Abstract
Objective
This case report describes a patient with severe lower limb spasticity treated with botulinum toxin type A (BoNT-A) and neurodynamic mobilization (NM).
Clinical Features
An 81-year-old male patient presented with a severe spastic lower limbs after total right hip replacement and severe alcoholic polyneuropathy. After the right hip replacement, he presented with generalized spasticity, crouched posture, and a large sacral pressure sore. The severe spasticity in his knees prevented walking.
Intervention and Outcome
The patient underwent combined treatment with BoNT-A and NM of the lower limb in 4 weekly applications. Evaluations were performed pretreatment, 4 weeks after the injection, and at a follow-up session 9 months after finishing treatment. We measured the following outcomes: pain by the Numerical Rating Scale, spasticity by the Modified Ashworth Scale for Grading Spasticity, acceptance and emotional reaction to the treatment by the Hospital Anxiety and Depression Scale, and functionality by ranges of motion. We found that the patient improved in all of the outcomes after treatment, and these results were maintained during the follow-up. After treatment, the patient was able to improve wound healing by properly positioning himself in bed or on his wheelchair and walking with help. At the follow-up evaluation, the results were maintained. The patient showed good acceptance and decreased anxiety/depression after treatment.
Conclusion
For this patient, the combination of NM and BoNT-A treatment decreased pain and spasticity and improved joint ranges of motion.
Key indexing terms: Botulinum toxin type A, Physiotherapy, Spasticity, Lower limb
Introduction
Alcoholic polyneuropathy is a disorder of the peripheral nervous system that interferes with sensory, motor, and autonomic nerve function. In the Western hemisphere, alcoholic peripheral neuropathy is most prevalent in the 40- to 70-year-old age group and is encountered in both men and women.1,2 Long-term excessive alcohol consumption affects the peripheral and autonomic nervous systems.1 Severe alcoholic polyneuropathy may cause difficulty walking, frequent falls, or paralysis.3
Spasticity is a state of hypertonicity with exaggeration of the tendon reflexes mediated by a loss of inhibitory control of the upper neurons.4 It is often associated with pain, spasms, and functional disability. Botulinum toxin treatment offers a targeted approach to managing spasticity. Botulinum toxin type A (BoNT-A) is a neurotoxin derived from the bacterium Clostridium botulinum. Lower limb spasticity causes severe functional limitations and pain.5-7 Botulinum toxin type A derived from C botulinum, when injected into a spastic muscle, inhibits acetylcholine release, causing blockade of the neuromuscular patches without affecting the antagonist muscles.8 Preliminary reports have suggested a role for botulinum toxin in the treatment of spasticity because it can reduce the degree of spasticity in both the upper and lower limbs.4 Botulinum toxin type A has already been shown to be effective in reducing poststroke upper limb spasticity in some double-blind, placebo-controlled, randomized, controlled trials.8-12 Moreover, BoNT-A has been used in conjunction with physiotherapeutics to improve function and decrease pain13,14 and spasticity.6,15,16
Neurodynamic mobilization (NM) is a form of manual therapy that directs force to neural structures through positioning and movement of multiple joints.17
Preliminary evidence suggests that NM interventions claim to improve pain and function in patients with neural symptoms.18,19 However, NM in combination with BoNT-A therapy has never been presented as a treatment for patients with polyneuropathy-related spasticity; and whether there are effects on anxiety and depression using these techniques has not been studied. Therefore, this case report describes a patient with severe lower limb spasticity and severe alcoholic polyneuropathy who was treated with BoNT-A and NM and the possible effects on anxiety and depression, particularly in regard to reducing distress and facilitating patient compliance.
Case report
An 81-year-old white man, weighing 57 kg with a height of 165 cm, presented with severe alcoholic polyneuropathy and severe spasticity of both lower limbs after prolonged immobilization due to total right hip replacement (RHT) surgery. The alcoholic polyneuropathy condition had been previously diagnosed and showed sensory, motor, and autonomic nerve function sensory disorders associated with this condition. Currently, the patient has not shown nutrient deficiencies (in laboratory tests). He was bedridden and caregiver dependent because of multiple dynamic contractures of both legs. Spasticity in his knees prevented ischial sitting, and he developed a pressure sore over the sacral region because of the prolonged crouched position. The patient did not walk for 1 year. He had generalized pain in his legs that was movement related, was aching, and increased during spastic cramps. Opioid analgesics were not effective, and his sleep was disturbed. In addition to the direct toxic effect of alcohol on the autonomic and peripheral nerves, the symptoms of alcoholic polyneuropathy also result from nutrient deficiencies. Verbal and written consents were obtained. Evaluations were performed before the BoNT-A inoculation, at the end of NM treatment (4 weeks after the injection), and 9 months after the end of treatment. The patient's response to therapy was monitored using the following assessment tools: the Numerical Rating Scale (NRS) to measure pain intensity,20,21 the Modified Ashworth Scale (MAS) for grading spasticity,22,23 the Hospital Anxiety and Depression Scale (HADS) to measure acceptance and emotional reaction to the treatment,24,25 and range of motion (ROM) with a goniometer to measure functionality.26 On pretreatment, the NRS was 7, the MAS scores at both hamstrings and adductors were 5, the HADS was 5, and both knees’ passive ROM (extension) was − 90° and active ROM was − 120°.
The treatment consisted of 1 BoNT-A injection (Dysport; Ipsen SpA, Milan, Italy) by intramuscular injection to the hamstring and adductor muscles of both legs (1000 IU). The provider of the BoNT-A injection is a medical doctor with prescriptive rights. The contents of each vial were reconstituted with 2.0-mL sodium chloride injection BP (0.9%) to give a total injection volume of 8.0 mL. The provider of the BoNT-A injection is a medical doctor with prescriptive rights. Muscles were located by palpation and were injected at the standard site used for electromyography (EMG).7 The NM technique consisted of a sliding mobilization of the sciatic nerve. The NM treatment started the day after the BoNT-A injection and continued for 4 sessions per week for a total period of 4 weeks. At each session, the NM techniques were applied to the injured lower limb 3 times for a 4-minute period with a 1-minute pause between each application. Neurodynamic mobilization of the sciatic nerve consisted of the following movements: (1) proximal slider, a straight leg raise, and the application of plantar flexion without producing symptoms and (2) distal slider; during the phase in which the leg is lowered, the foot is dorsiflexed.27,28 These motions were alternated at a rate of approximately 2 seconds per cycle (1 second into extension and 1 second into flexion). Neurodynamic mobilization was followed by stretching the lower limb extensor muscles for 20 seconds each (both hamstrings and both adductors) to prevent contractures (Fig 1).
Fig 1.
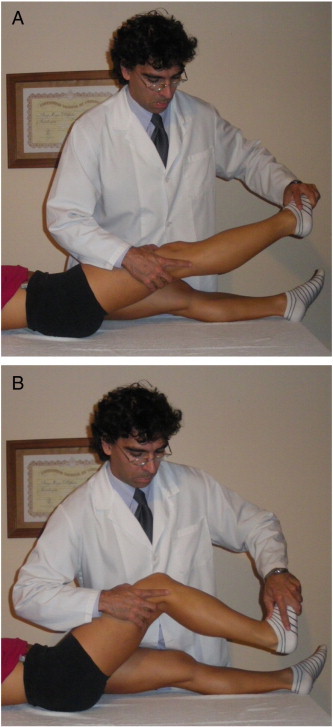
A, Proximal slider, straight leg raise, and application of plantar-flexion without producing symptoms. B, Distal slider, leg flexed, foot dorsiflexed. (Color version of figure is available online.)
The outcomes are summarized in Table 1. The combined therapy produced clinical changes, with major improvements in function and patient comfort. The patient's clinical presentation was improved following treatment when compared with his clearly deteriorated lower limb function after RHT. The patient's treatment compliance was 100% with full treatment tolerance. In addition, signs of anxiety or depression decreased during and after treatment.
Table 1.
Changes in outcome measures of the patient (pretreatment, posttreatment, and follow-up)
| Pretreatment (0 wk) | Posttreatment (4 wk) | Follow-up (10 mo) | |
|---|---|---|---|
| MAS | |||
| Both hamstrings | 5 | 2 | 1 |
| Both adductors | 5 | 2 | 1 |
| HADS | |||
| AS | 5 | 1 | 1 |
| DS | 5 | 2 | 2 |
| NRS | 7 | 2 | 1 |
| Passive both ROM knees | |||
| Passive extension | − 90° | − 20° | − 15° |
AS, anxiety scale; DS, depression scale; HADS, Hospital Anxiety and Depression Scale; MAS, Modified Ashworth Scale; NRS, Numerical Rating Scale; ROM, range of motion.
Spasticity also decreased after treatment. The MAS scores at the hamstrings and adductors were 2 and 1, respectively. In addition, after 9 months of follow-up, the hamstrings and adductor muscles both decreased to 1. Therefore, the spasticity was reduced by 60% by the end of the treatment and by 80% during the follow-up period.
Passive ROM improved after treatment. At baseline, the patient's both knees’ ROM (extension) was − 90°. In contrast, 4 weeks after the BoNT-A injection, the knees’ ROM increased to − 20°. At the follow-up, these results were maintained; and the knees’ ROM was − 15°, indicating a further increase in functionality (Table 1). The patient was able to sleep better (Fig 2), feed himself using adaptive aids, and transfer himself with minimal assistance.
Fig 2.
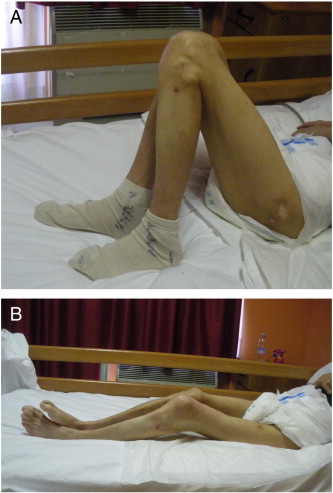
Applied combinatory treatment. A, Lower limb: view of the patient before injection. B, Lower limb: view of the patient 2 weeks after injection. (Color version of figure is available online.)
Similarly, his pain intensity decreased. At baseline, the patient's NRS was 7. His NRS score improved to 2 after 4 weeks of treatment. In addition, after 9 months of follow-up, his pain levels were maintained at 1. Therefore, general pain was decreased by 71% by the end of treatment and by 86% at the end of the follow-up period.
The anxiety and depression components were also reduced. The HADS scores for anxiety and depression were 5 and 5, respectively. In contrast, after 4 weeks of treatment, anxiety and depression decreased to 1 and 2, respectively. In addition, 9 months following the formal treatment period, the HADS scores for anxiety and depression were maintained at 1 and 2, respectively.
Four weeks after the BoNT-A injection, the patient could better position himself in a wheelchair and walk with a care provider (Fig 3); he also had reduced spasm-related pain and improved mobility and activities of daily living. The sacral pressure sore began to heal, indicated by a measured improvement in the National Pressure Ulcer Advisory Panel's staging system from 2 to 1. It healed completely a few months later.
Fig 3.
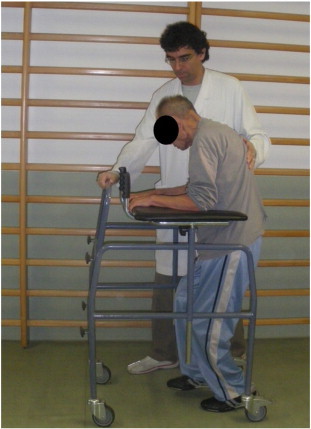
After treatment, the patient was able to improve walking with help. (Color version of figure is available online.)
Discussion
The pain and spasticity experienced by the patient were unchanged by the use of standard therapies (eg, pharmacological therapy, stretching, passive mobilization); and 4 weeks after treatment, the patient had not improved. However, the application of BoNT-A in conjunction with NM of the sciatic nerve brought about a reduction of pain and spasticity. A follow-up visit 9 months after treatment completion confirmed that the patient had improved. The sacral pressure sore has been improved after spasticity reduction and better positioning, and it healed completely a few months later.
Various treatment modalities have been reported to be useful for severe spasticity and related disorders, but there is no consensus on the best therapeutic option.29,30 Ryuji Kaji et al8 found that BoNT-A significantly reduced spasticity in the lower limb muscles in a study in which 120 patients with lower limb spasticity were randomized to a single treatment with 300 U BoNT-A or placebo. The application of Dysport (BoNT-A) in 74 patients reduced the degree of hip adductor spasticity associated with multiple sclerosis.4 The effects of BoNT-A extended beyond spasticity reduction in a man who became quadriplegic, bed bound, and caregiver dependent secondary to the development of cervical osteomyelitis.31 Botulinum toxin type A alone was shown to be effective for reducing spasticity in several double-blind, placebo-controlled, randomized controlled trials.8-12
Botulinum toxin type A was shown to be an effective treatment of painful localized contractures in RHT, even without central nervous system lesions.32 Therefore, BoNT-A may be a useful option for painful localized contractures that do not respond to standard treatment.32
Several studies investigating the effects of benfotiamine (a lipid-soluble derivative of vitamin B1 with high bioavailability) on polyneuropathy have shown significant improvements in vibration perception threshold,33 nerve conduction velocity,34 neuropathy score, and pain.35 These studies highlight the improvements in sensory and motor function in patients with polyneuropathy when treated with mono- or polyvitamin formulations. A specific vitamin B complex (with and without folic acid) significantly improved symptoms of alcoholic polyneuropathy over a 12-week treatment period; malnutrition is common in alcoholic persons.36
Neurodynamic mobilization could have led to improvements in ROM because sliding techniques are aimed at inducing biomechanical effects that permit the optimal movement of the nerve and its surrounding tissues. Regarding these effects, there is supporting evidence from postmortem studies showing that a sliding technique for the median nerve could improve longitudinal nerve excursion.17 Both of the NM techniques used in this study were effective for reducing pain in previous studies in participants with thumb carpometacarpal osteoarthritis.18,19 Some neurophysiological mechanisms can be used to explain the effects of NM on pain and spasticity. For instance, NM is hypothesized to stimulate the periaqueductal gray in a key area in the descending and ascending control of nociception.37 However, future studies are required to validate this theory for NM.
To our knowledge, this is the first report in which NM of the sciatic nerve in combination with BoNT-A therapy was shown to have promising results by improving spasticity and ROM. On the whole, our results are consistent with previous work by our group and others showing that the intervention had an immediate effect on mechanical restoration of function, decreased pain, and increased general relief of the patient.
Our findings suggest that BoNT-A in combination with NM may be useful for improving spasticity and quality of life in patients who develop lower limp spasticity after alcoholic polyneuropathy. This preliminary finding opens avenues for future research in the modulation of pain and spasticity pathways, perhaps offering targets to optimize manual and physical therapies for pain and spasticity management in alcoholic polyneuropathy. The results of this case are promising enough to justify further clinical studies.
Limitations
The results of combined NM and BoNT-A for spasticity reported in this study cannot be generalized to other patients. A limitation of this case report may be the lack of a functionality outcome measurement. As with any case report, the findings may be attributable to the natural history of the condition or concurrent life events.
Conclusion
For this elderly patient, the combination of NM and BoNT-A treatment decreased pain and spasticity and improved joint ROMs. At the follow-up evaluation, the same results were maintained. In addition, the patient showed good acceptance and decreased anxiety and/or depression after treatment. The treatment seemed to facilitate his rehabilitation; and its effects extended beyond spasticity and pain reduction, including acceptance and decreased anxiety/depression after treatment.
Funding sources and potential conflicts of interest
No external funding sources or conflicts of interest were reported for this study.
References
- 1.Zambelis T., Karandreas N., Tzavellas E., Kokotis P., Liappas J. Large and small fiber neuropathy in chronic alcohol-dependent subjects. J Peripher Nerv Syst. 2005;10(4):375–381. doi: 10.1111/j.1085-9489.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Ammendola A., Tata M.R., Aurilio C., Ciccone G., Gemini D., Ammendola E. Peripheral neuropathy in chronic alcoholism: a retrospective cross-sectional study in 76 subjects. Alcohol Alcohol. 2001;36(3):271–275. doi: 10.1093/alcalc/36.3.271. [DOI] [PubMed] [Google Scholar]
- 3.Peters T.J., Kotowicz J., Nyka W., Kozubski W., Kuznetsov V., Vanderbist F. Treatment of alcoholic polyneuropathy with vitamin B complex: a randomised controlled trial. Alcohol Alcohol. 2006;41(6):636–642. doi: 10.1093/alcalc/agl058. [DOI] [PubMed] [Google Scholar]
- 4.Hyman N., Barnes M., Bhakta B., Cozens A., Bakheit M., Kreczy-Kleedorfer B. Botulinum toxin (Dysport) treatment of hip adductor spasticity in multiple sclerosis: a prospective, randomised, double blind, placebo controlled, dose ranging study. J Neurol Neurosurg Psychiatry. 2000;68(6):707–712. doi: 10.1136/jnnp.68.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim J.Y., Koh J.H., Paik N.J. Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: a randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke. 2008;39(1):126–131. doi: 10.1161/STROKEAHA.107.484048. [DOI] [PubMed] [Google Scholar]
- 6.Yelnik A.P., Colle F.M., Bonan I.V., Vicaut E. Treatment of shoulder pain in spastic hemiplegia by reducing spasticity of the subscapular muscle: a randomised, double blind, placebo controlled study of botulinum toxin A. J Neurol Neurosurg Psychiatry. 2007;78(8):845–848. doi: 10.1136/jnnp.2006.103341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakta B.B., Cozens J.A., Bamford J.M., Chamberlain M.A. Use of botulinum toxin in stroke patients with severe upper limb spasticity. J Neurol Neurosurg Psychiatry. 1996;61(1):30–35. doi: 10.1136/jnnp.61.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaji R., Osako Y., Suyama K., Maeda T., Uechi Y., Iwasaki M. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J Neurol. 2010;257(8):1330–1337. doi: 10.1007/s00415-010-5526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson D.M., Alexander D.N., O'Brien C.F., Tagliati M., Aswad A.S., Leon J.M. Botulinum toxin type A in the treatment of upper extremity spasticity: a randomized, double-blind, placebo-controlled trial. Neurology. 1996;46(5):1306–1310. doi: 10.1212/wnl.46.5.1306. [DOI] [PubMed] [Google Scholar]
- 10.Bakheit A.M., Thilmann A.F., Ward A.B., Poewe W., Wissel J., Muller J. A randomized, double-blind, placebo-controlled, dose-ranging study to compare the efficacy and safety of three doses of botulinum toxin type A (Dysport) with placebo in upper limb spasticity after stroke. Stroke. 2000;31(10):2402–2406. doi: 10.1161/01.str.31.10.2402. [DOI] [PubMed] [Google Scholar]
- 11.Bhakta B.B., Cozens J.A., Chamberlain M.A., Bamford J.M. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: a randomised double blind placebo controlled trial. J Neurol Neurosurg Psychiatry. 2000;69(2):217–221. doi: 10.1136/jnnp.69.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrory P., Turner-Stokes L., Baguley I.J., De Graaff S., Katrak P., Sandanam J. Botulinum toxin A for treatment of upper limb spasticity following stroke: a multi-centre randomized placebo-controlled study of the effects on quality of life and other person-centred outcomes. J Rehabil Med. 2009;41(7):536–544. doi: 10.2340/16501977-0366. [DOI] [PubMed] [Google Scholar]
- 13.Prager E.M., Birkenmeier R.L., Lang C.E. Exploring expectations for upper-extremity motor treatment in people after stroke: a secondary analysis. Am J Occup Ther. 2011;65(4):437–444. doi: 10.5014/ajot.2010.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers H., Shaw L., Price C., van Wijck F., Barnes M., Graham L. Study design and methods of the BoTULS trial: a randomised controlled trial to evaluate the clinical effect and cost effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Trials. 2008;9:59. [Google Scholar]
- 15.Sun S.F., Hsu C.W., Hwang C.W., Hsu P.T., Wang J.L., Yang C.L. Application of combined botulinum toxin type A and modified constraint-induced movement therapy for an individual with chronic upper-extremity spasticity after stroke. Phys Ther. 2006;86(10):1387–1397. doi: 10.2522/ptj.20050262. [DOI] [PubMed] [Google Scholar]
- 16.Wolf S.L., Winstein C.J., Miller J.P., Taub E., Uswatte G., Morris D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 17.Coppieters M.W., Butler D.S. Do 'sliders' slide and 'tensioners' tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther. 2008 Jun;13(3):213–221. doi: 10.1016/j.math.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Villafañe J.H., Silva G.B., Bishop M.D., Fernandez-Carnero J. Radial nerve mobilization decreases pain sensitivity and improves motor performance in patients with thumb carpometacarpal osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2012;93(3):396–403. doi: 10.1016/j.apmr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Villafañe J.H., Silva G.B., Fernandez-Carnero J. Short-term effects of neurodynamic mobilization in 15 patients with secondary thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther. 2011;34(7):449–456. doi: 10.1016/j.jmpt.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Price D.D., Bush F.M., Long S., Harkins S.W. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994 Feb;56(2):217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 21.Wood B.M., Nicholas M.K., Blyth F., Asghari A., Gibson S. Assessing pain in older people with persistent pain: the NRS is valid but only provides part of the picture. J Pain. 2010;11(12):1259–1266. doi: 10.1016/j.jpain.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Kong K.H., Chua K.S., Lee J. Symptomatic upper limb spasticity in patients with chronic stroke attending a rehabilitation clinic: frequency, clinical correlates and predictors. J Rehabil Med. 2010;42(5):453–457. doi: 10.2340/16501977-0545. [DOI] [PubMed] [Google Scholar]
- 23.Lundstrom E., Smits A., Terent A., Borg J. Time-course and determinants of spasticity during the first six months following first-ever stroke. J Rehabil Med. 2010;42(4):296–301. doi: 10.2340/16501977-0509. [DOI] [PubMed] [Google Scholar]
- 24.Mykletun A., Stordal E., Dahl A.A. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Snaith R.P. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adrienne C., Manigandan C. Inpatient occupational therapists hand-splinting practice for clients with stroke: A cross-sectional survey from Ireland. J Neurosci Rural Pract. 2011;2(2):141–149. doi: 10.4103/0976-3147.83579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shacklock M. Clinical neurodynamics. Ed Elsevier. 2005.
- 28.Butler D, Jones M, Gore R. Mobilisation of the nervous system. Ed Churchill Livingstone. 1991.
- 29.Villafañe J.H., Silva G.B., Chiarotto A., Ragusa Orazio L.F. Botulinum toxin type A combined with neurodynamic mobilization for upper limb spasticity after stroke. J Chiropr Med. 2012;11(3):186–191. doi: 10.1016/j.jcm.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villafañe J.H., Fernandez-de-las-Peñas C., Pillastrini P. Botulinum toxin type-A combined with manual therapy at the cervical spine for masseteric hypertrophy: a case report. J Chiropr Med. 2012;11(4):280–285. doi: 10.1016/j.jcm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naicker A.S., Roohi S.A., Chan J.L. Botulinum toxin type A for rehabilitation after a spinal cord injury: a case report. J Orthop Surg (Hong Kong) 2009;17(1):96–99. doi: 10.1177/230949900901700121. [DOI] [PubMed] [Google Scholar]
- 32.Bertoni M., Castagna A., Baricich A., Berti G., Lazzaretti S., Morandi C. Administration of type A botulinum toxin after total hip replacement. Eur J Phys Rehabil Med. 2008;44(4):461–465. [PubMed] [Google Scholar]
- 33.Woelk H., Lehrl S., Bitsch R., Kopcke W. Benfotiamine in treatment of alcoholic polyneuropathy: an 8-week randomized controlled study. Alcohol Alcohol. 1998;33(6):631–638. doi: 10.1093/alcalc/33.6.631. [DOI] [PubMed] [Google Scholar]
- 34.Stracke H., Lindemann A., Federlin K. A benfotiamine vitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes. 1996;104(4):311–316. doi: 10.1055/s-0029-1211460. [DOI] [PubMed] [Google Scholar]
- 35.Haupt E., Ledermann H., Kopcke W. Benfotiamine in the treatment of diabetic polyneuropathy—a three-week randomized, controlled pilot study (BEDIP study) Int J Clin Pharmacol Ther. 2005;43(2):71–77. doi: 10.5414/cpp43071. [DOI] [PubMed] [Google Scholar]
- 36.Peters T.J., Kotowicz J., Nyka W., Kozubski W., Kuznetsov V., Vanderbist F., de Niet S., Marcereuil D., Coffiner M. Treatment of alcoholic polyneuropathy with vitamin B complex: a randomised controlled trial. Alcohol Alcohol. 2006;41(6):636–642. doi: 10.1093/alcalc/agl058. [DOI] [PubMed] [Google Scholar]
- 37.Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther. 1995 Nov;1(1):11–16. doi: 10.1054/math.1995.0244. [DOI] [PubMed] [Google Scholar]


