Abstract
Objective
Betaine supplementation has been shown to be an effective agent for decreasing plasma homocysteine in healthy adults. Studies in healthy volunteers show that 6 g/d of betaine lowers plasma homocysteine concentrations by 5% to 20%. The purpose of this study was to perform a meta-analysis of randomized placebo-controlled trials that used daily betaine supplementation to identify the range in betaine's effects on lowering homocysteine.
Methods
Five randomized controlled trials published between 2002 and 2010 were identified using MEDLINE and a manual search. All 5 studies used health adult participants who were supplemented with at least 4 g/d of betaine for between 6 and 24 weeks. A meta-analysis was carried out using a random-effects model, and the overall effect size was calculated for changes in plasma homocysteine.
Results
The pooled estimate of effect for betaine supplementation on plasma homocysteine was a reduction of 1.23 μmol/L, which was statistically significant (95% confidence interval, − 1.61 to − 0.85; P = .01).
Conclusion
Supplementation with at least 4g/d of betaine for a minimum of 6 weeks can lower plasma homocysteine.
Key indexing terms: Betaine, Homocysteine, Meta-analysis
Introduction
Homocysteine is a sulfur-containing amino acid formed during the metabolism of methionine. Homocysteine can be converted back to methionine using the enzyme methionine synthase, a vitamin B-12 and folate–dependent reaction, or the enzyme betaine-homocysteine methyltransferase (BHMT), a betaine (trimethylglycine)–dependent reaction. Betaine donates methyl groups to homocysteine, which in turn is metabolized back to methionine. In the liver, BHMT catalyzes up to 50% of homocysteine metabolism.1
Betaine is found naturally in most living organisms and is formed in cells as an oxidation product of choline and can be obtained externally from foods such as spinach, beets, and wheat products. An inverse association between dietary betaine intakes and homocysteine concentrations has been observed in the sixth examination of the Framingham Offspring Study.2 As well, low plasma betaine concentrations have been shown to be related to an unfavorable cardiovascular risk profile3 and an increased risk of secondary heart failure and acute myocardial infarction.4 Plasma homocysteine concentration has been associated with cardiovascular disease and stroke,5 as elevated homocysteine promotes atherosclerosis and stroke through increased oxidant stress, impaired endothelial functions, and induction of thrombosis.6 Plasma homocysteine concentrations are usually around 8 to 10 μmol/L, with the 95th percentile at approximately 15 μmol/L.7 Elevated plasma levels of homocysteine greater than 15 μmol/L are present in 5% of the general population and in as many as 50% of those with cardiovascular disease and stroke.6
Supplementation with betaine decreases plasma homocysteine concentrations substantially in patients with hyperhomocystinemia.8 Studies in healthy volunteers show that 6 g/d of betaine lowers plasma homocysteine concentrations by 5% to 20%.9-20 The purpose of this study was to perform a meta-analysis of randomized placebo-controlled trials that used daily betaine supplementation to identify the range in betaine's effects on lowering homocysteine.
Methods
Selection of studies
A comprehensive MEDLINE literature search was performed to locate relevant randomized controlled trials published between 1966 and August 2012. The following headings were combined using the following Boolean operation: (“betaine” OR “trimethylglycine”) AND “homocysteine.” The search was restricted to key terms located in the title/abstract and was also restricted to studies published in English-language journals. Only full-length original journal articles were considered. No attempt was made to include abstracts or unpublished studies. A manual search was also conducted by using reference lists from original research articles and review articles.
To be included in the meta-analysis, a study had to meet the following criteria: (1) there was at least a single-blind, random allocation of study participants to either betaine treatment or placebo-controlled groups; (2) the intervention was equal to or greater than 4 weeks and less than or equal to 24 weeks; and (3) the study reported the mean plasma homocysteine changes in both the treatment and control groups.
Five studies met the eligibility criteria and were included in the meta-analysis.9-13 Although 17 potentially relevant studies were identified and screened, 12 trials did not meet the eligibility criteria for the meta-analysis. Major reasons for exclusion of studies were as follows: not being randomized placebo-controlled trials (5 trials), utilization of patient populations with serious diseases such as chronic renal failure and steatohepatitis (4 trials), absence of data to calculate the net mean change in plasma homocysteine (2 trials), and involvement of methionine loading (1 trial). Fig 1 shows the number of studies that were identified and excluded at different stages of the selection process.
Fig 1.
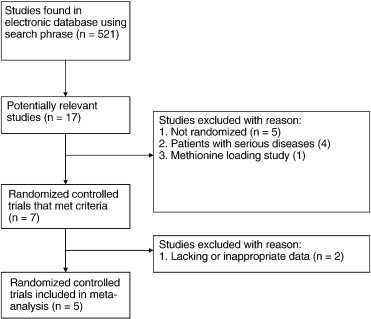
Study selection process for inclusion in the meta-analysis investigating the effects of betaine supplementation on plasma homocysteine.
Data abstraction and statistical analysis
Information on sample size, participant characteristics, study design, betaine dosage, duration, and treatment results were abstracted from the 5 clinical trials and entered into an EXCEL spreadsheet. To calculate the overall effect size, each study was weighted by the reciprocal of the variance for plasma homocysteine. Variances for homocysteine were calculated using the variances at baseline and at the end of follow-up based on the methodology of Follmann et al.21 In this method, a correlation coefficient of 0.5 between initial and final measures was assumed. Within each trial, equal variance was assumed between the control and intervention groups, as well as between the beginning and end of each trial. For parallel and cross-over trials, net changes in homocysteine were calculated as (homocysteine at end of follow-up in the treatment group − homocysteine at baseline in the treatment group) − (homocysteine at end of follow-up in the control group − homocysteine at baseline in the control group).
Estimates of the mean effect of betaine on homocysteine, as well as the corresponding 95% confidence intervals (CIs), were calculated using random-effects models. The assumption of heterogeneity implied by the use of the random-effects model was plausible because of differences between trials in such aspects as duration of the trial, dosages used, and sample populations that differed by age and sex.
Results
Participant characteristics and study designs
Participant and study design characteristics for the 5 randomized controlled trials included in the meta-analysis are presented in Table 1. Collectively, the 5 trials that were conducted between 2002 and 2010 included a total of 206 participants (124 in the betaine group and 119 in the control group). All of the trials were conducted in adults, with an age range of 27 to 59 years. Women were the majority in 3 of the 5 trials, with the pooled population being made up of 60.2% women. Four trials had a parallel double-blind design, and 1 used a crossover double-blind design. The study duration varied from 6 to 24 weeks, with a median duration of 6 weeks. Betaine supplementation for 4 of the 5 trials was 6 g/d, whereas 1 trial used 4 g/d. All 5 studies used participant populations with normal plasma homocysteine levels, as the baseline plasma homocysteine concentrations ranged from 8.4 to 12.7 μmol/L.
Table 1.
Population and baseline characteristics of the 5 trials included in the meta-analyses
| Source and year (reference) | Sample size | Mean age, y | Male, % | Study design | Betaine per day, g | Duration, wk | Plasma homocysteine, μmol/L |
|---|---|---|---|---|---|---|---|
| Schwab et al, 20029 | 42 | 44 | 33 | PD | 6 | 12 | 8.4 |
| Steenge et al, 200310 | 24 | 44.5 | 42 | PD | 6 | 6 | 12.7 |
| Olthof et al, 200311 | 38 | ND | 58 | PD | 6 | 6 | 10.6 |
| Olthof et al, 200612 | 39 | 59 | 59 | XD | 6 | 6 | 12.0 |
| Schwab et al, 201013 | 63 | 27 | 21 | PD | 4 | 24 | 8.8 |
ND, not determined; PD, parallel double-blind; XD, crossover double-blind.
Net change in homocysteine
The individual trial results along with the 95% CI and the overall pooled estimates of effect of betaine supplementation on plasma homocysteine are shown in Fig 2. Betaine's effect on plasma homocysteine illustrates that all 5 trials presented with an intervention-related trend toward a reduction in homocysteine, with 4 of the 5 showing statistically significant reductions (P < .05) in plasma homocysteine when compared with the control group (Fig 2). The overall pooled estimate of effect for betaine supplementation on plasma homocysteine resulted in a reduction of 1.23 μmol/L, which was statistically significant (95% CI, − 1.61 to − 0.85; P = .01).
Fig 2.
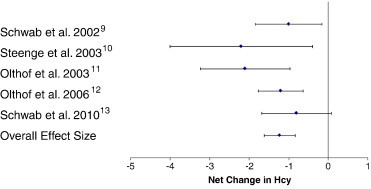
Net change (and 95% CI) in plasma homocysteine associated with betaine supplementation. The overall effect size is weighted by the inverse of the total variance of each trial.
Discussion
This meta-analysis shows that supplementation of betaine at 4 to 6 g/d significantly lowers plasma homocysteine concentration in healthy adults by 1.23 μmol/L or 11.8% of baseline values. A reduction in plasma homocysteine of 5 μmol/L is estimated to reduce the risk of cardiovascular disease by 20% to 30% and stroke by 40% to 60%.22 Based on this current meta-analysis, a person who consumes 4 to 6 g/d of betaine would have a 1.23-μmol/L lower plasma homocysteine concentration with an estimated concurrent reduction in cardiovascular disease risk of approximately 5% to 8% and a reduction in stroke risk of approximately 10% to 15%.
The homocysteine-lowering effects of betaine can most likely be ascribed to an increase in betaine-dependent methylation of homocysteine into methionine due to increased betaine availability and enhanced activity of the enzyme BHMT in both the liver and kidney. Betaine supplementation lowers plasma homocysteine levels almost immediately, with maximum results obtained within 4 to 6 weeks.11,15,17,18 It has been shown that betaine doses of 3 and 6 g decreased plasma homocysteine concentrations within 2 hours in healthy adults.17
No serious adverse effects were noted in any of the studies; but it is important to note that, in 2 studies, betaine supplementation significantly increased total serum cholesterol and low-density lipoprotein (LDL) cholesterol, which are both independent risk factors for cardiovascular disease.9,13 However, the changes were small and of minor clinical significance, with increases in both total serum cholesterol and LDL cholesterol of approximately 5 mg/dL. These 2 studies parallel another study that found significant increases in both total serum cholesterol and LDL cholesterol of approximately 10 mg/dL.16 However, in 2 other betaine-supplementation studies, the authors did not find any significant change in total serum cholesterol and LDL cholesterol after betaine supplementation.10,19 No significant changes in high-density lipoprotein cholesterol or triglycerides were noted in any of the studies that reported these values. The mechanism by which betaine might increase total serum cholesterol and LDL cholesterol concentration has been proposed to be the increase in synthesis and export of lipids in very low density lipoprotein from the liver into the circulation.23 Betaine is formed from choline; and so betaine supplementation spares this use of choline so that more is available for phosphatidylcholine biosynthesis in the liver, thereby making more available for very low density lipoprotein formation.
In regard to the risk of developing cardiovascular disease, it is important to point out that the unfavorable effects on serum lipids with betaine supplementation may undo the favorable homocysteine-lowering effects, as a 5- to 10-mg/dL increase in total serum cholesterol can increase the risk of developing cardiovascular disease by 3% to 6%.24 Therefore, in patients with normal plasma homocysteine levels, betaine supplementation may have a negligible effect on cardiovascular health; however, for patients with hyperhomocystinemia, the benefit of a homocysteine-lowering effect outweighs the effect of any modest lipid-related changes.
Many studies to date have shown that dietary supplementation with folic acid lowers plasma homocysteine concentrations in participants with either normal or elevated plasma homocysteine concentrations, with reductions of plasma homocysteine of 10% to 20%.25,26 Therefore, a decrease in plasma homocysteine of 3 μmol/L (achievable by daily intake of 800 μg folic acid) should reduce the risk of heart disease by 16% and stroke by 24%.22 When comparing betaine vs folic acid, it has been noted that folic acid supplementation lowers plasma homocysteine more than betaine supplementation.27 However, it has also been shown that the association of betaine with plasma homocysteine concentration is more pronounced in participants with low folate serum concentrations.28 Thus, betaine takes over as a methyl donor and sustains methionine synthesis under conditions of impaired folate status. Furthermore, folic acid supplementation has been shown to increase plasma betaine concentration by 15%, which indicates that the 2 remethylation pathways are interconnected.29
Folic acid has no adverse effects on blood lipids; and therefore, folic acid supplementation should remain the preferred homocysteine-lowering treatment in healthy humans.30 However, betaine may enhance homocysteine metabolism when the folic acid response is weak because of genetic polymorphisms such as MTHFR 677C→T. This is a point mutation that leads to an alanine to valine substitution in the enzyme methylenetetrahydrofolate reductase and is associated with approximately 40% higher plasma homocysteine concentration in carriers of the TT genotype compared with those with the CC wild type.31 The allele frequency of the MTHFR 677C→T mutation is 35%, with a homozygous rate of 12%. Betaine supplementation may be a useful adjunct along with folic acid for those 12% of patients who are TT homozygotes for the genetic mutation MTHFR 677C→T. This may be especially true in light of the possibility that patients with this genetic polymorphism are recommended to increase folic acid, but a few studies have shown that too much folic acid may increase the incidence of colorectal cancer.32
The overall purpose of supplementing with betaine is to decrease plasma homocysteine concentrations, which should ultimately lead to a reduction in the incidence of heart disease and stroke. Although no studies to date have concluded that betaine supplementation directly reduces the risk of developing cardiovascular disease and stroke, a recent review concluded that the long-term consumption of betaine may prevent cardiovascular disease mortality.33 More importantly, if homocysteine is elevated in response to a betaine insufficiency, it will not be corrected by folic acid supplementation alone; and this could help to explain why folic acid therapies do not lead to the expected reduction in vascular events.34 Therefore, the elevated homocysteine concentrations in these studies could be an indication of betaine insufficiency; and hence, using folic acid supplementation to lower plasma homocysteine concentrations would not lead to a sufficient decrease in plasma homocysteine to ultimately reduce the incidence of cardiovascular disease. It is important that future studies in this area control for both the betaine and folic acid intakes with respect to their influence on reducing the incidence of cardiovascular disease so that the effects of one can be carefully identified in relation to the other.
Limitations
One limitation in this meta-analysis is that all 5 studies used participants whose initial plasma homocysteine concentrations before intervention were normal. It has been shown that the extent of the decrease is smaller in healthy volunteers than in patients with hyperhomocystinemia.20 Therefore, future short-term studies must be conducted on patients with hyperhomocystinemia to assess the effects of betaine supplementation on plasma homocysteine, with additional long-term studies to assess the effects of betaine supplementation on reducing cardiovascular disease risk. Other limitations include that only one indexing system was searched and thus it is possible that some studies were not identified, and that only one author performed the selection of the articles included in this study and analysis of the data.
Conclusion
This meta-analysis shows that betaine supplementation of 4 to 6 g/d significantly lowers plasma homocysteine concentration in healthy adults by 1.23 μmol/L or 11.8% of baseline values. As new evidence continues to confirm that plasma homocysteine is a cause of cardiovascular disease, these results suggest that betaine could be used effectively to lower plasma homocysteine levels, which may potentially lead to a reduction in the risk of cardiovascular disease and stroke. However, there are few studies that directly test the connection between betaine and vascular disease, especially on patient populations with hyperhomocystinemia. Finally, betaine supplementation may be a useful adjunct along with folic acid for patients who are positive for the MTHFR 677C→T genetic mutation.
Funding sources and potential conflicts of interest
No funding sources or conflicts of interest were reported for this study.
References
- 1.Feng Q., Kalari K., Fridley B.L., Jenkins G., Ji Y., Abo R. Betaine-homocysteine methyltransferase: human liver genotype-phenotype correlation. Mol Genet Metab. 2011;102(2):126–133. doi: 10.1016/j.ymgme.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J.E., Jacques P.F., Dougherty L., Selhub J., Giovannucci E., Zeisel S.H., Cho E. Are dietary choline and betaine intakes determinants of total homocysteine concentration? Am J Clin Nutr. 2010;91(5):1303–1310. doi: 10.3945/ajcn.2009.28456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinova S.V., Tell G.S., Vollset S.E., Nygård O., Bleie Ø., Ueland P.M. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138(5):914–920. doi: 10.1093/jn/138.5.914. [DOI] [PubMed] [Google Scholar]
- 4.Lever M., George P.M., Atkinson W., Molyneux S.L., Elmslie J.L., Slow S. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PLoS One. 2011;6(7):e21666. doi: 10.1371/journal.pone.0021666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lonn E. Homocysteine in the prevention of ischemic heart disease, stroke and venous thromboembolism: therapeutic target or just another distraction? Curr Opin Hematol. 2007;14(5):481–487. doi: 10.1097/MOH.0b013e3282c48bd8. [DOI] [PubMed] [Google Scholar]
- 6.Guthikonda S., Haynes W.G. Homocysteine: role and implications in atherosclerosis. Curr Atheroscler Rep. 2006;8(2):100–106. doi: 10.1007/s11883-006-0046-4. [DOI] [PubMed] [Google Scholar]
- 7.Ganji V., Kafai M.R. Population reference values for plasma total homocysteine concentrations in US adults after the fortification of cereals with folic acid. Am J Clin Nutr. 2006;84(5):989–994. doi: 10.1093/ajcn/84.5.989. [DOI] [PubMed] [Google Scholar]
- 8.Matthews A., Johnson T.N., Rostami-Hodjegan A., Chakrapani A., Wraith J.E., Moat S.J. An indirect response model of homocysteine suppression by betaine: optimising the dosage regimen of betaine in homocystinuria. Br J Clin Pharmacol. 2002;54(2):140–146. doi: 10.1046/j.1365-2125.2002.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwab U., Törrönen A., Toppinen L., Alfthan G., Saarinen M., Aro A., Uusitupa M. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002;76(5):961–967. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- 10.Steenge G.R., Verhoef P., Katan M.B. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133(5):1291–1295. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 11.Olthof M.R., van Vliet T., Boelsma E., Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003;133(12):4135–4138. doi: 10.1093/jn/133.12.4135. [DOI] [PubMed] [Google Scholar]
- 12.Olthof M.R., Bots M.L., Katan M.B., Verhoef P. Effect of folic acid and betaine supplementation on flow-mediated dilation: a randomized, controlled study in healthy volunteers. PLoS Clin Trials. 2006;1(2):e10. doi: 10.1371/journal.pctr.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwab U., Alfthan G., Aro A., Uusitupa M. Long-term effect of betaine on risk factors associated with the metabolic syndrome in healthy subjects. Eur J Clin Nutr. 2011;65(1):70–76. doi: 10.1038/ejcn.2010.230. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer I.A., Verhoef P., Urgert R. Betaine supplementation and plasma homocysteine in healthy volunteers. Arch Intern Med. 2000;160(16):2546–2547. doi: 10.1001/archinte.160.16.2546-a. [DOI] [PubMed] [Google Scholar]
- 15.Alfthan G., Tapani K., Nissinen K., Saarela J., Aro A. The effect of low doses of betaine on plasma homocysteine in healthy volunteers. Br J Nutr. 2004;92(4):665–669. doi: 10.1079/bjn20041253. [DOI] [PubMed] [Google Scholar]
- 16.Olthof M.R., van Vliet T., Verhoef P., Zock P.L., Katan M.B. Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans. PLoS Med. 2005;2(5):e135. doi: 10.1371/journal.pmed.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab U., Törrönen A., Meririnne E., Saarinen M., Alfthan G., Aro A., Uusitupa M. Orally administered betaine has an acute and dose-dependent effect on serum betaine and plasma homocysteine concentrations in healthy humans. J Nutr. 2006;136(1):34–38. doi: 10.1093/jn/136.1.34. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson W., Elmslie J., Lever M., Chambers S.T., George P.M. Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am J Clin Nutr. 2008;87(3):577–585. doi: 10.1093/ajcn/87.3.577. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson W., Slow S., Elmslie J., Lever M., Chambers S.T., George P.M. Dietary and supplementary betaine: effects on betaine and homocysteine concentrations in males. Nutr Metab Cardiovasc Dis. 2009;19(11):767–773. doi: 10.1016/j.numecd.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Lawson-Yuen A., Levy H.L. The use of betaine in the treatment of elevated homocysteine. Mol Genet Metab. 2006;88(3):201–207. doi: 10.1016/j.ymgme.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Follmann D., Elliott P., Suh I., Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45(7):769–773. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 22.Wald D.S., Law M., Morris J.K. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharbanda K.K., Todero S.L., Ward B.W., Cannella J.J., Tuma D.J. Betaine administration corrects ethanol-induced defective VLDL secretion. Mol Cell Biochem. 2009;327(1–2):75–78. doi: 10.1007/s11010-009-0044-2. [DOI] [PubMed] [Google Scholar]
- 24.Menotti A., Lanti M., Nedeljkovic S., Nissinen A., Kafatos A., Kromhout D. The relationship of age, blood pressure, serum cholesterol and smoking habits with the risk of typical and atypical coronary heart disease death in the European cohorts of the Seven Countries Study. Int J Cardiol. 2006;106(2):157–163. doi: 10.1016/j.ijcard.2004.12.092. [DOI] [PubMed] [Google Scholar]
- 25.Homocysteine Lowering Trialists' Collaboration Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005;82(4):806–812. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer I.A., van Dusseldorp M., Thomas C.M., Duran M., Hautvast J.G., Eskes T.K. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomized trial. Am J Clin Nutr. 1999;69(1):99–104. doi: 10.1093/ajcn/69.1.99. [DOI] [PubMed] [Google Scholar]
- 27.van Oort F.V., Melse-Boonstra A., Brouwer I.A., Clarke R., West C.E., Katan M.B., Verhoef P. Folic acid and reduction of plasma homocysteine concentrations in older adults: a dose-response study. Am J Clin Nutr. 2003;77(5):1318–1323. doi: 10.1093/ajcn/77.5.1318. [DOI] [PubMed] [Google Scholar]
- 28.Ueland P.M., Holm P.I., Hustad S. Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin Chem Lab Med. 2005;43(10):1069–1075. doi: 10.1515/CCLM.2005.187. [DOI] [PubMed] [Google Scholar]
- 29.Melse-Boonstra A., Holm P.I., Ueland P.M., Olthof M., Clarke R., Verhoef P. Betaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrations. Am J Clin Nutr. 2005;81(6):1378–1382. doi: 10.1093/ajcn/81.6.1378. [DOI] [PubMed] [Google Scholar]
- 30.Liem A., Reynierse-Buitenwerf G.H., Zwinderman A.H., Jukema J.W., van Veldhuisen D.J. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol. 2003;41(12):2105–2113. doi: 10.1016/s0735-1097(03)00485-6. [DOI] [PubMed] [Google Scholar]
- 31.Miyaki K. Genetic polymorphisms in homocysteine metabolism and response to folate intake: a comprehensive strategy to elucidate useful genetic information. J Epidemiol. 2010;20(4):266–270. doi: 10.2188/jea.JE20100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer J., Mason J.B., Choi S.W. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care. 2009 Jan;12(1):30–36. doi: 10.1097/MCO.0b013e32831cec62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajaie S., Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86. [PMC free article] [PubMed] [Google Scholar]
- 34.Albert C.M., Cook N.R., Gaziano J.M., Zaharris E., MacFadyen J., Danielson E. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]


