Background: PACSIN adapter proteins couple clathrin-mediated endocytosis with regulation of actin polymerization.
Results: PACSIN 1 is phosphorylated at Ser358 by CK2, which leads to the dissociation of a protein complex of PACSIN 1, Rac1, and NADRIN and thereby releases Rac1 GTP.
Conclusion: CK2 phosphorylation of PACSIN 1 promotes Rac1-dependent dendritic spine formation.
Significance: Phosphorylation of PACSIN 1 is required for dendritic spine adaption in synaptic plasticity.
Keywords: Dendrite, Phosphorylation, Rac1, Synapses, Synaptic Plasticity, NADRIN, PACSIN 1, Spine Formation
Abstract
The PACSIN (protein kinase C and casein kinase 2 substrate in neurons) adapter proteins couple components of the clathrin-mediated endocytosis machinery with regulators of actin polymerization and thereby regulate the surface expression of specific receptors. The brain-specific PACSIN 1 is enriched at synapses and has been proposed to affect neuromorphogenesis and the formation and maturation of dendritic spines. In studies of how phosphorylation of PACSIN 1 contributes to neuronal function, we identified serine 358 as a specific site used by casein kinase 2 (CK2) in vitro and in vivo. Phosphorylated PACSIN 1 was found in neuronal cytosol and membrane fractions. This localization could be modulated by trophic factors such as brain-derived neurotrophic factor (BDNF). We further show that expression of a phospho-negative PACSIN 1 mutant, S358A, or inhibition of CK2 drastically reduces spine formation in neurons. We identified a novel protein complex containing the spine regulator Rac1, its GTPase-activating protein neuron-associated developmentally regulated protein (NADRIN), and PACSIN 1. CK2 phosphorylation of PACSIN 1 leads to a dissociation of the complex upon BDNF treatment and induces Rac1-dependent spine formation in dendrites of hippocampal neurons. These findings suggest that upon BDNF signaling PACSIN 1 is phosphorylated by CK2 which is essential for spine formation.
Introduction
Casein kinase 2 (CK2)5 is a pleiotropic serine/threonine kinase that is ubiquitously expressed and highly conserved during evolution (1). CK2 is most abundant in the brain, particularly in cortex and hippocampus, and many of its >300 substrates are implicated in synaptic plasticity, learning, and memory. Interestingly, CK2 activity in the hippocampus is developmentally regulated (2) and can be triggered by stimuli that drive synapse development such as induction of long term potentiation and BDNF (1, 3).
One CK2 substrate in neurons is PACSIN 1 (protein kinase C and casein kinase 2 substrate in neurons 1, also called syndapin 1) which belongs to a family of accessory proteins involved in actin dynamics and endocytosis (4–6). PACSIN 1, like the other two protein family members, PACSIN 2 and 3, is a multidomain protein which contains an N-terminal Fes-Cip4 homology-Bin-Amphiphysin-Rvs (F-BAR) domain and a C-terminal Src homology 3 (SH3) (Fig. 1A). The SH3 domain mediates protein-protein interactions with proline-rich regions of dynamin and N-WASP (neural Wiskott-Aldrich syndrome protein) (5, 7, 8). Dimerization and membrane binding via the F-BAR domain activate N-WASP and induce Arp2/3 complex-dependent actin nucleation. Through these interactions, PACSIN 1 connects the actin cytoskeleton to sites of clathrin-coated vesicle formation and regulates synaptic vesicle trafficking and the surface localization of cargo receptors (6, 9, 10).
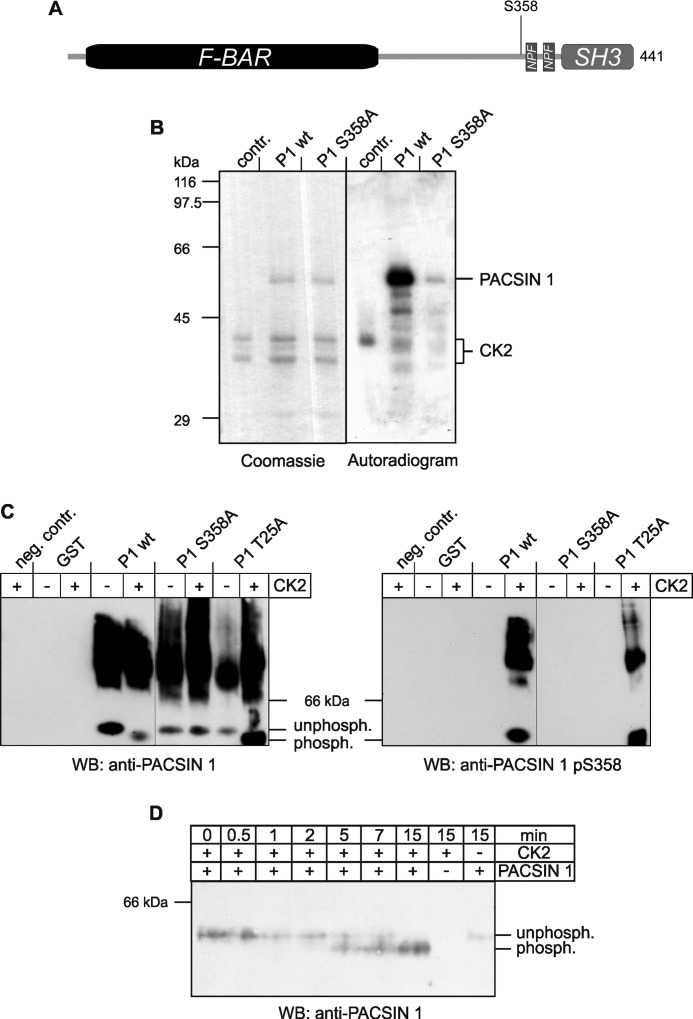
FIGURE 1.
Phosphorylation of PACSIN 1 at serine 358 by CK2. A, scheme of PACSIN 1 represents its domain structure and location of Ser358. B, recombinant wild-type PACSIN 1 (P1 wt) and PACSIN 1 S358A (P1 S358A) were purified as GST fusion proteins, and GST was removed by proteolytic cleavage. The proteins were incubated with CK2 and [γ-32P]ATP for 20 min and analyzed by SDS-PAGE. The SDS-polyacrylamide gel was stained with Coomassie Brilliant Blue, dried, and exposed to an x-ray film. C, recombinant proteins including GST and an unrelated mutant PACSIN 1 T25A (P1 T25A) as controls were incubated with (+) or without (−) CK2, resolved by native PAGE, and immunoblotted with antibodies specific for PACSIN 1 or PACSIN 1 pS358. D, for kinetic analysis wild-type PACSIN 1 (P1 wt) was incubated with CK2 for the indicated time periods, separated by native PAGE and immunoblotted (WB) for PACSIN 1.
During neuronal development, tightly balanced PACSIN 1 expression is necessary for proper neuromorphogenesis and axon development. Overexpression of PACSIN 1 triggers neurite and dendrite branching of hippocampal neurons which depends on PACSIN 1 associating with phosphatidylserine-containing membranes and binding to N-WASP, leading to actin nucleation at the plasma membrane (11). At synapses, PACSIN 1 regulates the surface expression of inhibitory NMDA receptors containing NR3A subunits and thereby promotes maturation of spines in an activity-dependent manner. Spines are small protrusions on dendritic branches of most neurons and constitute major sites of excitatory synapse formation (12, 13). The number, size, and shape of these actin-filled spines undergo continuous remodeling in response to changes in activity, and this type of structural plasticity is the basis for long lasting modifications of synaptic strength underlying learning and memory. Long term depression leads to actin depolymerization and thereby spine shrinkage whereas long term potentiation increases spine stability (14). Several modulators of spine morphology are known such as actin-binding proteins like cofilin and profilin (14) and BDNF (15). Loss of BDNF in hippocampal dentate gyrus neurons leads to an aberrant morphological development including a lower density of dendritic spines and failure to maintain mature synaptic spines. Little is known about the role of CK2 in dendritic spines; however, a recent publication indicates that activity of CK2 is required in a pathway that controls synaptic potentials via muscarinic cholinergic receptor stimulation of glutamate receptor-dependent long term potentiation (16). Here, we show that PACSIN 1 is phosphorylated in vitro and in vivo at serine 358, located within a classical CK2 phosphorylation motif (pS/TDDE) (17) and that this phosphorylation is important for proper spine formation in hippocampal neurons.
EXPERIMENTAL PROCEDURES
Expression and Purification of PACSIN 1
Glutathione S-transferase (GST) fusion proteins of PACSIN 1 were produced by cloning cDNAs corresponding to the complete coding region into the pGEX-6P vector (GE Healthcare) followed by expression in Escherichia coli (BL21). The fusion proteins were purified by affinity chromatography on glutathione-Sepharose 4B, and GST was removed by cleavage with PreScission protease (GE Healthcare). The mutants of PACSIN 1, S358A and S358D, were obtained by site-directed mutagenesis of the PACSIN 1 wild-type construct using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). Stably expressed eukaryotic StrepII-PACSIN 1 was purified from HEK293 cells via a Strep-Tactin-Sepharose (IBA) (18).
Enrichment of Phosphoproteins
Mouse brains were homogenized in cold 0.32 m sucrose, 5 mm HEPES, pH 7.4, containing protease and phosphatase inhibitors (HEPES-buffer) using 12 strokes with a homogenizer (Braun). After centrifugation at 1,400 × g for 10 min the resulting supernatant was spun at 14,000 × g for 10 min to obtain a crude synaptosomal pellet (P2) and a nonsynaptosomal supernatant (S2). P2 was homogenized with 10 strokes in HEPES-buffer and centrifuged at 140,000 × g for 2 h to give a high speed pellet (P3). S2 was centrifuged separately under the same conditions to produce pellet P3′. P3 contains synaptic proteins whereas the pellet P3′ contains nonsynaptic vesicle proteins. The supernatants of both high speed centrifugations contain cytosolic proteins. The three samples where used to enrich the contained phosphoproteins using the PhosphoProtein Purification Kit (Qiagen) and concentrated with Nanosep Ultrafiltration Columns (Pall Corporation). Protein concentrations were measured using the Pierce BCA Kit (Pierce) and proteins identified by immunoblotting.
Immunoblotting and Antibodies
Samples were solubilized in Laemmli sample buffer (19), resolved on SDS-polyacrylamide gels, and transferred to polyvinylidene fluoride (Millipore) or nitrocellulose membranes (Sigma). Membranes were blocked in 3% bovine serum albumin and incubated with primary and HRP-conjugated secondary antibodies (Dako) diluted in Tris-buffered saline containing 0.1% Tween 20 (TBST) using enhanced chemiluminescence. The following antibodies were used: anti-myc 9E10 (Santa Cruz Biotechnology), anti-dynamin (Transduction Laboratories), anti-PACSIN 1 (4), anti-SNAP-47,6 anti-GABA A-2052 (Sigma), anti-Rac1 23A8 (Upstate), anti-GRIP-1 (Upstate), anti-CK2α 1AD9 (Jena Bioscience), and anti-hemagglutinin (HA) 3F10 (Roche Applied Science). A polyclonal antibody against PACSIN 1 pS358 (CRGQTYATEW(pSer)DDES) was raised in a rabbit by GenScript Corporation (Piscataway, NJ).
CK2 Phosphorylation Assay
Samples of recombinant proteins (0.5 μg) were incubated at 30 °C for the indicated time periods in CK2 buffer (20 mm Tris, pH 7.5, 50 mm KCl, 10 mm MgCl2 with 200 μm ATP (or 125 μm ATP + 0.2 megabecquerel of [γ-32P]ATP)) and 250 units of CK2 (New England Biolabs). After separation by SDS-PAGE the gel was stained with Coomassie Brilliant Blue, dried, and exposed to an x-ray film, or alternatively, the gel was immunoblotted. For native PAGE nonreducing sample buffer (62.5 mm EDTA, 87.5 mm Tris, pH 7.5, 15% sucrose, 0.125% bromphenol blue) was added to the samples before loading onto a 8.5% native gel and detection by immunoblotting.
Culture of Primary Neurons and N2a Cells
Primary hippocampal neurons were isolated and cultured as described before (9). Briefly, hippocampi were dissected from embryonic days (E) 19–20 rat or E18.5 mouse pups and dissociated with papain. Neurons were plated onto poly-d-lysine (BD Biosciences)-coated 10-cm dishes at a density of 2.5 × 106 cells/dish and grown in neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen) and 5% FBS (Hyclone). In some experiments N2a cells (obtained from the American Type Culture Collection) were seeded at a density of 1 × 105 onto 6-cm dishes or 3 × 106 onto 10-cm dishes and grown in DMEM GlutaMAXTM (Invitrogen) until used for transfections.
Transfection and Immmunofluorescence
For immunofluorescence microscopy hippocampal neurons were plated onto poly-d-lysine-coated coverslips, co-transfected using calcium phosphate after 9 days in vitro with myc-tagged PACSIN 1 constructs and EGFP, and 4 days later stained using anti-myc and anti-GABA antibodies. Only excitatory neurons showing low to moderate levels of overexpression were chosen for analysis. In other experiments untransfected primary neurons were used to specifically inhibit CK2 with tetrabromocinnamic acid (TBCA, 50 or 100 μm, Calbiochem) and stained with the plasma membrane dye DiI (Invitrogen). Briefly, neurons were fixed in 4% paraformaldehyde with 4% sucrose, permeabilized in phosphate-buffered saline (PBS) containing 0.1% Triton X-100, and incubated for 2 h at room temperature with anti-myc and anti-GABA or anti-PACSIN 1 pS358 and anti-Rac1 followed by incubation with Cy3/Cy5- or Alexa Fluor 488/Cy3-conjugated secondary antibodies (Jackson Immunoresearch). N2A cells were transfected using XtremeGENE HP (Roche Applied Science) with 4 μg of plasmid on a 6-cm dish or 10 μg of plasmid on a 10-cm dish and incubated for 24 h before further use.
siRNA-mediated Silencing of CK2
Double-stranded RNA oligonucleotides specifically targeting mouse CK2 were obtained from Invitrogen. The sense sequences were as follows: 5′-GGGCCAGAGUUUACACAGAUGUUAA-3′ (siRNA1), 5′-CAGCGCCAAUAUGAUGUCAGGGAUU-3′ (siRNA2), 5′-UUGAACAGGUAUCCCAAGUGAGUUG-3′ (siRNA3). The Stealth medium GC siRNA was used as a control. N2A cells were transfected with CK2α-specific siRNAs (60 nm) or control siRNA (80 nm) using HiPerfect (Qiagen) and grown further up to 72 h. Expression of CK2 was determined for each experiment by SDS-PAGE (12% gel) and subsequent immunoblotting using a monoclonal mouse antibody specific for the α subunit of CK2.
Quantification of Dendritic Spines
For quantification of dendritic spines, image stacks were acquired with a confocal microscope (Leica TCS SP5). The total spine number of five dendrites were counted from 12 cells per independent experiment.
Subcellular Fractionation
For sucrose gradient centrifugation hippocampal neurons were homogenized in cold Tris-buffered sucrose (30 mm Tris-HCl, pH 7.4, 250 mm sucrose, 10 mm EGTA, 5 mm EDTA, 1 mm dithiothreitol (DTT), protease. and phosphatase inhibitors). After centrifugation at 1,000 × g for 10 min at 4 °C pellets were homogenized in Tris-buffered sucrose with 1% Triton X-100 and incubated for 1 h at 4 °C. The lysates were loaded on a 10–40% continuous sucrose gradient and centrifuged using a Beckman SW41TI rotor for 16 h at 38,000 rpm, as described previously (20). After centrifugation, fractions of 1 ml were collected, proteins precipitated with trichloroacetic acid, neutralized with NaOH, and dissolved in 100 μl of Laemmli sample buffer. Equal amounts of each protein fraction were separated by SDS-PAGE and analyzed by subsequent immunoblotting.
Liposome Sedimentation Assay
Recombinant PACSIN 1 protein was dialyzed into liposome buffer (20 mm HEPES, pH 7.4, 100 mm KCl, 1 mm EDTA), and precipitates were removed by ultracentrifugation. Liposomes containing 35% phosphatidylethanolamine, 20% phosphatidylserine, and 10% phosphatidylinositol 4,5-bisphosphate (all from Sigma) were resuspended in liposome buffer containing 0.1 m sucrose and 5 μg of the indicated protein added for 15 min. After centrifugation at 100,000 × g for 25 min pellet and supernatant were analyzed by SDS-PAGE and the gel stained with Coomassie Brilliant Blue.
Immunoprecipitation Using N2A Cells
N2A cells were transfected with myc-PACSIN 1 wild-type and HA-NADRIN 24 h after seeding. 24 h after transfection, cells were treated either with 10 nm BDNF or with H2O as control for 5 h and lysed in a buffer containing 20 mm HEPES pH 7.4, 150 mm NaCl, 1 mm EDTA, and 0.5% Nonidet P-40 supplemented with protease and phosphatase inhibitors. For preclearing equal amounts of lysate were incubated for 1 h with protein G-agarose (Roche Applied Science) at 4 °C. After centrifugation the resulting supernatants were incubated with antibodies either directed against specific proteins or, as a control, against mouse immunoglobulin G for 3 h at 4 °C. After addition of fresh protein G-agarose samples, were incubated overnight and extensively washed in lysis buffer, and proteins were resolved by SDS-PAGE and immunoblotting using specific antibodies.
Immunoprecipitation for Two-dimensional PAGE
Mouse brains were homogenized in deoxycholate buffer (1% deoxycholate, 5 mm Tris, pH 9.0, protease and phosphatase inhibitors) and dialyzed against PBS overnight. The sample was centrifuged at 10,000 × g for 15 min, and the supernatant was precleared with protein A-agarose (Roche Applied Science) and PACSIN 1 immunoprecipitated with protein A-agarose and anti-PACSIN 1. After washing, the beads were incubated for 1 h with two-dimensional PAGE equilibration buffer (8 m urea, 2% CHAPS, 20 mm DTT, 0.5% Immobilines pH 3–10 NL, and 0.0002% bromphenol blue) and centrifuged at 10,000 × g for 5 min. IPG strips (pH 3–10 NL, 18 cm, GE Healthcare) were rehydrated with 350 μl of the sample on an IPGphor apparatus (GE Healthcare) before proteins were focused. After completion the IEF strips were equilibrated in SDS-PAGE transfer buffer (50 mm Tris, pH 8.8, 6 m urea, 30% v/v glycerol, and 0.0002% bromphenol blue) including 1% w/v DTT or 2.5% iodoacetamide instead. Subsequently the equilibrated strips were placed and fixed on a 10% SDS-polyacrylamide gel. After electrophoresis the gels were stained with Gelcode Blue Coomassie stain (Pierce).
Rac1 GTPase Pulldown Assay
N2A cells were transfected with either myc-PACSIN 1 wild-type or myc-PACSIN 1 S358A 24 h after seeding on tissue culture plates. After 24 h, adherent cells were lysed and activated Rac1 specifically precipitated with the p21 binding domain of human Pak1 fused to GST (GST-Pak1) using the Active GTPase Pulldown Kit (Thermo Fisher). Briefly, the N2A cells were lysed in the provided buffer supplemented with protease and phosphatase inhibitors (Roche Applied Science). Lysates were cleared by centrifugation, the protein concentrations of the supernatants determined by the BCA assay (Pierce), and equal amounts were used to pull down Rac1 with GST-Pak1. Lysate and pulldown fractions were separated by SDS-PAGE on 15% acrylamide gels under reducing conditions and immunoblotted with a mouse monoclonal antibody specific for Rac1 (clone 23A8; Thermo Fisher) followed by detection by enhanced chemoluminescence.
In Gel Digestion and Phosphopeptide Enrichment
Excised protein spots were washed, dried, and rehydrated in an ice-cold solution of 10 ng/μl AspN (sequencing grade; Roche Applied Science) in 10 mm ammonium bicarbonate. After 45 min on ice, excessive AspN solution was replaced by 20 μl of buffer without enzyme, and proteins were digested at 37 °C for 4 h. The digestion was stopped by the addition of 10% trifluoroacetic acid (TFA), and peptides were extracted for 30 min at 37 °C. For phosphopeptide enrichment TopTips (Glygene) were used according to the manufacturer's protocol. Briefly, after pretreatment and peptide sample loading the tips were washed with 50 μl of water, and phosphopeptides were eluted in 20 μl of 50 mm triethanolamine, 50 mm ammonium bicarbonate. The eluate was acidified by the addition of 30 μl of 1% TFA and loaded onto a C18 micropurification tip (Eppendorf) equilibrated in 1% TFA. The C18 tip was washed three times with 10 μl of 1% TFA, and peptides were eluted in 10 μl of 1% TFA in 50% acetonitrile.
MALDI-TOF MS of in Gel Digested Proteins
Positive ion spectra of spotted peptide solution were acquired by mass spectrometry (Bruker Daltonics) using a peptide calibration standard for external calibration of the corresponding mass range. Interpretation of mass spectra was performed using Biotools 3.0 (Bruker Daltonics).
LC-MS/MS of in Gel Digested Proteins
Liquid chromatography (LC)-MS data were acquired on a HCT ETD II ion trap mass spectrometer (Bruker Daltonics) equipped with a nano-ESI source (Bruker Daltonics). Samples were introduced by an easy nano-LC system (Proxeon) using a vented column setup comprising a 0.1- × 20-mm trapping column and a 0.075- × 100-mm analytical column, both self- packed with ReproSil-Pur C18-AQ, 5 μm (Dr. Maisch). 5–18-μl samples were aspirated into the sample loop, and a total of 25 μl was loaded onto the trap column using a flow rate of 6 μl/min. Loading pump buffer was 0.1% formic acid. Peptides were eluted with a gradient of 0–35% acetonitrile in 0.1% formic acid over 20 min and a column flow rate of 300 nl/min. Subsequently, the acetonitrile content was raised to 100% over 2 min, and the column was regenerated in 100% acetonitrile for additional 8 min.
Data-dependent acquisition of MS and tandem MS (MS/MS) spectra was controlled by the Compass 3.0 software. MS1 scans were acquired in standard enhanced mode. Five single scans in the mass range from m/z 350 to m/z 1400 were combined for one survey scan. Up to two doubly and triply charged ions rising above a given threshold were selected for alternating collision-induced dissociation and electron transfer dissociation (ETD) MS/MS experiments. In both fragmentation modes the ultrascan mode was used for the acquisition of MS2 scans in the mass range from m/z 100 m/z 1600, and three single scans were added up. The ion charge control value was set to 250,000 for MS1 and collision-induced dissociation scans and to 100,000 for ETD scans. ETD reactant was the fluoranthene anion. Combined peaklists were generated from the raw data by using the Data Analysis software module (Bruker Daltonics)
Database Searches
Peptides and phosphopeptides were identified by searching expected protein sequences in a custom database using a local installation of MASCOT 2.2 (Matrix Science Ltd.). Searches were submitted via Proteinscape 2.0 (Bruker Daltonics) with the following parameter settings: enzyme, AspN; fixed modifications, carbamidomethyl; optional modifications, methionine oxidation and phosphorylation ST; and missed cleavages, 1. The mass tolerance was set to 0.2 Da for peptide and fragment spectra.
RESULTS
PACSIN 1 was originally identified as a phosphoprotein phosphorylated by CK2 in vitro (4). Inositol 6-phosphate specifically induces PACSIN 1 phosphorylation in a low speed supernatant extract from rat brain (21), probably via CK2 whose activity is regulated by higher inositol phosphates (22). However, several issues remaining to be addressed are: (i) whether CK2 is the relevant kinase in vivo; (ii) at which site(s) PACSIN 1 is phosphorylated by CK2; and (iii) what the role of phosphorylated PACSIN 1 is. Here we addressed these questions and examined how CK2-mediated phosphorylation regulates PACSIN 1 function.
Casein Kinase 2 Phosphorylates PACSIN 1 at Serine 358
We first confirmed PACSIN 1 phosphorylation by CK2 and identified the relevant phosphorylation site by conducting in vitro phosphorylation assays with recombinant full-length wild-type PACSIN 1 or mutant proteins containing single amino acid exchanges (Fig. 1A). Wild-type PACSIN 1 was phosphorylated in vitro in the presence of CK2, as visualized by autoradiography (Fig. 1B). Mutation of serine 358 to alanine (S358A) resulted in a dramatic loss of signal. To confirm the specificity of the phosphorylation at residue 358 we analyzed the assay products by native PAGE followed by immunoblotting (Fig. 1C). CK2 phosphorylation led to an increase in the mobility of the monomeric wild-type PACSIN 1 and an unrelated mutant PACSIN 1 T25A due to the negative charge added by the phosphate, whereas CK2 did not alter the mobility of PACSIN 1 S358A (Fig. 1C, left).
To further characterize the role of PACSIN 1 phosphorylation, we generated a specific antibody against phosphorylated serine 358 in PACSIN 1. Analogous native PAGE experiments probed with the phospho-PACSIN 1-specific antibody detected only wild-type PACSIN 1 and the T25A mutant in the presence of CK2 (Fig. 1C, right). The S358A mutant that is not phosphorylated in vitro by CK2 was not detected. All wild-type PACSIN 1 molecules were phosphorylated after a 15-min incubation with CK2, as shown by the characteristic mobility shift (Fig. 1, C and D). To test the specificity of the novel antibody, we purified recombinantly expressed PACSIN 1, 2, and 3 and, as a control, GST from bacteria and incubated half of the protein preparations with CK2. All proteins were subjected pairwise (phosphorylated and phosphate-free) to SDS-PAGE and analyzed by immunoblotting. The phospho-specific antibody recognized only the phosphorylated variants of all three PACSIN proteins (supplemental Fig. 1A). The signal appears strongest for PACSIN 1, but the antibody also recognizes PACSIN 2 and 3. This can be explained by the high conservation of the residues neighboring the serine corresponding to Ser358 in PACSIN 1 (supplemental Fig. 1B).
To determine the in vivo relevance of PACSIN 1 phosphorylation in neurons and to confirm our results in other systems, we enriched synaptosomal and nonsynaptosomal vesicles from brain homogenates by subcellular fractionation. Affinity chromatography was then used to enrich the phosphoproteins in both vesicle-associated protein fractions and a cytosolic fraction, and the resulting extracts were analyzed by SDS-PAGE followed by immunoblotting (Fig. 2A). The efficiency of our method for phosphoprotein enrichment had been validated before with in vitro phosphorylated PACSIN 1 (supplemental Fig. 2). Whereas only a subpopulation of dynamin was phosphorylated, the majority of vesicle-associated, but not cytosolic, PACSIN 1 was enriched in the phosphoprotein fractions. In contrast to PACSIN 1, dynamin was not phosphorylated in the cytosol. SNAP-47, used as a negative control, was not phosphorylated (23).
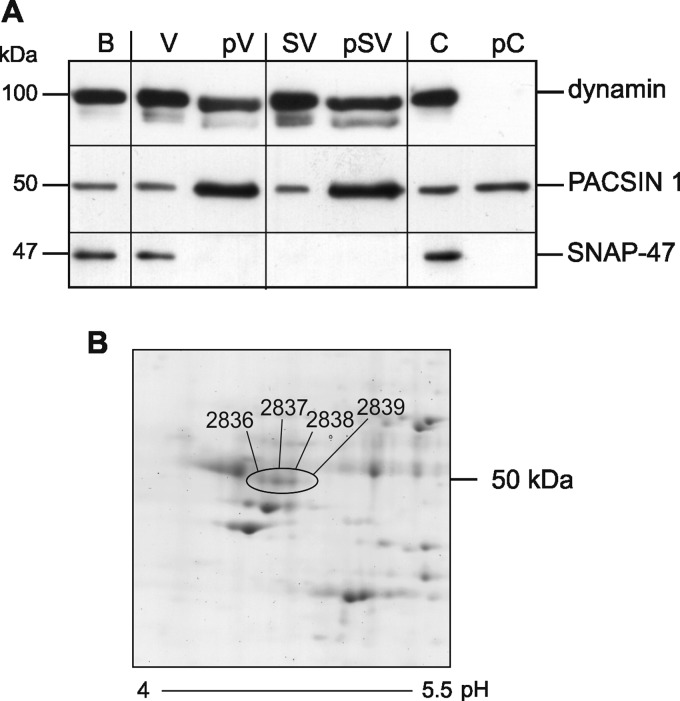
FIGURE 2.
In vivo phosphorylation of PACSIN 1 at serine 358. A, subcellular fraction of mouse brain homogenates (B) was used to produce a synaptosomal pellet and a nonsynaptosomal supernatant. Both fractions were separately subjected to ultracentrifugation to give the two high speed pellets SV (synaptosomal vesicles), V (nonsynaptosomal vesicles), and the combined supernatant C (cytosol). The three samples were further enriched for phosphorylated proteins (pV, pSV, and pC) by affinity chromatography. 8 μg of protein before and after phosphoprotein enrichment was separated by SDS-PAGE and immunoblotted for dynamin, PACSIN 1, and SNAP-47 as a negative control. B, PACSIN 1 was immunoprecipitated from a murine brain lysate and resolved by two-dimensional PAGE. Spots were manually picked and analyzed by LC-MS/MS. Four spots (encircled) were identified as PACSIN 1, and all spots contained PACSIN 1 pS358.
To confirm the phosphorylation of serine 358 in another system we purified PACSIN 1 from HEK293 cells stably expressing this protein. By MALDI-TOF mass spectrometry we detected the unmodified peptide 348DRGQTYATEWS358 at 1313 m/z (mass to charge ratio) and the phosphorylated peptide 348DRGQTYATEW(phos)S358 at 1393 m/z (supplemental Fig. 3B). Recombinant PACSIN 1 expressed in E. coli was not phosphorylated (supplemental Fig. 3, A and B). A second phosphorylated peptide 348DRGQTYATEW(phos)SD359 at 1508 m/z was also identified, but we could not detect the corresponding phosphate-free peptide. After phosphopeptide enrichment of eukaryotic PACSIN 1 on TiO2-columns, the peaks corresponding to 348DRGQTYATEW(phos)S358 at 1393 m/z and 348DRGQTYATEW(phos)SD359 at 1508 m/z became even more pronounced (supplemental Fig. 3, C and D).
To prove that the serine 358 phosphorylation occurs in vivo, we immunoprecipitated PACSIN 1 from mouse brain homogenates and analyzed the precipitate by two-dimensional-PAGE. PACSIN 1 was detected in four separate spots as determined by mass spectrometry peptide mass fingerprinting (Fig. 2B). The extracted ion chromatograms of PACSIN 1 peptides containing Ser358 revealed the presence of the unmodified peptide 348DRGQTYATEWS358 at 657.3 m/z (mass to charge ratio) and the monophosphorylated peptide 348DRGQTYATEW(phos)S358 at 697.3 m/z in all four spots. The ratio of the phospho-peptide/unphosphorylated peptide decreases with increasing pH (supplemental Fig. 4). Nano-LC-MS/MS was used to fragment the monophosphorylated peptide of spot 2837 (supplemental Fig. 5). B- and Y-type ion series throughout the sequence were used to confirm the sequence of the peptide and to determine the phosphorylation site. The ions Y1 and B11 unequivocally localize the phosphorylation to serine 358. Taken together, the results indicate that PACSIN 1 is phosphorylated at serine 358 in vitro. They further show that phosphorylation occurs in neurons in vivo and that CK2 is likely to be the responsible kinase.
PACSIN 1 Lipid Association Is Not Affected by Serine 358 Phosphorylation
Serine 358 is located within the unstructured linker sequence connecting the N-terminal F-BAR domain with the C-terminal SH3 domain (Fig. 1A). This linker acts as a hinge region that allows a conformational change from the autoinhibitory clamped conformation to an open conformation (24). In the clamped conformation the SH3 domain interacts with the F-BAR domain and prevents membrane binding, whereas the open conformation enables SH3 interactions with proline-rich regions of binding partners. Because phosphorylated PACSIN 1 is found predominantly in vesicular fractions (Fig. 2A) we asked whether phosphorylation at serine 358 promotes lipid binding. We therefore compared the liposome binding behavior of the phospho-defective PACSIN 1 mutant S358A and a mutant S358D that potentially mimics phosphorylation of PACSIN 1, with that of wild-type PACSIN 1 (supplemental Fig. 6A). No differences were observed between the ability of the mutants and wild-type PACSIN 1 to bind liposomes. Similar results were obtained when in vitro phosphorylated recombinant wild type and S358A PACSIN 1 were compared in the same assay (supplemental Fig. 6B). In all cases, >50% of all proteins were found in the pellet fraction (containing liposome-binding proteins), whereas a considerable fraction was in the supernatant, indicating that Ser358 phosphorylation does not regulate lipid binding.
BDNF Treatment Enhances PACSIN 1 Phosphorylation
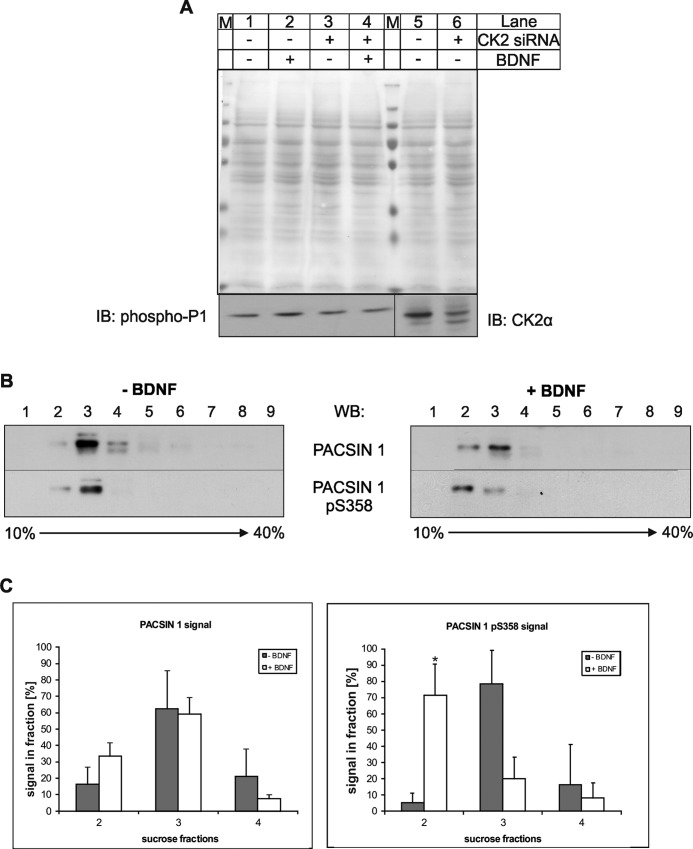
Previous studies demonstrated that activity-induced long term potentiation is also accompanied by BDNF-mediated signaling for increased actin polymerization in dendritic spines (25). To examine whether CK2 activity is regulated by growth factors we treated N2a cells with BDNF and tested whether the treatment affects the phosphorylation of PACSIN 1 or modulates its neuronal localization (3). BDNF treatment only slightly increased PACSIN 1 phosphorylation (Fig. 3A, lanes 1 and 2), which might be a consequence of the high constitutive CK2 activity observed in brain (26). When CK2 was depleted by siRNA to approximately 20% (Fig. 3A, lanes 5 and 6) this BDNF-dependent increase was no longer observed (Fig. 3A, lanes 3 and 4). We also compared the localization of phosphorylated PACSIN 1 and total PACSIN 1 on a continuous sucrose gradient, where plasma membranes in contrast to most organelles band at lower buoyant densities (27). Here, the majority of phosphorylated and unphosphorylated PACSIN 1 was found in fraction 3 (Fig. 3B, left). Treatment with BDNF led to a shift of PACSIN 1 pS358 to the less dense fraction 2 and a concomitant reduction in fraction 3 (Fig. 3B, right). The bands corresponding to PACSIN 1 proteins in fractions 2–4 of three independent experiments were individually scanned and quantified as percentage of total signal in all three fractions. The results clearly support a modulation of the localization of phosphorylated PACSIN 1 by BDNF (Fig. 3C).
FIGURE 3.
Phospho-PACSIN 1 levels and localization in neuronal cells. A, N2a cells were transfected with a CK2α-specific siRNA mixture for 72 h and then incubated with (+) or without (−) BDNF (100 ng/ml) for 5 h and lysed. Comparable amounts of the individual lysates (50 μg/lane) were resolved by SDS-PAGE and immunoblotted (IB) with antibodies specific for the indicated proteins. As a control, the Ponceau staining of the membrane is shown. M, marker proteins. B, protein extracts from cultured mouse hippocampal neurons were subjected to centrifugation through a continuous sucrose density gradient and fractions analyzed for the distribution of total and phosphorylated PACSIN 1. Immunoblot (WB) analysis shows the shift of phosphorylated PACSIN 1 to lighter fractions upon BDNF-treatment of the neurons. C, quantification of immunoblot signal intensities for total (left graph) and phosphorylated (right graph) PACSIN 1 with Image Quant 5.0 for the relevant fractions (2, 3, and 4) of three independent experiments is shown (*, p < 0.05, t test). Error bars, S.D.
Phosphorylation of PACSIN 1 Is Required for Dendritic Spine Formation
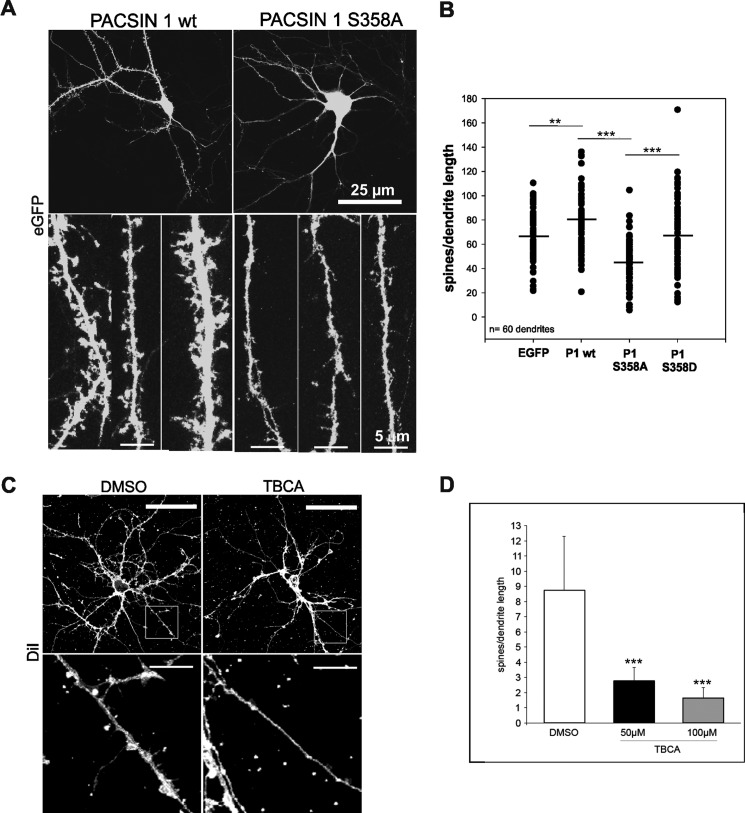
Neurotrophins such as BDNF are known to regulate dendrite development through multiple signaling pathways, including reorganization of the cytoskeleton and/or initiation of gene expression programs required for dendritic growth (28). To investigate a potential involvement of PACSIN 1 phosphorylation in primary hippocampal neurons, we determined the effects of the phospho-deficient S358A mutant on the dendritic morphology (Fig. 4). For visualization of neuronal branches and spines, we expressed the green fluorescent protein (GFP) with either the phospho-deficient mutant S358A, the phospho-mimic mutant S358D, or with wild-type PACSIN 1 in hippocampal neurons (9–13 days in vitro). After fixation, image stacks were acquired with a confocal microscope, and the number of dendritic spines was counted on dendritic branches of transfected neurons. Co-expression of the dominant negative PACSIN 1 S358A mutant caused a dramatic reduction of spine numbers in these neurons compared with neurons transfected with wild-type PACSIN 1 (Fig. 4A, B). Average total spine numbers were 66 ± 22 (EGFP), 80 ± 24 (wild-type), 46 ± 18 (S358A) and 67 ± 32 (S358D) corresponding to a spine reduction of almost 50% in the presence of PACSIN 1 S358A (p < 0.001). In contrast, co-expression of the S358D mutant did not lead to significant changes in neuron morphology (Fig. 4C).
FIGURE 4.
Reduced spine formation in the presence of the PACSIN 1 S358A mutant or after CK2 inhibition. A, rat hippocampal neurons were transfected with either PACSIN 1 wt (left). the mutant protein S358A (right) or the mutant protein S358D (data not shown) in combination with EGFP to visualize spines (upper panel). Enlargements of three dendrite segments of neurons transfected with either PACSIN 1 wt (left) or S358A mutant (right) are shown. Fewer spines can be detected in neurons expressing PACSIN 1 S358A (lower panel). B, quantification of two independent experiments. For each group five dendrites were counted on each of 12 cells, n = 60 dendrites; **, p < 0.01; ***, p < 0.001, t test. C, untransfected hippocampal neurons were treated either with dimethyl sulfoxide (DMSO) or the CK2 inhibitor TBCA in dimethyl sulfoxide and stained with the DiI plasma membrane dye to visualize the spines (upper panel). Enlargements of the indicated dendrite segments of neurons are shown (lower panel). D, quantification of three independent experiments is shown. For each group three dendrites were counted on each of five cells, n = 45 dendrites; ***, p < 0.001, t test. Error bars, S.D.
To demonstrate that CK2 phosphorylation of PACSIN 1 at Ser358 is required for dendritic spine formation we tried to suppress CK2α expression in the neurons by siRNA; however, the knockdown efficiencies were very low. We therefore instead inhibited CK2 activity by treating the cells with the specific CK2 inhibitor TBCA and observed a concentration-dependent drastic reduction in spine formation upon BDNF stimulation (Fig. 4, C and D). These results indicate that CK2 phosphorylation of PACSIN 1 at serine 358 is required for dendritic spine formation.
PACSIN 1 and Rac1 Act in a Complex
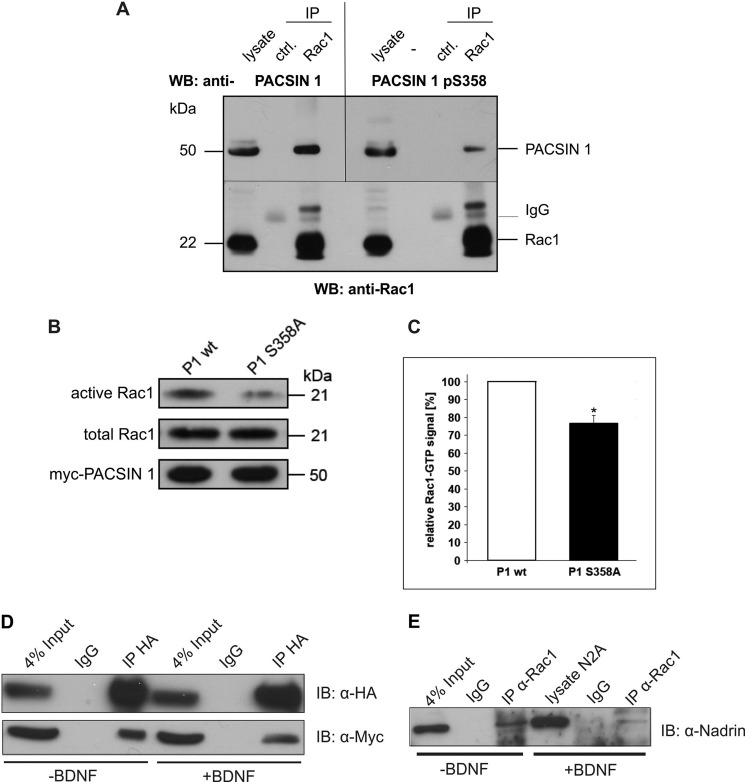
Small GTPases are key regulators of actin remodeling in postsynaptic spines. In particular, Rac1 regulates morphogenesis of dendritic spines by affecting actin dynamics (29, 30). Recently, we identified the PACSIN proteins as novel Rac1 interactors and showed that reciprocal regulation affects both Rac1 and PACSIN 2 (31). Given the role of Rac1 in spine morphology we tested whether this is true for PACSIN 1 as well. We thus performed immunoprecipitation experiments to test whether the two proteins act together in one complex in neurons. When Rac1 was immunoprecipitated from brain lysates PACSIN 1 was co-precipitated (Fig. 5A, left). The Rac1-interacting fraction contained PACSIN 1 phosphorylated at serine 358; however, only a subpopulation of phospho-PACSIN 1 co-precipitated (Fig. 5A, right). We therefore tested whether PACSIN 1 Ser358 phosphorylation affects Rac1 activity. When we transfected N2a cells with either wild-type PACSIN 1 or the phospho-defective PACSIN 1 mutant S358A approximately 25% less GTP-bound Rac1 was precipitated from cells expressing the phospho-deficient mutant (Fig. 5B), indicating that PACSIN 1 phosphorylation affects Rac1 hydrolysis.
FIGURE 5.
PACSIN 1 pS358 is present in a complex with the spine regulator Rac1 and the GAP NADRIN. A, mouse brain homogenate was used for immunoprecipitation (IP) with an antibody specific for Rac1 (lower panel) and co-precipitated proteins analyzed by immunoblotting (WB). The bands marked IgG represent immunoglobulin light chains. Total PACSIN 1 (upper panel left) as well as PACSIN 1 pS358 (upper panel right) are detected in the Rac1-IP lane. B, the levels of GTP-bound Rac1 were determined in PACSIN 1 wt or PACSIN 1 S358A-transfected N2a cells which were lysed 24 h after transfection. Pulldown experiments were performed, and equal amounts of bead fractions and lysate fractions were resolved on SDS-polyacrylamide gels (15%). The GTP-bound forms of Rac1 in bead fractions and total Rac1 in lysate fractions were stained with mouse anti-Rac1 antibody (top and middle panels). Transfection efficiencies of both constructs were comparable (bottom panel). C, quantification of three independent experiments was performed using ImageJ. *, p < 0.05, t test. Error bar, S.D. D, N2a cells were co-transfected with hemagglutinin (HA)-tagged NADRIN and Myc-tagged PACSIN 1 wt, treated with (+) or without (−) BDNF (100 ng/ml) for 5 h, and lysed. NADRIN was precipitated with an antibody specific for HA, and comparable amounts of the individual precipitates were resolved by SDS-PAGE. After immunoblotting (IB), the precipitated proteins were visualized with antibodies specific for the indicated tags. E, N2a cells were treated with (+) or without (−) BDNF (100 ng/ml) for 5 h and lysed. Endogenous Rac1 was precipitated with a specific antibody, and comparable amounts of the individual precipitates were resolved by SDS-PAGE. After immunoblotting co-precipitated endogenous NADRIN was visualized with a specific antibody.
Because PACSIN proteins lack a RhoGAP domain we asked whether the presence of a GTPase-activating protein (GAP) may be required in the PACSIN 1-Rac1 complex to mediate Rac1 activity. A previous study identified NADRIN (also named RICH1) as a Rac1 GAP, which also interacts with the SH3 domain of PACSIN 1 (32, 33). We confirmed the binding of PACSIN 1 to NADRIN (Fig. 5D, lane 3) and tested whether BDNF modulates the interaction by co-immunoprecipitation (Fig. 5D, lane 6). In the presence of BDNF less PACSIN 1 was co-precipitated with NADRIN (Fig. 5D, lanes 3 and 6). Furthermore, BDNF treatment also reduced the interaction of NADRIN with Rac1 (Fig. 5E, lanes 3 and 6). These results indicate that PACSIN 1 phosphorylation disrupts the PACSIN 1-NADRIN-Rac1 complex, which is essential for Rac1 hydrolysis and may explain the loss of spines in the presence of the PACSIN 1 mutant S358A.
DISCUSSION
Protein phosphorylation plays a critical role in the regulation of synapse function (34, 35). For instance, synapse plasticity is regulated by phosphorylation of glutamate receptors and postsynaptic density scaffold proteins, which controls their addition to or removal from the postsynaptic density and the subsequent regulation of synaptic strength (reviewed in 36). Dynamic changes in spine morphology, which accompany functional plasticity and contribute to the permanence of the changes in synaptic strength, are achieved by F-actin stabilization or destabilization leading to spine enlargement or spine loss, respectively. Rho GTPases, e.g. Rac1, Rho, and Cdc42, are known regulators of the actin cytoskeleton, with Rac1 triggering spine growth via WAVE (WASP family verprolin homologous protein) and Arp2/3-mediated actin polymerization (37, 38). The Rac1 activation state is regulated by its GTP exchange factor Tiam 1 (T lymphoma invasion and metastasis 1), which binds to stimulated NMDA and Trk receptor leading to phosphorylation and activation of Tiam 1 and, thereby, Rac1 (39, 40).
In this study we characterized the CK2 phosphorylation of PACSIN 1, a regulator of the glutamate receptor chain NR3A (9), using site-specific PACSIN 1 mutants and a phospho-specific antibody. We previously demonstrated that PACSIN 1 is phosphorylated by CK2 (4, 21), and we now identify serine 358 as the site used by this kinase (Figs. 1 and 2 and supplemental Fig. 5). This site is part of a CK2 substrate consensus sequence (17) which is conserved in all three PACSIN proteins. Serine 358 is located within the flexible hinge region that connects the N-terminal F-BAR and the C-terminal SH3 domain (4), suggesting that phosphorylation could affect the conformational switch required for efficient lipid binding (24). However, no changes in the liposome binding capacity were observed when testing different phosphorylation site mutants (supplemental Fig. 6A) or in vitro phosphorylated or phosphate-free recombinant PACSIN 1 (supplemental Fig. 6B).
To further study the role of PACSIN 1 phosphorylation we produced an antibody that specifically recognizes PACSIN 1 pS358. The antibody also recognized the corresponding sites in PACSIN 2 and 3. However, in immunohistochemistry PACSIN 2 and 3 give only weak signals in a few neuronal populations and were not expressed in most neurons, which makes this antibody a useful tool to investigate the phosphorylation status of PACSIN 1 in the brain.
Redistribution of PACSIN 1 pS358 was seen in sucrose density gradient centrifugation (Fig. 3B). This was caused by an increased CK2 activity and subsequent activation of PACSIN 1 phosphorylation upon BDNF treatment (3) because the addition of growth factor did significantly increase the amount of phosphorylated PACSIN 1 (Fig. 3A). Furthermore, we found that overexpression of PACSIN 1 increases spine density in hippocampal neurons; however, here a phosphorylation-deficient (S358A) mutant had the opposite effect and caused a drastic reduction in spine formation (Fig. 4).
The sedimentation shift of PACSIN 1 pS358 upon BDNF treatment as well as its effect on spine growth led us to the hypothesis that a role of PACSIN 1 in spine formation requires at least its partial presence in Rac1 protein complexes. Indeed, immunoprecipitation experiments revealed the presence of PACSIN 1 in Rac1 complexes (Fig. 5), suggesting that PACSIN 1 phosphorylation via BDNF initiates the complex disassembly, which appears to be required for Rac1-dependent spine formation.
Interestingly, we also observed a reduction of Rac-GTP upon overexpression of PACSIN 1 S358A (Fig. 5, B and C). This indicates that phosphorylation of PACSIN 1 positively regulates the Rac-GTP-level in the cell. Furthermore, Rac1 also co-immunoprecipitates its GAP NADRIN in the absence of BDNF. Induction of the Ser358 phosphorylation via CK2 by BDNF treatment leads to a destabilization of the Rac1 protein complex, including less NADRIN binding to Rac1 and also less PACSIN 1 binding to NADRIN. As a consequence more Rac1-GTP is available for the initiation of spine formation.
The changes observed were rather moderate, which could be explained by the following: (i) NADRIN is not the only GAP acting on Rac1 hydrolysis in brain (41); (ii) Rac1 is a key regulator of many cellular processes involving a remodeling of the actin cytoskeleton (summarized in Ref. 42); (iii) CK2 is only one of several kinases activated by BDNF (43); and (iv) CK2 shows high constitutive activity in brain (25).
Along the same lines, Rac1 and PACSIN 1 could act in concert to support spine growth after BDNF induction by endocytic removal of “juvenile” NMDAR subunit and strengthening “mature” NMDAR subunits on spines (9, 44). This is consistent with previous findings that PACSIN 1 regulates spine morphology and number by driving the removal of immature NR3A-containing NMDARs from synapses (45).
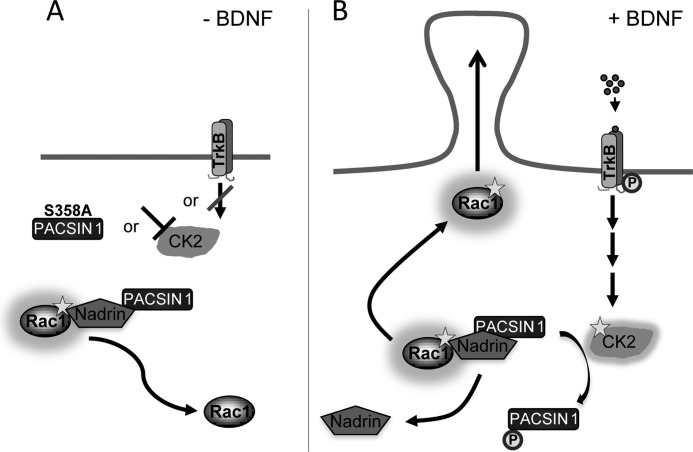
We propose the following model for the role of PACSIN 1 in spine formation (Fig. 6). A protein complex containing Rac1, NADRIN, and PACSIN 1 leading to a steady Rac1 GTP hydrolysis is stabilized under three different conditions: either in the absence of BDNF, or after specific inhibition of CK2, or after overexpression of the PACSIN 1 mutant S358A (Fig. 6A). In contrast, BDNF treatment increases CK2 activity, which leads to a destabilization of the Rac1-NADRIN-PACSIN 1 protein complex by phosphorylation of PACSIN 1 at Ser358. NADRIN no longer interacts with Rac1, and the resulting higher Rac1 GTP levels induce spine formation. Thus, PACSIN 1, through phosphorylation by CK2 and interactions with Rac1 and NADRIN, appears critical for the plasticity of dendritic spines.
FIGURE 6.
Model of the mechanism by which PACSIN 1 affects spine formation. A, in the absence of BDNF, overexpression of PACSIN 1 S358A or inhibition of CK2 supports a stable Rac1-NADRIN-PACSIN 1 complex which leads to Rac1 GTP hydrolysis. B, in the presence of BDNF higher levels of activated CK2 phosphorylate PACSIN 1 at Ser358 which causes a destabilization of the protein complex. This leads to higher concentrations of Rac1 GTP which induces spine formation. TrkB, tropomyosin-related kinase B.
Acknowledgment
We thank D. Mörsdorf for helpful discussions.
This work was supported by the Center for Molecular Medicine Cologne (CMMC) and the Köln Fortune program of the Medical Faculty of the University of Cologne (to M. Plomann).

This article contains supplemental Figs. 1–6.
S. Schael, J. Nüchel, S. Müller, P. Petermann, J. Kormann, I. Pérez-Otaño, S. Marco Martínez, M. Paulsson, and M. Plomann, unpublished data.
- CK2
- casein kinase 2
- BDNF
- brain-derived neurotrophic factor
- ETD
- electron transfer dissociation
- F-BAR
- Fes-Cip4 homology-Bin-Amphiphysin-Rvs
- GAP
- GTPase-activating protein
- MS/MS
- tandem MS
- N-WASP
- neural Wiskott-Aldrich syndrome protein
- NADRIN
- neuron-associated developmentally regulated protein
- PACSIN
- protein kinase C and casein kinase 2 substrate in neurons
- SH3
- Src homology 3
- TBCA
- tetrabromocinnamic acid.
REFERENCES
- 1. Blanquet P. R. (2000) Casein kinase 2 as a potentially important enzyme in the nervous system. Prog. Neurobiol. 60, 211–246 [DOI] [PubMed] [Google Scholar]
- 2. Sanz-Clemente A., Matta J. A., Isaac J. T., Roche K. W. (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67, 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanquet P. R. (1998) Neurotrophin-induced activation of casein kinase 2 in rat hippocampal slices. Neuroscience 86, 739–749 [DOI] [PubMed] [Google Scholar]
- 4. Plomann M., Lange R., Vopper G., Cremer H., Heinlein U. A., Scheff S., Baldwin S. A., Leitges M., Cramer M., Paulsson M., Barthels D. (1998) PACSIN, a brain protein that is upregulated upon differentiation into neuronal cells. Eur. J. Biochem. 256, 201–211 [DOI] [PubMed] [Google Scholar]
- 5. Qualmann B., Roos J., DiGregorio P. J., Kelly R. B. (1999) Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell 10, 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plomann M., Mörgelin M., Schael S. (2009) in The Pombe Cdc15 Homology Proteins (Aspenström P., ed) 1st Ed., pp. 39–48 Landes Bioscience, Austin, TX [Google Scholar]
- 7. Modregger J., Ritter B., Witter B., Paulsson M., Plomann M. (2000) All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J. Cell Sci. 113, 4511–4521 [DOI] [PubMed] [Google Scholar]
- 8. Qualmann B., Kelly R. B. (2000) Syndapin isoforms participate in receptor-mediated endocytosis and actin organization. J. Cell Biol. 148, 1047–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pérez-Otaño I., Luján R., Tavalin S. J., Plomann M., Modregger J., Liu X. B., Jones E. G., Heinemann S. F., Lo D. C., Ehlers M. D. (2006) Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat. Neurosci. 9, 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anggono V., Smillie K. J., Graham M. E., Valova V. A., Cousin M. A., Robinson P. J. (2006) Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat. Neurosci. 9, 752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dharmalingam E., Haeckel A., Pinyol R., Schwintzer L., Koch D., Kessels M. M., Qualmann B. (2009) F-BAR proteins of the syndapin family shape the plasma membrane and are crucial for neuromorphogenesis. J. Neurosci. 29, 13315–13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tashiro A., Yuste R. (2003) Structure and molecular organization of dendritic spines. Histol. Histopathol. 18, 617–634 [DOI] [PubMed] [Google Scholar]
- 13. Ethell I. M., Pasquale E. B. (2005) Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 75, 161–205 [DOI] [PubMed] [Google Scholar]
- 14. Bourne J. N., Harris K. M. (2008) Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 31, 47–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao X., Smith G. M., Chen J. (2009) Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp. Neurol. 215, 178–190 [DOI] [PubMed] [Google Scholar]
- 16. Giessel A. J., Sabatini B. L. (2010) M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron 68, 936–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meggio F., Pinna L. A. (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17, 349–368 [DOI] [PubMed] [Google Scholar]
- 18. Halbach A., Mörgelin M., Baumgarten M., Milbrandt M., Paulsson M., Plomann M. (2007) PACSIN 1 forms tetramers via its N-terminal F-BAR domain. FEBS J. 274, 773–782 [DOI] [PubMed] [Google Scholar]
- 19. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 20. Jones C., Hammer R. E., Li W. P., Cohen J. C., Hobbs H. H., Herz J. (2003) Normal sorting but defective endocytosis of the low density lipoprotein receptor in mice with autosomal recessive hypercholesterolemia. J. Biol. Chem. 278, 29024–29030 [DOI] [PubMed] [Google Scholar]
- 21. Hilton J. M., Plomann M., Ritter B., Modregger J., Freeman H. N., Falck J. R., Krishna U. M., Tobin A. B. (2001) Phosphorylation of a synaptic vesicle-associated protein by an inositol hexakisphosphate-regulated protein kinase. J. Biol. Chem. 276, 16341–16347 [DOI] [PubMed] [Google Scholar]
- 22. Solyakov L., Cain K., Tracey B. M., Jukes R., Riley A. M., Potter B. V., Tobin A. B. (2004) Regulation of casein kinase-2 (CK2) activity by inositol phosphates. J. Biol. Chem. 279, 43403–43410 [DOI] [PubMed] [Google Scholar]
- 23. Holt M., Varoqueaux F., Wiederhold K., Takamori S., Urlaub H., Fasshauer D., Jahn R. (2006) Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J. Biol. Chem. 281, 17076–17083 [DOI] [PubMed] [Google Scholar]
- 24. Rao Y., Ma Q., Vahedi-Faridi A., Sundborger A., Pechstein A., Puchkov D., Luo L., Shupliakov O., Saenger W., Haucke V. (2010) Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proc. Natl. Acad. Sci. U.S.A. 107, 8213–8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rex C. S., Lin C. Y., Kramár E. A., Chen L. Y., Gall C. M., Lynch G. (2007) Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J. Neurosci. 27, 3017–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerra B., Siemer S., Boldyreff B., Issinger O. G. (1999) Protein kinase CK2: evidence for a protein kinase CK2β subunit fraction, devoid of the catalytic CK2α subunit, in mouse brain and testicles. FEBS Lett. 462, 353–357 [DOI] [PubMed] [Google Scholar]
- 27. Hinton R. H., Mullock B. M. (1997) in Subcellular Fractionation: A Practical Approach (Graham J. M., Rickwood D., eds) 1st Ed., pp. 31–69, IRL Press, Oxford, UK [Google Scholar]
- 28. Gorski J. A., Zeiler S. R., Tamowski S., Jones K. R. (2003) Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J. Neurosci. 23, 6856–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakayama A. Y., Luo L. (2000) Intracellular signaling pathways that regulate dendritic spine morphogenesis. Hippocampus 10, 582–586 [DOI] [PubMed] [Google Scholar]
- 30. Tashiro A., Minden A., Yuste R. (2000) Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb. Cortex 10, 927–938 [DOI] [PubMed] [Google Scholar]
- 31. de Kreuk B.-J., Nethe M., Fernandez-Borja M., Anthony E. C., Hensbergen P. J., Deelder A. M., Plomann M., Hordijk P. L. (2011) The F-BAR domain protein PACSIN2 associates with Rac1 and regulates cell spreading and migration. J. Cell Sci. 124, 2375–2388 [DOI] [PubMed] [Google Scholar]
- 32. Richnau N., Aspenström P. (2001) Rich, a rho GTPase-activating protein domain-containing protein involved in signaling by Cdc42 and Rac1. J. Biol. Chem. 276, 35060–35070 [DOI] [PubMed] [Google Scholar]
- 33. Furuta B., Harada A., Kobayashi Y., Takeuchi K., Kobayashi T., Umeda M. (2002) Identification and functional characterization of nadrin variants, a novel family of GTPase activating protein for rho GTPases. J. Neurochem. 82, 1018–1028 [DOI] [PubMed] [Google Scholar]
- 34. Roche K. W., Tingley W. G., Huganir R. L. (1994) Glutamate receptor phosphorylation and synaptic plasticity. Curr. Opin. Neurobiol. 4, 383–388 [DOI] [PubMed] [Google Scholar]
- 35. Moss S. J., Smart T. G. (1996) Modulation of amino acid-gated ion channels by protein phosphorylation. Int. Rev. Neurobiol. 39, 1–52 [DOI] [PubMed] [Google Scholar]
- 36. Newpher T. M., Ehlers M. D. (2009) Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 19, 218–227 [DOI] [PubMed] [Google Scholar]
- 37. Haditsch U., Leone D. P., Farinelli M., Chrostek-Grashoff A., Brakebusch C., Mansuy I. M., McConnell S. K., Palmer T. D. (2009) A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Mol. Cell. Neurosci. 41, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steffen A., Rottner K., Ehinger J., Innocenti M., Scita G., Wehland J., Stradal T. E. (2004) Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J. 23, 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tolias K. F., Bikoff J. B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R. J., Greenberg M. E. (2005) The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron 45, 525–538 [DOI] [PubMed] [Google Scholar]
- 40. Miyamoto Y., Yamauchi J., Tanoue A., Wu C., Mobley W. C. (2006) TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc. Natl. Acad. Sci. U.S.A. 103, 10444–10449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tolias K. F., Duman J. G., Um K. (2011) Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 94, 133–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ridley A. J. (2006) Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16, 522–529 [DOI] [PubMed] [Google Scholar]
- 43. Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., Kunugi H. (2010) BDNF function and intracellular signaling in neurons. Histol. Histopathol. 25, 237–258 [DOI] [PubMed] [Google Scholar]
- 44. Jourdi H., Iwakura Y., Narisawa-Saito M., Ibaraki K., Xiong H., Watanabe M., Hayashi Y., Takei N., Nawa H. (2003) Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev. Biol. 263, 216–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roberts A. C., Díez-García J., Rodriguiz R. M., López I. P., Luján R., Martínez-Turrillas R., Picó E., Henson M. A., Bernardo D. R., Jarrett T. M., Clendeninn D. J., López-Mascaraque L., Feng G., Lo D. C., Wesseling J. F., Wetsel W. C., Philpot B. D., Pérez-Otaño I. (2009) Downregulation of NR3A-containing NMDARs is required for synapse maturation and memory consolidation. Neuron 63, 342–356 [DOI] [PMC free article] [PubMed] [Google Scholar]