Background: Toll-like receptors (TLR) are key components in autoimmune-mediated pathophysiology.
Results: Complement receptor 3 (CR3) suppresses TLR7/8 mediated-inflammation by degrading MyD88. The anti-inflammatory function of CR3 is negated if TLR7/8 ligation occurs prior to CR3 activation or in macrophages expressing a genetic variant of CD11b.
Conclusion: Environmental and genetic factors influence CR3 signaling.
Significance: CR3-specific agonists may be applicable for preventing pathologic TLR7/8 signaling.
Keywords: Genetic Polymorphism, Integrin, Macrophages, Nucleic Acid, Toll-like Receptors (TLR), Complement Receptor 3, Neonatal Lupus, Systemic Lupus Erythematosus
Abstract
Toll-like receptor (TLR) signaling is an important component in the inflammatory response generated in diseases characterized by autoantibody reactivity to proteins such as SSA/Ro in complex with endogenous nucleic acids. Complement receptor 3 (CR3), a genetic variant of which has been identified as a risk factor in systemic lupus erythematosus, has been shown to induce tolerogenic responses in dendritic cells and suppress TLR4 responses in a murine sepsis model. Accordingly, this study addressed the hypothesis that activation of CR3, influenced by genotype of CD11b, negatively regulates TLR7/8-dependent effector function. Allosteric activation of CD11b via pretreatment with the small molecule, leukadhedrin 1 (LA1), significantly attenuated TLR7/8-induced (hY3 RNA, R848) secretion of TNFα in THP-1 cells and human macrophages isolated from donors homozygous for the ancestral common ITGAM allele at rs1143679. This inhibition was accompanied by profound degradation of the adaptor protein MyD88, an effect not observed with direct inhibition of TLR ligation by an antagonist oligonucleotide. In contrast, the addition of LA1 after incubation with the TLR agonists did not result in MyD88 degradation and subsequent attenuation of TNFα secretion. In TLR7/8-stimulated macrophages isolated from donors heterozygous for the CD11b variant, pretreatment with LA1 did not down-regulate TNFα release. These novel findings support a negative cross-talk between CR3 and TLR pathways likely to be induced by antibodies reactive with ribonucleoproteins and point to the development of CR3-specific agonists as potential therapeutics for diseases such as neonatal lupus.
Introduction
Systemic lupus erythematosus (SLE)2 is characterized by both continuous and cyclic stimulation of the innate immune system by endogenous nucleic acids released from apoptotic or necrotic cells. This pathologic process is facilitated by circulating autoantibodies against nucleic antigens such as nucleosomes, histones, DNA, and ribonucleoproteins, a hallmark of disease (1–3). Autoantibody-dependent, FcγR-mediated uptake permits nucleic acids to activate dendritic cells and macrophages through the ligation of endosomal Toll-like receptors (TLR) (1, 4). For example, the SSA/Ro autoantigen is an RNA binding protein targeted by 40–60% of patients with lupus (5), and, together as a complex with hY3 RNA, it has been shown to activate TLR7/8 in macrophages (4, 6). One of the strongest clinical associations with reactivity to this ribonucleoprotein is observed in mothers of children with neonatal lupus, a fetal/neonatal disease that may share in common with SLE the pathologic activation of effector cells by nucleic acids. The identification of a biologic break to the inflammatory response induced by TLR7/8 signaling would be an important advance.
Activation of the complement receptor CR3, a leukocyte type I integral membrane heterodimer comprised of a 165-kDa α chain (CD11b), noncovalently linked to a 95-kDa β chain (CD18), although associated with inflammatory responses, may also deliver a negative signal (7, 8). CR3 engagement by its ligands, which include iC3b, intracellular cell adhesion molecule-1, -2, and fibrinogen, results in a stereotypical program of proinflammatory responses in the efferent limb of immunity, including leukocyte adhesion, migration, recruitment, and the promotion of superoxide anion release (9). However, it has also been shown that CR3 mediates uptake of iC3b-coated red blood cells and suppresses IL-12 production by human monocytes (10). Additionally, CR3 activation on dendritic cells delivers tolerogenic signals (11, 12). Specific cross-talk between TLR and CR3 has been provided by the observation that CR3 participates in a negative feedback loop to suppress TLR4-mediated inflammation in a murine sepsis model (13). CD11b/CD18 knock-out mice exhibited enhanced TLR-induced activation, confirming the interrelationship between CR3 and TLR (13). Moreover, genetic variation at ITGAM, the gene that encodes CD11b, has been identified as a risk factor for SLE. A missense single nucleotide polymorphism (SNP) results in an amino acid change from arginine to histidine at position 77 (R77H) in the extracellular I binding domain of CD11b and may affect phagocytosis or neutrophil adhesion (14).
Accordingly, this study was initiated to explore the hypothesis that activation of CR3 specifically inhibits TLR7/8 signaling because this should be particularly applicable to diseases in which the dominant autoantibody is reactive with a ribonucleoprotein. The approach exploited leukadhedrin 1 (LA1), a novel small molecule compound that engages CD11b via a highly specific interaction, allosterically activating CR3, and stimulating binding to its known ligands, including iC3b (15, 16). Both TLR7/8 agonists and the SSA/Ro-associated RNA, hY3, were used to stimulate TLR7/8 in macrophages generated from the THP-1 cell line and human donors. All cells used were genotyped to specifically account for any functional effects conferred by the SLE candidate genetic variant of CD11b compared with the common (ancestral) form.
EXPERIMENTAL PROCEDURES
Reagents
LA1 was selected based on its utility as a compound, which emerged from a screen of CD11b agonists (15). Human Y3 RNA was prepared from hY3 plasmids, kindly provided by Dr. Sandra Wolin (Yale University School of Medicine), as described (4, 17). Single-stranded oligoribonucleotides that specifically activate TLR7 or TLR8 and a modified oligodeoxynucleotide that inhibits TLR7/8/9 were synthesized and purified at Idera Pharmaceuticals as described previously (18–20). The imidazoquinoline compound, R848 (ligates TLR7/8) was from Invitrogen. Anti-MyD88 (4283) was from Cell Signaling Technology. Anti-CD11b antibody (functional grade-purified, 16-0113) and mouse IgG1 κ isotype control (16–4714) were from eBioscience. Recombinant interleukin-1β (IL-1β) containing <0.2 endotoxin units/μg was from Genscript. Anti-α tubulin antibody was from Sigma Aldrich.
Cells
Peripheral blood mononuclear cells (PBMCs) were obtained from white blood cell concentrates from random, unidentified healthy donors (New York Blood Center, New York, NY) by centrifugation on Ficoll-Hypaque gradients. Monocytes (CD14-positive cells) were isolated using anti-CD14 microbeads (Miltenyi Biotech) and cultured in Teflon beakers (RPMI 1640/10% FBS) for 7 days in the presence of 10 ng/ml GM-CSF to obtain macrophages (4, 21). For in vitro assays, monocyte-derived macrophages (4 × 105/ml) were plated in growth medium and incubated 37 °C for 48 h. Neutrophils were obtained from a healthy donor using an isolate from dextran sedimentation and Ficoll-Hypaque separation. The human monocytic cell line THP-1 was obtained from the ATCC and cultured in RPMI 1640/10% FBS. THP-1 cells (4 × 105/ml) were differentiated into a “macrophage-like” phenotype in 12-well plates with 0.2 μm phorbol 12-myristate 13-acetate (PMA) for 3 days followed by 48 h in growth medium without PMA (22). Typing of ITGAM (rs1143679) was performed by the allelic exclusion technique using assays and reagents purchased from Applied Biosciences, and assignments were confirmed by direct sequencing.
Polymorphonuclear Leukocyte Adhesion Assay
Preparation of fibrinogen-coated plates involved two steps, including an incubation of fibrinogen (0.1 μg/well) overnight at 4 °C and saturation of nonspecific sites with gelatin (100 μg/ml, 1 h, 22 °C). Polymorphonuclear leukocytes (3 × 104/well) were placed in the wells in the absence and presence of LA1 (15 μm, 10 min, 37 °C). Nonadherent cells were removed, and adherent cells were counterstained and enumerated.
TLR7/8 Activation Experiments and Assessments
PBMC-derived macrophages and PMA-differentiated THP-1 cells were incubated 37 °C in serum-free RPMI in the presence or absence of LA1 or a TLR7/8/9 inhibitor. After 30 min, cells were stimulated with a TLR7 agonist, TLR8 agonist, R848, or transfected with hY3, as described (4), for various time points (30 min–18 h). In some experiments, LA1 and a TLR7/8/9 inhibitor were added 30 min after TLR stimulation. Conditioned medium from the culturing conditions were centrifuged to remove cell debris. The readout of TLR signaling was the release of TNFα which was measured by ELISA (R&D Systems). Cell viability was evaluated by a lactate dehydrogenase cytotoxicity detection kit (Takara Bio, Inc.). To evaluate a TLR-independent signaling pathway that also requires MyD88, IL-1β (0.5 μg/ml) was used to ligate the IL-1 receptor (IL-1R) on THP-1 cells. These experiments were conducted in the presence or absence of LA1 as detailed for the TLR agonists.
Immunoblot
Cell monolayers from TLR7/8 activation assays were washed twice in Tris-buffered saline and lysed with 1% Nonidet P-40/Tris-buffered saline containing protease inhibitors (Roche). Total protein content of lysates was determined by BCA protein quantification kit (Pierce). Lysates were separated by SDS-PAGE and transferred to PVDF membranes. Primary antibodies were incubated at 4 °C overnight, and immunoreactive proteins were detected by use of a species-specific infrared dye-conjugated secondary antibody. The Odyssey Infrared Imager (LI-COR Biosciences) was used to analyze and quantitate protein. MyD88 expression was calculated by the following equation: (quantification of pixels for MyD88 immunoblot/quantification of pixels for α tubulin immunoblot) × 100.
Statistical Analysis
For each assay (TNFα secretion), the effect due to different conditions (i.e. LA1, TLR inhibitor, ITGAM variation) was analyzed using the Mann-Whitney U test. A p value of < 0.05 was considered significant.
RESULTS
LA1, a CR3 Agonist, Negatively Regulates TLR7/8 Signaling
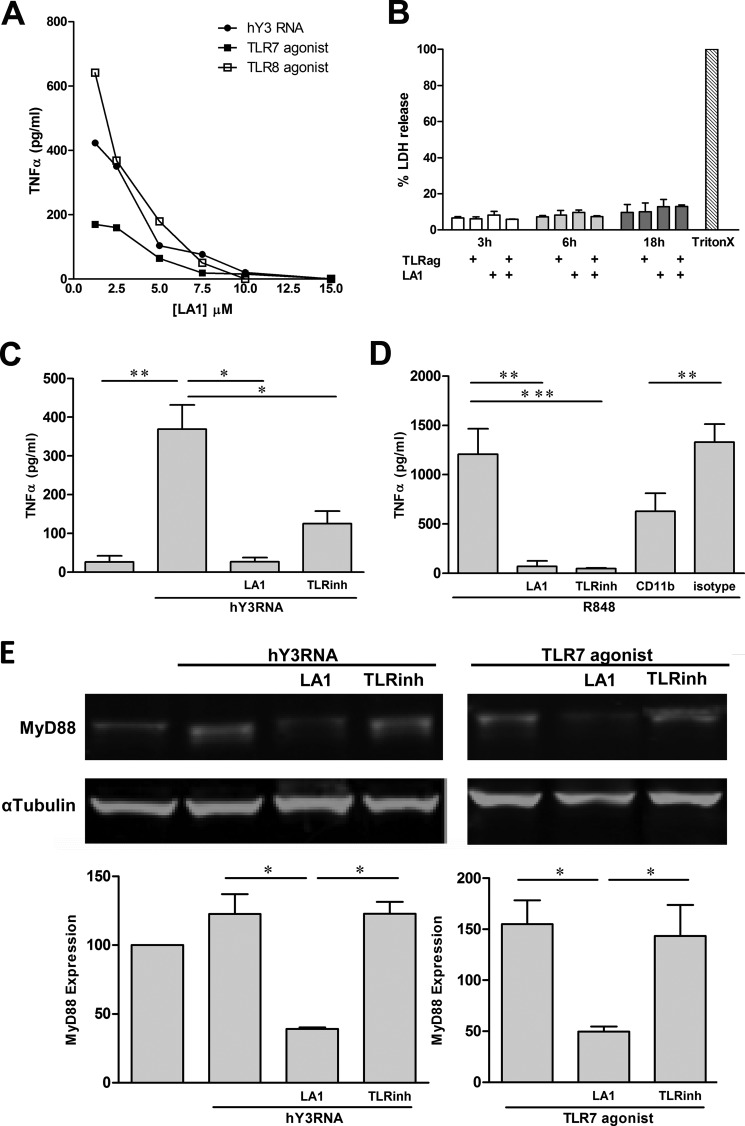
To determine whether CR3 negatively regulates TLR7/8 signaling, LA1, a novel small molecule compound that engages CD11b and allosterically activates CR3 was employed. In initial experiments, LA1 (15 μm, 10 min) stimulated polymorphonuclear leukocytes from a healthy donor to adhere to fibrinogen, thereby confirming properties related to the activity and nontoxicity of the compound (data not shown). Using PMA-differentiated THP-1 cells, TLR7/8 activation assays were then conducted in the presence or absence of LA1 to evaluate the effect of CR3 activation on TLR7/8 signaling. Based on genotyping of THP-1 cells, it was determined that the cell line was derived from a donor homozygous for the common R77 form. Treatment of THP-1 cells with either hY3 RNA, a TLR7 agonist, or a TLR8 agonist resulted in TNFα release. Irrespective of stimuli, pretreatment with LA1 (30 min, concentration varied), resulted in a dose-dependent inhibition of TNFα secretion (Fig. 1A). Evidence against cytotoxicity of LA1 was provided by the absence of lactate dehydrogenase in culture supernatants from untreated, TLR7/8 agonist, and LA1-exposed THP-1 cells (versus positive control, Triton X-100 treated THP-1 cells, Fig. 1B). Regarding hY3 RNA, in three separate experiments, this condition resulted in a significant increase of TNFα compared with unstimulated cells (369 ± 63 versus 26 ± 16 pg/ml, respectively (p = 0.005). The addition of LA1 prior to hY3-mediated TLR stimulation profoundly decreased TNFα secretion (26 ± 11 pg/ml, p = 0.01 versus hY3) and exceeded the inhibition observed with an oligonucleotide-based TLR7/8/9 inhibitor (125 ± 32 pg/ml, p = 0.02 versus hY3, Fig. 1C). Moreover, R848-stimulated TNFα release was also significantly decreased by pretreatment with LA1 (1208 ± 258 versus 68 ± 47 pg/ml, respectively (p = 0.003)). Pretreatment of THP-1 cells with an antibody reactive with the activation epitope of CD11b but not an isotype control resulted in a 50% inhibition of TNFα release by R848-stimulated THP-1 cells (Fig. 1D), further supporting the inhibitory effects of CR3 activation on TLR7/8 signaling.
FIGURE 1.
LA1, a CR3 agonist, attenuates TNFα release by THP-1 cells stimulated with hY3, TLR7, and TLR8 agonists via the degradation of MyD88. A, TNFα levels measured in supernatants generated from THP-1 after exposure to either hY3 RNA transfection, a TLR7 agonist, and a TLR8 agonist (18 h, 37 °C) in the presence of LA1 (pretreatment of 30 min, concentration varied). B, lactate dehydrogenase (LDH) release relative to a positive control (a condition employing lysis using Triton X-100 (TritonX)) in supernatants from THP-1 cells stimulated with a TLR7/8 agonist for 3, 6, and 18 h with or without LA1. TNFα levels from THP-1 cells stimulated with hY3 (C) or R848 (D) in the presence or absence of LA1; an oligonucleotided-based TLR7/8/9 inhibitor (TLRinh); an anti-CD11b antibody; or isotype control. Bars represent mean pg/ml TNFα ± S.E. (A, n = 6; B, n = 4). E, immunoblot analysis of MyD88 in lysates generated from THP-1 cells stimulated with hY3 (left) or a TLR7 agonist (right) in the presence or absence of LA1 or a TLR7/8/9 inhibitor. α-Tubulin serves as a loading control. Bars represent the ratio of MyD88 to α-tubulin for each condition (mean value ±S.E. (n = 3). *, p < 0.05, **, p ≤ 0.005; ***, p ≤ 0.0005.
LA1 Inhibits TLR7/8 Signaling by Promoting the Degradation of MyD88
To identify the potential mechanism for down-regulation of TLR7/8 signaling, the adaptor protein MyD88 was evaluated in lysates of hY3 or TLR7 agonist-stimulated THP-1 cells. The LA1-mediated inhibition of both hY3 and TLR7 agonist-stimulated TNFα release was accompanied by degradation of MyD88 as assessed by immunoblot. In contrast, degradation of MyD88 was not observed in THP-1 cells treated with an oligonucleotide-based TLR7/8/9 inhibitor. Reprobe of blot demonstrated that the expression of α-tubulin did not vary with treatment condition. A quantification of pixels confirmed a reduction of MyD88 relative to α-tubulin expression in THP-1 cells treated with LA1 prior to TLR stimulation compared with all other treatments (Fig. 1E).
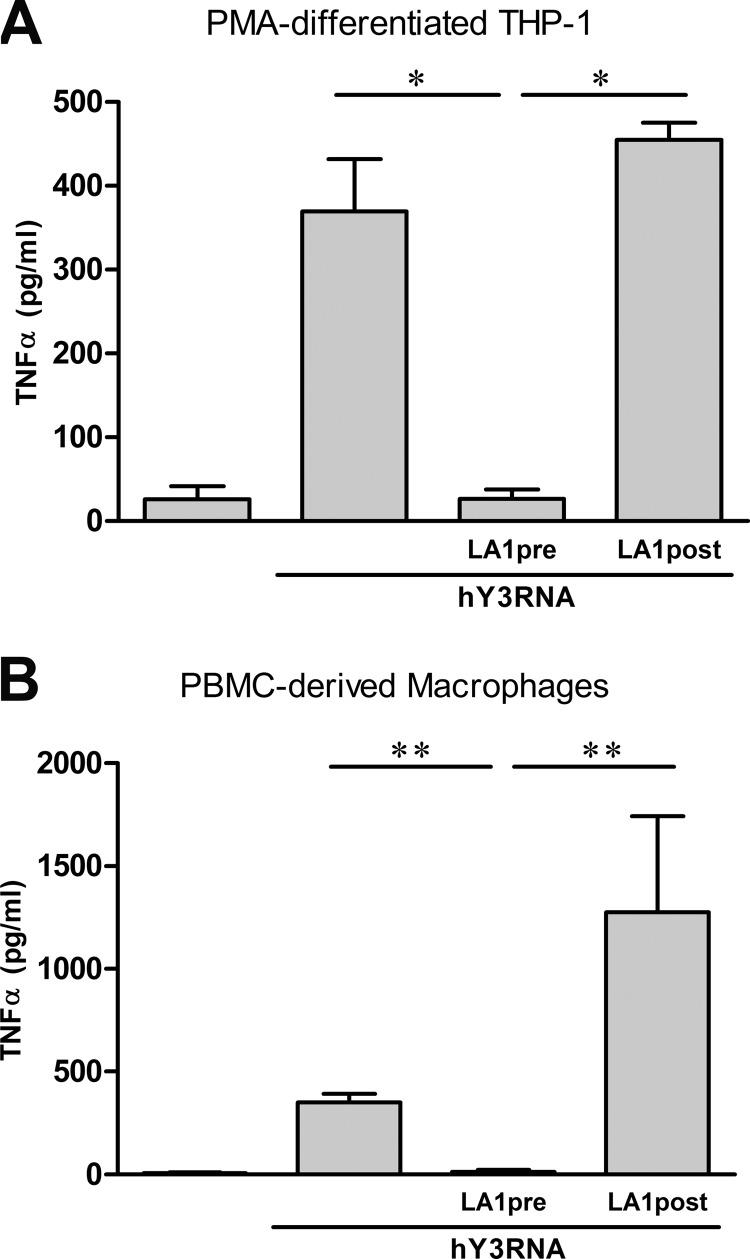
TNFα Release by hY3-stimulated Macrophages Is Attenuated by Pre- but Not Post-Treatment with LA1
In contrast to the LA1-mediated inhibition of TLR signaling, the addition of LA1 to THP-1 cells after stimulation with hY3 did not attenuate TNFα secretion (36 ± 11 versus 441 ± 21 pg/ml pre- versus postaddition of LA1, respectively (p = 0.02); Fig. 2A). In parallel with the results for hY3-stimulated THP-1 cells, TNFα was induced after exposure of human PBMC-derived macrophages to hY3 (350 ± 42 versus 7 ± 5 pg/ml stimulated versus unstimulated, respectively (p = 0.002)). Although pretreatment with LA1 profoundly decreased hY3-induced TNFα secretion (13 ± 9 pg/ml, p = 0.002 versus hY3), LA1 treatment following hY3 stimulation negated the LA1 inhibitory effect and increased TNFα secretion (1275 ± 468 pg/ml, p = 0.002 versus pre-LA1 treatment) (Fig. 2B).
FIGURE 2.
The effect of LA1 on TLR signaling varies depending on the timing of administration. TNFα was measured in the supernatants generated from PMA-differentiated THP-1 cells (A) or human PBMC-derived macrophages (B) transfected with hY3 RNA either 30 min after (LA1pre) or 30 min prior to (LA1post) the addition of LA1. Bars represent the mean ± S.E. (A, n = 4; B, n = 7). *, p < 0.05; **, p ≤ 0.005.
The Discordant Effect of Pre- and Post-treatment with LA1 Is Specific to TLR7/8 Ligation
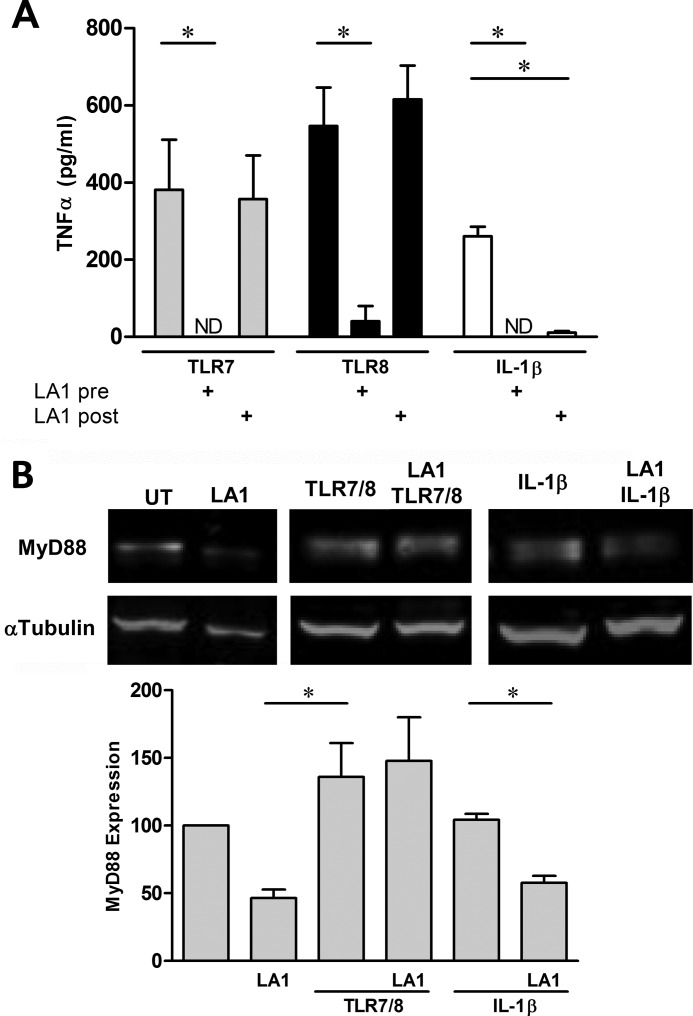
The next evaluations addressed whether the pre- and post-effects of LA1 extend to other TLR7/8 ligands and IL-1R engagement. TNFα release by THP-1 cells stimulated with a TLR7 agonist was inhibited by pretreatment but not post-treatment with LA1. Similarly, an agonist of TLR8 was sensitive to preaddition of LA1 but not postaddition (Fig. 3A). In contrast to LA1, TLR7- or TLR8-mediated TNFα release by THP-1 cells was abrogated by an oligonucleotide-based TLR7/8/9 inhibitor, irrespective of pre- or postaddition (763 ± 122 pg/ml versus 7 ± 6 and 25 ± 20 pg/ml, stimulated versus pre- and postaddition, respectively). To evaluate whether the timing of administration of LA1 was relevant to other molecules that signal through MyD88, IL-1β was used to engage the IL-1R in the presence or absence of LA1. Exposure of THP-1 cells to IL-1β resulted in a significant increase of TNFα compared with unstimulated cells (261 ± 25 versus 7 ± 3 pg/ml, respectively (p = 0.01)). In contrast to the temporal dependence of CR3 activation on TLR signaling, LA1 significantly decreased IL-1β-mediated TNFα secretion regardless of whether it was added before (TNFα not detected) or after stimulation (11 ± 4 pg/ml, p = 0.02 versus IL-1β) (Fig. 3A).
FIGURE 3.
The discordant effect of LA1 on hY3 stimulation extends to agonists of TLR7 and TLR8 but not the IL-1 receptor. A, TNFα was measured in the supernatants generated from PMA-differentiated THP-1 exposed to a TLR7 agonist, a TLR8 agonist, or IL-1β either 30 min after (LA1 pre) or 30 min prior to (LA1 post) the addition of LA1. Bars represent mean ± S.E. (n = 4). B, immunoblot analysis of MyD88 from THP-1 cells with or without a TLR7/8 agonist or IL-1β stimulation in the absence and presence of LA1 (5 μm added 30 min post-stimuli). α-Tubulin serves as a loading control. Aggregate data from three experiments utilizing the ratio of MyD88 to α-tubulin are reported as described in Fig. 1. *, p < 0.05. ND, not detected. UT, untreated.
TLR7/8 Engagement Prior to LA1-mediated CR3 Activation Protects MyD88 from Degradation
The next series of experiments addressed the role of MyD88 to explain the different effects of LA1 on TLR and IL-1R signaling. Specifically, MyD88 degradation was evaluated in LA1 exposed THP-1 cells in the presence or absence of prior stimulation with a TLR7/8 agonist or IL-1β. In the absence of TLR7/8 or IL-1R engagement, LA1-mediated degradation of MyD88 was apparent (Fig. 3B). In contrast, when LA1 was added 30 min post-TLR7/8 agonist, MyD88 was protected from degradation. The protective effect of TLR engagement on MyD88 was specific to TLR7/8 as degradation of MyD88 was evident in lysates from THP-1 cells pretreated with IL-1β 30 min prior to LA1 (Fig. 3B).
LA1-mediated Inhibition of TLR7/8 Varies Depending on Carrier Status for the rs114379 ITGAM Polymorphism
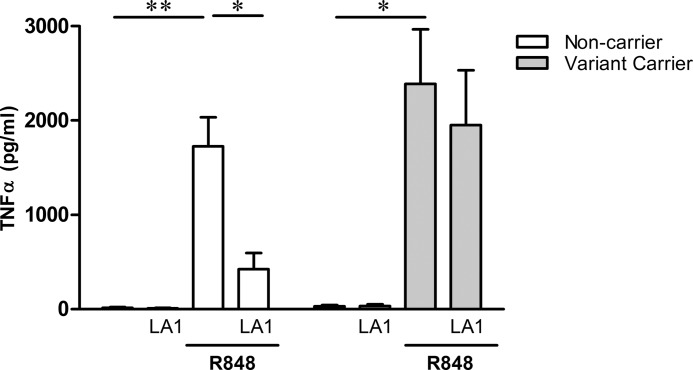
Given that the assignment of ITGAM for THP-1 cells was homozygous common, TLR7/8 activation assays were repeated with human PBMC-derived macrophages from donors that were either common (ancestral) or carried a variation at rs1143679. Macrophages isolated from donors homozygous for the ancestral R77 form secreted TNFα in response to the TLR7/8 agonist, R848 (1,400 ± 308 versus 10 ± 7 pg/ml stimulated versus unstimulated, respectively (p = 0.005)) and pretreatment with LA1-inhibited TNFα secretion (331 ± 172 pg/ml, p = 0.002 versus R848 stimulation). Macrophages derived from donors heterozygous for the variant at ITGAM also secreted TNFα upon stimulation with R848 (2,248 ± 510 versus 35 ± 15 pg/ml stimulated versus unstimulated, respectively (p = 0.01)). Not only was TNFα secretion by variant carriers enhanced, but in contrast to those homozygous common, treatment with LA1 only marginally attenuated TLR7/8 signaling (1996 ± 580 pg/ml, p = NS versus R848 stimulation). Overall, pretreatment of macrophages with LA1 prior to TLR7/8 stimulation inhibited TNFα secretion by 80 ± 10% for non-carriers and only 11 ± 7% for variant carriers, p = 0.02 (Fig. 4), further supporting that the inhibitory effect of LA1 was applicable to the dominant genotype.
FIGURE 4.
The inhibitory effect of LA1 varies depending on carrier status for the ITGAM polymorphism R77H. TNFα was measured in the supernatants generated from non-carrier (open bars, n = 6) or variant carrier (shaded bars, n = 3) macrophages stimulated with R848 in the presence or absence of LA1. Bars represent mean pg/ml TNFα ± S.E. *, p < 0.05; **, p ≤ 0.005.
DISCUSSION
TLR signaling is an important component of the inflammatory response generated in diseases characterized by autoantibody reactivity to proteins in complex with endogenous nucleic acids. Allosteric activation of CD11b via a small molecule, LA1, significantly attenuated TLR7/8 induced secretion of TNFα via the degradation of MyD88 in macrophages homozygous common for ITGAM polymorphism at rs114379. This inhibition was significantly reduced in macrophages isolated from donors carrying the R77H ITGAM variant.
LA1 is a novel small molecule compound that engages CD11b via a highly specific interaction, allosterically activating CR3, and stimulating binding to its known ligands: fibrinogen, intracellular cell adhesion molecule, and iC3b. In vitro, LA1 promotes CR3-dependent leukocyte adhesion to fibrinogen-coated surfaces while also deterring cell de-adhesion with the net effect being a decrease in chemotaxis and trans-endothelial migration (15, 16). In vivo, LA1 decreases leukocyte recruitment in rat peritonitis and preserves kidney function in a mouse model of experimental anti-glomerular basement nephritis. Furthermore, LA1 stimulates CR3-dependent phagocytosis of iC3b-coated sheep red blood cells (16), an effect exploited in the current study. Additionally, we have shown that LA1 promotes the degradation of MyD88, a finding in accord with that demonstrated in a murine model of sepsis (13). In that model, TLR3 and TLR4 ligation results in phosphatidylinositol-3-OH kinase and RapL-dependent intracellular signals, which trigger a conformational shift in CR3 from a quiescent low affinity to an active high affinity state capable of transducing intracellular signals analogous to the molecular transition we posit is induced by pretreatment with LA1. A feedback loop may then follow, whereby active CR3 promotes downstream phosphorylation of MyD88 and TIR-domain-containing adapter-inducing interferon-β by the Src and Syk tyrosine kinases. Phosphorylation of MyD88 and TIR-domain-containing adapter-inducing interferon-β targets these adaptors for proteolysis and inhibition of further inflammation with the ultimate effect inhibition of TLR-dependent proinflammatory cytokine secretion. In contrast, pretreatment of hY3-stimulated THP-1 cells with the oligonucleotide TLR7/8/9 inhibitor did not result in the degradation of MyD88, consistent with its role in directly interrupting TLR ligation upstream of MyD88. This finding suggests that LA1 and TLR inhibitors act to attenuate TLR7/8-mediated TNFα release by a different mechanism. Regarding an alternative explanation for the anti-inflammatory effects of CR3 on TLR signals, Bai and co-workers (11, 12) described a CR3-dependent up-regulation of microRNA miR-146a, which served as a break to a TLR-dependent proinflammatory pathway that is mediated by Notch1.
The clinical relevance of negative cross-talk between CR3 and TLR7/8 is evident based on the proposed pathogenesis of cardiac manifestations of neonatal lupus, which initiates with the placental transfer of maternal anti-SSA/Ro antibodies. The potential of SSA/Ro-associated hY3 RNA to perpetuate an inflammatory step via a TLR7/8 pathway has been experimentally substantiated by the transdifferentiation of human fetal cardiac fibroblasts to a scarring phenotype following exposure to supernatants generated by incubation of macrophages with surrogate immune complexes comprised of SSA/Ro, hY3, and affinity-purified anti-SSA/Ro antibody (4, 21). Consistent with the observation that chloroquine, an inhibitor of endosomal acidification, decreased the RNA-induced TLR signaling, two retrospective studies suggest that use of hydroxychloroquine may reduce the frequency of congenital heart block in anti-SSA/Ro-exposed mothers (23, 24). Thus, inhibition of TLR signaling via a distinct mechanism that depends on MyD88 degradation may represent a novel therapeutic approach to treatment or prevention of cardiac neonatal lupus. However, it was demonstrated that LA1 given after TLR7/8 stimulation was unable to degrade MyD88 and may enhance TNFα secretion, a paradox that should be considered when designing therapeutics to target CR3.
The prevention of LA1-mediated MyD88 degradation when LA1 was added post-TLR stimulation was specific to TLR7/8 ligation because MyD88 degradation and subsequent attenuation of TNFα secretion was observed upon engagement of IL-1R, regardless of whether LA1 was added pre- or post-treatment with IL-1β. A potential explanation for this finding is that TLR7/8 signaling alters CR3 perhaps by inhibiting phosphatidylinositol-3-OH kinase and RapL intracellular signaling to prevent the conformational shift in CR3 necessary for activation. Alternatively, TLR7/8 signaling may promote a conformational change in CR3 that prevents binding and activation by LA1. In addition, it is possible that TLR7/8 ligation directly prevents the phosphorylation of MyD88 by the tyrosine kinases Src and Syk independent of CR3.
The contribution of CR3 to the pathogenesis of SLE has been recently heightened by exciting advances in the search for genetic candidates. The locus that encodes ITGAM on chromosome 16 provided a strong association with susceptibility to SLE in separate genome-wide association studies (25, 26) and replication studies of multiple populations (27) reaching an odds ratio of 1.78 (1.56–2.03). The missense SNP, rs1143679, results in an amino acid change from arginine to histidine at position 77 (R77H) in the extracellular I/A ligand-binding domain of CD11b. The reduced ability of LA1 to inhibit TNFα secretion by variant carrier macrophages stimulated with the TLR7/8 agonist suggests that the R77H SNP prevents CD11b from forming its active high affinity epitope and/or prevents binding of LA1. This defect in CD11b may prevent the negative regulation of inflammatory TLR responses by CR3 and may also explain the enhanced TNFα secretion upon TLR7/8 stimulation by macrophages from donor variant carriers compared with homozygous common. These data further substantiate a recently published study in which sheep erythrocytes opsonized with iC3b were used to ligate CD11b in human macrophages. CR3 engagement resulted in an attenuation of TLR7/8-mediated cytokine release, which was less profound in cells isolated from donors homozygous for the 77H ITGAM variant (28).
In summary, these data suggest that activation of CR3 down-regulates macrophage TLR7/8 signaling via degradation of MyD88. These findings may be particularly relevant in disease states such as SLE and neonatal lupus in which inflammatory cells are triggered by RNA containing immune complexes.
Acknowledgments
We thank Tejaskumar Patel for technical assistance. J. B. and R. C. thank the Slottje family for the gift for lupus research.
This work was supported by an Alliance for Lupus Research grant (to R. C.), American Heart Association grants (to J. R. and V. G.), Arthritis Foundation grant (to J. R.), and National Institutes of Health Grants AR-42455 (to J. B.) and DK-084195 (to V. G.).
- SLE
- systemic lupus erythematosus
- TLR
- Toll-like receptor(s)
- CR3
- complement receptor 3
- LA1
- leukadhedrin 1
- PBMC
- peripheral blood mononuclear cell
- PMA
- phorbol 12-myristate 13-acetate
- IL-1R
- IL-1 receptor.
REFERENCES
- 1. Lövgren T., Eloranta M.L., Båve U., Alm G. V., Rönnblom L. (2004) Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 [DOI] [PubMed] [Google Scholar]
- 2. Lau C. M., Broughton C., Tabor A. S., Akira S., Flavell R. A., Mamula M. J., Christensen S. R., Shlomchik M. J., Viglianti G. A., Rifkin I. R., Marshak-Rothstein A. (2005) RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202, 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guiducci C., Gong M., Xu Z., Gill M., Chaussabel D., Meeker T., Chan J. H., Wright T., Punaro M., Bolland S., Soumelis V., Banchereau J., Coffman R. L., Pascual V., Barrat F. J. (2010) TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature 465, 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clancy R. M., Alvarez D., Komissarova E., Barrat F. J., Swartz J., Buyon J. P. (2010) Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J. Immunol. 184, 2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M. H., Trembleau S., Muller S., Steiner G. (2010) Nucleic acid-associated autoantigens: pathogenic involvement and therapeutic potential. J. Autoimmun. 34, J178–206 [DOI] [PubMed] [Google Scholar]
- 6. Vollmer J., Tluk S., Schmitz C., Hamm S., Jurk M., Forsbach A., Akira S., Kelly K. M., Reeves W. H., Bauer S., Krieg A. M. (2005) Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 202, 1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 8. Abram C. L., Lowell C. A. (2009) The ins and outs of leukocyte integrin signaling. Annu. Rev. Immunol. 27, 339–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merrill J. T., Slade S. G., Weissmann G., Winchester R., Buyon J. P. (1990) Two pathways of CD11b/CD18-mediated neutrophil aggregation with different involvement of protein kinase C-dependent phosphorylation. J. Immunol. 145, 2608–2615 [PubMed] [Google Scholar]
- 10. Marth T., Kelsall B. L. (1997) Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185, 1987–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skoberne M., Somersan S., Almodovar W., Truong T., Petrova K., Henson P. M., Bhardwaj N. (2006) The apoptotic-cell receptor CR3, but not αvβ5, is a regulator of human dendritic-cell immunostimulatory function. Blood 108, 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai Y., Qian C., Qian L., Ma F., Hou J., Chen Y., Wang Q., Cao X. (2012) Integrin CD11b negatively regulates TLR9-triggered dendritic cell cross-priming by upregulating microRNA-146a. J. Immunol. 188, 5293–5302 [DOI] [PubMed] [Google Scholar]
- 13. Han C., Jin J., Xu S., Liu H., Li N., Cao X. (2010) Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 11, 734–742 [DOI] [PubMed] [Google Scholar]
- 14. MacPherson M., Lek H. S., Prescott A., Fagerholm S. C. (2011) A systemic lupus erythematosus-associated R77H substitution in the CD11b chain of the Mac-1 integrin compromises leukocyte adhesion and phagocytosis. J. Biol. Chem. 286, 17303–17310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faridi M. H., Maiguel D., Barth C. J., Stoub D., Day R., Schürer S., Gupta V. (2009) Identification of novel agonists of the integrin CD11b/CD18. Bioorg. Med. Chem. Lett. 19, 6902–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maiguel D., Faridi M. H., Wei C., Kuwano Y., Balla K. M., Hernandez D., Barth C. J., Lugo G., Donnelly M., Nayer A., Moita L. F., Schürer S., Traver D., Ruiz P., Vazquez-Padron R. I., Ley K., Reiser J., Gupta V. (2011) Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci. Signal. 4, ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Brien C. A., Wolin S. L. (1994) A possible role for the 60 kD Ro autoantigen in a discard pathway for defective 5S ribosomal RNA precursors. Genes Dev. 8, 2891–2903 [DOI] [PubMed] [Google Scholar]
- 18. Lan T., Kandimalla E. R., Yu D., Bhagat L., Li Y., Wang D., Zhu F., Tang J. X., Putta M. R., Cong Y., Trombino A. F., Sullivan T., Agrawal S. (2007) Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc. Natl. Acad. Sci. U.S.A. 104, 13750–13755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lan T., Dai M., Wang D., Zhu F. G., Kandimalla E. R., Agrawal S. (2009) Toll-like receptor 7 selective synthetic oligoribonucleotide agonists: synthesis and structure-activity relationship studies. J. Med. Chem. 52, 6871–6879 [DOI] [PubMed] [Google Scholar]
- 20. Yu D., Wang D., Zhu F. G., Bhagat L., Dai M., Kandimalla E. R., Agrawal S. (2009) Modifications incorporated in CpG motifs of oligodeoxynucleotides lead to antagonist activity of toll-like receptors 7 and 9. J. Med. Chem. 52, 5108–5114 [DOI] [PubMed] [Google Scholar]
- 21. Alvarez D., Briassouli P., Clancy R. M., Zavadil J., Reed J. H., Abellar R. G., Halushka M., Fox-Talbot K., Barrat F. J., Buyon J. P. (2011) A novel role of endothelin-1 in linking TLR7-mediated inflammation to fibrosis in congenital heart block. J. Biol. Chem. June 5 (Epub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maess M. B., Sendelbach S., Lorkowski S. (2010) Selection of reliable reference genes during THP-1 monocyte differentiation into macrophages. BMC Mol. Biol. 11, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izmirly P. M., Kim M. Y., Llanos C., Le P. U., Guerra M. M., Askanase A. D., Salmon J. E., Buyon J. P. (2010) Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann. Rheum. Dis. 69, 1827–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izmirly P. M., Costedoat-Chalumeau N., Pisoni C. N., Khamashta M. A., Kim M. Y., Saxena A., Friedman D., Llanos C., Piette J. C., Buyon J. P. (2012) Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation 126, 76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath S. K., Han S., Kim-Howard X., Kelly J. A., Viswanathan P., Gilkeson G. S., Chen W., Zhu C., McEver R. P., Kimberly R. P., Alarcón-Riquelme M. E., Vyse T. J., Li Q. Z., Wakeland E. K., Merrill J. T., James J. A., Kaufman K. M., Guthridge J. M., Harley J. B. (2008) A nonsynonymous functional variant in integrin-α(M) (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat. Genet. 40, 152–154 [DOI] [PubMed] [Google Scholar]
- 26. Fan Y., Li L. H., Pan H. F., Tao J. H., Sun Z. Q., Ye D. Q. (2011) Association of ITGAM polymorphism with systemic lupus erythematosus: a meta-analysis. J. Eur. Acad. Dermatol. Venereol. 25, 271–275 [DOI] [PubMed] [Google Scholar]
- 27. Han S., Kim-Howard X., Deshmukh H., Kamatani Y., Viswanathan P., Guthridge J. M., Thomas K., Kaufman K. M., Ojwang J., Rojas-Villarraga A., Baca V., Orozco L., Rhodes B., Choi C. B., Gregersen P. K., Merrill J. T., James J. A., Gaffney P. M., Moser K. L., Jacob C. O., Kimberly R. P., Harley J. B., Bae S. C., Anaya J. M., Alarcón-Riquelme M. E., Matsuda K., Vyse T. J., Nath S. K. (2009) Evaluation of imputation-based association in and around the integrin-α-M (ITGAM) gene and replication of robust association between a non-synonymous functional variant within ITGAM and systemic lupus erythematosus (SLE). Hum. Mol. Genet. 18, 1171–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rhodes B., Fürnrohr B. G., Roberts A. L., Tzircotis G., Schett G., Spector T. D., Vyse T. J. (2012) The rs1143679 (R77H) lupus associated variant of ITGAM (CD11b) impairs complement receptor 3 mediated functions in human monocytes. Ann. Rheum. Dis. 71, 2028–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]