Background: A crystal structure of Escherichia coli RNA polymerase (RNAP) has not been determined.
Results: The σ1.1 and α subunit C-terminal domain structures have been determined in the context of an intact RNAP.
Conclusion: σ1.1 localizes within the RNAP DNA-binding channel and must disengage from this site to form an open complex.
Significance: This work enables future structure determination of bacterial RNAP mutants.
Keywords: Bacteria, Escherichia coli, RNA Polymerase, Transcription, X-ray Crystallography, σ70 Holoenzyme
Abstract
Escherichia coli RNA polymerase (RNAP) is the most studied bacterial RNAP and has been used as the model RNAP for screening and evaluating potential RNAP-targeting antibiotics. However, the x-ray crystal structure of E. coli RNAP has been limited to individual domains. Here, I report the x-ray structure of the E. coli RNAP σ70 holoenzyme, which shows σ region 1.1 (σ1.1) and the α subunit C-terminal domain for the first time in the context of an intact RNAP. σ1.1 is positioned at the RNAP DNA-binding channel and completely blocks DNA entry to the RNAP active site. The structure reveals that σ1.1 contains a basic patch on its surface, which may play an important role in DNA interaction to facilitate open promoter complex formation. The α subunit C-terminal domain is positioned next to σ domain 4 with a fully stretched linker between the N- and C-terminal domains. E. coli RNAP crystals can be prepared from a convenient overexpression system, allowing further structural studies of bacterial RNAP mutants, including functionally deficient and antibiotic-resistant RNAPs.
Introduction
RNA polymerase (RNAP)2 is the central enzyme of gene expression, and all life forms have RNAPs that function as multisubunit protein complexes (multisubunit cellular RNAP). The common core of the multisubunit RNAPs is composed of five subunits that are conserved in bacteria, archaea, and eukaryotes. Bacterial RNAP is the simplest form of this family (composed of the core enzyme αIαIIββ′ω subunits), whereas in eukaryotes and archaea, RNAP possesses additional polypeptides to form 11∼15-subunit complexes (1).
In bacteria, one of several different σ factors binds to the core enzyme to form the holoenzyme, which is responsible for recognizing promoter DNA. σ70 in Escherichia coli and SigA in other bacteria belong to the group 1 (primary or housekeeping) σ factor family (2). These σ factors contain distinct regions of highly conserved amino acid sequence and are composed of four domains: σ1.1 (region 1.1), σ2 (regions 1.2–2.4), σ3 (regions 3.0–3.2), and σ4 (regions 4.1–4.2) (3). Group 1 σ factors can bind to promoter DNA as part of the holoenzyme; once it binds to the core enzyme, the σ2, σ3, and σ4 domains are ideally positioned to recognize the promoter DNA sequences of −10, extended −10, and −35, respectively (4, 5).
In addition to the σ2, σ3, and σ4 domains, the group 1 σ family contains an ∼100-amino acid N-terminal extension, σ1.1, which is a negatively charged α helical domain (6). The σ1.1 domain has been shown to accelerate the formation of the open complex at some promoters and suggested to reside inside the RNAP main channel (7). This channel is positively charged to accommodate nucleic acids in the open complex and the transcription elongation complex. It has been proposed that during open complex formation, signals from DNA may induce opening and closing of the RNAP clamp, causing σ1.1 to eject from the RNAP main channel (4, 8). Given its flexible nature, σ1.1 has not been solved in all Thermus RNAP holoenzyme crystal structures that have been reported (5, 9–12). Only an NMR structure of σ1.1 from Thermotoga maritima has been reported, and it consists of three α helices with a compact hydrophobic core formed by highly conserved hydrophobic residues (6).
Since the first discovery of RNAP in the early 1960s (13), the RNAP from E. coli has been the primary model system of choice for understanding functions of cellular RNAPs for many reasons. For example, active E. coli RNAP can be conveniently reconstituted in vitro from its individual subunits using either wild-type or mutant proteins (14, 15), and its mechanism can be easily probed in vitro in the presence of purified template DNA, σ factors, and transcription factors. A simple and robust E. coli transcription system also makes it an excellent model for single-molecule studies of RNAPs (16).
X-ray crystal structures of bacterial RNAPs have been determined only from the Thermus genus. Because of the high sequence conservation among RNAPs from all species of bacteria, the most insight derived from the Thermus RNAP has been generalized to represent the transcription apparatus in all bacteria (4, 5, 9–12, 17–19). Nevertheless, without the structure of E. coli RNAP available, it is difficult to fully interpret the enormous amount of data that have been collected on E. coli RNAP. The structure of E. coli RNAP will also generate new insight about structural domains and motifs, as well as interactions with some ligands (e.g. ppGpp) and antibiotics (e.g. lipiarmycin) that specifically affect E. coli but not the Thermus RNAPs (20, 21). These structural insights are important to identify their binding sites and to understand the mechanisms of action.
EXPERIMENTAL PROCEDURES
Preparation and Crystallization of the E. coli RNAP Holoenzyme
The polycistronic plasmid pGEMABC was created for overexpressing the rpoA (encoding the α subunit), rpoB (encoding the β subunit), and rpoC (encoding the β′ subunit) genes as follows. The plasmid pGEMA185 expressing rpoA under the control of an IPTG-inducible T7 RNAP promoter (22) was digested at a BamHI site located downstream of rpoA. A DNA fragment containing the rpoB-rpoC genes was isolated from the pPNE2017 plasmid3 by BamHI digestion and inserted at the BamHI site of pGEMA185. pGEMABC expresses a single mRNA containing the rpoA-rpoB-rpoC genes.
All core RNAP subunits were expressed in E. coli BL21(DE3) cells transformed with pGEMABC (encoding rpoA, rpoB, and rpoC) and pACYCDuet-1_Ec_rpoZ (encoding rpoZ). Core RNAP was purified as follows. ∼16 g of cell paste was suspended in 50 ml of lysis buffer (50 mm Tris-HCl (pH 8 at 4 °C), 1 mm EDTA, 5 mm 2-mercaptoethanol, 1× protease inhibitor mixture, and 2 mm PMSF), and cells were lysed using an EmulsiFlex C3 homogenizer (Avestin Inc.) at 20,000 p.s.i. After a low-speed spin, RNAP in the soluble fraction was precipitated by adding 10% polyethyleneimine (Polymin P) solution (final concentration of 0.6%), and the pellet was recovered by low-speed centrifugation. RNAP was eluted from the pellet by suspension in TGED buffer (10 mm Tris-HCl (pH 8 at 4 °C), 10% glycerol, 0.1 mm EDTA, and 2 mm DTT) + 1 m NaCl and then precipitated by ammonium sulfate (final 60% saturation). The pellet was suspended in TGED buffer and dialyzed against TGED buffer + 50 mm NaCl. Core RNAP was purified by Bio-Rex 70 (Bio-Rad), Resource Q (GE Healthcare), and Superdex 200 (GE Healthcare) column chromatography. E. coli σ70 was expressed in BL21(DE3) cells transformed with pGEMD (22). After cells were lysed by sonication, σ70 was purified by HiTrap Q HP (GE Healthcare) and Superdex 200 column chromatography.
The RNAP holoenzyme was prepared by adding a 3-fold excess of σ70 to core RNAP, followed by incubation at 30 °C for 30 min and purification by Superdex 200 column chromatography. Crystals were obtained by hanging drop vapor diffusion by mixing equal volumes of RNAP holoenzyme solution (∼20 mg/ml) and crystallization solution (0.1 m HEPES-HCl (pH 7.0), 0.2 m calcium acetate, and ∼15% PEG 400) and incubating at 22 °C over the same crystallization solution. For cryocrystallography, crystals were soaked in crystallization solution containing 25% PEG 400. Selenomethionyl-substituted proteins, including core RNAP and σ70, were prepared by suppression of methionine biosynthesis (23). The crystals belong to the primitive orthorhombic space group (Table 1) containing two 440-kDa RNAP holoenzymes per asymmetric unit, and these RNAPs have almost identical structures (0.643-Å root mean square deviations by a structure alignment using the β′ subunit), with some minor deviations in the position of the σ non-conserved and σ4 domains.
TABLE 1.
Data collection and refinement statistic of the E. coli RNA polymerase σ70 holoenzyme
Data sets were collected at MacCHESS beamline A1. PDB, Protein Data Bank; r.m.s.d., root mean square deviations.
| PDB code | 4IGC |
| Data collection | |
| Space group | P212121 |
| Cell dimensions (Å) | |
| a | 187.308 |
| b | 205.901 |
| c | 309.185 |
| Resolution (Å) | 30–3.60 |
| Total reflections | 592,860 |
| Unique reflections | 123,448 |
| Redundancy | 4.5 (2.9)a |
| Completeness (%) | 96.81 (93.67)a |
| I/σ | 10.13 (2.04)a |
| Rsym | 0.091 (0.469)a |
| Refinement | |
| Resolution (Å) | 30–3.70 |
| Rwork | 0.242 |
| Rfree | 0.285 |
| r.m.s.d. | |
| Bond length (Å) | 0.003 |
| Bond angles | 0.77° |
a The highest resolution shell (3.66 to 3.6 Å) is shown in parentheses.
X-ray Data Collections and Structure Determination
The native data set was collected at Macromolecular Diffraction at the Cornell High Energy Synchrotron Source (MacCHESS) beamline A1 (Cornell University, Ithaca, NY). The data sets of SeMet-labeled crystals were collected at Berkley Center for Structural Biology (BCSB) beamline 8.2.1 (Lawrence Berkeley National Laboratory, Berkley, CA). The E. coli core RNAP model (24) was used as a search model for the molecular replacement (25). The data were processed by HKL2000 (26). Anomalous signals from SeMet were located by phase obtained from molecular replacement. Rigid body refinements were performed, and further adjustments to the model were performed manually. The resulting model phases allowed me to position E. coli σ70 structures (27, 28) in the electron density map. Positional refinement with non-crystallographic symmetry and secondary structure restraints was performed using the program PHENIX (29), and deformable elastic network (DEN) refinement was performed using Crystallography & NMR System (CNS) version 1.3 (30). The resulting map allowed segments that were not present in the search model to be built manually by Coot (31). The final coordinates and structure factors were submitted to the Protein Data Bank with code 4IGC.
RESULTS AND DISCUSSION
E. coli σ70 RNAP Holoenzyme Preparation and Crystallization
Endogenous E. coli RNAP can be purified from cells by a combination of RNAP-DNA co-precipitation using Polymin P and column chromatography (32). However, the yield and purity of endogenous E. coli RNAP are inadequate to obtain high-quality crystals for x-ray crystallography (<1 mg of RNAP is generated from 1 liter of cell culture). Therefore, I developed a co-overexpression plasmid (pGEMABC) that expresses the rpoA (encoding the α subunit), rpoB (encoding the β subunit), and rpoC (encoding the β′ subunit) genes under a single T7 RNAP promoter. This overexpression system drastically improves the yield and purity of RNAP (10 mg of RNAP from 1 liter of cell culture). The σ70 holoenzyme can be prepared by adding recombinant σ70 to core RNAP. Both the core and holoenzyme formed crystals, but neither diffracted beyond 10 Å resolution. pGEMABC overexpresses the α, β, and β′ subunits but not the ω subunit; thus, purified RNAP contains a substoichiometric amount of the ω subunit. The importance of the ω subunit for RNAP assembly and formation was suggested by a biochemical experiment (33) and by the Thermus RNAP crystal structure, which shows that the ω subunit binds the C-terminal tail of the β′ subunit (see Fig. 3b and supplemental Movie S4) (17).
FIGURE 3.
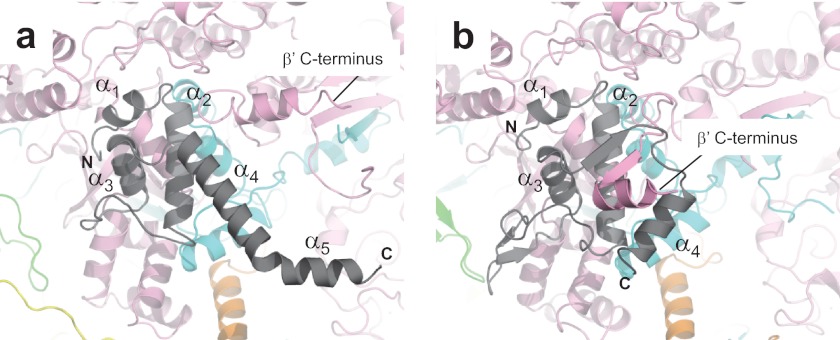
Structure comparisons of the ω subunits of the E. coli and Thermus RNAPs. Shown are close-up views of the ω (gray) and β′ (pink) subunit interactions of the E. coli (a) and Thermus (b) RNAPs. The positions of α helices (E. coli, α1–α5; and Thermus, α1–α4) of the ω subunits are indicated, and the C termini of the β′ subunits are also indicated.
To prepare RNAP containing a stoichiometric amount of the ω subunit, all RNAP subunits were overexpressed by pGEMABC and pACYCDuet-1_Ec_rpoZ, which overexpresses the ω subunit. The E. coli RNAP holoenzyme was prepared in vitro by addition of σ70, which produced better quality crystals that allowed determination of the structure by x-ray crystallography.
Structure Determination of the E. coli σ70 RNAP Holoenzyme
The crystals contain two 440-kDa RNAP holoenzyme molecules, designated RNAPA and RNAPB, per asymmetric unit. The structure was solved by molecular replacement with an E. coli RNAP core enzyme model (24). After density modification, the resulting electron density map had several deviations from the molecular replacement solution, including the following regions: 1) β insert 4 (βi4, residues 225–343, previously named β dispensable region 1/βDR1/SI1), 2) β insert 9 (βi9, residues 938–1042, previously named β dispensable region 2/βDR2/SI2), 3) β insert 11 (βi11, residues 1122–1180, present between β conserved regions H and I), 4) β′ insert 6 (β′i6, residues 942–1129, present in the middle of the highly conserved β′ trigger loop/helix), 5) β′ residues 515–597 (present between β′ conserved regions B and C), and 6) the C-terminal tails of the β′ and ω subunits (Fig. 1; see Fig. 3a). The overall structures of βi4 and βi9 are similar to the structures in the previously reported E. coli RNA core enzyme model (24), but their orientations relative to the main body of the RNAP are different. The crystal structures of the E. coli σ70 domains (27, 28) were manually placed in the Fo − Fc map, resulting in good fits of σ2, σ3, and σ4. Anomalous signals from SeMet sites from both the core enzyme and σ70 were used as guides for model building and refinement.
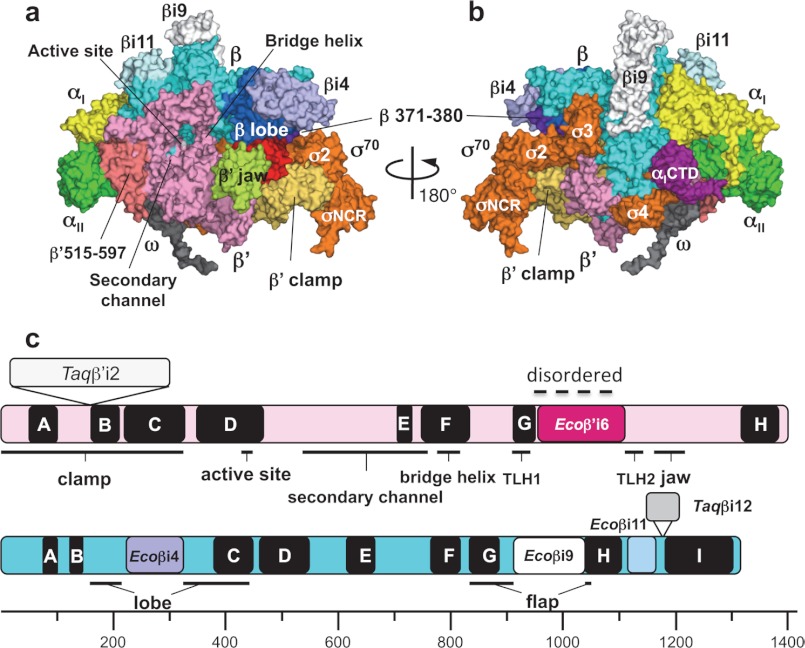
FIGURE 1.
Three-dimensional crystal structure of the E. coli RNAP σ70 holoenzyme. a and b, surface representation of the E. coli RNAP holoenzyme. Panel a shows a view from the RNAP secondary channel leading to the active site, and panel b shows the σ-binding site. Each subunit of RNAP is denoted by a unique color: yellow, αI; green, αII; cyan, β; pink, β′; gray, ω; and orange, σ70. Several domains described under “Results and Discussion” are also denoted by a unique color and are indicated. c, linear maps of the β′ (upper) and β (lower) subunits. Conserved regions of the β′ subunit (A–H) and the β subunit (A–I) are shown as black boxes with the structural domains of RNAP. Specific insertions of the E. coli and Thermus RNAPs are shown by the same colors as in panels a and b and in Fig. 2.
Structure of the E. coli σ70 RNAP Holoenzyme
The overall structure of the E. coli RNAP holoenzyme is similar to the structure of Thermus RNAP, resembling a crab claw with two pincers that constitute the DNA-binding cleft and the active site (Fig. 1 and supplemental Movie S1) (17). The β′ subunit forms one pincer, called the “clamp,” and the β subunit forms the other pincer. The clamp changes its position by swinging between open and closed states (34). Comparison of the E. coli RNAP structure with the Thermus RNAP structures, including the core enzyme (17, 35), holoenzyme (4, 5), and transcription elongation complex (18), revealed that the E. coli RNAP clamp is in a more closed conformation compared with any other RNAP crystal structure solved to date. The gap is narrow (∼7.5 Å) between the Cα atoms of σ2 and the tip of the β subunit pincer (residues 371–380) (Fig. 1; see Fig. 4a and supplemental Movie S1). The sequences and structures of σ2 and the β subunit pincer are highly conserved in the E. coli and Thermus RNAPs; therefore, the narrow gap between σ2 and the β subunit pincer observed in the E. coli RNAP crystal structure is due to closing of the entire clamp. The σ3.2 region formed a well ordered loop in the Thermus thermophilus holoenzyme (5), but it was disordered in the Thermus aquaticus holoenzyme (4). The E. coli holoenzyme shows a well ordered σ3.2 structure (residues 509–519) (see Fig. 4a and supplemental Movie S1).
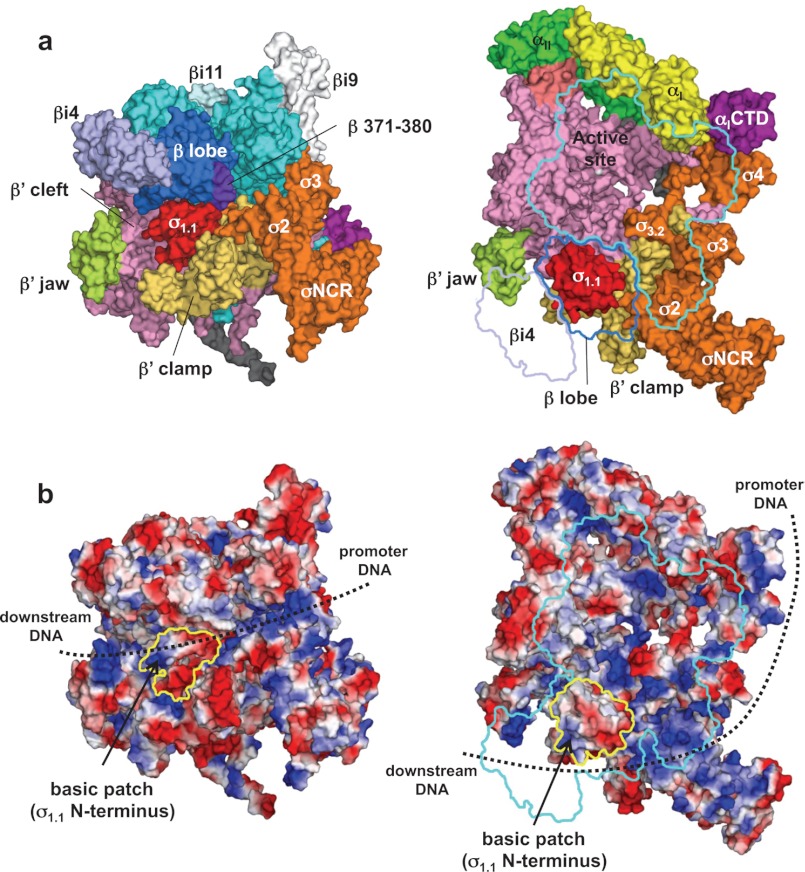
FIGURE 4.
Structure and function of σ1.1. a, molecular surface of the holoenzyme with σ1.1. Left, front view; right, side view. In the right panel, β subunit has been removed and outlined for clarity. b, electrostatic distribution of the holoenzyme. Left, front view; right, side view (orientations are the same as in a). Positive electrostatic potential is blue, and negative potential is red. The positions of σ1.1 in these views are indicated by yellow outlines. A basic patch found at the σ1.1 N terminus is shown. The potential DNA pathway during open complex formation is shown by dotted lines.
Structural Comparison of E. coli and Thermus RNAPs
The structures of the E. coli α subunit dimer and σ70 domains σ2 and σ4 have been determined previously (27, 28, 36) and have already been compared with their counterparts in the Thermus RNAP (17, 37). However, the E. coli RNAP structure from this study enables a direct comparison of the β, β′, and ω subunits between E. coli and Thermus (Fig. 2).
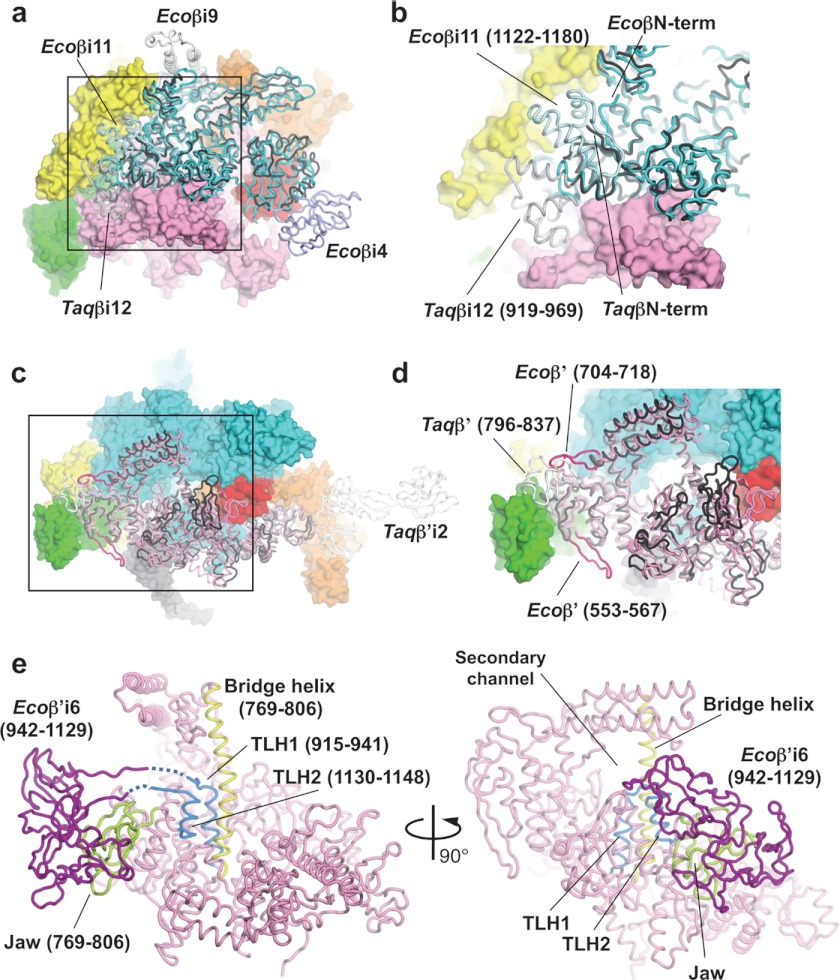
FIGURE 2.
Structure comparisons of the β and β′ subunits of the E. coli and T. aquaticus RNAPs. a, superposition of Ecoβ and Taqβ RNAPs. Ecoβ (cyan) and Taqβ (black) are shown as α-carbon backbones in addition to the molecular surfaces of other E. coli RNAP subunits (αI, yellow; αII, green; β′, pink; σ70, orange; and σ1.1, red). b, magnified view of the boxed region in a. c, superposition of Ecoβ and Taqβ RNAPs. Ecoβ′ (pink and magenta), Taqβ′ (black and white) are shown as α-carbon backbones in addition to the molecular surfaces of other E. coli RNAP subunits (αI, yellow; αII, green; β′, pink; σ70, orange; and σ1.1, red). d, magnified view of the boxed region in c. e, the bridge helix (yellow), TLH (light blue), and jaw (yellow green) are highlighted on the α-carbon backbone of the Ecoβ′ (pink) structure. Ecoβ′i6 (purple) was modeled using the E. coli core enzyme model (24).
The entire architecture of the E. coli β subunit (Ecoβ) can be superimposed on the T. aquaticus β subunit (Taqβ), with deviations around Ecoβi4 (residues 225–343), Ecoβi9 (residues 938–1042), and Ecoβi11 (residues 1122–1180) (Fig. 2, a and b). Ecoβi11 comprises three α helices, with a long loop connecting the second and third α helices, and it is located near the β subunit N terminus. The Ecoβi11 structural homolog in the Thermus RNAP is βi12 (Taqβi12, residues 919–969), but it is located ∼20 Å away from the relative position of Ecoβi11 and does not associate with the N-terminal tail of Taqβ (Fig. 2b). The structures of Ecoβi4 and Ecoβi9 have been determined and described previously (24).
In the case of the β′ subunits of E. coli and Thermus, there is structural conservation distributed throughout the entire subunit (Fig. 2c). However, Ecoβ′ has several insertions that are not present in Taqβ′ and vice versa. These insertions include a 13-amino acid insertion between Thr-553 and Thr-567 of Ecoβ′, a 13-amino acid insertion between Glu-704 and Ser-718 of Ecoβ′, and a domain inserted between Arg-796 and Gly-837 of Taqβ′ (Fig. 2d). Taqβ′ also has a large insert (Taqβ′i2) between conserved regions A and B (Figs. 1c and 2c) (24, 38).
The β′ subunit trigger loop/helix (TLH) plays a critical role in the nucleotide addition cycle (39, 40). The front edge of the TLH (residues 930–941 and 1130–1137) is highly flexible, but it becomes a rigid “trigger helix” structure when an incoming nucleotide is present at the active site. The middle of the E. coli TLH has a large insert (β′i6, residues 942–1129) that separates the TLH into two regions (TLH1, residues 915–941; and TLH2, residues 1130–1148) (Fig. 2e). The edges of TLH1 and TLH2 of E. coli RNAP are in loop conformations (residues 930–933 in TLH1 and residues 1133–1138 in TLH2; residues 934–941 and 1130–1132 are disordered). Ecoβ′i6 plays an important role in all stages of transcription, including open complex formation, transcription pausing, and termination, and its location was proposed to be near the β′ subunit jaw (41). However, β′i6 in the E. coli holoenzyme structure is completely disordered, without any trace of electron density map, indicating that β′i6 is highly mobile in this crystal structure and possibly in an apo-form holoenzyme.
The β′ subunit bridge helix separates the deep groove of RNAP into a DNA-binding main channel and an NTP entry secondary channel (Figs. 1 and 2e) (17). The eukaryotic RNA polymerase II structure shows a straight-form bridge helix (39, 42), whereas the Thermus RNAP structures show a bent-form bridge helix (5, 17). Further crystallographic studies of the Thermus RNAP complex with the antibiotic streptolydigin (11), as well as a transcription elongation complex (18), have shown that an alternative straight-form bridge helix can exist in the Thermus RNAP. Based on these structures, it was proposed that alternate straight-form and bent-form bridge helix conformations are important for the nucleotide additional cycle, including NTP binding and DNA/RNA hybrid translocation (43, 44). The E. coli RNAP holoenzyme structure presented here possesses a straight bridge helix (Fig. 2e).
Structure and Function of the ω Subunit of E. coli RNAP
The ω subunit of E. coli RNAP is composed of five α helices (α1–α5) (Fig. 3a), and the first three α helices (α1–α3) can be overlaid with the first three α helices of the Thermus ω subunit (Fig. 3b). The folding of the ω subunit N-terminal tail in the E. coli RNAP is different in the Thermus RNAP structure. The E. coli ω subunit C-terminal tail, including α4 and α5, is fully extended; the E. coli ω subunit makes no interaction with the C-terminal tail of the β′ subunit, in contrast to the Thermus RNAP, which has an extensive interaction between the ω subunit and the C-terminal tail of the β′ subunit (supplemental Movie S4).
Functionally, the ω subunit is the least understood subunit, but there is a clear link between the ω subunit and ppGpp-dependent transcription (45, 46). The finding that the ω subunit structure is so different in the E. coli and Thermus RNAPs may be related to the observation that E. coli RNAP can respond to ppGpp only in the presence of the ω subunit (46, 47). Thus, the E. coli holoenzyme structure can be used as an ideal system for understanding the relationship between the ω subunit and ppGpp-dependent transcription regulation and may finally reconcile 4 decades of experimental data, especially in understanding the cause of the stringent response and growth control by ppGpp in E. coli cells (48–51).
Structure and Function of σ1.1
Strong and traceable electron density maps of σ70 were attainable from σ1.2 to the C terminus. In one of two E. coli RNAP molecules (RNAPA) in the asymmetric unit, the Fo − Fc electron density map calculated using CNS version 1.3 (30) showed rod-like densities for σ1.1, which is adjacent to σ1.2. A homology model of E. coli σ1.1, which was constructed by SWISS-MODEL (52) based on the T. maritima σ1.1 NMR structure (6), was placed on the σ1.1 electron density map, and the positions of three α helices were manually adjusted. An additional α helix (H4) was then built based on a rod-like density next to the third α helix (H3). The σ1.1 structure was refined in the holoenzyme. The final σ1.1 structure contains four α helices (residues 6–64), and the electron density of residues from position 65 to σ1.2 (residue 95) is completely disordered. The higher B-factor and weak electron density map of σ1.1 in the E. coli holoenzyme structure indicate that σ1.1 is highly mobile in the holoenzyme.
The structure shows that σ1.1 is surrounded by σ2, the β lobe, the β′ clamp, and the β′ cleft (Fig. 4a and supplemental Movie S3). The σ1.1 location in the E. coli RNAP crystal structure is consistent with the E. coli RNAP model derived from systematic FRET and distance-constrained docking (8).
The acidic residues of σ1.1 mask the basic residues of the β lobe and β′ clamp, and σ1.1 fits snugly in the DNA-binding main channel of RNAP, thereby preventing access of either double- or single-stranded DNA to the RNAP active site. Therefore, σ1.1 must disengage from this binding site, or the RNAP clamp must open further (34) to form an open complex.
The structure shows that the three basic residues (Lys-10, Arg-15, and Lys-17) found at the σ1.1 N terminus are surface-exposed and face the outside of the RNAP main channel (Fig. 4b and supplemental Movie S3). These σ1.1 basic residues, together with other positively charged regions, including σ2, the β lobe, the β′ clamp, and the β′ jaw, form a continuous path of negative electrostatic potential for promoter DNA and downstream DNA binding. This region may also serve an important role in bending DNA to form the early stage intermediates between the closed and open promoter complexes (7). Although the presence of basic residues at the σ1.1 N terminus is common in the group 1 σ family, the function of this basic region for transcription has not been tested. This basic region in σ1.1 could make a contribution to open complex formation.
The α Subunit C-terminal Domain within E. coli RNAP
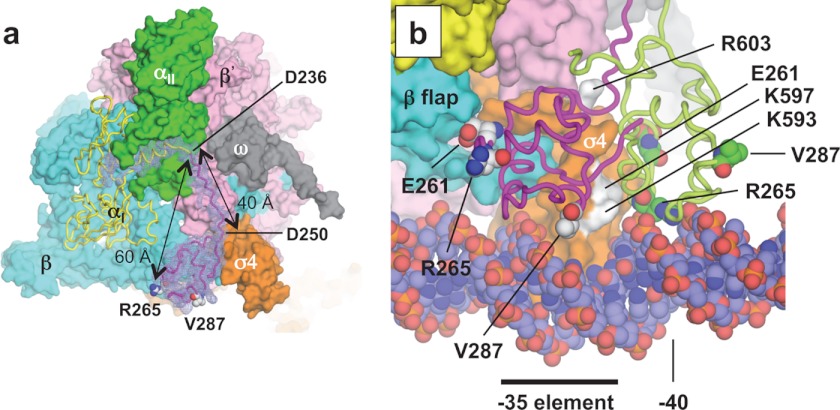
The C-terminal domain of the α subunit (α-CTD, residues 250–329) is a DNA-binding element and a major target of transcription factors for regulation (22, 53). The two α-CTDs of the RNAP holoenzyme, connected to their N-terminal domains (α-NTD) by linkers (54), can interact independently with transcription factors that bind to DNA 40–100 bp upstream from the transcription start site (55, 56). The structure of an α-CTD in the context of an intact RNAP has not been solved because it is dynamic. In the E. coli RNAP structure presented in this study, electron density was visible for only one of the four α-CTDs in the asymmetric unit (RNAPA αI). The map enabled a model of the α-CTD to be fitted (57). Furthermore, the map included density of the linker region that allows modeling of the linker (Fig. 5a). Arg-265 in the α-CTD is ∼60 Å away from the α-NTD (residue 233), with the linker fully stretched and without any secondary structure (Fig. 5a and supplemental Movie S2), indicating that the near-maximum length that Arg-265 in the α-CTD can reach DNA from its N-terminal domain is ∼60 Å.
FIGURE 5.
α-CTD structure. a, difference (Fo − Fc) electron density map between the holoenzyme and holoenzyme without αI residues 215–315 (including the last α-NTD α helix, linker, and α-CTD), shown in mesh, overlaid on the final holoenzyme structure (αII, β, β′, ω, and σ, surface representation; and αI, ribbon model). The positions of two key residues for DNA (Arg-265) and transcription factor (Val-287) interaction on the α-CTD are indicated. Glu-261, another key residue of the α-CTD for the σ4 interaction, positioned between the α-CTD and β subunit, cannot be seen from this view. The distance between the α-NTD and Arg-295 and the length of the linker are shown. b, superimposed holoenzyme crystal structure (this study) and holoenzyme·catabolite activator protein·DNA model (Protein Data Bank code 3IYD). The two structures were aligned by superimposing their σ4 domains. The α-CTD from the crystal structure (magenta) and the model (light green) are shown as ribbons, and key α-CTD residues are shown as spheres. Surface-exposed σ4.2 residues that are predicted for direct interaction with the α-CTD are colored white and are indicated.
Previous biochemical studies suggested that surface-exposed residues in σ4.2 interact directly with the α-CTD (Fig. 5b) (58, 59). Although these residues are partially involved in making the α-CTD·σ4 complex in the E. coli RNAP crystal structure, the orientation of the α-CTD relative to σ4 is different compared with the cryo-EM model of the RNAP·catabolite activator protein·DNA complex (60) and the predicted models of the α-CTD·σ4·DNA complex based on biochemical studies (Fig. 5b and supplemental Movie S2) (58, 59). The structure of the α-CTD in this holoenzyme structure may be one of several possible conformations of free holoenzyme and would have to rearrange itself for promoter DNA binding.
Concluding Remarks
The crystal structure of the E. coli RNAP holoenzyme presented here provides an ideal model for analyzing the functional data that have been generated for over 50 years and for designing future experiments that will uncover the transcription mechanisms. My E. coli RNAP structure reveals the molecular features of the α-CTD and σ1.1 for the first time in the context of an intact bacterial RNAP. Furthermore, I have shown that the E. coli RNAP prepared from a co-overexpression vector can generate sufficient quantities of active RNAP for crystallization and high-quality diffraction. This methodology will facilitate the structure determination of the large collection of mutant RNAPs that have been generated for E. coli transcription and regulation studies. Finally, because the sequence and antibiotic sensitivity of E. coli RNAP are similar to those of pathogen-related RNAPs, including Mycobacterium tuberculosis and Staphylococcus aureus, E. coli RNAP can now be used to readily study RNAP-antibiotic interactions by x-ray crystallography.
Acknowledgments
I thank Scott Pandya, Tomoko Bowser, and Taiki for preparing crystals and Ritwika Basu for synchrotron data collection. I thank the staff at MacCHESS and BCSB for support crystallographic data collection. I thank Seth Darst for providing pACYCDuet-1_Ec_rpoZ. I am grateful to Paul Babitzke, Lucia Rothman-Denes, and Rieko Yajima for critical reading of the manuscript, and Thomas Record, Jr., Amanda Drennan, Pieter de Haseth, Deborah Hinton, and Akira Ishihama for helpful discussions. I thank Nobuyuki Fujita and Shoko Murakami for technical advice for constructing an RNAP-overexpressing vector (pGEMABC). PyMOL was used for preparing figures and calculating the electrostatic potential. This work was initiated in the laboratories of Yasuo Shirakihara at the National Institute of Genetics in Japan and Seth Darst at The Rockefeller University.
This work was supported, in whole or in part, by National Institutes of Health Grant GM087350-A1. This work was also supported by a research fellowship from the Center of Excellence of the Japan Society for the Promotion of Science and a long-term postdoctoral research fellowship from the Human Frontier Science Program.

This article contains supplemental Movies S1–S4.
The atomic coordinates and structure factors (code 4IGC) have been deposited in the Protein Data Bank (http://wwpdb.org/).
N. Fujita and R. E. Glass, personal communication.
- RNAP
- RNA polymerase
- βi
- β insert
- Ecoβ
- E. coli β subunit
- Taqβ
- T. aquaticus β subunit
- TLH
- trigger loop/helix
- α-CTD
- α subunit C-terminal domain
- α-NTD
- α subunit N-terminal domain.
REFERENCES
- 1. Werner F., Grohmann D. (2011) Evolution of multisubunit RNA polymerases in the three domains of life. Nat. Rev. Microbiol. 9, 85–98 [DOI] [PubMed] [Google Scholar]
- 2. Gruber T. M., Gross C. A. (2003) Multiple σ subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57, 441–466 [DOI] [PubMed] [Google Scholar]
- 3. Murakami K. S., Darst S. A. (2003) Bacterial RNA polymerases: the wholo story. Curr. Opin. Struct. Biol. 13, 31–39 [DOI] [PubMed] [Google Scholar]
- 4. Murakami K. S., Masuda S., Darst S. A. (2002) Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science 296, 1280–1284 [DOI] [PubMed] [Google Scholar]
- 5. Vassylyev D. G., Sekine S., Laptenko O., Lee J., Vassylyeva M. N., Borukhov S., Yokoyama S. (2002) Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature 417, 712–719 [DOI] [PubMed] [Google Scholar]
- 6. Schwartz E. C., Shekhtman A., Dutta K., Pratt M. R., Cowburn D., Darst S., Muir T. W. (2008) A full-length group 1 bacterial σ factor adopts a compact structure incompatible with DNA binding. Chem. Biol. 15, 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saecker R. M., Record M. T., Jr., Dehaseth P. L. (2011) Mechanism of bacterial transcription initiation: RNA polymerase-promoter binding, isomerization to initiation-competent open complexes, and initiation of RNA synthesis. J. Mol. Biol. 412, 754–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mekler V., Kortkhonjia E., Mukhopadhyay J., Knight J., Revyakin A., Kapanidis A. N., Niu W., Ebright Y. W., Levy R., Ebright R. H. (2002) Structural organization of bacterial RNA polymerase holoenzyme and the RNA polymerase-promoter open complex. Cell 108, 599–614 [DOI] [PubMed] [Google Scholar]
- 9. Murakami K. S., Masuda S., Campbell E. A., Muzzin O., Darst S. A. (2002) Structural basis of transcription initiation: an RNA polymerase holoenzyme-DNA complex. Science 296, 1285–1290 [DOI] [PubMed] [Google Scholar]
- 10. Mukhopadhyay J., Das K., Ismail S., Koppstein D., Jang M., Hudson B., Sarafianos S., Tuske S., Patel J., Jansen R., Irschik H., Arnold E., Ebright R. H. (2008) The RNA polymerase “switch region” is a target for inhibitors. Cell 135, 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuske S., Sarafianos S. G., Wang X., Hudson B., Sineva E., Mukhopadhyay J., Birktoft J. J., Leroy O., Ismail S., Clark A. D., Jr., Dharia C., Napoli A., Laptenko O., Lee J., Borukhov S., Ebright R. H., Arnold E. (2005) Inhibition of bacterial RNA polymerase by streptolydigin: stabilization of a straight-bridge-helix active-center conformation. Cell 122, 541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belogurov G. A., Vassylyeva M. N., Sevostyanova A., Appleman J. R., Xiang A. X., Lira R., Webber S. E., Klyuyev S., Nudler E., Artsimovitch I., Vassylyev D. G. (2009) Transcription inactivation through local refolding of the RNA polymerase structure. Nature 457, 332–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurwitz J. (2005) The discovery of RNA polymerase. J. Biol. Chem. 280, 42477–42485 [DOI] [PubMed] [Google Scholar]
- 14. Fujita N., Ishihama A. (1996) Reconstitution of RNA polymerase. Methods Enzymol. 273, 121–130 [DOI] [PubMed] [Google Scholar]
- 15. Tang H., Kim Y., Severinov K., Goldfarb A., Ebright R. H. (1996) Escherichia coli RNA polymerase holoenzyme: rapid reconstitution from recombinant α, β, β′, and σ subunits. Methods Enzymol. 273, 130–134 [DOI] [PubMed] [Google Scholar]
- 16. Larson M. H., Landick R., Block S. M. (2011) Single-molecule studies of RNA polymerase: one singular sensation, every little step it takes. Mol. Cell 41, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang G., Campbell E. A., Minakhin L., Richter C., Severinov K., Darst S. A. (1999) Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell 98, 811–824 [DOI] [PubMed] [Google Scholar]
- 18. Vassylyev D. G., Vassylyeva M. N., Perederina A., Tahirov T. H., Artsimovitch I. (2007) Structural basis for transcription elongation by bacterial RNA polymerase. Nature 448, 157–162 [DOI] [PubMed] [Google Scholar]
- 19. Ho M. X., Hudson B. P., Das K., Arnold E., Ebright R. H. (2009) Structures of RNA polymerase-antibiotic complexes. Curr. Opin. Struct. Biol. 19, 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tupin A., Gualtieri M., Leonetti J. P., Brodolin K. (2010) The transcription inhibitor lipiarmycin blocks DNA fitting into the RNA polymerase catalytic site. EMBO J. 29, 2527–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vrentas C. E., Gaal T., Berkmen M. B., Rutherford S. T., Haugen S. P., Vassylyev D. G., Ross W., Gourse R. L. (2008) Still looking for the magic spot: the crystallographically defined binding site for ppGpp on RNA polymerase is unlikely to be responsible for rRNA transcription regulation. J. Mol. Biol. 377, 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Igarashi K., Ishihama A. (1991) Bipartite functional map of the E. coli RNA polymerase α subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell 65, 1015–1022 [DOI] [PubMed] [Google Scholar]
- 23. Doublié S. (1997) Preparation of selenomethionyl proteins for phase determination. Methods Enzymol. 276, 523–530 [PubMed] [Google Scholar]
- 24. Opalka N., Brown J., Lane W. J., Twist K. A., Landick R., Asturias F. J., Darst S. A. (2010) Complete structural model of Escherichia coli RNA polymerase from a hybrid approach. PLoS Biol. 10.1371/journal.pbio.1000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otwinowski Z., Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 27. Malhotra A., Severinova E., Darst S. A. (1996) Crystal structure of a σ70 subunit fragment from E. coli RNA polymerase. Cell 87, 127–136 [DOI] [PubMed] [Google Scholar]
- 28. Blanco A. G., Canals A., Bernués J., Solà M., Coll M. (2011) The structure of a transcription activation subcomplex reveals how σ70 is recruited to PhoB promoters. EMBO J. 30, 3776–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Afonine P. V., Mustyakimov M., Grosse-Kunstleve R. W., Moriarty N. W., Langan P., Adams P. D. (2010) Joint X-ray and neutron refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 66, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schröder G. F., Levitt M., Brunger A. T. (2010) Super-resolution biomolecular crystallography with low-resolution data. Nature 464, 1218–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 32. Hager D. A., Jin D. J., Burgess R. R. (1990) Use of Mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry 29, 7890–7894 [DOI] [PubMed] [Google Scholar]
- 33. Mukherjee K., Nagai H., Shimamoto N., Chatterji D. (1999) GroEL is involved in activation of Escherichia coli RNA polymerase devoid of the ω subunit in vivo. Eur. J. Biochem. 266, 228–235 [DOI] [PubMed] [Google Scholar]
- 34. Chakraborty A., Wang D., Ebright Y. W., Korlann Y., Kortkhonjia E., Kim T., Chowdhury S., Wigneshweraraj S., Irschik H., Jansen R., Nixon B. T., Knight J., Weiss S., Ebright R. H. (2012) Opening and closing of the bacterial RNA polymerase clamp. Science 337, 591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Campbell E. A., Pavlova O., Zenkin N., Leon F., Irschik H., Jansen R., Severinov K., Darst S. A. (2005) Structural, functional, and genetic analysis of sorangicin inhibition of bacterial RNA polymerase. EMBO J. 24, 674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang G., Darst S. A. (1998) Structure of the Escherichia coli RNA polymerase α subunit amino-terminal domain. Science 281, 262–266 [DOI] [PubMed] [Google Scholar]
- 37. Campbell E. A., Muzzin O., Chlenov M., Sun J. L., Olson C. A., Weinman O., Trester-Zedlitz M. L., Darst S. A. (2002) Structure of the bacterial RNA polymerase promoter specificity σ subunit. Mol. Cell 9, 527–539 [DOI] [PubMed] [Google Scholar]
- 38. Chlenov M., Masuda S., Murakami K. S., Nikiforov V., Darst S. A., Mustaev A. (2005) Structure and function of lineage-specific sequence insertions in the bacterial RNA polymerase β′ subunit. J. Mol. Biol. 353, 138–154 [DOI] [PubMed] [Google Scholar]
- 39. Wang D., Bushnell D. A., Westover K. D., Kaplan C. D., Kornberg R. D. (2006) Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell 127, 941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vassylyev D. G., Vassylyeva M. N., Zhang J., Palangat M., Artsimovitch I., Landick R. (2007) Structural basis for substrate loading in bacterial RNA polymerase. Nature 448, 163–168 [DOI] [PubMed] [Google Scholar]
- 41. Artsimovitch I., Svetlov V., Murakami K. S., Landick R. (2003) Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. J. Biol. Chem. 278, 12344–12355 [DOI] [PubMed] [Google Scholar]
- 42. Cramer P., Bushnell D. A., Kornberg R. D. (2001) Structural basis of transcription: RNA polymerase II at 2.8 Å resolution. Science 292, 1863–1876 [DOI] [PubMed] [Google Scholar]
- 43. Svetlov V., Nudler E. (2009) Macromolecular micromovements: how RNA polymerase translocates. Curr. Opin. Struct. Biol. 19, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brueckner F., Ortiz J., Cramer P. (2009) A movie of the RNA polymerase nucleotide addition cycle. Curr. Opin. Struct. Biol. 19, 294–299 [DOI] [PubMed] [Google Scholar]
- 45. Mathew R., Chatterji D. (2006) The evolving story of the ω subunit of bacterial RNA polymerase. Trends Microbiol. 14, 450–455 [DOI] [PubMed] [Google Scholar]
- 46. Igarashi K., Fujita N., Ishihama A. (1989) Promoter selectivity of Escherichia coli RNA polymerase: ω factor is responsible for the ppGpp sensitivity. Nucleic Acids Res. 17, 8755–8765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vrentas C. E., Gaal T., Ross W., Ebright R. H., Gourse R. L. (2005) Response of RNA polymerase to ppGpp: requirement for the ω subunit and relief of this requirement by DksA. Genes Dev. 19, 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Terui Y., Akiyama M., Sakamoto A., Tomitori H., Yamamoto K., Ishihama A., Igarashi K., Kashiwagi K. (2012) Increase in cell viability by polyamines through stimulation of the synthesis of ppGpp regulatory protein and ω protein of RNA polymerase in Escherichia coli. Int. J. Biochem. Cell Biol. 44, 412–422 [DOI] [PubMed] [Google Scholar]
- 49. Chatterji D., Ogawa Y., Shimada T., Ishihama A. (2007) The role of the ω subunit of RNA polymerase in expression of the relA gene in Escherichia coli. FEMS Microbiol. Lett. 267, 51–55 [DOI] [PubMed] [Google Scholar]
- 50. Cashel M. (1969) The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J. Biol. Chem. 244, 3133–3141 [PubMed] [Google Scholar]
- 51. Potrykus K., Cashel M. (2008) (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 [DOI] [PubMed] [Google Scholar]
- 52. Kiefer F., Arnold K., Künzli M., Bordoli L., Schwede T. (2009) The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 37, D387–D392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ross W., Gosink K. K., Salomon J., Igarashi K., Zou C., Ishihama A., Severinov K., Gourse R. L. (1993) A third recognition element in bacterial promoters: DNA binding by the α subunit of RNA polymerase. Science 262, 1407–1413 [DOI] [PubMed] [Google Scholar]
- 54. Blatter E. E., Ross W., Tang H., Gourse R. L., Ebright R. H. (1994) Domain organization of RNA polymerase α subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell 78, 889–896 [DOI] [PubMed] [Google Scholar]
- 55. Murakami K., Owens J. T., Belyaeva T. A., Meares C. F., Busby S. J., Ishihama A. (1997) Positioning of two α subunit carboxy-terminal domains of RNA polymerase at promoters by two transcription factors. Proc. Natl. Acad. Sci. U.S.A. 94, 11274–11278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tebbutt J., Rhodius V. A., Webster C. L., Busby S. J. (2002) Architectural requirements for optimal activation by tandem CRP molecules at a class I CRP-dependent promoter. FEMS Microbiol. Lett. 210, 55–60 [DOI] [PubMed] [Google Scholar]
- 57. Jeon Y. H., Negishi T., Shirakawa M., Yamazaki T., Fujita N., Ishihama A., Kyogoku Y. (1995) Solution structure of the activator contact domain of the RNA polymerase α subunit. Science 270, 1495–1497 [DOI] [PubMed] [Google Scholar]
- 58. Ross W., Schneider D. A., Paul B. J., Mertens A., Gourse R. L. (2003) An intersubunit contact stimulating transcription initiation by E coli RNA polymerase: interaction of the α C-terminal domain and σ region 4. Genes Dev. 17, 1293–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen H., Tang H., Ebright R. H. (2003) Functional interaction between RNA polymerase α subunit C-terminal domain and σ70 in UP-element- and activator-dependent transcription. Mol. Cell 11, 1621–1633 [DOI] [PubMed] [Google Scholar]
- 60. Hudson B. P., Quispe J., Lara-González S., Kim Y., Berman H. M., Arnold E., Ebright R. H., Lawson C. L. (2009) Three-dimensional EM structure of an intact activator-dependent transcription initiation complex. Proc. Natl. Acad. Sci. U.S.A. 106, 19830–19835 [DOI] [PMC free article] [PubMed] [Google Scholar]