inflammation and atherosclerosis. J Am Coll Cardiol 59: 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, et al. (2008) Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 118: 1172–1182. [DOI] [PubMed] [Google Scholar]
- 29. Epps KC, Wilensky RL (2011) Lp-PLA(2)- a novel risk factor for high-risk coronary and carotid artery disease. J Intern Med 269: 94–106. [DOI] [PubMed] [Google Scholar]
- 30. Grallert H, Dupuis J, Bis JC, Dehghan A, Barbalic M, et al. (2012) Eight genetic loci associated with variation in lipoprotein-associated phospholipase A2 mass and activity and coronary heart disease: meta-analysis of genome-wide association studies from five community-based studies. Eur Heart J 33: 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Qi L, Lv N, Gao Q, Cheng Y, et al. (2011) Association between lipoprotein-associated phospholipase A2 gene polymorphism and coronary artery disease in the Chinese Han population. Ann Hum Genet 75: 605–611. [DOI] [PubMed] [Google Scholar]
- 32. Zhou J, Huang Y, Huang RS, Wang F, Xu L, et al. (2012) A case-control study provides evidence of association for a common SNP rs974819 in PDGFD to coronary heart disease and suggests a sex-dependent effect. Thromb Res 130: 602–606. [DOI] [PubMed] [Google Scholar]
- 33. Lopes-Virella MF, Stone P, Ellis S, Colwell JA (1977) Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem 23: 882–884. [PubMed] [Google Scholar]
- 34. Chen C (2001) A survey of the dietary nutritional composition of centenarians. Chin Med J (Engl) 114: 1095–1097. [PubMed] [Google Scholar]
- 35. Schumann G, Bonora R, Ceriotti F, Ferard G, Ferrero CA, et al. (2002) IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin Chem Lab Med 40: 718–724. [DOI] [PubMed] [Google Scholar]
- 36. Schumann G, Canalias F, Joergensen PJ, Kang D, Lessinger JM, et al. (2010) IFCC reference procedures for measurement of the catalytic concentrations of enzymes: corrigendum, notes and useful advice. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)–IFCC Scientific Division. Clin Chem Lab Med 48: 615–621. [DOI] [PubMed] [Google Scholar]
- 37. Schumann G, Bonora R, Ceriotti F, Ferard G, Ferrero CA, et al. (2002) IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 6. Reference procedure for the measurement of catalytic concentration of gamma-glutamyltransferase. Clin Chem Lab Med 40: 734–738. [DOI] [PubMed] [Google Scholar]
- 38. Doumas BT, Peters T Jr (1997) Serum and urine albumin: a progress report on their measurement and clinical significance. Clin Chim Acta 258: 3–20. [DOI] [PubMed] [Google Scholar]
- 39. Sharma P, Kumar J, Garg G, Kumar A, Patowary A, et al. (2008) Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol 27: 357–365. [DOI] [PubMed] [Google Scholar]
- 40. Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, et al. (2008) Intra-individual change over time in DNA methylation with familial clustering. JAMA 299: 2877–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Q, Hao Y, Mo X, Wang L, Lu X, et al. (2010) PLA2G7 gene polymorphisms and coronary heart disease risk: a meta-analysis. Thromb Res 126: 498–503. [DOI] [PubMed] [Google Scholar]
- 42. Casas JP, Ninio E, Panayiotou A, Palmen J, Cooper JA, et al. (2010) PLA2G7 genotype, lipoprotein-associated phospholipase A2 activity, and coronary heart disease risk in 10 494 cases and 15 624 controls of European Ancestry. Circulation 121: 2284–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corti MC, Guralnik JM, Bilato C (1996) Coronary heart disease risk factors in older persons. Aging (Milano) 8: 75–89. [DOI] [PubMed] [Google Scholar]
- 44. Zhao Y, Tian F, Hu SY, Wang J, Zhang T, et al. (2012) [Characteristics of traditional risk factors and coronary lesions on coronary heart disease among different sex populations]. Zhonghua Liu Xing Bing Xue Za Zhi 33: 423–427. [PubMed] [Google Scholar]
- 45. Hyvarinen M, Qiao Q, Tuomilehto J, Soderberg S, Eliasson M, et al. (2010) The difference between acute coronary heart disease and ischaemic stroke risk with regard to gender and age in Finnish and Swedish populations. Int J Stroke 5: 152–156. [DOI] [PubMed] [Google Scholar]
- 46.van der Meer MG, Cramer MJ, van der Graaf Y, Doevendans PA, Nathoe HM (2012) Gender difference in long-term prognosis among patients with cardiovascular disease. Eur J Prev Cardiol. [DOI] [PubMed]
- 47. Yasuda K, Furukawa M, Johnston JM (1996) Effect of estrogens on plasma platelet-activating factor acetylhydrolase and the timing of parturition in the rat. Biol Reprod 54: 224–229. [DOI] [PubMed] [Google Scholar]
- 48. Yoshimura T, Ohshige A, Maeda T, Ito M, Okamura H (1999) Estrogen replacement therapy decreases platelet-activating factor-acetylhydrolase activity in post-menopausal women. Maturitas 31: 249–253. [DOI] [PubMed] [Google Scholar]
- 49. Yasuda K, Johnston JM (1992) The hormonal regulation of platelet-activating factor-acetylhydrolase in the rat. Endocrinology 130: 708–716. [DOI] [PubMed] [Google Scholar]
- 50. Fang C, Chen Y, Nie R, Li G, Xu G, et al. (2009) Retrospective analysis of risk factors in young patients with coronary artery disease in Guangdong and Zhejiang, China. Acta Cardiol 64: 195–199. [DOI] [PubMed] [Google Scholar]
- 51. Ajmal M, Ahmed W, Sadeque A, Ali SH, Bokhari SH, et al. (2010) Identification of a recurrent insertion mutation in the LDLR gene in a Pakistani family with autosomal dominant hypercholesterolemia. Mol Biol Rep 37: 3869–3875. [DOI] [PubMed] [Google Scholar]
- 52. Grabner R, Lotzer K, Dopping S, Hildner M, Radke D, et al. (2009) Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE−/− mice. J Exp Med 206: 233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mosher MJ, Lange LA, Howard BV, Lee ET, Best LG, et al. (2008) Sex-specific interaction between APOE genotype and carbohydrate intake affects plasma HDL-C levels: the Strong Heart Family Study. Genes Nutr 3: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bekris LM, Millard SP, Galloway NM, Vuletic S, Albers JJ, et al. (2008) Multiple SNPs within and surrounding the apolipoprotein E gene influence cerebrospinal fluid apolipoprotein E protein levels. J Alzheimers Dis 13: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nie G-n, Wang X-y, Yang H-y, Ou A-h (2011) The research on the factors affecting the timing of natural menopause in Chinese city women. Maternal and Child Health Care of China 26: 3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of PLA2G7 methylation levels between cases and controls in different age groups in femalesa. a) P values were adjusted for age, the history of smoking, diabetes and hypertension.
(TIF)
Correlation between PLA2G7 methylation and agea.
(TIF)
Primer information of PLA2G7 methylation assay.
(DOC)