Background: Vac14 binds negative and positive regulators of phosphatidylinositol 3,5-bisphosphate to control endolysosome function.
Results: Vac14 dimerizes through its C terminus. Monomeric Vac14 mutants cannot interact with Fab1 and Fig4 and cannot complement vac14Δ cells.
Conclusion: Vac14 dimerization is a prerequisite for the assembly and function of the Fab1 complex.
Significance: The Vac14 dimer but not the monomer integrates regulators of PtdIns(3,5)P2.
Keywords: Lysosomes, Membrane Trafficking, Phosphatidylinositol Kinase, Phosphatidylinositol Signaling, Protein Assembly, Organelle Identity, Phosphoinositides, Yeast Vacuole
Abstract
Phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2) helps control various endolysosome functions including organelle morphology, membrane recycling, and ion transport. Further highlighting its importance, PtdIns(3,5)P2 misregulation leads to the development of neurodegenerative diseases like Charcot-Marie-Tooth disease. The Fab1/PIKfyve lipid kinase phosphorylates PtdIns(3)P into PtdIns(3,5)P2 whereas the Fig4/Sac3 lipid phosphatase antagonizes this reaction. Interestingly, Fab1 and Fig4 form a common protein complex that coordinates synthesis and degradation of PtdIns(3,5)P2 by a poorly understood process. Assembly of the Fab1 complex requires Vac14/ArPIKfyve, a multimeric scaffolding adaptor protein that coordinates synthesis and turnover of PtdIns(3,5)P2. However, the properties and function of Vac14 multimerization remain mostly uncharacterized. Here we identify several conserved C-terminal motifs on Vac14 required for self-interaction and provide evidence that Vac14 likely forms a dimer. We also show that monomeric Vac14 mutants do not support interaction with Fab1 or Fig4, suggesting that Vac14 multimerization is likely the first molecular event in the assembly of the Fab1 complex. Finally, we show that cells expressing monomeric Vac14 mutants have enlarged vacuoles that do not fragment after hyperosmotic shock, which indicates that PtdIns(3,5)P2 levels are greatly abated. Therefore, our observations support an essential role for the Vac14 homocomplex in controlling PtdIns(3,5)P2 levels.
Introduction
Phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2)4 controls endolysosome function in eukaryotes (1). For instance, cells deficient in PtdIns(3,5)P2 have dramatically enlarged vacuoles in Saccharomyces cerevisiae (yeast) and endolysosomes in higher eukaryotes (2–6). It is postulated that this may be due to defective membrane recycling from vacuoles and late endosomal organelles (7, 8). PtdIns(3,5)P2 also seems to play a role in cargo sorting into multivesicular bodies, autophagy, and endolysosome acidification (3, 9–11). However, it should be stressed that these functions are not universal to all organisms or cell types; for example, autophagy defects are only perceived in higher eukaryotes but not in yeast (1). Interestingly, PtdIns(3,5)P2 has emerged as an apparent novel regulator of various Ca2+ channels, including the ryanodine receptor in cardiac tissue and the lysosomal TRPML1 channel defective in mucolipodosis type IV (12–14).
PtdIns(3,5)P2 is thought to localize predominantly to late endosomes in mammals and to vacuoles in yeast (1, 15). Fab1/PIKfyve (yeast/mammal nomenclature) converts PtdIns(3)P into PtdIns(3,5)P2 by phosphorylation at the 5-position (3, 16). Conversely, Fig4/Sac3 dephosphorylates PtdIns(3,5)P2 back to PtdIns(3)P (17, 18). Surprisingly, Fab1 and Fig4 form a conserved protein complex (19–21). The Fab1/PIKfyve complex also contains the Vac14/ArPIKfyve adaptor protein, a protein predicted to be composed entirely of HEAT repeats (19–21).
Vac14/ArPIKfyve is necessary for PtdIns(3,5)P2 synthesis; in vac14Δ yeast cells, only 10% of PtdIns(3,5)P2 remains relative to wild-type levels, which leads to swollen vacuoles (22, 23). In mammals, Vac14 knockdown and knock-out mice result in at least 50% reduction in this lipid and enlarged endolysosomes, and consequently, vac14−/− mice develop severe neurodegeneration (24, 25). Unexpectedly, Vac14 is also necessary for the turnover of PtdIns(3,5)P2 by binding and recruiting Fig4 to the membrane (18, 26). In fact, Vac14 is necessary for the interaction between Fab1/PIKfyve and the Fig4/Sac3 phosphatase, which led to the notion that Vac14 is a core scaffold of the Fab1/PIKfyve complex (19, 20, 27). Interestingly, Fig4 is also necessary to stabilize the Vac14 interaction with Fab1, and consequently, it is necessary for PtdIns(3,5)P2 synthesis as well (17, 19, 26, 28).
Vac14/ArPIKfyve is a self-interacting protein (19, 20, 23, 27). ArPIKfyve has been suggested to form either a dimer and/or a trimer and to require its C-terminal region to mediate self-interaction (27). However, the relevance of Vac14/ArPIKfyve multimerization toward the interaction with Fab1 and Fig4, and ultimately Vac14 function, has not yet been determined. Here we identify several motifs necessary for Vac14 self-interaction and provide evidence that monomeric Vac14 mutants do not support interaction with Fab1 or Fig4 and cannot rescue the vacuolar defects in vac14Δ cells.
EXPERIMENTAL PROCEDURES
Yeast and Bacteria Strains
All yeast strains were grown in either YPD medium or selective SD medium as appropriate. DH5α and BL21 DE3 bacteria were grown in selective LB medium. All yeast strains used in this study are listed in Table 1 along with their genotype.
TABLE 1.
S. cerevisiae strains employed in this study
| Strain name | Genotype | Reference or source |
|---|---|---|
| SEY6210 | Matα leu2–3, 112 ura3–52 his3-Δ200 trp1-Δ901 lys2–801 suc2-Δ9 | Robinson et al. (31) |
| JGY145 | SEY6210; vac14Δ::TRP1 | Gary et al. (32) |
| RBY24 | SEY6210; vac14Δ::TRP1 FAB1-Myc::HIS3 | 19 |
| RBY64 | SEY6210; VAC14-HA::TRP1 | 19 |
| RBY52 | SEY6210; VAC14-FLAG::TRP1 | 19 |
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Winzeler et al (33), Giaever et al. (34) |
| vac14Δ | BY4741; vac14Δ::KanMX | Winzeler et al. (33), Giaever et al. (34) |
Nucleic Acid Manipulation, Plasmids, and Bioinformatics Analysis
All plasmids made and employed in this study are shown in Table 2. Yeast expression plasmids based on the pRB415A-FLAG backbone and encoding Vac14Δ600–880, Vac14Δ1–350, and Vac14Δ1–500 were generated by amplification of the appropriate portions of VAC14. Similarly, pET23d(+) vectors encoding recombinant T7-Vac14Δ1–334-His6 and T7-Vac14Δ557–880-His6 were cloned by PCR amplification of the appropriate VAC14 portions. In addition, VAC14 was cloned into pETDuet-1 to express recombinant Vac14-S·Tag.
TABLE 2.
Plasmids employed in this study
| Plasmid name | Backbone and insert | Reference or source |
|---|---|---|
| pAS4 | pRB415A-FLAG::vac14Δ1–350 | This study |
| pAS6 | pRB415A-FLAG::vac14Δ1–500 | This study |
| pDT5 | pRB415A-FLAG::vac14Δ600–880 | This study |
| pTA4 | pRB415A-FLAG::VAC14SS | This study |
| pTA7 | pRB415A-FLAG::VAC14NG | This study |
| pTA5 | pRB415A-FLAG::VAC14SIA | This study |
| pTA6 | pRB415A-FLAG::VAC14CRY | This study |
| pTA1 | pET23d::vac14SS | This study |
| pTA9 | pET23d::vac14NG | This study |
| pTA2 | pET23d::vac14SIA | This study |
| pTA3 | pET23d::vac14CRY | This study |
| pSH8 | pET23d::Vac14Δ557–880 | This study |
| pSH10 | pET23d::Vac14Δ1–334 | This study |
| pSH11 | pETDuet-1::VAC14 | This study |
| pJE1811.5 | pBP73G::ATG18 | Efe et al. (35) |
| pRB415::HA-VAC14 | 19 | |
| pRB415A-FLAG::VAC14 | 19 | |
| pET23d (+)::VAC14 | 19 | |
| pRS413::VAC14L149R | Jin et al. (20) |
To generate the Vac14 site-directed mutants, we used the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies), following the manufacturer's instructions. We employed pET23d::VAC14 as the template, which encodes the T7-Vac4-His6 recombinant chimera (19). The bacterial vectors expressing vac14560SS, vac14582NG, vac146623SIA, and vac14651CRY were then used to PCR-amplify the entire mutant vac14 using primers containing a 5′-BglII and a 3′-XhoI restriction site for cloning into pRB415A-FLAG. All cloning and mutations were verified by sequencing and Western blot analysis. The construct expressing VAC14L149R-HA were described previously (20).
Co-immunoprecipitation
Yeast cultures were grown overnight in appropriate medium to an A600 of ≈0.6. Cells were resuspended in spheroplasting medium (2% glucose, 1× amino acids, 1 m sorbitol, 20 mm Tris, pH 7.5, 1× Yeast Nitrogen Base) with 15 units of zymolyase. Spheroplasted cells were lysed in a 2-ml Dounce homogenizer in 1 ml of HEPES-lysis buffer (20 mm HEPES, pH 7.2, 50 mm potassium acetate, 200 mm sorbitol, and 2 mm EDTA), supplemented with 3× Complete protease inhibitor mixture, 0.3 mm 4-(2-aminoethyl)benzenesulfonyl fluoride, and 3 mm benzamide. Cell lysates were solubilized with 0.25% Tween 20, rotating at 4 °C for 15 min. Detergent-insoluble material was spun down at 13,000 × g. Input samples were removed and precipitated with 10% cold trichloroacetic acid (TCA), acetone-washed, and dissolved in Laemmli buffer. Immunoprecipitation (IP) was done by adding 1 μg/ml monoclonal antibodies, anti-FLAG antibodies (M2; Sigma-Aldrich) to the cleared lysates and rotating for 1 h at 4 °C. Approximately 200 μl of washed GammaBind G-linked Sepharose beads (GE Healthcare) was then added, rotating for 1–2 h at 4 °C. Beads were washed three times with HEPES-lysis buffer containing 0.25% Tween 20 and two times without detergent. Bound proteins were extracted with 2× sample buffer and heated at 95 °C for 5 min. Proteins were then analyzed by SDS-PAGE and Western blotting.
Recombinant Protein Expression and Purification
Recombinant proteins were expressed in BL21 DE3star cells in Super Broth supplemented with 100 μg/ml ampicillin medium overnight at 16 °C. Cells were lysed with 0.5 mg/ml lysozyme for 2 h at 4 °C and sonicated in an ice bath for 4 pulses of 15 s (output 50) in HIS-lysis buffer (50 mm Tris-HCl, pH 8.0, 300 mm NaCl, and 20 mm imidazole) supplemented with 30 mg/ml EDTA, 2 mm β-mercaptoethanol, 0.2 mm AEBSF and bacterial protease mixture A (Bio-basic Ltd.). Recombinant proteins were isolated with Ni2+-NTA agarose (Bio-basic Ltd.) or S-protein agarose (Novagen) batch affinity chromatography and dialyzed overnight in d-Tube Dialyzer Maxi (Novagen) at 4 °C in dialysis buffer (50 mm Tris-HCl, pH 8.0, 200 mm NaCl) supplemented with 2 mm β-mercaptoethanol.
In Vitro Protein Binding Assays
Recombinant Vac14-S·Tag immobilized on S protein-agarose was incubated with purified recombinant T7-Vac14-His6 for 2 h at 4 °C with 0.05% Tween 20. After washing, bound recombinant proteins were eluted with 2× sample buffer (100 mm Tris, pH 6.8, 4% SDS, 10% glycerol, 1% bromphenol blue, and 10% β-mercaptoethanol).
Velocity Sedimentation Ultracentrifugation
Ten to 40 percent continuous glycerol gradients were prepared in dialysis buffer in 11-ml polyallomer tubes. One milliliter of samples containing 300 μg of phosphorylase b (Sigma), catalase (Sigma), ferritin (Calbiochem), thyroglobulin (Sigma), and purified recombinant T7-Vac14-His6, were loaded on top of individual glycerol gradients. Samples were centrifuged at 30,000 rpm for 4 h at 4 °C in a swinging bucket SW41 Ti rotor (Beckman Coulter) using a floor-top ultracentrifuge (Optima L-100K, Beckman Coulter). One-milliliter sample fractions were then collected. Protein standards were detected by reading the absorbance at A280, and recombinant T7-Vac14-His6 fractions were concentrated with 10% TCA and detected by Western blotting.
Fast Protein Liquid Chromatography
FPLC was performed using the AKTA FPLC system and a Sephacryl S300 column (GE Healthcare). All FPLC analyses were performed at 4 °C, and the flow rate was 0.250 ml/min in dialysis buffer. Recombinant T7-Vac14-His6 and T7-Vac14SS-His6 elutions were collected in 2-ml fractions and concentrated with 10% TCA for detection with Western blotting. Standard samples containing 300 μg of phosphorylase b (Sigma), catalase (Sigma), ferritin (Calbiochem), and thyroglobulin (Sigma) were injected into the FPLC individually.
SDS-PAGE and Western Blotting
Protein samples in 2× sample buffer were heated at 100 °C for 5 min and vortexed for 10 min at 4 °C. Protein samples were separated in a discontinuous polyacrylamide gel with 5% stacking and 9% separating gels in 1× Tris/SDS running buffer (Bio-basic Ltd.). Separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane (Pall Corporation). Incubations with antibodies were performed in 0.5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20.
Monoclonal anti-FLAG antibodies (M2; Sigma-Aldrich) were used at 1:2500 dilutions. Monoclonal anti-HA antibodies (HA.C5; Abcam) were used at 1:2000 dilutions. Monoclonal anti-Myc antibodies (Bioshop) were used at 1:1500 dilutions. Fig4 was detected with rabbit anti-Fig4 polyclonal antibodies at a concentration of 1:20,000 (18). Membranes were probed with primary antibodies overnight at 4 °C. Horseradish peroxidase (HRP)-linked secondary anti-mouse antibodies were used at a concentration of 1:5000, and HRP goat anti-rabbit IgG were used at 1:25,000. Membranes were incubated with secondary antibodies for 1 h at room temperature, followed by four washes with 1× Tris-buffered saline with 0.2% Tween-20 for 1 h at room temperature. Membranes were then exposed to Immun-Star WesternC chemiluminescence reagents (Bio-Rad) and exposed to film or using the Gel-Doc System (Bio-Rad). Band densitometry was done using ImageJ software or with Image Lab (Bio-Rad).
Vacuolar Staining, Hyperosmotic Shock, Acidification Assays, and Fluorescence Microscopy
Vacuoles were labeled with a 20-min endocytic pulse of 15 μm FM4-64 (Invitrogen), followed by a 60-min chase at 30 °C. After FM4-64 labeling, cells were exposed to 0.9 m NaCl for 10 min to induce hyperosmotic shock. Fluorescence and differential interference contrast images of FM4-64-labeled cells were obtained with a LSM 510 Zeiss laser scanning confocal microscope. Background intensity levels were linearly adjusted and assembled using Adobe Photoshop and Illustrator CS5 (Adobe Systems, San Jose, CA).
RESULTS
The C-terminal Region of Vac14 Is Necessary and Sufficient for Self-interaction
It was shown previously that the C-terminal third of ArPIKfyve, the mammalian ortholog of Vac14, was necessary for self-interaction (27). To confirm that this was conserved in yeast, we generated and expressed a FLAG-tagged Vac14 mutant lacking the C-terminal 609–880 residues (FLAG-Vac14Δ609–880). We co-expressed FLAG-Vac14Δ609–880 with full-length HA-tagged Vac14 (Vac14-HA) and performed co-immunoprecipitation assays from yeast whole cell lysates. As expected, immunoprecipitation with anti-FLAG antibodies successfully co-recovered wild-type FLAG-Vac14 and Vac14-HA (Fig. 1A). In contrast, anti-FLAG antibodies recovered FLAG-Vac14Δ609–880 but failed to co-IP Vac14-HA (Fig. 1A). We also assayed whether the N-terminal region played a role in self-interaction by designing several FLAG-tagged N-terminal truncations of Vac14. We observed that IP of Vac14Δ1–350 and Vac14Δ1–500 with anti-FLAG antibodies co-recovered Vac14-HA (Fig. 1B). Although Vac14Δ1–500 did not express as well as Vac14Δ1–350, normalization of the recovered HA signal to the corresponding FLAG-IP signal using quantitative volumetric analysis confirms that the first 500 residues of Vac14 are dispensable for self-interaction (see Fig. 1B). Larger N-terminal truncations of Vac14 failed to express (data not shown). Therefore, the N-terminal region of Vac14 appears to be dispensable for self-interaction.
FIGURE 1.
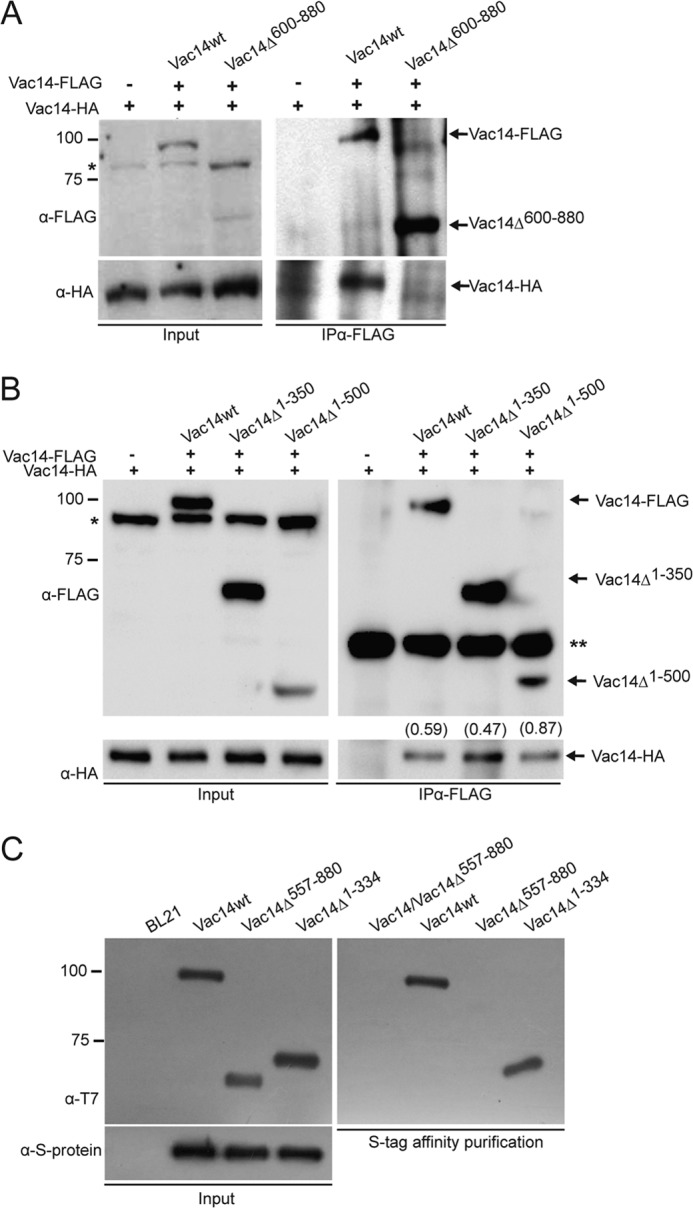
The C terminus of Vac14 is necessary and sufficient for Vac14 self-interaction. A and B, FLAG-Vac14, or its C-terminal (A) and N-terminal truncations (B) were immunoprecipitated with monoclonal anti-FLAG antibodies from whole cell lysates. Input and IP samples were then separated by SDS-PAGE and immunoblotted with anti-FLAG or anti-HA antibodies to, respectively, detect FLAG-tagged Vac14 and recovered Vac14-HA. The values in parentheses in B show the ratio of Vac14-HA signal to the respective FLAG-Vac14 signal obtained by volumetric densitometry. * denotes a nonspecific band whereas ** denotes the antibody heavy chain. C, purified recombinant wild-type Vac14-S·Tag bound to S protein-agarose beads were incubated with purified recombinant T7-Vac14-His6 or its recombinant truncation mutants. As a negative control, recombinant T7-His6-tagged Vac14 proteins were incubated with S protein-agarose beads alone. In all interaction assays, the input lanes represent 20% of protein or cell lysates used.
To further support our co-IP results from yeast lysates, we tested Vac14 self-interaction using recombinant Vac14 truncations. We generated recombinant Vac14Δ1–334 and Vac14Δ557–880 fused to the T7 and His6 epitopes on the N and C termini, respectively. These proteins expressed well in bacteria relative to the full-length T7-Vac14-HIS6, which was used as a control (19). We also attempted other truncations, but these did not express well in bacteria (data not shown). In addition, we expressed and purified recombinant S-tagged wild-type Vac14, which was bound to S protein-agarose beads (Fig. 1C). Our observations show that recombinant Vac14Δ1–334 but not Vac14Δ557–880 binds to recombinant full-length Vac14-S·Tag. In sum, our data suggest that the N terminus of Vac14 is not necessary to mediate self-interaction whereas the C terminus of Vac14 is necessary and sufficient for Vac14-Vac14 interaction.
Design and Expression of Vac14 C-terminal Point Mutants
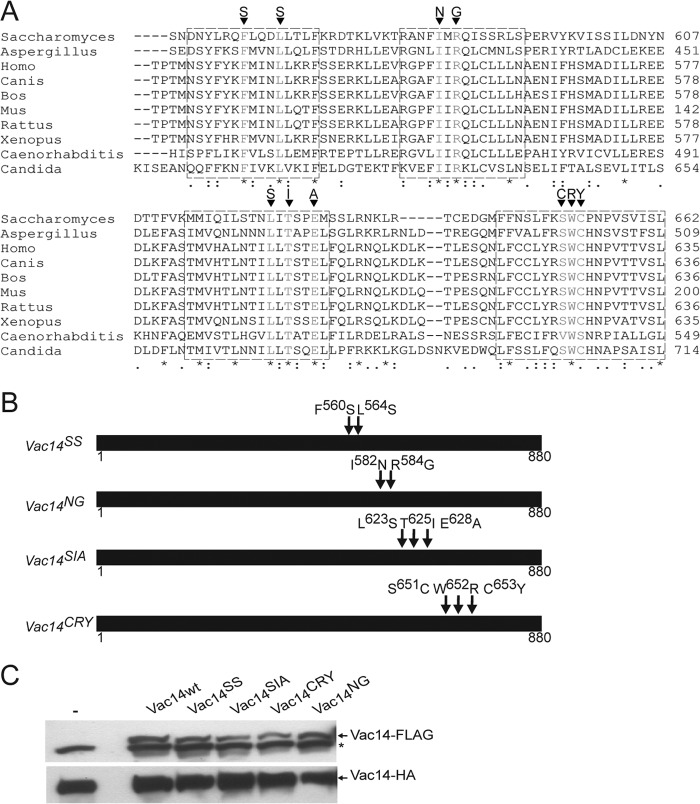
To better define the role of Vac14 self-interaction, we next designed point mutants in several conserved C-terminal motifs that we postulated might mediate Vac14 self-interaction. We identified at least four highly conserved motifs by comparing residues 500–880 from yeast Vac14 against evolutionary divergent species that included fungi, vertebrates, and nematodes (Fig. 2A).
FIGURE 2.
Identification of conserved Vac14 C-terminal motifs. A, sequence alignment of yeast Vac14 and various eukaryotic orthologs using the ClustalW algorithm. * indicates fully conserved residues, a colon indicates strong conservation between groups of similar properties, and a period indicates partial residue conservation. Boxed regions represent selected conserved motifs for site-directed mutagenesis. The targeted mutations are shown for each motif. B, schematic representation of Vac14 point mutants. Arrows show the relative positions of the point mutations in Vac14. C, whole cell lysates from cells genomically expressing Vac14-HA alone (−) or co-expressing FLAG-tagged wild-type Vac14 (Vac14wt) or Vac14 point mutants, were subjected to SDS-PAGE and Western blotting. Monoclonal anti-FLAG and monoclonal anti-HA antibodies were used to, respectively, detect the FLAG-tagged (top) and HA-tagged (bottom) Vac14 proteins. An * (asterisk) indicates a nonspecific band detected by the anti-FLAG antibody in whole cell lysates.
Previously, Vac14 was predicted to be composed entirely of HEAT repeats (20). To gain insight into functionally relevant surfaces and residues on Vac14, we attempt to model the three-dimensional structure of the Vac14 C terminus, using protein sequences from a diverse panel of species via SWISS-MODEL. Our attempts to generate a reliable model were unsuccessful due to the cryptic nature of the HEAT consensus that resides within the C terminus, if at all present. Given this, we relied on sequence conservation alone to design and generate Vac14 point mutants by site-directed mutagenesis as follows: Phe-560 and Leu-564 were converted to Ser-560 and Ser-564 (Vac14SS); Ile-582 and Arg-584 were changed to Asn-582 and Gly-584 (Vac14NG); Leu-623, Thr-625, and Glu-628 were mutated to Ser-623, Ile-625, and Ala-628 (Vac14SIA); and Ser-651, Trp-652, and Cys-653 were converted to Cys-651, Arg-652, and Tyr-653 (Vac14CRY; Fig. 2B).
The FLAG-tagged Vac14 mutants were then co-expressed with Vac14-HA in yeast. Importantly, the steady-state expression level of Vac14SS and Vac14NG mutants was similar to that of wild-type FLAG-tagged Vac14, suggesting that these mutants were stable (Fig. 2C). Vac14CRY and Vac14SIA were also expressed in yeast cells but displayed 20–30% lower steady-state expression levels relative to the wild-type protein (Fig. 2C).
Identification of Motifs Required for Vac14 Self-interaction
We next examined the ability of each of the generated Vac14 point mutants to interact with wild-type Vac14-HA. As before, we used anti-FLAG antibodies to IP FLAG-tagged Vac14 isoforms and examined for co-recovery of Vac14-HA. As anticipated, Vac14-HA was specifically recovered with wild-type FLAG-Vac14 (Fig. 3A). In contrast, co-IP of Vac14-HA with the FLAG-tagged mutants was significantly abated (Fig. 3A). The most disruptive mutants were Vac14SS and Vac14CRY, with >90% loss of Vac14 self-interaction, whereas Vac14SIA and Vac14NG recovered ∼50 and ∼20% of Vac14-HA, respectively, and relative to wild-type Vac14 (Fig. 3A). Semiquantitative densitometry of Western blots also showed that the lower steady-state expression level of Vac14CRY and Vac14SIA was not sufficient to account for the extensive loss of Vac14-HA recovered with these mutants relative to wild-type FLAG-Vac14 (Fig. 3B). As a general control for co-IP of Vac14 point mutants, we also tested and showed that Vac14L149R-HA, an N-terminal Vac14 point mutant that is unable to interact with Fab1 (20), retained its ability to self-interact with Vac14-FLAG (Fig. 3C).
FIGURE 3.
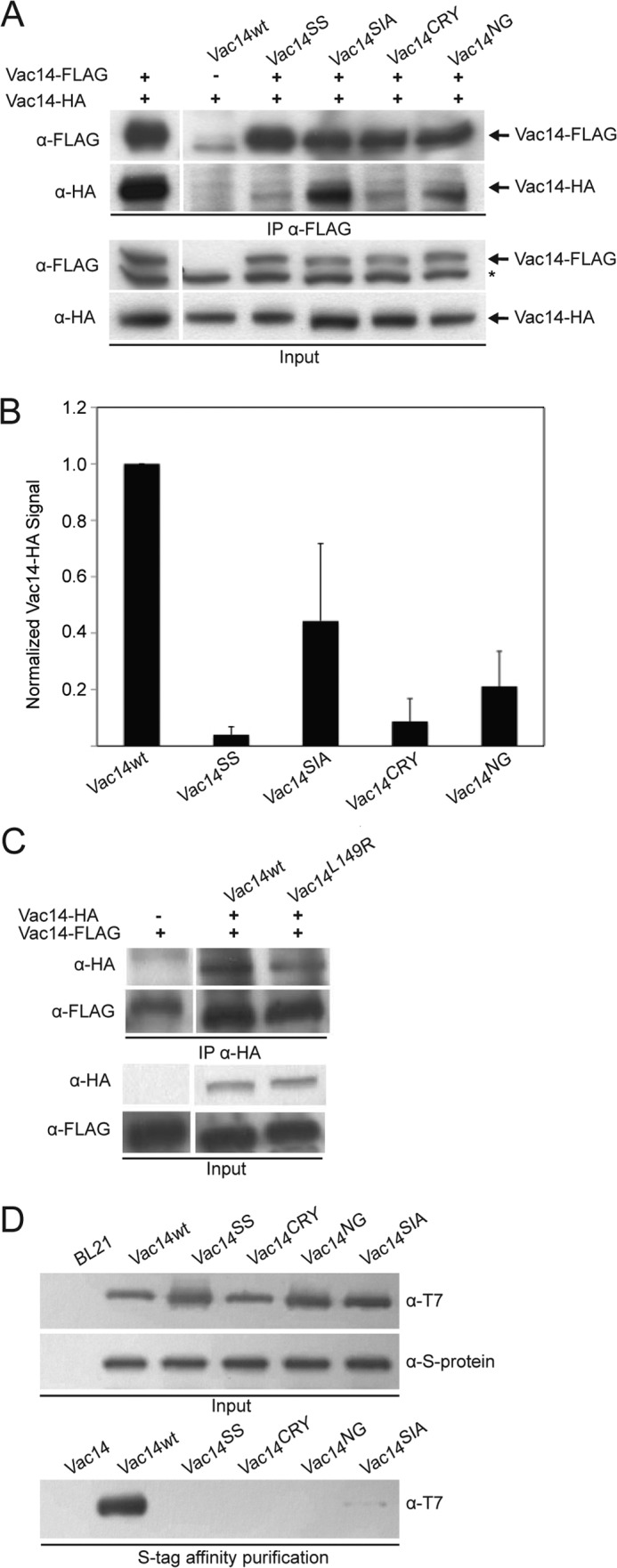
Identification of four conserved C-terminal motifs necessary for Vac14 self-interaction. A, co-IP from whole cell lysates expressing Vac14-HA and transformed with empty vector (−), or with vectors expressing FLAG-tagged wild-type or Vac14 point mutants. IP was done with monoclonal anti-FLAG antibodies and subjected to SDS-PAGE and Western blotting with anti-FLAG or anti-HA antibodies. The * indicates nonspecific bands. B, semiquantification of Vac14-HA co-immunoprecipitated with FLAG-tagged Vac14. Integrated pixel intensity of Vac14-HA was normalized to integrated pixel intensity of inputted FLAG-tagged Vac14. All pixel intensity values were background-corrected and employed nonsaturated signals. Shown is the normalized mean ± S.D. All mutant Vac14 means were statistically analyzed against wild-type Vac14 using Student's t test (n = 3, p < 0.05). C, IP of HA-tagged wild-type and L149R Vac14 with anti-HA antibodies, followed by SDS-PAGE and immunoblotting against genomically encoded Vac14-FLAG. D, recombinant Vac14-S·Tag bound to S protein-agarose beads were incubated with T7-His6-tagged recombinant wild-type and point mutant Vac14 proteins. In all cases, input lanes represent 20% of total whole cell lysates or protein used in affinity precipitation.
To corroborate the co-IP assays, we next performed in vitro protein binding assays using recombinant proteins. We used site-directed mutagenesis to generate bacterial expression vectors encoding Vac14SS, Vac14NG, Vac14SIA, and Vac14CRY fused to the T7 and His6 epitopes on the N and C termini, respectively, by using a previously described plasmid encoding wild-type T7-Vac14-His6 (19). We then expressed and purified the T7-tagged Vac14 point mutants and tested for interaction with recombinant wild-type Vac14-S·Tag immobilized onto S protein-agarose beads (Fig. 3D). As expected, T7-Vac14-His6 readily interacted and was recovered with Vac14-S·Tag but not with beads alone (Fig. 3D). Strikingly, all recombinant Vac14 mutants bound very poorly to Vac14-S·Tag, albeit recombinant Vac14SIA retained detectable interaction (Fig. 3D). This difference in binding is not attributable to differences in expression or inputs because all Vac14 recombinant mutants expressed as well as the wild-type T7-Vac14-His6 (Fig. 3D). Together, our data suggest that we have identified four key motifs in the C terminus of Vac14 ranging from residues Phe-560 to Cys-653 that help mediate the Vac14-Vac14 intermolecular interaction.
Recombinant Vac14 Is Likely a Dimer
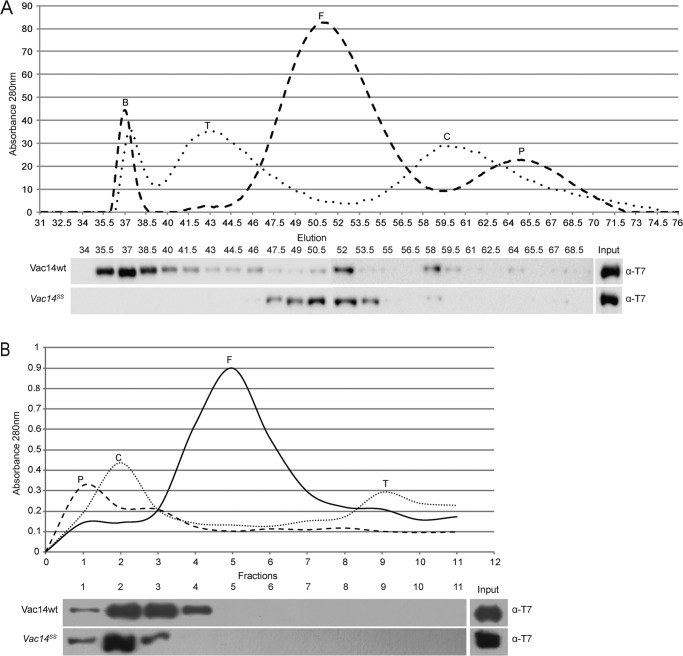
Following, we attempted to identify the multimeric state of recombinant Vac14. We first employed FPLC-controlled gel filtration using a S300 Sephacryl size-exclusion column to fractionate soluble recombinant Vac14. However, Vac14 was recovered predominantly in the void volume, suggesting that the Vac14 multimer had a very large hydrodynamic radius, possibly due to a molecular mass >1000 kDa and/or an elongated molecular shape (Fig. 4A). Strikingly, the void volume peak was no longer observed when the recombinant Vac14SS mutant was fractionated (Fig. 4A). Instead, Vac14SS protein was mainly found in fractions corresponding to ferritin (∼450 kDa); the wild-type Vac14 also exhibited a minor peak in these fractions (Fig. 4A). Overall, these data suggest that monomeric Vac14 has a very large Stokes radius due to an extended conformation because the monomer behaves like the ferritin standard instead of the phosphorylase b standard (∼100 kDa). To further help differentiate whether Vac14 forms a very large multimer and/or whether Vac14 has a very large hydrodynamic radius due to a nonglobular conformation, we analyzed recombinant Vac14 by velocity sedimentation through a glycerol gradient (29).
FIGURE 4.
The multimeric state of Vac14 and Vac14SS. A, gel-exclusion chromatography through a Sephacryl S300 column. Three hundred micrograms of each protein standard, purified recombinant wild type T7-Vac14-His6, and purified recombinant T7-Vac14SS-His6 were separately injected and fractionated through the S300 column. Eluted standards were detected by A280 nm (top). The elutions of recombinant T7-Vac14-His6 and T7-Vac14SS-His6 were collected in 2-ml fractions, followed by SDS-PAGE, and immunoblotted with anti-T7 antibodies. Input lane represents 10% of the total recombinant protein injected. B, differential velocity ultracentrifugation through a 10–40% glycerol gradient. Approximately 300 μg of each protein standard and purified recombinant T7-Vac14-His6 or T7-Vac14SS-His6 were layered onto individual glycerol gradients and centrifuged at 30,000 rpm for 5 h. Fractionated protein standards and recombinant Vac14 were collected in 1-ml fractions. Protein standards were detected at A280 nm, and recombinant T7-Vac14-His6 was detected by immunoblotting with anti-T7 antibodies. Input lane represents 10% of the total recombinant protein. The protein standards used were: blue dextran (B), thyroglobulin (T), ferritin (F), catalase (C), and phosphorylase b (P).
We employed four molecular mass standards for velocity sedimentation and obtained the following pattern: phosphorylase b (100 kDa) peaked at fraction 1, catalase (250 kDa) peaked at fractions 2–3, ferritin (450 kDa) was concentrated in fractions 4–6, and thyroglobulin (660 kDa) predominated in fractions 9–10 (Fig. 4B). Compared with these standards, recombinant wild-type Vac14 was consistently enriched in fractions 2 and 3, corresponding to the catalase pattern (250 kDa; Fig. 4B). Importantly, fractionation of recombinant Vac14SS clearly demonstrated a shift to fraction 2, consistent with a loss of Vac14 self-interaction (Fig. 4B). These results suggest that Vac14 does not form a very large multimer, contrary to what the FPLC experiments suggest. Furthermore, because monomeric Vac14SS still co-migrates with catalase, a 250-kDa protein, and does not readily accumulate in fraction 1, which corresponds to phosphorylase b (100 kDa), this suggests that Vac14 migration is still affected by a putative large hydrodynamic radius, consistent with the gel filtration of Vac14SS. Therefore, whereas wild-type recombinant protein co-migrates to apparent sizes of ∼300 kDa, Vac14 is likely a dimer that appears to migrate as a heavier complex due to the putative larger Stokes radius (29).
Monomeric Vac14 Mutants Do Not Interact with Fab1 or Fig4
Vac14 was previously shown to interact with the Fig4 phosphatase in a Fab1-independent manner (19). Conversely, Vac14 also interacts with Fab1 but requires Fig4 for stable binding (19, 20). However, it remained unknown whether Vac14 multimerization was a necessary step for Vac14 to bind Fab1 and/or Fig4. For example, it was conceivable that Fig4 helped to stabilize the Fab1-Vac14 interaction by securing the formation of a Vac14 multimer, which might in turn complete the Fab1 binding interface on Vac14. Thus, we next chose to test whether the monomeric Vac14SS and Vac14CRY mutants could support interaction with Fab1 and/or Fig4 by co-IP. As previously demonstrated, wild-type FLAG-Vac14 specifically co-immunoprecipitated together with chromosomally expressed Fab1-Myc (Fig. 5A) and Fig4 (Fig. 5B). In sharp contrast, co-IP of FLAG-tagged Vac14SS and Vac14CRY failed to recover both Fab1-Myc (Fig. 5A) and Fig4 (Fig. 5B). Therefore, our data suggest that Vac14 multimerization is an essential step for interaction with both Fab1 and Fig4 and further suggest that Vac14 multimerization is a prerequisite for Fab1 complex assembly.
FIGURE 5.
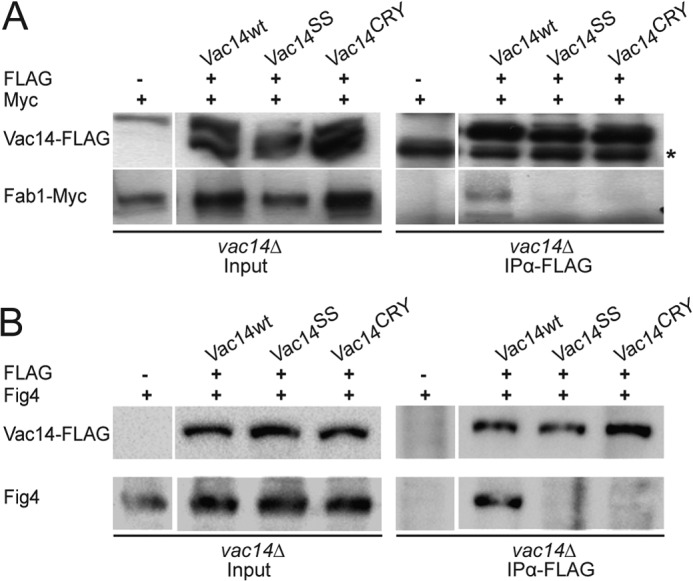
Monomeric Vac14 mutants do not interact with Fab1 or Fig4. vac14Δ FAB1-Myc cells were transformed with plasmids encoding FLAG-tagged Vac14, Vac14SS, or Vac14CRY mutants. FLAG-Vac14 proteins were then immunoprecipitated with monoclonal anti-FLAG antibodies and subjected to SDS-PAGE and immunoblotting. A, co-IP of Fab1-Myc with FLAG-tagged wild-type Vac14 but not with corresponding mutants. B, co-IP of Fig4 with FLAG-tagged wild-type Vac14 but not with corresponding Vac14 mutants. vac14Δ FAB1-Myc cells carrying an empty vector (−) were used as a negative control. Input lanes represent 20% of total protein used in IP. * (asterisk) indicates nonspecific.
Expression of Monomeric Vac14 Mutants Does Not Rescue vac14Δ Phenotypes
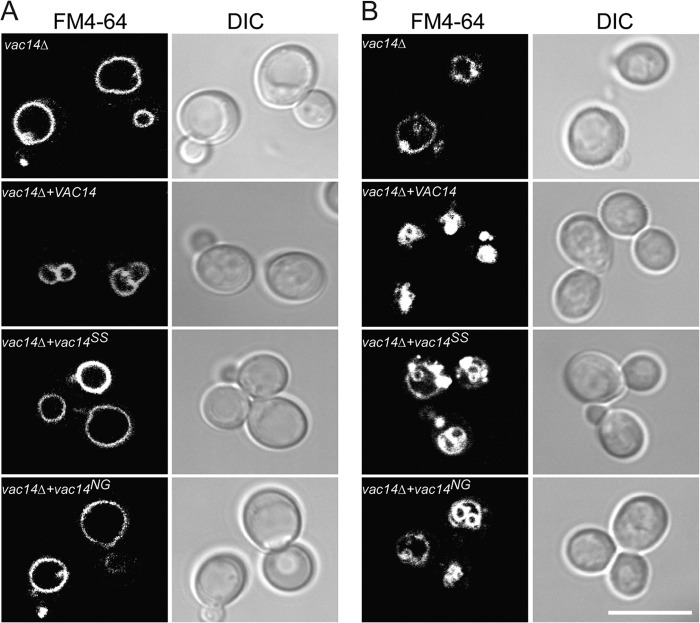
We finally examined the functional consequence of expressing monomeric Vac14 mutants by investigating their ability to rescue vac14Δ cells. Plasmids expressing Vac14SS, Vac14CRY, Vac14SIA, and Vac14NG were transformed into vac14Δ cells. We then examined the vacuolar morphology of these cells by staining the vacuoles with FM4-64. As expected, vac14Δ cells had very large vacuoles that were rescued when VAC14 was expressed (Fig. 6A). In contrast, vac14Δ cells expressing monomeric Vac14 mutant proteins retained the swollen vacuole phenotype (Vac14SS and Vac14NG results are shown, Fig. 6A). We also investigated whether monomeric Vac14 mutants could support hyperosmotic shock-mediated vacuole fragmentation in yeast cells, which is caused by a 5–10-fold increase in the levels of PtdIns(3,5)P2 (22). Indeed, whereas vac14Δ cells expressing wild-type Vac14 exhibited extremely fragmented vacuoles, parental vac14Δ cells and those expressing Vac14 point mutants resisted vacuole fragmentation (Fig. 6B, Vac14SS and Vac14NG are shown). Instead, the vacuoles in these cells collapsed or infolded to form apparent invaginations instead of fragmenting after hyperosmotic shock (Fig. 6B). Together, our results indicate that monomeric Vac14 mutants poorly support PtdIns(3,5)P2 synthesis.
FIGURE 6.
Monomeric Vac14 mutants display vacuolar defects. A and B, vac14Δ cells transformed with either empty vector or vectors expressing Vac14, Vac14SS, or Vac14NG were grown to log phase. Vacuoles were then labeled with FM4-64 as described under “Experimental Procedures.” A, vacuolar morphology of log-phase cells. B, vacuolar morphology of cells exposed to a 10-min hyperosmotic shock with 0.9 m NaCl. The respective FM4-64 (left) and differential interference contrast (DIC, right) are shown for each condition. The scale bar represents 10 μm.
DISCUSSION
Vac14 is important for cells to maintain proper PtdIns(3,5)P2 levels; vac14Δ cells possess only 10% of the wild-type levels of this lipid (22, 23). At first glance, the abated PtdIns(3,5)P2 levels in vac14Δ cells imply that Vac14 is simply necessary for the synthesis of PtdIns(3,5)P2. However, elegant studies performed by Duex et al. showed that Vac14 also plays a role in the degradation of PtdIns(3,5)P2 (26). Indeed, Vac14 not only binds to the Fab1 kinase and Vac7, likely to support PtdIns(3,5)P2 synthesis, but it also associates directly with the Fig4 phosphatase and possibly with Atg18; these interactions likely support down-modulation of PtdIns(3,5)P2 (19, 20). Thus, Vac14 has been proposed to form a scaffolding core for the Fab1 complex that integrates Fab1, Fig4, Vac7, and Atg18 signaling. Of course, this interesting superimposition of the positive and negative regulatory arms of PtdIns(3,5)P2 complicates our interpretation of how Vac14 functions, requiring a systematic analysis of Vac14 (20).
Vac14 is a self-interacting protein as indicated by yeast two-hybrid assays, co-IP, and in vitro recombinant protein binding assays (19, 20, 23). We speculated and provide evidence here that Vac14 multimerization is a prerequisite for Vac14 to govern PtdIns(3,5)P2 levels. In mammals, ArPIKfyve was shown to self-interact through the C-terminal third (27). Our observations using endogenous and recombinant yeast Vac14 confirm that the C-terminal region of Vac14 is not only necessary, but is also sufficient for Vac14 self-interaction. This excludes the possibility that Vac14 self-interacts in an anti-parallel fashion with the C terminus binding to the N terminus.
We identified at least four highly conserved motifs in the C terminus of Vac14 that appear to contribute to Vac14 self-interaction because Vac14SS, Vac14CRY, Vac14SIA, and Vac14NG mutants were all compromised for interaction with wild-type Vac14 as determined by in vitro binding assays and co-IP with yeast lysates. Although we cannot rule out generalized disruption of the Vac14 structure, it is unlikely that all four of these mutants disrupt Vac14 self-interaction in this manner because both yeast and recombinant Vac14 mutant proteins were soluble and expressed at steady-state levels similar to the corresponding yeast and recombinant wild-type Vac14 proteins. Nevertheless, the mutated residues may locally disrupt secondary structures such as an α-helix causing a local unfolding of the Vac14, which in turn could displace the residues that make direct contact with other Vac14 molecules. Thus, we stress that the mutated residues are not necessarily the ones making direct contact with other Vac14 molecules. Unfortunately, our attempts to generate an unbiased molecular model of the Vac14 C terminus did not produce any useful data. Because the Vac14 C terminus is not built using classical HEAT repeats, it diminishes our confidence in an in silico approach. The nature of the C terminus requires a comprehensive biophysical characterization to better define its composition and three-dimensional structure.
ArPIKfyve was previously proposed to form a homodimer and/or homotrimer (27). However, this conclusion relied on formaldehyde cross-linked products from mammalian cell lysates overexpressing ArPIKfyve; arguably, ArPIKfyve may have been cross-linked to endogenous binding partners even if not Sac3 or PIKfyve; in fact, at least one additional band other than the bands consistent with a dimer and trimer is observable (27). Therefore, we attempted to determine the Vac14 multimeric state by utilizing recombinant Vac14; using this system, we believe our data better support the existence of a Vac14 homodimer, although we cannot conclusively exclude the possibility of a homotrimer or a difference between yeast Vac14 and ArPIKfyve. First, our data suggest that Vac14 has a large conformation-dependent hydrodynamic radius because (i) recombinant monomeric Vac14SS behaved as a ∼400-kDa and ∼200-kDa protein by gel filtration and velocity sedimentation, respectively, instead of the expected 100-kDa protein; and (ii) whereas wild-type Vac14 eluted in the void volume of a S300 Sephacryl column, which has a fractionation resolution up to 1500 kDa, the fractionation pattern of wild-type Vac14 by ultracentrifugation was most compatible with a complex <400 kDa, the difference in apparent sizes is likely because gel filtration is more greatly affected by the hydrodynamic radius of a molecule than velocity ultracentrifugation. Second, given the apparent large hydrodynamic radius, we would have expected a homotrimeric Vac14 to fractionate closer to ferritin by ultracentrifugation; however, we did not observe this pattern. Hence, we deem that recombinant yeast Vac14 forms a homodimer.
We also showed that monomeric Vac14 mutants do not interact with Fab1 or Fig4. This suggests that the dimerization of Vac14 is necessary to form the stable binding interface for Fig4 and Fab1, and consequently, assembly of the Fab1 complex. Consistent with this, monomeric Vac14 mutants are incapable of rescuing vac14Δ defects including vacuolar morphology and fragmentation after hyperosmotic shock; these observations intimate that monomeric Vac14 mutants have low PtdIns(3,5)P2 levels. In fact, the vacuolar morphology of vac14Δ and vac14Δ cells expressing monomeric Vac14 alleles resembled the recently observed invaginated vacuoles that form in PtdIns(3,5)P2-deficient cells exposed to salt shock (30).
Based on our work, we suggest a model for the sequential assembly of the Fab1 complex (Fig. 7). First, we propose that the Vac14 forms a homodimer subcomplex, although we cannot yet distinguish whether Vac14 homodimerizes via its C termini in a parallel or in an anti-parallel fashion. This generates an interface that binds Fig4 to form the Vac14-Fig4 subcomplex, which occurs independently of Fab1 (19). Binding of Fig4 then induces a conformational change in the Vac14 homodimer that better complements and binds Fab1. Because Vac7 and Atg18 are not required for Fab1-Vac14-Fig4 interactions, they likely assemble last (19, 20).
FIGURE 7.
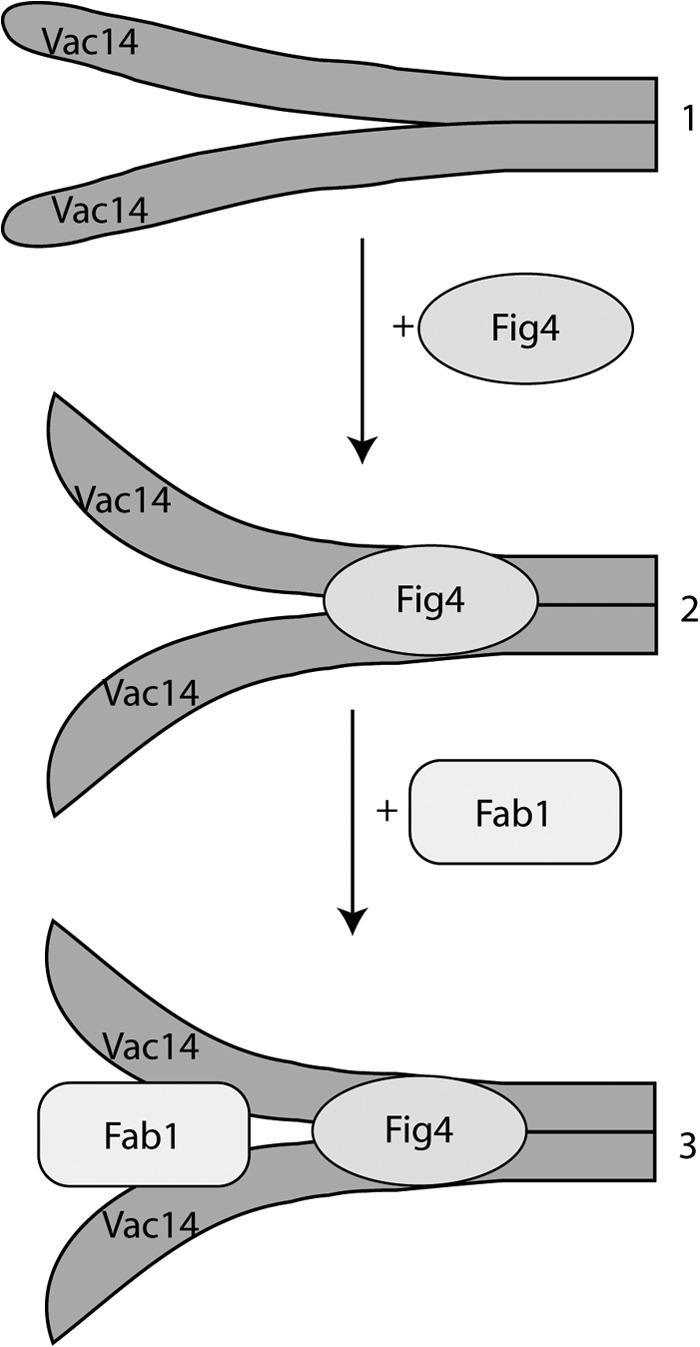
Model for the assembly of the Fab1 complex. Step 1, Vac14 is dimerized through the C-terminal tails, either in parallel (shown) or anti-parallel (not shown). Step 2, the Vac14 homodimer recruits the Fig4 phosphatase to form the Vac14-Fig4 subcomplex. We postulate that Fig4 association induces a conformational change on the N-terminal regions of the Vac14 dimer that better binds Fab1 because (i) Fig4 is necessary for Fab1-Vac14 association and (ii) there is no evidence to suggest that Fab1 and Fig4 bind directly to each other. Step 3, the Fab1 kinase finally binds to the N termini of the Vac14 dimer. Although not shown, we postulate that Vac7 and possibly Atg18 interact last with the Fab1 complex because neither of these proteins is necessary for the Fab1 complex assembly.
In conclusion, we present evidence that Vac14 forms a homodimer that is essential for the assembly and function of the Fab1 complex. Future work will depend on structural analysis to better understand how Vac14 dimerizes and how the molecular changes that ensue permit Vac14, Fig4, and Fab1 to communicate and govern PtdIns(3,5)P2.
Acknowledgments
We thank Dr. Brenda Andrews for the BY4741 and BY4741 vac14Δ and Dr. Lois Weisman for the plasmid encoding HA-Vac14L149R.
Footnotes
- PtdIns(3,5)P2
- phosphatidylinositol 3,5-bisphosphate
- IP
- immunoprecipitation.
REFERENCES
- 1. Ho C. Y., Alghamdi T. A., Botelho R. J. (2012) Phosphatidylinositol 3,5-bisphosphate: no longer the poor PIP2. Traffic 13, 1–8 [DOI] [PubMed] [Google Scholar]
- 2. Bonangelino C. J., Catlett N. L., Weisman L. S. (1997) Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol. Cell. Biol. 17, 6847–6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gary J. D., Wurmser A. E., Bonangelino C. J., Weisman L. S., Emr S. D. (1998) Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 143, 65–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikonomov O. C., Sbrissa D., Mlak K., Shisheva A. (2002) Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology 143, 4742–4754 [DOI] [PubMed] [Google Scholar]
- 5. Rusten T. E., Rodahl L. M., Pattni K., Englund C., Samakovlis C., Dove S., Brech A., Stenmark H. (2006) Fab1 phosphatidylinositol-3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol. Biol. Cell 17, 3989–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicot A. S., Fares H., Payrastre B., Chisholm A. D., Labouesse M., Laporte J. (2006) The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol. Biol. Cell 17, 3062–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dove S. K., Piper R. C., McEwen R. K., Yu J. W., King M. C., Hughes D. C., Thuring J., Holmes A. B., Cooke F. T., Michell R. H., Parker P. J., Lemmon M. A. (2004) Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 23, 1922–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rutherford A. C., Traer C., Wassmer T., Pattni K., Bujny M. V., Carlton J. G., Stenmark H., Cullen P. J. (2006) The mammalian phosphatidylinositol-3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J. Cell Sci. 119, 3944–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rusten T. E., Vaccari T., Lindmo K., Rodahl L. M., Nezis I. P., Sem-Jacobsen C., Wendler F., Vincent J. P., Brech A., Bilder D., Stenmark H. (2007) ESCRTs and Fab1 regulate distinct steps of autophagy. Curr. Biol. 17, 1817–1825 [DOI] [PubMed] [Google Scholar]
- 10. Ferguson C. J., Lenk G. M., Meisler M. H. (2009) Defective autophagy in neurons and astrocytes from mice deficient in PI(3,5)P2. Hum. Mol. Genet. 18, 4868–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odorizzi G., Babst M., Emr S. D. (1998) Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95, 847–858 [DOI] [PubMed] [Google Scholar]
- 12. Dong X. P., Shen D., Wang X., Dawson T., Li X., Zhang Q., Cheng X., Zhang Y., Weisman L. S., Delling M., Xu H. (2010) PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen J., Yu W. M., Brotto M., Scherman J. A., Guo C., Stoddard C., Nosek T. M., Valdivia H. H., Qu C. K. (2009) Deficiency of MIP/MTMR14 phosphatase induces a muscle disorder by disrupting Ca2+ homeostasis. Nat. Cell. Biol. 11, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Romero-Suarez S., Shen J., Brotto L., Hall T., Mo C., Valdivia H. H., Andresen J., Wacker M., Nosek T. M., Qu C. K., Brotto M. (2010) Muscle-specific inositide phosphatase (MIP/MTMR14) is reduced with age and its loss accelerates skeletal muscle aging process by altering calcium homeostasis. Aging 2, 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michell R. H., Heath V. L., Lemmon M. A., Dove S. K. (2006) Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem. Sci. 31, 52–63 [DOI] [PubMed] [Google Scholar]
- 16. Ikonomov O. C., Sbrissa D., Shisheva A. (2001) Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J. Biol. Chem. 276, 26141–26147 [DOI] [PubMed] [Google Scholar]
- 17. Duex J. E., Nau J. J., Kauffman E. J., Weisman L. S. (2006) Phosphoinositide 5-phosphatase Fig4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryot. Cell 5, 723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rudge S. A., Anderson D. M., Emr S. D. (2004) Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol. Biol. Cell 15, 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Botelho R. J., Efe J. A., Teis D., Emr S. D. (2008) Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Mol. Biol. Cell 19, 4273–4286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin N., Chow C. Y., Liu L., Zolov S. N., Bronson R., Davisson M., Petersen J. L., Zhang Y., Park S., Duex J. E., Goldowitz D., Meisler M. H., Weisman L. S. (2008) VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 27, 3221–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sbrissa D., Ikonomov O. C., Fu Z., Ijuin T., Gruenberg J., Takenawa T., Shisheva A. (2007) Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport: novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J. Biol. Chem. 282, 23878–23891 [DOI] [PubMed] [Google Scholar]
- 22. Bonangelino C. J., Nau J. J., Duex J. E., Brinkman M., Wurmser A. E., Gary J. D., Emr S. D., Weisman L. S. (2002) Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 156, 1015–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dove S. K., McEwen R. K., Mayes A., Hughes D. C., Beggs J. D., Michell R. H. (2002) Vac14 controls PtdIns(3,5)P(2) synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr. Biol. 12, 885–893 [DOI] [PubMed] [Google Scholar]
- 24. Sbrissa D., Ikonomov O. C., Strakova J., Dondapati R., Mlak K., Deeb R., Silver R., Shisheva A. (2004) A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity. Mol. Cell. Biol. 24, 10437–10447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y., Zolov S. N., Chow C. Y., Slutsky S. G., Richardson S. C., Piper R. C., Yang B., Nau J. J., Westrick R. J., Morrison S. J., Meisler M. H., Weisman L. S. (2007) Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc. Natl. Acad. Sci. U.S.A. 104, 17518–17523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duex J. E., Tang F., Weisman L. S. (2006) The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J. Cell Biol. 172, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sbrissa D., Ikonomov O. C., Fenner H., Shisheva A. (2008) ArPIKfyve homomeric and heteromeric interactions scaffold PIKfyve and Sac3 in a complex to promote PIKfyve activity and functionality. J. Mol. Biol. 384, 766–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chow C. Y., Zhang Y., Dowling J. J., Jin N., Adamska M., Shiga K., Szigeti K., Shy M. E., Li J., Zhang X., Lupski J. R., Weisman L. S., Meisler M. H. (2007) Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448, 68–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erickson H. P. (2009) Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 11, 32–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zieger M., Mayer A. (2012) Yeast vacuoles fragment in an asymmetrical two-phase process with distinct protein requirements. Mol. Biol. Cell 23, 3438–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. (1988) Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gary J. D., Sato T. K., Stefan C. J., Bonangelino C. J., Weisman L. S., Emr S. D. (2002) Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol. Biol. Cell 13, 1238–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J. D., Bussey H., et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 [DOI] [PubMed] [Google Scholar]
- 34. Giaever G., Chu A. M., Ni L., Connelly C., Riles L., Véronneau S., Dow S., Lucau-Danila A., Anderson K., André B., et al. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 [DOI] [PubMed] [Google Scholar]
- 35. Efe J. A., Botelho R. J., Emr S. D. (2007) Atg18 regulates organelle morphology and Fab1 kinase activity independent of its membrane recruitment by phosphatidylinositol 3,5-bisphosphate. Mol. Biol. Cell 18, 4232–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]