Abstract
The 2-micron plasmid of the budding yeast Saccharomyces cerevisiae encodes copy-number amplification and partitioning systems that enable the plasmid to persist despite conferring no advantage to its host. Plasmid partitioning requires interaction of the plasmid Rep1 and Rep2 proteins with each other and with the plasmid-partitioning locus STB. Here we demonstrate that Rep1 stability is reduced in the absence of Rep2, and that both Rep proteins are sumoylated. Lysine-to-arginine substitutions in Rep1 and Rep2 that inhibited their sumoylation perturbed plasmid inheritance without affecting Rep protein stability or two-hybrid interaction between Rep1 and Rep2. One-hybrid and chromatin immunoprecipitation assays revealed that Rep1 was required for efficient retention of Rep2 at STB and that sumoylation-deficient mutants of Rep1 and Rep2 were impaired for association with STB. The normal co-localization of both Rep proteins with the punctate nuclear plasmid foci was also lost when Rep1 was sumoylation-deficient. The correlation of Rep protein sumoylation status with plasmid-partitioning locus association suggests a theme common to eukaryotic chromosome segregation proteins, sumoylated forms of which are found enriched at centromeres, and between the yeast 2-micron plasmid and viral episomes that depend on sumoylation of their maintenance proteins for persistence in their hosts.
Introduction
The 2 µm plasmid is a benign parasitic plasmid harbored in the nucleus of most strains of the budding yeast, Saccharomyces cerevisiae (For review see [1] and [2]). Despite providing no selective advantage to the host, the plasmid is faithfully maintained at ∼60 copies per cell [3]. The 2 µm plasmid achieves this by encoding copy-number amplification and partitioning systems [1], [2], and by borrowing host cell machinery for its replication [4] and segregation [5]–[9]. Retention of the 2 µm plasmid at normal copy number is also dependent on the host cell process of sumoylation [10]–[13], the post-translational modification of target proteins with the small ubiquitin-related modifier protein SUMO [14].
Sumoylation is an essential conserved eukaryotic function known to regulate diverse cellular processes by modulating the interactions, localization, or post-translational stability of substrate proteins (reviewed in [14]–[16]). Like ubiquitin, SUMO must be activated in a series of enzyme-catalyzed steps before being conjugated to target proteins by the E2 conjugating enzyme Ubc9. Some target proteins require an E3 ligase [17], [18] for recruitment to Ubc9. In yeast, two SUMO-specific deconjugating enzymes, Ulp1 and Ulp2, remove SUMO conjugates from distinct subsets of target proteins [19], [20]. Ulp1 also processes the SUMO primary translation product to its mature form. Combined loss of the SUMO E3 ligases Siz1 and Siz2 [11], loss of the SUMO-targeted ubiquitin-ligase Slx5-Slx8 [13], or mutation or mislocalization of Ulp1 [10], [12] has been found to lead to elevated levels of the 2 µm plasmid, indicating that plasmid maintenance is dependent on host cell sumoylation.
Amplification of the plasmid copy number is mediated by the plasmid site-specific recombinase, Flp, and in wild type yeast occurs only when the copy number drops below the normal level [21], [22]. Flp is directly targeted for sumoylation [11]. SUMO conjugates have also been observed for one of the two plasmid-encoded partitioning proteins, Rep2, and suggested for the other, Rep1 [11], [12]. When Flp sumoylation is impaired, an aberrant high-molecular weight form of the plasmid accumulates [23], demonstrating that Flp function is normally regulated by this host cell post-translational modification. We have previously observed mis-segregation of a fluorescently-tagged 2 µm plasmid in yeast defective for the SUMO-specific protease Ulp1 [12], suggesting that plasmid partitioning might be regulated by sumoylation, but the effect of Rep protein sumoylation on plasmid maintenance has not been investigated.
Equal partitioning of the multiple copies of the 2 µm plasmid during mitosis depends on interaction of Rep1 and Rep2 with each other, and with a tandemly-repeated sequence at the plasmid STB locus ([24] and reviewed in [2] and [25]). Mutations in Rep1 that impair these interactions [24], or absence of any one of these three elements results in most newly budded daughter cells failing to receive copies of the plasmid [26]. Association of Rep1 and Rep2 with STB aggregates the multiple copies of the plasmid into a small number of nuclear foci that persist throughout the cell cycle [5], [27], and localize near spindle pole bodies [5], [7]. Equal partitioning of these clustered plasmids between the mother and daughter cell at mitosis is dependent on conversion of the STB chromatin to a form that shares some features with centromeres. The STB-associated Rep proteins recruit the kinesin-related motor protein Kip1 [8] which is required for exchange of the canonical histone H3 in the STB-bound nucleosome for the centromere-specific histone H3 variant Cse4 [9], for association of the RSC2 chromatin remodeling complex [24], [28], [29], and for recruitment of the cohesin complex to STB [6], [24], a process that is also dependent on the integrity of the mitotic spindle [7]. The precise mechanism by which the Rep proteins perform these functions is unknown. Here we investigate the contribution of sumoylation of both Rep1 and Rep2 to plasmid inheritance. We demonstrate that mutations in Rep1 and Rep2 that impair sumoylation also impede their stable association with STB, and plasmid inheritance, suggesting similarity to the sumoylation-dependent localization of eukaryotic host chromosome segregation proteins to centromeres, and to viral episomes that depend on sumoylation of their maintenance proteins for faithful persistence in the host.
Experimental Procedures
Yeast strains and media
Standard methods were used for growth and manipulation of yeast and bacteria [30], [31]. Yeast were cultured in YPAD (1% yeast extract, 2% Bacto Peptone, 0.003% adenine, 2% glucose), synthetic defined (0.67% Difco yeast nitrogen base without amino acids, 2% glucose, 0.003% adenine, 0.002% uracil, and all required amino acids), or synthetic complete medium (0.67% Difco yeast nitrogen base without amino acids, 2% glucose, 1% Difco casamino acids, 0.003% adenine, 0.002% uracil, 0.002% tryptophan) [30]. For induction of GAL promoters, glucose was replaced with galactose (2%). Media were supplemented with 200 µg/mL geneticin (G418) (Sigma) and 100 µg/mL nourseothricin (clonNAT) (Werner BioAgents) for selection of KanMX6- and NatMX6-tagged gene replacements, respectively. Yeast were transformed by the LiAC/SS-DNA/PEG method [32].
Yeast strains used in this study are given in Table 1. Strains lacking the 2 µm circle, designated cir 0, were derived from strains containing the 2 µm circle, cir+, by expression of a defective Flp recombinase from the plasmid pBIS-GALkFLP-(TRP1) [33]. Yeast gene deletion strains were created by targeted replacement of wild-type alleles with KanMX6 gene deletion alleles amplified by PCR from appropriate strains in the EUROSCARF yeast gene deletion strain collection [34] using recommended primers and conditions. Transformed yeast were selected for G418-resistance and gene deletions confirmed by PCR. NatMX6-tagged deletion strains were derived from the corresponding KanMX6-tagged deletion strains by targeted gene replacement.
Table 1. Yeast strains used in this study.
| Name | Relevant genotype | Reference or parental strain |
| W303a/α [cir +] | MAT a /MATα ade2-1/ade2-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 his3-11his3-11,15 trp1-1,trp1-1 [cir+] | [79] |
| W303a/α [cir 0] | MAT a /MATα ade2-1/ade2-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 his3-11/his3-11,15 trp1-1,trp1-1 [cir0] | W303a/α [cir +] |
| W303/1a | MAT a ade2-1 ura3-1 leu2-3,112 his3-11,15 trp1-1 [cir+] | [79] |
| MD83/1b | MAT a ade2-1 ura3-1 leu2-3,112 his3-11,15 trp1-1 [cir0] | [12] |
| MD83/1c | MATα ade2-1 ura3-1 leu2-3,112 his3-11,15 trp1-1 [cir0] | [12] |
| JP91/4 | MATα smtΔ:KanMX6 [pRS426-CUP1p-HA3-SMT3(GG)] [cir0] | this study |
| CTY10/5d | MAT a gal4 gal80 his3-200 trp1-901 ade2 ura3-52 leu2-3,112 met thr URA3:(lexAop)8-lacZ [cir+] | [80] |
| CTMD/3a | MAT a his3 trp1 leu2-3,112 ade2-1 ura3 met URA3:(lexAop)8-lacZ | this study |
| MD83/29 | MAT a GFP-lacI:HIS3 [cir0] | this study |
| EP4 | MAT a gal4 gal80 his3-200 trp1-901 ade2 ura3-52 leu2-3.112 met thr URA3:STB-p-HIS3 [cir+] | CTY10/5d |
| EP4MD [cir +] | MAT a/ MATα gal4/GAL4 gal80/GAL80 his3-11,-15/his3-200 trp1-1/trp1-901 ade2-1/ade2 ura3-1/ura3-52 leu2-3,-112/leu2-3,-112 MET/met THR/thr URA3: STB-p-HIS3 [cir+] | this study |
| EP4MD [cir 0] | MAT a/ MATα gal4/GAL4 gal80/GAL80 his3-11,-15/his3-200 trp1-1/trp1-901 ade2-1/ade2 ura3-1/ura3-52 leu2-3,-112/leu2-3,-112 MET/met THR/thr URA3: STB-p-HIS3 [cir0] | EP4MD [cir +] |
| EGY48 [cir +] | MATα ura3 his3 trp1 (lexAop)6:LEU2 [cir+] | [81] |
| EGY48 [cir 0] | MATα ura3 his3 trp1 (lexAop)6:LEU2 [cir0] | EGY48 [cir +] |
| JP98/2 | MAT a ade2-1 ura3-1 leu2-3,112 his3-11,15 trp1-1 rsc2Δ:NatMX6 [cir0] | MD83/1b |
| IRS10 | MAT a pAS10ΔORI:ADE2 [cir0] | MD83/1b |
| IRS133 | MAT a pAS-rep13RΔORI:ADE2 [cir0] | MD83/1b |
| IRS135 | MAT a pAS-rep213RΔORI:ADE2 [cir0] | MD83/1b |
One-hybrid and two-hybrid assay expression plasmids
pGAD424- and pSH2-1-derived plasmids expressing Rep1, Rep2, or mature yeast SUMO fused to the Gal4 transcriptional activation domain (Gal4AD) or to the bacterial LexA repressor protein, respectively, have been previously described [12]. The genomic DNA encoding the Nis1 SUMO interaction-motif-containing domain [35] inserted in plasmid pSH-NIS1357–408 was isolated in a library screen (unpublished data). A mutant version of REP1 encoding an I202T substitution (REP1I202T) was isolated in screen for mutagenized REP genes unable to maintain a 2 µm-based plasmid (unpublished data). The REP1 I202T open reading frame (ORF) was amplified by PCR and inserted in pGAD424 and pSH2-1 to create pGAD-REP1I202T and pSH-REP1I202T, respectively, as previously described for the wild type REP1 ORF [36]. TRP1 gene-based plasmids to direct galactose-inducible expression of the Rep proteins as HA-epitope-tagged B42 transcriptional activation domain (B42AD-HA)-fusions in yeast and that were either 2 µm-based (pMM2) or single-copy CEN/ARS (pMM3) were created by digestion of pJG4-5 [37] with BamHI and NotI. The overhangs were made flush and the plasmid self-ligated to create pMM1. A 6.1-kb EcoRI/SphI fragment from pMM1 was ligated with EcoRI/SphI fragments from pGAD424, pGAD-REP1, pGAD-REP2 [36], and pGAD- REP1I202T, to yield pMM2, pMM2-REP1, pMM2-REP2, and pMM2- REP1I202T, respectively. The 2 µm backbone in the pMM2-based plasmids was removed by digestion with KpnI and EagI and then replaced with KpnI/EagI-digested pRS314 [38] to generate the single copy CEN/ARS plasmids pMM3-based versions of these plasmids. All PCR-amplified genes were checked after cloning by sequencing. Plasmid pGAD-SUMOΔGG was created by subcloning a SmaI/PstI fragment from pGBD-smt3ΔGG [35] (a generous gift from M. Hochstrasser) encoding conjugation-defective SUMO, into pGAD424.
2 µm-based plasmids
Plasmid pAS4, a flp- ADE2-tagged version of the 2 µm circle that can be propagated in yeast and E. coli, has been previously described [36]. The ADE2 gene insertion disrupts the FLP gene, and the Flp target site between the REP1 and REP2 genes has been deleted and replaced with the E.coli vector pTZ18R (Pharmacia). pAS10 is identical to pAS4 but with pTZ18R inserted in the opposite orientation. Site-directed mutagenesis of REP1 and REP2 was carried out by gap repair of plasmids pAS10 and pAS4, respectively (primer sequences are available upon request). PCR amplicons containing either the REP1 ORF flanked by ∼650 bp upstream and ∼450 bp downstream or the REP2 ORF flanked by ∼900 bp upstream and ∼600 bp downstream and containing the designated point mutation(s) were created by assembly PCR, and co-transformed into yeast with NruI/SalI-digested pAS10 and SphI-digested pAS4, respectively. Plasmids were isolated in E. coli, mutations confirmed by sequencing, and plasmids re-transformed into yeast for subsequent experiments. In order to combine various REP1 and REP2 alleles in a single ADE2 flp- 2 µm plasmid, a PCR product encoding from ∼900 bp upstream of the REP2 ORF to ∼980 bp into the REP1 ORF in a pAS4-based plasmid was used for gap repair of BamHI/SphI-digested pAS10-based plasmids. ADE2-tagged 2 µm plasmids directing expression of Ubc9- or His6-N-terminally-tagged Rep1 and Rep2 proteins expressed from their own promoters were created by assembly PCR and gap repair in a similar fashion to plasmids encoding point-mutant REP alleles. Ubc9 fusion proteins contained five glycine residues between the carboxy-terminal lysine residue in Ubc9 and the first residue of Rep1 or Rep2. To create ADE2-tagged 2 µm-based plasmids lacking the REP genes, pAS4 was digested with SphI and the plasmid self-ligated to make pAS4ΔREP2, and an XhoI site was introduced upstream of the REP1 coding region in pAS10 by PCR, and the resulting plasmid digested with XhoI and SalI and self-ligated to create pAS10ΔREP1. To create pKan4 in which the ADE2 gene in pAS4 is replaced with KanMX6, yeast harboring pAS4 were transformed to G418-resistance with a PCR product encoding the ade2Δ:KanMX6 allele. pKan4 was isolated in E. coli and re-transformed into yeast for all subsequent experiments. An identical strategy was used to derive all other KanMX6-tagged 2 µm plasmids from pAS4/pAS10-based plasmids. To create yeast strains containing the REP1 and REP2 genes with their flanking 2 µm sequences chromosomally-integrated at the ADE2 locus, pAS4 was digested with BclI and SnaBI to remove the origin of replication, the ends were made flush and the plasmids self-ligated to create pAS10ΔORI. pAS10ΔORI was linearized with NdeI and used to transform cir 0 strains to adenine prototrophy.
Other plasmids
To create TRP1- or LEU2-based CEN/ARS plasmids for expression of untagged Rep proteins from the GAL1 promoter, the REP1 and REP2 ORFs, encoding wild-type or mutant Rep1 and Rep2 alleles, were amplified by PCR and cloned into plasmids pRSGAL-TRP and pRSGAL-LEU, which were created by replacement of the PvuII fragment encoding the multiple cloning site of pRS314 and pRS315 [38], respectively, with the 1.4-kb PvuII fragment from pESC-URA (Stratagene) containing the GAL1 promoter.
Plasmids for galactose-inducible expression of untagged (pJP2-SMT3) and HA-tagged (pJP2-HA-SMT3) SUMO were constructed by using assembly PCR to synthesize products encoding untagged or HA-tagged mature SUMO that were used to gap repair pMM2, with deletion of the B42AD-HA coding region.
The URA3-based integrative one-hybrid vector pJL638 was a kind gift from J. Li [39]. The integrative one-hybrid HIS3 reporter gene plasmid pEP1 was created from pJL638 by replacing the MscI/SstI fragment encoding the lacZ ORF, which lies downstream of a basal yeast promoter, with an MscI/SstI fragment encoding the HIS3 gene ORF with its transcription termination sequences. The repeated sequence from the 2 µm STB locus (a 295-bp AvaI/HpaI fragment) was converted to an XhoI fragment by addition of linkers and inserted at the XhoI site upstream of the HIS3 reporter gene in pEP1 to create pEP1-STB. STB one-hybrid HIS3 reporter strains were created by using StuI-linearized pEP1-STB to transform yeast to uracil prototophy.
One-hybrid and two-hybrid assays
To test for interaction of the Rep proteins with STB, the one-hybrid reporter strain EP4MD was transformed to tryptophan prototrophy with pMM2- or pMM3-based plasmids encoding various Rep1 and Rep2 alleles. Transformants were grown overnight in selective liquid medium containing glucose, and were serially diluted, spotted on solid medium containing galactose, and imaged after seven days incubation at 28°C. For two-hybrid assays testing for interaction of the Rep proteins with each other and with SUMO, co-transformants in the cir 0 two-hybrid reporter strain CTMD/3a were assayed for β-galactosidase expression by a filter assay. Specificity of interactions was confirmed by co-expressing LexA-fusion proteins with the Gal4AD or Gal4AD-Snf4, and Gal4AD-fusion proteins with LexA [40]. Two-hybrid interactions were also assayed in the cir 0 reporter yeast strain EGY48 by spotting serial dilutions of co-transformants onto solid medium lacking leucine to assess expression of the LEU2 reporter gene.
Plasmid loss assays
To determine the rate of loss of KanMX6-tagged 2 µm plasmids, yeast transformants were initially grown in YPAD medium containing the antibiotic G418 to select for retention of the plasmid. The proportion of plasmid-containing cells was then determined both before and after ∼15 generations of growth in medium that did not select for the retention of the plasmid (YPAD) by comparing plating efficiency on solid YPAD medium containing or lacking G418. Statistical significance was determined using an unpaired t test.
Protein analysis
For all applications protein was extracted from yeast by alkaline lysis as previously described [11], [41]. Briefly, ∼108 cells were pelleted, resuspended in 200 µL of lysis solution (1.85 M NaOH, 7.4% β-mercaptoethanol) and incubated for 10 min on ice. Protein was precipitated for 10 min by addition of 200 µL cold 50% trichloroacetic acid, pelleted by centrifugation, washed twice with 1 mL cold acetone and thoroughly dried.
For western blot analysis, protein was resuspended in equal volumes of urea extraction buffer (8 M urea, 100 mM NaH2PO4/Na2HPO4, 50 mM Tris) and 2× protein gel loading buffer (125 mM Tris pH 6.8, 4.0% SDS, 20% glycerol, 4.0% β-mercaptoethanol, 1 M urea, 0.05% bromophenol blue, 0.05% xylene cyanol). Protein suspensions were briefly vortexed, centrifuged at 16 000× g for 1 min and supernatants analyzed as previously described [36]. Primary antibodies were rabbit-derived polyclonal anti-Rep1 or anti-Rep2 [36], and mouse anti-Pgk1 (Molecular Probes), or mouse anti-HA (Sigma). Secondary antibodies for chemiluminescent detection were horseradish peroxidase (HRP)-conjugated goat anti-rabbit and anti-mouse IgG (KPL), and for fluorescent detection were anti-mouse Dylight 488, anti-mouse Dylight 549, and anti-rabbit Dylight 649 (Rockland). Chemiluminescence was generated using an ImmunStar Western C kit (BioRad) and captured either by X-ray film or digitally using a charge-coupled device (CCD) camera in a VersaDoc 4000 MP imaging system (BioRad). Fluorescence was digitally captured using the VersaDoc 4000 MP system equipped with the appropriate LED/filter combination.
For metal ion affinity chromatography, dried protein pellets were resuspended in 1 mL of binding buffer (6 M guanidine HCl, 300 mM NaCl, 50 mM sodium phosphate, pH 8.0) supplemented with 10 mM N-ethylmaleimide, vortexed briefly, and clarified by centrifugation at 16 000× g for 15 min. Supernatants were transferred to a microfuge tube containing ∼20 µL TALON resin (Clontech) and rocked at room temperature for 2 h. Resin was briefly washed three times with wash buffer (8 M urea, 300 mM NaCl, 50 mM sodium phosphate, 5 mM imidazole, pH 7.5), and then 4 µL of 1.5 M imidazole was added, followed by 25 µL of 2× protein gel loading buffer. Resin suspensions were boiled for 5 min, clarified by centrifugation and ∼5 µL analyzed by western blotting.
Chromatin immunoprecipitation (ChIP)
For ChIP analysis of Rep protein association with the plasmid STB locus, 2 µm-based plasmids tagged with an ADE2 adenine biosynthetic gene were used. The ADE2-tagged plasmids are maintained at higher copy number than the equivalent KanMX6-tagged plasmids when yeast are grown under conditions selecting for the presence of the plasmids, with a copy number closer to that of the native 2 µm plasmid. ChIP assays were performed essentially as described [9], [42] with the following modifications. Yeast cultures (50 mL) were grown to OD600 ∼1–2 and fixed with 1% formaldehyde for 15 min at room temperature. Crosslinking was quenched by addition of 125 mM glycine and after 5 min cells were harvested, washed with water three times and resuspended in 400 µL cold lysis buffer D (50 mM HEPES-KOH pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1× complete protease inhibitor cocktail (Roche)). Yeast were kept chilled for the following steps. Cells were lysed using glass beads, and chromatin was sheared to 0.1–1 kbp fragments by 8 rounds of sonication for 12 s each using a Branson 250 sonifier set to an output level of 3 and 50% duty cycle. Sonicated lysates were clarified by centrifugation at 16 000× g for 15 min, the supernatant transferred to a new tube, centrifuged again at 16 000× g for 10 min, and 20–100 µL of supernatant (whole cell extract) brought up to 400 µL with lysis buffer D, and incubated for 4–16 h with polyclonal anti-Rep1, anti-Rep2, or monoclonal anti-FLAG (Sigma) antibodies at 4 °C. Protein A Sepharose CL-4B (GE Healthcare) was blocked with 20 µg of sonicated salmon sperm DNA and 100 µg bovine serum albumin for ≥ 1 h, and 20 µL of beads added to the immunoprecipitation mixtures and incubated for 1 h. Beads were washed six times at room temperature as follows: twice with 1 mL ChIP wash buffer I (50 mM Hepes-KOH, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% sodium deoxycholate, and 1% Triton X-100) for 5 min each, twice with 1 ml of ChIP wash buffer II (50 mM Hepes-KOH, pH 7.5, 500 mM NaCl, 1 mM EDTA, 0.1% sodium deoxycholate, and 1% Triton X-100) for 5 min each, once with 1 ml of ChIP wash buffer III (10 mM Tris-HCl, pH 8.0, 250 mM LiCl, 1 mM EDTA, 0.5% sodium deoxycholate, and 0.5% NP-40) for 5 min, and once with 1 mL of TE for 5 min. Chromatin was eluted in SDS, decrosslinked, digested with proteinase K, and DNA extracted with phenol and chloroform as described [30]. DNA was resuspended in 100 µL of TE, and serial dilutions of template DNA were amplified by 30 cycles of PCR with primers flanking STB, which were 5′- ATTATAGAGCGCACAAAGGAGA-3′ and 5′- TGCACTTCAATAGCATATCTTTG -3′. PCR products were resolved by agarose gel electrophoresis, stained with ethidium bromide, imaged using a VersaDoc MP 4000 imaging system (BioRad) and quantified by densitometry using QuantityOne software (BioRad). Specificity of co-immunoprecipitation was assessed by comparing the amount of STB DNA immunoprecipitated by anti-Rep1 and anti-Rep2 antibodies to that pulled down by the anti-FLAG antibody, and by assessing the degree of non-specific immunoprecipitation of a chromosomal target (CEN3) (not shown).
Fluorescence microscopy
All images were digitally captured using a Nikon80i fluorescent microscope with a Nikon DS-Qi1Mc digital camera and processed using NIS-Elements Basic Research software. Immunofluorescence analysis of yeast was carried out essentially as described [30]. Logarithmically-growing yeast were fixed in 3.7% formaldehyde for 1 h, digested with zymolyase 20T (ICN), and spotted to Superfrost Plus slides (Fisher). After 30 min the fixed spheroplasts were dried in cold methanol for 6 min and cold acetone for 30 s, and rehydrated in blocking buffer (PBS with 2% BSA). Slides were incubated at 4°C overnight with affinity-purified anti-Rep1 or anti-Rep2 antibodies diluted in blocking buffer. Slides were washed twice in PBS, incubated at room temperature for 1 h with 1∶500 AlexaFluor594-conjugated goat anti-rabbit secondary antibody (Invitrogen) in blocking buffer, and washed twice in PBS. Mounting media containing 100 ng/mL 4′,6-diamidino-2-phenylindole (DAPI) was added prior to imaging. To visualize the localization of the TRP1-marked 2 µm reporter plasmid pSV5 containing the 2 µm origin of replication, STB, and 256 lac operator sequences [6], a GFP-LacI repressor fusion protein was expressed from a chromosomally-integrated gene as previously described [5]. All images are representative of at least two independent experiments in which >200 cells were scored.
Results
Multiple lysine substitutions in Rep1 and Rep2 are required to abolish their two-hybrid interaction with SUMO
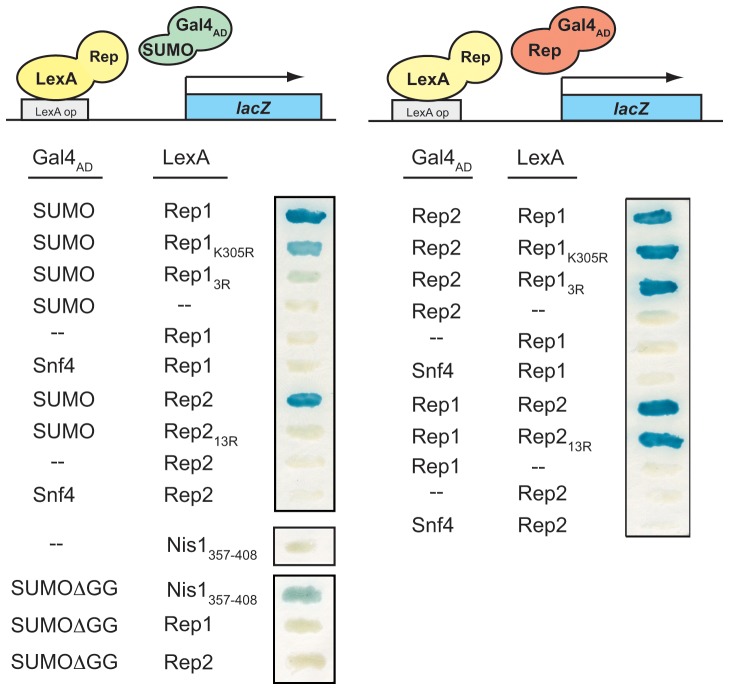
We have previously observed that both 2 µm plasmid partitioning proteins, Rep1 and Rep2, interact with SUMO in vivo in a two-hybrid assay, suggesting they might be direct targets of sumoylation [12]. SUMO-conjugated forms of Rep2 have been observed [11], while sumoylated forms of Rep1 have not been definitively identified to date. Although both Rep proteins might be directly targeted for sumoylation, a two-hybrid interaction would also be observed if either Rep protein could interact with SUMO indirectly. To determine whether the two-hybrid association with SUMO reflected non-covalent recognition of SUMO through a SUMO-interaction motif (SIM), both proteins were tested for their ability to interact with a truncated version of SUMO lacking the two C-terminal glycine residues required for covalent attachment (SUMOΔGG) [35]. Neither Rep1 nor Rep2 interacted with SUMOΔGG in a two-hybrid assay, while a fusion protein containing the previously characterized SIM domain from the yeast Nis1 protein [35] did display interaction with this truncated form of SUMO (Fig. 1, bottom left). The lack of interaction of the Rep proteins with SUMOΔGG indicates that neither Rep protein contains a SIM, consistent with the results of in vitro protein interaction assays in which SUMO did not bind to E. coli-expressed Rep1 or Rep2 (unpublished data).
Figure 1. Lysine mutations in Rep1 and Rep2 impair two-hybrid interaction with SUMO but do not affect Rep1-Rep2 interaction.
A cir0 two-hybrid reporter yeast strain was co-transformed with plasmids expressing the indicated Gal4AD and LexA fusion proteins. Co-transformants were grown for 24 h on a nitrocellulose membrane and interaction of the fusion proteins was assessed by monitoring expression of the lacZ reporter gene using a β-galactosidase filter assay with the substrate X-gal, which produces a blue precipitate upon cleavage. Vector with no insert is indicated (--). Assays for interaction with conjugation-competent SUMO and conjugation-defective SUMOΔGG are shown on left and those for interaction between Rep1 and Rep2 on right.
Although the two-hybrid association of the Rep proteins with SUMO could be due to interaction of Rep1 and Rep2 with a host protein that is conjugated to SUMO, previously reported observations of SUMO-conjugated forms of Rep2 [11] suggested that at least for Rep2, the two-hybrid interaction with SUMO might result from covalent modification. A mutational approach was undertaken based on the premise that mutation of lysine residues in Rep1 and Rep2 normally targeted for sumoylation should lead to loss of the two-hybrid interaction with SUMO if the interaction solely reflected covalent SUMO modification. We first identified potential sumoylation sites in Rep1 and Rep2. Rep2 lacks a canonical sumoylation motif, (I/V/L)-K-x-(E/D) [43], while Rep1 contains one consensus site at K348. Rep1 and Rep2 mutants containing one or more lysine-to-arginine substitutions were created by site-directed mutagenesis and tested for two-hybrid interaction with SUMO in a yeast reporter strain that lacked the native 2 µm plasmid (cir 0) to exclude endogenous Rep proteins from participating in the interactions. No single mutation, including Rep1-K348R, completely abolished two-hybrid association with SUMO for either protein, indicating that target lysines were in non-canonical sites and suggesting that more than one lysine residue could be targeted for sumoylation in both Rep1 and Rep2 (Tables 2 and 3, and Fig. 1, top left). The two-hybrid interaction of Rep1 with SUMO was partially reduced for the Rep1-K305R mutant, suggesting this might be a major SUMO acceptor site, and was almost completely abolished in the triple lysine mutant Rep1-K305,315,328R (Rep13R), consistent with these three sites being direct targets for covalent addition of SUMO. Similarly, although different combinations of multiple lysine-to-arginine substitutions in Rep2 slightly reduced two-hybrid interaction with SUMO, simultaneous mutation of thirteen lysine residues in Rep2-K42,44,92,124,130,134,146,148,149,177,208,226,227R (Rep213R) was required to virtually eliminate the two-hybrid interaction with SUMO (unpublished data and Fig. 1). Western blot analysis established that the loss of interaction in our assay was not due to a reduction in steady-state levels of the respective mutant Rep fusion proteins (unpublished data). The mutant Rep proteins also retained their normal two-hybrid interaction with each other (Fig. 1, right) suggesting that the proteins were not grossly misfolded. Taken together, the data suggest that the loss of two-hybrid interaction between the Rep13R and Rep213R mutant proteins and SUMO might be due to loss of the lysine residues normally targeted for sumoylation.
Table 2. Two-hybrid interactions of Rep1 alleles with SUMO.
| Rep1 allele | SUMO interaction |
| WT | +++ |
| K11, 44, 45, 47, 68, 105, 117, 125, 131, 146, 159, 169, 190, 204, 212, 261, 290, 295, 297, 305, 315, 328, 348R | − |
| K11, 44, 45, 47, 68, 105, 117, 125R | +++ |
| K146, 159, 169, 190, 204, 212, 261, 290, 295, 297, 305, 315, 328, 348R | − |
| K146, 159, 169, 190, 204, 212R | +++ |
| K290, 295, 297, 305, 315, 328, 348R | − |
| K290, 295, 297, 305, 315R | + |
| K305, 315, 328, 348R | − |
| K305, 315, 328R ( = 3R) | − |
| K305, 315, 348R | + |
| K305, 315R | + |
| K305R | ++ |
| K315R | +++ |
| K328R | +++ |
Table 3. Two-hybrid interactions of Rep2 alleles with SUMO.
| Rep2 allele | SUMO interaction |
| WT | +++ |
| K8, 13R | +++ |
| K42, 44R | +++ |
| K92R | +++ |
| K95R | +++ |
| K124R | +++ |
| K130R | +++ |
| K134R | +++ |
| K146, 148, 149R | ++ |
| K158R | +++ |
| K177R | +++ |
| K208R | +++ |
| K226, 227R | +++ |
| K42, 44, 92, 124, 130, 134, 146, 148, 149, 177, 208, 226, 227R ( = 13R) | − |
Lysine substitutions in the Rep13R and Rep213R mutants impair conjugation to SUMO
If the lysine-to-arginine mutations in Rep13R and Rep213R that led to loss of interaction with SUMO in the two-hybrid assay impaired sumoylation, we would expect a reduction in their respective sumoylated forms. While we did observe slower-migrating species of wild-type Rep1 or Rep2 by western blot analysis of protein extracted from yeast containing the native 2 µm plasmid, these species were often barely detectable above background, probably due to the relatively low steady-state levels of the native Rep proteins (unpublished data) and consistent with previously reported observations that Rep1 SUMO conjugates could not be definitively detected, even after Rep1 was affinity-purified [11].
To artificially enhance the stoichiometry of Rep protein sumoylation, Rep1 and Rep2 were expressed as fusions with the SUMO-conjugating enzyme Ubc9. Ubc9 fusion-directed sumoylation (UFDS) is typically used to enhance auto-sumoylation of the Ubc9-chimera [44]–[46]. However, because Ubc9 itself is sumoylated [35], it would be difficult to discern whether a SUMO conjugate of the Ubc9-Rep fusion protein represented SUMO-modification of the Ubc9 moiety rather than of Rep1 or Rep2. Because Rep1 and Rep2 directly interact in vivo, we tested whether fusion of Ubc9 to Rep1 could enhance sumoylation of Rep2 and vice-versa. Yeast were transformed with marker-tagged 2 µm plasmids encoding one Rep protein fused with Ubc9, and the other fused to a hexahistidine tag. Total protein was extracted, and His6-tagged Rep proteins were affinity-purified using Co2+ resin and analyzed by western blotting. Slower-migrating species of both Rep1 (Fig. 2A, bottom panel, lane 1) and Rep2 (Fig. 2B, bottom panel, lane 1) were detected that had mobilities consistent with these being sumoylated forms. To determine whether these species were SUMO-conjugates of Rep1 and Rep2, the proteins were expressed in yeast over-expressing either untagged or HA epitope-tagged SUMO (HA-SUMO). As expected, the major slower-migrating forms of Rep1 and Rep2 displayed lower mobility when SUMO was HA-tagged (bottom panels of Fig. 2A and 2B, lane 5), a shift consistent with the additional molecular weight of the three added HA epitopes. These species were verified to be HA-SUMO conjugates by their reactivity with an anti-HA antibody (top panels in Fig. 2A and 2B, lane 5). As expected for HA-SUMO conjugates of Rep1 and Rep2, respectively, these species were not detected by the anti-HA antibody in metal-ion affinity-purified protein extracted from yeast that did not express Rep1 or Rep2 (Fig. 2A and 2B, lane 7). Notably, the lysine-to-arginine mutations in Rep13R and Rep213R that impaired two-hybrid interaction with SUMO also reduced the levels of the HA-tagged SUMO-conjugates of Rep1 and Rep2 (top panels in Fig. 2A and 2B, lane 6), suggesting that Rep13R and Rep213R are sumoylation-deficient mutants.
Figure 2. Lysine-to-arginine mutations significantly reduce levels of SUMO-conjugated Rep1 and Rep2.
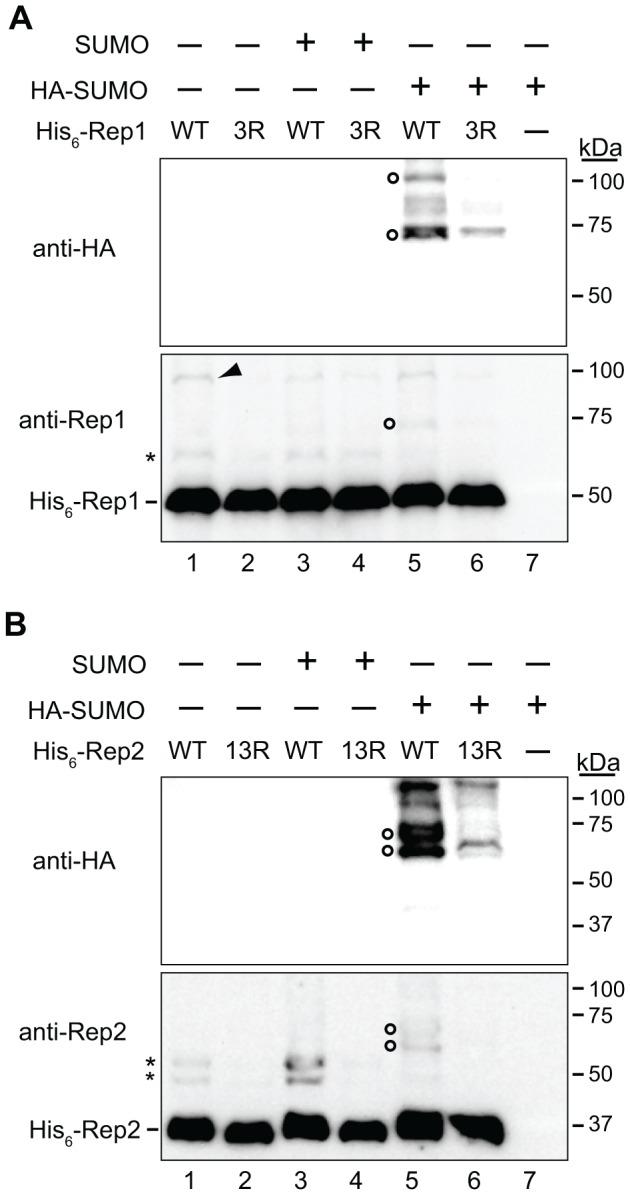
Yeast lacking the native 2 µm plasmid were co-transformed with a plasmid expressing untagged SUMO, HA-epitope-tagged SUMO (HA-SUMO), or no protein (-) from a galactose-inducible promoter, and an ADE2-tagged 2 µm plasmid encoding (A) Ubc9-tagged Rep2 and His6-tagged Rep1 (wild-type (WT) (lanes 1, 3 and 5), or Rep13R (lanes 2, 4 and 6)) or (B) Ubc9-tagged Rep1 and His6-tagged Rep2 (wild type (WT) (lanes 1, 3, and 5) or Rep213R (lanes 2, 4, and 6)). Yeast were also transformed solely with the plasmid expressing HA-epitope-tagged SUMO (A and B, lane 7). Yeast were cultured in medium containing galactose for 20 h to induce expression of SUMO proteins. Protein was extracted and His6-tagged proteins were affinity purified with Co2+ resin and analyzed by western blotting with anti-Rep1, anti-Rep2, or anti-HA antibodies. Species consistent with SUMO-conjugated (asterisks) and HA-SUMO-conjugated (open circles) forms of Rep1 and Rep2, and an unknown Rep1 species (arrowhead) are indicated. Sizes of molecular weight standards resolved in the same gels are indicated.
In addition to the slower-migrating forms of Rep1 and Rep2 that were reduced in abundance for the Rep13R and Rep213R mutants, a species of Rep1 migrating at ∼100 kDa was also observed (Fig. 2A, bottom panel, lane 1). We do not know the nature of this species. The species co-migrates with one of the high-molecular weight Rep1-HA-SUMO-conjugates detected by the anti-HA antibody (Fig. 2A, top panel, lane 5) but was observed even when all Rep1 lysine residues with the exception of the three most carboxy-terminal were mutated to arginine and was not consistently reduced in abundance by the mutations in Rep13R (data not shown). We speculate that this species might represent a post-translational modification to Rep1 other than sumoylation.
Lysine-to-arginine substitutions in Rep13R and Rep213R that impair SUMO conjugation also perturb 2 µm plasmid inheritance
To investigate whether the mutations in Rep13R and Rep213R affect the function of the Rep proteins in partitioning of the 2 µm plasmid, we compared the inheritance of 2 µm-based plasmids encoding either wild-type Rep proteins or the Rep13R and Rep213R mutants. Since the native 2 µm plasmid confers no phenotype, we introduced the KanMX6 gene that confers resistance to the aminoglycoside antibiotic G418 [50] into the plasmids. The FLP gene was inactivated in these plasmids so that defects in plasmid partitioning would not be obscured by the copy-number amplification activity of Flp. To examine inheritance of the KanMX6-tagged 2 µm plasmids encoding wild-type or mutant Rep1 and Rep2, the plasmids were introduced into cir 0 yeast. The fraction of cells in the population containing plasmid was measured after ∼15 generations of growth in the presence of G418, a condition under which only cells that have inherited plasmid can continue to proliferate. Under this selective pressure, the fraction of plasmid-free cells in the population should remain consistently low as plasmid-containing cells out-compete plasmid-free cells. However, if plasmid partitioning is sufficiently impaired, daughter cells will more frequently receive no copies of the high copy number plasmid, and the proportion of G418-sensitive cells in the population will be increased. As a control for the assay, inheritance of the KanMX6-tagged 2 µm plasmid encoding wild-type Rep proteins was monitored in cir0 yeast lacking the RSC2 gene (rsc2Δ). RSC2 encodes a regulatory subunit of one form of the RSC chromatin remodeling complex [51], and has previously been shown to be required for maintenance of the native 2 µm plasmid [28]. In the assay used here, the fraction of G418-resistant cells in the rsc2Δ cell population (0.49 ±.02) was reduced compared to that of RSC2 yeast (0.56 ±.03) (Fig. 3). This is consistent with a measured loss rate of 8.0% (± 1.2) of plasmid-containing cells per generation for the KanMX6-tagged 2 µm plasmid in rsc2Δ cells when cultured in the absence of G418 (data not shown). When the inheritance of the KanMX6-tagged 2 µm plasmid encoding lysine-to-arginine mutant versions of Rep1 and Rep2 was assayed in wild-type ciro yeast, the fraction of plasmid-containing cells was significantly lower than when Rep1 and Rep2 were wild type, with the amino acid substitutions in Rep1 having a more significant impact on plasmid inheritance than those in Rep2 (Fig. 3). The reduction in the proportion of cells containing the plasmid when only Rep2 was mutant was similar to that observed for rsc2Δ yeast containing the KanMX6-tagged 2 µm plasmid encoding wild type Rep1 and Rep2. When both Rep1 and Rep2 were mutant, the fraction of plasmid-containing cells was reduced further still, indicating that an even higher proportion of daughter cells was consistently receiving no copies of the plasmid during cell division. The more severe inheritance defect caused by the lysine-to-arginine mutations in Rep13R and Rep213R relative to that caused by the chromosomal rsc2Δ mutation known to cause spontaneous loss of the endogenous 2 µm plasmid suggests that a native 2 µm plasmid with these mutations would also be lost at high frequency.
Figure 3. Mutations that inhibit sumoylation of Rep1 and Rep2 also impair plasmid maintenance.
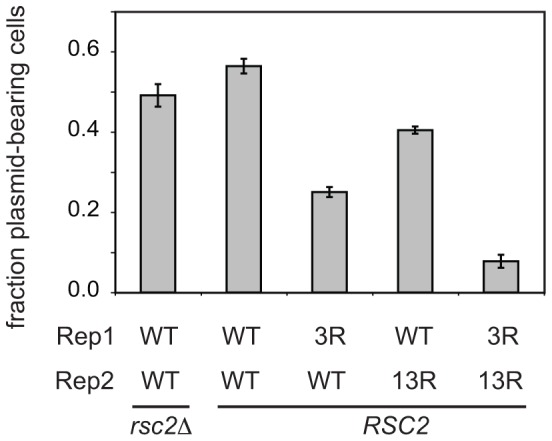
Yeast lacking the native 2 µm plasmid were transformed with KanMX6-tagged 2 µm plasmids encoding the indicated Rep1 and Rep2 alleles, grown in medium containing G418 and the proportion of plasmid-containing (G418-resistant) cells (mean ± SEM from six independent yeast transformants) was determined.
Mutations in Rep13R and Rep213R do not alter their post-translational stability, or the ability of Rep2 to chaperone Rep1
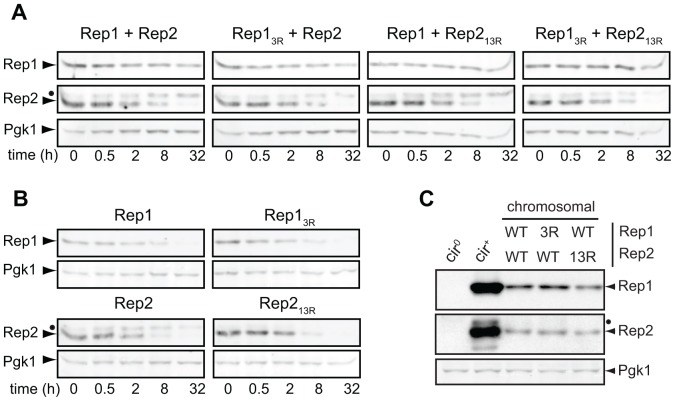
The defect in 2 µm plasmid maintenance associated with the Rep13R and Rep213R mutants could be due to altered steady-state levels of the mutant Rep proteins. Mutation of lysine residues in the Rep proteins could alter their half-life if sumoylation of these sites contributes to Rep1 and Rep2 post-translational stability. Alternatively, the lysine residues might normally be directly targeted for ubiquitin-mediated proteolysis, or mutation of these residues could significantly perturb protein folding or other post-translational modifications of the Rep proteins. To examine Rep1 and Rep2 post-translational stability, cir 0 yeast were transformed with single-copy (CEN/ARS) plasmids encoding REP1 or REP2 under control of a galactose-inducible promoter. Expression from non-2 µm-based plasmids using a heterologous promoter ensured that Rep1 and Rep2 protein levels would not be affected by differences in plasmid copy number caused by mis-segregation, or altered Rep protein-dependent regulation of REP gene expression [52], [53]. The transformed yeast were transferred to medium containing galactose to activate GAL1 promoter-driven expression of the REP genes. After a brief period sufficient for Rep protein expression, cycloheximide was added to inhibit translation, and Rep1 and Rep2 protein levels were monitored by western blotting. The levels of a highly stable glycolytic enzyme, 3-phosphoglycerate kinase (Pgk1) [54], were simultaneously examined (Fig. 4A). We did not observe any significant difference in the stability of wild-type Rep1 and Rep2 compared to their respective sumoylation-deficient mutants. However, we did notice that Rep1 had a much shorter half-life when expressed in the absence of Rep2 (Fig. 4B), suggesting that interaction of Rep2 with Rep1 contributes to Rep1 post-translational stability. We have previously observed that point mutations in Rep1 that abolish association with Rep2 produce a similar reduction in Rep1 steady-state levels (unpublished data), consistent with Rep2 association protecting Rep1 from degradation.
Figure 4. Effects of lysine-to-arginine mutations in Rep1 and Rep2 on post-translational stability.
Rep1 and Rep2 were (A) co-expressed or (B) expressed in the absence of one another and protein was extracted from yeast at the indicated time points following addition of cycloheximide. A closed circle indicates a phosphorylated species of Rep2 (unpublished data). (C) Yeast with the indicated alleles of REP1 and REP2 integrated in the genome were cultured and protein extracted. Rep protein levels were examined by western blot analysis.
Although mutation of the sites required for sumoylation did not reduce the half-life of the Rep proteins expressed from the GAL1 promoter, the high level of expression might have masked proteolytic turnover that would alter steady-state levels if expressed from their native promoters. Comparison of the levels of wild-type versus sumoylation-deficient Rep proteins expressed from 2 µm-based plasmids was precluded by differences in plasmid copy number resulting from defective partitioning of plasmids encoding Rep13R and Rep213R (data not shown). To circumvent the plasmid copy number differences, the REP1 and REP2 genes or their lysine-to-arginine mutant derivatives were integrated into the chromosome in a cir 0 yeast strain, and steady-state Rep protein levels were examined by western blotting (Fig. 4C). The level of Rep13R did not significantly differ from wild-type Rep1, indicating that the lysine substitutions did not affect Rep1 protein stability, and suggesting that no critical ubiquitination sites were destroyed in the process of eliminating Rep1 SUMO attachment sites. A slight reduction in the level of both Rep1 and Rep2 was observed when Rep2 was mutant. Since the REP genes were expressed from their own promoters, and both REP genes are regulated by Rep1 and Rep2 [52], [53], reduced Rep protein levels could indicate a slight reduction in transcription of both REP genes in the presence of Rep213R. Alternatively, the slightly lower level of both proteins could indicate increased turnover of the mutant Rep213R protein, which would result in less Rep2 being available to stabilize Rep1.
The Rep13R and Rep213R mutants are defective for association with the plasmid-partitioning locus
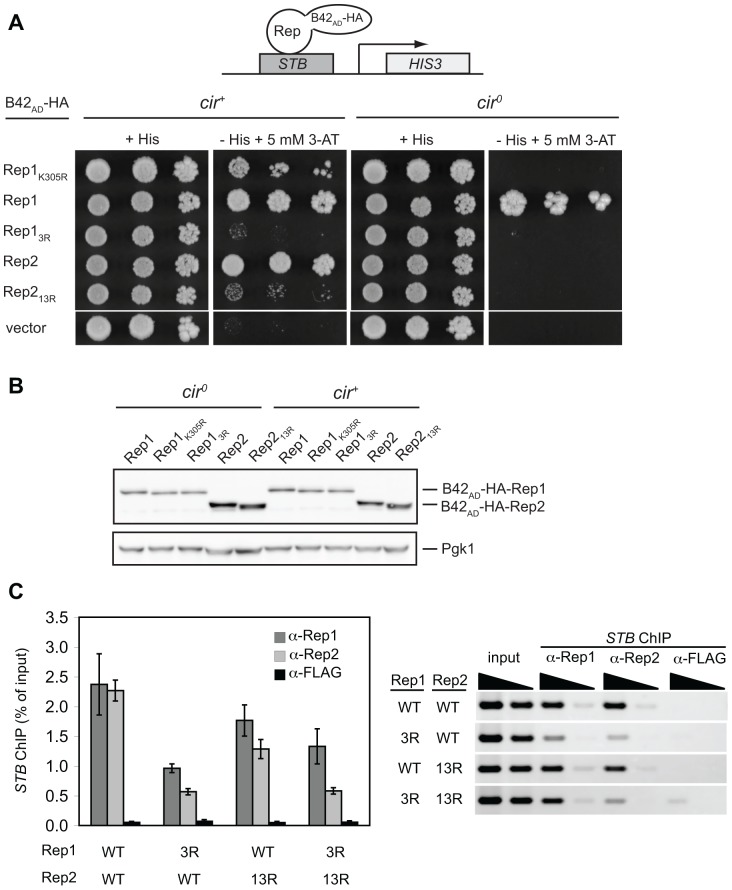
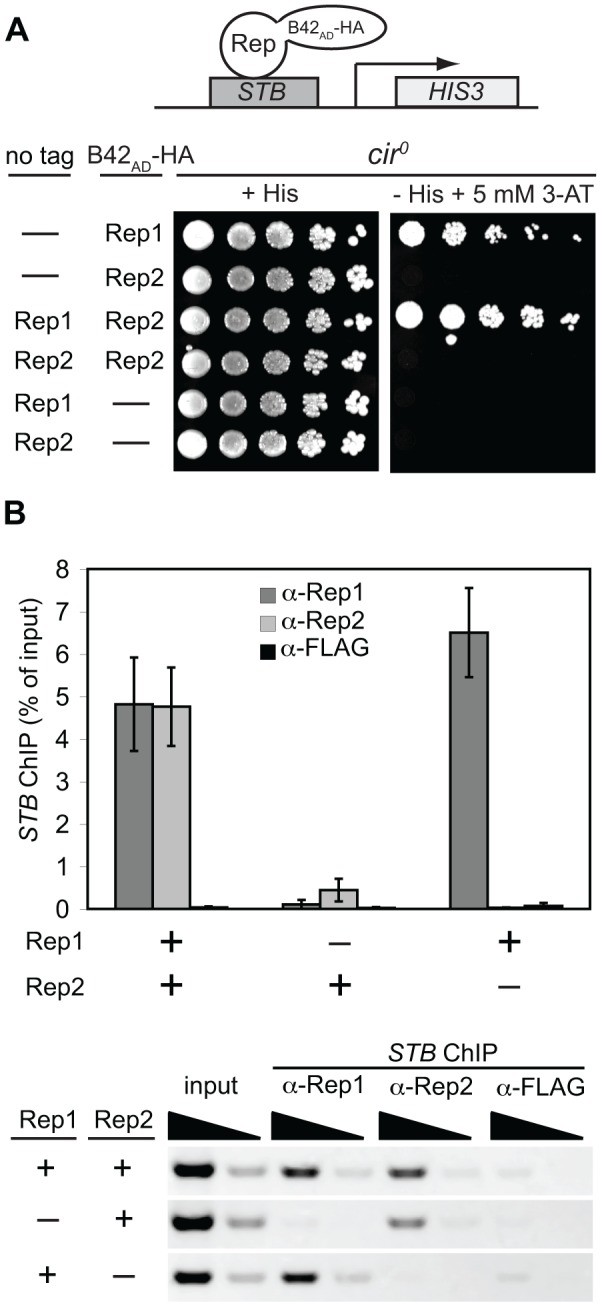
Point mutations in Rep1 that prevent its recruitment to the 2 µm plasmid STB locus have previously been shown to result in plasmid mis-segregation [24]. To examine the ability of Rep13R and Rep213R to associate with STB in vivo, we used a one-hybrid assay. Rep proteins were expressed as HA epitope-tagged, viral B42 activation domain (B42AD-HA) fusion proteins in a yeast strain in which the plasmid STB locus was integrated in the genome upstream of a histidine biosynthetic reporter gene (HIS3). In this assay, expression of HIS3 is dependent on interaction of the respective Rep fusion protein with STB and can be assessed by monitoring growth of the yeast on medium lacking histidine and supplemented with 3-aminotriazole (3-AT), a competitive inhibitor of the HIS3 gene product [55] (Fig. 5A). Yeast expressing B42AD-HA alone (vector) exhibited no growth, indicating that the viral activation domain cannot be recruited to STB by itself. As previously reported, in a cir + reporter strain, expression of wild-type Rep1 or Rep2 fused to B42AD-HA led to robust growth, indicating that both Rep proteins are recruited to STB [55]. The single K305R point mutation in Rep1 that only slightly diminished its interaction with SUMO in a two-hybrid assay modestly reduced one-hybrid STB association, while the mutations in Rep13R and Rep213R that virtually abolished two-hybrid interaction with SUMO severely impaired their ability to associate with STB in the one-hybrid assay. We also examined the ability of the Rep proteins to associate with STB in an isogenic cir 0 strain where any contribution to the interaction from endogenous plasmid proteins would be lost (Fig. 5A). The difference in STB interaction between wild-type Rep1 and the two sumoylation-deficient Rep1 mutants was more pronounced in the cir 0 reporter strain. The Rep1-K305R mutant expressed as a B42AD-HA fusion protein did not result in growth of the reporter strain, suggesting that loss of this single sumoylation site was sufficient to significantly reduce association of Rep1 with the STB locus in the absence of native plasmid proteins. Expression of native Rep1 or Rep2 individually from a second plasmid in the cir 0 reporter strain also expressing B42AD-HA-Rep1K305R or B42AD-HA-Rep13R did not result in any growth, suggesting that the combined presence of endogenous Rep1 and Rep2 proteins was required for the limited association of the sumoylation-deficient Rep1 mutants with STB observed in the cir + reporter strain (data not shown). Interestingly, although wild-type Rep2 expressed as a B42AD-HA fusion protein could activate the STB-driven HIS3 reporter gene in the cir + strain, no growth was observed for the cir 0 reporter strain, suggesting B42AD-HA-Rep2 was dependent on endogenous plasmid proteins for association with STB.
Figure 5. Lysine mutations in Rep13R and Rep213R impair Rep-STB association but do not reduce levels of Rep fusion proteins.
(A) Five-fold serial dilutions of a cir + or cir 0 yeast one-hybrid reporter strain encoding STB upstream of a HIS3 reporter gene and transformed with plasmids encoding the indicated Rep1 and Rep2 proteins fused to B42AD-HA were spotted onto galactose media to induce expression of fusion proteins. Recruitment of the Rep proteins to STB was monitored by growth on medium lacking histidine supplemented with 5 mM 3-aminotriazole. (B) Levels of the B42AD-HA fusion proteins were monitored by western blot analysis of total protein extracted from the yeast transformants 24 h after galactose induction. (C) ChIP assays were performed with anti-Rep1, anti-Rep2, or anti-FLAG antibodies and the precipitated DNA amplified using primers specific for STB. ChIP efficiency is indicated by the percent of input DNA immunoprecipitated (avg ± sd from triplicate assays) (left) and ethidium-stained agarose gels of PCR products from a representative assay are shown (right). Template DNA amplified in “input” PCR reactions represented 40% of the DNA that was immunoprecipitated and used as template in “ChIP” PCR reactions.
A reduction in the steady-state levels of the mutant Rep1 and Rep2 fusion proteins could also explain the reduced one-hybrid association with STB. However, no significant differences in Rep fusion protein levels in the cir + or cir 0 reporter strains were observed (Fig. 5B), suggesting that reduced activation of the reporter gene by the mutant Rep fusion proteins was a consequence of reduced interaction with the STB sequence in the promoter rather than being due to lower abundance.
To determine whether Rep13R and Rep213R were impaired for interaction with STB in a native context, the efficiency of chromatin immunoprecipitation (ChIP) of the STB locus with anti-Rep1 or anti-Rep2 antibodies was examined for 2 µm-based plasmids encoding wild-type or mutant Rep proteins (Fig. 5C). Consistent with results of the one-hybrid assays, STB DNA co-immunoprecipitated less efficiently when the plasmid encoded Rep13R or Rep213R rather than wild-type Rep1 and Rep2. Taken together, the results of the one-hybrid and ChIP assays indicate that defects in the inheritance of plasmids encoding Rep13R and Rep213R may be due to impaired interaction of the mutant Rep proteins with the plasmid partitioning locus. STB was also less efficiently co-immunoprecipitated with anti-Rep2 antibodies in yeast expressing Rep13R, suggesting that association of Rep2 with STB is, in part, dependent on the stable association of Rep1 with STB. In contrast, STB was efficiently co-immunoprecipitated by anti-Rep1 antibodies when the plasmid encoded wild-type Rep1 and the Rep213R mutant, suggesting Rep1 association with STB was not affected by the mutations in Rep213R.
Rep2 depends on Rep1 for robust association with STB
The impaired association of Rep2 with STB when Rep1 was absent (Fig. 5A) or when Rep1 interaction with STB was reduced due the lysine mutations in Rep13R (Fig. 5C) suggested that Rep2 may depend on interaction with Rep1 for stable association with STB. Although previous work has shown that Rep2, when over-expressed, is able to interact with STB in the absence of Rep1 [6], the degree to which Rep1 and Rep2 associate with STB in the absence of the partner protein has not been examined. To investigate this, we assessed the interaction of B42AD-HA-Rep2 with STB in a cir 0 one-hybrid reporter strain in which either untagged Rep1 or untagged Rep2 was expressed from the GAL1 promoter (Fig. 6A). In this assay, expression of Rep1, but not Rep2, promoted association of B42AD-HA-Rep2 with STB. To examine the dependence of Rep1 and Rep2 on each other for their association with STB in a native context, we performed ChIP assays using yeast transformed with a marker-tagged 2 µm plasmid encoding Rep1 and Rep2, or derivatives that lacked either the REP1 or REP2 gene (Fig. 6B). While STB did co-immunoprecipitate with anti-Rep2 antibodies when Rep1 was absent, the yield was significantly lower than when Rep1 was present. In contrast, absence of Rep2 did not reduce association of Rep1 with STB. These results suggest that STB-bound Rep1 may promote more stable association of Rep2 with the plasmid-partitioning locus.
Figure 6. Stable association of Rep2 with STB depends on Rep1.
(A) A cir 0 yeast one-hybrid reporter strain was co-transformed with single-copy plasmids allowing for galactose-inducible expression of untagged and B42AD-HA-tagged Rep1 or Rep2. Expression of the STB-driven HIS3 reporter gene was monitored by growth on solid galactose media as described in the legend to Fig. 5. (B) ChIP assays were performed on extracts from yeast expressing Rep1, Rep2, or both Rep1 and Rep2 from an ADE2-tagged 2 µm plasmid as detailed in the legend to Fig. 5.
Mutations in Rep13R cause loss of normal Rep1 punctate nuclear staining pattern
The multiple copies of the 2 µm plasmid are organized in a small number of nuclear foci that co-localize with Rep1 and Rep2 [5], [27]. In rsc2Δ yeast, impaired association of Rep1 with STB [24] is accompanied by loss of the normal Rep1 punctate nuclear staining pattern [28]. The impaired interaction of Rep13R and Rep213R with the plasmid STB locus observed in the one-hybrid and ChIP assays suggested that localization to the plasmid foci might be affected by these mutations. To assess this, we used indirect immunofluorescence to compare localization of wild-type Rep proteins and the Rep13R and Rep213R mutants expressed from marker-tagged 2 µm plasmids (Fig. 7A). As previously reported, wild-type Rep1 was present in distinct nuclear foci [5], [27], [56] (Fig. 7A, column 1); however, Rep13R showed diffuse nuclear staining (Fig. 7A, columns 2 and 4). The absence of Rep1 foci when the protein had lysine substitutions that impaired sumoylation was consistent with the results of the one-hybrid and ChIP assays and suggests either that sumoylation is required for Rep1 localization to the distinct sub-nuclear plasmid-containing domains or that association with the plasmid STB locus is required for Rep protein sumoylation. Wild-type Rep2, when co-expressed with wild-type Rep1, exhibited a less sharply punctate but still uneven nuclear staining pattern as has been previously reported [5], [56] (Fig. 7A, column 3). However, Rep2 expressed with Rep13R displayed a more uniform, pan-nuclear staining pattern (Fig. 7A, column 2), consistent with results of ChIP assays that demonstrated that association of Rep2 with STB was impaired when Rep2 was co-expressed with Rep13R (Fig. 5C). When Rep213R was expressed with wild-type Rep1 (Fig. 7A, column 3), the Rep2 punctate staining pattern was similar to that of wild-type Rep2, suggesting Rep2 is not dependent on being sumoylated for its nuclear distribution. The respective staining patterns of Rep1 and Rep2 when both were mutant (Fig. 7A, column 4) were similar to those observed when only Rep1 was mutant.
Figure 7. Localization of Rep13R and Rep213R.
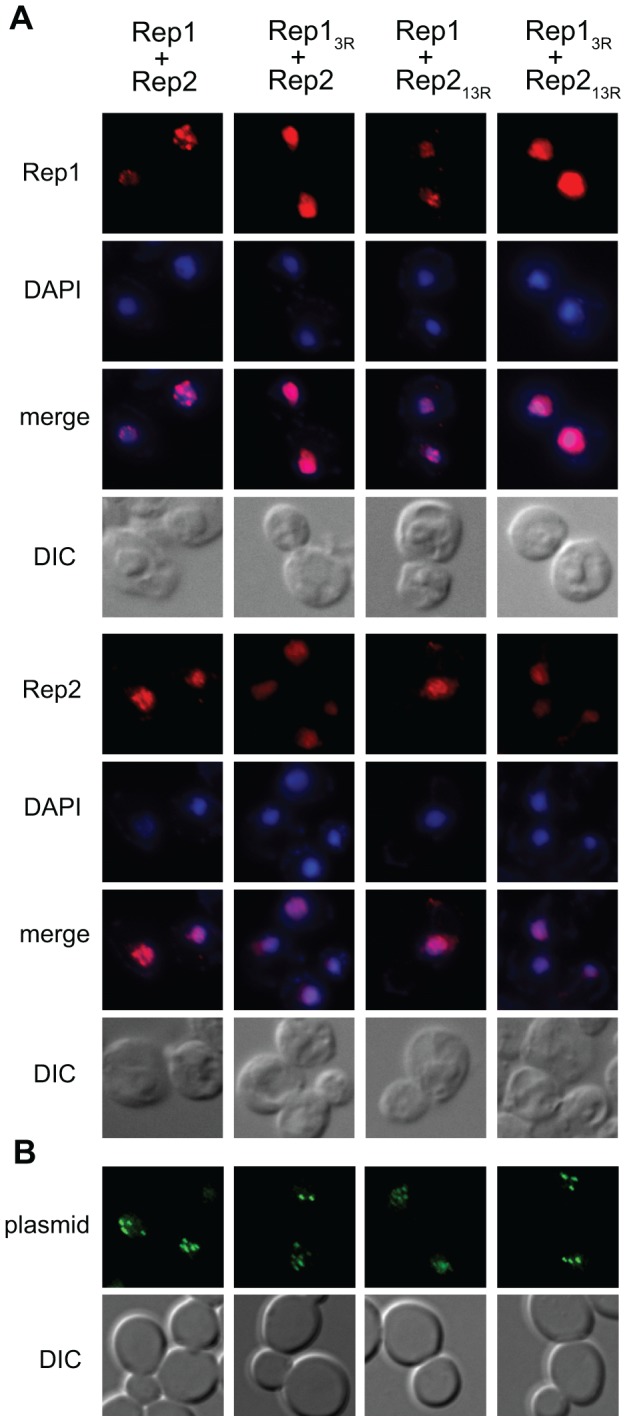
(A) Spheroplasts were prepared from cir 0 yeast expressing wild-type or mutant Rep1 and Rep2 from an ADE2-tagged 2 µm plasmid and the Rep proteins were visualized by indirect immunofluorescence. Bulk chromatin was visualized by DAPI staining and cells by light microscopy (DIC). (B) Yeast were co-transformed with an ADE2-tagged 2 µm plasmid encoding the indicated Rep1 and Rep2 alleles and a plasmid containing the STB locus and 256 lacO repeats. Plasmid localization was visualized by fluorescence microscopy following induction of GFP-LacI repressor fusion protein expression.
The mislocalization of Rep1 and Rep2 when Rep1 was sumoylation-deficient prompted us to examine plasmid localization in the presence of Rep13R and Rep213R mutants. To visualize 2 µm plasmid foci, we introduced a 2 µm reporter plasmid containing 256 lac operator sequences in yeast that expressed a GFP-tagged LacI repressor protein (Fig. 7B) [5]. As has been previously reported, the plasmid was localized into a small number of distinct nuclear foci in cells expressing wild-type Rep1 and Rep2 [5], [27]. These foci were still observed when both Rep proteins were mutant, suggesting that clustering of plasmid copies is not dependent on Rep protein sumoylation.
Rep1 with an I202T substitution loses association with the STB locus but interacts with SUMO in a two-hybrid assay
Our data suggest that the lysine-to-arginine mutations in Rep13R and Rep213R impair their ability to be sumoylated and their interaction with STB, implying that for both Rep1 and Rep2, sumoylation status correlates with the ability to associate with STB. Sumoylation of Rep1 and Rep2 may be required for their stable association with STB. Alternatively, association with STB may promote sumoylation of Rep1 and Rep2. To attempt to distinguish between these two possibilities, we made use of a mutant REP1 allele isolated in a screen for mutations in REP1 that impaired plasmid partitioning (unpublished data). The mutant Rep1 has threonine substituted for isoleucine at residue 202 (Rep1I202T), and was chosen because the altered residue lies outside the domain required for Rep1 self-association and Rep2 interaction [36], [55], was not within the region containing the three lysine-to-arginine substitutions in Rep13R, and leads to loss of association of Rep1 with STB in a one-hybrid assay in both cir+ and cir0 strains (unpublished data and Fig. 8A). To determine whether this mutant version of Rep1 could be sumoylated despite loss of STB association, Rep1I202T was assessed for interaction with SUMO in a two-hybrid assay (Fig. 8B). Interaction between Rep1I202T and SUMO was observed although expression of the β-galactosidase reporter gene was weaker than when Rep1 was wild type (Fig. 8B). A similar reduction in the expression of the reporter gene was also observed for interaction of Rep1I202T with Rep2 when compared to interaction of wild-type Rep1 with Rep2 (Fig. 8B). The similar degree of reduction for both types of interaction suggested that the steady-state level of the Rep1I202T fusion protein might be lower that that of the wild type Rep1 fusion. Western blotting analysis confirmed reduced steady-state levels for the Rep1I202T fusion proteins in both the one-hybrid (Fig. 8C) and two-hybrid reporter strains (Fig. 8D). Weaker activation of the reporter gene by the mutant Rep1I202T in the two-hybrid assays would be expected from the reduced level of the fusion protein (Fig. 8D) and does not necessarily represent impaired interaction of Rep1I202T with SUMO or Rep2. While the complete loss of detectable STB-association for Rep1I202T in the one-hybrid assay might be due to the reduced level of the fusion protein, the observed loss of association of Rep1I202T with STB is not sufficient to abolish interaction of Rep1I202T with SUMO in the two-hybrid assay. This result is consistent with our observations that Rep1 and Rep2 both retain two-hybrid interaction with SUMO in yeast lacking an STB sequence (data not shown).
Figure 8. Rep1I202T does not associate with the plasmid STB locus but retains interaction with SUMO.
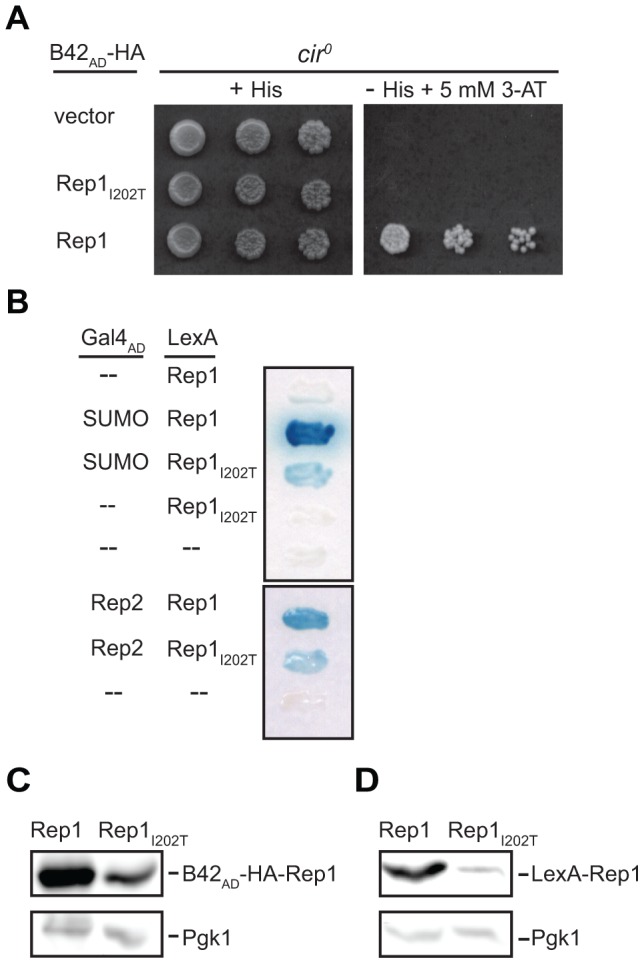
(A) Association of wild type and I202T mutant Rep1 (Rep1I202T) with STB in cir0 yeast was monitored using a one-hybrid assay as described in the legend to Figure 5. (B) Interaction of Rep1 and Rep1I202T with SUMO and with Rep2 was monitored in cir0 yeast using a two-hybrid assay as described in the legend to Figure 1. Levels of the Rep1 and Rep1I202T fusion proteins in the (C) one-hybrid and (D) two-hybrid reporter yeast strains, in A and B respectively, were monitored by western blotting analysis with antibodies specific for Pgk1 (C and D, bottom), anti-HA (C, top) and anti-LexA (D, top).
Discussion
Equal partitioning of the multiple copies of the yeast 2 µm plasmid at mitosis is dependent on the association of plasmid proteins Rep1 and Rep2 with each other, and with the plasmid-partitioning locus, STB [5], [36], [55]–[58]. Here we have identified distinct roles for Rep1 and Rep2 in this process; Rep1 stabilizes Rep2 association with STB, while Rep2 association with Rep1 increases Rep1 post-translational stability. We have demonstrated that like Flp, the 2 µm-encoded site-specific recombinase required for plasmid copy number amplification, and Rep2 [11], Rep1 is sumoylated, and that lysine-to-arginine mutations in Rep1 and Rep2 that inhibit their sumoylation also impair their association with STB and lead to defective plasmid partitioning.
Both Rep1 and Rep2 required simultaneous mutation of multiple lysine residues to effectively block their sumoylation (Rep13R and Rep213R mutants, respectively). Based on two-hybrid assays, the three sites mutated in Rep13R each likely represent sites targeted for SUMO conjugation; however, it seems unlikely that the thirteen residues mutated in Rep213R each normally serve as a SUMO acceptor site. Proteins targeted for sumoylation often contain multiple SUMO acceptor sites [59], but in other studies, abolishing major sumoylation sites has been shown to lead to increased SUMO conjugation at less preferred sites [60], [61] and this may also be the case for Rep1 and Rep2. Despite the multiple mutations in Rep13R and Rep213R that abolished two-hybrid interaction with SUMO, faint slower-migrating Rep protein species were detected in western blot analysis, suggesting sumoylation was still occurring, albeit at a low level, on remaining lysine residues. However, since inheritance of 2 µm plasmids encoding Rep13R or Rep213R mutants was perturbed, the residual sumoylation was not sufficient for normal Rep protein function.
Sumoylation can have diverse effects, altering the activity, interactions, localization or stability of a targeted protein [14]. Our analyses demonstrated that Rep1 and Rep2 were not dependent on sumoylation for their interaction with each other, consistent with observations that bacterially-expressed Rep1 and Rep2 interact in vitro [36], [56], [57]. However, the Rep13R and Rep213R mutants were defective for association with the plasmid STB partitioning locus. Even mutation of a single sumoylation site in Rep1, K305, modestly reduced Rep1-STB association. Since efficient partitioning of the 2 µm plasmid has been shown to depend on association of Rep1 and Rep2 with STB [24], loss of association of Rep1 and Rep2 lysine-to-arginine mutants with STB is likely to be the primary defect leading to loss of efficient plasmid partitioning.
While the Rep13R and Rep213R mutants were both impaired for association with STB, the mutations in Rep13R led to more severe defects in maintenance of a 2 µm plasmid compared to those in Rep213R. This result was somewhat unexpected in light of observations that absence of either Rep1 or Rep2 results in an equally severe defect in plasmid partitioning [58], [62], [63]. However, ChIP assays also supported this difference, showing that the amount of STB DNA that co-immunoprecipitated with Rep2 was markedly reduced when Rep2 was expressed with Rep13R or in the absence of Rep1, while Rep1 association with STB was not dependent on the presence of Rep2. Our results, combined with those from earlier studies [24], [36], [64] suggest that Rep2 may recognize STB in the absence of Rep1, but this limited association is inadequate for function. The increased sensitivity of STB chromatin to micrococcal nuclease in yeast lacking Rep1, or both Rep1 and Rep2, but not when only Rep2 was absent [65], supports the hypothesis that Rep1 is either more directly or more tightly associated with STB than Rep2. Co-evolution of STB and Rep1 sequence variants in 2 µm plasmids isolated from several laboratory and industrial S. cerevisiae strains [66], [67] also supports a model in which Rep1 recognition of STB is a critical prerequisite to productive association of Rep2 with STB.
Although Rep1 association may be an essential first stage in establishing the plasmid-partitioning complex, Rep2 association with STB also correlated with plasmid inheritance. Mutations that led to loss of Rep1 sumoylation perturbed both Rep2-STB association and plasmid partitioning more severely than mutations associated with loss of Rep2 sumoylation. The milder defect in plasmid partitioning associated with the Rep213R mutant suggests that Rep213R, recruited to STB through its interaction with wild-type Rep1, can confer some partitioning function. Although the partitioning defect produced by the lysine-to-arginine substitutions in Rep213R was mild compared to when Rep13R was expressed, it was similar to that observed when the RSC2 chromatin remodeling complex was impaired, a defect severe enough to make yeast unable to maintain the native 2 µm plasmid [28]. We also identified another key role for Rep2 in this process, namely the stabilization of the Rep1 protein. This chaperoning activity of Rep2 may explain why loss of Rep2 leads to an equally severe defect in plasmid inheritance as loss of Rep1 [58], [62], [63]. A reduction in Rep1 levels when Rep2 is absent and a requirement for Rep1 to stabilize Rep2 association with STB would explain why absence of either would result in a similar failure to recruit critical host proteins to STB [6], [8], [9], [24].
A hierarchical assembly of Rep protein sub-complexes has previously been proposed to mediate early events required for establishing the functional 2 µm plasmid-partitioning complex. Recruitment of the motor protein Kip1 to STB was shown to be dependent on both Rep1 and Rep2, but Kip1 was found to co-immunoprecipitate only with Rep2, and not with Rep1 [8]. Downstream events, including recruitment of the centromere-specific histone H3 variant Cse4, the RSC2 complex, and ultimately the cohesin complex [6], [8], [9], [24], [28] are dependent on Kip1 recruitment to STB [8].
Our data indicate a correlation between sumoylation status of the Rep proteins and their ability to associate with STB. Is sumoylation of Rep1 and Rep2 required for association with STB, or does recruitment to STB lead to their sumoylation? For the replication clamp protein, PCNA, sumoylation has been shown to be dependent on PCNA association with DNA [68]. In Xenopus, sumoylation of centromere-associated proteins is dependent on their co-localization with SUMO E3 ligase PIASy at centromeres of mitotic chromosomes [69]. Therefore, for the yeast plasmid Rep proteins we cannot exclude the possibility that the lysine substitutions in Rep13R and Rep213R directly impair interactions with STB DNA or with host proteins in a manner that consequently leads to reduced sumoylation of the Rep proteins. Rep1 has not been shown to have intrinsic DNA-binding activity, precluding in vitro assessment of the effect of the lysine substitutions on DNA association. However, the observation that a Rep1 mutant (Rep1I202T) impaired for in vivo association with STB could still interact with SUMO in a two-hybrid assay supports a model in which sumoylation promotes an early stage in the assembly of the Rep partitioning complex, enabling stable association of the Rep proteins at STB. SUMO-regulated association with DNA has been demonstrated for heat shock proteins Hsf1 and Hsf2, which are dependent on sumoylation for their DNA-binding activity [70], [71].
Consistent with the hypothesis that impaired sumoylation of Rep1 correlates with defective assembly of the Rep1-Rep2 complex at STB, when Rep1 was sumoylation-deficient, both Rep proteins lost their localization to discrete nuclear foci previously shown to contain clusters of the 2 µm plasmid [5], [27], [56]. The uniform nuclear staining pattern observed for sumoylation-deficient Rep1 is reminiscent of that observed for wild-type Rep1 in rsc2Δ yeast [28], in which association of Rep1 with STB is also impaired [24], supporting Rep1 interaction with STB as critical for Rep1 sub-nuclear localization. Sumoylation has been shown to regulate the sub-nuclear localization of other proteins, a notable example being the SUMO-dependent localization of promyelocytic leukemia (PML) protein to PML nuclear bodies in mammalian cells [72]. In this study, 2 µm plasmid foci were observed even when Rep1 and Rep2 were both sumoylation-deficient. Further investigation is needed to assess whether localization of plasmid clusters in their normal spindle pole-proximal nuclear address [5], [7] is altered by changes in Rep1 and Rep2 sumoylation status.
Our data suggest that Rep protein sumoylation may promote stable association of the Rep proteins with 2 µm plasmid DNA, an association reminiscent of sumoylation-dependent targeting of proteins to centromeres in yeast and in higher eukaryotes. In human cells, proteins conjugated with SUMO-2/3 are enriched at centromeres [73]. In yeast, topoisomerase II is robustly targeted to pericentric DNA when translationally fused to SUMO [74], and yeast kinetochore proteins Ndc10, Cep3, Bir1, and Ndc80 are all SUMO targets [75], with sumoylation of Ndc10 being functionally relevant. Sumoylation-deficient Ndc10 fails to localize to the mitotic spindle, resulting in defective chromosome segregation [75]. The short defined point centromeres to which Ndc10 binds are unique to the Saccharomycetaceae family of budding yeast, and were recently proposed to have arisen by replacement of a typical epigenetic fungal centromere with an ancestral 2 µm plasmid-derived partitioning system [76]. While rapid evolution may have obscured sequence homology between the 2 µm plasmid and chromosomal segregation proteins, it is tempting to speculate that post-translational modification of segregation proteins with SUMO might be a conserved process, essential for their common function.
The yeast 2 µm plasmid is not the only parasitic DNA element to exploit the host cell SUMO pathway for its maintenance [77]. Many viral proteins involved in maintenance of the episomes that encode them are SUMO-modified. Members of the human papillomavirus E2 family of proteins are dependent on sumoylation for their ability to tether viral genomes to host chromosomes to ensure faithful segregation [78]. Host sumoylation has therefore frequently been exploited to ensure maintenance of parasitic genomes in eukaryotic cells, and here we have presented evidence that suggests the yeast 2 µm plasmid may also co-opt this essential cellular process to ensure its efficient segregation during host cell division.
Acknowledgments
We are very grateful to Joyce S. K. Chew for providing technical assistance, Elizabeth Polvi for pEP1 plasmid constructions, Arpita Sengupta for the REP1 mutant screen, Nisa Renault for in vitro SUMO-Rep protein interaction assays, Graham Dellaire for comments on the manuscript, and members of the Dalhousie University Yeast Molecular Biology Group for helpful discussions. We thank Huilin Zhou for kindly sending us yeast strain HZY1017 encoding the HIS6-FLAG-SMT3:KanMX6 at the endogenous SMT3 locus, and Mark Hochstrasser for plasmids pRS426-CUP1p-3HA-SMT3gg and pGBD-smt3ΔGG.
Funding Statement
This research was supported by an NSERC Discovery grant 155268 to MJD, NSERC CGSD and Killam pre-doctoral scholarship to JBP, and Killam pre-doctoral and NSERC CGSM and PGSD scholarships to MEM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Futcher AB (1988) The 2 micron circle plasmid of Saccharomyces cerevisiae . Yeast Chichester, England: 4: 27–40 10.1002/yea.320040104. [DOI] [PubMed] [Google Scholar]
- 2.Broach JR, Volkert FC. (1991) Circular DNA plasmids of yeasts: Genome dynamics, protein synthesis and energetics. In: Broach JR, Pringle JR, Jones EW, editors. The molecular and cellular biology of the yeast Saccharomyces.Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory press. pp. 297-331. [Google Scholar]
- 3. Futcher AB, Cox BS (1983) Maintenance of the 2 micron circle plasmid in populations of Saccharomyces cerevisiae . Journal of Bacteriology 154: 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Livingston DM (1977) Inheritance of the 2 micrometer DNA plasmid from Saccharomyces . Genetics 86: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Velmurugan S, Yang XM, Chan CS, Dobson M, Jayaram M (2000) Partitioning of the 2-micron circle plasmid of Saccharomyces cerevisiae: functional coordination with chromosome segregation and plasmid-encoded Rep protein distribution. The Journal of Cell Biology 149: 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehta S, Yang XM, Chan CS, Dobson MJ, Jayaram M, et al. (2002) The 2 micron plasmid purloins the yeast cohesin complex: A mechanism for coupling plasmid partitioning and chromosome segregation? The Journal of Cell Biology 158: 625–637 10.1083/jcb.200204136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta S, Yang XM, Jayaram M, Velmurugan S (2005) A novel role for the mitotic spindle during DNA segregation in yeast: Promoting 2 micron plasmid-cohesin association. Molecular and Cellular Biology 25: 4283–4298 10.1128/MCB.25.10.4283-4298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cui H, Ghosh SK, Jayaram M (2009) The selfish yeast plasmid uses the nuclear motor Kip1p but not Cin8p for its localization and equal segregation. The Journal of Cell Biology 185: 251–264 10.1083/jcb.200810130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hajra S, Ghosh SK, Jayaram M (2006) The centromere-specific histone variant Cse4p (CENP-A) is essential for functional chromatin architecture at the yeast 2-micron circle partitioning locus and promotes equal plasmid segregation. The Journal of Cell Biology 174: 779–790 10.1083/jcb.200603042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao X, Wu CY, Blobel G (2004) Mlp-dependent anchorage and stabilization of a desumoylating enzyme is required to prevent clonal lethality. The Journal of Cell Biology 167: 605–611 10.1083/jcb.200405168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen XL, Reindle A, Johnson ES (2005) Misregulation of 2 micron circle copy number in a SUMO pathway mutant. Molecular and Cellular Biology 25: 4311–4320 10.1128/MCB.25.10.4311-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dobson MJ, Pickett AJ, Velmurugan S, Pinder JB, Barrett LA, et al. (2005) The 2 micron plasmid causes cell death in Saccharomyces cerevisiae with a mutation in Ulp1 protease. Molecular and Cellular Biology 25: 4299–4310 10.1128/MCB.25.10.4299-4310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burgess RC, Rahman S, Lisby M, Rothstein R, Zhao X (2007) The Slx5-Slx8 complex affects sumoylation of DNA repair proteins and negatively regulates recombination. Molecular and Cellular Biology 27: 6153–6162 10.1128/MCB.00787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson ES (2004) Protein modification by SUMO. Annual Review of Biochemistry 73: 355–382 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 15. Makhnevych T, Sydorskyy Y, Xin X, Srikumar T, Vizeacoumar FJ, et al. (2009) Global map of SUMO function revealed by protein-protein interaction and genetic networks. Molecular Cell 33: 124–135 10.1016/j.molcel.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson KA, Henley JM (2010) Mechanisms, regulation and consequences of protein SUMOylation. The Biochemical Journal 428: 133–145 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Johnson ES, Gupta AA (2001) An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106: 735–744. [DOI] [PubMed] [Google Scholar]
- 18. Zhao X, Blobel G (2005) A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proceedings of the National Academy of Sciences of the United States of America 102: 4777–4782 10.1073/pnas.0500537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li SJ, Hochstrasser M (1999) A new protease required for cell-cycle progression in yeast. Nature 398: 246–251 10.1038/18457. [DOI] [PubMed] [Google Scholar]
- 20. Li SJ, Hochstrasser M (2000) The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Molecular and Cellular Biology 20: 2367–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Futcher AB (1986) Copy number amplification of the 2 micron circle plasmid of Saccharomyces cerevisiae . Journal of Theoretical Biology 119: 197–204. [DOI] [PubMed] [Google Scholar]
- 22. Volkert FC, Broach JR (1986) Site-specific recombination promotes plasmid amplification in yeast. Cell 46: 541–550. [DOI] [PubMed] [Google Scholar]
- 23. Xiong L, Chen XL, Silver HR, Ahmed NT, Johnson ES (2009) Deficient SUMO attachment to Flp recombinase leads to homologous recombination-dependent hyperamplification of the yeast 2 micron circle plasmid. Molecular Biology of the Cell 20: 1241–1251 10.1091/mbc.E08-06-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang XM, Mehta S, Uzri D, Jayaram M, Velmurugan S (2004) Mutations in a partitioning protein and altered chromatin structure at the partitioning locus prevent cohesin recruitment by the Saccharomyces cerevisiae plasmid and cause plasmid missegregation. Molecular and Cellular Biology 24: 5290–5303 10.1128/MCB.24.12.5290-5303.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghosh SK, Hajra S, Paek A, Jayaram M (2006) Mechanisms for chromosome and plasmid segregation. Annual Review of Biochemistry 75: 211–241 10.1146/annurev.biochem.75.101304.124037. [DOI] [PubMed] [Google Scholar]
- 26. Murray AW, Szostak JW (1983) Pedigree analysis of plasmid segregation in yeast. Cell 34: 961–970. [DOI] [PubMed] [Google Scholar]
- 27. Scott-Drew S, Wong CM, Murray JA (2002) DNA plasmid transmission in yeast is associated with specific sub-nuclear localisation during cell division. Cell Biology International 26: 393–405. [DOI] [PubMed] [Google Scholar]
- 28. Wong MC, Scott-Drew SR, Hayes MJ, Howard PJ, Murray JA (2002) RSC2, encoding a component of the RSC nucleosome remodeling complex, is essential for 2 micron plasmid maintenance in Saccharomyces cerevisiae . Molecular and Cellular Biology 22: 4218–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang J, Hsu JM, Laurent BC (2004) The RSC nucleosome-remodeling complex is required for cohesin's association with chromosome arms. Molecular Cell 13: 739–750. [DOI] [PubMed] [Google Scholar]
- 30.Burke D, Dawson D, Stearns T. (2000) Methods in yeast genetics. a Cold Spring Harbor laboratory course manual.Cold Spring Harbor,N.Y.:Cold Spring Harbor Laboratory Press.
- 31.Sambrook J, Fritsch EF, Maniatis T. (1989)Molecular cloning: A laboratory manual, 2nd ed.Cold Spring Harbor,N.Y.: Cold Spring Harbor Laboratory Press.
- 32. Gietz RD, Schiestl RH, Willems AR, Woods RA (1995) Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast Chichester, England: 11: 355–360 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 33. Tsalik EL, Gartenberg MR (1998) Curing Saccharomyces cerevisiae of the 2 micron plasmid by targeted DNA damage. Yeast Chichester, England: 14: 847–852.2-9. [DOI] [PubMed] [Google Scholar]
- 34. Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science New York, N.Y. 285: 901–906. [DOI] [PubMed] [Google Scholar]
- 35. Hannich JT, Lewis A, Kroetz MB, Li SJ, Heide H, et al. (2005) Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae . The Journal of Biological Chemistry 280: 4102–4110 10.1074/jbc.M413209200. [DOI] [PubMed] [Google Scholar]
- 36. Sengupta A, Blomqvist K, Pickett AJ, Zhang Y, Chew JS, et al. (2001) Functional domains of yeast plasmid-encoded Rep proteins. Journal of Bacteriology 183: 2306–2315 10.1128/JB.183.7.2306-2315.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gyuris J, Golemis E, Chertkov H, Brent R (1993) Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell 75: 791–803. [DOI] [PubMed] [Google Scholar]
- 38. Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae . Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li JJ, Herskowitz I (1993) Isolation of ORC6, a component of the yeast origin recognition complex by a one-hybrid system. Science New York, N.Y. 262: 1870–1874. [DOI] [PubMed] [Google Scholar]
- 40. Fields S, Song O (1989) A novel genetic system to detect protein-protein interactions. Nature 340: 245–246 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 41. Yaffe MP, Schatz G (1984) Two nuclear mutations that block mitochondrial protein import in yeast. Proceedings of the National Academy of Sciences of the United States of America 81: 4819–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang J, Cheeseman IM, Kallstrom G, Velmurugan S, Barnes G, et al. (2001) Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. The Journal of Cell Biology 155: 763–774 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodriguez MS, Dargemont C, Hay RT (2001) SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. The Journal of Biological Chemistry 276: 12654–12659 10.1074/jbc.M009476200. [DOI] [PubMed] [Google Scholar]
- 44. Jakobs A, Himstedt F, Funk M, Korn B, Gaestel M, et al. (2007) Ubc9 fusion-directed SUMOylation identifies constitutive and inducible SUMOylation. Nucleic Acids Research 35: e109 10.1093/nar/gkm617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niedenthal R (2007) Ubc9 fusion-directed SUMOylation (UFDS). Biochemical Society Transactions 35: 1430–1432 10.1042/BST0351430. [DOI] [PubMed] [Google Scholar]
- 46. Niedenthal R (2009) Enhanced detection of in vivo SUMO conjugation by Ubc9 fusion-dependent sumoylation (UFDS). . Methods in Molecular Biology Clifton, N.J. 497 63–79 10.1007/978-1-59745-566-4_5. [DOI] [PubMed] [Google Scholar]
- 47. Zhou W, Ryan JJ, Zhou H (2004) Global analyses of sumoylated proteins in Saccharomyces cerevisiae: induction of protein sumoylation by cellular stresses. The Journal of Biological Chemistry 279: 32262–32268 10.1074/jbc.M404173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao Y, Kwon SW, Anselmo A, Kaur K, White MA (2004) Broad spectrum identification of cellular small ubiquitin-related modifier (SUMO) substrate proteins. The Journal of Biological Chemistry 279: 20999–21002 10.1074/jbc.M401541200. [DOI] [PubMed] [Google Scholar]
- 49. Wohlschlegel JA, Johnson ES, Reed SI, Yates JR,3rd (2004) Global analysis of protein sumoylation in Saccharomyces cerevisiae . The Journal of Biological Chemistry 279: 45662–45668 10.1074/jbc.M409203200. [DOI] [PubMed] [Google Scholar]
- 50. Wach A, Brachat A, Pohlmann R, Philippsen P (1994) New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae . Yeast Chichester, England: 10: 1793–1808. [DOI] [PubMed] [Google Scholar]
- 51. Cairns BR, Schlichter A, Erdjument-Bromage H, Tempst P, Kornberg RD, et al. (1999) Two functionally distinct forms of the RSC nucleosome-remodeling complex, containing essential AT hook, BAH, and bromodomains. Molecular Cell 4: 715–723. [DOI] [PubMed] [Google Scholar]
- 52. Som T, Armstrong KA, Volkert FC, Broach JR (1988) Autoregulation of 2 micron circle gene expression provides a model for maintenance of stable plasmid copy levels. Cell 52: 27–37. [DOI] [PubMed] [Google Scholar]
- 53. Veit BE, Fangman WL (1988) Copy number and partition of the Saccharomyces cerevisiae 2 micron plasmid controlled by transcription regulators. Molecular and Cellular Biology 8: 4949–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belle A, Tanay A, Bitincka L, Shamir R, O′Shea EK (2006) Quantification of protein half-lives in the budding yeast proteome. Proceedings of the National Academy of Sciences of the United States of America 103: 13004–13009 10.1073/pnas.0605420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Velmurugan S, Ahn YT, Yang XM, Wu XL, Jayaram M (1998) The 2 micrometer plasmid stability system: Analyses of the interactions among plasmid- and host-encoded components. Molecular and Cellular Biology 18: 7466–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Scott-Drew S, Murray JA (1998) Localisation and interaction of the protein components of the yeast 2 micron circle plasmid partitioning system suggest a mechanism for plasmid inheritance. Journal of Cell Science 111 (Pt13) 1779–1789. [DOI] [PubMed] [Google Scholar]
- 57. Ahn YT, Wu XL, Biswal S, Velmurugan S, Volkert FC, et al. (1997) The 2 micron plasmid-encoded Rep1 and Rep2 proteins interact with each other and colocalize to the Saccharomyces cerevisiae nucleus. Journal of Bacteriology 179: 7497–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kikuchi Y (1983) Yeast plasmid requires a cis-acting locus and two plasmid proteins for its stable maintenance. Cell 35: 487–493. [DOI] [PubMed] [Google Scholar]
- 59. Maison C, Bailly D, Roche D, Montes de Oca R, Probst AV, et al. (2011) SUMOylation promotes de novo targeting of HP1α to pericentric heterochromatin. Nature Genetics 43: 220–227 10.1038/ng.765. [DOI] [PubMed] [Google Scholar]
- 60. Lin DY, Huang YS, Jeng JC, Kuo HY, Chang CC, et al. (2006) Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Molecular Cell 24: 341–354 10.1016/j.molcel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 61. Eladad S, Ye TZ, Hu P, Leversha M, Beresten S, et al. (2005) Intra-nuclear trafficking of the BLM helicase to DNA damage-induced foci is regulated by SUMO modification. Human Molecular Genetics 14: 1351–1365 10.1093/hmg/ddi145. [DOI] [PubMed] [Google Scholar]
- 62. Cashmore AM, Albury MS, Hadfield C, Meacock PA (1986) Genetic analysis of partitioning functions encoded by the 2 micron circle of Saccharomyces cerevisiae . Molecular and General Genetics 203: 154–162. [Google Scholar]
- 63. Jayaram M, Li YY, Broach JR (1983) The yeast plasmid 2mu circle encodes components required for its high copy propagation. Cell 34: 95–104. [DOI] [PubMed] [Google Scholar]
- 64. Hadfield C, Mount RC, Cashmore AM (1995) Protein binding interactions at the STB locus of the yeast 2 micron plasmid. Nucleic Acids Research 23: 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Veit BE, Fangman WL (1985) Chromatin organization of the Saccharomyces cerevisiae 2 micron plasmid depends on plasmid-encoded products. Molecular and Cellular Biology 5: 2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xiao W, Pelcher LE, Rank GH (1991) Evidence for cis- and trans-acting element coevolution of the 2-micron circle genome in Saccharomyces cerevisiae . Journal of Molecular Evolution 32: 145–152. [DOI] [PubMed] [Google Scholar]
- 67. Xiao W, Pelcher LE, Rank GH (1991) Sequence diversity of yeast 2 microns RAF gene and its co-evolution with STB and REP1 . Gene 101: 75–80. [DOI] [PubMed] [Google Scholar]
- 68.Parker JL, Bucceri A, Davies AA, Heidrich K, Windecker H, et al. (2008) SUMO modification of PCNA is controlled by DNA. The EMBO Journal 27: :2422- 2431.10.1038/emboj.2008.162; 10.1038/emboj.2008.162. [DOI] [PMC free article] [PubMed]
- 69. Ryu H, Azuma Y (2010) Rod/Zw10 complex is required for PIASy-dependent centromeric SUMOylation. The Journal of Biological Chemistry 285: 32576–32585 10.1074/jbc.M110.153817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Goodson ML, Hong Y, Rogers R, Matunis MJ, Park-Sarge OK, et al. (2001) Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor. The Journal of Biological Chemistry 276: 18513–18518 10.1074/jbc.M008066200. [DOI] [PubMed] [Google Scholar]
- 71. Hong Y, Rogers R, Matunis MJ, Mayhew CN, Goodson ML, et al. (2001) Regulation of heat shock transcription factor 1 by stress-induced SUMO-1 modification. The Journal of Biological Chemistry 276: 40263–40267 10.1074/jbc.M104714200. [DOI] [PubMed] [Google Scholar]
- 72. Zhong S, Muller S, Ronchetti S, Freemont PS, Dejean A, et al. (2000) Role of SUMO-1-modified PML in nuclear body formation. Blood 95: 2748–2752. [PubMed] [Google Scholar]
- 73. Zhang XD, Goeres J, Zhang H, Yen TJ, Porter AC, et al. (2008) SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Molecular Cell 29: 729–741 10.1016/j.molcel.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takahashi Y, Yong-Gonzalez V, Kikuchi Y, Strunnikov A (2006) SIZ1/SIZ2 control of chromosome transmission fidelity is mediated by the sumoylation of topoisomerase II. Genetics 172: 783–794 10.1534/genetics.105.047167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Montpetit B, Hazbun TR, Fields S, Hieter P (2006) Sumoylation of the budding yeast kinetochore protein Ndc10 is required for Ndc10 spindle localization and regulation of anaphase spindle elongation. The Journal of Cell Biology 174: 653–663 10.1083/jcb.200605019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Malik HS, Henikoff S (2009) Major evolutionary transitions in centromere complexity. Cell 138: 1067–1082 10.1016/j.cell.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 77. Boggio R, Chiocca S (2006) Viruses and sumoylation: Recent highlights. Current Opinion in Microbiology 9: 430–436 10.1016/j.mib.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu YC, Roark AA, Bian XL, Wilson VG (2008) Modification of papillomavirus E2 proteins by the small ubiquitin-like modifier family members (SUMOs). Virology 378: 329–338 10.1016/j.virol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rothstein R (1991) Targeting, disruption, replacement, and allele rescue: Integrative DNA transformation in yeast. Methods in Enzymology 194: 281–301. [DOI] [PubMed] [Google Scholar]
- 80.Bartel P, Chien CT, Sternglanz R, Fields S. (1993) Using the two-hybrid system to detect protein-protein interactions. In:Anonymous Cellular interactions in development: a practical approach. Oxford,United Kingdom:IRL Press.pp. 153-179.
- 81. Estojak J, Brent R, Golemis EA (1995) Correlation of two-hybrid affinity data with in vitro measurements. Molecular and Cellular Biology 15: 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]