Background: 4-AP treats the symptoms of MS because it inhibits Kv channels. Deg/ENaC channels contribute to the progression of MS.
Results: 4-AP also inhibits Deg/ENaC channels.
Conclusion: Effects on both Kv and Deg/ENaC channels should be considered when evaluating the actions of 4-AP.
Significance: 4-AP may influence the symptoms and progression of MS because of inhibitory actions on Kv and Deg/ENaC channels, respectively.
Keywords: Acid-sensing Ion Channel (ASIC), ENaC, Multiple Sclerosis, Neurodegeneration, Potassium Channel
Abstract
The voltage-gated K+ (Kv) channel blocker 4-aminopyridine (4-AP) is used to target symptoms of the neuroinflammatory disease multiple sclerosis (MS). By blocking Kv channels, 4-AP facilitates action potential conduction and neurotransmitter release in presynaptic neurons, lessening the effects of demyelination. Because they conduct inward Na+ and Ca2+ currents that contribute to axonal degeneration in response to inflammatory conditions, acid-sensing ion channels (ASICs) contribute to the pathology of MS. Consequently, ASICs are emerging as disease-modifying targets in MS. Surprisingly, as first demonstrated here, 4-AP inhibits neuronal degenerin/epithelial Na+ (Deg/ENaC) channels, including ASIC and BLINaC. This effect is specific for 4-AP compared with its heterocyclic base, pyridine, and the related derivative, 4-methylpyridine; and akin to the actions of 4-AP on the structurally unrelated Kv channels, dose- and voltage-dependent. 4-AP has differential actions on distinct ASICs, strongly inhibiting ASIC1a channels expressed in central neurons but being without effect on ASIC3, which is enriched in peripheral sensory neurons. The voltage dependence of the 4-AP block and the single binding site for this inhibitor are consistent with 4-AP binding in the pore of Deg/ENaC channels as it does Kv channels, suggesting a similar mechanism of inhibition in these two classes of channels. These findings argue that effects on both Kv and Deg/ENaC channels should be considered when evaluating the actions of 4-AP. Importantly, the current results are consistent with 4-AP influencing the symptoms of MS as well as the course of the disease because of inhibitory actions on Kv and ASIC channels, respectively.
Introduction
Multiple sclerosis (MS)3 is a progressive autoimmune neuroinflammatory disease of the central nervous system characterized by demyelination of neurons (1–4). Demyelination impedes conduction of actions potentials by axons disrupting the function of presynaptic neurons and ultimately, diminishing the activity of postsynaptic cells to include skeletal muscle. The 4-amino derivative of pyridine, 4-AP, is an emerging drug used to target the symptoms of MS (1–3).
4-AP is a K+ channel blocker targeting Kv channels in particular (3–5). The inhibitory action of 4-AP on Kv channels is complex but includes entry into the channel pore by the ionized blocker through the intracellular mouth of the channel (4–6). Once in the pore, 4-AP reduces the amount of time the channel is physically open to allow movement of K+.
The Kv channel family contains a large number of distinct but related ion channels that serve a wide array of functions in diverse types of cells to include neurons, muscle, and immune cells (3). Because they conduct outward hyperpolarizing K+ currents to affect membrane potential, these channels, in general, influence the shape and conduction of action potentials, the amount of neurotransmitter released at a synapse, and influence muscle contraction. They also influence ionic gradients and cell signaling to modulate immune cell function.
Details about the cellular mechanism by which 4-AP improves symptoms in MS are currently being studied. It has been proposed that 4-AP betters symptoms primarily by overcoming axonal block to increase action potential conduction in neurons that have undergone demyelination (1–4). Alternatives, which are not necessarily mutually exclusive, are that 4-AP enhances neurotransmitter release from presynaptic neurons, improves end organ function by increasing skeletal muscle twitch, and enhances CNS motor-evoked brain activity and/or exerts immunomodulatory actions. Nevertheless, it is commonly held that 4-AP improves symptoms in MS by targeting Kv channels with debate focused on the specific cell type and cellular locale of these Kv channel targets, and the specific Kv isoform(s) targeted by the drug. Because of its wide recognition as a Kv channel blocker and because Kv channels are well positioned to mediate the beneficial cellular actions of 4-AP in treating symptoms of MS, possible additional and/or alternative protein targets for 4-AP in this regard have not attracted much attention.
In addition to the primary feature of CNS lesions with hallmark inflammation and demyelination, MS is associated with axonal degeneration. In many instances, it is the latter that correlates best with clinical deficits (7, 8). Recent findings support that inflammatory insult in MS lesions leads to axonal degeneration by causing neuronal mitochondrial dysfunction and associated energy deficits with concomitant imbalance in cellular ion exchange mechanisms (9–12). The consequent improper influx of Na+ and Ca2+ into neurons in MS lesions results in a cellular pathology akin to that in ischemia leading to axonal degeneration (13–16).
A recent study finds that the expression of the acid-sensing ion channel (ASIC), ASIC1, is enhanced in the axons of mouse and human neurons in inflammatory lesions of acute and chronic experimental autoimmune encephalomyelitis (EAE), and MS spinal cord and optic nerve tissues, respectively (17). In this study, the expression of ASIC1 was associated with axonal damage as indicated by co-localization with the axonal injury maker β-amyloid precursor protein.
Acid-sensing ion channels are members of the degenerin/epithelial Na+ channel (Deg/ENaC) family. Deg/ENaC channels are expressed in all metazoan species with ASICs first emerging in chordates, animals that possess the hollow dorsal nerve cord, the notochord (18–21). ASICs are expressed predominantly in neurons of the CNS and peripheral nervous system with distinct isoforms enriched in central neurons and peripheral sensory neurons. Four genes encode ASIC channels, ACCN1–4 with the closely related brain, liver, intestine Na+ channel, BLINaC, encoded by ACCN5 (22–24). BLINaC, like ASIC, is expressed in CNS neurons but also in epithelial cells. There are six ASIC channel subunits: ASIC1a, 1b, 2a, 2b, 3, and 4, where a and b note alternative splice variants producing proteins with distinct NH2 termini (18, 25). ASIC subunits oligomerize into homotrimeric and heterotrimeric channels; whereas ASIC2b seems only able to contribute to heterotrimeric channels, and little is known about ASIC4 (26–28).
ASICs function as plasmalemmal incidence detectors capable of sensing and transforming changes in extracellular pH into changes in intracellular Na+ and Ca2+ concentrations (18, 20, 21, 25). Thus, these molecular transducers are proton receptors positioned to influence cellular ion exchange in neurons. Physiologically, ASIC1 has been described as a postsynaptic proton receptor that influences intracellular Ca2+ concentration in CA1 hippocampal neurons (29). Pathologically, ASIC1 has been proposed to increase neuronal Na+ and Ca2+ influxes during tissue acidosis resulting from ischemia in experimental models of stroke (16).
As discussed above, expression of ASIC1 is increased in inflammatory lesions of MS and in the EAE animal model of this disease (17). That inhibition of ASIC1 with the Deg/ENaC channel blocker, amiloride, protected both myelin and neurons from damage in mice with EAE when provided at disease onset, and ameliorated disability when provided at first relapse is consistent with ASIC1 serving a causative role in the pathology and progression of this disease (17, 30). Additional support for this comes from the fact that ASIC1-null mice show markedly reduced clinical deficit and reduced axonal degeneration compared with wild type mice after induction of EAE (30). The mechanism of protection in ASIC1-null mice is related to loss of function of this channel in central neurons as a proton receptor capable of conducting debilitating inward Na+ and Ca2+ currents in response to localized tissue acidosis. Thus, ASIC1 is emerging as a target to modify the course of MS with inhibition of this channel retarding axonal degeneration.
No published evidence, to date, suggests that 4-AP affects Deg/ENaC channels, including ASIC. However, in our study of the Drosophila ASIC homolog, pickpocket1 (PPK1 (31), we found that 4-AP decreased the magnitude of acid-sensitive PPK1 currents in polymodal class IV multidendritic (md) peripheral sensory neurons (see “Results”). This led us to test the hypothesis that 4-AP blocks ASIC and other neuronal Deg/ENaC channels in a manner similar to its effects on Kv channels.
The current results demonstrate that 4-AP is an inhibitor of the Deg/ENaC channels expressed in neurons. This effect is evolutionarily conserved in flies to mammals with 4-AP being equally potent for neuronal Deg/ENaC and Kv channels. Also similar to Kv channels, 4-AP affects Deg/ENaC channels in an isoform-specific manner. In mammals, 4-AP has a larger inhibitory effect on ASICs expressed in central neurons, as represented by ASIC1, compared with those enriched in peripheral sensory neurons, as typified by ASIC3. 4-AP inhibition of Deg/ENaC channels is voltage-sensitive and most consistent with ionized 4-AP occupying a binding site within the channel pore. Consequently, the current results argue that the cellular effects of 4-AP should be considered from the perspective that this drug inhibits both Kv and ASIC channels. With this in mind, 4-AP may be a drug capable of influencing both the symptoms as well as the course of MS because of its action on these two distinct classes of ion channels.
EXPERIMENTAL PROCEDURES
Materials
All chemicals and materials were purchased from Sigma and Calbiochem unless noted otherwise. The effects of 4-AP on endogenous ASIC and PPK1 expressed in native tissue were determined in mouse and rat CA1 hippocampal neurons, and Drosophila class IV md sensory neurons, respectively. The effects of 4-AP and other agents on recombinant rat ASIC and rat BLINaC were determined in Chinese hamster ovary (CHO) cells. The subclone CHO-K1 cell line used in these studies was purchased from ATTC (32). Expression vectors encoding rASIC1a, rASIC2a, and rASIC3 were a gift from Dr. P. K. Ahring (NeuroSearch A/S, Ballerup, Denmark) and have been described previously (33). The expression vector encoding YFP-tagged rASIC1b was a gift from Drs. D. J. Benos and C. Fuller (University of Alabama-Birmingham, AL) and has been described previously (34, 35). The expression vector encoding constitutively active rat BLINaC harboring the A443T mutation was a gift from Dr. E. Lingueglia (Institut de Pharmacologie Moleculaire et Cellulaire, Valbonne, France) and has been described previously (24).
Cell Culture
Primary CA1 hippocampal neurons were obtained from newborn (P1–P3) Sprague-Dawley rats and C57BL/6 mice using standard procedures (34, 36, 37). All animal use and welfare adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals following a protocol reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. In brief, the hippocampus was dissected from meninges in ice-cold Hanks' Balanced Salt Solution and dissociated into a cellular suspension using 20 units/ml papain (Worthington Corp) combined with mechanical trituration through a Pasteur pipette. Dissociated hippocampal cells were plated on poly-d-lysine-coated glass coverslips. Hippocampal neurons were cultured using Neurobasal culture medium supplemented with B-27 supplement (1:50), 0.5 mm l-glutamine, 10% FBS, and 1% penicillin-streptomycin in a humidified atmosphere of 5% CO2 at 37 °C for 3–5 days. A homogeneous population of CA1 hippocampal interneurons was used for all experiments. These interneurons were identified by morphology, expression of the GluR2 subunit of the AMPA receptor, and other criteria as described previously (37).
Primary Drosophila class IV md neuronal cultures were created using midgastrula stage embryos as described previously (31, 38). In brief, 3–4-h-old embryos were collected and dechorionated with 50% bleach. The content of three to four embryos was removed and dispersed onto a glass coverslip in a drop of culture medium. Neurons were grown in culture in a defined bicarbonate-based medium in 5% CO2 at 23 °C for 2–3 days. Neurons arise from neuroblast precursors in midgastrula stage embryo cultures. To facilitate identification of md neurons, cultures were generated using embryos that expressed GFP in a restrictive manner in class IV md neurons. The genotype of this fly line is ppk1-Gal4, UAS-mCD8-GFP. This line was a gift from Darren Williams (King's College London, UK).
CHO cells were cultured using standard procedures (32). In brief, CHO cells were cultured at 37 °C in a humidified atmosphere of 5% CO2. Cells were maintained with standard culture conditions and medium (DMEM + 10% FBS and 1% penicillin-streptomycin).
Expression of Recombinant ASIC and BLINaC in CHO Cells
CHO cells were transfected with plasmids encoding BLINaC or ASIC subunits using Polyfect reagent (Qiagen) following the manufacturer's protocol as described previously (34). For expression of homomeric channels, cells were transfected with 1 μg of rASIC1a, rASIC1b, rASIC2a, rACIS3, or rBLINaC cDNA/35-mm2 dish. For expression of heteromeric channels, a subunit cDNA ratio of 1:1 was used with 500 ng of each subunit cDNA/35-mm2 dish transfected. Electrophysiological experiments were performed 48–72 h after transfection.
Patch Clamp Electrophysiology
Acid-sensing macroscopic currents conducted by ASIC in hippocampal CA1 neurons were measured in the whole cell patch clamp configuration under voltage clamp conditions using standard methods (34). In brief, neurons were clamped to −60 mV, and currents were activated by rapid solution exchange from control (7.4) to acidic (5.0) pH. For these experiments, the extracellular bath solution contained 143 mm NaCl, 5 mm KCl, 2.5 mm CaCl2, 10 mm d-glucose, 10 mm HEPES, 10 mm MES (pH was adjusted with HCl before each experiment). The pipette solution contained 100 mm CsF, 40 mm CsCl, 5 mm NaCl, 0.5 mm CaCl2, 5 mm EGTA, 10 mm HEPES (pH was adjusted to 7.2 with CsOH).
Class IV md sensory neurons were identified in primary midgastrula neuronal cultures with epifluorescence for GFP expression, as driven by ppk1-Gal4, UAS-mCD8-GFP. Macroscopic PPK1 currents in these neurons were recorded in voltage clamp experiments using the whole cell patch clamp configuration following standard procedures (31). Neurons were clamped to −60 mV with PPK1 currents evoked using a standard protocol, as described previously by us (31), entailing relief from amiloride blockade subsequent to an activating acid (pH 4.5) pre-prepulse. For these experiments, the extracellular bath solution contained 140 mm NaCl, 5 mm KCl, 1 mm CaCl2, 10 mm d-glucose, 10 mm HEPES, and 10 mm MES (pH was adjusted with HCl before each experiment). The pipette solution contained 120 mm cesium gluconate, 20 mm NaCl, 0.1 mm CaCl2, 1 mm EGTA, and 10 mm HEPES (pH was adjusted to 7.2 with CsOH).
Whole cell macroscopic current recordings of ASIC expressed in CHO cells were performed under voltage-clamp conditions (Vm = −10 mV) using standard methods (34). In these experiments, ASIC currents were evoked by rapid solution exchange from control (7.6) to acidic (5.0) pH. The intracellular pipette solution contained 120 mm CsCl, 5 mm NaCl, 2 mm MgCl2, 5 mm EGTA, 2.0 mm ATP, 0.1 mm GTP, and 40 mm HEPES (pH 7.4). The extracellular bath solution was 150 mm NaCl, 1 mm CaCl2, 2 mm MgCl2, 10 mm HEPES, and 10 mm MES adjusted to pH 7.6. For these and all other experiments involving acid-sensing channels, desensitization in response to repetitive stimulation by acid was minimized using the standard procedure of applying brief (4-s) acidic pH pulses at 2-min intervals. With such an approach current run-down is <1% in response to repetitive stimulation (34).
Whole cell macroscopic current recordings of BLINaC expressed in CHO cells also were performed under voltage clamp conditions with standard methods (24, 39). Mutant BLINaC harboring the A443T substitution is constitutively active (24). For BLINaC experiments using asymmetrical solutions, the intracellular pipette solution was 120 mm CsCl, 5 mm NaCl, 2 mm MgCl2, 5 mm EGTA, 2.0 mm ATP, 0.1 mm GTP, and 10 mm HEPES (pH 7.4). The extracellular bath solution was 150 mm NaCl, 1 mm CaCl2, 2 mm MgCl2, and 10 mm HEPES adjusted to pH 7.4. In BLINaC experiments using symmetrical solutions, the intracellular pipette and extracellular bath solutions were 150 mm NaCl, 2 mm MgCl2, 5 mm EGTA, 2.0 mm ATP, 0.1 mm GTP, and 10 mm HEPES (pH 7.4); and 150 mm NaCl, 1 mm CaCl2, 2 mm MgCl2, and 10 mm HEPES (pH 7.4), respectively.
Single-channel current recordings of recombinant BLINaC expressed in CHO cells were acquired in the excised, outside-out patch configuration under voltage clamp conditions using standard methods (24, 40). Symmetrical 150 mm LiCl, 1 mm CaCl2, 2 mm MgCl2, 10 mm HEPES (pH 7.4) extracellular bath and 150 mm LiCl, 2 mm MgCl2, 5 mm EGTA, 2.0 mm ATP, 0.1 mm GTP, and 10 mm HEPES (pH 7.4) intracellular pipette solutions were used in these experiments.
Macroscopic currents were acquired with an Axopatch 200B (Axon Instruments) patch clamp amplifier interfaced via a Digidata 1400A (Molecular Devices) to a PC running the pClamp 10.3 suite of software (Molecular Devices). Macroscopic currents were filtered at 1 kHz and digitized at 2 kHz. Whole cell capacitance and series resistances were routinely compensated. Only recordings where access resistance and capacitance changed <10% over the course of the experiment were used.
Gap-free single-channel current recordings from gigaohm seals were acquired (and subsequently analyzed) with an Axopatch 200B patch clamp amplifier interfaced via a Digidata 1440A to a PC running the pClamp 10.3 suite software. Currents were low pass-filtered at 100 Hz with an eight-pole Bessel filter (Warner Instruments) and digitized at 500 Hz. For these experiments, pipette resistance was 7–8 megohms.
Data Analysis and Comparison
For single-channel recordings, unitary current (i) was determined from all-point amplitude histograms fitted with single- or multi-Gaussian curves using the standard 50% threshold criterion to differentiate between events. Channel activity, defined as NPo, was calculated using the standard equation: NPo = (t1 + 2t2 + … + ntn), where N and Po are the channel number in a patch and the mean open probability of these channels, respectively, and tn is the fractional open time spent at each of the observed current levels.
The Hill equation was used for the analytical description of the 4-AP block. Dose-response curves describing the inhibition of macroscopic and single channel currents, respectively, were fitted with percentage inhibition
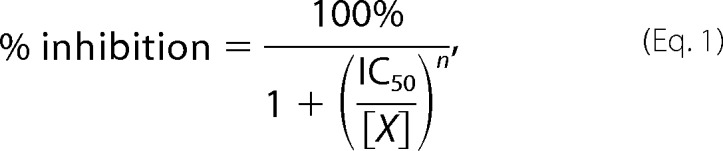 |
and
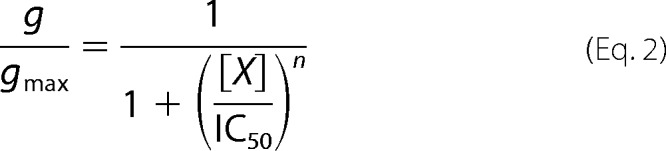 |
where percentage inhibition and g/gmax are the blocked fraction of current and nonblocked fraction of conductance, respectively, in the presence of blocker at concentration [X]; IC50 is the half-maximal concentration of blocker; and n is the Hill coefficient.
For the analytical description of the voltage dependence of the 4-AP block data were fitted with the Boltzmann equation
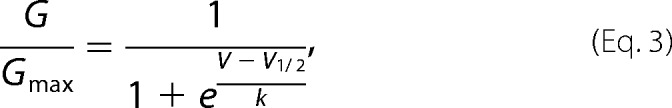 |
where G/Gmax is normalized conductance at the membrane potential V, V1/2 is the membrane potential resulting in half-activation, and k as normal is the Boltzmann constant. To understand further the voltage dependence of the 4-AP block, the modified Hill equation
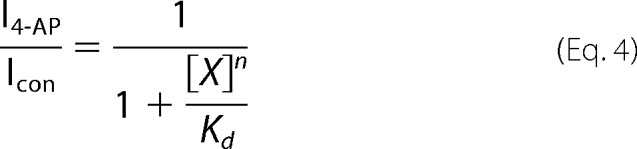 |
was used to determine the equilibrium constant, Kd, for 4-AP at concentration [X]. The change in Kd as a function of voltage then was fitted with a logarithmic form of the Arrhenius equation
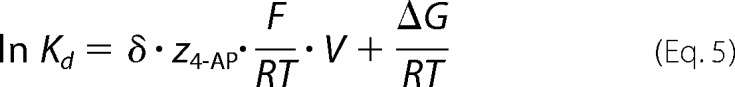 |
where Kd is the equilibrium constant, δ is the fraction of the membrane potential sensed by 4-AP when interacting with the channel, z4-AP is the effective valence of 4-AP, F is the Faraday constant, R is the gas constant, T is absolute temperature, V is membrane potential, and ΔG is change in free energy. The pKa of 4-AP is ∼9 (6). Consequently, at a physiological pH the effective valence for ∼90% of 4-AP is +1.
All summarized data are reported as mean ± S.E. Paired data are compared with a two-tailed paired t test. The criterion for significance was p ≤ 0.05.
RESULTS
4-AP Decreases the Magnitude of Acid-sensing Na+ Currents in Central and Peripheral Neurons
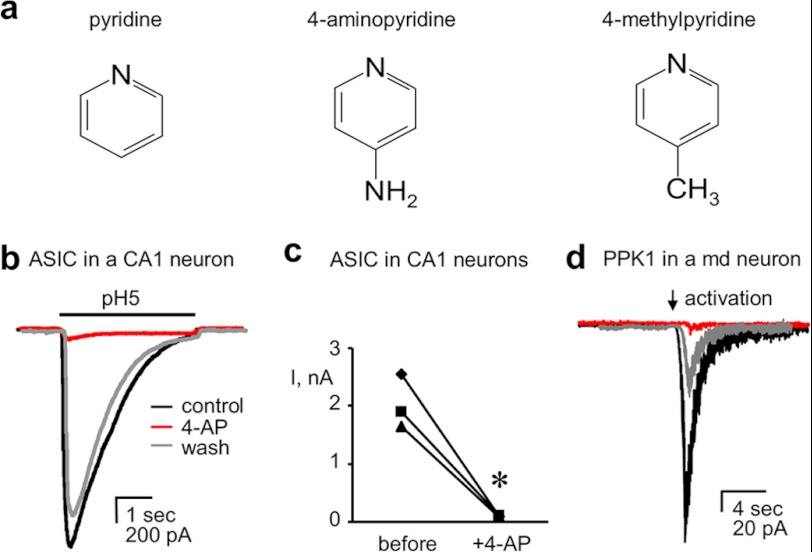
The chemical structures of 4-AP and related compounds used in the current study are shown in Fig. 1a. The effect of 4-AP on the acid-sensing inward Na+ current endogenous to rat CA1 hippocampal neurons is shown in Fig. 1b. As revealed in this representative current trace (Fig. 1b) and summarized in Fig. 1c, 4-AP significantly decreases the magnitude of the depolarizing, acid-sensing inward Na+ current in this CNS neuron. An identical observation was made for acid-sensing (pH 5) Na+ currents in murine CA1 hippocampal neurons (data not shown), where the magnitude of inward current at −60 mV decreased 88.5 ± 1.3% (n = 7) in response to 10 mm 4-AP. Similarly, as supported by the representative current trace shown in Fig. 1d, 4-AP decreases the magnitude of macroscopic acid-sensing currents in voltage-clamped peripheral sensory neurons. Fig. 1d shows the effect of 4-AP on the endogenous acid-sensing current conducted by PPK1 in Drosophila polymodal class IV md peripheral sensory neurons.
FIGURE 1.
4-AP decreases endogenous acid-sensing Deg/ENaC currents in neurons. a, structure of pyridine and its 4-amino and 4-methyl derivatives. b and c, overlay of representative traces (b) and the corresponding summary graph (c) showing the effects of 10 mm 4-AP on macroscopic acid-sensing Na+ currents in rat hippocampal CA1 interneurons. Traces before, after, and following wash of 4-AP are black, red, and gray, respectively. Inward current is downward with currents activated with pH5 (noted with bar). Membrane potential was clamped to −60 mV. Physiological bath and pipette solution were used in these experiments. *, significant decrease compared with before 4-AP. d, macroscopic PPK1 currents in a Drosophila class IV md peripheral sensory neuron before, after, and following wash of 4-AP. PPK1 current was evoked using a relief from inhibition protocol as described under “Experimental Procedures” (and Ref. 31; noted with an arrowhead). Membrane potential was clamped to −60 mV with physiological solutions used for these experiments.
4-AP Inhibits ASIC1a in a Dose-dependent Manner
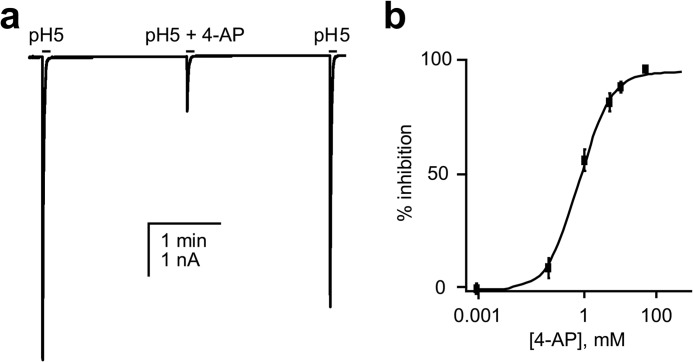
The acid-sensing, inward Na+ current in CA1 hippocampal neurons is believed to be conducted primarily by channels containing the ASIC1a subunit (16, 18, 29). As shown in Fig. 2a, recombinant rat ASIC1a channels also are sensitive to 4-AP when expressed in CHO cells. As summarized in Fig. 2b, 4-AP decreases the magnitude of macroscopic recombinant rat ASIC1a currents in a dose-dependent manner with an IC50 of 0.76 ± 0.08 mm and a Hill coefficient of 1.02 ± 0.10 at −10 mV.
FIGURE 2.
4-AP decreases the activity of recombinant rat ASIC1a expressed in CHO cells in a dose-dependent manner. a, representative macroscopic current trace of recombinant rat ASIC1a expressed in a CHO cell before, after, and subsequent to washing 10 mm 4-AP with the acid stimulus (pH 5) added in the absence and presence of blocker at 2-min intervals. Cell membrane was clamped to −10 mV with physiological solutions used in these experiments. b, dose-response curve showing the effects of 4-AP (IC50 = 0.76 ± 0.08 mm at −10 mV, n ≥ 7) on recombinant rat ASIC1a expressed in CHO cells. Data from experiments are similar to that shown in a.
4-AP Has Differential Effects on Distinct Acid-sensing Ion Channels
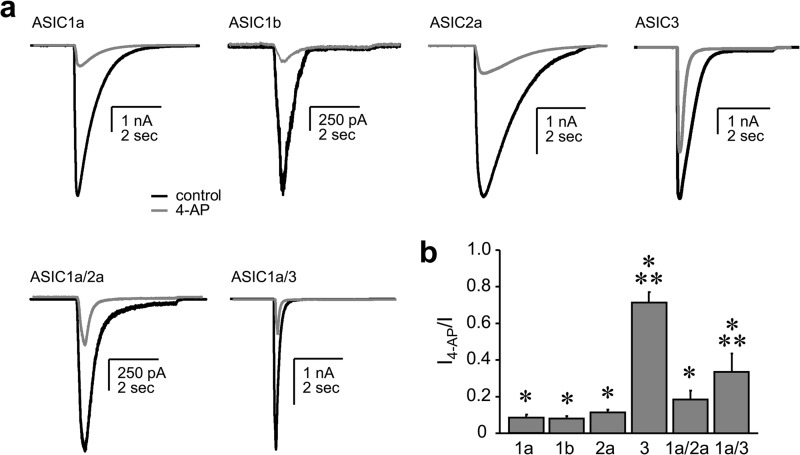
In mammals, distinct ASIC isoforms are differentially expressed in central and peripheral neurons (18, 20, 25). We wondered whether 4-AP was equally effective at inhibiting all Deg/ENaC channels within the spectrum of those expressed in neurons. The representative macroscopic currents conducted by homomeric ASIC1a, 1b, 2a, and 3, and heteromeric ASIC1a/2a and ASIC1a/3 in voltage-clamped CHO cells as shown in Fig. 3a, and the associated summary graph in 3b demonstrate that 4-AP has isoform-specific effects on the Deg/ENaC channels expressed in neurons. Channels containing ASIC1a, 1b, and 2a subunits are sensitive to 4-AP. In comparison, those containing ASIC3 are markedly less sensitive such that 10 mm 4-AP has no effect on homomeric ASIC3 channels. (The effect of 4-AP on BLINaC is addressed below.)
FIGURE 3.
4-AP has differential effects on homo- and heteromeric acid-sensing ion channels. Overlays of representative traces (a) and corresponding summary graph (b) show the effects of 4-AP (10 mm at −10 mV) on macroscopic acid-sensing currents in CHO cells expressing recombinant ASIC1a, 1b, 2a, 3, and 1a plus 2a, and 1a plus 3. Activation in response to an acid stimulus (pH 5) provided in the absence and presence of 4-AP is shown with black and gray lines, respectively. At least four observations were made for each channel subtype. Physiologic bath and pipette solutions were used. *, significant decrease compared with before inhibitor when assessed with a paired t test. **, significantly less compared with the ASIC1a control group (ANOVA + Dunnett subtest).
Compounds Related to 4-AP Differentially Affect ASIC1a
4-AP is a derivative of pyridine (see Fig. 1a). 4-Methpyridine (4-MP) is a structurally related analog where the primary amino group is replaced by a primary methyl group. We asked what specifically about the structure of 4-AP allows it to inhibit ASIC currents by comparing the effects of pyridine and 4-MP on recombinant rat ASIC1a. As shown by the representative current traces and accompanying summary graph in Fig. 4, neither pyridine nor 4-MP affected ASIC1a currents.
FIGURE 4.
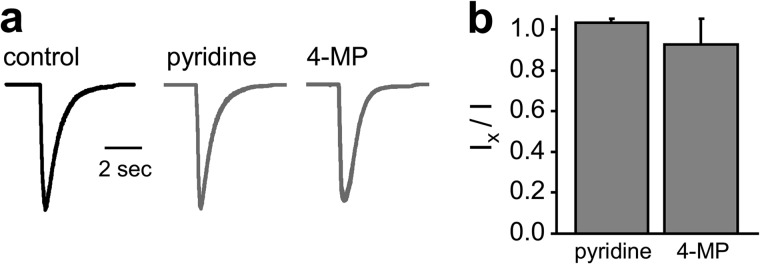
Differential effects of compounds related to 4-AP on recombinant ASIC1a. Representative traces (a) and corresponding summary graph (b, n ≥5) show the effects of vehicle (black) and pyridine and 4-MP (gray; at −10 mV) on macroscopic ASIC1a currents in CHO cells. ASIC1a was activated with pH 5. All compounds were used at 10 mm. Asymmetrical physiological solutions were used in these experiments. Neither pyridine nor 4-MP significantly affected the magnitude of macroscopic ASIC1a current.
4-AP Inhibits BLINaC
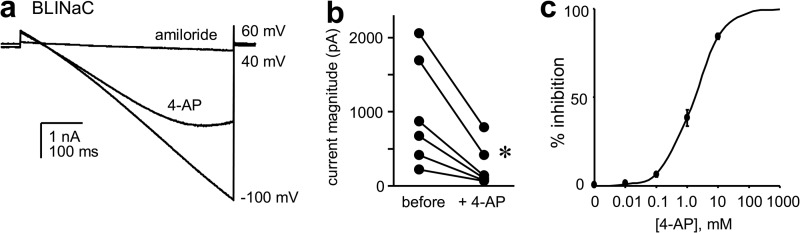
We tested next the effects of 4-AP on BLINaC. Like ASIC this closely related Deg/ENaC channel is expressed in CNS neurons of mammals (19, 24, 25). BLINaC is expressed also in epithelial cells, including those lining the hepatic bile duct (41). In contrast to ASIC, BLINaC is not activated by acidic pH but rather is activated by bile acids and possibly other ligands (41). As shown in Fig. 5, 4-AP decreases the current conducted by recombinant rat (A443T) BLINaC expressed in CHO cells in a dose-dependent manner (IC50 = 1.70 ± 0.18 mm and Hill coefficient = 0.94 ± 0.03 at −60 mV).
FIGURE 5.
4-AP inhibits BLINaC. a, overlay of representative macroscopic current traces showing the effects of 1 mm 4-AP and 100 μm amiloride on recombinant rat BLINaC in CHO cells. cDNA encoding constitutively active BLINaC harboring the A443T mutation was used for these experiments. Inward current, as above, is downward. Current was evoked with a voltage ramp from 60 to −100 mV from a holding potential of 40 mV. Asymmetrical physiological solutions were used in these experiments. b, summary graph (n = 6) showing the effect of 4-AP (10 mm at −60 mV) on recombinant BLINaC in paired experiments. Data from experiments are similar to those in a. *, significant decrease compared with before 4-AP. c, dose-response curve showing the effects of 4-AP (at −60 mV) on recombinant BLINaC in CHO cells (IC50 = 1.70 ± 0.18 mm, n = 5). Data from experiments are similar to those in a.
Voltage Influences Inhibition of BLINaC by 4-AP
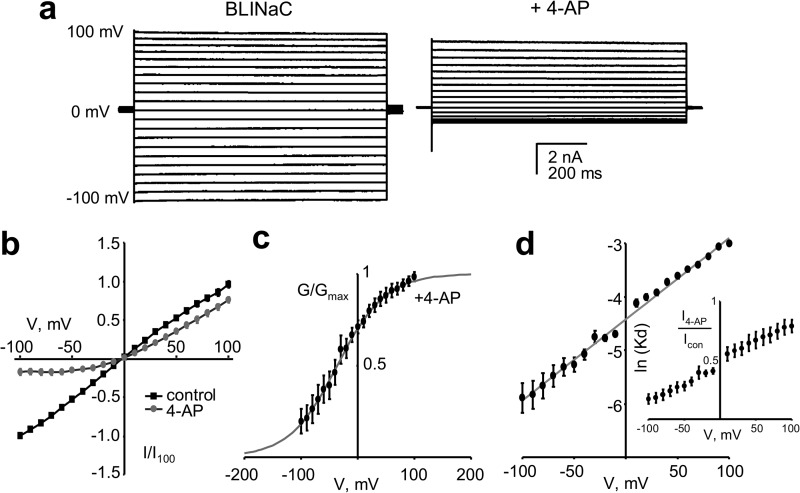
We noticed (see Fig. 5a) that voltage influenced inhibition of BLINaC by 4-AP. To better understand this, we quantified the voltage dependence of the inhibitory actions of 4-AP on macroscopic BLINaC currents using voltage steps. As shown in Fig. 6a, 4-AP strongly inhibits BLINaC currents at hyperpolarizing but not depolarizing potentials. This is clear in the summary I–V relation for BLINaC in the absence and presence of 4-AP shown in Fig. 6b, and the corresponding G-V relation for BLINaC in the presence of 4-AP shown in Fig. 6c. This voltage dependence is a manifestation of the 4-AP block for in the absence of this inhibitor, BLINaC is not sensitive to voltage. As shown in Fig. 6d, the Kd for inhibition by 4-AP decreases as a function of hyperpolarization. Assuming that this dependence on voltage arises from 4-AP binding within the pore of the channel (see below), the fraction of the transmembrane electric field sensed by the blocker is ∼39% (δ = 0.39 ± 0.01).
FIGURE 6.
4-AP inhibits BLINaC in a voltage-dependent manner. a, representative macroscopic current traces of rat BLINaC (A443T) in a voltage-clamped CHO cell before (left) and after (right) 10 mm 4-AP. Current was evoked with a 10-mV step protocol ranging from 100 to −100 mV from a holding potential of 0 mV. Symmetrical NaCl solutions were used in these experiments. b, summary graph of the macroscopic current-voltage (I-V) relation for recombinant rat BLINaC expressed in CHO cells in the absence (black) and presence (gray; 10 mm) of 4-AP. Data are from experiments identical to that in a (n = 5). c, corresponding conductance-voltage (G-V) curve for rat BLINaC in CHO cells in the presence of 10 mm 4-AP. Normalized conductance was fit with a Boltzmann distribution. d, summary graph showing the voltage dependence of the Kd for 10 mm 4-AP on BLINaC. The normalized (I4-AP/Icon) I-V relation for BLINaC used to calculate Kd is shown in the inset. Data from experiments are identical to those in a.
4-AP Seems to Decrease the Unitary Conductance of BLINaC
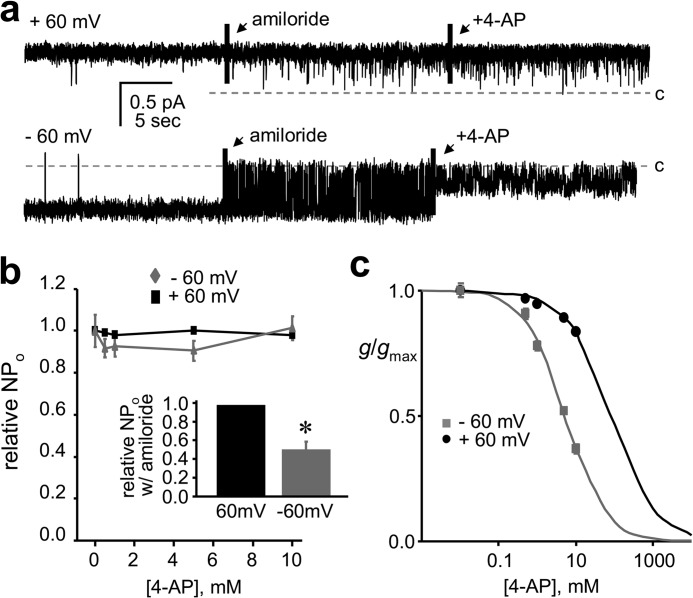
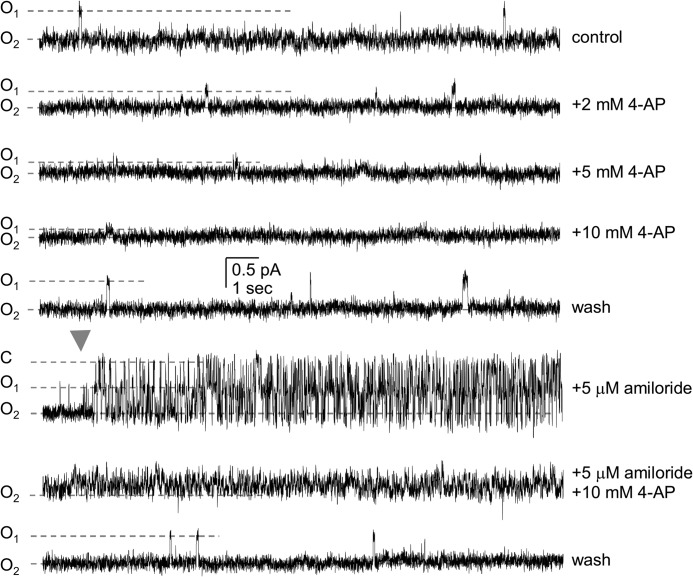
To better understand the molecular mechanism of 4-AP action on Deg/ENaC channels, we investigated the effects of this inhibitor at the single-channel level. ASIC rapidly actives and inactivates in response to an acid challenge (18, 20). This complicates study of mechanism at the single-channel level. Consequently, we used constitutively active BLINaC as a surrogate for Deg/ENaC channels expressed in neurons to investigate mechanism of block. As demonstrated by the single-channel current traces of recombinant BLINaC shown in Fig. 7a and summarized in 7, b and c, 4-AP has a marked effect on the current conducted by BLINaC at hyperpolarizing but not depolarizing potentials. Surprisingly, and seemingly different compared with amiloride, 4-AP does not appear to affect single-channel open probability but rather seems to decrease unitary conductance. As shown in Figs. 7 and 8, the unitary current and relative NPo of BLINaC under control conditions and in the presence of 2, 5, and 10 mm 4-AP is 0.54 ± 0.01, 0.39 ± 0.01, 0.28 ± 0.01, and 0.21 ± 0.01 pA; and 0.95 ± 0.02, 0.97 ± 0.01, 0.99 ± 0.01, and 0.99 ± 0.01, respectively (n = 4). (Moreover, the unitary current (at −60 mV) before and after amiloride in these paired studies was 0.59 ± 0.01 and 0.58 ± 0.03 pA (n = 3), respectively). The consequence of this, as revealed in Fig. 7c, is that the dose-response curve describing 4-AP-dependent decreases in the unitary conductance of BLINaC is shifted to the left by hyperpolarizing voltages where the IC50 at 60 mV of 37.6 mm is almost 10-fold greater than that of 4.8 mm at −60 mV.
FIGURE 7.
Voltage-sensitive changes in unitary conductance underpin the inhibitory actions of 4-AP on BLINaC. a, representative single-channel current traces of recombinant BLINaC (A443T) in excised, outside-out patches from CHO cells clamped to 60 (top) or −60 mV (bottom) before and after 5 μm amiloride and 10 mm 4-AP added in the presence of amiloride. Data are from continuous traces with representative segments shown for each condition. Breaks in the record are noted by solid vertical bars. Drugs were added to the extracellular bath solution. The open channel pore blocker, amiloride, was included with 4-AP at a submaximal dose (to increase channel “flicker”) in the latter part of the trace to facilitate calculation of unitary current (i) and conductance (g). Also for these experiments, the pipette and bath contained symmetrical LiCl solutions. Inward current is down, and the closed state (zero current level) is indicated with a dashed line and c. Summary graphs (n = 4) show the dose response of 4-AP on the single-channel activity (NPo; b) and unitary conductance (g; c) of BLINaC at 60 (black) and −60 mV (gray). For these graphs, g and NPo were normalized to that in the absence of 4-AP at the respective voltage. The inset in b shows the effect of submaximal amiloride on normalized NPo at 60 (black) and −60 (gray) mV.
FIGURE 8.
4-AP decreases the unitary current of BLINaC in a dose-dependent manner. Representative segments from a typical (n = 4) continuous single-channel current trace of recombinant BLINaC (A443T) expressed in CHO cells in an excised, outside-out patch clamped to −60 mV before and after 4-AP in the absence and presence of amiloride. This patch contains at least two BLINaC channels. The pipette and bath contained symmetrical LiCl solutions. Inward current is down, and closed and open 1 and open 2 states are indicated with dashed lines and C, O1, and O2, respectively. The point at which amiloride was added is indicated by a solid gray arrowhead.
DISCUSSION
The current results demonstrate that like Kv channels, Deg/ENaC channels expressed in neurons, including ASIC, are sensitive to 4-AP. This fits with other recent findings showing that the purportedly selective-agent, 4-AP, affects several different types of ion channels (42). The inhibitory action of 4-AP on Deg/ENaC channels is isoform-specific, voltage-dependent, and the blocker likely binds to a single binding site in the channel perhaps within the pore. These findings argue that in addition to effects on Kv and N-type Ca2+ channels, targeting of neuronal Deg/ENaC channels must be considered when evaluating the consequences of 4-AP. With regard to the clinical use of this drug, this means that 4-AP may possibly influence the symptoms and disease progression of MS because it inhibits Kv and ASIC channels, both of which are involved in the sequelae of this disease.
Because 4-AP is considered to be a selective pore blocker of Kv channels in the Shaker ion channel family (3, 6), which are structurally distinct from channels in the Deg/ENaC family, the current finding that this drug inhibits Deg/ENaC channels expressed in neurons was surprising. We argue that this action of 4-AP on Deg/ENaC channels is a direct effect for several reasons. First, 4-AP decreases macroscopic currents conducted by disparate members of the Deg/ENaC channel family expressed in several distinct types of neurons from different species and in a heterologous system. Second, 4-AP inhibited Deg/ENaC currents under voltage clamp conditions excluding secondary effects due to changes in membrane potential mediated by other channels. Moreover, 4-AP inhibited BLINaC in excised, outside-out patches, which were uncoupled from cell signaling pathways and the influences of other channels on membrane potential. Moreover, the biophysical description of 4-AP inhibition of Deg/ENaC channels, in particular, the fact that it is voltage-sensitive and best described by a single binding site within the channel pore, is most consistent with a direct effect of this drug.
Whereas the effects of 4-AP on Deg/ENaC channels is evolutionarily conserved as supported by effects on dm PPK1, and mammalian ASIC and BLINaC channels, the action of this drug on certain members within this ion channel family are isoform-specific. For instance, in mammals 4-AP has the greatest inhibitory effect on ASIC isoforms, as typified by ASIC1a, enriched in CNS neurons, and a markedly less effect on isoforms, such as ASIC3, enriched in peripheral sensory neurons (18, 20, 25). The differential effects of 4-AP on mammalian ASIC perhaps can be used to selectively target the function of these channels in distinct neurons.
This isoform-specific effect on Deg/ENaC channels parallels the actions of this drug on Kv channels where certain family members are highly responsive to the inhibitor and others less so (3). In general, the concentration of 4-AP that inhibits Deg/ENaC channels expressed in CNS neurons overlaps that for inhibition of most Kv channels: 0.1–10 mm. This is consistent with 4-AP being equally potent as an inhibitor of Kv and Deg/ENaC channels.
The targeted serum level of 4-AP in MS patients is ∼60 ng/ml (43). This dose is several orders of magnitude less than the IC50 for 4-AP on Kv and ASIC channels. The significance of this as yet is unclear. Perhaps a distinct protein is the primary target for 4-AP in MS. However, caution is called for here because little is actually understood about how 4-AP works in vivo to better MS, and the targeted dose of 4-AP used in the clinic is limited by the side effects of this drug rather than its maximal efficacy in treating disease (1–3, 43). The current findings add neuronal Deg/ENaC channels to the list of possible targets that should be considered when probing how 4-AP betters MS and other diseases and pathologies of the nervous system.
We find that the organic heterocyclic ring of 4-AP, pyridine, alone has no effect on Deg/ENaC channels. Similarly, the 4-MP derivative of pyridine, which is structurally close to 4-AP, has no effect on Deg/ENaC channels. These findings argue that the primary amine of 4-AP plays a key role in inhibition of Deg/ENaC channels but only in the presence of the conjugated heterocyclic (pyridine) ring, which by itself has no effect. This structural requirement is reminiscent of the conjugation of the active inhibitory guanidinium moiety of amiloride to a pyrazine heterocyclic ring (44–46). Recall that amiloride is the prototypic pore blocker of Deg/ENaC channels (19).
The pKa of 4-AP compared with that of pyridine and 4-MP mandates that the bulk (>90%) of the former but not necessarily the latter compounds is ionized in physiological solutions (pH 7.4). The importance of this with respect to actions on Deg/ENaC channels is as yet unknown; however, it is recognized that ionization of 4-AP is critical to its blockade of Kv channels (3, 6, 45). Similarly, the positive charge of the guanidinium cation is critical to blockade of Deg/ENaC channels by amiloride (46).
The atomic structure of the homomeric chicken ASIC1 channel recently was solved (26, 27). This channel is trimeric, having 3-fold symmetry perpendicular to the plasma membrane. It is widely held that all Deg/ENaC channels assume a related quaternary structure. In the current studies, the Hill coefficient describing 4-AP inhibition of Deg/ENaC channels, as typified by ASIC1a and BLINaC, approaches 1. This is consistent but not definitive of these channels having a single binding site for this inhibitor. Again, this parallels 4-AP inhibition and binding to Kv channels (3). The crystal structure of Kv channels also has been solved (47). Kv channels comprise four subunits having a central axis with 4-fold symmetry perpendicular to the plasma membrane. In consideration of the 3-fold symmetry of Deg/ENaC channels, a single binding site for 4-AP is best accommodated within the central axis of the channel at the junction of all three subunits. Perhaps not a coincidence, such a description also defines the conductive pore of the channel as it runs through the plasma membrane. Kv channels also have a single binding site for 4-AP (4, 6, 48, 49). This binding site is within the pore, enabling 4-AP to be a pore blocker. Thus, the current findings are most consistent with 4-AP binding to a Deg/ENaC channel within its central axis perhaps within the pore with each of the three channel subunits likely contributing equally to this binding site.
Cationic 4-AP enters the pore of a Kv channel through its intracellular mouth (1, 3, 6). Once in the pore, this drug has complex effects leading to pore block resulting in a decrease in the open probability of the channel. The charge of 4-AP, that the binding site for this drug is within the Kv channel pore and that it enters the pore from the intracellular compartment cause the inhibitory effects of 4-AP on Kv channels to be sensitive to voltage. The inhibitory actions of 4-AP on Deg/ENaC channels similarly are sensitive to voltage. 4-AP inhibits Deg/ENaC channels greatest at hyperpolarizing potentials. This suggests that if the 4-AP binding site is within the Deg/ENaC pore that it enters this site through the extracellular mouth of the channel.
Akin to 4-AP, the effects on Deg/ENaC channels of the pore blocker, amiloride, are voltage-sensitive, being greatest at hyperpolarizing potentials (18, 19, 46). As 4-AP, amiloride has a single binding site within the pore, entering Deg/ENaC channels through their extracellular mouths. Because amiloride enters the Deg/ENaC pore and the active group of this drug with respect to inhibition, the guanidinium moiety, is cationic, amiloride senses the transmembrane electrical field across the channel. This explains why blockade of Deg/ENaC by amiloride is sensitive to voltage (18, 46). A recent study reported that amiloride senses ∼35% of the electric field across BLINaC (39). Whereas interpretation of such a finding is complicated and often dependent on variables that have not been fully quantified, a simple explanation is that the amiloride binding site or the inhibitory effector site for the cationic guanidinium moiety of amiloride lies about a third of the way through the BLINaC pore referenced from its extracellular mouth. Here we find that 4-AP senses ∼39% of the electric field across BLINaC. If 4-AP binds in the pore, then this suggests that the binding/effector site for this drug overlaps to some degree that of amiloride.
Compared with amiloride on Deg/ENaC channels and 4-AP on Kv channels, which decrease open probability (1, 3, 6, 18, 19, 46), we find that 4-AP appears to decrease the unitary conductance of BLINaC. Although this could represent a true effect on conductance, it more likely reflects a change in open probability stemming from a rapid, flickery block of the pore, for if Deg/ENaC channels have a single binding site for 4-AP and this binding site lies within the pore, it is challenging to rationalize how 4-AP could influence unitary conductance in a dose-dependent manner when considering contemporary understanding of ion channels as gated pores. If the decrease in conductance is real, then 4-AP must exert an allosteric action on the channel regardless of the location of its binding site, or the calculated Hill coefficient of one describing the 4-AP block reflects only the lower limit with the actual number being much greater than 1. Alternatively, and we believe more likely, experimental limitations often require the use of corner frequencies that can introduce aliasing when collecting single-channel current data for channels that have a small unitary conductance, such as Deg/ENaC channels. This can cause a real effect on open probability to appear as an effect on conductance (50, 51). This is particularly problematic when investigating the mechanism of a fast pore blocker, such as 4-AP is for Kv channels (6, 52). Although attempts were made to mitigate this, such a possibility cannot be fully excluded. The corner frequencies used here mandate that for a real effect on open probability to appear as an apparent effect on conductance, the block time of BLINaC by 4-AP must be substantially briefer than 1.0 ms.
Regardless of molecular mechanism, the primary importance of the current studies lies in the observation that 4-AP inhibits Deg/ENaC channels to include neuronal ASIC expressed in CNS neurons in a manner similar to the effects of this drug on Kv channels. This is important because activation of ASIC has been implicated in the disease progression of MS and other neuroinflammatory and ischemic diseases of the brain; and consequently, inhibition of ASIC is an emerging target to counter the pathology of such diseases (16–18, 30, 53). Moreover, targeting Kv channels with 4-AP is an emerging treatment to address symptoms of MS (1–3). The current findings argue this action of 4-AP, presumably mediated by Kv channels, needs to be evaluated in the context that this drug also targets ASIC. Thus, 4-AP may influence both the symptoms and progression of MS by influencing both Kv and ASIC channels.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK059594 (to J. D. S.).
- MS
- multiple sclerosis
- 4-AP
- 4-aminopyridine
- ASIC
- acid-sensing ion channel
- BLINaC
- brain, liver, intestine Na(+) channel
- CHO
- Chinese hamster ovary
- Deg/ENaC
- degenerin/epithelial Na+ channel
- EAE
- experimental autoimmune encephalomyelitis
- Kv channel
- voltage-gated K+ channel
- md
- multidendritic
- 4-MP
- 4-methpyridine
- PPK1
- pickpocket1.
REFERENCES
- 1. Korenke A. R., Rivey M. P., Allington D. R. (2008) Sustained-release fampridine for symptomatic treatment of multiple sclerosis. Ann. Pharmacother. 42, 1458–1465 [DOI] [PubMed] [Google Scholar]
- 2. Solari A., Uitdehaag B., Giuliani G., Pucci E., Taus C. (2002) Aminopyridines for symptomatic treatment in multiple sclerosis. Cochrane Database Syst. Rev. 4, CD001330. [DOI] [PubMed] [Google Scholar]
- 3. Judge S. I., Bever C. T., Jr. (2006) Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol. Ther. 111, 224–259 [DOI] [PubMed] [Google Scholar]
- 4. Wulff H., Zhorov B. S. (2008) K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem. Rev. 108, 1744–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong C. M., Loboda A. (2001) A model for 4-aminopyridine action on K channels: similarities to tetraethylammonium ion action. Biophys. J. 81, 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choquet D., Korn H. (1992) Mechanism of 4-aminopyridine action on voltage-gated potassium channels in lymphocytes. J. Gen. Physiol. 99, 217–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lovas G., Szilágyi N., Majtényi K., Palkovits M., Komoly S. (2000) Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain 123, 308–317 [DOI] [PubMed] [Google Scholar]
- 8. Kornek B., Storch M. K., Weissert R., Wallstroem E., Stefferl A., Olsson T., Linington C., Schmidbauer M., Lassmann H. (2000) Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am. J. Pathol. 157, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waxman S. G. (2006) Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat. Rev. Neurosci. 7, 932–941 [DOI] [PubMed] [Google Scholar]
- 10. Lassmann H. (2007) Multiple sclerosis: is there neurodegeneration independent from inflammation? J. Neurol. Sci. 259, 3–6 [DOI] [PubMed] [Google Scholar]
- 11. Dutta R., Trapp B. D. (2007) Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 68, S22–31; discussion S43–54 [DOI] [PubMed] [Google Scholar]
- 12. Dutta R., McDonough J., Yin X., Peterson J., Chang A., Torres T., Gudz T., Macklin W. B., Lewis D. A., Fox R. J., Rudick R., Mirnics K., Trapp B. D. (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann. Neurol. 59, 478–489 [DOI] [PubMed] [Google Scholar]
- 13. Petrescu N., Micu I., Malek S., Ouardouz M., Stys P. K. (2007) Sources of axonal calcium loading during in vitro ischemia of rat dorsal roots. Muscle Nerve 35, 451–457 [DOI] [PubMed] [Google Scholar]
- 14. Nikolaeva M. A., Mukherjee B., Stys P. K. (2005) Na+-dependent sources of intra-axonal Ca2+ release in rat optic nerve during in vitro chemical ischemia. J. Neurosci. 25, 9960–9967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stys P. K. (2005) General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 233, 3–13 [DOI] [PubMed] [Google Scholar]
- 16. Xiong Z. G., Zhu X. M., Chu X. P., Minami M., Hey J., Wei W. L., MacDonald J. F., Wemmie J. A., Price M. P., Welsh M. J., Simon R. P. (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118, 687–698 [DOI] [PubMed] [Google Scholar]
- 17. Vergo S., Craner M. J., Etzensperger R., Attfield K., Friese M. A., Newcombe J., Esiri M., Fugger L. (2011) Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain 134, 571–584 [DOI] [PubMed] [Google Scholar]
- 18. Sherwood T. W., Frey E. N., Askwith C. C. (2012) Structure and activity of the acid-sensing ion channels. Am. J. Physiol. Cell Physiol. 303, C699-C710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kellenberger S., Schild L. (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767 [DOI] [PubMed] [Google Scholar]
- 20. Krishtal O. (2003) The ASICs: signaling molecules? Modulators? Trends Neurosci. 26, 477–483 [DOI] [PubMed] [Google Scholar]
- 21. Waldmann R., Champigny G., Lingueglia E., De Weille J. R., Heurteaux C., Lazdunski M. (1999) H+-gated cation channels. Ann. N.Y. Acad. Sci. 868, 67–76 [DOI] [PubMed] [Google Scholar]
- 22. Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. (1997) A proton-gated cation channel involved in acid-sensing. Nature 386, 173–177 [DOI] [PubMed] [Google Scholar]
- 23. Schaefer L., Sakai H., Mattei M., Lazdunski M., Lingueglia E. (2000) Molecular cloning, functional expression and chromosomal localization of an amiloride-sensitive Na+ channel from human small intestine. FEBS Lett. 471, 205–210 [DOI] [PubMed] [Google Scholar]
- 24. Sakai H., Lingueglia E., Champigny G., Mattei M. G., Lazdunski M. (1999) Cloning and functional expression of a novel degenerin-like Na+ channel gene in mammals. J. Physiol. 519, 323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lingueglia E., Deval E., Lazdunski M. (2006) FMRFamide-gated sodium channel and ASIC channels: a new class of ionotropic receptors for FMRFamide and related peptides. Peptides 27, 1138–1152 [DOI] [PubMed] [Google Scholar]
- 26. Jasti J., Furukawa H., Gonzales E. B., Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449, 316–323 [DOI] [PubMed] [Google Scholar]
- 27. Gonzales E. B., Kawate T., Gouaux E. (2009) Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460, 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lingueglia E., de Weille J. R., Bassilana F., Heurteaux C., Sakai H., Waldmann R., Lazdunski M. (1997) A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 272, 29778–29783 [DOI] [PubMed] [Google Scholar]
- 29. Zha X. M., Wemmie J. A., Green S. H., Welsh M. J. (2006) Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc. Natl. Acad. Sci. U.S.A. 103, 16556–16561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Friese M. A., Craner M. J., Etzensperger R., Vergo S., Wemmie J. A., Welsh M. J., Vincent A., Fugger L. (2007) Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 13, 1483–1489 [DOI] [PubMed] [Google Scholar]
- 31. Boiko N., Kucher V., Stockand J. D., Eaton B. A. (2012) Pickpocket1 is an ionotropic molecular sensory transducer. J. Biol. Chem. 287, 39878–39886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puck T. T., Cieciura S. J., Robinson A. (1958) Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J. Exp. Med. 108, 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hesselager M., Timmermann D. B., Ahring P. K. (2004) pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 279, 11006–11015 [DOI] [PubMed] [Google Scholar]
- 34. Staruschenko A., Dorofeeva N. A., Bolshakov K. V., Stockand J. D. (2007) Subunit-dependent cadmium and nickel inhibition of acid-sensing ion channels. Dev. Neurobiol. 67, 97–107 [DOI] [PubMed] [Google Scholar]
- 35. Berdiev B. K., Xia J., Jovov B., Markert J. M., Mapstone T. B., Gillespie G. Y., Fuller C. M., Bubien J. K., Benos D. J. (2002) Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J. Biol. Chem. 277, 45734–45740 [DOI] [PubMed] [Google Scholar]
- 36. Filippova N., Sedelnikova A., Tyler W. J., Whitworth T. L., Fortinberry H., Weiss D. S. (2001) Recombinant GABAC receptors expressed in rat hippocampal neurons after infection with an adenovirus containing the human rho1 subunit. J. Physiol. 535, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bolshakov K. V., Essin K. V., Buldakova S. L., Dorofeeva N. A., Skatchkov S. N., Eaton M. J., Tikhonov D. B., Magazanik L. G. (2002) Characterization of acid-sensitive ion channels in freshly isolated rat brain neurons. Neuroscience 110, 723–730 [DOI] [PubMed] [Google Scholar]
- 38. Sicaeros B., O'Dowd D. K. (2007) Preparation of neuronal cultures from midgastrula stage Drosophila embryos. J. Vis. Exp. 5, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wiemuth D., Gründer S. (2011) The pharmacological profile of brain liver intestine Na+ channel: inhibition by diarylamidines and activation by fenamates. Mol. Pharmacol. 80, 911–919 [DOI] [PubMed] [Google Scholar]
- 40. Kucher V., Boiko N., Pochynyuk O., Stockand J. D. (2011) Voltage-dependent gating underlies loss of ENaC function in pseudohypoaldosteronism type 1. Biophys. J. 100, 1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wiemuth D., Sahin H., Falkenburger B. H., Lefèvre C. M., Wasmuth H. E., Gründer S. (2012) BASIC: a bile acid-sensitive ion channel highly expressed in bile ducts. FASEB J. 26, 4122–4130 [DOI] [PubMed] [Google Scholar]
- 42. Wu Z. Z., Li D. P., Chen S. R., Pan H. L. (2009) Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel β subunit. J. Biol. Chem. 284, 36453–36461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Diemen H. A., Polman C. H., Koetsier J. C., Van Loenen A. C., Nauta J. J., Bertelsmann F. W. (1993) 4-Aminopyridine in patients with multiple sclerosis: dosage and serum level related to efficacy and safety. Clin. Neuropharmacol. 16, 195–204 [DOI] [PubMed] [Google Scholar]
- 44. Hunt T., Atherton-Watson H. C., Axford J., Collingwood S. P., Coote K. J., Cox B., Czarnecki S., Danahay H., Devereux N., Howsham C., Hunt P., Paddock V., Paisley D., Young A. (2012) Discovery of a novel chemotype of potent human ENaC blockers using a bioisostere approach. Part 1. Quaternary amines. Bioorg. Med. Chem. Lett. 22, 929–932 [DOI] [PubMed] [Google Scholar]
- 45. Kalia J., Swartz K. J. (2011) Elucidating the molecular basis of action of a classic drug: guanidine compounds as inhibitors of voltage-gated potassium channels. Mol. Pharmacol. 80, 1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pietra F. (2012) Putative binding sites, and pathways to them, for amidine and guanidine current inhibitors on acid-sensing ion channels (ASIC): a theoretical approach with hASIC1a homology model. Chem. Biodivers. 9, 331–351 [DOI] [PubMed] [Google Scholar]
- 47. Long S. B., Campbell E. B., Mackinnon R. (2005) Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 [DOI] [PubMed] [Google Scholar]
- 48. Campbell D. L., Qu Y., Rasmusson R. L., Strauss H. C. (1993) The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. II. Closed state reverse use-dependent block by 4-aminopyridine. J. Gen. Physiol. 101, 603–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson S. (1982) Aminopyridine block of transient potassium current. J. Gen. Physiol. 80, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yellen G. (1984) Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J. Gen. Physiol. 84, 157–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heinemann S. H., Sigworth F. J. (1988) Open channel noise. IV. Estimation of rapid kinetics of formamide block in gramicidin A channels. Biophys. J. 54, 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kirsch G. E., Drewe J. A. (1993) Gating-dependent mechanism of 4-aminopyridine block in two related potassium channels. J. Gen. Physiol. 102, 797–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leng T. D., Xiong Z. G. (2013) The pharmacology and therapeutic potential of small molecule inhibitors of acid-sensing ion channels in stroke intervention. Acta Pharmacol. Sin. 34, 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]