Background: Cell signaling induced by pathogenic IgG in the human autoimmune skin disease pemphigus vulgaris contributes to loss of adhesion.
Results: PV IgG activate EGFR downstream of p38 and inhibiting EGFR blocks antibody induced desmoglein endocytosis, keratin retraction, and blistering.
Conclusion: PV IgG-mediated transactivation of EGFR contributes mechanistically to loss of keratinocyte cell-cell adhesion.
Significance: This study provides the mechanistic rationale for using EGFR inhibitors in PV.
Keywords: Adhesion, Desmosome, Epidermal Growth Factor Receptor (EGFR), p38 MAPK, Signal Transduction
Abstract
The pemphigus family of autoimmune bullous disorders is characterized by autoantibody binding to desmoglein 1 and/or 3 (dsg1/dsg3). In this study we show that EGF receptor (EGFR) is activated following pemphigus vulgaris (PV) IgG treatment of primary human keratinocytes and that EGFR activation is downstream of p38 mitogen-activated protein kinase (p38). Inhibition of EGFR blocked PV IgG-triggered dsg3 endocytosis, keratin intermediate filament retraction, and loss of cell-cell adhesion in vitro. Significantly, inhibiting EGFR prevented PV IgG-induced blister formation in the passive transfer mouse model of pemphigus. These data demonstrate cross-talk between dsg3 and EGFR, that this cross-talk is regulated by p38, and that EGFR is a potential therapeutic target for pemphigus. Small-molecule inhibitors and monoclonal antibodies directed against EGFR are currently used to treat several types of solid tumors. This study provides the experimental rationale for investigating the use of EGFR inhibitors in pemphigus.
Introduction
Pemphigus is a group of human autoimmune blistering diseases in which autoantibodies bind to desmosomal cadherins in the skin and induce keratinocyte cell-cell detachment (e.g. acantholysis). Pathogenic IgG binds to the ectodomain of desmoglein (dsg)2 1 in pemphigus foliaceus (PF) (1, 2) and/or dsg3 in pemphigus vulgaris (PV) (3, 4). Accumulating evidence suggests an essential role for intracellular signaling in the mechanism by which pemphigus IgG induces acantholysis. A number of intracellular signaling events have been observed when keratinocytes are treated with pemphigus IgG (5–8). PV as well as PF autoantibodies activate p38 MAPK in keratinocyte cultures and in both the PV and PF passive transfer mouse models of pemphigus (9–11). Pretreatment with p38 inhibitors blocked PV and PF IgG-induced actin reorganization, keratin intermediate filament retraction (11, 12), dsg endocytosis (13), and blistering in vivo (9, 10), suggesting a pivotal role for p38 signaling in the mechanism of acantholysis. Unfortunately, clinical trials of p38 inhibitors for pemphigus have been hindered by the hepatotoxicity of these compounds3.
Epidermal growth factor receptor (EGFR) is a prominent signaling complex whose repertoire of functions is increased by cross-communication with other signaling pathways (15). In addition to its ligand-induced activity, EGFR can be transactivated by various signaling pathways in a ligand-independent manner (16–18). Activation of EGFR can lead to a variety of biological outcomes including cell growth, migration, and suppression of apoptosis. In the skin, EGFR signaling plays a major role in regulating keratinocyte proliferation, and deregulation of EGFR signaling has been observed in skin disorders such as psoriasis, squamous cell carcinoma, and melanoma (19).
EGFR has been implicated in modulating cell adhesion junctions, including desmosomes (20, 21). EGFR-regulated adhesion plays an important role in modulating epithelial adhesion and motility. For example, EGFR phosphorylation of β-catenin regulates adherens junction assembly/disassembly (22). An increasing number of reports implicate a role for desmoglein-EGFR interactions. Desmoglein 1 has been shown to suppress EGFR-Erk 1/2 (extracellular signal-regulated kinase 1/2) signaling in skin (23). In keratinocytes, EGF-EGFR-mediated plakoglobin phosphorylation has been shown to decrease the association of desmoplakin with the desmosome, thereby reducing cell-cell adhesion (21, 24). EGFR has been shown to regulate dsg2 endocytosis in a squamous cell carcinoma cell line. EGFR promoted dsg2 depletion from the membrane (25). Conversely, EGFR inhibition increased membrane levels of dsg2 (26) and cell adhesion in an oral squamous cell carcinoma cell line (27). Collectively, these studies predict that EGFR inhibition in normal human keratinocytes might similarly stabilize desmosome assembly and cell-cell adhesion. whereas EGFR activation might promote desmosome disassembly and reduce cell-cell adhesion.
Pemphigus IgG-induced EGFR activation has been suggested to contribute to acantholysis by induction of apoptosis. EGFR inhibition blocked the observed induction of apoptosis by PV IgG in the immortalized HaCaT keratinocyte line and A431 squamous cell carcinoma cultures (28). However, time course studies in normal human keratinocyte cultures and in mice suggest that apoptosis is not required for blistering (11, 29). Additionally, more recent studies did not detect EGFR activation in pemphigus IgG-treated keratinocyte cultures (30). Another group observed EGFR activation in keratinocytes treated with pemphigus IgG (8). However, their studies suggest that p38 activation occurs downstream of, and at time points subsequent to, EGFR activation. The EGFR inhibitor erlotinib has been used to block blistering in the passive transfer mouse model. However, the authors of this study interpreted their results to implicate a role for apoptosis in the mechanism of acantholysis (31).
Because of the role of EGFR in dsg trafficking and the seemingly conflicting reports on the role of EGFR in pemphigus acantholysis, we explored the potential for EGFR to contribute to the mechanism by which pemphigus IgG induce loss of adhesion in keratinocytes. In this study we not only attempt to further elucidate the mechanism of PV IgG-induced acantholysis but to clarify the involvement of EGFR in this cascade. We show that the basal activity of the EGFR contributes to desmosome stability; that EGFR is activated by PV IgG; that EGFR activation is upstream of PV IgG-induced keratin intermediate filament retraction and dsg3 endocytosis and downstream of PV IgG-induced p38 activation; and that EGFR inhibitors block PV IgG-mediated keratin intermediate filament retraction, dsg internalization, and blistering in vivo using the passive transfer mouse model of pemphigus. Collectively, these observations identify EGFR as a potential target for pemphigus disease management.
EXPERIMENTAL PROCEDURES
Materials
Rabbit polyclonal anti-dsg3 antibodies were from Serotec (Oxford, UK). Mouse monoclonal E-cadherin and cytokeratin AE5/8 antibodies were from BD Biosciences. Phospho-EGFR (pY845) antibodies and human recombinant EGF were from Invitrogen. EEA1, total EGFR receptor antibodies, and rabbit polyclonal phospho-ERK antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). Rabbit polyclonal ERK-1 and ERK-2 antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse and rabbit anti-sheep horseradish peroxidase-conjugated secondary antibodies were from GE Healthcare. Fluorescent secondary antibodies were from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). The p38 inhibitor SB202190 and the EGFR inhibitors AG1478; CL387,785; PD15330; BPIQ-II; erlotinib; and gefitinib were from Calbiochem (La Jolla, CA). Normal primary human keratinocytes, Epilife keratinocyte growth medium, human keratinocyte growth supplement, and antibiotics were from Invitrogen.
IgG Preparation
AK 23 antibodies were generated in hybridoma cells as described previously (32). PV IgG was prepared from the serum of a single patient with confirmed mucocutaneous PV (indirect immunofluorescence titer 1:640). Both PV IgG and AK23 were prepared by ammonium sulfate precipitation followed by affinity chromatography on protein G (HiTrap, Pharmacia, Piscataway, NJ) as described previously (12). IgG fractions were dialyzed against PBS and sterile-filtered. Purity was confirmed by SDS-PAGE, and activity was assayed by indirect immunofluorescence and ELISA. NH IgG (no activity by indirect immunofluorescence) was prepared in parallel from normal human sera.
Tissue Culture
Normal primary human keratinocytes were passaged and expanded as described (12). Third-passage keratinocytes were grown to 80–90% confluence. Keratinocyte medium was supplemented with CaCl2 to a final concentration of 0.5 mm 3 h prior to treating cells. For some experiments, keratinocytes were preincubated with the p38 inhibitor SB202190 or the EGFR inhibitors AG1478; CL387,785; PD15330; BPIQ-II; erlotinib; gefitinib; or DMSO vehicle control at 37 °C as described in the figure legends. Cells were then treated with PBS, NH IgG (2 mg/ml), PV IgG (2 mg/ml), or AK23 (2 mg/ml) for the indicated times and harvested.
Confocal Microscopy
Confocal microscopy was performed as described previously (11–13). Keratinocytes were grown on glass coverslips to 90% confluence, treated, fixed in 3.7% paraformaldehyde at 4 °C for 10 min, and then washed three times in 2% BSA in PBS for 10 min. Cells were then permeabilized using 0.5% Triton X-100 for 10 min at 4 °C followed by three 5-min washes using 2% BSA in PBS. After blocking the cells in 5% goat serum in PBS for 1 h at room temperature, cells were probed with primary antibodies overnight at 4 °C as described in the figure legends. The following morning, cells were washed using 2% BSA in PBS for three 10-min washes. Cells were then incubated with fluorescent secondary antibodies at room temperature for 1 h as described in the figure legends. Coverslips were mounted on glass slides, and images were analyzed using a Leica SP2 AOBS confocal microscope using excitation wavelengths of 488, 512, and 561 nm. Images were viewed using a ×63 objective with a numerical aperture of 1.4 at ×2 magnification. Double- and triple-labeled samples were checked for bleed-through by turning off the various lasers and assaying for the absence of an image. Independent representative images were assembled using Adobe Photoshop. Brightness and contrast were adjusted uniformly.
shRNA Transfection
Primary keratinocytes were grown to 60–80% confluence and transfected with shGFP (as control shRNA) or with shRNA against EGFR (clone identification TRCN0000121204, ThermoScientific, Rockford, IL) in medium containing 10 μm polybrene and incubated at 37 °C according to the recommendations of the manufacturer. After 4–6 h, the medium was aspirated, and fresh cell culture medium was added, incubated for an additional 3 days, and then utilized for experiments.
Cell Surface Biotinylation
Following treatment, keratinocyte cell surface proteins were labeled using EZ Link Sulfo NHS SS biotin (Pierce) at a concentration of 1 mg/ml at 4 °C on a rocking platform. After 1 h, the biotin was quenched using 500 mm ammonium chloride, and cells were lysed in buffer A (50 mm NaCl, 10 mm PIPES, 3 mm MgCl2, 1% Triton X-100) using probe sonication. Lysates were clarified by centrifugation at 14,000 rpm for 10 min at 4 °C. Clarified lysates were passed over NeutraAvidin-agarose beads (Pierce) and incubated at room temperature for 1 h in an end-over-end mixer. Following three washes with buffer A, cell surface proteins were eluted using 1× Laemmli buffer with 50 mm DTT. Western blot analysis was performed using anti-dsg3 and anti-E-cadherin antibodies.
Preparation of Detergent-soluble and Detergent-insoluble Fractions
Monolayer cells grown to confluence were extracted in cell lysis buffer (1% Nonidet P-40, 150 mm NaCl, 50 mm Tris-HCl (pH 7.4), 1 mm EDTA, 10 μm E64, 100 μm leupeptin, 10 μm pepstatin, and 1 mm phenylmethylsulfonyl fluoride) at 4 °C for 1 h with rotating and then centrifuged at 13,700 × g for 15 min at 4 °C. The supernatants were collected as detergent-soluble fractions. The pellets were washed twice with PBS, resuspended by incubation in urea lysis buffer (8 m urea, 4% CHAPS, 10 mm Tris-HCl (pH 7.4)) for 1 h at 4 °C, and then centrifuged as above. The supernatant was used as the detergent-insoluble fraction. Samples were loaded equally on and separated by SDS-PAGE. Immunoblotting was performed according to established protocols and developed by ECL reaction (Amersham Biosciences).
Dispase Assay
Keratinocytes were grown in 12-well plates until confluent. Cells were washed, and medium containing 0.5 mm CaCl2 was added to the cells with or without 10 μm AG1478. Two hours later, 2 mg/ml PV IgG was added to the appropriate wells. Alternatively, cells were treated with 100 ng/ml EGF. After 24 h, cells were washed and incubated in dispase II (2 units/ml, Roche) for 30 min at 37 °C. To subject the cell sheet to shear force, the floating cell sheet was pipetted up and down three times with a 1-ml pipette. The fragments were counted under the light microscope at ×2.5 magnification.
Passive Transfer Mouse Model
Breeding pairs of C57BL-6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at the University of North Carolina Division of Laboratory Animal Medicine Facility in accordance with International Animal Care and Use Committee protocols. Neonatal mice (24- to 36-h-old with body weights between 1.4 and 1.6 g) were used for passive transfer experiments. Neonates were injected intradermally with a sterile solution of either control IgG or PV IgG as described (9–11). For in vivo inhibitor studies, mice were pretreated with 4 μg of AG1478 in 50 μl intradermally 2 h prior to injection with PV IgG. After clinical examination, animals were sacrificed, and skin specimens were obtained for routine histological examination (hematoxylin and eosin staining) using light microscopy and direct IF assays to detect keratinocyte cell surface-bound human IgG. No increased mortality was observed in the inhibitor versus control mice. Statistical significance was determined using the non-parametric chi square test.
RESULTS AND DISCUSSION
Pemphigus Autoantibody-induced Activation of the EGFR Is p38-dependent
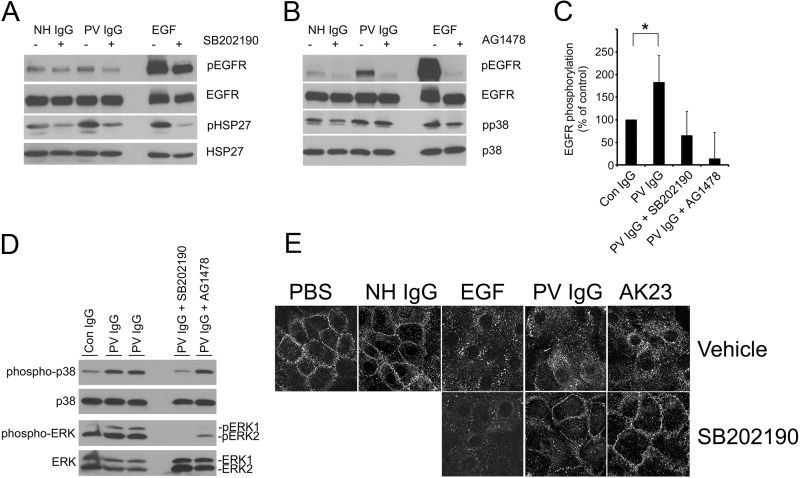
To investigate the role of the EGFR in pemphigus, primary human keratinocytes were grown to confluency and treated for 30 min with either control NH IgG or PV IgG. EGF was used as a positive control for EGFR activation. PV IgG stimulated EGFR phosphorylation (1.8 ± 0.6-fold compared with the control, n = 4, p = 0.031), although not to the extent of EGF (Fig. 1, A–C). One of the mediators of EGFR signaling is ERK, whose phosphorylation occurs downstream of EGFR activation. Thus, we next investigated whether PV IgG induced ERK phosphorylation. Consistent with the activation of EGFR by PV IgG, PV IgG induced phosphorylation of ERK (Fig. 1D), and PV IgG-induced ERK phosphorylation was inhibited with the EGFR inhibitor AG1478. Activated EGFRs are rapidly endocytosed (33). We utilized this property of the EGFR as a third assay for EGFR activation (Fig. 1E). Keratinocytes were grown to confluency and treated for 4 h with either control NH IgG or PV IgG. Confocal immunofluorescent staining using EGFR-specific antibodies demonstrated PV IgG-induced endocytosis of the EGFR. EGF-stimulated EGFR endocytosis was used as a positive control.
FIGURE 1.
p38 is upstream of EGFR activation by PV IgG. A and B, immunoblot analysis of keratinocyte lysates probed with antibodies to phospho-EGFR (pEGFR), total EGFR (EGFR), phospho-HSP27 (pHSP27), total HSP27 (HSP27), phospho-p38 (pp38), and total p38 (p38). Unlike NH IgG, PV IgG stimulated EGFR, p38, and HSP27 phosphorylation. The p38 inhibitor SB202190 blocked PV IgG-induced phosphorylation of EGFR (A) as well as HSP27, previously identified to be downstream of PV IgG-induced p38 activity. In contrast, the EGFR inhibitor AG1478 blocked PV IgG-induced EGFR phosphorylation but not PV IgG-induced p38 phosphorylation (B), indicating that p38 acts upstream of EGFR in PV IgG-treated keratinocytes. C, quantitation of PV IgG-stimulated EGFR phosphorylation in the presence and absence of SB202190 and AG1478. PV IgG stimulated EGFR phosphorylation 1.8 ± 0.6-fold compared with the control (Con). *, p = 0.031. Primary human keratinocytes were grown to confluence, serum-starved overnight, preincubated for 3 h with 0.5 mm calcium and for 1 h in vehicle (dimethyl sulfoxide), the EGFR inhibitor AG1478 (10 μm) (A), or the p38 inhibitor SB202190 (10 μm) (B) and then treated with NH IgG or PV IgG for 30 min or EGF (100 ng/ml, positive control) for 5 min. Cell lysates were prepared, and immunoblot analysis was performed. In addition to EGFR, each blot was also probed for HSP27 (A) or p38 (B) as loading controls. Results shown are representative of three independent experiments. D, PV IgG induced ERK phosphorylation. Keratinocyte monolayers were treated with normal human control IgG (Con IgG, 2 mg/ml) or PV IgG (2 mg/ml) for 30 min. Alternatively, keratinocytes were pretreated for 1 h with either SB202190 (10 μm) or AG1478 (10 μm) and then treated with PV IgG for 30 min. Lysates were prepared, separated by SDS-PAGE, and probed by immunoblot analysis with antibodies to phosphorylated p38 (phospho-p38), total p38 (p38), phosphorylated ERK (phospho-ERK), and ERK1 and ERK2. E, confocal immunofluorescent images of EGFR-stained keratinocytes. Keratinocytes were grown as above on coverslips; treated with PBS, EGF (100 ng/ml), NH IgG (2 mg/ml), PV IgG (2 mg/ml), or AK23 (2 mg/ml) for 4 h, and then fixed and stained with Cy-2 (1:100)-conjugated anti-EGFR secondary antibodies. In contrast to buffer (PBS)- and NH IgG-treated controls, EGF, PV IgG and AK23 induced EGFR endocytosis. SB202190 blocked both PV IgG- and AK23-induced EGFR endocytosis but not EGF-triggered EGFR endocytosis. Results shown are representative of at least three independent experiments.
Activation of EGFR can be p38-dependent. For example, in several human cell lines, including colon carcinoma cells, pancreatic carcinoma cells, and HeLa cells, the EGFR has been shown to be activated in a p38-dependent manner (34–36). In keratinocytes, disruption of lipid rafts by cholesterol depletion stimulated EGFR phosphorylation and internalization, which was also p38-dependent (37). Because of the demonstrated role for p38 activation in pemphigus, we next investigated the relationship between PV IgG-triggered EGFR activation and p38 using the p38 inhibitor SB202190 and the EGFR inhibitor AG1478. PV IgG-induced phosphorylation of the small heat shock protein (HSP) 27 is p38-dependent (12). As expected, inhibition of p38 blocked PV IgG-induced HSP27 phosphorylation (Fig. 1A). Significantly, p38 inhibition blocked PV IgG-induced EGFR phosphorylation (Fig. 1A), PV IgG-induced EGFR endocytosis (E), and PV IgG-induced ERK phosphorylation (D). Although the EGFR inhibitor AG1478 blocked PV IgG-induced ERK phosphorylation, it did not inhibit PV IgG-induced p38 phosphorylation, consistent with p38 being upstream of EGFR and ERK (Fig. 1D). These observations indicate that PV IgG-mediated EGFR phosphorylation and internalization occur downstream of and are dependent on p38 activity. Interestingly, p38 inhibition failed to block EGF-induced EGFR endocytosis (Fig. 1E), indicating different mechanisms by which PV IgG and EGF induce EGFR endocytosis. Consistent with the ordering of p38 upstream of EGFR activation, the EGFR inhibitor AG1478 blocked PV IgG-induced EGFR phosphorylation but not p38 phosphorylation (Fig. 1B). It has been suggested that pemphigus patient serum may also contain non-dsg-targeted autoantibodies that activate “non-desmoglein” signaling (8). To confirm that EGFR activation was a consequence of autoantibody binding to dsg3, we utilized the pathogenic monoclonal antibody AK23, which binds specifically within the EC1 extracellular domain of dsg3. Similar to PV IgG, AK23 stimulated EGFR internalization, and AK23-stimulated EGFR internalization was blocked with the p38 inhibitor SB202190, confirming that EGFR activation directly follows autoantibody binding to dsg3 (Fig. 1E).
EGFR Inhibition Blocks Pemphigus Autoantibody-triggered Keratin Intermediate Filament Retraction and Desmoglein Internalization
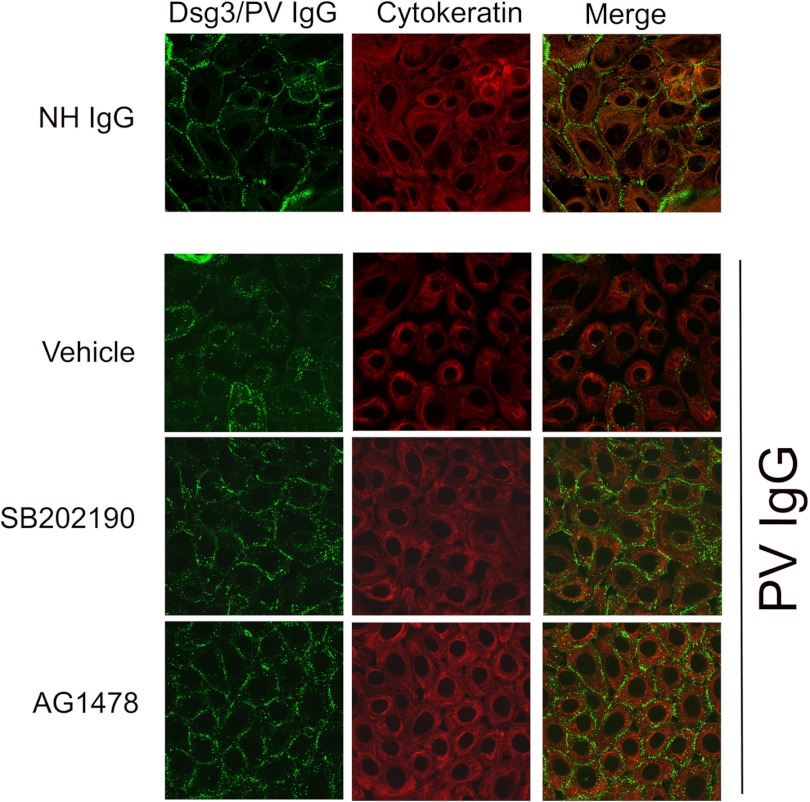
Following pemphigus autoantibody binding to dsg3, the keratin intermediate filament network retracts from the cell membrane. Significantly, p38 inhibition blocks PV IgG-induced keratin intermediate filament retraction (11–13). We next examined the relationship between PV IgG-induced EGFR activation and keratin intermediate filament retraction utilizing the EGFR inhibitor AG1478 (Fig. 2). Consistent with results published previously, PV IgG causes the marked redistribution and retraction of keratin in keratinocytes pretreated with vehicle control, whereas p38 inhibition prevented PV IgG-induced keratin retraction. Significantly, EGFR inhibition also blocked PV IgG-induced keratin retraction.
FIGURE 2.
The EGFR inhibitor blocks PV IgG-induced keratin intermediate filament retraction. Confocal immunofluorescent images of keratinocytes treated with NH or PV IgG ± the p38 inhibitor SB202190 or the EGFR inhibitor AG1478. Primary human keratinocytes were grown as described above; pretreated for 1 h with either vehicle (dimethyl sulfoxide), SB202190 (10 μm), or AG1478 (10 μm) as indicated; and then treated with NH IgG or PV IgG for 4 h. To study the effects on dsg3 and cytokeratin, Cy2-conjugated (1:100) and Cy3-conjugated (1:75) secondary antibodies were used to stain for PV IgG/Dsg3 (green) and keratin 5 (red), respectively. As expected, when compared with NH IgG (top panel), PV IgG caused dsg3 endocytosis and keratin intermediate filament retraction. In contrast, both SB202190 and AG1478 blocked PV IgG-induced dsg3 endocytosis and keratin retraction, indicating that both p38 and EGFR activation occur upstream of these acantholysis-related events.
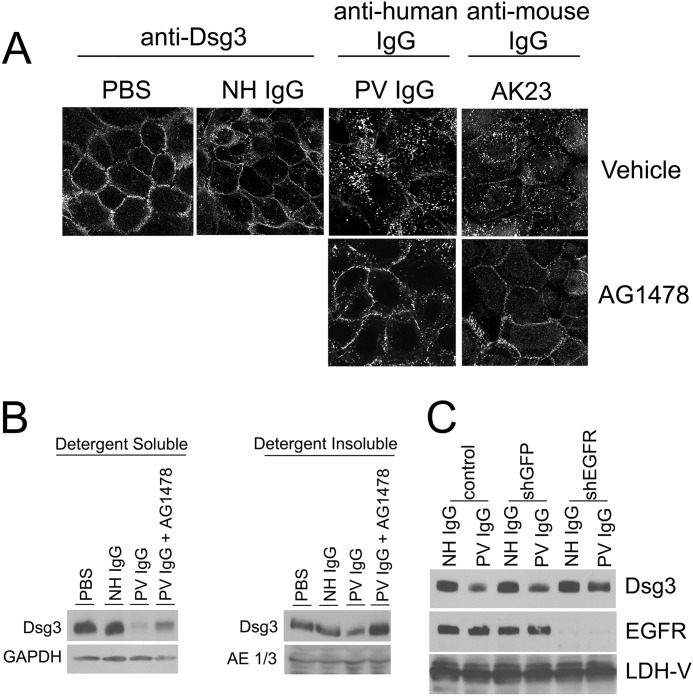
PV IgG stimulates the internalization of dsg3 into endosomes and its subsequent degradation (13, 38–41). PV IgG-induced keratin intermediate filament retraction and dsg3 endocytosis may be mechanistically linked events that lead to the loss of cell-cell adhesion (13). Confocal immunofluorescence and cellular fractionation studies were utilized to investigate the relationship of EGFR to dsg3 endocytosis. In PBS- or NH IgG-treated keratinocytes, dsg3 is localized primarily at the plasma membrane (Fig. 3A). Consistent with previous studies, both PV IgG and AK23 caused the internalization of dsg3 within 4 h. Pretreating keratinocytes with the EGFR inhibitor AG1478 blocked the ability of both PV IgG and AK23 to stimulate the endocytosis of dsg3.
FIGURE 3.
The EGFR inhibitor AG1478 blocks PV IgG- and AK23-triggered dsg3 internalization. A, no dsg3 endocytosis was observed in PBS or NH IgG controls. Both PV IgG- and AK23-stimulated internalization was blocked by AG1478. Keratinocytes were preincubated with AG1478 or vehicle (as described in Fig. 2) and treated with PBS, NH IgG, PV IgG, or AK23, as indicated, for 4 h. B, the EGFR inhibitor AG1478 antagonized PV IgG-induced dsg3 depletion from the detergent-soluble and detergent-insoluble cell fractions. Primary human keratinocytes were grown as described above; preincubated with either vehicle control (dimethyl sulfoxide) or AG1478 (10 μm); and then treated with PBS, NH IgG, or PV IgG. After 18 h, detergent-soluble and detergent-insoluble lysates were prepared, and Western blot analysis was performed. In detergent-soluble fractions, PV IgG treatment caused a marked depletion of dsg3 that was blocked by AG1478. Similarly, PV IgG-induced dsg3 depletion from the detergent-insoluble fraction was blocked by AG1478. C, down-regulating the EGFR prevents dsg3 depletion by PV IgG. Primary keratinocytes were transfected with shRNA against GFP or EGFR. After 3 days, cells were treated with PBS, NH IgG, or PV IgG. After 4 h, cell lysates were prepared, and Western blot analysis was performed with antibodies to dsg3, EGFR, or lactate dehydrogenase V (LDH-V) as a loading control. Results shown are representative of three independent experiments.
PV IgG-induced depletion of dsg3 from the cell surface likely contributes to the loss of cell adhesion seen in pemphigus. Because EGFR inhibition blocked PV IgG-mediated dsg3 endocytosis, we examined the relationship of EGFR inhibition to the depletion of dsg3 from the membrane. Consistent with reports published previously, pemphigus autoantibodies caused the depletion of dsg3 from both the detergent-soluble and detergent-insoluble pools (Fig. 3B). In agreement with the above immunofluorescence studies, EGFR inhibition similarly blocked pemphigus autoantibody-triggered loss of dsg3 from both the detergent-soluble and detergent-insoluble cell fractions (Fig. 3B). To further substantiate the finding that inhibiting EGFR impairs PV IgG-mediated dsg3 depletion, we down-regulated the EGFR using shRNA. Similar to the use of EGFR inhibitors, shRNA knockdown of the EGFR reduces the effect of PV IgG on dsg3 depletion (Fig. 3C).
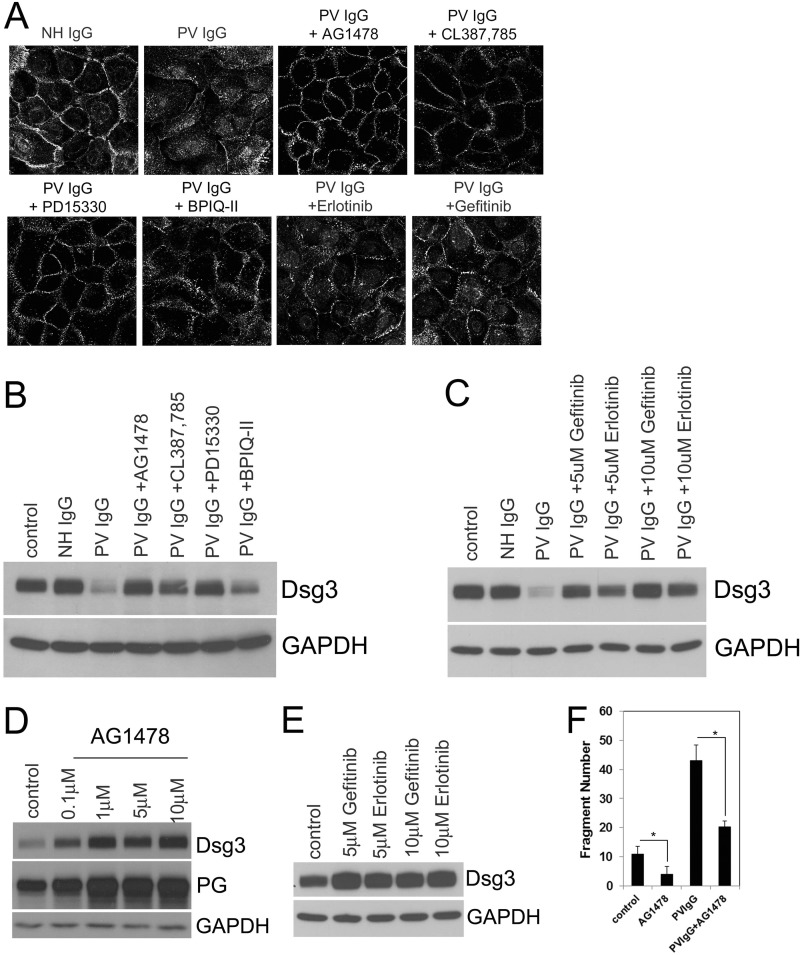
To confirm the effect of EGFR inhibition on PV IgG-mediated dsg3 depletion, we tested additional EGFR inhibitors, including those approved for use in patients. As seen in the confocal images of Fig. 4A, the EGFR inhibitors AG1478; CL387,785; PD15330; BPIQ-II; erlotinib; and gefitinib prevent PV IgG-induced dsg3 redistribution from the membrane to the cytosol. When analyzed by immunoblot, the EGFR inhibitors similarly blocked PV IgG-induced membrane depletion of dsg3 (Fig. 4, B and C).
FIGURE 4.
EGFR inhibitors block dsg3 depletion. A, keratinocytes grown on coverslips were serum-starved overnight and preincubated with 0.5 mm calcium and vehicle ± 10 μm AG1478; CL387,785; PD15330; BPIQ-II; erlotinib; or gefitinib; treated as indicated for 4 h; and then fixed and stained with Cy-2-conjugated (1:100) secondary antibodies against dsg3 in NH IgG-treated cells or Cy-2-conjugated anti-human IgG for PV IgG-treated cells. In contrast to NH IgG-treated controls, PV IgG caused the endocytosis of dsg3. EGFR inhibitors blocked PV IgG-induced dsg3 internalization. B and C, EGFR inhibitors blocked PV IgG-induced dsg3 depletion. Keratinocytes were grown and preincubated with 0.5 mm calcium and the EGFR inhibitor as indicated. NH IgG or PV IgG were added 2 h later. After 18 h, cell lysates were prepared and subjected to immunoblot analysis with antibodies to dsg3 and GAPDH (loading control). D and E, EGFR inhibitors increase basal dsg3 levels. Keratinocytes were cultured as above and incubated in the presence of AG1478, gefitinib, or erlotinib at the indicated concentrations for 18 h. Immunoblot analysis of cell lysates with antibodies to dsg3, plakoglobin (PG), and GAPDH show increased levels of dsg3 and PG with EGFR inhibition. F, EGFR inhibition stabilizes cell-cell adhesion and antagonizes the acantholytic effects of PV IgG in vitro. Cells were grown to confluence and pretreated with 0.5 mm calcium and 10 μm AG1478. Two hours later, 2 mg/ml PVIgG was added. 24 h later, a dispase assay was performed. Data are expressed as the number of fragments/well + S.D. n = 3. *, p < 0.05.
Inhibiting Basal EGFR Activity Stabilizes Desmosome Adhesion
Inhibition of basal EGFR activity has been demonstrated to increase cell adhesion in an oral squamous cell carcinoma cell line (27), suggesting a potential for basal EGFR activity to contribute to desmosomal adhesion in primary keratinocytes. Therefore, we next investigated the potential for inhibition of basal EGFR activity to increase desmosome adhesion and contribute to the mechanism by which EGFR inhibitors make desmosomes more resistant to PV IgG-mediated disruption. Because EGFR inhibition prevented PV IgG-induced loss of cell surface dsg3, we next tested whether EGFR inhibition altered the amount of membrane-associated dsg3 under basal conditions. A dose-dependent increase in cell membrane-associated dsg3 was observed when primary human keratinocytes were incubated with the EGFR inhibitor AG1478 (Fig. 4D). Similarly, two additional EGFR inhibitors, gefitinib and erlotinib, also increased the amount of cell membrane-associated dsg3 in a dose-dependent manner (Fig. 4E). To link the increase of dsg3 protein to enhanced desmosome formation and stability, we also looked at plakoglobin expression, an essential component of the desmosomal cadherin complex in AG1478-treated cells. As shown in Fig. 4D, plakoglobin protein levels also increase with escalating concentrations of AG1478. The functional consequence of up-regulated dsg3 and plakoglobin levels should be stronger cell-cell adhesion. Therefore, we next utilized the dispase assay to investigate the ability of EGFR inhibitors to functionally stabilize cell adhesion. Consistent with the hypothesis that EGFR inhibitors stabilize cell-cell adhesion, cells treated with AG1478 displayed fewer fragments than control cells. Furthermore, pretreatment with AG1478 significantly decreased the number of fragments induced by PV IgG (Fig. 4F).
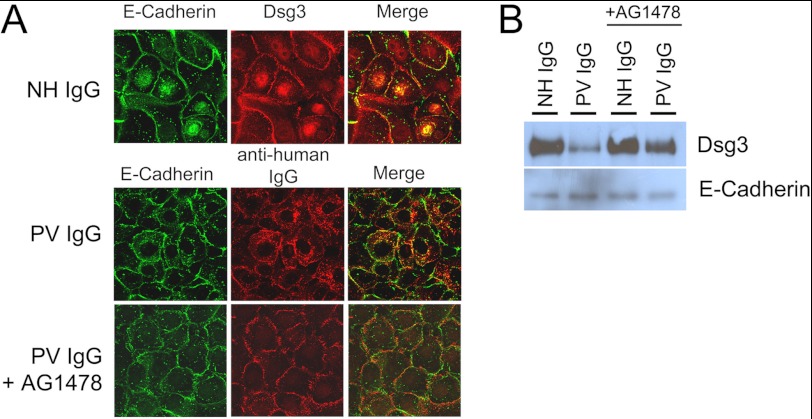
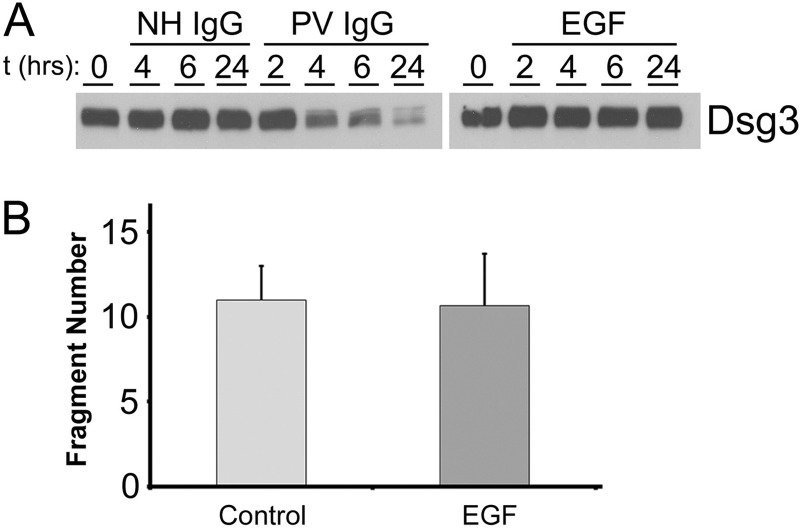
The specificity of the effect of EGFR inhibition for dsg3 was demonstrated in confocal immunofluorescence studies, showing that PV IgG caused EGFR-dependent dsg3 endocytosis but had no effect on the localization of the adherens junction adhesion protein E-cadherin (Fig. 5A). As an additional means to examine this effect, we next employed cell surface biotinylation studies. Consistent with the above immunofluorescence results, the EGFR inhibitor AG1478 partially blocked the PV IgG-induced loss of dsg3 from the cell surface (Fig. 5B). In contrast to PV IgG, no effect on dsg3 depletion was observed when keratinocytes were incubated with EGF (Fig. 6A), indicating that dsg3 depletion is downstream of PV IgG-mediated EGFR signaling but not downstream of EGF-mediated EGFR activation. Furthermore, EGF did not induce loss of cell-cell adhesion because no additional fragmentation was observed in the dispase assay when keratinocyte monolayers were incubated with EGF relative to control monolayers (Fig. 6B).
FIGURE 5.
The effect of PV IgG and EGFR inhibition is dsg3-specific. PV IgG-induced cell surface depletion of dsg3 was blocked by the EGFR inhibitor AG1478. In contrast, PV IgG did not induce E-cadherin depletion. A, keratinocytes were treated with NH IgG, PV IgG, or PV IgG and the EGFR inhibitor AG1478 and then examined by confocal immunofluorescent microscopy. PV IgG stimulated the endocytosis of dsg3 (Cy2, 1:100) but not E-cadherin (Cy3, 1:75). PV IgG-stimulated dsg3 endocytosis was blocked with AG1478. B, cell surface biotinylation studies. Keratinocytes were treated with NH IgG or PV IgG ± AG1478 and labeled with a membrane-impermeable biotin cell surface label. Biotin-labeled proteins were purified from lysates on NeutraAvidin-agarose beads and subjected to immunoblot analysis with antibodies to dsg3 or E-cadherin.
FIGURE 6.
In contrast to PV IgG, EGF does not induce dsg3 depletion or loss of cell-cell adhesion. A, keratinocyte monolayers were treated with NH IgG (2 mg/ml), PV IgG (2 mg/ml), or EGF (100 ng/ml) for the indicated times. Lysates were prepared and probed by immunoblot analysis with antibodies to dsg3. B, no additional cell fragments were observed by the dispase assay in EGF-treated versus control keratinocyte monolayers. Cells were grown to confluence and incubated for 24 h in the presence (EGF) or absence (Control) of 100 ng/ml EGF, after which the dispase assay was performed. The data are expressed as number of fragments/well + S.D. (n = 6).
PV IgG Causes the Colocalization of EGFR, dsg3, and Plakoglobin into Endosomes
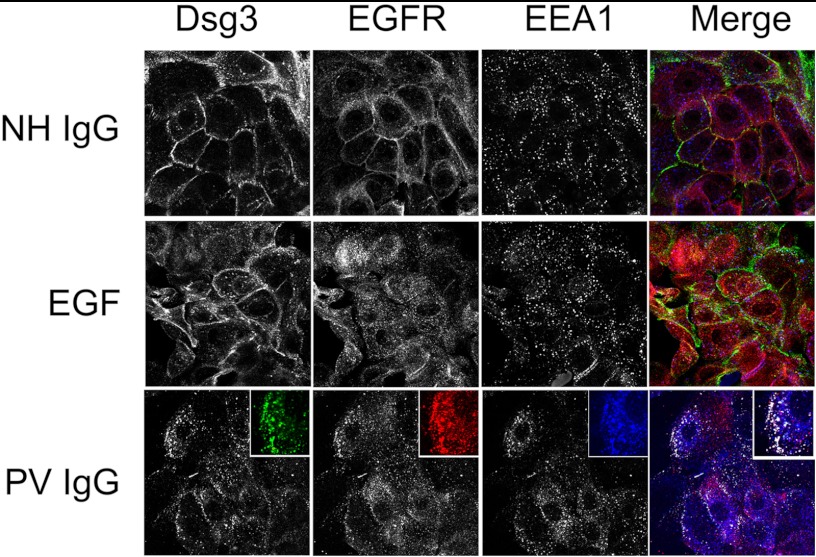
In this study, both EGFR and dsg3 were shown separately to be internalized following PV IgG treatment. Using confocal immunofluorescent microscopy, we then determined whether EGFR and dsg3 colocalized into endosomes in PV IgG-treated keratinocytes (Fig. 7). Control NH IgG failed to induce internalization of either dsg3 or EGFR, whereas EGF treatment caused the internalization and colocalization of EGFR with the early endosome marker EEA1 but did not induce dsg3 internalization. In contrast, in PV IgG-treated keratinocytes, both EGFR and dsg3 partially colocalized with EEA1, indicating that EGFR and dsg3 colocalize into early endosomes.
FIGURE 7.
EGFR and dsg3 colocalize in early endosomes following PV IgG treatment. Confocal images of control NH IgG, EGF, and PV IgG-treated keratinocytes labeled with antibody specific for dsg3 (green), EGFR (red), and the early endosome marker EEA1 (blue). Primary human keratinocytes were treated for 4 h with NH IgG, EGF, or PV IgG and probed for PV IgG (dsg3-Cy2, 1:100), EGFR (Cy3, 1:75), and EEA1 (Cy5, 1:50). In NH IgG-treated cells, both dsg3 and EGFR remained at the cell surface and did not colocalize with EEA1. In EGF-treated cells, EGFR, but not dsg3, was internalized. EGFR and EEA1 appeared to partially colocalize in early endosomes. In PV IgG-treated cells, dsg3 and EGFR undergo endocytosis and partially colocalize with EEA1 in early endosomes.
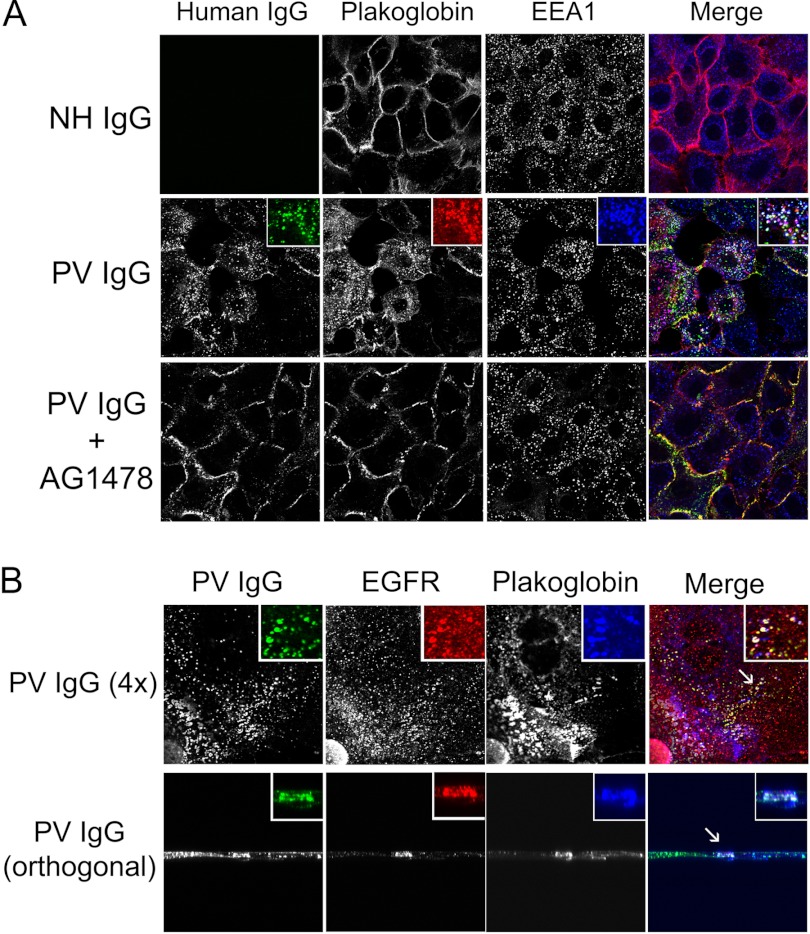
It has been proposed that EGFR can phosphorylate plakoglobin, leading to the destabilization of desmosomes (24). Additionally, pemphigus autoantibody treatment of keratinocytes has been shown to induce the colocalization of plakoglobin and EGFR (41). Therefore, we next investigated the dependence of plakoglobin-EGFR colocalization on EGFR activity. Keratinocytes were treated with NH IgG, PV IgG, or PV IgG and the EGFR inhibitor AG1478, and the distribution of bound PV IgG (to detect dsg3), plakoglobin, EGFR, and EEA1 was examined by confocal immunofluorescent microscopy (Fig. 8). After treatment with PV IgG, but not control NH IgG, colocalization of plakoglobin, EGFR, and PV IgG (dsg3) was detected in internal vesicular structures that stained with the early endosome marker EEA1. PV IgG-induced plakoglobin internalization was blocked in cells treated with the EGFR inhibitor AG1478, indicating that this internalization event was EGFR-dependent.
FIGURE 8.
Plakoglobin colocalizes with dsg3 and EGFR in early endosomes following PV IgG treatment. Primary human keratinocytes were treated for 4 h with NH IgG, PV IgG, or PV IgG and the EGFR inhibitor AG1478 and probed for PV IgG (dsg3-Cy2, 1:100), plakoglobin (Cy3, 1:75), and EEA1 (Cy5, 1:50). In PV IgG-treated cells, dsg3 and plakoglobin partially colocalized in early endosomes (A). In addition, following PV IgG treatment, dsg3, EGFR, and plakoglobin also partially colocalized (B). Conventional (A) and orthogonal (B) views are shown.
The EGFR Inhibitor AG1478 Prevents Blistering in the PV Passive Transfer Mouse Model
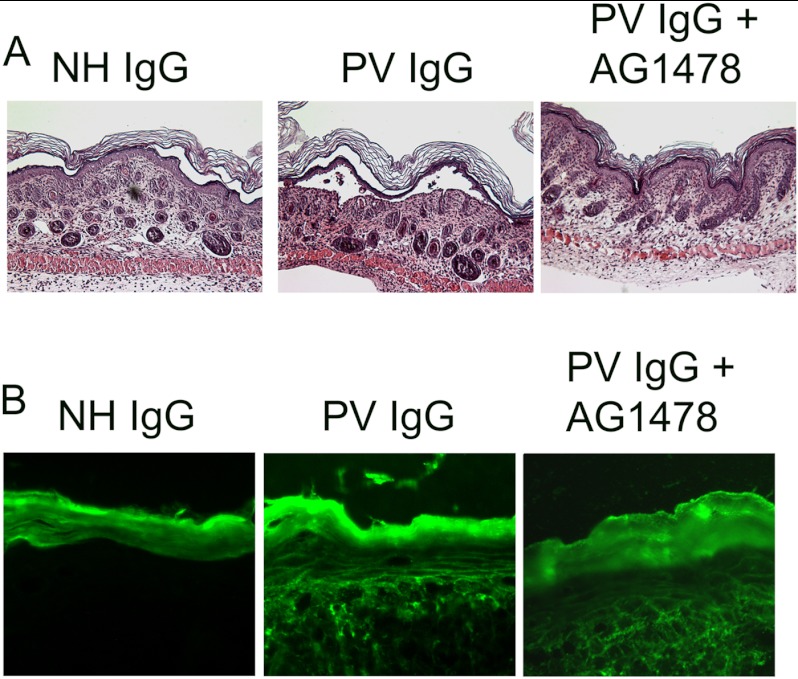
The observation that EGFR inhibition blocked PV IgG-induced dsg3 internalization and depletion, plakoglobin internalization, keratin intermediate filament retraction, and loss of cell-cell adhesion suggested a mechanistic role for EGFR activation in pemphigus-induced acantholysis. We next tested the contribution of EGFR activity to blistering in vivo utilizing the PV IgG passive transfer mouse model. Neonatal mice were treated with NH IgG, PV IgG, or PV IgG and the EGFR inhibitor AG1478 (n = 3 per treatment group) (Fig. 9A). The EGFR inhibitor prevented PV IgG-induced epidermal blistering. PV IgG bound to keratinocyte cell surfaces was detected in skin biopsies from PV IgG as well as PV IgG + inhibitor-treated mice, indicating that the EGFR inhibitor was functioning downstream of PV IgG binding to keratinocyte cell surface dsg3 (Fig. 9B).
FIGURE 9.
The EGFR inhibitor AG1478 blocks blister formation in vivo. Neonatal mice were preinjected with either vehicle or AG1478 for 2 h and then treated by intradermal injection with either NH IgG or PV IgG (n = 3 per treatment group). After 18 h, the mice were sacrificed, and skin from the injection site was harvested and stained by H&E (A) or examined by direct immunofluorescence using anti-human IgG to detect bound PV IgG (B). PV IgG-treated but not NH IgG-treated mice demonstrated characteristic suprabasilar acantholysis. PV IgG-induced blistering was blocked in mice pretreated with AG1478. Mice were examined for the presence of human anti-dsg3 PV IgG by direct immunofluorescence using a mouse anti-human Cy-2-conjugated monoclonal antibody. A honeycomb pattern of staining in the epidermis is seen in both PV IgG-treated and PV IgG + AG1478-treated mice, demonstrating that the EGFR inhibitor does not prevent binding of PV autoantibodies to the keratinocyte cell surface. No bound human IgG was detected in skin biopsies of NH IgG-treated mice. The statistical significance between the PV IgG-treated and PV IgG+AG1478-treated groups was determined using the chi square test. p = 0.014.
DISCUSSION
In keratinocytes treated with PV IgG or the pathogenic anti-dsg3 monoclonal antibody AK23, we observed EGFR phosphorylation and endocytosis. The dependence of these events on EGFR activity was evidenced by the ability of pharmacologic EGFR inhibition to block PV IgG-induced endocytosis of dsg3 and plakoglobin, keratin intermediate filament retraction, and PV IgG-induced loss of keratinocyte cell-cell adhesion both in vitro and in vivo. Additionally, shRNA knockdown of the EGFR similarly prevented PV IgG-induced endocytosis of dsg3.
PV IgG-mediated activation of the EGFR was dependent on p38 activity because pharmacologic inhibition of p38 with SB202190 blocked PV IgG-induced phosphorylation and endocytosis of the EGFR. In contrast to PV IgG, no effect on dsg3 depletion or cell-cell adhesion was observed when keratinocytes were incubated with EGF, providing additional support for mechanistically distinguishing activation of EGFR by PV IgG versus EGF. EGFR inhibitors did not block PV IgG-mediated phosphorylation of p38 and HSP27. However, p38 inhibitors blocked EGFR activation as well as phosphorylation of ERK, a downstream mediator of EGFR signaling. This suggests that for PV IgG-induced signaling, p38 activation is upstream of EGFR activation. Furthermore, inhibition of basal EGFR activity in primary keratinocytes resulted in increased levels of membrane-associated dsg3 and plakoglobin as well as increased cell-cell adhesion. Thus, in addition to activation of EGFR by PV IgG, our data also support a contribution of basal EGFR activity to desmosome adhesion in primary human keratinocytes. Collectively, these observations support a mechanistic role for EGFR in the cellular events leading to acantholysis in pemphigus.
Previous studies examining the potential role of EGFR in pemphigus have produced mixed results. For example, in a prior study it was suggested that EGFR might function to potentiate the proapoptotic effect of PV IgG (28). However, apoptosis does not appear to be required for pemphigus-induced blistering. Results from our laboratory have confirmed that although markers of apoptosis are elevated following pemphigus autoantibody treatment of keratinocytes, apoptosis occurs as a secondary event that may have the ability to sensitize cells to, but is not required for, pemphigus IgG-mediated acantholysis (11), an observation confirmed by other investigators (29). A second report suggested that although EGFR is activated, it is done so independently of autoantibody binding to dsg3, a process referred to as “desmoglein-independent signaling” (8). To study whether EGFR activation was a result of desmoglein-independent signaling, we used the pathogenic anti-dsg3 monoclonal antibody AK23 (42). Internalized dsg3 is targeted for degradation, which leads to its depletion and likely contributes to the loss of cell adhesion. Similar to PV IgG, we found that AK23 induced the endocytosis of both dsg3 and EGFR and that EGFR inhibitors blocked AK23-stimulated dsg3 endocytosis. Because AK23 is a monoclonal antibody-specific for dsg3, we interpret these results to indicate that the activation of EGFR following PV IgG treatment is due to autoantibody binding to dsg3 and subsequent downstream signaling events.
A third report proposed that pemphigus pathogenesis is independent of EGFR (30). In this third report, positive signals from antibody-based detection systems such as Western blotting, ELISA, and immunofluorescence were suggested to result from cross-reactivity with secondary antibodies to high amounts of human pemphigus autoantibody. Our results from this study indicate that nonspecific cross-reactivity in our experimental system is an unlikely explanation for our ability to detect EGFR activation by PV IgG. For example, the increased signal we see on Western blot analysis from phospho-EGFR antibodies is sensitive to AG1478. If cross-reactivity were the reason for an increased signal, the signal should remain high regardless of the application of EGFR inhibitors. Additionally, we demonstrate that these specific EGFR inhibitors block several in vitro events associated with acantholysis, including PV IgG-induced dsg3 membrane depletion, dsg3 endocytosis, keratin intermediate filament retraction, and loss of cell-cell adhesion, as well as blistering in mice. Prior studies have shown that EGFR could directly phosphorylate plakoglobin in A431 squamous cell carcinoma keratinocytes, which destabilized desmosomes (24). Following PV IgG binding, dsg3 is internalized in a clathrin- and dynamin-independent mechanism (40, 41). Interestingly, the pan-tyrosine kinase inhibitor genistein was also able to block PV IgG-triggered dsg3 internalization (40), perhaps in part because of EGFR inhibition. We suggest that the detection by immunoblot of the transient, relative to EGF ligand, weak phosphorylation of EGFR is suboptimal and that this may in part explain the conflicting data in the literature on the role of EGFR in pemphigus. Despite this limitation, two additional assays of EGFR activation, EGFR endocytosis and phosphorylation of the downstream signaling mediator ERK, similarly support the activation of EGFR by PV IgG. Furthermore, the inhibition of EGFR, whether by pharmacologic inhibition or genetic knockdown, gave consistent results inhibiting PV IgG mediated dsg3 endocytosis.
Our results are consistent with these prior studies. p38 is activated in diverse cell processes, including cell stress responses, apoptosis, proliferation, and receptor endocytosis (43). We have previously shown p38 to be activated following PV IgG binding to dsg3 (12). Inhibiting p38 blocks PV IgG-induced keratin intermediate filament retraction, actin reorganization, and dsg3 endocytosis in primary keratinocytes. Furthermore, p38 inhibition blocked blistering in both PV and PF passive transfer mouse models (9, 10). There are several reports in the literature suggesting that p38 can modulate EGFR activity and internalization. Cells subjected to cytotoxic stress such as UV irradiation and chemotherapeutic agents activate p38 which, in turn, was shown to be responsible for EGFR phosphorylation and internalization (44). Unlike EGF-induced EGFR internalization, p38-dependent EGFR internalization did not lead to receptor degradation in lysosomes but rather to its accumulation in early endosomes. Vergarajauregui and colleagues (14) demonstrated that activation of p38 by anisomycin leads to ligand-independent internalization of EGFR. Interestingly, they further show that EGF and anisomycin-induced p38-dependent EGFR internalization differ in the way the receptor is trafficked intracellularly. Another report demonstrates, in keratinocytes, that following disruption of detergent-resistant lipid rafts, both p38 and EGFR are activated and that p38 is upstream of EGFR (37). Our results also indicate that in the PV IgG signal transduction cascade, p38 is activated upstream of EGFR because p38 inhibition also inhibited PV IgG-induced EGFR activation as measured by EGFR phosphorylation, EGFR internalization, and ERK phosphorylation. We interpret the data to indicate that these events contribute to the subsequent loss of dsg3 from desmosomes and, in turn, to keratinocyte acantholysis. As such, this study suggests a therapeutic role for EGFR inhibitors in pemphigus and provides a biologic rationale for pursuing clinical trials of EGFR inhibitors in PV.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 AI49427 (to D. S. R.).
D. S. Rubenstein, V. Werth, B. Strober, F. Kerdel, M. Kolodney, N. Korman, and A. G. Pandya. Use of KC706 for the treatment of pemphigus vulgaris. clinicaltrials.gov.
- dsg
- desmoglein
- PF
- pemphigus foliaceus
- PV
- pemphigus vulgaris
- EGFR
- EGF receptor
- NH
- normal human
- HSP
- heat shock protein.
REFERENCES
- 1. Stanley J. R., Koulu L., Klaus-Kovtun V., Steinberg M. S. (1986) A monoclonal antibody to the desmosomal glycoprotein desmoglein I binds the same polypeptide as human autoantibodies in pemphigus foliaceus. J. Immunol. 136, 1227–1230 [PubMed] [Google Scholar]
- 2. Koulu L., Kusumi A., Steinberg M. S., Klaus-Kovtun V., Stanley J. R. (1984) Human autoantibodies against a desmosomal core protein in pemphigus foliaceus. J. Exp. Med. 160, 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amagai M., Klaus-Kovtun V., Stanley J. R. (1991) Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell 67, 869–877 [DOI] [PubMed] [Google Scholar]
- 4. Eyre R. W., Stanley J. R. (1988) Identification of pemphigus vulgaris antigen extracted from normal human epidermis and comparison with pemphigus foliaceus antigen. J. Clin. Invest. 81, 807–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seishima M., Iwasaki-Bessho Y., Itoh Y., Nozawa Y., Amagai M., Kitajima Y. (1999) Phosphatidylcholine-specific phospholipase C, but not phospholipase D, is involved in pemphigus IgG-induced signal transduction. Arch. Dermatol. Res. 291, 606–613 [DOI] [PubMed] [Google Scholar]
- 6. Williamson L., Raess N. A., Caldelari R., Zakher A., de Bruin A., Posthaus H., Bolli R., Hunziker T., Suter M. M., Müller E. J. (2006) Pemphigus vulgaris identifies plakoglobin as key suppressor of c-Myc in the skin. EMBO J. 25, 3298–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waschke J., Spindler V., Bruggeman P., Zillikens D., Schmidt G., Drenckhahn D. (2006) Inhibition of Rho A activity causes pemphigus skin blistering. J. Cell Biol. 175, 721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chernyavsky A. I., Arredondo J., Kitajima Y., Sato-Nagai M., Grando S. A. (2007) Desmoglein versus non-desmoglein signaling in pemphigus acantholysis. Characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J. Biol. Chem. 282, 13804–13812 [DOI] [PubMed] [Google Scholar]
- 9. Berkowitz P., Chua M., Liu Z., Diaz L. A., Rubenstein D. S. (2008) Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen-activated protein kinase-dependent signaling in the skin. Am. J. Pathol. 173, 1628–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berkowitz P., Hu P., Warren S., Liu Z., Diaz L. A., Rubenstein D. S. (2006) p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc. Natl. Acad. Sci. U.S.A. 103, 12855–12860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H. E., Berkowitz P., Jolly P. S., Diaz L. A., Chua M. P., Rubenstein D. S. (2009) Biphasic activation of p38MAPK suggests that apoptosis is a downstream event in pemphigus acantholysis. J. Biol. Chem. 284, 12524–12532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berkowitz P., Hu P., Liu Z., Diaz L. A., Enghild J. J., Chua M. P., Rubenstein D. S. (2005) Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J. Biol. Chem. 280, 23778–23784 [DOI] [PubMed] [Google Scholar]
- 13. Jolly P. S., Berkowitz P., Bektas M., Lee H. E., Chua M., Diaz L. A., Rubenstein D. S. (2010) p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. J. Biol. Chem. 285, 8936–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vergarajauregui S., San Miguel A., Puertollano R. (2006) Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 7, 686–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hackel P. O., Zwick E., Prenzel N., Ullrich A. (1999) Epidermal growth factor receptors. Critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol. 11, 184–189 [DOI] [PubMed] [Google Scholar]
- 16. Keely S. J., Uribe J. M., Barrett K. E. (1998) Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen-activated protein kinase in T84 cells. Implications for carbachol-stimulated chloride secretion. J. Biol. Chem. 273, 27111–27117 [DOI] [PubMed] [Google Scholar]
- 17. Li X., Lee J. W., Graves L. M., Earp H. S. (1998) Angiotensin II stimulates ERK via two pathways in epithelial cells. Protein kinase C suppresses a G-protein coupled receptor-EGF receptor transactivation pathway. EMBO J. 17, 2574–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsai W., Morielli A. D., Peralta E. G. (1997) The m1 muscarinic acetylcholine receptor transactivates the EGF receptor to modulate ion channel activity. EMBO J. 16, 4597–4605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schneider M. R., Werner S., Paus R., Wolf E. (2008) Beyond wavy hairs. The epidermal growth factor receptor and its ligands in skin biology and pathology. Am. J. Pathol. 173, 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoschuetzky H., Aberle H., Kemler R. (1994) β-Catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J. Cell Biol. 127, 1375–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin T., Getsios S., Caldelari R., Godsel L. M., Kowalczyk A. P., Müller E. J., Green K. J. (2005) Mechanisms of plakoglobin-dependent adhesion. Desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J. Biol. Chem. 280, 40355–40363 [DOI] [PubMed] [Google Scholar]
- 22. Miravet S., Piedra J., Castaño J., Raurell I., Francí C., Duñach M., García de Herreros A. (2003) Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates β-catenin-mediated transcription. Mol. Cell. Biol. 23, 7391–7402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Getsios S., Simpson C. L., Kojima S., Harmon R., Sheu L. J., Dusek R. L., Cornwell M., Green K. J. (2009) Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 185, 1243–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaudry C. A., Palka H. L., Dusek R. L., Huen A. C., Khandekar M. J., Hudson L. G., Green K. J. (2001) Tyrosine-phosphorylated plakoglobin is associated with desmogleins but not desmoplakin after epidermal growth factor receptor activation. J. Biol. Chem. 276, 24871–24880 [DOI] [PubMed] [Google Scholar]
- 25. Klessner J. L., Desai B. V., Amargo E. V., Getsios S., Green K. J. (2009) EGFR and ADAMs cooperate to regulate shedding and endocytic trafficking of the desmosomal cadherin desmoglein 2. Mol. Biol. Cell 20, 328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lorch J. H., Klessner J., Park J. K., Getsios S., Wu Y. L., Stack M. S., Green K. J. (2004) Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. J. Biol. Chem. 279, 37191–37200 [DOI] [PubMed] [Google Scholar]
- 27. Lorch J. H., Thomas T. O., Schmoll H. J. (2007) Bortezomib inhibits cell-cell adhesion and cell migration and enhances epidermal growth factor receptor inhibitor-induced cell death in squamous cell cancer. Cancer Res. 67, 727–734 [DOI] [PubMed] [Google Scholar]
- 28. Frusi-Zlotkin M., Raichenberg D., Wang X., David M., Michel B., Milner Y. (2006) Apoptotic mechanism in pemphigus autoimmunoglobulins-induced acantholysis. Possible involvement of the EGF receptor. Autoimmunity 39, 563–575 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt E., Gutberlet J., Siegmund D., Berg D., Wajant H., Waschke J. (2009) Apoptosis is not required for acantholysis in pemphigus vulgaris. Am. J. Physiol. Cell Physiol. 296, C162–172 [DOI] [PubMed] [Google Scholar]
- 30. Heupel W. M., Engerer P., Schmidt E., Waschke J. (2009) Pemphigus vulgaris IgG cause loss of desmoglein-mediated adhesion and keratinocyte dissociation independent of epidermal growth factor receptor. Am. J. Pathol. 174, 475–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pretel M., España A., Marquina M., Pelacho B., López-Picazo J. M., López-Zabalza M. J. (2009) An imbalance in Akt/mTOR is involved in the apoptotic and acantholytic processes in a mouse model of pemphigus vulgaris. Exp. Dermatol. 18, 771–780 [DOI] [PubMed] [Google Scholar]
- 32. Kawasaki Y., Aoyama Y., Tsunoda K., Amagai M., Kitajima Y. (2006) Pathogenic monoclonal antibody against desmoglein 3 augments desmoglein 3 and p38 MAPK phosphorylation in human squamous carcinoma cell line. Autoimmunity 39, 587–590 [DOI] [PubMed] [Google Scholar]
- 33. Sorkin A., Von Zastrow M. (2002) Signal transduction and endocytosis. Close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 3, 600–614 [DOI] [PubMed] [Google Scholar]
- 34. Adachi S., Natsume H., Yamauchi J., Matsushima-Nishiwaki R., Joe A. K., Moriwaki H., Kozawa O. (2009) p38 MAP kinase controls EGF receptor down-regulation via phosphorylation at Ser-1046/1047. Cancer Lett. 277, 108–113 [DOI] [PubMed] [Google Scholar]
- 35. Nishimura M., Shin M. S., Singhirunnusorn P., Suzuki S., Kawanishi M., Koizumi K., Saiki I., Sakurai H. (2009) TAK1-mediated serine/threonine phosphorylation of epidermal growth factor receptor via p38/extracellular signal-regulated kinase: NF-κB-independent survival pathways in tumor necrosis factor α signaling. Mol. Cell. Biol. 29, 5529–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sloss C. M., Wang F., Palladino M. A., Cusack J. C., Jr. (2010) Activation of EGFR by proteasome inhibition requires HB-EGF in pancreatic cancer cells. Oncogene 29, 3146–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jans R., Atanasova G., Jadot M., Poumay Y. (2004) Cholesterol depletion upregulates involucrin expression in epidermal keratinocytes through activation of p38. J. Invest. Dermatol. 123, 564–573 [DOI] [PubMed] [Google Scholar]
- 38. Aoyama Y., Kitajima Y. (1999) Pemphigus vulgaris-IgG causes a rapid depletion of desmoglein 3 (Dsg3) from the Triton X-100 soluble pools, leading to the formation of Dsg3-depleted desmosomes in a human squamous carcinoma cell line, DJM-1 cells. J. Invest. Dermatol. 112, 67–71 [DOI] [PubMed] [Google Scholar]
- 39. Cirillo N., Gombos F., Lanza A. (2007) Changes in desmoglein 1 expression and subcellular localization in cultured keratinocytes subjected to anti-desmoglein 1 pemphigus autoimmunity. J. Cell. Physiol. 210, 411–416 [DOI] [PubMed] [Google Scholar]
- 40. Delva E., Jennings J. M., Calkins C. C., Kottke M. D., Faundez V., Kowalczyk A. P. (2008) Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin- and dynamin-independent mechanism. J. Biol. Chem. 283, 18303–18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calkins C. C., Setzer S. V., Jennings J. M., Summers S., Tsunoda K., Amagai M., Kowalczyk A. P. (2006) Desmoglein endocytosis and desmosome disassembly are coordinated responses to pemphigus autoantibodies. J. Biol. Chem. 281, 7623–7634 [DOI] [PubMed] [Google Scholar]
- 42. Tsunoda K., Ota T., Aoki M., Yamada T., Nagai T., Nakagawa T., Koyasu S., Nishikawa T., Amagai M. (2003) Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3. J. Immunol. 170, 2170–2178 [DOI] [PubMed] [Google Scholar]
- 43. Cuadrado A., Nebreda A. R. (2010) Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429, 403–417 [DOI] [PubMed] [Google Scholar]
- 44. Zwang Y., Yarden Y. (2006) p38 MAP kinase mediates stress-induced internalization of EGFR. Implications for cancer chemotherapy. EMBO J. 25, 4195–4206 [DOI] [PMC free article] [PubMed] [Google Scholar]