Background: Wnt3a-dependent neurite outgrowth is associated with Dishevelled phosphorylation.
Results: PKCι is required for Wnt3a-induced neurite extension. PKCι binds wild-type Dishevelled, but not an inactive phosphorylation-deficient Dishevelled mutant.
Conclusion: PKCι mediates Wnt3a-dependent neurite outgrowth; Dishevelled phosphorylation is required for PKCι interaction.
Significance: PKCι has key role in Wnt3a-induced neurite outgrowth; Dvl phosphorylation at defined sites is critical for PKCι association.
Keywords: Neurite Outgrowth, Protein Kinase C (PKC), Signal Transduction, Wnt Pathway, Wnt Signaling, Atypical PKC, Casein Kinase 1, Dishevelled, Ewing Sarcoma, PKC Iota
Abstract
Previously we reported that Wnt3a-dependent neurite outgrowth in Ewing sarcoma family tumor cell lines was mediated by Frizzled3, Dishevelled (Dvl), and c-Jun N-terminal kinase (Endo, Y., Beauchamp, E., Woods, D., Taylor, W. G., Toretsky, J. A., Uren, A., and Rubin, J. S. (2008) Mol. Cell. Biol. 28, 2368–2379). Subsequently, we observed that Dvl2/3 phosphorylation correlated with neurite outgrowth and that casein kinase 1δ, one of the enzymes that mediate Wnt3a-dependent Dvl phosphorylation, was required for neurite extension (Greer, Y. E., and Rubin, J. S. (2011) J. Cell Biol. 192, 993–1004). However, the functional relevance of Dvl phosphorylation in neurite outgrowth was not established. Dvl1 has been shown by others to be important for axon specification in hippocampal neurons via an interaction with atypical PKCζ, but the role of Dvl phosphorylation was not evaluated. Here we report that Ewing sarcoma family tumor cells express PKCι but not PKCζ. Wnt3a stimulated PKCι activation and caused a punctate distribution of pPKCι in the neurites and cytoplasm, with a particularly intense signal at the centrosome. Knockdown of PKCι expression with siRNA reagents blocked neurite formation in response to Wnt3a. Aurothiomalate, a specific inhibitor of PKCι/Par6 binding, also suppressed neurite extension. Wnt3a enhanced the co-immunoprecipitation of endogenous PKCι and Dvl2. Although FLAG-tagged wild-type Dvl2 immunoprecipitated with PKCι, a phosphorylation-deficient Dvl2 derivative did not. This derivative also was unable to rescue neurite outgrowth when endogenous Dvl2/3 was suppressed by siRNA (González-Sancho, J. M., Greer, Y. E., Abrahams, C. L., Takigawa, Y., Baljinnyam, B., Lee, K. H., Lee, K. S., Rubin, J. S., and Brown, A. M. (2013) J. Biol. Chem. 288, 9428–9437). Taken together, these results suggest that site-specific Dvl2 phosphorylation is required for Dvl2 association with PKCι. This interaction is likely to be one of the mechanisms essential for Wnt3a-dependent neurite outgrowth.
Introduction
The Wnts comprise a large family of secreted glycoproteins that have many activities during embryonic development and promote tissue homeostasis in the adult. Wnt signals control cell proliferation, differentiation, survival, motility, and polarity. They also have higher order effects on the developing embryo by regulating tissue patterning, organogenesis, and specification of the body plan (1, 2).
Wnts are particularly important in the development of the nervous system, where they contribute to neural tube closure, formation of specific brain structures, and the induction and migration of neural crest cells (3, 4). Wnt signaling stimulates axonal remodeling, pathfinding, dendritic arborization, and neuronal connectivity in the central nervous system (5–7). Many Wnt signaling components have been implicated in neurite outgrowth (7–9). For instance, Wnt7b induces dendritic morphogenesis in mouse hippocampal neurons via a noncanonical pathway including Dvl, Rac, and JNK (10). The gene encoding the Wnt receptor, Frizzled3 (Fzd3), is required for the development of major fiber tracts in the rostral central nervous system (11), and targeted disruption of Fzd3 caused axonal growth and guidance defects (12). Derailed/Ryk is another Wnt receptor that regulates axon guidance in a variety of contexts (13–16).
Neurons have highly polarized structures, and establishment of cell polarity is an essential aspect of neurite outgrowth (17). It is widely accepted that the polarity complex consisting of atypical protein kinase C (aPKC, that is PKC isoforms PKCζ or PKCλ/ι),3 Par3, and Par6 has a critical role in neuronal polarity (18–21), a process that involves neurite extension followed by the differentiation of a neurite into an axon (22). Recent studies have indicated that the aPKC-Par3-Par6 complex participates in neurite outgrowth before and after axon differentiation, as well as in dendrite formation (23–25). Of note, Wnt4 attracts the outgrowth of commissural axons by a mechanism that relies on aPKC (24). Furthermore, Wnt5a promotes axon differentiation in cultured hippocampal neurons via an interaction of Dvl1 with PKCζ (25).
Previously, we used an Ewing sarcoma family of tumors (ESFT) cell line, TC-32, to study Wnt3a-dependent neurite outgrowth (26). ESFT cells are small, round, and poorly differentiated with characteristics of primitive neuroectoderm (27–29). Gene microarray data indicated that ESFT cells have expression patterns that resemble those of neuroectoderm and endothelial cells (30). A small fraction (10–15%) of TC-32 cells have long neurites in the basal state, whereas Wnt3a treatment elicits neurite outgrowth in 50–75% of cells within 3 h. This response is mediated by noncanonical Wnt rather than β-catenin signaling and involves a mechanism that requires Fzd3 and Dvl2/3 expression, as well as JNK activation (26). The centrosomal localization of casein kinase 1δ (CK1δ), a kinase that phosphorylates Dvl proteins, was also shown to be necessary for neurite outgrowth (31), although the functional relevance of Dvl phosphorylation was not established.
The present study was undertaken to further delineate the mechanisms responsible for Wnt3a-dependent neurite outgrowth in ESFT cells. We determined that PKCι, but not PKCζ, was expressed in TC-32 cells. Wnt3a induced the phosphorylation of PKCι, and siRNA knockdown of PKCι-blocked neurite extension. Furthermore, neuritogenesis was suppressed by aurothiomalate (ATM), a specific inhibitor of PKCι binding to Par6 (32). Wnt3a stimulated an association of both endogenous and FLAG-tagged WT Dvl2 with PKCι. However, a FLAG-tagged Dvl2 mutant with alanine substitutions at a cluster of CK1 phosphorylation sites in the C-terminal domain failed to interact with PKCι. In an accompanying manuscript (43), we demonstrated that this mutant was unable to support neurite outgrowth when expression of endogenous Dvl2/3 was suppressed. These results strongly suggest that Dvl phosphorylation at these sites is necessary for the interaction with PKCι and reinforce the idea that the Dvl/aPKC interaction is important for Wnt-dependent neurite outgrowth.
EXPERIMENTAL PROCEDURES
Recombinant Protein and Chemicals
Recombinant Wnt3a was purchased from R&D Systems (Minneapolis, MN). ATM was from Taylor Pharmaceuticals (Decatur, IL).
Cell Culture
TC-32 cells were seeded on cell culture dishes, cluster plates, or glass coverslips that had been precoated with type I collagen solution (Sigma-Aldrich) as described (26). HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum.
Antibodies and Reagents Used for Immunostaining
Alexa Fluor 568 Phalloidin (catalog no. A12380), Alexa Fluor 488 goat anti-mouse IgG (catalog no. A11001), Alexa Fluor 568 goat anti-mouse IgG (catalog no. A11004), and Alexa Fluor 488 goat anti-rabbit IgG (catalog no. A11008) were from Invitrogen. Pericentrin antibody (ab28144) was from Abcam (Cambridge, MA). DAPI (catalog no. D9542) was from Sigma-Aldrich. Rabbit anti-phospho-PKCζ/λ/ι (catalog no. 9378) was from Cell Signaling Technology, Inc. (Danvers, MA). Mouse anti-PKCι (catalog no. 610175) was from BD Transduction Laboratories, and rabbit anti-PKCζ (sc-216) was from Santa Cruz Biotechnology (Santa Cruz, CA).
Immunofluorescent Analysis
TC-32 cells were cultured in complete growth medium on 12-mm-diameter glass coverslips (Fisher) coated with collagen. To visualize phalloidin and phospho-PKCζ/ι, formaldehyde fixation and staining were performed as previously described (31). Anti-phospho-PKCζ/ι was used at a 1:250 dilution. Double staining of phospho-PKCι and pericentrin was performed either with formaldehyde or methanol (MeOH) fixation (31). Total PKCι was detected with mouse anti-PKCι (1:500 dilution) when cells were fixed in formaldehyde and with rabbit anti-PKCζ (sc-216, 1:500), which recognizes PKCι, when cells were fixed in methanol because of high background with mouse anti-PKCι antibody following methanol fixation.
Cell Imaging
Fluorescent images were collected with a Zeiss 510 LSCM, using a 63× objective (Carl Zeiss Inc., Thornton, NY). Zeiss LSM image browser Version 4.0.0.157 was used for image processing, and composite figures were prepared with Adobe PhotoShop CS3 version 10.0.1 (Adobe Systems Inc., San Jose, CA).
Quantitative Analysis of Neurite Outgrowth
Stimulation of neurite outgrowth was monitored as previously described (26).
Antibodies Used for Western Blotting
Rabbit anti-PKCζ (sc-216), mouse CK1δ (sc-55553), and mouse anti-HSP70 (sc-7298) antibodies were obtained from Santa Cruz Biotechnology. Mouse anti-PKCι (catalog no. 610175) and mouse anti-CK1ϵ (catalog no. 610445) were from BD Transduction Laboratories. Rabbit anti-phospho-PKCζ/λ/ι (catalog no. 9378), rabbit anti-PKCζ (catalog no. 9372), and rabbit anti-Dvl2 (catalog no. 3216) were from Cell Signaling Technology, Inc. Mouse anti-tubulin (catalog no. A11126) was from Invitrogen. Mouse anti-FLAG (M2) was from Sigma-Aldrich.
Immunoblotting
To examine the effect of Wnt3a on PKCι phosphorylation, 80–90% confluent monolayers of TC-32 cells that had been seeded in 6- or 12-well cell culture plates were serum-starved overnight. After incubation with Wnt3a for the indicated time, the cells were rinsed twice with PBS, lysed with buffer, and processed for SDS-PAGE and Western blot analysis as previously described (26). For immunoblot analysis to verify siRNA knockdown of endogenous proteins, TC-32 cells that had been transfected with siRNA were seeded in 6- or 12-well cell culture plates and harvested 48 h after transfection.
Immunoprecipitation
TC-32 cells were harvested with immunoprecipitation buffer (20 mm Tris-HCl, pH 8.0, 10 mm EDTA, 1 mm EGTA, 150 mm NaCl, 0.2% Triton-X, 0.2% Nonidet P-40), and cell lysates were homogenized by passing through 23-gauge syringe needles. Cell lysates were centrifuged at 20,817 × g for 15 min at 4 °C. Supernatant was transferred to another tube, and protein concentration was determined. Cell lysates (1 mg) were precleared by incubation with 1 μg of rabbit IgG (sc-2027; Santa Cruz Biotechnology) and 20 μl of protein A/G Plus-agarose (sc-2003; Santa Cruz Biotechnology) for 2 h at 4 °C, followed by centrifugation at 20,817 × g for 10 min. At the same time, 1 μg of rabbit Dvl2 antibody and 20 μl of protein A/G-agarose were combined and incubated for 2 h at 4 °C with rotation in immunoprecipitation buffer, followed by centrifugation at 956 × g for 5 min. Precleared cell lysate was combined with resuspended Dvl2 antibody/protein A/G-agarose mixture and incubated overnight at 4 °C with rotation. After 18 h, the samples were washed with immunoprecipitation buffer three times, combined with sample loading buffer, heated at 95 °C for 10 min, and resolved by SDS-PAGE.
siRNA Transfection
PKCζ pooled siRNA (catalog no. L-003526-00), PKCι pooled siRNA (catalog no. L-004656-00), and luciferase siRNA (target sequence: CGUACGCGGAAUACUUCGA) were from Dharmacon (Lafayette, CO). CK1δ siRNA (catalog no. SI00287413) and CK1ϵ siRNA (catalog no. SI00287728) were from Qiagen. siRNA transfection experiments in TC-32 cells were performed with the Amaxa system (Amaxa, Cologne, Germany) according to the manufacturer's protocol, using 200 pmol of siRNA/106 cells, with Nucleofector R (catalog no. VCA-0001), program O-017. The effects of siRNA treatment were analyzed 48 h after transfection.
Recombinant DNA
pCS2+ FLAG-mDvl2 WT was kindly provided by Dr. Xi He (Harvard University). pCS2+FLAG-mDvl2 P4m (S594A, S595T, S597A, T604A) was generated by site-directed mutagenesis according to manufacturer's protocol (QuikChange II; Agilent Technologies, Inc. Santa Clara, CA). Mutation was verified by DNA sequence analysis in the DNA sequencing mini core facility at the National Cancer Institute. Lentiviral constructs, pCMV32 FLAG-tagged mouse Dvl2 WT and Dvl2 P4m were generated as described (43).
Lentiviral Expression
Lentiviral particles were prepared as previously described (31).
Statistical Analysis
Except where indicated, the significance of differences in data were determined with Student's t test. The differences were considered to be significant when the p value was less than 0.05.
RESULTS
PKCι Is Expressed in ESFT Cells and Phosphorylated in Response to Wnt3a
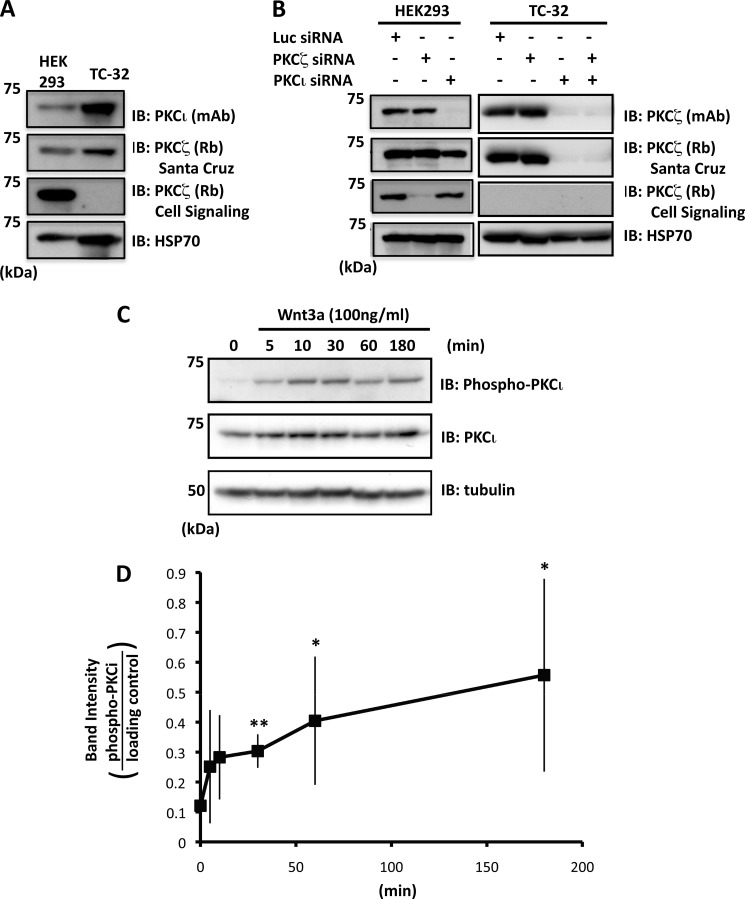
To investigate the potential role of aPKC isoforms in Wnt3a-dependent neurite outgrowth in ESFT cells, we initially examined the expression of these proteins using antisera intended to recognize PKCζ or PKCι. Immunoblotting of TC-32 cell lysates with PKCι mAb revealed a band of the expected size (74 kDa) (Fig. 1A). A comparable band was observed with one PKCζ polyclonal antiserum but not with another (Fig. 1, A and B). To resolve this ambiguity, the cells were treated with siRNA reagents specific for PKCζ versus PKCι, and Western blot analysis of the TC-32 lysates revealed that the bands recognized by the antibodies corresponded to PKCι (Fig. 1B).
FIGURE 1.
PKCι is expressed by TC-32 cells and phosphorylated in response to Wnt3a. A, immunoblot analysis of PKCι and PKCζ in HEK293 and TC-32 cell lysates. PKCι was detected in TC-32 cells, but contradictory results were obtained for PKCζ with two different antibodies. B, combination of immunoblot analysis and isoform-specific siRNA indicated that PKCι, but not PKCζ, is expressed by TC-32 cells. PKCζ antiserum from Santa Cruz reacts with both PKCζ and PKCι. Note that knockdown in HEK293 confirmed the specificity of the PKCι and PKCζ siRNA reagents. C, time course of PKCι phosphorylation stimulated by Wnt3a (antibody recognizes Thr412 in PKCι and Thr410 in PKCζ). D, quantitative results of Wnt3a-dependent PKCι phosphorylation. The band intensity of phospho-PKCι was normalized to loading control (HSP70 or tubulin). The graph shows average values ± S.D. of five independent experiments. *, p < 0.05; **, p < 0.01 compared with zero time point. Similar results were obtained when data were normalized to total PKCι. However, because of fluctuation in total PKCι levels, the statistical significance was evident only at the 3-h time point. IB, immunoblot.
Incubation of TC-32 cells with recombinant Wnt3a at 100 ng/ml stimulated the phosphorylation of PKCι, which was variably detected within 5–10 min and consistently seen at 30 min through 3 h (Fig. 1, C and D). The phospho-specific PKCζ/λ/ι antibody was directed against T412 in PKCι, a residue in the activation loop that is phosphorylated in the activated form of the enzyme (33, 34). These results suggested that exposure to Wnt3a activated PKCι in a time frame consistent with its possible function in neurite extension (26).
Intracellular Distribution of Phosphorylated PKCι
Confocal microscopy of TC-32 cells showed little evidence of phosphorylated PKCι (pPKCι) in control cells (Fig. 2A, upper row). However, cells treated for 1 h with 100 ng/ml of Wnt3a displayed a punctate pattern of staining in the cell body and the neurite (Fig. 2A, lower row). The specificity of pPKCι immunofluorescent signal was confirmed by knockdown with PKCι siRNA (Fig. 2B). A punctate pattern also was seen with antibody to total PKCι (Fig. 2C) and was absent following treatment with PKCι siRNA (Fig. 2D). Interestingly, signal for pPKCι and total PKCι exhibited a cytosolic and pericentrosomal distribution (Fig. 3). Wnt3a treatment increased the percentage of cells with pericentrosomal pPKCι and the intensity of the stain there (Fig. 3B and data not shown). Previously, we reported that CK1δ staining was strongest at the centrosome, where it was required for neurite outgrowth induced by Wnt3a (31). Although CK1δ siRNA did not block PKCι phosphorylation following Wnt3a treatment, it did inhibit the pericentrosomal distribution of pPKCι (data not shown). Knockdown of both CK1δ and the highly related isoform CK1ϵ increased the basal level of phospho-PKCι in some experiments, but CK1ϵ siRNA had no effect on the pericentrosomal localization of PKCι (data not shown).
FIGURE 2.
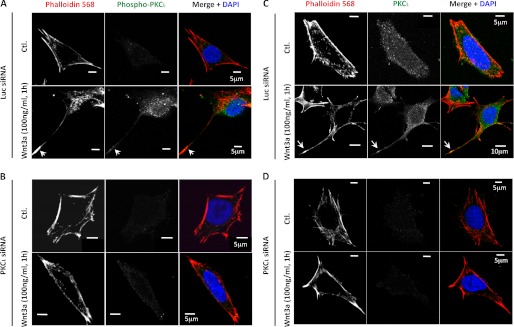
Immunofluorescent analysis of pPKCι and total PKCι in TC-32 cells with or without Wnt3a treatment. The cells were transfected with luciferase (Luc; A and C) or PKCι (B and D) siRNA reagents and 48 h later incubated with serum-free culture medium ± Wnt3a (100 ng/ml) for 1 h, fixed in formaldehyde, and then stained for phalloidin, DNA (DAPI), and pPKCι (A and B) or total PKCι (C and D). A scale bar is indicated. Ctl., control.
FIGURE 3.
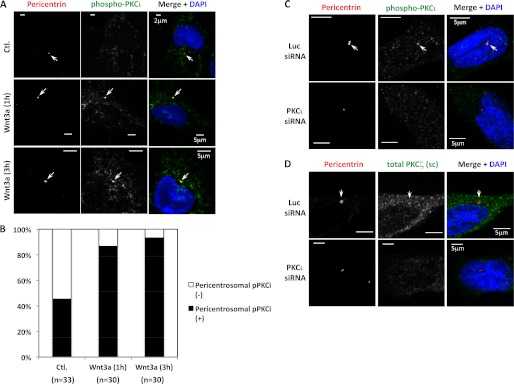
Co-localization of pPKCι and total PKCι with the centrosomal marker pericentrin. A, confocal micrographs of TC-32 cells treated in the absence or presence of Wnt3a (100 ng/ml) for the indicated times and subsequently stained with DAPI and antibodies to pPKCζ/ι and pericentrin. Arrows point to centrosomes. B, percentage of cells in different treatment groups with pericentrosomal distribution of pPKCι. The cumulative number (n) of cells analyzed in more than three experiments is indicated. Wnt3a treatment for 1 and 3 h increased the percentage of cells with pericentrosomal distribution of pPKCι. p < 0.001 for both time points compared with negative control, Fisher's exact test in SigmaPlot (Systat Software Inc., San Jose, CA). C and D, the specificity of pPKCι (C) and total PKCι (D) immunofluorescent staining was tested by comparing signal obtained after treatment with luciferase (Luc, upper rows) versus PKCι (lower rows) siRNA as described in the Fig. 2 legend (Wnt3a was not used in this experiment). The cells were fixed in methanol. A scale bar is indicated. Ctl., control.
Inhibition of PKCι Expression or Interaction with Par6 Blocked Wnt3a-dependent Neurite Outgrowth
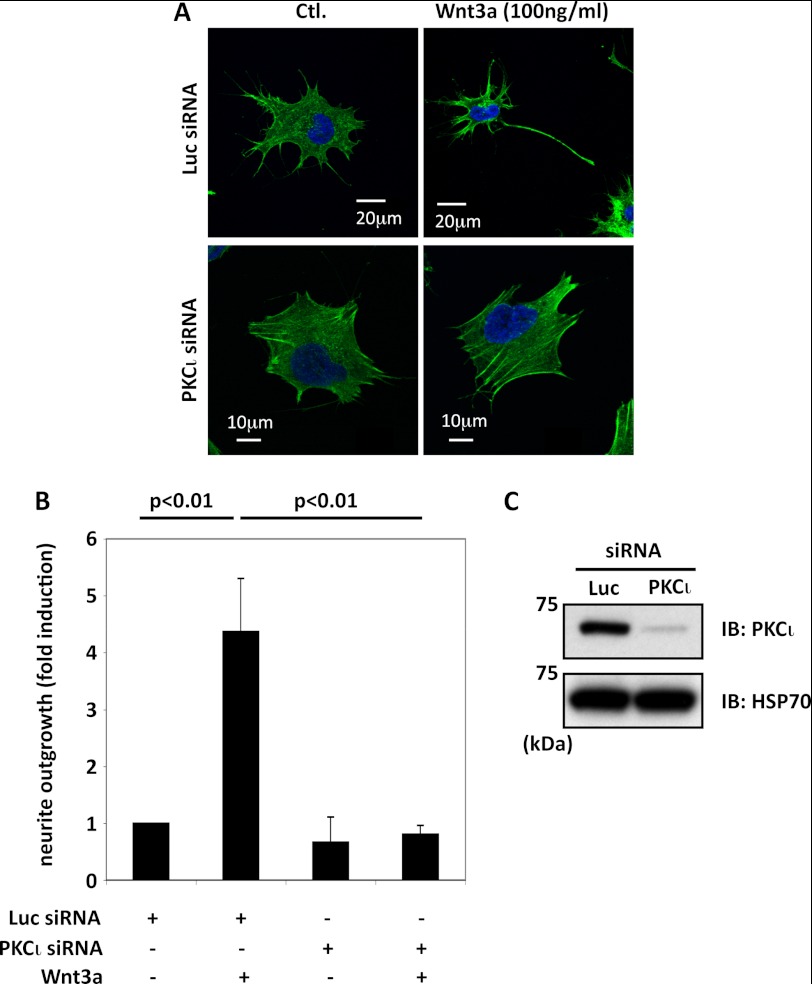
In the absence of Wnt3a, ∼10% of TC-32 cells have a neurite extending at least one cell diameter in length, and this proportion was unaffected by a negative control siRNA directed against luciferase. When cells that had been treated with luciferase siRNA were subsequently exposed to Wnt3a (100 ng/ml) for 3 h, the percentage with a long neurite increased 4–5-fold (Fig. 4, A and B). In contrast, the induction of neurite outgrowth by Wnt3a was completely abrogated in cells that had received PKCι siRNA (Fig. 4, A and B). Efficient and specific knockdown of PKCι protein was confirmed by Western blot analysis (Figs. 1B and 4C). These results indicate that expression of PKCι is required for Wnt3a-dependent neurite outgrowth in TC-32 cells.
FIGURE 4.
Knockdown of PKCι expression with siRNA blocked Wnt3a-dependent neurite outgrowth. A, representative confocal micrographs, PKCι siRNA versus luciferase siRNA control. TC-32 cells were treated with the indicated siRNA reagents and 48 h later incubated for 3 h with serum-free culture medium ± Wnt3a (100 ng/ml). B, quantitative summary of the percentage of cells with a neurite at least one cell diameter in length, normalized to luciferase siRNA control without Wnt3a treatment (typically ∼10% cells have a long neurite in this control). The bar graph shows the results of three independent experiments (mean ± S.D.). C, immunoblot confirmed knockdown of endogenous PKCι protein. Ctl., control; IB, immunoblot; Luc, luciferase.
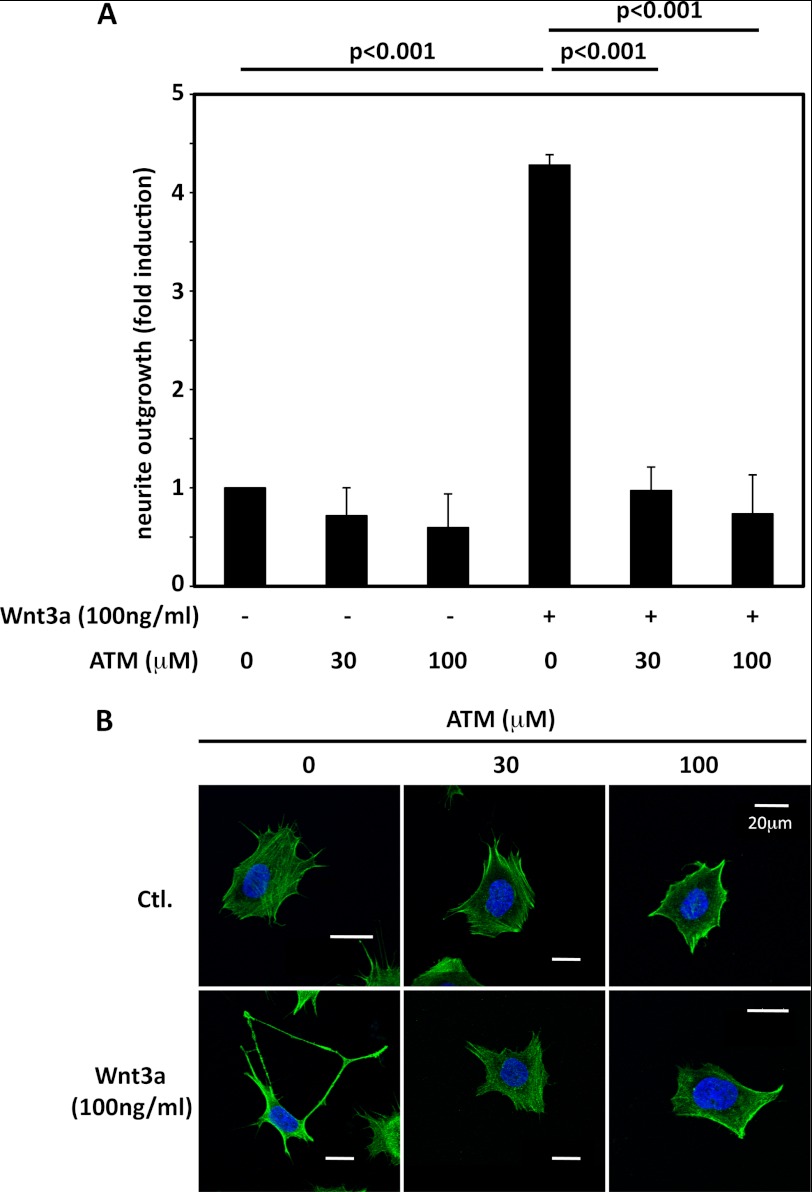
ATM is a small molecule that disrupts the interaction of PKCι with Par6 and thereby blocks the activity of the PKCι-Par3-Par6 polarity complex (32). Using ATM at concentrations shown to inhibit the polarity complex in other cells, we observed a marked suppression of Wnt3a-dependent neurite extension without any negative impact on the viability of the cells (Fig. 5). This reinforces the evidence from siRNA experiments that PKCι activity is necessary for neurite outgrowth in ESFT cells following Wnt3a treatment.
FIGURE 5.
ATM blocked Wnt3a-dependent neurite outgrowth. A, TC-32 cells were preincubated for 30 min with the indicated concentrations of ATM in serum-free culture fluid and then treated with Wnt3a (0 or 100 ng/ml) for 3 h. Quantification of neurite outgrowth was performed as described in the Fig. 4 legend. The bar graph shows the results of three independent experiments (mean ± S.D.). B, representative confocal micrographs of cells from the different treatment groups. Ctl., control.
Wnt3a Stimulated the Interaction of PKCι with Dvl2
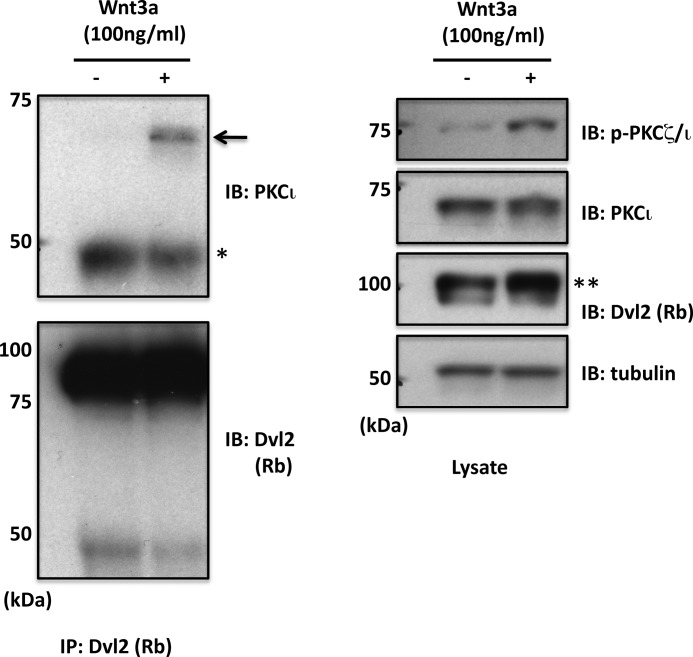
Prompted by a report that PKCζ interacts with Dvl1 to mediate axonal specification in hippocampal neurons (25), we explored the possibility that Wnt3a causes an association of PKCι with Dvl. Although Dvl1 protein was not detected in TC-32 cells, Wnt3a promoted the co-immunoprecipitation of endogenous PKCι with Dvl2 (Fig. 6). After a 3-h treatment with Wnt3a (100 ng/ml), the cells were lysed, and proteins were precipitated with Dvl2 antiserum and immunoblotted with anti-PKCι. Little or no PKCι was seen in the pellet from control cells, but PKCι was observed in the immunoprecipitated sample from cells that had been incubated with Wnt3a. As previously observed (31), Wnt3a treatment also elicited an upward shift in the electrophoretic mobility of Dvl2 that corresponds to Dvl2 phosphorylation (Fig. 6). Occasionally the mobility shift was accompanied by an increase in Dvl2 band intensity, although that was not a consistent finding.
FIGURE 6.
Co-immunoprecipitation of endogenous PKCι and Dvl2 following Wnt3a treatment. TC-32 cells were incubated ± Wnt3a for 3 h, and cell lysates were prepared for immunoprecipitation with Dvl2 rabbit polyclonal antiserum. Pelleted protein and cell lysates were immunoblotted as indicated. An arrow points to immunoprecipitated PKCι. *, IgG heavy chain. **, note increased relative intensity of slower migrating Dvl2 band. IB, immunoblot; IP, immunoprecipitation.
Phosphorylation-deficient Dvl2 Derivative Did Not Co-immunoprecipitate with PKCι
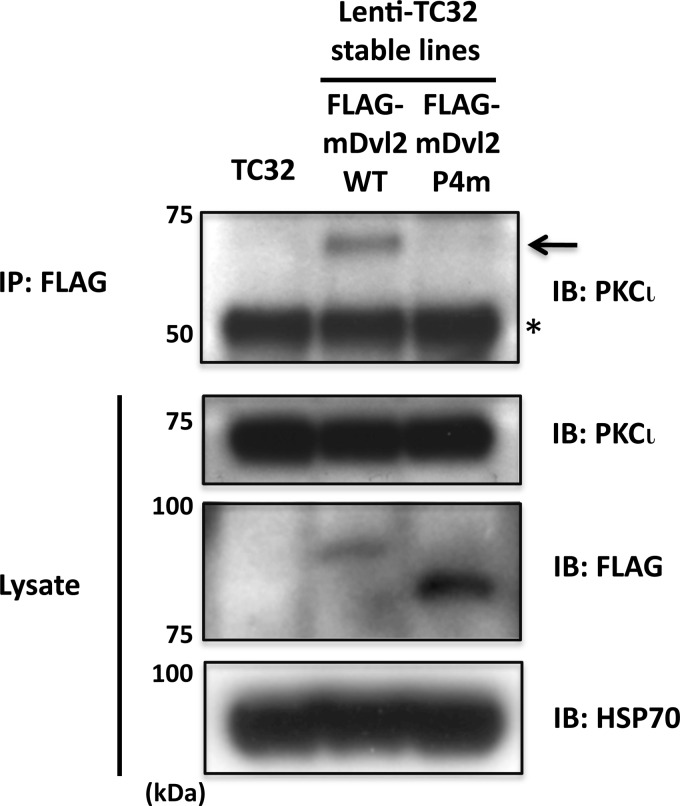
In a parallel study, we identified CK1 phosphorylation sites in the C-terminal domain of Dvl2 that were critical for its Wnt-induced electrophoretic mobility shift (43). In contrast to Dvl2 WT, a mutant containing alanine substitutions at Ser594, Thr595, Ser597, and Thr604 (FLAG-Dvl2 P4m) was unable to rescue the neurite outgrowth phenotype when expression of endogenous Dvl was suppressed by siRNA (43). This implied that phosphorylation at one or more of the sites is essential for neurite outgrowth. To test the hypothesis that phosphorylation of these residues is required for Dvl2 interaction with PKCι, we compared the co-immunoprecipitation of PKCι with FLAG-tagged Dvl2 WT versus Dvl2 P4m. The WT protein exhibited a slower electrophoretic mobility than the mutant protein (Fig. 7), consistent with the former being phosphorylated, presumably by endogenous Wnt/Frizzled signaling (43). Despite the fact that the mutant protein was expressed at a higher level than Dvl2 WT, PKCι only precipitated with Dvl2 WT (Fig. 7). This result strongly suggests that Dvl phosphorylation at one or more of the four mutated sites is required for interaction with PKCι. Because Dvl2 P4m was unable to substitute for native protein in the neurite outgrowth assay, this finding also implied that the interaction of PKCι with Dvl2 was necessary for Wnt3a-dependent neurite extension in ESFT cells.
FIGURE 7.
Co-immunoprecipitation of FLAG-tagged Dvl2 derivatives and endogenous PKCι. FLAG-tagged mouse Dvl2 WT or a Wnt-induced phosphorylation mutant containing four alanine substitutions (Dvl2 P4m) was stably expressed in TC-32 using a lentiviral vector. The cells were lysed, and the proteins were precipitated with FLAG antibody. Pellets and cell lysates were immunoblotted as indicated. An arrow points to immunoprecipitated PKCι. *, IgG heavy chain. IB, immunoblot; IP, immunoprecipitation.
DISCUSSION
In this report we demonstrate that PKCι is required for Wnt3a-dependent neurite outgrowth in TC-32 cells. This is consistent with earlier studies showing that aPKC was essential for commissural axon guidance induced by Wnt4 and that PKCζ was necessary for axon specification in hippocampal neurons treated with Wnt5a (24, 25). Ectopically expressed Dvl1 co-precipitated with PKCζ and other members of the Par complex, and their association was important for axon specification (25). We also observed the co-precipitation of epitope-tagged Dvl2 with PKCι, as well as a physical association of the endogenous proteins that was stimulated by Wnt3a. Our findings indicate that a Wnt-induced phosphorylation-deficient Dvl2 derivative with mutated CK1 phosphorylation sites in its C-terminal domain failed to precipitate with PKCι. This reveals a functional significance of Dvl phosphorylation at these sites that is reinforced by the inability of the mutant protein to mediate neurite outgrowth (43). We propose that the interaction between Dvl2 and PKCι is required for Wnt3a-dependent neurite outgrowth in TC-32 cells, just as the Dvl1/PKCζ interaction was found to be critical for axon specification in hippocampal neurons.
The implied significance of Dvl phosphorylation in binding to PKCι and in neurite outgrowth is noteworthy because there are few instances in which Dvl phosphorylation at specific sites has been shown to be functionally important. One group reported that phosphorylation of Dvl2 at Thr206 was necessary for proper orientation of the mitotic spindle (35), and another showed that Dvl2 phosphorylation at Ser143 and Thr224 enables binding to polo-like kinase 1 to facilitate primary cilia disassembly (36). Dvl2 phosphorylation mediates binding to Ror2, although the sites of phosphorylation were not determined (37). A recent extensive analysis identified dozens of phosphorylated Ser/Thr residues in Dvl (or the Drosophila ortholog, Dsh). However, these modifications were dispensable for β-catenin and planar cell polarity signaling in the Drosophila assays (38). The present study and our accompanying report (43) suggest that Dvl2 phosphorylation, particularly at Ser594, Thr595, and Ser597, is relevant for signaling in a Wnt/aPKC pathway.
In addition to the work by Zhang and co-workers (25), another group identified an interaction between Dvls and Par6, although aPKC was not reported to be part of the complex (39). Wnt5a stimulated Dvl/Par6 binding to the E3-ubiquitin ligases Smurf1 and Smurf2, which facilitated the ubiquitination and proteasomal degradation of Prickle, a core component of planar cell polarity signaling. Smurf binding was dependent on Dvl2 phosphorylation via a PY motif in the Dvl2 C-terminal domain. The association of Dvl/Par6/Smurfs is intriguing because Smurf1 and Smurf2 are essential for neurite outgrowth and neuronal polarity, respectively, due to their regulation of the GTPases RhoA and Rap1B (40, 41). This raises the possibility that Wnt-dependent neurite outgrowth involves protein turnover mediated by Dvl2-Par6-Smurf complexes with or without aPKC.
The pericentrosomal localization of pPKCι that we observed in TC-32 cells following Wnt3a treatment may have mechanistic relevance. CK1δ also is concentrated at the centrosome, where it functions in neurite extension (31). Although we have not detected Dvl2 at this site, a transient substrate/kinase interaction might occur there and facilitate Dvl2/PKCι binding. Knockdown of CK1δ by siRNA did not affect Wnt3a-induced PKCι phosphorylation, but it did disrupt the pericentrosomal localization of pPKCι. This suggests that CK1δ might interact with pPKCι through a direct or indirect mechanism. The pericentrosomal localization of pPKCι is provocative because PKCζ also was identified at the centrosome in hippocampal neurons where it promoted neurite outgrowth in a pathway that involved Aurora A, NDEL1, and the regulation of microtubule dynamics (42).
In summary, we have shown that PKCι is required for Wnt3a-dependent neurite outgrowth in the ESFT line TC-32. Combined with its utility as a cell line, our study emphasizes the relevance of the TC-32 model for the study of Wnt-dependent neurite extension. Wnt3a stimulated PKCι phosphorylation and its distribution to the neurite and the centrosome. We believe that PKCι acts at the tip of the neurite to directly mediate protrusion through established mechanisms (18–21), although its pericentrosomal distribution might promote neurite outgrowth via effects on microtubule dynamics. We suspect that PKCι activity depends on its association with Dvl2. This interaction was blocked when four Ser/Thr residues in the C-terminal domain of Dvl2 were replaced with alanine. Our results strongly suggest that Dvl phosphorylation at these sites, particularly CK1-mediated phosphorylation at Ser594, Thr595, and Ser597, is functionally significant and reinforces the idea that Dvl/aPKC interactions have an important role in Wnt-dependent neurite extension.
Acknowledgments
We thank Xi He for providing the FLAG-mDvl2 construct and Dom Esposito (Advanced Technology Program, SAIC-Frederick) for preparation of Gateway entry clones and lentiviral expression constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA123238 (to A. M. C. B.) and R01 CA081436-15 (to A. P. F.). This work was also supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Florida Biomedical Research Program Grant KG05-339710-03 (to A. P. F.).
- aPKC
- atypical protein kinase C
- ATM
- aurothiomalate
- CK1
- casein kinase 1
- ESFT
- Ewing sarcoma family of tumors
- pPKCι
- phosphorylated PKCι.
REFERENCES
- 1. Logan C. Y., Nusse R. (2004) The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 2. Fuerer C., Nusse R., Ten Berge D. (2008) Wnt signalling in development and disease. Max Delbruck Center for Molecular Medicine meeting on Wnt signaling in Development and Disease. EMBO Rep. 9, 134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malaterre J., Ramsay R. G., Mantamadiotis T. (2007) Wnt-Frizzled signalling and the many paths to neural development and adult brain homeostasis. Front. Biosci. 12, 492–506 [DOI] [PubMed] [Google Scholar]
- 4. Montcouquiol M., Crenshaw E. B., 3rd, Kelley M. W. (2006) Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 29, 363–386 [DOI] [PubMed] [Google Scholar]
- 5. Bovolenta P., Rodriguez J., Esteve P. (2006) Frizzled/RYK mediated signalling in axon guidance. Development 133, 4399–4408 [DOI] [PubMed] [Google Scholar]
- 6. Ciani L., Salinas P. C. (2005) WNTs in the vertebrate nervous system. From patterning to neuronal connectivity. Nat. Rev. Neurosci. 6, 351–362 [DOI] [PubMed] [Google Scholar]
- 7. Endo Y., Rubin J. S. (2007) Wnt signaling and neurite outgrowth. Insights and questions. Cancer Sci. 98, 1311–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salinas P. C., Zou Y. (2008) Wnt signaling in neural circuit assembly. Annu. Rev. Neurosci. 31, 339–358 [DOI] [PubMed] [Google Scholar]
- 9. Sánchez-Camacho C., Bovolenta P. (2009) Emerging mechanisms in morphogen-mediated axon guidance. Bioessays 31, 1013–1025 [DOI] [PubMed] [Google Scholar]
- 10. Rosso S. B., Sussman D., Wynshaw-Boris A., Salinas P. C. (2005) Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat. Neurosci. 8, 34–42 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y., Thekdi N., Smallwood P. M., Macke J. P., Nathans J. (2002) Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J. Neurosci. 22, 8563–8573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y., Zhang J., Mori S., Nathans J. (2006) Axonal growth and guidance defects in Frizzled3 knock-out mice. A comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J. Neurosci. 26, 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keeble T. R., Halford M. M., Seaman C., Kee N., Macheda M., Anderson R. B., Stacker S. A., Cooper H. M. (2006) The Wnt receptor Ryk is required for Wnt5a-mediated axon guidance on the contralateral side of the corpus callosum. J. Neurosci. 26, 5840–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y., Shi J., Lu C. C., Wang Z. B., Lyuksyutova A. I., Song X. J., Zou Y. (2005) Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat. Neurosci. 8, 1151–1159 [DOI] [PubMed] [Google Scholar]
- 15. Lu W., Yamamoto V., Ortega B., Baltimore D. (2004) Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119, 97–108 [DOI] [PubMed] [Google Scholar]
- 16. Schmitt A. M., Shi J., Wolf A. M., Lu C. C., King L. A., Zou Y. (2006) Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature 439, 31–37 [DOI] [PubMed] [Google Scholar]
- 17. Higginbotham H. R., Gleeson J. G. (2007) The centrosome in neuronal development. Trends Neurosci. 30, 276–283 [DOI] [PubMed] [Google Scholar]
- 18. Shi S. H., Jan L. Y., Jan Y. N. (2003) Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112, 63–75 [DOI] [PubMed] [Google Scholar]
- 19. Nishimura T., Kato K., Yamaguchi T., Fukata Y., Ohno S., Kaibuchi K. (2004) Role of the PAR-3-KIF3 complex in the establishment of neuronal polarity. Nat. Cell Biol. 6, 328–334 [DOI] [PubMed] [Google Scholar]
- 20. Nishimura T., Yamaguchi T., Kato K., Yoshizawa M., Nabeshima Y., Ohno S., Hoshino M., Kaibuchi K. (2005) PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat. Cell Biol. 7, 270–277 [DOI] [PubMed] [Google Scholar]
- 21. Higginbotham H., Tanaka T., Brinkman B. C., Gleeson J. G. (2006) GSK3β and PKCζ function in centrosome localization and process stabilization during Slit-mediated neuronal repolarization. Mol. Cell Neurosci. 32, 118–132 [DOI] [PubMed] [Google Scholar]
- 22. Yoshimura T., Arimura N., Kaibuchi K. (2006) Signaling networks in neuronal polarization. J. Neurosci. 26, 10626–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanabe K., Kani S., Shimizu T., Bae Y. K., Abe T., Hibi M. (2010) Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J. Neurosci. 30, 16983–16992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf A. M., Lyuksyutova A. I., Fenstermaker A. G., Shafer B., Lo C. G., Zou Y. (2008) Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J. Neurosci. 28, 3456–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang X., Zhu J., Yang G. Y., Wang Q. J., Qian L., Chen Y. M., Chen F., Tao Y., Hu H. S., Wang T., Luo Z. G. (2007) Dishevelled promotes axon differentiation by regulating atypical protein kinase C. Nat. Cell Biol. 9, 743–754 [DOI] [PubMed] [Google Scholar]
- 26. Endo Y., Beauchamp E., Woods D., Taylor W. G., Toretsky J. A., Uren A., Rubin J. S. (2008) Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol. Cell. Biol. 28, 2368–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cavazzana A. O., Miser J. S., Jefferson J., Triche T. J. (1987) Experimental evidence for a neural origin of Ewing's sarcoma of bone. Am. J. Pathol. 127, 507–518 [PMC free article] [PubMed] [Google Scholar]
- 28. Lipinski M., Braham K., Philip I., Wiels J., Philip T., Goridis C., Lenoir G. M., Tursz T. (1987) Neuroectoderm-associated antigens on Ewing's sarcoma cell lines. Cancer Res. 47, 183–187 [PubMed] [Google Scholar]
- 29. Noguera R., Triche T. J., Navarro S., Tsokos M., Llombart-Bosch A. (1992) Dynamic model of differentiation in Ewing's sarcoma cells. Comparative analysis of morphologic, immunocytochemical, and oncogene expression parameters. Lab. Invest. 66, 143–151 [PubMed] [Google Scholar]
- 30. Staege M. S., Hutter C., Neumann I., Foja S., Hattenhorst U. E., Hansen G., Afar D., Burdach S. E. (2004) DNA microarrays reveal relationship of Ewing family tumors to both endothelial and fetal neural crest-derived cells and define novel targets. Cancer Res. 64, 8213–8221 [DOI] [PubMed] [Google Scholar]
- 31. Greer Y. E., Rubin J. S. (2011) Casein kinase 1δ functions at the centrosome to mediate Wnt-3a-dependent neurite outgrowth. J. Cell Biol. 192, 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stallings-Mann M., Jamieson L., Regala R. P., Weems C., Murray N. R., Fields A. P. (2006) A novel small-molecule inhibitor of protein kinase Ciota blocks transformed growth of non-small-cell lung cancer cells. Cancer Res. 66, 1767–1774 [DOI] [PubMed] [Google Scholar]
- 33. Frederick M. J., VanMeter A. J., Gadhikar M. A., Henderson Y. C., Yao H., Pickering C. C., Williams M. D., El-Naggar A. K., Sandulache V., Tarco E., Myers J. N., Clayman G. L., Liotta L. A., Petricoin E. F., 3rd, Calvert V. S., Fodale V., Wang J., Weber R. S. (2011) Phosphoproteomic analysis of signaling pathways in head and neck squamous cell carcinoma patient samples. Am. J. Pathol. 178, 548–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirai T., Chida K. (2003) Protein kinase Cζ (PKCζ). Activation mechanisms and cellular functions. J. Biochem. 133, 1–7 [DOI] [PubMed] [Google Scholar]
- 35. Kikuchi K., Niikura Y., Kitagawa K., Kikuchi A. (2010) Dishevelled, a Wnt signalling component, is involved in mitotic progression in cooperation with Plk1. EMBO J. 29, 3470–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee K. H., Johmura Y., Yu L. R., Park J. E., Gao Y., Bang J. K., Zhou M., Veenstra T. D., Kim B. Y., Lee K. S. (2012) Identification of a novel Wnt5a-CK1e-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J. 31, 3104–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Witte F., Bernatik O., Kirchner K., Masek J., Mahl A., Krejci P., Mundlos S., Schambony A., Bryja V., Stricker S. (2010) Negative regulation of Wnt signaling mediated by CK1-phosphorylated Dishevelled via Ror2. FASEB J. 24, 2417–2426 [DOI] [PubMed] [Google Scholar]
- 38. Yanfeng W. A., Berhane H., Mola M., Singh J., Jenny A., Mlodzik M. (2011) Functional dissection of phosphorylation of Disheveled in Drosophila. Dev. Biol. 360, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Narimatsu M., Bose R., Pye M., Zhang L., Miller B., Ching P., Sakuma R., Luga V., Roncari L., Attisano L., Wrana J. L. (2009) Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell 137, 295–307 [DOI] [PubMed] [Google Scholar]
- 40. Bryan B., Cai Y., Wrighton K., Wu G., Feng X. H., Liu M. (2005) Ubiquitination of RhoA by Smurf1 promotes neurite outgrowth. FEBS Lett. 579, 1015–1019 [DOI] [PubMed] [Google Scholar]
- 41. Schwamborn J. C., Müller M., Becker A. H., Püschel A. W. (2007) Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J. 26, 1410–1422 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42. Mori D., Yamada M., Mimori-Kiyosue Y., Shirai Y., Suzuki A., Ohno S., Saya H., Wynshaw-Boris A., Hirotsune S. (2009) An essential role of the aPKC-Aurora A-NDEL1 pathway in neurite elongation by modulation of microtubule dynamics. Nat. Cell Biol. 11, 1057–1068 [DOI] [PubMed] [Google Scholar]
- 43. González-Sancho J. M., Greer Y. E., Abrahams C. L., Takigawa Y., Baljinnyam B., Lee K. H., Lee K. S., Rubin J. S., Brown A. M. (2013) Functional consequences of Wnt-induced Dishevelled 2 phosphorylation in canonical and noncanonical Wnt signaling. J. Biol. Chem. 288, 9428–9437 [DOI] [PMC free article] [PubMed] [Google Scholar]