Background: Lgr4 as a transmembrane receptor binds with R-spondins to modulate Wnt signaling.
Results: Deletion of Lgr4 in mice leads to loss of epithelial barrier function and high susceptibility to inflammatory bowel disease.
Conclusion: Lgr4-mediated Wnt/β-catenin signaling is required for intestinal homeostasis and regeneration.
Significance: Lgr4 has potential implications for diagnosis or drug discovery for human colitis.
Keywords: G Protein-coupled Receptors (GPCR), Inflammatory Bowel Disease, Regeneration, Stem Cells, Wnt Signaling
Abstract
Lgr4/Gpr48 is one of the newly identified R-spondins receptors and potentiates Wnt signaling, which regulates intestinal homeostasis. We used a hypomorphic mouse strain to determine the role of Lgr4 in intestinal inflammation and recovery. Intestinal inflammation was induced with dextran sulfate sodium (DSS) followed by a recovery period. Intestinal inflammation symptoms and molecular mechanisms were examined. We found that Lgr4−/− mice exhibited dramatically higher susceptibility to and mortality from DSS-induced inflammatory bowel disease than WT mice. Lgr4 deficiency resulted in greatly reduced numbers of either Paneth cells or stem cells in the intestine. During the intestinal regeneration process, cell proliferation but not apoptosis of intestinal epithelial cells was significantly impaired in Lgr4−/− mice. When Wnt/β-catenin signaling was reactivated by crossing with APCmin/+ mice or by treating with a GSK-3β inhibitor, the number of Paneth cells was partially restored and the mortality caused by DSS-induced inflammatory bowel disease was strikingly reduced in Lgr4-deficient animals. Thus, Lgr4 is critically involved in the maintenance of intestinal homeostasis and protection against inflammatory bowel disease through modulation of the Wnt/β-catenin signaling pathway.
Introduction
Inflammatory bowel disease (IBD)4 refers to several inflammatory conditions of the colon and small intestine. Clinically, there are two main forms of IBD: Crohn disease, which can affect any part of the gastrointestinal tract, and ulcerative colitis, which is restricted to the colon and the rectum (1, 2). The precise etiology of IBD remains unclear, but several factors have been identified to play major roles in intestinal homeostasis, including microbial defense, innate/adaptive immunity, and autophagy among others (3, 4). Disorders of barrier function and epithelial restitution are the major intrinsic pathogenic factors that are regulated by specific signaling pathways in response to harmful microenvironmental factors.
In mammals, intestinal epithelial cells establish tight contacts with numerous adjacent epithelial cells to form a single layer that functions as a physical barrier (5). The intestinal epithelium undergoes constant cell turnover through shedding into the intestinal lumen and is the most vigorously renewing adult tissue. Like other somatic stem cells, multipotent intestinal stem cells (ISCs) constitute a long-lived population of cells that have both self-renewal and differentiation abilities (6). Bmi1 and Lgr5 mark two distinct types of ISCs based on their location and cycling properties (7–10). Both of these two populations give rise to all the differentiated cell lineages of the intestinal epithelium, and the interconversion between +4 (quiescent) and crypt base columnar (fast-cycling) stem cells in their niches was reported (7–9). ISCs and their transit-amplifying daughter cells give rise to enterocytes, goblet cells, and enteroendocrine cells that occupy the villi and are renewed about every 3–5 days in mice (5). Paneth cells are terminally differentiated cells that move opposite to the crypt-villus flow and reside in the bottom of crypts for several weeks as a potential niche for ISCs (11). In addition, Paneth cells secrete numerous antimicrobial peptides, which include lysozymes, defensins, cathelicidins, and RegIIIγ, and play important roles in innate immune defense (12, 13). Several studies on mice deficient in different Crohn disease-associated genes involved in reactive oxygen species (14) generation, autophagy, and endoplasmic reticulum stress have implicated Paneth cell dysfunction in susceptibility to intestinal inflammation (15, 16).
As defective epithelial restitution is an important risk factor for IBD, it is not surprising that dysfunction of genes involved in intestinal development, proliferation, and differentiation will increase susceptibility to IBD. The Wnt/β-catenin signaling cascade is the single most dominant pathway in controlling proliferation and differentiation of intestinal epithelial cells (17). Genetic variants of TCF4, a Wnt signaling pathway transcription factor that is critical for the maintenance of the crypt progenitor phenotype, have been reported to be associated with Crohn disease (18). However, little is known about how Wnt signaling functions during intestinal inflammation.
Lgr4, also known as Gpr48, belongs to the leucine-rich, G protein-coupled receptor (LGR) family (19). Genetically modified mouse models have demonstrated that Lgr4 plays broad roles in embryonic development as well as postnatal physiological processes in multiple organs (20–22). With a gene trap strategy, we obtained an Lgr4 hypomorphic mouse strain, and 40% of Lgr4 hypomorphic mice are viable with no significant reduction of lifespan. Using this unique strain, we demonstrated that Lgr4 plays important roles in various organs, including the liver, reproductive tract, bone, and eye (23–25). Recently, Lgr4 and its homologue Lgr5 have been identified as receptors of R-spondins, secreted Wnt pathway agonists and potentiators of Wnt/β-catenin and Wnt/PCP signaling (14, 26). In the intestine, Lgr4 is required for Paneth cell differentiation (27) and maintenance of intestinal stem cells (26). Here, we demonstrate that Lgr4-deficient mice are more susceptible to DSS-induced colitis. When Wnt/β-catenin signaling is reactivated by crossing with APCmin/+ mice or by treating with a GSK-3β inhibitor, the decreased number of Paneth cell was partially restored and the mortality caused by DSS-induced IBD was dramatically reduced in Lgr4 mutant mice. Taken together, these results underscore the importance of Lgr4-mediated Wnt signaling in intestinal homeostasis and inflammation.
EXPERIMENTAL PROCEDURES
Mouse Lines
Lgr4−/− mice, initially generated on a mixed 129×C57BL/6 background as described previously (25), were backcrossed with C57BL/6 mice for at least six generations at the beginning of this study. Heterozygous mice were intercrossed to generate homozygous Lgr4−/− mice and wild-type littermate controls. Lgr5-EGFP-IRES-creERT2 mice (Stock Number 008875) were obtained from The Jackson Laboratory. APCmin mice (Stock Number J002020) were obtained from the National Resource Center of Mutant Mice (NRCMM). All experiments conformed to the regulations drafted by the Association for Assessment and Accreditation of Laboratory Animal Care in Shanghai and were approved by the East China Normal University Center for Animal Research.
Induction of DSS-induced Inflammation
Acute colitis was induced in mice by the administration of 2.5% (w/v) DSS (molecular mass, 36–50 kDa; MP Biomedicals, Irvine, CA) in the drinking water for 5 days and then changing to regular drinking water.
Colonic Injury Scoring
Entire colons were rolled up and fixed in 4% paraformaldehyde, embedded in paraffin, and stained with hematoxylin/eosin. The degree of colonic injury was assessed by a histology scoring system (28) with minor modifications. Briefly, the score was performed with a combined score of inflammatory cell infiltration (score, 0–3), loss of crypts (score, 0–4), and tissue damage (score, 0–6). The inflammatory cell infiltration score was defined as follows: 0 = inflammatory cells occasionally presenting in the lamina propria, 1 = increased number of inflammatory cells in the lamina propria, 2 = confluence of inflammatory cells, extending into the submucosa, 3 = transmural extension of the infiltrate. Loss of crypts: 0 = no involvement, 1 = <25%, 2 = <50%, 3 = <75%, 4 = <100%. Tissue damage including parameter of mucosal ulceration and depth of injury, for mucosal ulceration: 0 = no injury, 1 = focal injury, 2 = multifocal injury, 3 = diffuse ulceration/infiltration; for the depth of injury: 0 = no injury, 1 = mucosal involvement only, 2 = mucosal and submucosal involvement, 3 = transmural involvement.
Bone Marrow Transplantation
Wild-type (WT) mice were irradiated with 9 grays, received donor bone marrow (107 cells) by injection from the vein of tail, and then were reconstituted with donor bone marrow for 8 weeks. The success of bone marrow reconstitution was confirmed by PCR.
β-Galactosidase (LacZ) Staining
To perform LacZ staining, intestine was washed with ice-cold LacZ fixture buffer (2% formaldehyde, 0.2% glutaraldehyde, 0.02% Nonidet P-40 in PBS) and then incubated for 2 h in fixing buffer at 4 °C on a shaking platform. After washing in LacZ washing buffer (2 mm MgCl2, 0.01% deoxycholate, 0.02% Nonidet P-40 in PBS, pH 8.0) twice (10 min each time), intestines were incubated in LacZ staining buffer (0.5 mg/ml X-gal dissolved in LacZ wash buffer) overnight at room temperature. Tissues were then fixed and sectioned for histological analysis.
Immunohistochemistry (IHC)
The intestine was dissected and flushed gently with cold 2% paraformaldehyde and rolled up into a compact circle and then fixed in 4% paraformaldehyde-PBS at 4 °C in the dark on a rolling platform, dehydrated, embedded in paraffin, and cut into 4-μm sections. Sections were deparaffinized in xylene and rehydrated in gradient alcohols. Endogenous peroxidase activity was quenched with 3% H2O2 in methanol for 20 min, and sections were washed in PBS. Antigen retrieval was performed by boiling slides in 10 mm sodium citrate buffer, pH 6.0, or 10 mm Tris base, 1 mm EDTA solution, pH 9.0. Sections were blocked with 1% BSA and incubated with the primary antibodies overnight at 4 °C. After incubation with secondary antibody for 30 min, sections were developed with diaminobenzidine and counterstained with hematoxylin and then dehydrated and mounted in neutral resins. For BrdU staining, a similar procedure was performed with the addition of a 30-min treatment with 2 n HCl after antigen retrieval. Antibodies were as follows: lysozyme (1:2000; Dako), β-catenin (1:100; BD Transduction Laboratories), c-Myc (1:100; Santa Cruz Biotechnology), cyclin D1 (1:100; Cell Signaling), phospho-S6 (1:400; Cell Signaling), cleaved caspase3 (1:200; Cell Signaling), Ki67 (1:2000; NeoMarker), and Bmi1 (1:2000; Santa Cruz Biotechnology).
Flow Cytometry
A standardized 3-cm segment of duodenum was isolated, exposed longitudinally, and washed with cold PBS. Then tissue was chopped into ∼5-mm pieces, washed with cold PBS, and incubated in 5 mm EDTA in PBS for 5 min at room temperature. The tissue fragments were suspended vigorously, and the supernatant, including most villi, was discarded. The sediment was resuspended with 5 mm EDTA in PBS and incubated for 30 min on ice. After vigorous resuspension, the supernatant was filtered through a 70-μm cell mesh (BD Bioscience) and incubated in 1 mg/ml trypsin for 30 min at room temperature. For FACS, dead cells were excluded by scatter characteristics and propidium iodide. Lgr5+ ISCs were identified by their endogenous GFP expression.
Electron Microscopy
Small intestine tissue samples were fixed with 2.5% glutaraldehyde and postfixed in 1% osmium tetroxide in 100 mm phosphate buffer. Tissue was dehydrated, embedded in epoxy resin, and visualized with a JEOL transmission electron microscope at 120 kV (model JEM-1210).
Quantitative RT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen). RNA concentration was determined spectrophotometrically, and quality was assessed by agarose electrophoresis. cDNA was synthesized with the SuperScript RNase H2 reverse transcriptase kit (Invitrogen) using 2.5 mm random hexamers (Invitrogen). Real-time PCR was performed using SYBR Green probe with the iCycler IQ5 (Bio-Rad).
Statistical Analysis
Data are expressed as means ± S.E. Means of two groups were compared using Student's t test (unpaired, two-tailed). Comparisons between multiple groups were made using one-way analysis of variance followed by a post hoc Bonferroni's correction, and p < 0.05 was considered to be statistically significant. Unless indicated in the figure legends, all the experiments were performed at least three times with similar results.
RESULTS
Lgr4 in the Intestinal Epithelium Protects Mice from Susceptibility and Mortality to DSS-induced IBD
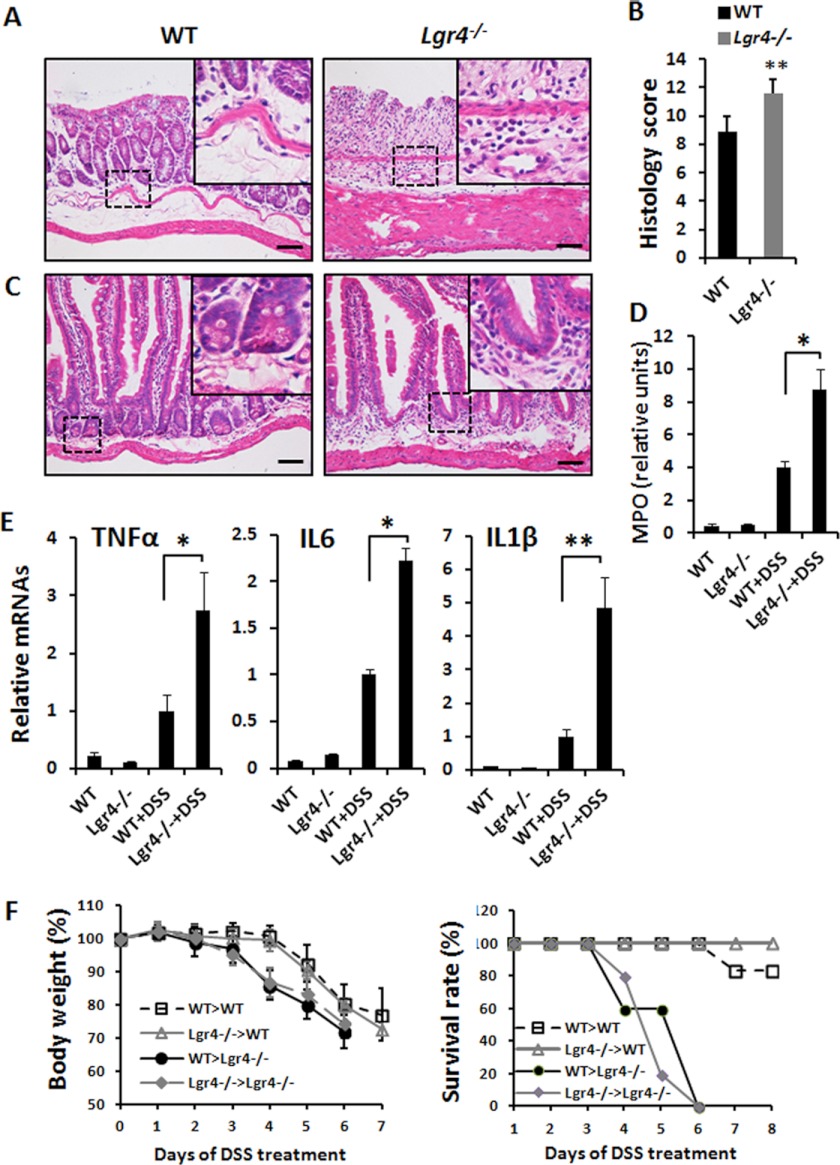
To explore the roles of Lgr4 in intestinal inflammation, Lgr4 hypomorphic mice were subjected to the DSS-induced experimental IBD model. Notably, after DSS administration, a more severe colitis developed in Lgr4 mutant mice than in WT sex-matched littermates. Strikingly, following 5 days of treatment with 2.5% DSS, no mortality was observed in WT mice during the experimental period; however, all Lgr4 mutant mice died within 8 days after treatment (Fig. 1A). None of the Lgr4 mutant mice could survive even when we decreased the DSS concentration to 1% (data not shown). Higher body weight loss, a hallmark of intestinal inflammation, was observed from day 4 in Lgr4 mutant mice, concordant with a more severe anemia as reflected by the decreased circulating peripheral blood hematocrit concentration (Fig. 1, B and C). Moreover, an increased daily activity index (as described in Table 1) was observed in Lgr4 mutant mice, indicating more severe clinical symptoms (Fig. 1D). Although the small intestine is not the major target of DSS-induced tissue damage (29), the relative length reduction after DSS-induced inflammation was significantly increased in the small intestine of Lgr4 mutant mice (Fig. 1E), indicating critical functions of Lgr4 in the small intestine. Histological examination showed dramatically increased signs of colitis, which is characterized by the loss of crypts and the infiltration of leukocytes into the colons of Lgr4 mutant mice (Fig. 2, A and B). Accordingly, we found that almost all crypts throughout the small intestine were lost in Lgr4 mutant mice but remained intact in WT littermates (Fig. 2C). Infiltration of neutrophils in the colon was quantified by the determination of MPO activities. At day 8, MPO levels were significantly higher in Lgr4 mutant mice than in WT mice (Fig. 2D), which is consistent with the increase of leukocytes visualized in H&E-stained sections (Fig. 2A). Additionally, inflammatory cytokines such as TNFα, IL6, and IL1β were significantly increased in Lgr4 mutant mice after DSS administration (Fig. 2E), suggesting a more severe inflammatory response in Lgr4-deficient mice.
FIGURE 1.
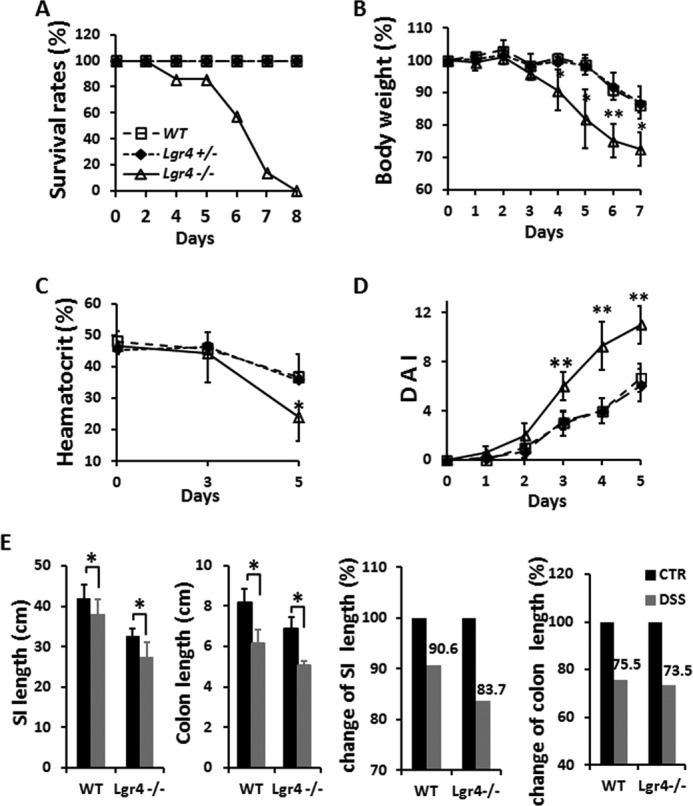
Deletion of Lgr4 increases susceptibility and mortality to DSS-induced inflammation. Mice were subjected to DSS-induced colitis for 5 days followed by regular drinking water. A–D, survival rate (A), body weight (B), hematocrit values of peripheral blood (C), and mean disease activity index (DAI) (D) were determined. WT, n = 10; Lgr4+/−, n = 6; Lgr4−/−, n = 12. Data are means ± S.E. *, p < 0.01; **, p < 0.001. Mice were euthanized, the entire intestine was obtained in both WT and Lgr4 mutant mice. The lengths of small intestines and colons were measured. E, the alteration of the length of both small intestines and colons was analyzed after DSS treatment when compared with regular drinking water control mice. SI, small intestine; CTR, control; DSS, 2.5% DSS in drinking water for 5 days followed by 3 days of regular drinking water. n ≥ 3 for each group. Data are means ± S.E.
TABLE 1.
Scoring of the disease activity index
The disease activity index is combined with weight loss, stool consistency, and rectal bleeding, leading to a maximum disease activity index of 12.
| Score | Weight loss | Stool consistency | Bleeding |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1–5% | Normal | Normal |
| 2 | 5–10% | Loose | Hemoccult + |
| 3 | 10–20% | Loose | Hemoccult + |
| 4 | >20% | Diarrhea | Gross bleeding |
FIGURE 2.
More severe intestine inflammation in DSS-induced Lgr4−/− mice. A and C, H&E staining revealed more severe disease activity in both colon (A) and jejunum (C) of Lgr4−/− mice on day 8. Note more infiltration of leukocytes in Lgr4 mutant mice. Scale bars: 50 μm. B, the histology score was measured. D, colon MPO activity as a measure of neutrophil infiltration. E, real-time PCR analysis revealed the relative expression of cytokines in colonic mucosa of WT and Lgr4−/− mice at day 5 of DSS administration. Data are means ± S.E. *, p < 0.01; **, p < 0.001. n ≥ 5. F, mice were reconstituted with donor bone marrow for 8 weeks, and then bowel inflammation was induced by 2.5% DSS (w/v) in drinking water for 5 days followed by normal drinking water; weight loss and survival rate were monitored every day. WT recipient mice (n = 6) and Lgr4−/− recipient mice (n = 5) were both studied. Data are means ± S.E.
Lgr4 in Nonhematopoietic Cells Is More Important for Protection against DSS-induced IBD
To determine the cell populations that are critical for Lgr4-dependent protection against DSS-induced bowel inflammation, bone marrow transplantation was employed to determine the contribution of immune and/or nonimmune cells to the IBD phenotype observed in Lgr4−/− mice. After 8 weeks of bone marrow reconstitution, bowel inflammation was induced by DSS administration. Interestingly, WT mice reconstituted with Lgr4−/− bone marrow did not exhibit an exacerbated inflammation response when compared with WT mice transplanted with WT bone marrow (Fig. 2F). Upon DSS-induced colitis, the body weight was decreased more dramatically in Lgr4−/− recipients than that of WT recipients despite the genotype of transplanted bone marrow. Furthermore, all the Lgr4−/− recipients died in 6 days, but most of the WT recipients survived (Fig. 2F). These results indicate that Lgr4 expression in nonhematopoietic cells is more important for protection against DSS-induced IBD.
Impaired Proliferation but Not Apoptosis of Intestinal Crypts in Lgr4 Mutant Mice during Tissue Regeneration
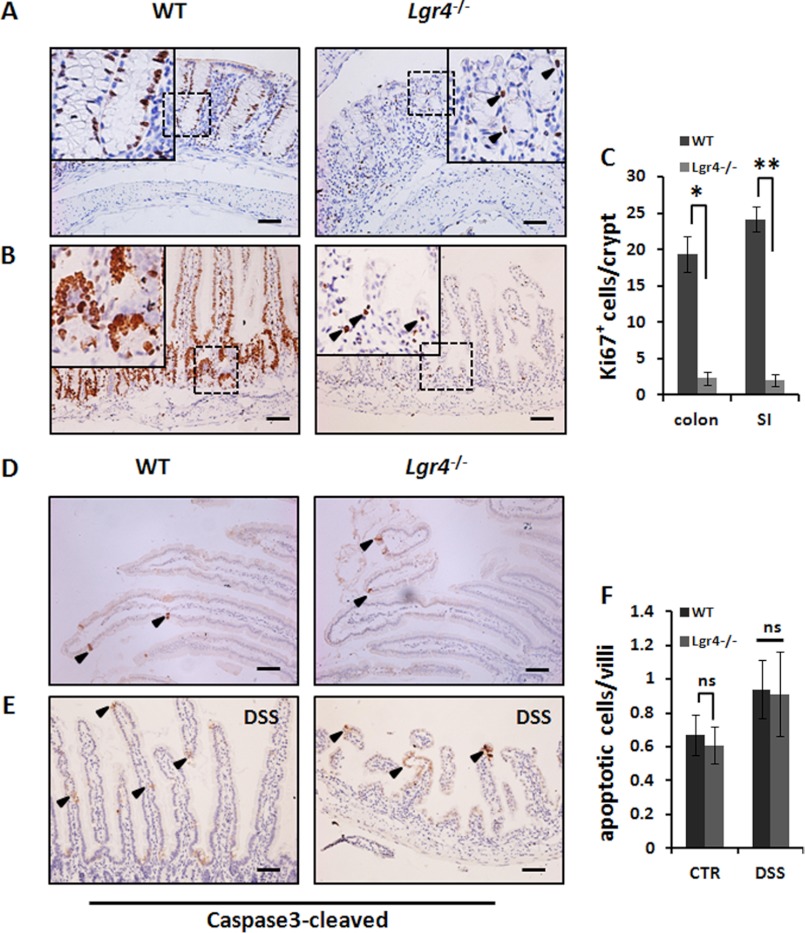
Lgr4 mutant mice exhibited much more severe disease symptoms and disrupted intestinal integrity in DSS-induced intestinal inflammation, suggesting a potential role of Lgr4 in regulation of epithelial cell behavior. In the DSS-induced IBD model, after 5 days of DSS treatment, the intestinal tissue transits to regeneration process. By IHC against Ki67-positive proliferating cells, we observed significantly decreased cell proliferation in Lgr4 mutant intestines during tissue regeneration (Fig. 3, A–C). However, we observed no significant alteration of apoptosis either in normal condition (Fig. 3, D and F) or in the recovery period between Lgr4 mutant and WT mice (Fig. 3, E and F). These results indicated that Lgr4 is responsible for epithelial cell proliferation but not apoptosis during DSS-induced tissue regeneration.
FIGURE 3.
Impaired proliferation but not apoptosis of intestinal crypts in Lgr4 mutant mice during recovery. A and B, colon (A) and jejunum (B) sections from WT and Lgr4−/− mice on day 8 of DSS-induced colitis; proliferation was identified by Ki67 staining (arrowhead). C, the numbers of Ki67-positive cells were analyzed. SI, small intestine. 40–60 crypts per mouse were examined; n = 3 per group. Data are means ± S.E. *, p < 0.01; **, p < 0.001. D and E, cleaved caspase3-positive cells (arrowhead) were detected through IHC with specific antibodies in mice in normal conditions (D) or after treatment with DSS (E). Scale bars: 50 μm. F, statistic indicated the numbers of apoptosis cells in WT and Lgr4−/− mice. CTR, control; DSS, 5 days of treatment with DSS followed by 3 days recovery. Data are means ± S.E. ns, not significant.
Lgr4 Is Required for Intestinal Homeostasis under Normal Conditions
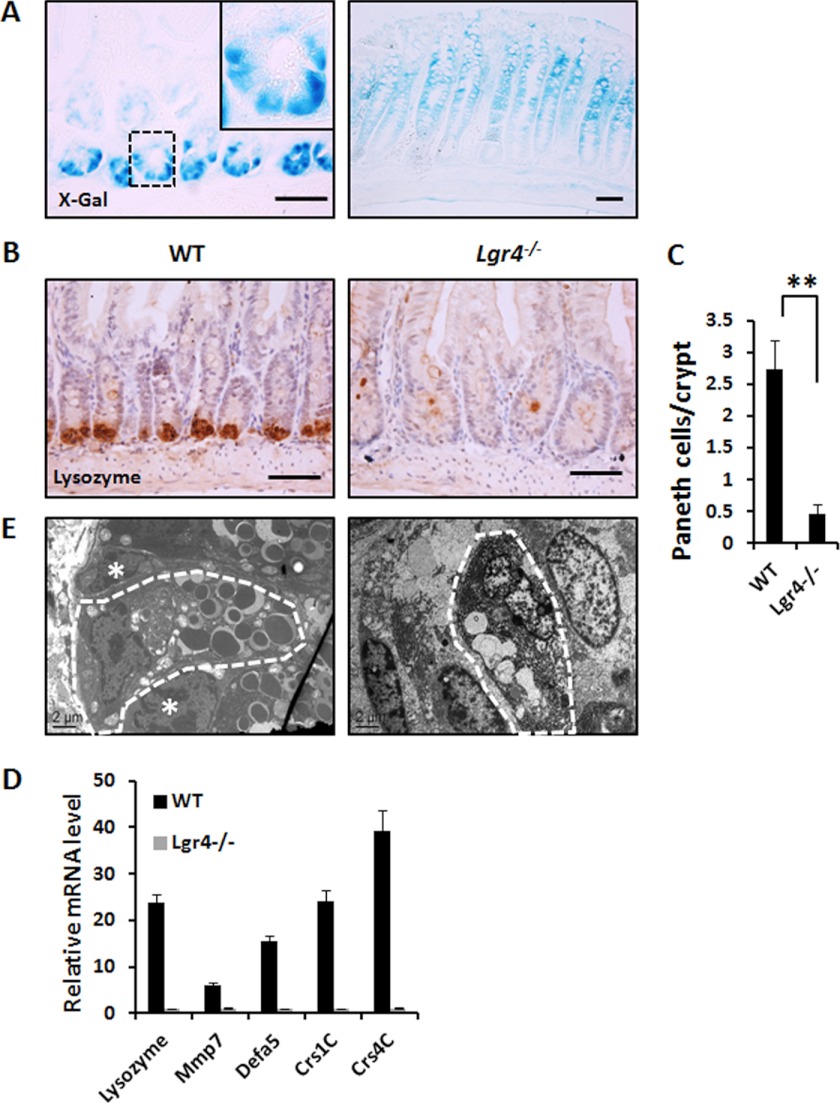
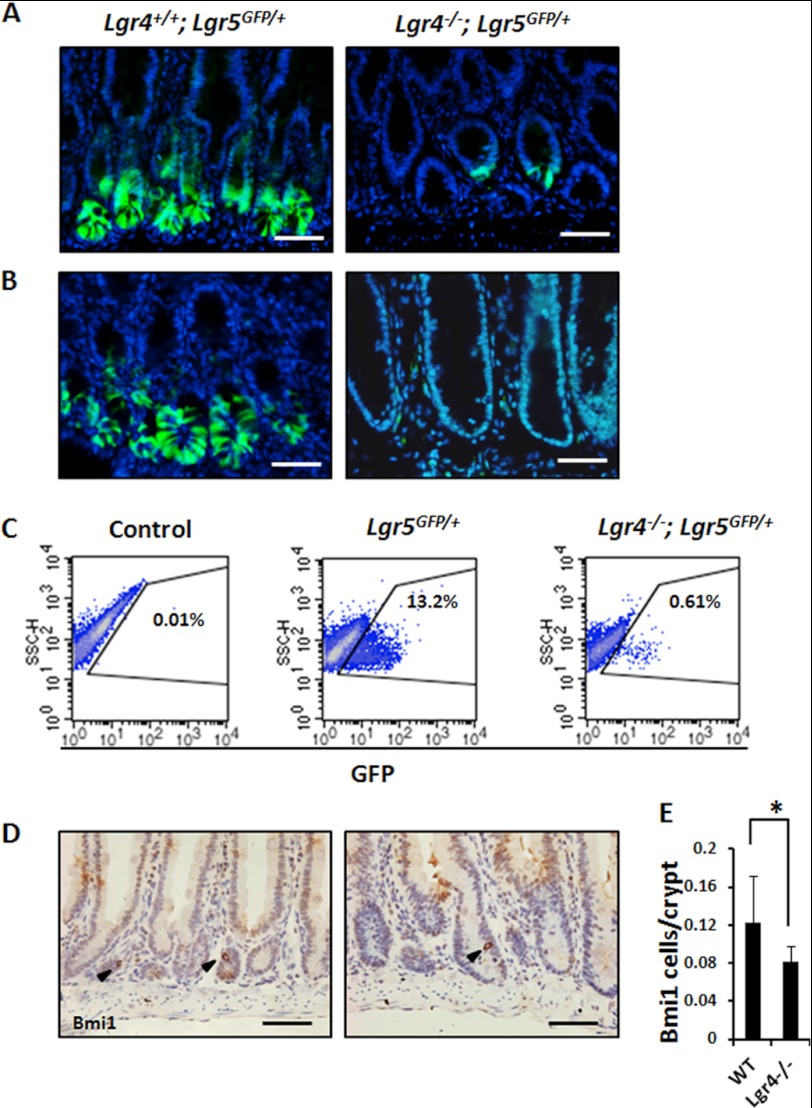
Taking advantage of our gene-trapped mice with the LacZ reporter integrated in the first intron of the Lgr4 locus (25), we examined the expression pattern of Lgr4 in the intestine by X-gal staining in heterozygous mice. In the small intestine, dense staining signals were observed in Paneth cells, which are easily distinguished by large secretory granules in the cytoplasm. In the colon, Lgr4 was mainly expressed in the zone of transit-amplifying cells (Fig. 4A). By using an unambiguous Paneth cell marker, we observed that the number of Paneth cells was 80–85% lower in Lgr4 mutant mice when compared with WT mice, which is consistent with a previous study (27) (Fig. 4, B and C). Furthermore, the mRNA levels of Paneth-specific antimicrobial genes, such as Lyz2, Mmp7, Defa5, Crs1c, and Crs4c, were also dramatically down-regulated (Fig. 4D). Transmission electron microscopy also confirmed that the ultrastructure of the few remaining Paneth cells in mutant mice was obviously altered, suggesting that the secretory function of the remaining Paneth cells in Lgr4 mutant mice was largely impaired (Fig. 4E). With different strategies, we also confirmed that the number of Lgr5+ ISCs was dramatically reduced (Fig. 5, A–C). Furthermore, we found that Bmi1+ cells were decreased slightly but significantly in Lgr4 mutant mice (Fig. 5, D and E). However, the other intestine cell types including Goblet cells and enteroendocrine cells were not significantly altered between WT and Lgr4 mutant mice (data not shown). These data suggest that Lgr4 modulates both Lgr5+ and Bmi1+ ISCs either directly or indirectly to maintain intestinal homeostasis, and the deficiencies of ISCs in Lgr4 mutant mice may be a reason for the impaired cell proliferation and intestine integrity during DSS-induced inflammation.
FIGURE 4.
Lgr4 is required for differentiation of Paneth cells. A, whole-mount X-gal staining was performed on the ileum (left) and colon (right) of Lgr4+/− mice; note the granular particles observed in Paneth cells in the enlargement. B, Paneth cells were assessed by IHC staining with anti-lysozyme antibody. C, the number of Paneth cells in mice was analyzed by IHC with anti-lysozyme antibody. 40–60 crypts per mouse were examined; n = 5 per group. Data are means ± S.E. **, p < 0.001. D, quantitative real-time PCR analysis of the expression level of Paneth cell marker genes. Data are means ± S.E. E, ultrastructure of Paneth cells was explored with electron microscopy. Crypt base columnar cells are easily recognized (white asterisks), and Paneth cells are indicated by the white dashed borders.
FIGURE 5.
Lgr4 is required for maintenance of intestinal stem cells in adult mice. A and B, endogenous GFP (green) driven by Lgr5 promoter indicates crypt base columnar stem cells in duodenum (A) or colon (B) of Lgr5GFP/+;Lgr4+/+(left) and Lgr5GFP/+;Lgr4−/− mice (right). C, FACS analysis demonstrated that the ratio of GFP-positive ISCs was significantly decreased in Lgr5GFP/+;Lgr4−/− mice. Lgr5+/− percentage was 13.2 ± 1.62% when compared with Lgr5+/−;Lgr4−/− (0.61 ± 0.11%). D, duodenum sections from WT or Lgr4−/− mice were prepared. Bmi1+ ISCs were detected by IHCs with Bmi1-specific antibodies (arrowhead). E, statistical data indicating the number of Bmi1+ ISCs in WT and Lgr4−/− mice. 300 crypts per mouse from four different pairs of WT and Lgr4−/− mice were examined. Data are means ± S.E. *, p < 0.01. Scale bars: 50 μm
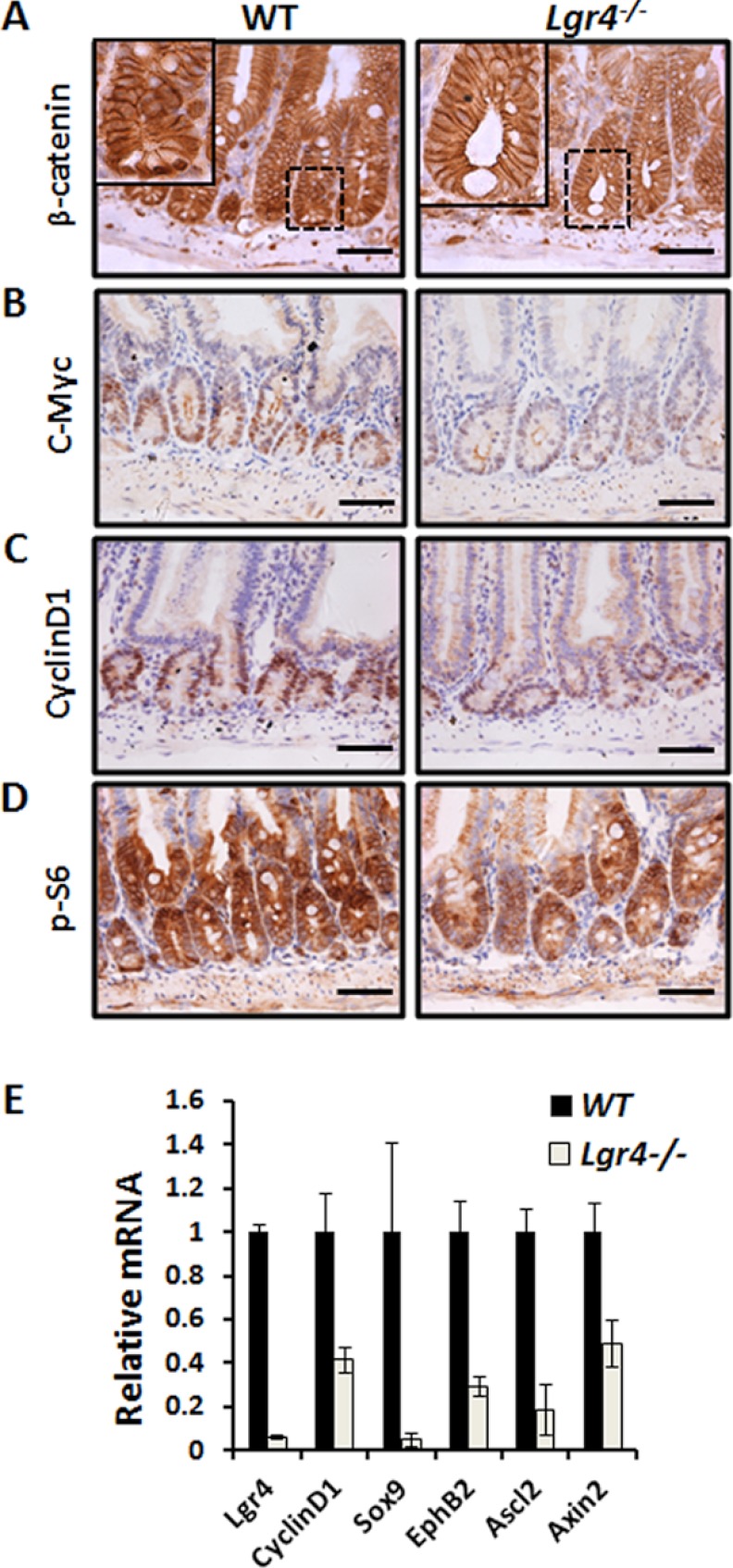
Lgr4 Deficiency Impairs Wnt/β-Catenin Signaling in Intestinal Epithelial Cells
Lgr4 is one of the receptors of R-spondins that amplify the Wnt/β-catenin signaling pathway (26, 30). Given that Wnt/β-catenin signaling is required for intestinal homeostasis and ISC self-renewal (31), we examined how Lgr4 regulates the activity of Wnt signaling in Lgr4 mutant mice. Using immunohistochemical staining, we found markedly fewer cells with nucleus-localized β-catenin (active form) at the bottom of crypts in Lgr4 mutant mice, whereas higher Wnt/β-catenin activity was observed in the intestinal epithelium in WT mice (Fig. 6A). The protein levels of the direct Wnt target genes, such as c-Myc and cyclin D1, were both down-regulated in Lgr4−/− mice (Fig. 6, B and C). Moreover, the activity of mTOR signaling, which is demonstrated to be downstream in the β-catenin pathway, was also inhibited, as shown by the decreased phospho-S6 level in Lgr4 mutants (Fig. 6D). To determine whether Lgr4 affects the mRNA levels of the target genes, we evaluated the expression of β-catenin target genes, including EphB2, Axin2, Ccnd1, Ascl2, and Sox9, using quantitative real-time PCR analysis. Our data indicate the mRNA levels of all these target genes decreased in Lgr4 mutant mice (Fig. 6E), suggesting that Lgr4 is required for the maintenance of Wnt/β-catenin signaling in the intestinal epithelium.
FIGURE 6.
Down-regulation of Wnt target genes in Lgr4−/− mice. A, nuclear β-catenin was observed at the bottom of crypts in WT mice (left) but not in those of Lgr4−/− mice (right). B and C, the Wnt/β-catenin targets, c-Myc and CylinD1, were dramatically down-regulated in Lgr4−/− mice as detected by IHC. D, mTOR downstream target, phospho-S6 (p-s6), was decreased in Lgr4 mutant mice. Scale bars: 50 μm. E, quantitative real-time PCR analysis of mRNA levels of Wnt target genes in WT and Lgr4−/− littermates. Data are means ± S.E.
The Defect of Lgr4−/− Mice Is Partially Rescued by the Reactivation of the Wnt/β-Catenin Signaling Cascade
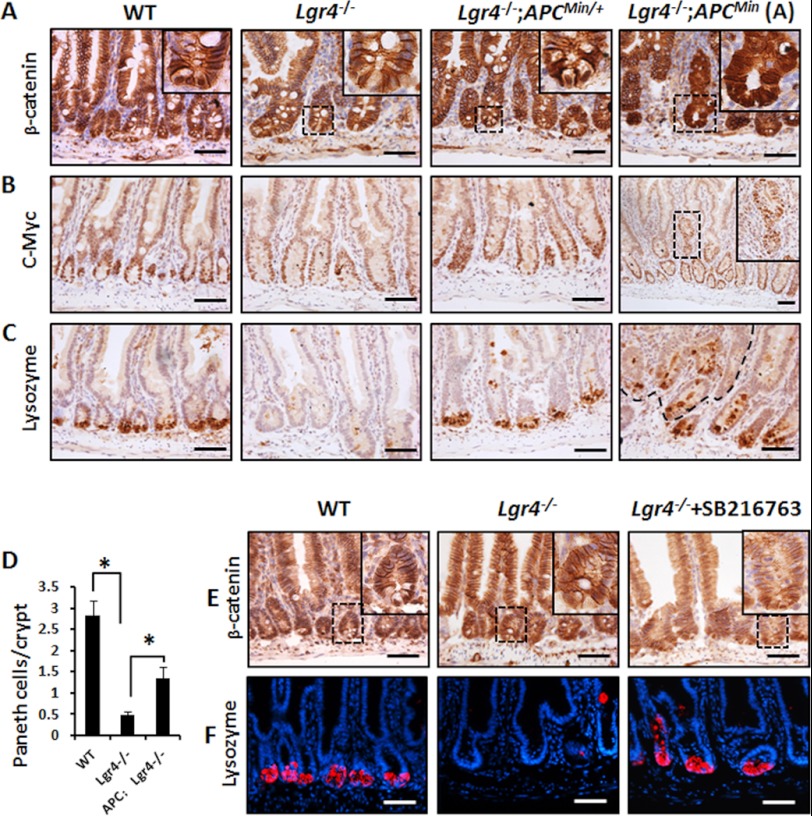
To determine whether the functions of Lgr4 in intestinal homeostasis and inflammation are mediated by the Wnt/β-catenin signaling pathway, we generated Lgr4−/−;APCmin/+ mice and examined the effects of APC mutation in restoring the defects caused by Lgr4 deletion. APCmin/+ mice contain a germ line mutation in the APC gene, which spontaneously activates β-catenin. As shown in Fig. 7A, mutation of APC partially restored the activation of β-catenin signaling in Lgr4−/−;APCmin/+ mice. The number of cells with nucleus-localized β-catenin increased significantly in Lgr4−/−;APCmin/+ mice. Furthermore, the APC mutation increased the expression of c-Myc in the Lgr4−/− crypts (Fig. 7B), suggesting reactivation of the Wnt/β-catenin target genes. Moreover, the number of Paneth cells was restored in Lgr4−/−;APCmin/+ mice when compared with Lgr4 mutant mice (Fig. 7, C and D). Interestingly, the deficiency of Lgr4 in Apcmin/+ background did not delay or decrease adenoma formation (Fig. 7, A–C, right panel). To further confirm that Lgr4 functions through Wnt/β-catenin signaling in intestines, we reactivated β-catenin by administration of a GSK-3β inhibitor SB216763 in Lgr4 mutant mice. As shown in Fig. 7E, the addition of SB216763 partially restored the nuclear localization and activation of β-catenin in Lgr4 mutant mice. Moreover, treatment of SB216763 largely restored the number of lysozyme-positive Paneth cells (Fig. 7F). These data suggest that reactivation of Wnt/β-catenin signaling in Lgr4 mutant mice partially rescued the intestinal homeostasis defects, and we also hypothesize that Wnt signaling reactivation helps protect the mice from intestinal inflammation.
FIGURE 7.
The phenotypes are partially restored in Wnt/β-catenin reactivated Lgr4 mutant mice. A, in Lgr4−/−;APCmin/+ double mutant mice, Wnt target genes were reactivated. The number of cells with active (nucleus-localized) β-catenin was increased in Lgr4−/−;APCmin/+ when compared with Lgr4−/− mice. B, the expression of c-Myc was increased in crypts in Lgr4−/−;APCmin/+ mice. C, IHC with antibody against lysozyme indicated the recovery of Paneth cells in the epithelium of Lgr4−/−;APCmin/+ mice. The right panel presents the microadenoma in small intestines. D, the number of Paneth cells in mice was analyzed by IHC with lysozyme-specific antibody. 40–60 crypts per mouse were examined; n = 3 per group. Data are means ± S.E. *, p < 0.01. E and F, Lgr4−/− mice received 2 mg/kg of SB216763 intraperitoneally every day for 5 days and were euthanized on day 8. Sections of the small intestine from mice of the indicated genotypes were stained with β-catenin antibody (E) or lysozyme (F). Scale bars: 50 μm.
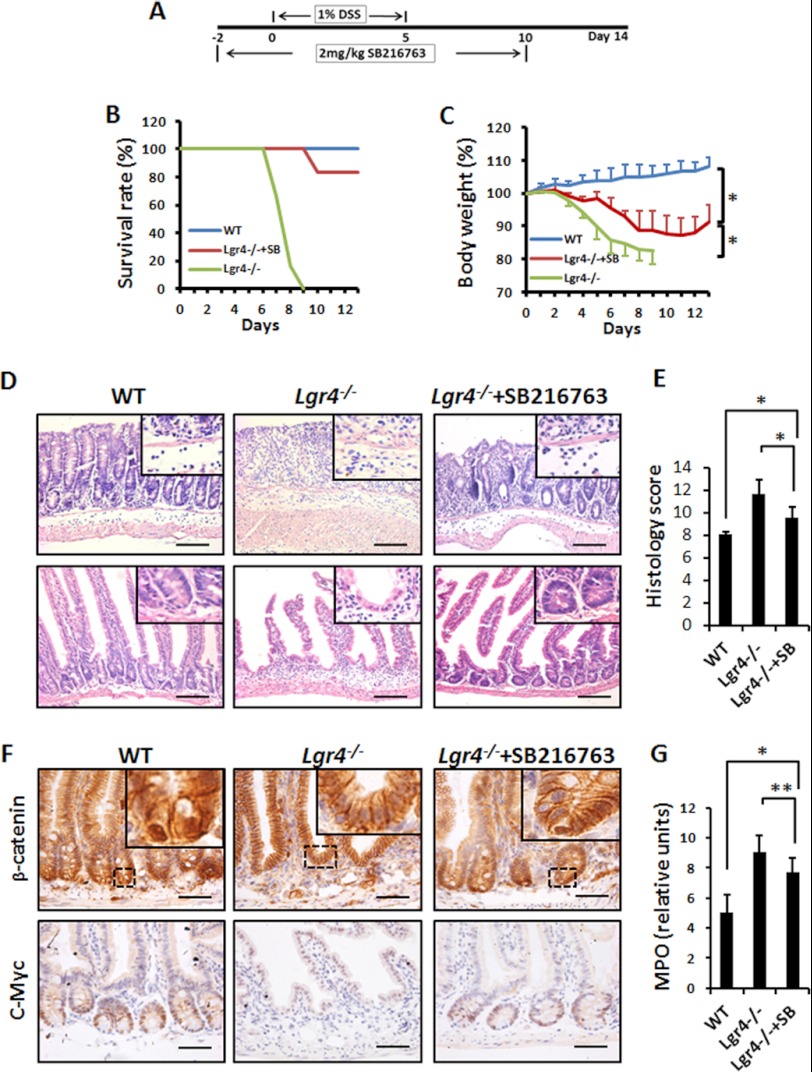
To address this issue, we pretreated Lgr4 mutant mice with SB216763 for 2 days and then subjected them to DSS treatment to induce IBD (Fig. 8A). The survival rate was significantly increased when compared with Lgr4 mutant mice injected with vehicle (83% versus 0) (Fig. 8B). The body weight of SB216763-treated Lgr4−/− mice increased slightly from days 10 to 11, indicating the improvement from colitis (Fig. 8C), which was also confirmed by histology analysis (Fig. 8, D and E). Accordingly, we observed reactivated Wnt/β-catenin signaling in SB216763-treated Lgr4−/− mice (Fig. 8F), and the infiltration of neutrophils also reduced in SB216763-treated Lgr4−/− mice when compared with controls through histological analysis (Fig. 8D, insets) and MPO studies (Fig. 8G). These observations strongly suggest that the defects in Lgr4−/− mice are mainly due to hypoactivation of Wnt/β-catenin signaling, and support the hypothesis that Lgr4 is a key receptor in intestinal homeostasis and inflammation.
FIGURE 8.
Inhibition of GSK-3β activity ameliorates experimental colitis in Lgr4 mutant mice. A, scheme of treatment with SB216763 in Lgr4−/− mice during DSS-induced colitis. B and C, Lgr4−/− mice were treated with SB216763 and then subjected to 1% DSS-induced colitis for 5 days. Survival rates (B) and body weight changes (C) were monitored. SB, SB216763. n = 6 per group. Data are means ± S.E. *, p < 0.01. D and E, on day 8 of DSS-induced colitis, intestinal sections from mice of WT, Lgr4−/−, and Lgr4−/− treated with SB216763 were stained with H&E (insets indicated the infiltration of leukocytes) (D), and the histological score was measured (E) (n = 3 per group). Data are means ± S.E. *, p < 0.01. F, IHC with antibodies of β-catenin and c-Myc indicates reactivation in SB216763-treated Lgr4−/− mice. Scale bars: 100 μm. G, colon MPO activity was measured for neutrophil infiltration. n = 3 per group. Data are means ± S.E. *, p < 0.01; **, p < 0.001.
DISCUSSION
Our studies demonstrate that Lgr4 plays a crucial role in recovery from inflammatory bowel disease. Using an Lgr4 hypomorphic gene trap mouse strain (25), we found a striking susceptibility to DSS-induced IBD in Lgr4−/− mice. We confirmed that Lgr4 deficiency causes a dramatic loss of Paneth cells as well as Lgr5+ ISCs in mice. Furthermore, when Wnt/β-catenin signaling is reactivated by using APCmin/+Lgr4−/− mice or by treating with a GSK-3β inhibitor, the number of Paneth cells was partially restored, and the mortality caused by DSS-induced IBD was strikingly reduced. Moreover, adenoma initiation is not altered between APCmin/+ and APCmin/+;Lgr4−/− double mutant mice, suggesting that not only is Lgr4 regulation of the Wnt/β-catenin signaling pathway important for intestinal homeostasis and wound repair, but also that Lgr4 functions upstream of the APC/GSK-3β complex.
Lgr4 is one of the receptors for R-spondin proteins that synergize with low levels of Wnt to enhance Wnt signaling activity by inhibition of ZNRF3-mediated turnover of frizzled and LRP6 (32). In Tcf4−/− neonatal mice, the crypt progenitor compartment is entirely absent (33), but in adult Lgr4−/− mice, only Paneth cells and Lgr5+ ISCs are affected. Because Lgr4 binds to R-spondins and is responsible for the hyperactivation of Wnt signaling, this suggests that Paneth cells and Lgr5+ ISCs are dependent on higher Wnt activities than other compartments of the crypt. Upon DSS-induced intestinal inflammation, all Lgr4 mutant mice died within 8 days even at the low DSS concentration of 1%, which usually does not induce significant IBD symptoms in wild-type mice. These observations again suggested that higher Wnt signaling activity is critical for intestinal regeneration and protection against IBD.
Lgr4 is required for Paneth cell differentiation and the maintenance of Lgr5+ ISCs in normal conditions (26, 27). Recently, several processes have been highlighted to be important to Paneth cell biology and susceptibility to IBD in both humans and mice, such as the regulation of necroptosis, granule exocytosis, and the endoplasmic reticulum stress response (15, 16, 34). However, ablation of Paneth cells in mice did not produce detectable effects on the proliferation or terminal differentiation programs of the other three lineages or on host-microbial interactions (35). We also found that loss of Paneth cells did not cause spontaneous inflammation. It is seemed that loss of Paneth cells in IBD patients is probably not the cause of IBD. We speculate that under stressed conditions such as DSS treatment, the severity of IBD in Lgr4 mutant mice is mainly due to the dysfunction of the epithelial barrier because during the recovery process, Lgr4 mutant mice exhibited dramatically decreased epithelial proliferation when compared with WT mice (Fig. 4). Additionally, although we have not examined the function of Lgr5+ ISCs during intestinal inflammation and regeneration, the decreased number of Lgr5+ ISCs in Lgr4 mutant mice might decrease epithelial cell turnover, which further leads to the loss of epithelial integrity because this stem cell is the source of epithelial cells in the intestine.
Interestingly, we observed a slightly decreased number of Bmi1+ ISCs, which is different from the studies suggesting that the number of Bmi1+ cells was elevated when Lgr5-positive stem cells were depleted (36, 37). Because the Lgr4 mutant mouse model is different from the Paneth cell depletion models, we speculate that Lgr4 could regulate Bmi1+ stem cell numbers, which was supported by Mustata et al. (27), who demonstrated that the Bmi1 mRNA level was decreased in Lgr4 mutant crypts. Moreover, Yan et al. (36) reported that Lgr5+ ISCs were dependent on R-spondin1, which is also essential for in vitro culture of Bmi1+ ISCs.
The Wnt/β-catenin signaling pathway has an essential role not only in intestinal development but also in homeostasis and regeneration after injury (31). A notable feature of intestinal inflammation is a persistently increased expression of mucosal cytokines that modulate intestinal homeostasis in a biphasic manner (38, 39). For example, at the onset of inflammation, INF-γ activates β-catenin through the PI3K/AKT pathway and promotes epithelial proliferation, but activation of β-catenin in turn facilitates the induction of the secreted Wnt antagonist Dkk1 to inhibit cell proliferation and promote apoptosis (38). Deletion of Dkk1 induces a strong proliferative response that promotes wound repair after colitis (40). Moreover, PI3K signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis (41). These studies reveal that Wnt/β-catenin signaling is an important regulator of inflammatory cytokine responses and homeostasis during inflammation (17). In the subsequent regeneration process, Wnt/β-catenin signaling also plays an important role by activating its downstream targets such as c-Myc and mTOR (42).
In summary, we have demonstrated that Lgr4 is essential for the maintenance of intestinal homeostasis and protection from DSS-induced IBD in mice. Using different strategies, we also demonstrated that Lgr4 directly modulates Wnt/β-catenin signaling in animal models. Because Paneth cell dysfunction or abnormal expression of Paneth cell-associated genes was reported to be correlated with both intestinal inflammation and cancer (34, 43), the identification of the essential roles of Lgr4 in IBD represents an advance that has implications for diagnosis or potential drug targets for human colitis.
Acknowledgments
We thank Meizhen Liu for technical assistance. We also thank Stefan Siwko for comments and advice.
This work was partially supported by grants from the State Key Development Programs of China (Grants 2012CB910400 and 2010CB945403), the National Natural Science Foundation of China (Grants 31171318 and 30930055), and the Science and Technology Commission of Shanghai Municipality (Grant 11DZ2260300).
This article was selected as a Paper of the Week.
- IBD
- inflammatory bowel disease
- ISC
- intestinal stem cell
- APC
- adenomatous polyposis coli
- DSS
- dextran sulfate sodium
- LGR
- leucine-rich, G protein-coupled receptor
- IHC
- immunohistochemistry
- MPO
- myeloperoxidase
- GSK-3β
- glycogen synthase kinase-3β
- mTOR
- mammalian target of rapamycin
- X-gal
- 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside.
REFERENCES
- 1. Kaser A., Zeissig S., Blumberg R. S. (2010) Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Podolsky D. K. (2002) Inflammatory bowel disease. N. Engl. J. Med. 347, 417–429 [DOI] [PubMed] [Google Scholar]
- 3. Maloy K. J., Powrie F. (2011) Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 [DOI] [PubMed] [Google Scholar]
- 4. Khor B., Gardet A., Xavier R. J. (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barker N., van de Wetering M., Clevers H. (2008) The intestinal stem cell. Genes Dev. 22, 1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weissman I. L. (2000) Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168 [DOI] [PubMed] [Google Scholar]
- 7. Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., Clevers H. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 8. Sangiorgi E., Capecchi M. R. (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeda N., Jain R., LeBoeuf M. R., Wang Q., Lu M. M., Epstein J. A. (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334, 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Flier L. G., van Gijn M. E., Hatzis P., Kujala P., Haegebarth A., Stange D. E., Begthel H., van den Born M., Guryev V., Oving I., van Es J. H., Barker N., Peters P. J., van de Wetering M., Clevers H. (2009) Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 [DOI] [PubMed] [Google Scholar]
- 11. Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., Clevers H. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaishnava S., Behrendt C. L., Ismail A. S., Eckmann L., Hooper L. V. (2008) Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. U.S.A. 105, 20858–20863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cash H. L., Whitham C. V., Behrendt C. L., Hooper L. V. (2006) Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glinka A., Dolde C., Kirsch N., Huang Y. L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C. M., Niehrs C. (2011) LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cadwell K., Liu J. Y., Brown S. L., Miyoshi H., Loh J., Lennerz J. K., Kishi C., Kc W., Carrero J. A., Hunt S., Stone C. D., Brunt E. M., Xavier R. J., Sleckman B. P., Li E., Mizushima N., Stappenbeck T. S., Virgin H. W., 4th (2008) A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Lau W., Barker N., Clevers H. (2007) WNT signaling in the normal intestine and colorectal cancer. Front. Biosci. 12, 471–491 [DOI] [PubMed] [Google Scholar]
- 18. Koslowski M. J., Kübler I., Chamaillard M., Schaeffeler E., Reinisch W., Wang G., Beisner J., Teml A., Peyrin-Biroulet L., Winter S., Herrlinger K. R., Rutgeerts P., Vermeire S., Cooney R., Fellermann K., Jewell D., Bevins C. L., Schwab M., Stange E. F., Wehkamp J. (2009) Genetic variants of Wnt transcription factor TCF-4 (TCF7L2) putative promoter region are associated with small intestinal Crohn's disease. PLoS One 4, e4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu S. Y., Kudo M., Chen T., Nakabayashi K., Bhalla A., van der Spek P. J., van Duin M., Hsueh A. J. (2000) The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol. Endocrinol. 14, 1257–1271 [DOI] [PubMed] [Google Scholar]
- 20. Mazerbourg S., Bouley D. M., Sudo S., Klein C. A., Zhang J. V., Kawamura K., Goodrich L. V., Rayburn H., Tessier-Lavigne M., Hsueh A. J. (2004) Leucine-rich repeat-containing, G protein-coupled receptor 4 null mice exhibit intrauterine growth retardation associated with embryonic and perinatal lethality. Mol. Endocrinol. 18, 2241–2254 [DOI] [PubMed] [Google Scholar]
- 21. Mendive F., Laurent P., Van Schoore G., Skarnes W., Pochet R., Vassart G. (2006) Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Dev. Biol. 290, 421–434 [DOI] [PubMed] [Google Scholar]
- 22. Song H., Luo J., Luo W., Weng J., Wang Z., Li B., Li D., Liu M. (2008) Inactivation of G-protein-coupled receptor 48 (Gpr48/Lgr4) impairs definitive erythropoiesis at midgestation through down-regulation of the ATF4 signaling pathway. J. Biol. Chem. 283, 36687–36697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo J., Zhou W., Zhou X., Li D., Weng J., Yi Z., Cho S. G., Li C., Yi T., Wu X., Li X. Y., de Crombrugghe B., Höök M., Liu M. (2009) Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development 136, 2747–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X. Y., Lu Y., Sun H. Y., Wang J. Q., Yang J., Zhang H. J., Fan N. G., Xu J., Jiang J. J., Liu R. Y., Li D. L., Liu M. Y., Ning G. (2010) G protein-coupled receptor 48 upregulates estrogen receptor α expression via cAMP/PKA signaling in the male reproductive tract. Development 137, 151–157 [DOI] [PubMed] [Google Scholar]
- 25. Weng J., Luo J., Cheng X., Jin C., Zhou X., Qu J., Tu L., Ai D., Li D., Wang J., Martin J. F., Amendt B. A., Liu M. (2008) Deletion of G protein-coupled receptor 48 leads to ocular anterior segment dysgenesis (ASD) through down-regulation of Pitx2. Proc. Natl. Acad. Sci. U.S.A. 105, 6081–6086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., Stange D. E., van Es J. E., Guardavaccaro D., Schasfoort R. B., Mohri Y., Nishimori K., Mohammed S., Heck A. J., Clevers H. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 [DOI] [PubMed] [Google Scholar]
- 27. Mustata R. C., Van Loy T., Lefort A., Libert F., Strollo S., Vassart G., Garcia M. I. (2011) Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 12, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Araki A., Kanai T., Ishikura T., Makita S., Uraushihara K., Iiyama R., Totsuka T., Takeda K., Akira S., Watanabe M. (2005) MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J. Gastroenterol. 40, 16–23 [DOI] [PubMed] [Google Scholar]
- 29. Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. (1995) Experimental models of inflammatory bowel disease. Gastroenterology 109, 1344–1367 [DOI] [PubMed] [Google Scholar]
- 30. Carmon K. S., Gong X., Lin Q., Thomas A., Liu Q. (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. U.S.A. 108, 11452–11457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Medema J. P., Vermeulen L. (2011) Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474, 318–326 [DOI] [PubMed] [Google Scholar]
- 32. Hao H. X., Xie Y., Zhang Y., Charlat O., Oster E., Avello M., Lei H., Mickanin C., Liu D., Ruffner H., Mao X., Ma Q., Zamponi R., Bouwmeester T., Finan P. M., Kirschner M. W., Porter J. A., Serluca F. C., Cong F. (2012) ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 [DOI] [PubMed] [Google Scholar]
- 33. Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P. J., Clevers H. (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383 [DOI] [PubMed] [Google Scholar]
- 34. Günther C., Martini E., Wittkopf N., Amann K., Weigmann B., Neumann H., Waldner M. J., Hedrick S. M., Tenzer S., Neurath M. F., Becker C. (2011) Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garabedian E. M., Roberts L. J., McNevin M. S., Gordon J. I. (1997) Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 272, 23729–23740 [DOI] [PubMed] [Google Scholar]
- 36. Yan K. S., Chia L. A., Li X., Ootani A., Su J., Lee J. Y., Su N., Luo Y., Heilshorn S. C., Amieva M. R., Sangiorgi E., Capecchi M. R., Kuo C. J. (2012) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U.S.A. 109, 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tian H., Biehs B., Warming S., Leong K. G., Rangell L., Klein O. D., de Sauvage F. J. (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nava P., Koch S., Laukoetter M. G., Lee W. Y., Kolegraff K., Capaldo C. T., Beeman N., Addis C., Gerner-Smidt K., Neumaier I., Skerra A., Li L., Parkos C. A., Nusrat A. (2010) Interferon-γ regulates intestinal epithelial homeostasis through converging β-catenin signaling pathways. Immunity 32, 392–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaiser G. C., Polk D. B. (1997) Tumor necrosis factor α regulates proliferation in a mouse intestinal cell line. Gastroenterology 112, 1231–1240 [DOI] [PubMed] [Google Scholar]
- 40. Koch S., Nava P., Addis C., Kim W., Denning T. L., Li L., Parkos C. A., Nusrat A. (2011) The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology 141, 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee G., Goretsky T., Managlia E., Dirisina R., Singh A. P., Brown J. B., May R., Yang G. Y., Ragheb J. W., Evers B. M., Weber C. R., Turner J. R., He X. C., Katzman R. B., Li L., Barrett T. A. (2010) Phosphoinositide 3-kinase signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139, 869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ashton G. H., Morton J. P., Myant K., Phesse T. J., Ridgway R. A., Marsh V., Wilkins J. A., Athineos D., Muncan V., Kemp R., Neufeld K., Clevers H., Brunton V., Winton D. J., Wang X., Sears R. C., Clarke A. R., Frame M. C., Sansom O. J. (2010) Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 19, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang D., Peregrina K., Dhima E., Lin E. Y., Mariadason J. M., Augenlicht L. H. (2011) Paneth cell marker expression in intestinal villi and colon crypts characterizes dietary induced risk for mouse sporadic intestinal cancer. Proc. Natl. Acad. Sci. U.S.A. 108, 10272–10277 [DOI] [PMC free article] [PubMed] [Google Scholar]