Background: Microglial activation contributes strongly to brain inflammation.
Results: The modulation of microglial cyclooxygenase-2, iNOS, and cytokine production by EP2 (PTGER2) activation is not blocked by a protein kinase A antagonist but is mimicked by an Epac agonist.
Conclusion: Epac signaling pathways prominently contribute to the modulation of microglial activation by EP2.
Significance: EP2 and Epac represent potential immunomodulatory targets during brain inflammation.
Keywords: Cytokine, Inflammation, Microglia, Prostaglandins, Protein Kinase A (PKA), EP2, Epac
Abstract
Activation of EP2 receptors by prostaglandin E2 (PGE2) promotes brain inflammation in neurodegenerative diseases, but the pathways responsible are unclear. EP2 receptors couple to Gαs and increase cAMP, which associates with protein kinase A (PKA) and cAMP-regulated guanine nucleotide exchange factors (Epacs). Here, we studied EP2 function and its signaling pathways in rat microglia in their resting state or undergoing classical activation in vitro following treatment with low concentrations of lipopolysaccharide and interferon-γ. Real time PCR showed that PGE2 had no effect on expression of CXCL10, TGF-β1, and IL-11 and exacerbated the rapid up-regulation of mRNAs encoding cyclooxygenase-2, inducible NOS, IL-6, and IL-1β but blunted the production of mRNAs encoding TNF-α, IL-10, CCL3, and CCL4. These effects were mimicked fully by the EP2 agonist butaprost but only weakly by the EP1/EP3 agonist 17-phenyl trinor PGE2 or the EP4 agonist CAY10598 and not at all by the EP3/EP1 agonist sulprostone and confirmed by protein measurements of cyclooxygenase-2, IL-6, IL-10, and TNF-α. In resting microglia, butaprost induced cAMP formation and altered the mRNA expression of inflammatory mediators, but protein expression was unchanged. The PKA inhibitor H89 had little or no effect on inflammatory mediators modulated by EP2, whereas the Epac activator 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate acetoxymethyl ester mimicked all butaprost effects. These results indicate that EP2 activation plays a complex immune regulatory role during classical activation of microglia and that Epac pathways are prominent in this role.
Introduction
As the tissue macrophages of the central nervous system (CNS), microglia play the key role in the innate immunity system of the brain, actively monitoring their environment and reacting quickly to local damage with a nuanced set of “activation responses” (1). About 10–20% of the total glial cells in the brain are microglia (2). Under resting conditions, they are characterized by a small cell body with fine, ramified processes and low expression of surface antigens (3). In response to brain injury, ischemia, or inflammatory stimuli, microglia rapidly transform into an activated phenotype associated with proliferation, migration to the site of injury, elaboration of a host of both neurotoxic and neurotrophic cytokines and chemokines, and phagocytosis of cellular debris (1, 4, 5). Activated microglia have enlarged cell bodies attached to a few short, thick processes and play key roles in neuroinflammation and neurodegenerative diseases.
Cyclooxygenase-2 (COX-2;2 PTGS2) is dramatically up-regulated in neurons following injury or seizures that engage the N-methyl-d-aspartate system (6, 7). Both COX-2 and PGE2 are functionally implicated in brain inflammation. Cytosolic prostaglandin E synthase and microsomal prostaglandin E synthase-2 are constitutively expressed, whereas microsomal prostaglandin E synthase-1 is inducible and is often coupled to COX-2, leading to PGE2 synthesis during inflammation (8–10). PGE2 activates four different G protein-coupled receptors designated EP1, EP2, EP3, and EP4 (11). Previous studies and our real time PCR results show that only EP1, EP2, and EP4 are expressed by rat microglia (12–14), and together they influence microglial function in complex ways. For example, PGE2 decreases microglial expression of IL-12 and B7-2 (necessary for antigen presentation) produced by high concentrations of lipopolysaccharide (LPS) and interferon-γ (IFN-γ) (15, 16). Moreover, PGE2 is reported to down-regulate iNOS, IL-1β, and TNF-α expression in LPS-activated microglia (14, 17) and at the same time to up-regulate the expression of anti-inflammatory IL-10 (18). Based on these findings, PGE2 appears to both interfere with inflammatory pathways and promote anti-inflammatory pathways in activated microglial cells.
EP2 receptors (PTGER2) are seven-transmembrane receptors that couple to Gαs and increase cAMP concentration. Previously, cAMP signaling had only been known to signal through PKA (19). Epac1 (RAPGEF3) and Epac2 (RAPGEF4) have been recently identified as alternative cAMP mediators that regulate physiological processes either alone and/or in concert with PKA (20, 21). Recent evidence based on EP2 gene ablation indicates that activation of EP2 receptors might promote inflammation in several mouse models of neurodegenerative diseases including Alzheimer disease (22), Parkinson disease (23), and amyotrophic lateral sclerosis (24). EP2 gene ablation also abrogates LPS-induced, microglially mediated neurotoxicity, and induction of COX-2 and iNOS (25). A selective and brain-permeant EP2 antagonist has recently been shown to blunt inflammation in a pilocarpine epilepsy model (26). Based on these results, we asked whether modulation of innate immunity by EP2 activation in microglia proceeds through PKA or Epac. Our findings establish an immunomodulatory role for EP2 that proceeds largely through Epac pathways.
EXPERIMENTAL PROCEDURES
Reagents and Solutions
Recombinant rat IFN-γ and granulocyte/macrophage colony-stimulating factor (GM-CSF) were purchased from R&D Systems. LPS was obtained from Sigma. Heat-inactivated fetal bovine serum (FBS), Dulbecco's modified Eagle's medium (high glucose; DMEM), and macrophage serum-free medium were from Invitrogen. PGE2, butaprost, and H89 were from Cayman Chemical. 8-pCPT-2′-O-Me-cAMP-AM was from BioLog Life Science Institute. The novel EP2 potentiator TG3-95-1 (referred to as compound 1 in Ref. 27) and EP2 antagonist TG4-155 were synthesized in our laboratory (27–29). All plasticware and reagents were endotoxin-free.
Animals and Microglial Cell Culture
Pregnant Sprague-Dawley rats were from Charles River Laboratories. Primary microglia were prepared from the cortex of newborn (p1–3) Sprague-Dawley rats as described previously (30, 31). In brief, cortical tissue was carefully freed from blood vessels and meninges, triturated, and washed. Cortical cells were cultured in DMEM, 10% FBS with penicillin/streptomycin plus 2 ng/ml GM-CSF for 11–21 days (medium was changed every 2–3 days). Microglia were separated from the underlying astrocytic monolayer by gentle agitation and spun down (300 × g for 10 min). The cell pellet was resuspended in DMEM, 10% FBS with penicillin/streptomycin plus 0.2 ng/ml GM-CSF and plated on Primaria culture dishes or plates (BD Biosciences). Non-adherent cells were removed after 30–60 min by changing the medium, and then adherent microglia were incubated for 24 h in culture medium before being serum-starved in macrophage serum-free medium plus 0.2 ng/ml GM-CSF for 24 h. Such cultures consist of >95% Ox42-positive microglia (29).
RNA Isolation, Reverse Transcription, and Quantitative Real Time PCR
RNA isolation (including on-column DNase digestion) and cDNA synthesis were done by using the PureLink RNA minikit and Superscript II reverse transcriptase from Invitrogen, and then simplex quantitative real time polymerase chain reaction (PCR) was performed using the iQTM5 Multicolor real time PCR system (Bio-Rad). The iQ SYBR Green SuperMix kit was used to amplify transcripts of interest and endogenous controls HPRT1, β-actin, and GAPDH. Normalization of quantitative real time PCR data was performed by subtracting the geometric average of these three internal control genes from the measured cycle threshold of each gene of interest (32). The following components were combined per 20-μl reaction volume: cDNA, 10 μl of SYBR Green SuperMix, and 400 nm mouse forward primer and reverse primer. Cycling conditions were 95 °C for 3 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Melting curve analysis was used to verify a single species PCR product. Fluorescence data were acquired at the 60 °C step. All experiments had a “no template” negative control, and most primers used were intron-spanning (supplemental Table 1). Data were analyzed by a relative quantification method as described previously (33, 34).
Time-resolved FRET cAMP Assay
cAMP was measured with a homogeneous time-resolved FRET method (Cisbio Bioassays). The assay is based on generation of a strong FRET signal upon the interaction of two molecules: an anti-cAMP antibody coupled to a FRET donor (cryptate) and cAMP coupled to a FRET acceptor (d2). Endogenous cAMP produced by cells competes with labeled cAMP for binding to the cAMP antibody and thus reduces the FRET signal. Briefly, microglia were seeded into 384-well plates in 30 μl of complete medium (4,000 cells/well) and grown overnight. The medium was thoroughly withdrawn, and 10 μl of Hanks' buffered salt solution (Hyclone) plus 20 μm rolipram was added into the wells to block phosphodiesterase. The cells were incubated at room temperature for 30 min and then treated with vehicle or TG4-155 for 30 min before addition of butaprost for 2 h. The cells were lysed in 10 μl of lysis buffer containing the FRET acceptor cAMP-d2, and 1 min later another 10 μl of lysis buffer with anti-cAMP-cryptate was added. After a 60–90-min incubation at room temperature, the time-resolved FRET signal was detected by an Envision 2103 multilabel plate reader (PerkinElmer Life Sciences) with laser excitation at 337 nm and dual emissions at 665 and 590 nm for d2 and cryptate, respectively. The FRET signal is expressed as F665/F590 × 104.
ELISA and Western Blot
After being seeded and then serum-starved, rat microglia received various treatments for 24 h, and then supernatants were collected and frozen at −80 °C. The levels of IL-6 and TNF-α were measured with commercial ELISA kits from R&D Systems. The cells were lysed in radioimmune precipitation assay buffer with proteinase and phosphatase inhibitors (Thermo Scientific). The lysate was cleared by centrifugation at 14,000 × g for 15 min and stored at −80 °C. The protein level of COX-2 was measured by Western blot. The polyclonal COX-2 antibody was from Cayman Chemical, and polyclonal iNOS antibody was from Abcam.
Statistical Analysis
Statistical evaluation was carried out using PRISM software (GraphPad, San Diego, CA). Multiple comparisons were made using one-way analysis of variance with Bonferroni post-test. Data are presented as mean ± S.E., and statistical significance was assumed if p < 0.05.
RESULTS
EP2 Activation Modulates Expression of Inflammatory Mediators in Rat Microglia
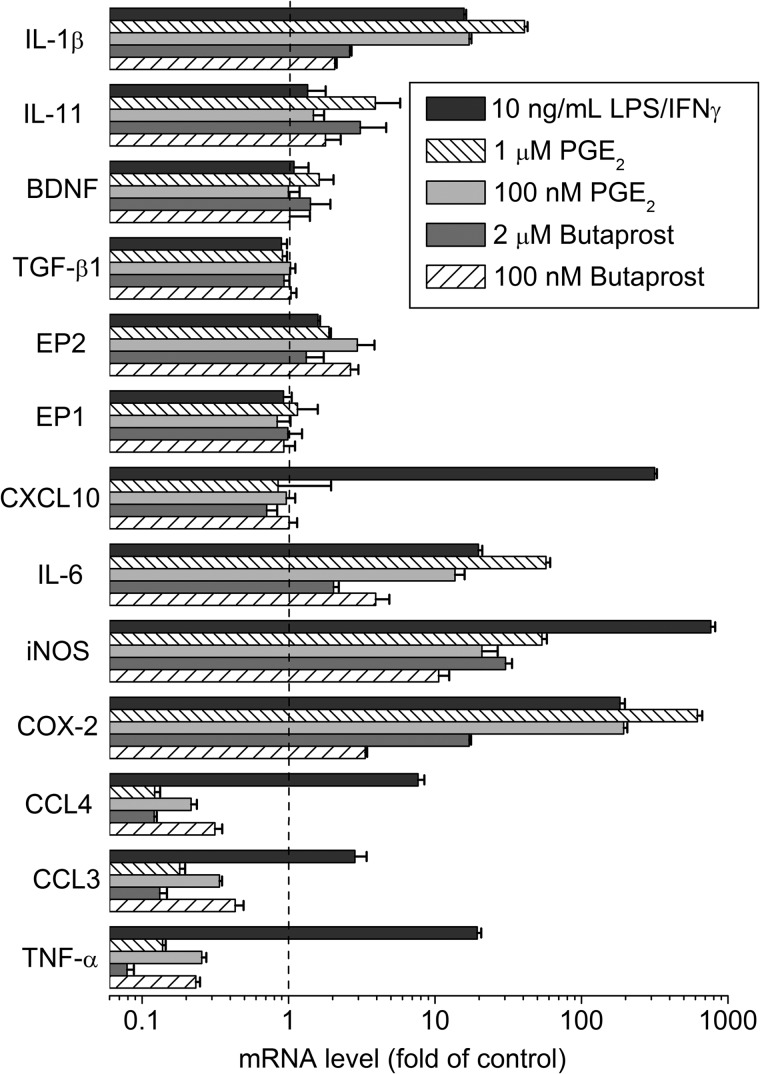
Resting state microglia were stimulated with 100 nm or 1 μm PGE2, 200 nm or 2 μm butaprost, or 10 ng/ml each LPS and IFN-γ for 2 h, and then the levels of mRNAs encoding inflammation-related genes were measured by RT-PCR. We selected 14 inflammatory modulators to study. COX-2; iNOS; the cytokines IL-1β, IL-6, IL-10, IL-11, and TNF-α; and the chemokines CXCL10, CCL3, and CCL4 are all important inflammatory mediators in the brain. Ablation of COX-2 in forebrain neurons dampens brain inflammation after status epilepticus in part by reducing the induction of CCL3, CCL4, CXCL10, IL-11, and TNF-α (6). For the remaining proteins, EP1 and EP2 are important prostanoid receptors that can be activated by PGE2, TGF-β1 appears to be neuroprotective in ischemic brain and stroke, and BDNF supports neuronal survival after injury and encourages the growth and differentiation of new neurons and synapses. Following treatment, the genes fell into four groups (Fig. 1): those that were induced by all three agents (COX-2, iNOS, IL-6, and IL-1β), those induced by LPS/IFN-γ but suppressed by PGE2 and butaprost (TNF-α, IL-10 (see Fig. 3), CCL3, and CCL4), one induced only by LPS/IFN-γ (CXCL10), and those that were affected little or were unaffected by any of the three agents (EP1, EP2, TGF-β1, BDNF, and IL-11). Based on these results, we selected COX-2, iNOS, IL-1β, IL-6, IL-10, TNF-α, CCL3, and CCL4 for additional study.
FIGURE 1.
Effect of PGE2, butaprost, and LPS/IFN-γ on the mRNA expression of inflammatory mediators in resting state rat microglia. Rat microglia were incubated with vehicle, 200 nm butaprost, 2 μm butaprost, 100 nm PGE2, 1 μm PGE2, or 10 ng/ml LPS/IFN-γ for 2 h, and mRNA levels were measured by quantitative real time PCR. The mRNA changes were normalized to the mean of the control group. Data are expressed as mean ± S.E. (error bars), n = 3.
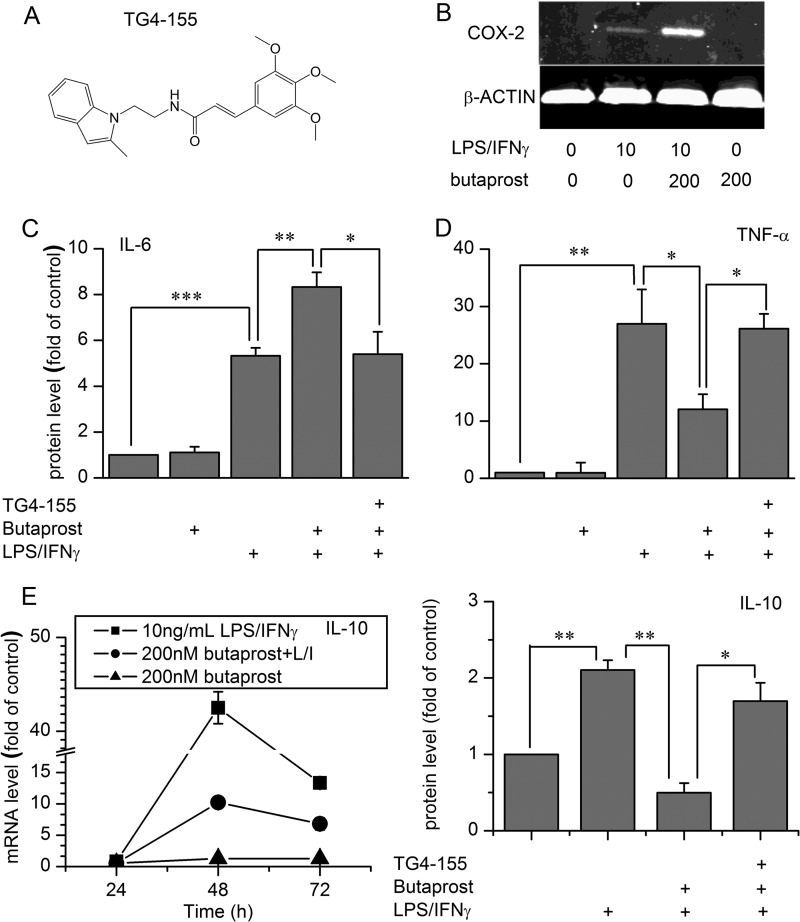
FIGURE 3.
Effect of EP2 activation on secreted cytokines and COX-2 expression in resting or classically activated rat microglia. After treatments as shown below each graph (1 μm TG4-155, 200 nm butaprost, or 10 ng/ml LPS/IFN-γ (L/I)), cell culture medium was harvested for cytokine ELISA, or cells were lysed to obtain total protein samples for Western blot. A, the structure of TG4-155. B, the changes in COX-2 protein induced by different treatments. The data shown are representative of three independent experiments. Different treatments induced changes in IL-6 (C) and TNF-α (D) secretion. Data were analyzed by analysis of variance followed by comparison of selected pairs with Bonferroni correction. Data are expressed as mean ± S.E. (error bars), n = 4. E, changes in IL-10 mRNA and protein under different treatments. Protein was tested by ELISA after treatments for 72 h. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
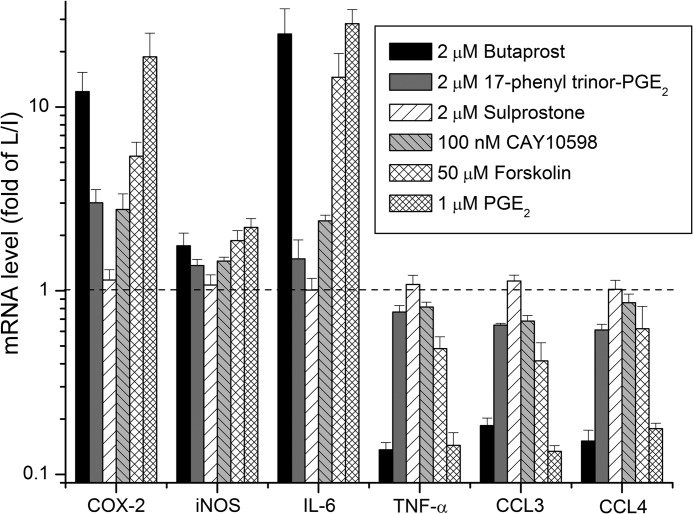
In rat microglia stimulated with 10 ng/ml each LPS and IFN-γ for 2 h to trigger classical activation (35), mRNA levels encoding all eight of these inflammatory mediators were increased as described above. Pretreatment with 1 μm PGE2 for 30 min dose-dependently potentiated the induction of COX-2, iNOS, IL-1β, and IL-6 mRNA and suppressed the LPS/IFN-γ-induced expression of TNF-α, CCL3, and CCL4 mRNA. PGE2 had little or no effect on expression of CXCL10 and EP2 mRNA in activated microglia (not shown). The EP2 agonist butaprost (2 μm) mimicked each PGE2 effect in microglia undergoing classical activation (Figs. 2 and 4). Agonists selective for the other PGE2 receptors were then used to determine whether other PGE2 receptors modulate microglial activation. 17-Phenyl trinor PGE2 activates EP1 and EP3 (36) and increases intracellular [Ca2+] in rat astrocytes with an EC50 of 69 nm.3 Sulprostone activates EP3 receptors with an EC50 <1 nm (36) and has a weaker effect on EP1. Finally, CAY10598 is a selective EP4 agonist (37) with an EC50 of 18 pm for elevating cAMP in human EP4-expressing HEK293 cells, whereas 10 μm CAY10598 elicits only 10% of the maximum cAMP signal in microglia produced by PGE2 (not shown). At saturating concentrations, 17-phenyl trinor PGE2 (2 μm) and CAY10598 (100 nm) were much less effective than butaprost in modulating expression of these inflammatory mediators, and sulprostone (2 μm) had no effect (Fig. 2). These data taken together indicate that EP2 receptors contribute strongly to the modulatory effects of PGE2 in classically activated rat microglia with EP1 and EP4 playing a minor role if any. The two other Gαs-coupled prostanoid receptors, DP1 and IP, appear to play no role in microglia because saturating concentrations of BW245C (1 μm) and iloprost (10 nm) had no effect on cAMP levels (not shown). We also tested the effect of forskolin, a direct adenylate cyclase activator, on expression of these inflammatory mediators in classically activated microglia. The EC50 of forskolin is 10 μm for elevating cAMP in primary rat microglia (not shown). A subsaturating concentration of forskolin (50 μm) mimicked the effect of butaprost and PGE2 on the three EP2-induced inflammatory mediators and partially mimicked their effect on the EP2-suppressed cytokines (Fig. 2). The lower efficacy of forskolin on the down-regulated cytokines suggests involvement of G proteins other than Gαs or perhaps a β-arrestin signaling pathway.
FIGURE 2.
Effect of PGE2 and its receptors on the mRNA expression of inflammatory mediators in classically activated rat microglia. Rat microglia pretreated with vehicle, 2 μm butaprost, 17-phenyl trinor PGE2, sulprostone,100 nm CAY 10598, 50 μm forskolin, or 1 μm PGE2 for 30 min were incubated with 10 ng/ml LPS/IFN-γ (L/I) for 2 h. The mRNA changes were normalized to the mean of the LPS/IFN-γ group. Data are expressed as mean ± S.E. (error bars), n = 3.
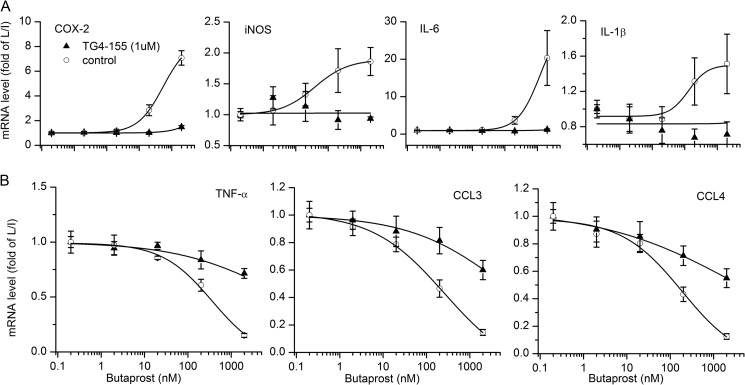
FIGURE 4.
Inhibition of EP2 receptors in classically activated microglia. EP2 antagonist TG4-155 at 1 μm was used to determine how EP2 inhibition affected rat microglia stimulated with 10 ng/ml LPS/IFN-γ (L/I) for 2 h (for iNOS, 1 ng/ml LPS/IFN-γ was used). In the presence of 1 μm TG4-155, the effect of butaprost was suppressed in classically activated microglia. Data are expressed as mean ± S.E. (error bars), n = 4.
ELISA and Western blot were then performed to determine whether secreted cytokine protein levels were also altered by EP2 activation. Induction of COX-2 protein by butaprost required LPS/IFN-γ treatment (Fig. 3B). The EP2 agonist butaprost (200 nm) increased IL-6 secretion in rat microglia stimulated with 10 ng/ml LPS/IFN-γ for 24 h (Fig. 3C) but decreased TNF-α secretion (Fig. 3D), and the EP2 inhibitor TG4-155 (1 μm; Fig. 3A) prevented these effects. EP2 activation by butaprost in the absence of LPS/IFN-γ, however, had no effect on 24-h inflammatory protein expression (Fig. 3, B–D) even though 2 h of butaprost treatment alone altered mRNA expression of these cytokines in resting microglia (Fig. 1). Similar effects were obtained when cytokine and COX-2 protein levels were measured 8 h after stimulation with butaprost (not shown), making it unlikely that a transient protein induction escaped notice. The EP2 antagonist did not alter inflammatory mediator levels beyond that of LPS/IFN-γ treatment itself (Fig. 3, C and D), suggesting that classical activation of microglia does not require EP2 receptor activation. Similarly, LPS/IFN-γ (10 ng/ml for 72 h) induced IL-10 mRNA and protein levels, and butaprost (200 nm) opposed IL-10 induction (Fig. 3E). These results indicate that EP2 activation rapidly modulates the mRNA expression of inflammatory mediators in both resting and activated microglia, but these effects are translated to changes in protein expression only in microglia undergoing classical activation.
EP2 Receptor Pharmacology in Rat Microglia
The results described above indicate that, of the four PGE2 receptors, EP2 is the most prominent modulator of inflammatory mediators in resting or activated microglia. To explore EP2 pharmacology in microglia, we examined the effects of an EP2 receptor antagonist (28) and allosteric potentiator (27).
Rat primary microglia cultures were preincubated with vehicle or 1 μm competitive EP2 antagonist TG4-155 (28) for 20 min followed by addition of the EP2 agonist butaprost at different concentrations. After 5 min, microglia were stimulated with 10 ng/ml LPS/IFN-γ for 2 h, and then mRNA was isolated to measure inflammatory gene expression. The expression of iNOS is more sensitive to LPS/IFN-γ so 1 ng/ml LPS/IFN-γ was used for iNOS. Butaprost dose-dependently potentiated the induction of COX-2, iNOS, IL-1β, and IL-6 mRNA expression in classically activated microglia, and TG4-155 at 1 μm completely blocked these effects (Fig. 4A). Additionally, butaprost dose-dependently down-regulated TNF-α, CCL3, and CCL4 mRNA expression in classically activated microglia, but TG4-155 at 1 μm appeared to be less potent at inhibiting the inhibitory effects of butaprost (Fig. 4B). Thus, TG4-155 antagonized the effect of EP2 receptors in classically activated microglia, although it appeared to be more potent against the EP2-inducible mediators than the EP2-down-regulated mediators, suggesting that different conformations of the EP2 receptor might exist.
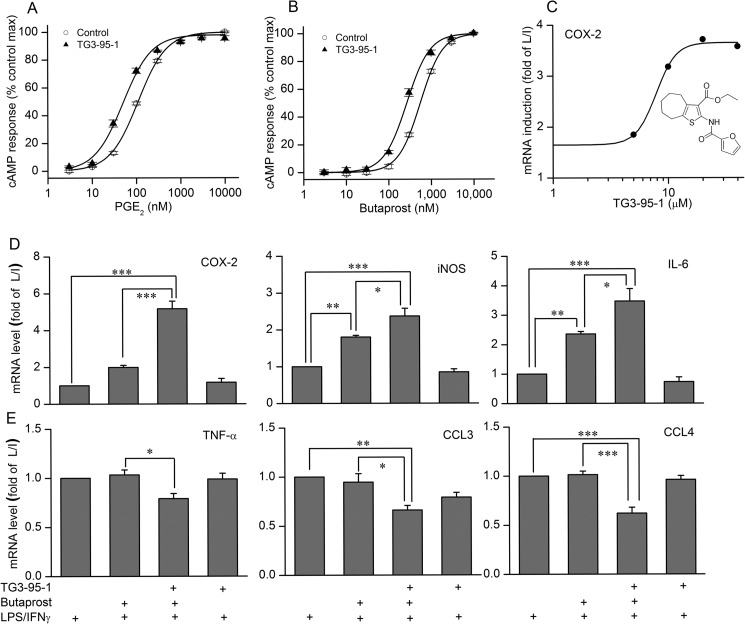
The thiophene carboxylate TG3-95-1 (Fig. 5C, inset; referred to as compound 1 in Ref. 27) was previously reported as an allosteric potentiator of recombinant human EP2 receptors expressed in C6 glioma cells (27). Similar to its mode of action on human EP2 receptors, TG3-95-1 also increased the potency of both PGE2 and butaprost 2.1-fold on endogenous EP2 receptors in rat microglia (Fig. 5, A and B). This molecule was then used to determine whether potentiation of EP2 affected the expression of inflammatory gene mRNA in rat microglia stimulated with 10 ng/ml LPS/IFN-γ for 2 h. TG3-95-1 at 20 μm potentiated both the induction of COX-2, iNOS, IL-1β (not shown), and IL-6 mRNA expression by 100 nm butaprost (Fig. 5D) and the reduced expression of TNF-α, CCL3, and CCL4 mRNA (Fig. 5E). The EC50 of TG3-95-1 for potentiating COX-2 induction is 7.9 μm (Fig. 5C), consistent with its potency on cAMP production (27). In the absence of butaprost, the EP2 potentiator had no effect, reinforcing the conclusion that microglial activation does not require EP2.
FIGURE 5.
Potentiation of EP2 receptors in classically activated microglia. A and B, EP2 potentiator TG3-95-1 at 20 μm was used to determine how EP2 potentiation affected cAMP levels induced by PGE2 and butaprost in rat primary microglia. C, effect of EP2 potentiator on the COX-2 mRNA expression in the presence of 100 nm butaprost in classically activated microglia. Data are expressed as mean ± S.E. (error bars), n = 4. Changes in COX-2, iNOS, and IL-6 mRNA expression (D) and TNF-α, CCL3, and CCL4 mRNA expression (E) caused by 20 μm EP2 potentiator TG3-95-1 in the presence of 100 nm butaprost in classically activated microglia. *, p < 0.05; **, p < 0.01; ***, p < 0.001. L/I, LPS/IFN-γ.
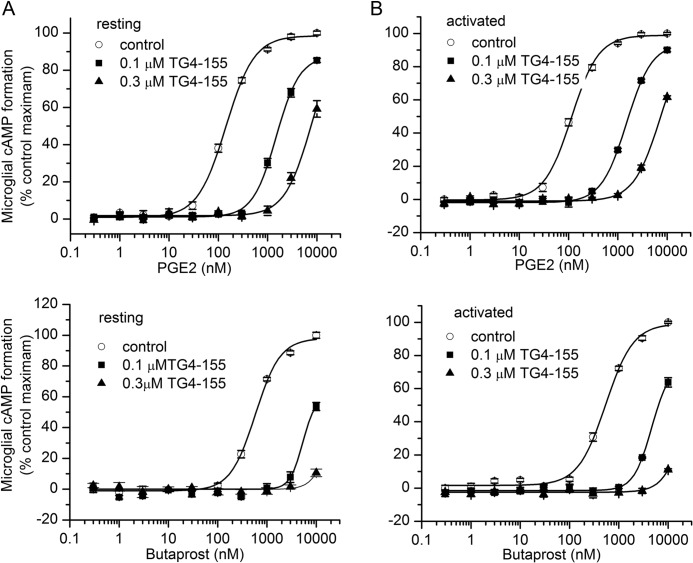
To determine whether classical activation alters the potency of EP2 agonists or antagonist, we preincubated rat primary microglia cultures with vehicle or 100 nm TG4-155 for 30 min followed by addition of butaprost or PGE2 for 2 h. Cellular cAMP levels were evaluated by a time-resolved FRET assay. Butaprost and PGE2 dose-dependently increased the cAMP level in rat primary microglia with similar potencies in the resting or activated state (Fig. 6, A–D). Likewise, 100 nm or 0.3 μm TG4-155 produced a parallel shift to the right in the concentration-response curves of both agonists with the magnitude of the shift being similar in both resting and activated state conditions. The estimated Schild KB is 14 nm against butaprost and 10 nm against PGE2, which is about a 5-fold lower potency than that measured in C6G cells overexpressing human EP2 receptors (28). These data together with pharmacological results described above demonstrate that PGE2 and butaprost up to at least 1 μm act entirely on EP2 receptors in both resting and activated rat microglia, that the weaker action of butaprost on EP3 receptors (38) does not come into play under our conditions, and that the only source of cAMP in PGE2-stimulated microglia is EP2.
FIGURE 6.
cAMP levels induced by EP2 activation in rat primary microglia. Rat microglia pretreated with vehicle or 0.1 or 0.3 μm TG4-155 for 30 min were incubated with different concentrations of PGE2 or butaprost for 2 h. TG4-155 caused a concentration-dependent shift to the right in the PGE2 or butaprost concentration-response curve in resting state microglia (A) and classically activated microglia (B). Data points represent mean ± S.E. (error bars) from a single experiment run in triplicate.
Epac Contributes to EP2 Signaling in Rat Microglia
Cyclic AMP produced by EP2 activation can bind to the regulatory subunit of PKA, causing dissociation of this subunit from the enzyme and thus activating the catalytic subunit, or bind to a regulatory subunit of Epac with a similar effect (21). To determine which of these pathways mediates the effect of EP2 activation on inflammatory mediators, we studied the effects of the PKA inhibitor H89 at 5 μm and Epac activator 8-pCPT-2′-O-Me-cAMP-AM up to 10 μm. These concentrations were chosen as being selective for PKA and Epac, respectively (39–42).
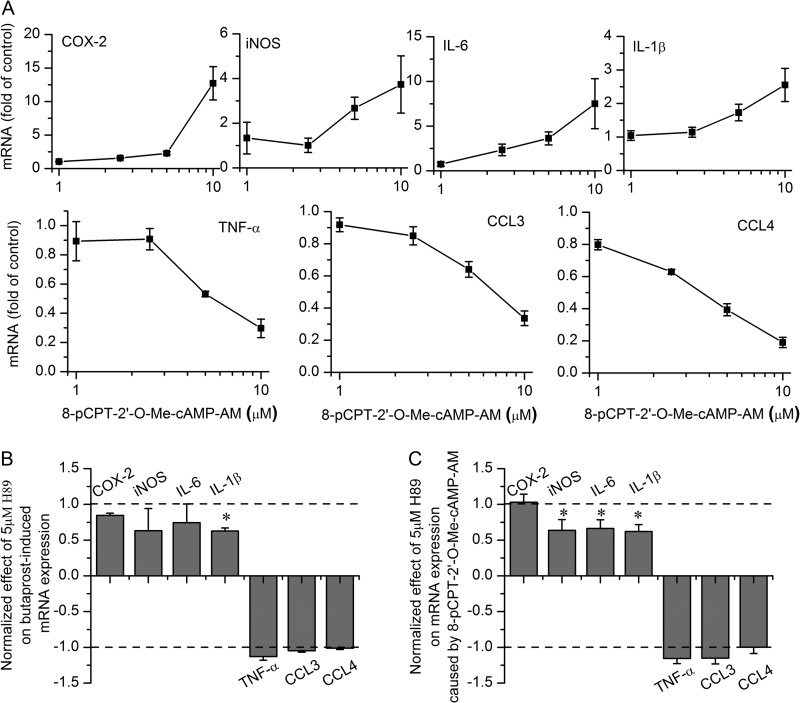
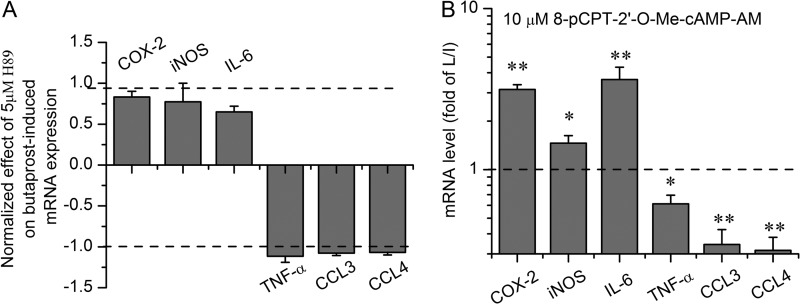
As shown in Fig. 7A, the Epac agonist had a qualitatively similar effect on the mRNA levels of seven inflammatory mediators in resting microglia as had been previously shown for PGE2 and butaprost (cf. Fig. 1). H89 (5 μm) exerted little or no inhibition of the effect of butaprost (Fig. 7B). Likewise, in microglia undergoing classical activation, H89 (5 μm) failed to block the modulation by butaprost of the mRNA expression of these inflammatory mediators (Fig. 8A).
FIGURE 7.
EP2 receptor signaling pathways in resting state microglia. Epac activator 8-pCPT-2′-O-Me-cAMP-AM (1–10 μm) caused changes in mRNA expression of the inflammatory mediators (A) in resting state microglia that mimicked those of EP2 activation. Data are expressed as mean ± S.E. (error bars), n = 5. In the presence of 5 μm H89, mRNA expression changes in the butaprost group (B) or 8-pCPT-2′-O-Me-cAMP-AM group (C) were normalized by setting the control group as 0 and the butaprost or 8-pCPT-2′-O-Me-cAMP-AM group as ±1. *, p < 0.05 versus butaprost or 8-pCPT-2′-O-Me-cAMP-AM group.
FIGURE 8.
EP2 receptor signaling pathways in classically activated microglia. H89 at 5 μm had little or no effect on expression of inflammatory mediator mRNA. The H89 effects were normalized by setting the LPS/IFN-γ (L/I) group as 0 and the butaprost with LPS/IFN-γ group as ±1 (A). Epac activator 8-pCPT-2′-O-Me-cAMP-AM at 10 μm induced changes in inflammatory mediator mRNA expression (B) in classically activated microglia that mimicked the effects of EP2 activation. Data are expressed as mean ± S.E. (error bars), n = 3. *, p < 0.05; **, p < 0.01 versus the LPS/IFN-γ group.
We next turned our attention to the effect of an Epac agonist (10 μm), which was found to mimic the effect of butaprost in microglia undergoing activation (Fig. 8B). H89 exerted similar small effects on the response to the Epac agonist (Fig. 7C) in resting microglia, suggesting that PKA impinges on the Epac pathway. An Epac antagonist has recently been reported (43, 44), but we found that this antagonist (10 μm) had no consistent effect on cytokine modulation by the Epac agonist in primary microglia, which precluded its testing against butaprost; we presume the antagonist has poor cell penetration in microglia under our conditions. These data are consistent with the notion that cAMP-activated Epac, not PKA, mediates much of the effect of the EP2 receptor on the mRNA expression of inflammatory genes in both resting and classically activated microglia.
DISCUSSION
We show that in classically activated or resting state microglia EP2 receptor activation by PGE2 or butaprost creates a mixed immune state by exacerbating the rapid induction of proinflammatory COX-2, iNOS, IL-1β, and IL-6 but blunting the induction of proinflammatory TNF-α and the chemotactic factors CCL3 and CCL4. Selective activation of the other PGE2 receptors, EP1, EP3, and EP4, has little or no effect on expression of these inflammatory mediators. Based on the effects of a selective PKA antagonist and a selective Epac activator, we conclude that cAMP-activated Epac, not PKA, appears to mediate the effect of EP2 receptors on these inflammatory genes in classically activated microglia.
COX-2 is an important component of neuroinflammation. In an Alzheimer disease model, a selective COX-2 inhibitor decreased inflammatory factors and attenuated astrogliosis and neuronal cell loss (45). Inhibition of COX-2 was able to prevent or slow down dopamine neuron degeneration either directly or through inhibition of microglial activation (46). Likewise, conditional ablation of COX-2 from principal forebrain neurons was neuroprotective and anti-inflammatory in a mouse model of epilepsy (6). iNOS, induced after inflammatory insults in the brain, mediates neurotoxicity because of the oxidative/nitrosative effects produced by NO release and the production of peroxynitrite. In stroke models, excessive glutamate receptor activation induces neurotoxicity partly by iNOS and peroxynitrite production (47). Pharmacological inhibition of iNOS delayed disease onset and extended survival in mutant SOD1 mice, showing that iNOS expression in amyotrophic lateral sclerosis contributed to disease progression (48). IL-6 is a multifunctional cytokine during neuroinflammation in the central nervous system. Mice with IL-6 deficiency were fully resistant to experimental allergic encephalomyelitis, and IL-6 is proposed to be a therapeutic target for autoimmune diseases (49). However, in traumatic brain injury, mice deficient for IL-6 showed increased oxidative stress, decreased cell survival, and delayed wound healing, indicating that IL-6 might also be protective (50). Here, we show that PGE2 and EP2 activation by butaprost exacerbates the induction of COX-2, iNOS, and IL-6, which should promote inflammation caused by microglial activation, in classically activated microglia.
COX-2 is present in cytosolic vesicle-like structures in macrophages (51), and its protein expression and PGE2 secretion are low in rat microglia even after treatment with 10 ng/ml LPS/IFN-γ for 24 h. Butaprost, however, can increase COX-2 expression at both the mRNA and protein levels (Figs. 1–3), confirming previous results in microglia (25) and suggesting that EP2 activation on microglia by PGE2 released from any source should produce a self-reinforcing proinflammatory effect. The major source of PGE2 in the early stages of inflammation is likely to be neurons (6), which would promote brain inflammation in part by activating EP2 receptors on nearby microglia.
Based on the effects of EP2 activation on activated microglia, the consequences of EP2 receptor activation at the systems level in the inflamed brain are expected to be complex. Prolonged incubation of rat microglia with LPS/IFN-γ induces delayed expression of the anti-inflammatory cytokine IL-10, which limits inflammation and promotes survival of neurons and glial cells in the brain (56). Induced IL-10 by LPS/IFN-γ is blunted by EP2 activation. The reduction in IL-10 expression is expected to be a secondary consequence of reduced TNF-α expression because TNF-α is a strong inducer of IL-10 (52). The cytokine TNF-α plays a multidimensional role in inflammation-induced neuronal damage in brain diseases. TNF-α expression in substantia nigra elicits progressive neurodegeneration, motor symptoms, and microglial activation (53), and TNF-α receptor activation enhances microglial phagocytic activity (54). Inhibition of TNF-α prevents cognitive decline and β-amyloid accumulation in a mouse model of Alzheimer disease (55). Chemokines CCL3 and CCL4 function to attract macrophages from blood in addition to microglia migrating to the injured site in the brain and thereby magnify local inflammation in neurodegenerative diseases (56). Our results show that EP2 activation blunts the induction of TNF-α and the chemotactic factors CCL3 and CCL4 in classically activated microglia, and this may inhibit chemotaxis of inflammatory cells in the brain.
Modulation of the immune response by cAMP in monocytes and macrophages had been attributed to PKA and PKA-mediated changes in protein expression and function (57). However, Epac can act as an alternative cAMP mediator in monocytes and macrophages (20). Cytokine and chemokine production in bone marrow-derived dendritic cells involves both Epac1 and PKA (58). It has also been reported that high concentrations of PKA and cell-impermeable Epac agonists play differential roles in the production of cytokines in primary cultured microglia stimulated with a high concentration of endotoxin (59). In our study, the actions of selective concentrations of both H89 (5 μm) and the cell-permeable Epac agonist (10 μm) suggest that cAMP-activated Epac rather than PKA mediates the effect of EP2 receptors on the expression of COX-2, iNOS, and inflammatory cytokines in classically activated rat microglia.
Our study demonstrates that EP2 activation promotes brain inflammation by exacerbating COX-2, iNOS, IL-1β, and IL-6 induction and by opposing IL-10 induction in classically activated rat microglia. At the same time, PGE2 and EP2 activation also plays a negative feedback role by decreasing TNF-α, CCL3, and CCL4 release to inhibit chemotaxis of inflammatory cells. In conclusion, PGE2-activated EP2 regulates innate immunity in the central nervous system in a nuanced manner by promoting many aspects of inflammation while reducing chemotaxis of macrophages and microglia to the inflamed area. Epac signaling pathways are engaged in these EP2 actions and may provide new drug targets for brain inflammation.
This work was supported, in whole or in part, by National Institutes of Health Grants U01NS058158 and R21NS074169 (both to R. D.) from the NINDS and the Countermeasures against Chemical Threats (CounterACT) Program, National Institutes of Health Office of the Director. This work was also supported by the Epilepsy Foundation (to J. J.).

This article contains supplemental Table 1.
S. J. Myers and R. Dingledine, unpublished data.
- COX-2
- cyclooxygenase-2
- PGE2
- prostaglandin E2
- Epac
- cAMP-regulated guanine nucleotide exchange factor
- iNOS
- inducible NOS
- 8-pCPT-2′-O-Me-cAMP-AM
- 8-(4-chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate acetoxymethyl ester.
REFERENCES
- 1. Hanisch U. K., Kettenmann H. (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 [DOI] [PubMed] [Google Scholar]
- 2. Carson M. J., Thrash J. C., Walter B. (2006) The cellular response in neuroinflammation: the role of leukocytes, microglia and astrocytes in neuronal death and survival. Clin. Neurosci. Res. 6, 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kreutzberg G. W. (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 [DOI] [PubMed] [Google Scholar]
- 4. Melchior B., Puntambekar S. S., Carson M. J. (2006) Microglia and the control of autoreactive T cell responses. Neurochem. Int. 49, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garden G. A., Möller T. (2006) Microglia biology in health and disease. J. Neuroimmune Pharmacol. 1, 127–137 [DOI] [PubMed] [Google Scholar]
- 6. Serrano G. E., Lelutiu N., Rojas A., Cochi S., Shaw R., Makinson C. D., Wang D., FitzGerald G. A., Dingledine R. (2011) Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J. Neurosci. 31, 14850–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamagata K., Andreasson K. I., Kaufmann W. E., Barnes C. A., Worley P. F. (1993) Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11, 371–386 [DOI] [PubMed] [Google Scholar]
- 8. Murakami M., Naraba H., Tanioka T., Semmyo N., Nakatani Y., Kojima F., Ikeda T., Fueki M., Ueno A., Oh S., Kudo I. (2000) Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275, 32783–32792 [DOI] [PubMed] [Google Scholar]
- 9. Tanioka T., Nakatani Y., Semmyo N., Murakami M., Kudo I. (2000) Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J. Biol. Chem. 275, 32775–32782 [DOI] [PubMed] [Google Scholar]
- 10. Murakami M., Nakashima K., Kamei D., Masuda S., Ishikawa Y., Ishii T., Ohmiya Y., Watanabe K., Kudo I. (2003) Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J. Biol. Chem. 278, 37937–37947 [DOI] [PubMed] [Google Scholar]
- 11. Narumiya S., Sugimoto Y., Ushikubi F. (1999) Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79, 1193–1226 [DOI] [PubMed] [Google Scholar]
- 12. Cimino P. J., Keene C. D., Breyer R. M., Montine K. S., Montine T. J. (2008) Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr. Med. Chem. 15, 1863–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi J., Johansson J., Woodling N. S., Wang Q., Montine T. J., Andreasson K. (2010) The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J. Immunol. 184, 7207–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caggiano A. O., Kraig R. P. (1999) Prostaglandin E receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1β production. J. Neurochem. 72, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aloisi F., Penna G., Cerase J., Menéndez Iglesias B., Adorini L. (1997) IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 159, 1604–1612 [PubMed] [Google Scholar]
- 16. Menèndez Iglesias B., Cerase J., Ceracchini C., Levi G., Aloisi F. (1997) Analysis of B7–1 and B7–2 costimulatory ligands in cultured mouse microglia: upregulation by interferon-γ and lipopolysaccharide and downregulation by interleukin-10, prostaglandin E2 and cyclic AMP-elevating agents. J. Neuroimmunol. 72, 83–93 [DOI] [PubMed] [Google Scholar]
- 17. Minghetti L., Nicolini A., Polazzi E., Créminon C., Maclouf J., Levi G. (1997) Inducible nitric oxide synthase expression in activated rat microglial cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia 19, 152–160 [PubMed] [Google Scholar]
- 18. Aloisi F., De Simone R., Columba-Cabezas S., Levi G. (1999) Opposite effects of interferon-γ and prostaglandin E2 on tumor necrosis factor and interleukin-10 production in microglia: a regulatory loop controlling microglia pro- and anti-inflammatory activities. J. Neurosci. Res. 56, 571–580 [DOI] [PubMed] [Google Scholar]
- 19. Cohen P. (2002) Protein kinases—the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 1, 309–315 [DOI] [PubMed] [Google Scholar]
- 20. Serezani C. H., Ballinger M. N., Aronoff D. M., Peters-Golden M. (2008) Cyclic AMP: master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 39, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gloerich M., Bos J. L. (2010) Epac: defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375 [DOI] [PubMed] [Google Scholar]
- 22. Shie F. S., Breyer R. M., Montine T. J. (2005) Microglia lacking E prostanoid receptor subtype 2 have enhanced Aβ phagocytosis yet lack Aβ-activated neurotoxicity. Am. J. Pathol. 166, 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jin J., Shie F. S., Liu J., Wang Y., Davis J., Schantz A. M., Montine K. S., Montine T. J., Zhang J. (2007) Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated α-synuclein. J. Neuroinflammation 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang X., Wang Q., Shi J., Lokteva L., Breyer R. M., Montine T. J., Andreasson K. (2008) The prostaglandin E2 EP2 receptor accelerates disease progression and inflammation in a model of amyotrophic lateral sclerosis. Ann. Neurol. 64, 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shie F. S., Montine K. S., Breyer R. M., Montine T. J. (2005) Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia 52, 70–77 [DOI] [PubMed] [Google Scholar]
- 26. Jiang J., Quan Y., Ganesh T., Pouliot W. A., Dudek F. E., Dingledine R. (February 11, 2013) Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.1218498110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang J., Ganesh T., Du Y., Thepchatri P., Rojas A., Lewis I., Kurtkaya S., Li L., Qui M., Serrano G., Shaw R., Sun A., Dingledine R. (2010) Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc. Natl. Acad. Sci. U.S.A. 107, 2307–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang J., Ganesh T., Du Y., Quan Y., Serrano G., Qui M., Speigel I., Rojas A., Lelutiu N., Dingledine R. (2012) Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc. Natl. Acad. Sci. U.S.A. 109, 3149–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang J., Dingledine R. (2013) Role of prostaglandin receptor EP2 in the regulations of cancer cell proliferation, invasion, and inflammation. J. Pharmacol. Exp. Ther. 344, 360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quan Y., Möller T., Weinstein J. R. (2009) Regulation of Fcγ receptors and immunoglobulin G-mediated phagocytosis in mouse microglia. Neurosci. Lett. 464, 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Quan Y., Jiang C. T., Xue B., Zhu S. G., Wang X. (2011) High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways. Acta Pharmacol. Sin. 32, 188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weinstein J. R., Zhang M., Kutlubaev M., Lee R., Bishop C., Andersen H., Hanisch U. K., Möller T. (2009) Thrombin-induced regulation of CD95(Fas) expression in the N9 microglial cell line: evidence for involvement of proteinase-activated receptor(1) and extracellular signal-regulated kinase 1/2. Neurochem. Res. 34, 445–452 [DOI] [PubMed] [Google Scholar]
- 34. Quan Y., Du J., Wang X. (2007) High glucose stimulates GRO secretion from rat microglia via ROS, PKC, and NF-κB pathways. J. Neurosci. Res. 85, 3150–3159 [DOI] [PubMed] [Google Scholar]
- 35. Martinez F. O., Helming L., Gordon S. (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 [DOI] [PubMed] [Google Scholar]
- 36. Kiriyama M., Ushikubi F., Kobayashi T., Hirata M., Sugimoto Y., Narumiya S. (1997) Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 122, 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Billot X., Chateauneuf A., Chauret N., Denis D., Greig G., Mathieu M. C., Metters K. M., Slipetz D. M., Young R. N. (2003) Discovery of a potent and selective agonist of the prostaglandin EP4 receptor. Bioorg. Med. Chem. Lett. 13, 1129–1132 [DOI] [PubMed] [Google Scholar]
- 38. Abramovitz M., Adam M., Boie Y., Carrière M., Denis D., Godbout C., Lamontagne S., Rochette C., Sawyer N., Tremblay N. M., Belley M., Gallant M., Dufresne C., Gareau Y., Ruel R., Juteau H., Labelle M., Ouimet N., Metters K. M. (2000) The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta 1483, 285–293 [DOI] [PubMed] [Google Scholar]
- 39. Engh R. A., Girod A., Kinzel V., Huber R., Bossemeyer D. (1996) Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J. Biol. Chem. 271, 26157–26164 [DOI] [PubMed] [Google Scholar]
- 40. Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vliem M. J., Ponsioen B., Schwede F., Pannekoek W. J., Riedl J., Kooistra M. R., Jalink K., Genieser H. G., Bos J. L., Rehmann H. (2008) 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chembiochem 9, 2052–2054 [DOI] [PubMed] [Google Scholar]
- 42. Chepurny O. G., Leech C. A., Kelley G. G., Dzhura I., Dzhura E., Li X., Rindler M. J., Schwede F., Genieser H. G., Holz G. G. (2009) Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM. J. Biol. Chem. 284, 10728–10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen H., Tsalkova T., Mei F. C., Hu Y., Cheng X., Zhou J. (2012) 5-Cyano-6-oxo-1,6-dihydro-pyrimidines as potent antagonists targeting exchange proteins directly activated by cAMP. Bioorg. Med. Chem. Lett. 22, 4038–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Almahariq M., Tsalkova T., Mei F. C., Chen H., Zhou J., Sastry S. K., Schwede F., Cheng X. (2013) A novel EPAC specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol. Pharmacol. 83, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dargahi L., Nasiraei-Moghadam S., Abdi A., Khalaj L., Moradi F., Ahmadiani A. (2011) Cyclooxygenase (COX)-1 activity precedes the COX-2 induction in Aβ-induced neuroinflammation. J. Mol. Neurosci. 45, 10–21 [DOI] [PubMed] [Google Scholar]
- 46. Sánchez-Pernaute R., Ferree A., Cooper O., Yu M., Brownell A. L., Isacson O. (2004) Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J. Neuroinflammation 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumar A., Singh R. L., Babu G. N. (2010) Cell death mechanisms in the early stages of acute glutamate neurotoxicity. Neurosci. Res. 66, 271–278 [DOI] [PubMed] [Google Scholar]
- 48. Chen K., Northington F. J., Martin L. J. (2010) Inducible nitric oxide synthase is present in motor neuron mitochondria and Schwann cells and contributes to disease mechanisms in ALS mice. Brain Struct. Funct. 214, 219–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka T., Katada Y., Higa S., Fujiwara H., Wang W., Saeki Y., Ohshima S., Okuda Y., Suemura M., Kishimoto T. (2001) Enhancement of T helper2 response in the absence of interleukin (IL-)6; an inhibition of IL-4-mediated T helper2 cell differentiation by IL-6. Cytokine 13, 193–201 [DOI] [PubMed] [Google Scholar]
- 50. Penkowa M., Giralt M., Carrasco J., Hadberg H., Hidalgo J. (2000) Impaired inflammatory response and increased oxidative stress and neurodegeneration after brain injury in interleukin-6-deficient mice. Glia 32, 271–285 [DOI] [PubMed] [Google Scholar]
- 51. Yamashita M., Tsuji S., Nishiyama A., Myrvik Q. N., Henriksen R. A., Shibata Y. (2007) Differential subcellular localization of COX-2 in macrophages phagocytosing heat-killed Mycobacterium bovis BCG. Am. J. Physiol. Cell Physiol. 293, C184–C190 [DOI] [PubMed] [Google Scholar]
- 52. Foey A. D., Parry S. L., Williams L. M., Feldmann M., Foxwell B. M., Brennan F. M. (1998) Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-α: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. 160, 920–928 [PubMed] [Google Scholar]
- 53. De Lella Ezcurra A. L., Chertoff M., Ferrari C., Graciarena M., Pitossi F. (2010) Chronic expression of low levels of tumor necrosis factor-α in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol. Dis. 37, 630–640 [DOI] [PubMed] [Google Scholar]
- 54. von Zahn J., Möller T., Kettenmann H., Nolte C. (1997) Microglial phagocytosis is modulated by pro- and anti-inflammatory cytokines. Neuroreport 8, 3851–3856 [DOI] [PubMed] [Google Scholar]
- 55. Chavant F., Deguil J., Pain S., Ingrand I., Milin S., Fauconneau B., Pérault-Pochat M. C., Lafay-Chebassier C. (2010) Imipramine, in part through tumor necrosis factor α inhibition, prevents cognitive decline and β-amyloid accumulation in a mouse model of Alzheimer's disease. J. Pharmacol. Exp. Ther. 332, 505–514 [DOI] [PubMed] [Google Scholar]
- 56. Ransohoff R. M. (2009) Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zambon A. C., Zhang L., Minovitsky S., Kanter J. R., Prabhakar S., Salomonis N., Vranizan K., Dubchak I., Conklin B. R., Insel P. A. (2005) Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc. Natl. Acad. Sci. U.S.A. 102, 8561–8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aronoff D. M., Carstens J. K., Chen G. H., Toews G. B., Peters-Golden M. (2006) Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J. Interferon Cytokine Res. 26, 827–833 [DOI] [PubMed] [Google Scholar]
- 59. Liu J., Zhao X., Cao J., Xue Q., Feng X., Liu X., Zhang F., Yu B. (2011) Differential roles of PKA and epac on the production of cytokines in the endotoxin-stimulated primary cultured microglia. J. Mol. Neurosci. 45, 186–193 [DOI] [PubMed] [Google Scholar]