Background: MEF/ELF4 can function as an oncogene. We demonstrated the role of MEF/ELF4 in acute myeloid leukemia.
Results: NPM1 inhibited the DNA binding and transcriptional activity of MEF/ELF4 on the HDM2 promoter, whereas NPM1 mutant protein enhanced these activities of MEF/ELF4.
Conclusion: MEF/ELF4 activity may be activated by NPM1 mutant protein.
Significance: NPM1 mutant proteins have a role in MEF/ELF4-dependent leukemogenesis.
Keywords: Ets Family Transcription Factor, Leukemia, Oncogene, Promoters, Transcription Regulation
Abstract
Myeloid ELF1-like factor (MEF/ELF4), a member of the ETS transcription factors, can function as an oncogene in murine cancer models and is overexpressed in various human cancers. Here, we report a mechanism by which MEF/ELF4 may be activated by a common leukemia-associated mutation in the nucleophosmin gene. By using a tandem affinity purification assay, we found that MEF/ELF4 interacts with multifactorial protein nucleophosmin (NPM1). Coimmunoprecipitation and GST pull-down experiments demonstrated that MEF/ELF4 directly forms a complex with NPM1 and also identified the region of NPM1 that is responsible for this interaction. Functional analyses showed that wild-type NPM1 inhibited the DNA binding and transcriptional activity of MEF/ELF4 on the HDM2 promoter, whereas NPM1 mutant protein (Mt-NPM1) enhanced these activities of MEF/ELF4. Induction of Mt-NPM1 into MEF/ELF4-overexpressing NIH3T3 cells facilitated malignant transformation. In addition, clinical leukemia samples with NPM1 mutations had higher human MDM2 (HDM2) mRNA expression. Our data suggest that enhanced HDM2 expression induced by mutant NPM1 may have a role in MEF/ELF4-dependent leukemogenesis.
Introduction
Myeloid ELF1-like factor (MEF/ELF4), a member of the ETS family of transcription factors, is characterized by an 85-amino acid ETS domain that recognizes a core sequence of GGAA or TTCC (1). MEF/ELF4 is expressed in various normal and malignant hematopoietic cells and regulates the expression of various cytokines (interleukin-3 (1), granulocyte-macrophage colony-stimulating factor (1), and interleukin-8 (2) as well as the cytolytic perforin molecule (3) and antibacterial peptides lysozyome and human β-defensin2 (4)) and matrix metalloproteinase-9 expression (5). Furthermore, analyses of MEF/ELF4-deficient mice have revealed the essential role of MEF/ELF4 in the development and function of NK (natural killer) cells and NK-T cells (3). Recently, Smith et al. (6) have shown that repression of Elf-4 by transcriptional repressor Gfi1b is important for the maturation of primary fetal liver erythroid cells. MEF/ELF4 also regulates the key aspects of hematopoietic stem cell behavior by controlling movement through the cell cycle from quiescence (G0) to G1 and from G1 to S as well as resistance to myelosuppression (7, 8).
MEF/ELF4 is expressed in cancers such as leukemia (9), lymphoma, and ovarian cancer (10). Recently, Totoki et al. (11) identified an intrachromosomal inversion (Xq25) in hepatocellular carcinoma that generated a BCORL1-MEF/ELF4 fusion transcript. Experiments in several mouse models have suggested that MEF/ELF4 plays a role in tumorigenesis. For example, models of retrovirus-induced insertional mutagenesis have identified MEF/ELF4 as a gene that is involved in leukemic transformation (12). Sashida et al. (13) have shown that overexpression of MEF/ELF4 enhances the expression of Mdm2, leading to decreased p53 expression and enhanced transformation. In experiments with MEF/ELF4-overexpressing cells, they demonstrated that Ets1-induced p16 induction is suppressed, resulting in senescence suppression and tumor promotion.
Nucleophosmin (NPM1) is a nucleolar phosphoprotein (14) and a frequent target of genetic alterations in hematopoietic malignancies. NPM1 gene mutations have been found in ∼60% of adult patients who have acute myeloid leukemia (AML)2 and a normal karyotype (15). These mutations lead to the aberrant cytoplasmic expression of NPM1 (NPMc+) due to nucleotide gain at the C terminus (16, 17), which results in the loss of tryptophan residues essential for nucleolar localization and the gain of a new nuclear export signal (18). Increased NPM1 export into the cytoplasm probably perturbs multiple cellular pathways by delocalizing the proteins that interact with NPM1. By using a transgenic mouse model expressing the human NPMc+ mutation, it has been shown that NPMc+ confers a proliferative advantage in the myeloid lineage, suggesting that NPM1 mutations can participate in leukemia development (19).
In the present study, we found that wild-type NPM1 (Wt-NPM1) down-regulates, whereas mutated NPM1 (Mt-NPM1) up-regulates, the transcriptional activity of MEF/ELF4 on the human MDM2 (HDM2) promoter. The expression of Mt-NPM1 in MEF/ELF4-overexpressing NIH3T3 cells resulted in enhanced malignant transformation. We also found that HDM2 mRNA expression in primary AML cells with NPM1 mutations is significantly higher compared with AML cells without NPM1 mutations. Taken together, our data suggest that NPM1 mutations may promote transformation by enhancing the oncogenic functions of MEF/ELF4.
EXPERIMENTAL PROCEDURES
Cell Culture
293T cells (CRL-11268, ATCC (Manassas, VA)) were maintained at 37 °C in DMEM (Invitrogen) with bovine calf serum. U937 cells (CRL-1593.2, ATCC) were maintained with 10% (v/v) FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin (Fisher). NIH3T3 cells (CRL-1658, ATCC) were maintained under identical conditions with 10% (v/v) FBS and grown in RPMI 1640 (Fisher) with 10% FCS (HyClone, Logan, UT), 100 units/ml penicillin G, and 100 μg/ml streptomycin. COS7 cells (CRL-1651, ATCC) were cultured in DMEM (Invitrogen) containing 10% FCS.
Tandem Affinity Purification Assay
The cDNA of MEF/ELF4 was inserted into InterPlay N-terminal mammalian TAP vector (pTAP/MEF/ELF4, Stratagene (San Diego, CA)) comprising two affinity tags (immunoglobulin G (IgG)-binding domain and calmodulin-binding peptide) separated by the cleavage site of tobacco etch virus protease (20). 293T cells were transfected with pTAP or pTAP/MEF/ELF4 plasmids in a 10-cm dish. Transfected cells were collected and lysed in a solution containing 100 mm Tris-HCl (pH 8.0), 300 mm NaCl, and 0.1% Nonidet P-40. The lysate was centrifuged at 15,000 rpm for 30 min at 4 °C. The resulting supernatant was incubated for 2 h at 4 °C with IgG-Sepharose 6 Fast Flow (GE Healthcare), after which the resin was washed and incubated with tobacco etch virus protease for 2 h at 16 °C. Purification on calmodulin affinity resin (Stratagene) was performed according to the manufacturer's instructions. Purified proteins were precipitated with trichloroacetic acid, resolved with 1× sample buffer and subjected to SDS-PAGE. Gels were stained with Coomassie Blue, and protein bands were cut out. Proteins were eluted with trypsin. The resulting peptides were analyzed with a Procise 49X cLC protein sequencer (Applied Biosystems, Foster City, CA) (20).
In Vitro Translation
The cDNA molecules of Wt-NPM1 and Mt-NPM1 (21) were inserted into the pTnT vector (pTnT-NPM, Promega (Madison, WI)) for in vitro translation. NPM1 protein (biotin-NPM1) was in vitro-translated with pTnT-NPM1 and labeled with biotinylated lysine (Transcend tRNA, Promega) by using the TNT Quick Coupled transcription/translation system (Promega). The cDNA of MEF/ELF4 was inserted into pET-3a (Novagen, VWR (Lisbon, Portugal)), which allows the introduction of a His tag into the N terminus of MEF/ELF4 (pET/MEF/ELF4). Overexpression of the recombinant protein (His-MEF/ELF4) was achieved in Escherichia coli BL21Gold (DE3) cells (Stratagene) transformed with the constructed plasmid pET/MEF/ELF4. His-MEF/ELF4 was isolated from cells broken in lysis buffer (STE buffer) with sonication and centrifuged at 15,000 × g for 10 min at 4 °C (1).
Biotin-NPM1 was incubated with His-MEF/ELF4 or His (as a control) proteins at 4 °C for 1 h. The mixture was loaded onto His spin traps (GE Healthcare) and eluted with 500 mm imidazole at pH 7.4. After SDS-PAGE and electroblotting, biotin-NPM1 in purified samples was detected by using the Transcend non-radioactive translation detection system (Promega).
Immunoprecipitation and Immunoblotting
MEF/ELF4 was cloned into p3xFLAG-CMV (Sigma) (FLAG-MEF/ELF4) from PCR products generated from pcDNA/MEF/ELF4 (1). Wt-NPM1 and Mt-A-NPM1 were cloned into pcDNA3.1/V5-His (pcDNA/V-Wt-NPM1 and Mt-A-NPM1, respectively) (Invitrogen) from PCR products generated from pcDNA/Wt-NPM1 and pcDNA/Mt-A (21). 293T cells were transfected with each plasmid by using Effectene transfection reagent (Qiagen, Berlin, Germany). After 48 h, cells were lysed by using the Universal Magnetic co-immunoprecipitation kit (Active Motif, Carlsbad, CA) following the manufacturer's instructions for nuclear extraction. Lysates were centrifuged at 15,000 rpm for 10 min at 4 °C to remove the resin. The resulting supernatants were incubated for 4 h at 4 °C with 5 μg of antibodies against FLAG (Sigma), 5 μg of antibodies against V5 (Invitrogen), or normal mouse IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA).
Immunoprecipitates were recovered, washed four times with ice-cold co-immunoprecipitation solution (Active Motif), and fractionated by SDS-PAGE. Separated proteins were transferred to a membrane. After incubation in blocking buffer, membranes were probed with peroxidase-labeled antibodies against FLAG (Sigma), V5 (Invitrogen), or tag (Invitrogen). Detection was achieved with an enhanced chemiluminescence system (ECL Advance Western blotting detection kit, GE Healthcare). Quantification of Western blotting bands was performed by using AE-6982/C/FC and CS Analyzer version 3.0 software (ATTO, Tokyo, Japan).
GST and His Pull-down Assay
Fusion protein of GST and Wt-NPM1 (GST-NPM1) and GST-NPM1 deletion mutant constructs (Fig. 1C) were generated by PCR with pcDNA/Wt-NPM1 as a template. PCR products were cloned in-frame into bacterial expression vector pGEX-T4. Plasmids that express GST fusion protein (GST-NPM1, GST-NPM1 deletion mutants) and His-MEF/ELF4 protein (pET/MEF/ELF4) or their controls were transfected into E. coli. Bacterial pellets were lysed in 1 ml of phosphate-buffered saline (PBS) with sonication. His-MEF/ELF4 or His alone was incubated with an equivalent amount of GST, GST-Wt-NPM1, or GST-Wt-NPM1 deletion mutants for 1 h at 4 °C. Proteins were purified by using GST columns (MicroSpin GST Purification Module, GE Healthcare) or His columns. Bound proteins were analyzed by using SDS-PAGE/immunoblot.
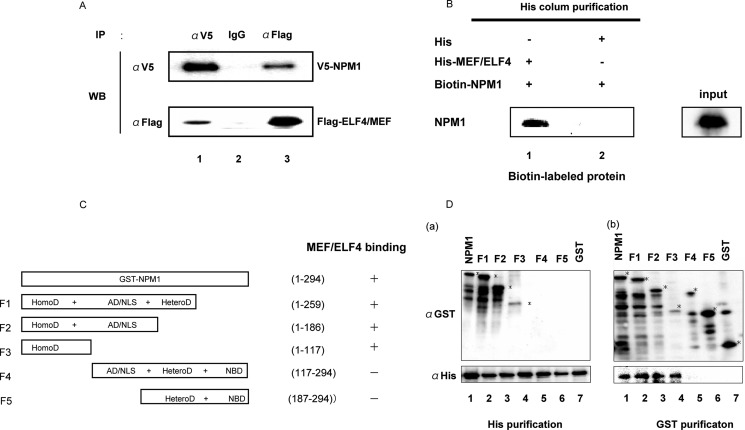
FIGURE 1.
NPM1 interacts with MEF/ELF4. A, 293T cells were transfected with the indicated expression plasmids. After 48 h, cell lysates were immunoprecipitated (IP) with anti-FLAG and anti-V5 antibodies. Immunoprecipitates were analyzed by 10% SDS-PAGE and subjected to immunoblotting (WB) with anti-V5 antibody (top row) or anti-FLAG antibody (bottom row). B, MEF/ELF4 interacts directly with NPM1 in vitro. In vitro association assays were undertaken by incubating His-MEF/ELF4 fusion protein immobilized by using a His-column with biotin-labeled MEF/ELF4 (lane 1). His alone was incubated with biotin-labeled NPM1 (lane 2) as a control. C, NPM1 structure and the relative binding of MEF/ELF4 (schematic). HomoD, homodimerization domain, residues 1–117; AD/NLS, acidic domain/nuclear localization sequence, residues 117–187; HeteroD, heterodimerization domain, residues 187–259; NBD, nucleic acid binding domain, residues 259–294. D, the N-terminal portion of NPM1 is the MEF/ELF4-interacting domain. Bacterially expressed and purified GST, GST-NPM1, and GST-NPM1 mutants with deletions were mixed with bacterially expressed and purified His or His-MEF/ELF4 protein. Recombinant proteins were subjected to His or GST affinity columns, followed by immunoblotting with anti-GST or anti-His antibodies. a, the reactive samples were subjected to analyses on a His affinity column followed by immunoblotting with anti-His antibodies (bottom left) or with anti-GST antibodies (top left). b, the reactive samples were subjected to GST affinity columns, followed by immunoblotting with anti-GST antibodies (top right) or with anti-His antibodies (bottom right).
EMSA
Recombinant proteins GST, GST-NPM1, His, and His-MEF/ELF4 were collected as described above. Nuclear protein from 293T cells transfected with pcDNA/MEF/ELF4, pcDNA/Wt-NPM1, or pcDNA/Mt-A-NPM1 was extracted with the NE-PER nuclear and cytoplasmic extraction kit (Pierce) according to the manufacturer's instructions. EMSA was performed by using the LightShift chemiluminescent EMSA kit (Pierce). Recombinant protein or nuclear extracts were incubated with 20 fmol of biotin 3′-end-labeled oligonucleotides containing APET (an ETS binding site in the IL-3 promoter that was shown to bind to MEF/ELF4) (1). After electrophoresis, transfer, and cross-linking, the signal was detected by a peroxidase/luminol system (chemiluminescent nucleic acid detection module, Pierce). To confirm specificity, a 200-fold excess amount of non-labeled oligonucleotides (APET competitor) (1) was added. The DNA sequence of the APET oligonucleotide is 5′-CCTCAGTGAGCTGAGTCAGGCTTCCCCTTCCTGCCACAGGG-3′.
RNA Interference
siRNA for NPM1 was transfected into 293T cells by using the GeneClip U1 hairpin cloning system (Promega) according to the manufacturer's instructions. The siRNA sequence-targeting NPM1 gene corresponded to nucleotides 103–125 of the coding region relative to the first nucleotide of the start codon, as described previously (22).
Luciferase Assay
A 0.5-μg aliquot of pcDNA/MEF/ELF4, pcDNA/Wt-NPM1, pcDNA/Mt-A-NPM1, pcDNA/Mt-I-NPM1, or pcDNA/Mt-J-NPM1 was transfected into U937, 293T, and COS7 cells seeded in 6-well dishes by using Nucleofectin (Qiagen) together with 0.1 μg of pGL4 reporter plasmid (pGL4/APET (1), pGL4/ETSm-APET (1), pGL4/HDM2, or pGL4/HDM2mut) and 0.05 μg of pLR-Bact vector. pGL4/ETSm-APET contains a mutation in the ETS binding site (ETSm-APET, 5′-CCTCAGTGAGCTGAGTCAGGCTgagCCTcgacGCCACAGGG-3′). pGL4/HDM2 contains a wild-type hdm2 (P2) promoter sequence from bp −82 to −122 (Wt-Ets, CAGGTTGACTCAGCTTTTCCTCTTGAGCTGGTCAAGTTCAG), and pGL4/HDM2mut contains an hdm2 (P2) promoter sequence with a mutated ETS site (Mt-Ets, CAGGTTGACTCAGCTTTTaCTCTTGAGCTGGTCAAGTTCAG) (23). Cell lysates were prepared 48 h after transfection, and luciferase activity was determined by using the Dual-Luciferase reporter assay system (Promega).
Anchorage-independent Growth Assay
NIH3T3 cells were plated on 24-well dishes in soft agar containing DMEM supplemented with 10% FCS after they were transfected with various combinations of empty vector, pcDNA/MEF/ELF4, pcDNA/Wt-NPM1, or pcDNA/Mt-A-NPM1 and cultured for 2 weeks. Images were taken with a Leica DM IRBE inverted microscope (Leica Microsystems GmbH, Mannheim, Germany) with a ×10 objective lens.
Immunochemistry
MEF/ELF4 was cloned into the pGFP-C3 vector (Clontech, Mountain View, CA) (pGFP-MEF/ELF4). 293T cells were transfected with the empty vector, pGFP-MEF/ELF4, pcDNA/V-Wt-NPM1, or pcDNA/V-Mt-A-NPM1. Cells were harvested 3 days after transfection. Cytospin samples were fixed for 15 min in PBS containing 4% paraformaldehyde. Fixed coverslips were washed twice in TBS, permeabilized in 0.5% Triton X-100 for 10 min, and incubated in Image-iT FX signal enhancer (Invitrogen) for 30 min. Cells were incubated with primary antibody for 1 h and then washed extensively in TBS before incubation with Alexa546-conjugated goat anti-mouse-IgG antibody (dilution 1:2000; Invitrogen) for 1 h. Cells were covered with a drop of ProLong Gold Antifade Reagent with DAPI (Invitrogen). Fluorescent images were obtained by using a confocal laser-scanning microscope (LSM 5 Pascal V3.2, Carl Zeiss).
ChIP Assay
293T cells were transfected with empty vector, pcDNA/MEF-FLAG, pcDNA/Wt-NPM1, or pcDNA/Mt-A-NPM1 by using a nucleofection kit (Qiagen). After 48 h of culture at 26 °C, cells were fixed by the addition of 1% formaldehyde in PBS for 10 min. Chromatin isolation and shearing were performed by using the OneDay ChIP kit (Diagenode, Liege, Belgium) and Shearing-ChIP kit (Diagenode) according to the manufacturer's instructions. Immunoprecipitation reactions were performed with anti-FLAG monoclonal antibody (Sigma) or isotype control IgG (BD Biosciences). Samples were analyzed by quantitative reverse transcription-polymerase chain reaction (RQ-PCR) by using the LightCycler DNA Master SYBR Green I kit (Roche Applied Science) as specified by the manufacturer. The primer sequences for the HDM2 promoter were 5′-GAACGCTGCGCGTAGTCTGG-3′ (forward) and 5′-ACTGCAGTTTCGGAACGTGT-3′ (reverse).
Clinical Samples
Informed consent for sample collection was obtained according to protocols approved by the International Review Board of Nagasaki University, Nagasaki, Japan (approval number 33-3). Bone marrow aspirates were collected from 22 AML patients before the initiation of chemotherapy. CD34-positive cells were isolated by using Ficoll density gradient centrifugation and magnetic beads (CD34 Isolation Kit, Miltenyi Biotec, Auburn, CA) to minimize the confounding effect of MEF/ELF4 and NPM1 expression by mature myeloid cells. For the screening of NPM1 mutations, genomic DNA corresponding to exon 12 was amplified by using forward primer 5′-TTAACTCTCTGGTGGTAGAATGAA-3′ and reverse primer 5′-CAAGACTATTTGCCATTCCTAAC-3′, as reported previously. Amplified products were separated by agarose gel electrophoresis, purified by using a QIAquick gel extraction kit (Qiagen), and directly sequenced by using a DNA sequencer (3100, Applied Biosystems) with the BigDye terminator cycle sequencing kit (Applied Biosystems). When mutations were found by direct sequencing, the fragments were cloned into a pTOPO vector (Invitrogen) and then transfected into the E. coli strain DH5A. At least four recombinant colonies were selected, and plasmid DNA samples were prepared by using the QIAprep Spin Miniprep kit (Qiagen). Cloned fragments were sequenced to confirm the mutation of the NPM1 gene.
Total RNA was harvested from purified CD34-positive cells by using an RNeasy minikit (Qiagen). cDNA synthesis was undertaken by using an oligo(dT) primer with the PrimeScript II first strand cDNA synthesis kit (Takara, Shiga, Japan). These cDNA molecules were measured by RQ-PCR with the primers listed under “RQ-PCR.”
RQ-PCR
RQ-PCR was performed by using a LightCycler TaqMan Master kit (Roche Applied Science) following the manufacturer's instructions. Twenty microliters of Universal ProbeLibrary probes (Exiqon, Vedbaek, Denmark) were added in the final reaction. Primers designed by using the Universal ProbeLibrary Assay Design Centre (available on the Roche Applied Science Web site) were synthesized by Sigma. PCR amplification was performed by using a LightCycler 350S instrument (Roche Applied Science). Thermal cycling conditions comprised 2 min at 40 °C and 10 min at 95 °C, followed by 45 amplification cycles at 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 s and then a 40 °C cooling cycle for 30 s. Specific primers and probes were as follows: for HDM2, forward (5′-TCTGATAGTATTTCCCTTTCCTTTG-3′), reverse (5′-TGTTCACTTACACCAGCATCAA-3′), and probe (5′-CGCCACTTTTTCTCTGCTGATCCAGG-3′); for human MEF/ELF4, forward (5′-TGGAGACTCTCAGGGTCGAAA-3′), reverse (5′-AAGCAACGGGATGGATGAT-3′), and probe (5′-TCACAGCTGGGAACACAGAG-3′); and for human G6PDH, forward (5′-AAGCAACGGGATGGATGAT-3′), reverse (5′-TCACAGCTGGGAACACAGAG-3′), and probe (5′-CGCCACTTTTTCTCTGCTGATCCAGG-3′).
Statistical Analyses
Comparisons of patient characteristics between two groups were performed with the Wilcoxon test. The results of in vivo experiments are presented as the mean ± S.D. of three independent experiments and compared by using one-way analysis of variance followed by Scheffe's multiple comparison test. A p value of 0.05 was considered statistically significant.
RESULTS
Identification of MEF/ELF4-binding Protein
To identify the proteins that bind to MEF/ELF4, we performed the tandem affinity purification (TAP) procedure and analyzed the amino acid sequence of the protein complex, thereby identifying 25 proteins (including NPM1). NPM1 is essential for embryonic development and is frequently translocated or mutated in hematological malignancies (24). Therefore, we decided to focus on the interaction between NPM1 and MEF/ELF4.
Wt-NPM1 Interacts with MEF/ELF4 in Vivo and in Vitro
To determine if Wt-NPM1 interacts with MEF/ELF4 in human cells, we transfected 293T cells with FLAG-MEF/ELF4 and V5-Wt-NPM1 expression plasmids and performed immunoprecipitations with mouse monoclonal anti-FLAG or anti-V5 antibody. As shown in Fig. 1A, FLAG-MEF/ELF4 protein co-precipitated with V5-Wt-NPM1 by the anti-V5 antibody (lane 1) but not by the isotype-matched control (lane 2). In reciprocal experiments, V5-Wt-NPM1 protein co-precipitated with FLAG-MEF/ELF4 protein by the anti-FLAG antibody (lane 3). These results showed the in vivo interaction between Wt-NPM1 and MEF/ELF4. To ascertain whether Wt-NPM1 protein interacted directly with MEF/ELF4, an in vitro association assay with biotin-labeled in vitro-translated Wt-NPM1 and bacterially recombinant His-MEF/ELF4 fusion protein was performed (Fig. 1B). Biotin-labeled Wt-NPM1 bound to His-MEF/ELF4 (lane 1) but not to His alone (lane 2). These results demonstrated that His-MEF/ELF4 bound directly to Wt-NPM1.
To characterize the region of Wt-NPM1 that binds MEF/ELF4, five distinct GST-NPM1 proteins were prepared (Fig. 1C). GST pull-down assays (Fig. 1D (a)) and His tag pull-down assays (Fig. 1D (b)) revealed that the N-terminal region of NPM1 (the F1, F2, and F3 fragments that contain the oligomerization domain) bound to His-MEF/ELF4, unlike the C-terminal region of NPM1 (F4 and F5).
Wt-NPM1 Interferes with MEF/ELF4 Binding to Target DNA Sequences
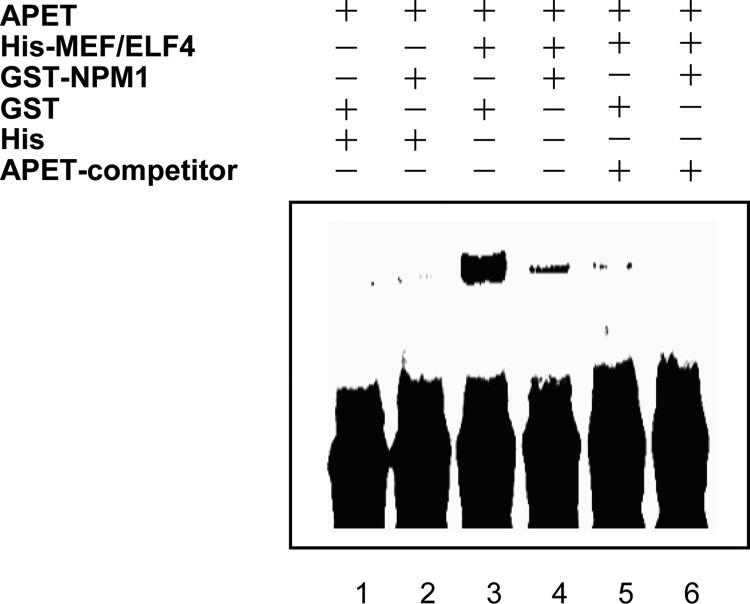
To assess the direct role of Wt-NPM1 in MEF/ELF4 action, we undertook EMSA. His-MEF/ELF4 bound to the APET probe (1), but no band was observed with His, GST, or GST-NPM1 (Fig. 2). The shifted band of MEF/ELF4 was diminished when the APET competitor was added to the reaction mixture. When Wt-NPM1 was added to the reaction mixture, the shifted band containing MEF/ELF4 was diminished. These results implied that Wt-NPM1 inhibits the DNA binding of MEF/ELF4 DNA through direct interactions.
FIGURE 2.
EMSA with recombinant His-MEF/ELF4, His, GST, and GST-Wt-NPM1. His-MEF/ELF4 was incubated with GST and GST-Wt-NPM1 at room temperature prior to EMSA by using a biotin-conjugated APET probe (lanes 1–4). An excess amount of unlabeled APET competitor was added to the reaction mixtures (lanes 5 and 6).
Wt-NPM1 Inhibits, whereas Mt-NPM1 Enhances, MEF/ELF4-dependent Transcriptional Activity
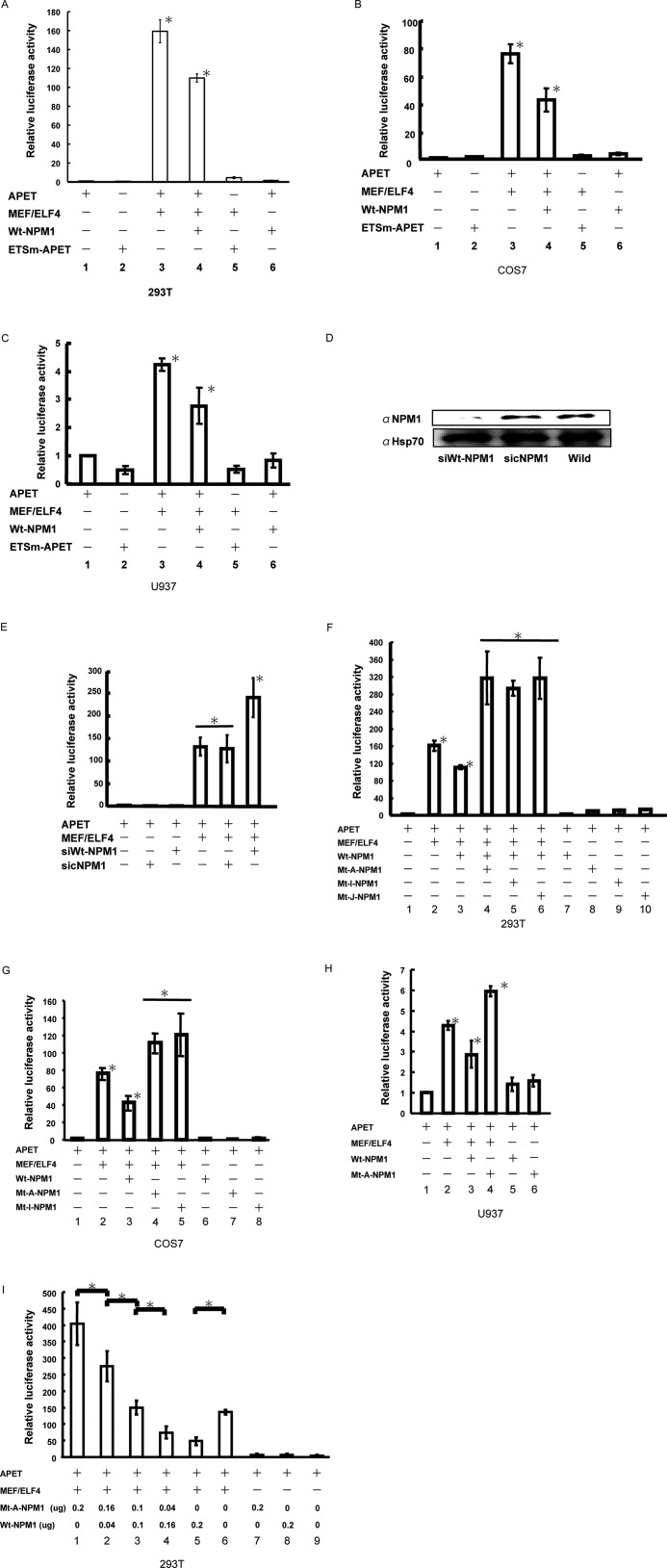
To study the functional relevance of the physical interaction between MEF/ELF4 and Wt-NPM1, we transfected pcDNA/MEF/ELF4 in combination with pcDNA/Wt-NPM1 and examined the activity of the APET promoter construct (1) in 293T cells (Fig. 3A). As reported previously, MEF/ELF4 activated the APET promoter by ∼159-fold. Co-expression of Wt-NPM1 with MEF/ELF4 led to a significant decrease in luciferase activity. Similar data were obtained by using COS7 cells (Fig. 3B) and a human leukemia cell line, U937 (Fig. 3C).
FIGURE 3.
Wt-NPM1 inhibits, whereas Mt-NPM1 enhances, MEF/ELF4-dependent APET promoter transactivation. 293T human kidney (A), COS7 monkey kidney (B), and U937 human hematological (C) cell lines were co-transfected with the luciferase reporter gene of an artificial MEF/ELF4 target promoter (APET) and effector genes. The target promoter and effector genes were as follows: pGL4/APET (lane 1); pGL4/ETSm-APET (lane 2); pGL4/APET and pcDNA/MEF/ELF4 (lane 3); pGL4/APET, pcDNA/MEF/ELF4, and pcDNA/Wt-NPM1 (lane 4); pGL4/ETSm-APET and pcDNA/MEF/ELF4 (lane 5); and pGL4/APET and pcDNA/Wt-NPM1 (lane 6). Luciferase activity by pGL4/APET alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. (error bars). *, p < 0.05. D, 293T cells transduced with siRNA encoding vector (siWt-NPM1) were harvested 72 h after transduction for Western blotting. Hsp90 is shown as a control. sicNPM1, control siRNA non-relevant to the expression of NPM1; Wild, without transduction. E, 293T cells were co-transfected with the luciferase reporter plasmid (pGL4/APET), expression plasmid (pcDNA MEF/ELF4), and siWt-NPM1 gene (pcDNA/siRNA-Wt-NPM1) or control. Luciferase activity by pGL4/APET alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. *, p < 0.05. F, 293T cells were co-transfected with the luciferase reporter gene of an artificial MEF/ELF4 target promoter and effector genes. Target promoter and effector genes were as follows: pGL4/APET (lane 1); pGL4/APET and pcDNA/MEF/ELF4 (lane 2); pGL4/APET, pcDNA/MEF/ELF4, and Wt-NPM1 (lane 3); pGL4/APET, pcDNA/MEF/ELF4, and Mt-A-NPM1, Mt-I-NPM1, or Mt-J-NPM1 (lanes 4–6, respectively); and pGL4/APET and pcDNA/Wt-NPM1, Mt-A-NPM1, Mt-I-NPM1, or Mt-J-NPM1 (lanes 7–10, respectively). Luciferase activity by pGL4/APET alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. *, p < 0.05. G, COS7 cells were co-transfected with the luciferase reporter gene of an artificial MEF/ELF4 target promoter and effector genes. The target promoter and effector genes were as follows: pGL4/APET (lane 1); pGL4/APET and pcDNA/MEF/ELF4 (lane 2); pGL4/APET, pcDNA/MEF/ELF4, and Wt-NPM1 (lane 3); pGL4/APET, pcDNA/MEF/ELF4, and Mt-A-NPM1 or Mt-I-NPM1 (lanes 4 and 5, respectively); and pGL4/APET and pcDNA/Wt-NPM1, Mt-A-NPM1, or Mt-I-NPM1 (lanes 6–8, respectively). Luciferase activity by pcDNA/APET alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. (*, p < 0.05). H, U937 cells were co-transfected with the luciferase reporter gene of an artificial MEF/ELF4 target promoter and effector genes. Target promoter and effector genes were as follows: pGL4/APET (lane 1); pGL4/APET and pcDNA/MEF/ELF4 (lane 2); pGL4/APET, pcDNA/MEF/ELF4, and Wt-NPM1 (lane 3); pGL4/APET, pcDNA/MEF/ELF4, and Mt-A-NPM1 (lane 4); and pGL4/APET and pcDNA/Wt-NPM1 or Mt-A-NPM1 (lanes 5 and 6, respectively). Luciferase activity by pcDNA/APET alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. *, p < 0.05. I, 293T cells were co-transfected with 0.1 μg of the luciferase reporter gene of an artificial MEF/ELF4 target promoter (lanes 1–9) and 0.1 μg of effector genes (pcDNA/MEF/ELF4) (lanes 1–6). The effector genes were as follows: 0.2 μg of Mt-A-NPM1 (lane 1); 0.16 μg of Mt-A-NPM1 and 0.04 μg of Wt-NPM1 (lane 2); 0.1 μg of Mt-A-NPM1 and 0.1 μg of Wt-NPM1 (lane 3); 0.04 μg of Mt-A-NPM1 and 0.16 μg of Wt-NPM1 or 0.2 μg of Wt-NPM1 (lanes 4 and 5, respectively); none (lane 6); pGL4/APET and 0.2 μg of Mt-A-NPM1 (lane 7); pGL4/APET and 0.2 μg of Wt-NPM1 (lane 8); and pGL4/APET (lane 9). Luciferase activity by pGL4/APET alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. *, p < 0.05.
Having shown that NPM1 expression attenuated the transcriptional activity of MEF/ELF4 in leukemia cells, we next assessed whether the inhibition of Wt-NPM1 expression in vivo enhanced MEF/ELF4-dependent transcriptional activity. The siRNA directed against Wt-NPM1 in 293T cells suppressed the expression of Wt-NPM1 protein by 60–70% (Fig. 3D). Transient transfections were performed by using NPM1-knockdown 293T cells with pcDNA/MEF/ELF4 and pGL4/APET reporter plasmids. A luciferase assay revealed that MEF/ELF4-dependent transcriptional activity was significantly elevated in Wt-NPM1-knockdown cells by 1.8-fold (Fig. 3E). These results implied that Wt-NPM1 functioned as an inhibitor of MEF/ELF4.
Mutated nucleophosmin (Mt-NPM1) has been found in 50% of adult AML patients with normal karyotypes (15). It has been suggested that the mutation is a critical event for leukemogenesis. To determine the effect of Mt-NPM1 on the transcription-activating properties of MEF/ELF4, we transfected pcDNA/MEF/ELF4 in combination with pcDNA/Mt-A-NPM1, pcDNA/Mt-I-NPM1, or pcDNA/Mt-J-NPM1 and then examined the activity of the APET promoter construct in 293T cells (Fig. 3F). Co-expression of Mt-NPM1 with MEF/ELF4 led to a 315-fold increase in luciferase activity. Similar data were obtained with COS7 (Fig. 3G) and U937 (Fig. 3H) cells. To show the effect of the coexistence of both Wt- and Mt-NPM1, we transfected 293T cells with various amounts of plasmids that expressed Wt-NPM1 and Mt-A-NPM1. The expression of Mt-NPM1 enhanced MEF/ELF4-dependent APET promoter activation in a dose-dependent manner, even in the presence of Wt-NPM1 (Fig. 3I). Taken together, our results suggest that Wt-NPM1 has an inhibitory effect, whereas Mt-NPM1 has enhancing effect, on the function of MEF/ELF4.
Mt-NPM1 Does Not Interact with MEF/ELF4 in Vivo
Because the mutated region of Mt-NPM1 was located outside the domain responsible for interaction with MEF/ELF4, we hypothesized that Mt-NPM1 might bind to MEF/ELF4. To test this hypothesis, we transfected 293T cells with FLAG-MEF/ELF4 and V5-Mt-A-NPM1 expression plasmids and performed immunoprecipitations with mouse monoclonal anti-FLAG or anti-V5 antibody. Contrary to our expectations, as shown in Fig. 4, FLAG-MEF/ELF4 protein and V5-Wt-A-NPM1 did not co-precipitate with each other (Fig. 4). These results showed that there is little in vivo interaction between Mt-A-NPM1 and MEF/ELF4.
FIGURE 4.
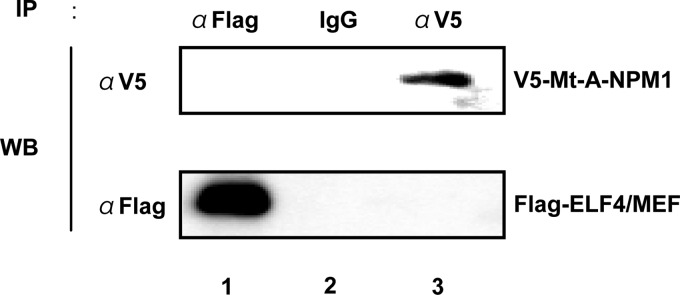
Mt-A-NPM1 does not interact with MEF/ELF4 in vivo. 293T cells were transfected with the indicated expression plasmids. After 48 h, cell lysates were immunoprecipitated (IP) with anti-FLAG and anti-V5 antibodies. Immunoprecipitates were analyzed by 10% SDS-PAGE and subjected to immunoblotting (WB) with anti-V5 antibody (top row) or anti-FLAG antibody (bottom row).
Localization of MEF/ELF4 Is Unaffected by Mt-NPM1
Having shown that Mt-NPM1 enhances the transcriptional activity of MEF/ELF4, we next assessed whether Mt-NPM1 dislocates MEF/ELF4 into the cytoplasm. We transiently co-transfected a MEF/ELF4-GFP fusion protein vector together with the pcDNA/V-Wt-NPM1 or pcDNA/V-Mt-A-NPM1 expression vector into 293T cells. Wt-NPM1 protein and MEF/ELF4 localized to the nucleus (Fig. 5A (a)), whereas Mt-A-NPM1 protein localized to the cytoplasm (Fig. 5A (b)). Contrary to our expectations, the presence of Mt-A-NPM1 did not affect the subcellular distribution of MEF/ELF4. Western blot analysis of MEF/ELF4 and Wt- or Mt-NPM1 in nuclear and cytoplasmic proteins confirmed the nuclear localization of MEF/ELF4 even with Mt-NPM1 (Fig. 5B).
FIGURE 5.
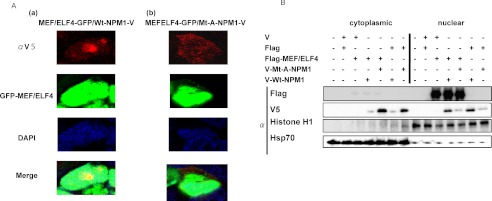
Localization of MEF/ELF4 was unaffected by the mutation of NPM1. A, 293T cells were transfected with the GFP-MEF/ELF4 fusion protein expression vector and pcDNA/V-Wt-NPM1 (a) or pcDNA/V-Mt-A-NPM1 (b). Forty-eight hours after transfection, cells were fixed and immunofluorescence-stained with anti-V tag antibody. B, Western blotting of FLAG-MEF/ELF4 subcellular distribution in 293T cells co-transfected with pFLAG-MEF/ELF4 and pcDNA/V-Wt-NPM1 or pcDNA/V-Mt-A-NPM1. Purity of the subcellular fractions was assessed by blotting with histone H1 (nuclear extraction) and Hsp70 (cytoplasmic extraction).
Wt-NPM1 Inhibits, whereas Mt-NPM1 Enhances, the Oncogenic Activity of MEF/ELF4
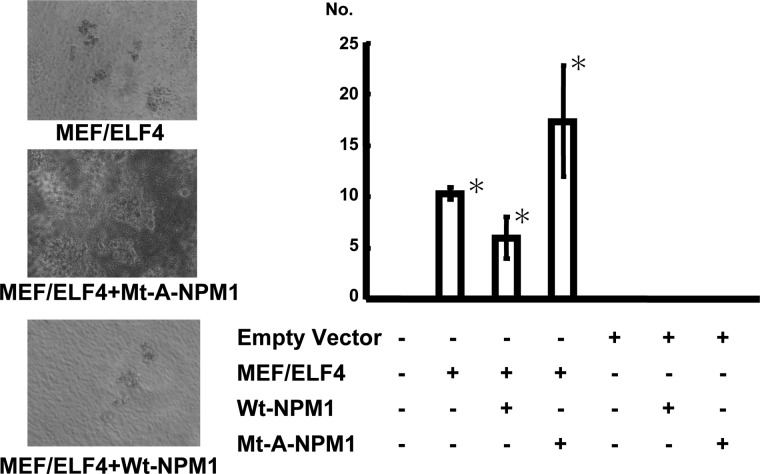
The overexpression of MEF/ELF4 in NIH3T3 cells increases the growth rate, enhances colony formation in soft agar, and promotes tumor formation in nude mice (10). To determine the effects of the interaction of NPM1 with MEF/ELF4 on cell behavior, we assessed the anchorage-independent growth of NIH3T3 cells after co-transfection of MEF/ELF4 with Wt-NPM1 or Mt-A-NPM1. Compared with NIH3T3 transfected with only MEF/ELF4, Wt-NPM1-coexpressing cells showed reduced anchorage-independent growth, whereas Mt-A-NPM1-coexpressing cells exhibited increased growth (Fig. 6).
FIGURE 6.
Mt-NPM1 stimulates MEF/ELF4-induced hyperproliferation and transformation. NIH3T3 cells transfected with various combinations of expression plasmids were plated in soft agar on 60-mm dishes and incubated for 2 weeks. A, microscopy of MEF/ELF4-transfected NIH3T3 cells with Wt-NPM1 or Mt-A-NPM1. B, the average number of colonies of three independent experiments with S.D. (error bars). *, p < 0.05.
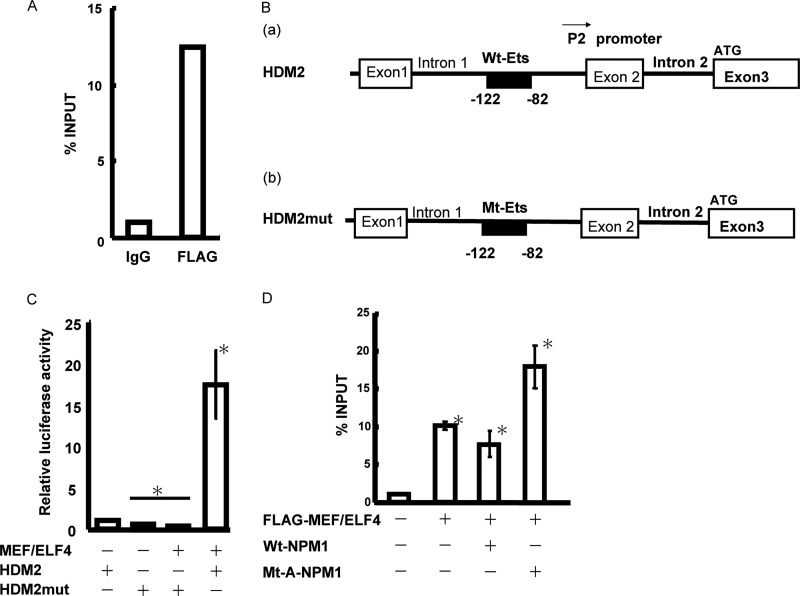
MEF/ELF4 Binds to the HDM2 Promoter and Activates Its Expression
In murine cells, MEF/ELF4 binds directly to the Mdm2 promoter, thereby promoting Mdm2 expression (12). To ascertain whether MEF/ELF4 also directly regulates the promoter activity of HDM2 (the human analog of Mdm2), we scrutinized the DNA sequence of the HDM2 gene and found a conserved putative MEF/ELF4 binding site in the P2 promoter (Fig. 7B). To establish the association of MEF/ELF4 with the HDM2 promoter, we performed a ChIP assay with nuclear lysates from 293T cells expressing FLAG-MEF/ELF4. Immunoprecipitation with the FLAG antibody (but not with the control IgG) and subsequent PCRs revealed the recruitment of overexpressed MEF/ELF4 to the promoter region of the HDM2 gene (Fig. 7A). The luciferase assay revealed that MEF/ELF4 strongly transactivated the wild-type HDM2 promoter (Fig. 7, B (a) and C) and that the effect was abrogated by mutation of the ETS site (−122 to −82) (Fig. 7, B (b) and C). Compared with Wt-NPM1, the expression of Mt-A-NPM1 in 293T cells enhanced the association of MEF/ELF4 with the HDM2 promoter, as detected by ChIP analysis (Fig. 7D). Taken together, these findings suggest that Mt-NPM1 up-regulates HDM2 transcription by increasing the recruitment of MEF/ELF4 to the HDM2 promoter by dislocating Wt-NPM1 that interferes with its binding to the promoter.
FIGURE 7.
MEF/ELF4 transactivates the HDM2 promoter. A, MEF/ELF4 binds to the HDM2 promoter in vivo. FLAG-MEF/ELF4-bound DNA from 293T cells was immunoprecipitated with FLAG antibody or normal mouse IgG. RQ-PCR amplification was performed on the corresponding templates by using primers for HDM2. B, structure of the HDM2 promoter region (−82 to −122) (schematic). C, 293T cells were transfected with HDM2 promoter-driven luciferase reporter plasmid encoding wild-type (B (a)) or mutant (B (b)) protein. Luciferase activity by pcDNA alone was assigned a value of 1.0. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. (error bars). D, 293T cells were co-transfected with pFLAG/MEF/ELF4 and pcDNA/Wt-NPM1 or pcDNA/Mt-A-NPM1. RQ-PCR amplification was undertaken on corresponding templates using primers for HDM2. The analysis was performed in triplicate assays, and the results were reproducible. The results are shown as the mean ± S.D. *, p < 0.05.
Higher Levels of HDM2 mRNA in Clinical Samples from AML Patients with Mt-NPM1 and Higher MEF/ELF4 Expression
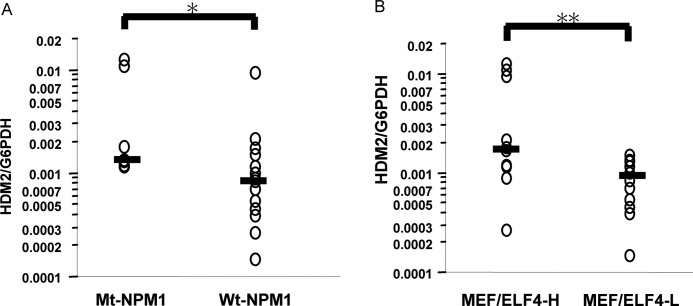
To determine the possible clinical relevance of MEF/ELF4, NPM1, and HDM2 in AML patients, we examined the mRNA levels of each in CD34-positive leukemic blasts from 22 AML patients with normal karyotypes. Fourteen patients had Wt-NPM1, and eight patients had Mt-A-NPM1. There was no significant difference between the clinical characteristics of the Wt-NPM1 group and those of the Mt-NPM1 group (Table 1). Samples from the Mt-NPM1 group had significantly higher levels of HDM2 expression as compared with the Wt-NPM1 group (p = 0.009) (Fig. 8A). In addition, patients with high expression levels of MEF/ELF4 (the MEF/ELF4-H group) had significantly higher HDM2 expression than patients with low expression levels of MEF/ELF4 (the MEF/ELF4-L group) (p = 0.03) (Fig. 8B).
TABLE 1.
Clinical and laboratory characteristics of patients (ranges shown in parentheses)
| Wt-NPM1 | Mt-NPM1 | p | |
|---|---|---|---|
| No. of patients | 14 | 8 | |
| Sex | |||
| Male | 5 | 5 | |
| Female | 9 | 3 | 0.60 |
| Median age (years) | 54.5 (18–78) | 62 (44–76) | |
| FAB classification | |||
| M0 | 1 | 0 | |
| M1 | 2 | 2 | |
| M2 | 4 | 2 | |
| M4 | 2 | 2 | |
| M5 | 2 | 2 | |
| M6 | 3 | 0 | 0.50 |
| TLD+ | 6 | 4 | 0.50 |
| Median white blood cell count/μl | 7300 (1300–556,000) | 47,500 (1700–114,700) | 0.10 |
| Median lactate dehydrogenase level | 647 (203–5325) | 669 (270–2391) | 0.07 |
| Median bone marrow cell count/μl | 337,000 (9000–738,000) | 475,000 (34,900–769,000) | 0.10 |
FIGURE 8.
Expression of Mt-NPM1 and higher expression of MEF/ELF4 are associated with the elevated expression of HDM2 in CD34-positive AML cells. Total RNA isolated from 22 AML patients (CD34-positive leukemia cells) was analyzed for the expression of HDM2 by RQ-PCR. Shown is stratification by the presence of the NPM1 mutation (A) and by the level of ELF4/MEF (B). These bars were median lines for each group. *, p < 0.009 against Wt-NPM1; **, p < 0.03 against MEF/ELF4-L, assessed by analysis of variance followed by Scheffe's multiple comparison test.
DISCUSSION
In the present study, we identified NPM1 to be a MEF/ELF4-binding protein. Wt-NPM1 inhibited the function of MEF/ELF4 (i.e. DNA binding and transcriptional activities), whereas Mt-NPM1 augmented its function. Some of these effects of Wt-NPM1 and Mt-NPM1 on MEF/ELF4 were reproducible on the HDM2 promoter (one of the target genes of MEF/ELF4), suggesting that HDM2 expression is influenced by NPM1. Furthermore, we found that the expression of Mt-NPM1 in MEF/ELF4-overexpressing NIH3T3 cells resulted in enhanced malignant transformation. We also found that the mRNA level of HDM2 in primary leukemia cells was higher in patients with NPM1 mutations. Mef/Elf4 directly activates Mdm2 expression (13). Therefore, NPM1 mutation could enhance HDM2 expression through the increased MEF/ELF4 activity, thereby promoting transformation by inhibiting the p53 pathway.
NPM1 is a multifunctional phosphoprotein that has been implicated in cell proliferation as well as regulation of transcription factors. It appears to repress or stimulate transcription. For example, Wt-NPM1 activates and inhibits p53 function through direct binding (22, 25). Interferon regulatory factor-1 (IRF-1), a transcriptional activator, binds to Wt-NPM1, resulting in the inhibition of DNA binding and transcriptional activity (26). Our findings with Wt-NPM1 and MEF/ELF4 are consistent with these observations. Wt-NPM1 interacts directly with c-Myc and regulates the expression of endogenous c-Myc target genes at the promoter, which enhances c-Myc-induced proliferation and transformation (27). In contrast, the present study suggests that Wt-NPM1 inhibits (whereas Mt-NPM1 facilitates) the transformation induced by MEF/ELF4, suggesting that there is a contradiction in terms of NPM1 function. However, the overexpression of Wt-NPM1 without c-Myc activation has only a small effect on proliferation and has no effect on transformation, so Wt-NPM1 may mainly have a role in c-Myc-driven tumors. Interestingly, c-Myc, IRF-1, and MEF/ELF4 are all regulated during the cell cycle, and the levels of these transcription factors are highest in the G1 phase (28, 29).
We found that Wt-NPM1 could interfere with the ability of MEF/ELF4 to bind to DNA, resulting in the inhibition of MEF/ELF4-dependent transcriptional activity. The mechanism by which Wt-NPM1 interferes with the DNA binding of MEF/ELF4 is unclear. We previously showed that the 120 amino acids N-terminal to the ETS domain in MEF/ELF4 (residues 87–206) are responsible for its binding to AML1 proteins (30); thus, MEF/ELF4 interacts with other proteins outside the DNA-binding domain. As mentioned above, the association of Wt-NPM1 and IRF-1 inhibits the DNA binding of IRF-1. Narayan et al. showed that IRF1 binds directly to Wt-NPM1 through a short linear motif in the nuclear localization sequence outside the DNA-binding domain (31). These results suggest that the inhibition of DNA binding by NPM1 may not be through simple interference with the DNA-binding domain of MEF/ELF4. Determining the protein-binding interface of MEF/ELF4 may help to reveal the mechanism of NPM1-mediated transcriptional regulation.
The heterodimerization domain (residues 186–259) of NPM1 is essential for its interaction with p53 (22), and the c-Myc-binding region is within the NPM1 heterodimerization domain (27). In the case of MEF/ELF4 and NPM1, the N-terminal regions of NPM1 (F1, F2, and F3) could bind to His-MEF/ELF4, implying that the oligomerization domain is important for the interaction.
Recently, it has been shown in vivo that NPM1 mutants actively contribute to leukemogenesis by conferring a proliferative advantage in the myeloid lineage. In zebrafish, forced expression of mutant NPM1 causes an increase in PU.1-positive primitive early myeloid cells (32). Furthermore, in a transgenic mouse expressing the human NPM1 mutant, although spontaneous AML was not found, myeloproliferation occurred in the bone marrow and spleen (33). Moreover, Vassiliou et al. (34) showed that activation of a humanized mouse NPM1 mutant knock-in allele in mouse hematopoietic stem cells caused overexpression of the Hox gene, enhanced self-renewal, and expanded myelopoiesis, resulting in delayed onset AML in one-third of the mice. Taken together, these data suggest that NPM1 mutations initiate leukemia by activating a set of proliferative pathways. Mt-NPM1 enhances the transcriptional activity of MEF/ELF4, so the up-regulation of HDM2 and subsequent down-regulation of p53 may also have a role in leukemogenesis.
In vitro transfection studies and immunohistochemical observations in samples from AML patients have demonstrated that NPM1 mutants recruit Wt-NPM1 from the nucleolus and delocalize it to the nucleoplasm and cytoplasm (18) and that aberrant NPM1 accumulation in the cytoplasm may have a critical role in leukemogenesis. While Wt-NPM1 protein co-localizes with tumor suppressor p19ARF in the nucleolus, Mt-NPM1 delocalizes p19ARF from the nucleolus to the cytoplasm, which results in reduced p19ARF activities (e.g. Mdm2 and p21cip1 induction, stimulation of NPM1) (35). Furthermore, by using OCI/AML3 human leukemia cells where mutant NPM1 is localized in the cytoplasm, Bhat et al. (36) have recently shown that NPM1-co-localizing nuclear transcription factor, FOXM1 (forkhead box M1), disappears from the cytoplasm following transient NPM1 knockdown. These data suggest that NPM1 may determine the intracellular localization of interacting transcription factors. However, in our experiments, Mt-NPM1 did not interact with MEF/ELF4 in vivo, and the subcellular distribution of MEF/ELF4 was not affected by the presence of Mt-NPM1. It seems that Mt-NPM1 binds and dislocates Wt-NPM1 into the cytoplasm of leukemia cells, which eventually leads to uncontrolled transactivation of MEF/ELF4. Wt-NPM1 knockdown with siRNA against NPM1 also enhanced MEF/ELF4 activity (Fig. 3E), suggesting that the depletion of an MEF/ELF4 inhibitor (i.e. Wt-NPM1) in the nucleus is responsible for the transactivation of MEF/ELF4. Taken together, it is likely that NPM1 mutants exert oncogenic functions at least in part through the up-regulation of the activities of oncogenic transcription factors, such as MEF/ELF4. The correlation between NPM1 mutations and the elevated expression of HDM2 in primary leukemia cells seems to support this theory.
In patients with AML, NPM1 mutations are mutually exclusive of recurrent genetic abnormalities. It can be speculated that the enhanced MEF/ELF4-HDM2-p53 pathway induced by NPM1 mutations may participate in leukemia development, especially in patients with a normal karyotype. The transactivation of MEF/ELF4 by E2F1 is inhibited by p53 (37), suggesting that p53 suppression induced by NPM1 mutation could lead to the activation of E2F1, resulting in the enhanced expression of MEF/ELF4. Our previous data showing the elevated expression of MEF/ELF4 in AML cells with a normal karyotype compared with that of AML cells carrying t(8;21) and t(15;17) seem to support this hypothesis. Our results suggest a new role for NPM1 and MEF/ELF4 in leukemia development.
This work was supported in part by a grant from the Ministry of Health, Welfare, and Labor and a grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
- AML
- acute myeloid leukemia
- Wt-NPM1
- wild type NPM1
- Mt-NPM1
- mutant NPM1
- RQ-PCR
- quantitative reverse transcription-polymerase chain reaction.
REFERENCES
- 1. Miyazaki Y., Sun X., Uchida H., Zhang J., Nimer S. (1996) A novel transcription factor with an Elf-1 like DNA binding domain but distinct transcriptional activating properties. Oncogene 13, 1721–1729 [PubMed] [Google Scholar]
- 2. Hedvat C. V., Yao J., Sokolic R. A., Nimer S. D. (2004) Myeloid ELF1-like factor is a potent activator of interleukin-8 expression in hematopoietic cells. J. Biol. Chem. 279, 6395–6400 [DOI] [PubMed] [Google Scholar]
- 3. Lacorazza H. D., Miyazaki Y., Di Cristofano A., Deblasio A., Hedvat C., Zhang J., Cordon-Cardo C., Mao S., Pandolfi P. P., Nimer S. D. (2002) The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity 17, 437–449 [DOI] [PubMed] [Google Scholar]
- 4. Lu Z., Kim K. A., Suico M. A., Shuto T., Li J. D., Kai H. (2004) MEF up-regulates human β-defensin 2 expression in epithelial cells. FEBS Lett. 561, 117–121 [DOI] [PubMed] [Google Scholar]
- 5. Seki Y., Suico M. A., Uto A., Hisatsune A., Shuto T., Isohama Y., Kai H. (2002) The ETS transcription factor MEF is a candidate tumor suppressor gene on the X chromosome. Cancer Res. 62, 6579–6586 [PubMed] [Google Scholar]
- 6. Smith A. M., Calero-Nieto F. J., Schütte J., Kinston S., Timms R. T., Wilson N. K., Hannah R. L., Landry J. R., Göttgens B. (2012) Integration of Elf-4 into stem/progenitor and erythroid regulatory networks through locus-wide chromatin studies coupled with in vivo functional validation. Mol. Cell Biol. 32, 763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacorazza H. D., Yamada T., Liu Y., Miyata Y., Sivina M., Nunes J., Nimer S. D. (2006) The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell 9, 175–187 [DOI] [PubMed] [Google Scholar]
- 8. Liu Y., Elf S. E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J. M., Deblasio A., Menendez S., Antipin J., Reva B., Koff A., Nimer S. D. (2009) p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 4, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukushima T., Miyazaki Y., Tsushima H., Tsutsumi C., Taguchi J., Yoshida S., Kuriyama K., Scadden D., Nimer S., Tomonaga M. (2003) The level of MEF but not ELF-1 correlates with FAB subtype of acute myeloid leukemia and is low in good prognosis cases. Leuk. Res. 27, 387–392 [DOI] [PubMed] [Google Scholar]
- 10. Yao J. J., Liu Y., Lacorazza H. D., Soslow R. A., Scandura J. M., Nimer S. D., Hedvat C. V. (2007) Tumor promoting properties of the ETS protein MEF in ovarian cancer. Oncogene 26, 4032–4037 [DOI] [PubMed] [Google Scholar]
- 11. Totoki Y., Tatsuno K., Yamamoto S., Arai Y., Hosoda F., Ishikawa S., Tsutsumi S., Sonoda K., Totsuka H., Shirakihara T., Sakamoto H., Wang L., Ojima H., Shimada K., Kosuge T., Okusaka T., Kato K., Kusuda J., Yoshida T., Aburatani H., Shibata T. (2011) High-resolution characterization of hepatocellular carcinoma genome. Nat. Genet. 43, 464–469 [DOI] [PubMed] [Google Scholar]
- 12. Du Y., Spence S. E., Jenkins N. A., Copeland N. G. (2005) Cooperating cancer-gene identification through oncogenic-retrovirus-induced insertional mutagenesis. Blood 106, 2498–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sashida G., Liu Y., Elf S., Miyata Y., Ohyashiki K., Izumi M., Menendez S., Nimer S. D. (2009) ELF4/MEF activates MDM2 expression and blocks oncogene-induced p16 activation to promote transformation. Mol. Cell Biol. 29, 3687–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. (1989) Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 56, 379–390 [DOI] [PubMed] [Google Scholar]
- 15. Falini B., Mecucci C., Tiacci E., Alcalay M., Rosati R., Pasqualucci L., La Starza R., Diverio D., Colombo E., Santucci A., Bigerna B., Pacini R., Pucciarini A., Liso A., Vignetti M., Fazi P., Meani N., Pettirossi V., Saglio G., Mandelli F., Lo-Coco F., Pelicci P. G., Martelli M. F., and GIMEMA Acute Leukemia Working Party (2005) Cytoplasmic nucleophosmin in acute myelogenous leukemia with normal karyotype. N. Engl. J. Med. 352, 254–266 [DOI] [PubMed] [Google Scholar]
- 16. Yu Y., Maggi L. B., Jr., Brady S. N., Apicelli A. J., Dai M. S., Lu H., Weber J. D. (2006) Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol. Cell Biol. 26, 3798–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mariano A. R., Colombo E., Luzi L., Martinelli P., Volorio S., Bernard L., Meani N., Bergomas R., Alcalay M., Pelicci P. G. (2006) Cytoplasmic localization of NPM in myeloid leukemias is dictated by gain-of-function mutations that create a functional nuclear export signal. Oncogene 25, 4376–4380 [DOI] [PubMed] [Google Scholar]
- 18. Falini B., Bolli N., Shan J., Martelli M. P., Liso A., Pucciarini A., Bigerna B., Pasqualucci L., Mannucci R., Rosati R., Gorello P., Diverio D., Roti G., Tiacci E., Cazzaniga G., Biondi A., Schnittger S., Haferlach T., Hiddemann W., Martelli M. F., Gu W., Mecucci C., Nicoletti I. (2006) Both carboxyl-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood 107, 4514–4523 [DOI] [PubMed] [Google Scholar]
- 19. Grisendi S., Bernardi R., Rossi M., Cheng K., Khandker L., Manovae K., Pandolfi P. P. (2005) Role of nucleophosmin in embryonic development and tumorigenesis. Nature 437, 147–153 [DOI] [PubMed] [Google Scholar]
- 20. Rigaut G., Shevchenko A., Rutz B., Wilm M., Mann M., Séraphin B. (1999) A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030–1032 [DOI] [PubMed] [Google Scholar]
- 21. Suzuki T., Kiyoi H., Ozeki K., Tomita A., Yamaji S., Suzuki R., Kodera Y., Miyawaki S., Asou N., Kuriyama K., Yagasaki F., Shimazaki C., Akiyama H., Nishimura M., Motoji T., Shinagawa K., Takeshita A., Ueda R., Kinoshita T., Emi N., Naoe T. (2005) Clinical characteristics and prognostic implications of NPM1 mutations in acute myeloid leukemia. Blood 106, 2854–2861 [DOI] [PubMed] [Google Scholar]
- 22. Colombo E., Marine J. C., Danovi D., Falini B., Pelicci P. G. (2002) Nucleophosmin regulates the stability and transcriptional activity of p53. Nat. Cell Biol. 4, 529–533 [DOI] [PubMed] [Google Scholar]
- 23. Phelps M., Darley M., Primrose J. N., Blaydes J. P. (2003) p53-independent activation of the hdm-P2 promoter through multiple transcription factor response elements results in elevated hdm2 expression in estrogen receptor α-positive breast cancer cells. Cancer Res. 63, 2616–2623 [PubMed] [Google Scholar]
- 24. Grisendi S., Mecucci C., Falini B., Pandolfi P. P. (2006) Nucleophosmin and cancer. Nat. Rev. Cancer 6, 493–505 [DOI] [PubMed] [Google Scholar]
- 25. Li J., Zhang X., Sejas D. P., Pang Q. (2005) Negative regulation of p53 by nucleophosmin antagonizes stress-induced apoptosis in human normal and malignant hematopoietic cells. Leuk. Res. 29, 1415–1423 [DOI] [PubMed] [Google Scholar]
- 26. Kondo T., Minamino N., Nagamura-Inoue T., Matsumoto M., Taniguchi T., Tanaka N. (1997) Identification and characterization of nucleophosmin/B23/numatrin which binds the anti-oncogenic transcription factor IRF-1 and manifests oncogenic activity. Oncogene 15, 1275–1281 [DOI] [PubMed] [Google Scholar]
- 27. Li Z., Boone D., Hann S. R. (2008) Nucleophosmin interacts directly with c-Myc and controls c-Myc-induced hyperproliferation and transformation. Proc. Natl. Acad. Sci. U.S.A. 105, 18794–18799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amati B., Alevizopoulos K., Vlach J. (1998) Myc and the cell cycle. Front. Biosci. 3, d250–d268 [DOI] [PubMed] [Google Scholar]
- 29. Miyazaki Y., Boccuni P., Mao S., Zhang J., Erdjument-Bromage H., Tempst P., Kiyokawa H., Nimer S. D. (2001) Cyclin A-dependent phosphorylation of the ETS-related protein, MEF, restricts its activity to the G1 phase of the cell cycle. J. Biol. Chem. 276, 40528–40536 [DOI] [PubMed] [Google Scholar]
- 30. Mao S., Frank R. C., Zhang J., Miyazaki Y., Nimer S. D. (1999) Functional and physical interactions between AML1 proteins and an ETS protein, MEF. Implications for the pathogenesis of t(8;21)-positive leukemias. Mol. Cell Biol. 19, 3635–3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Narayan V., Halada P., Hernychová L., Chong Y. P., Žáková J., Hupp T. R., Vojtesek B., Ball K. L. (2011) A multi-protein binding interface in an intrinsically disordered region of the tumor suppressor protein interferon regulatory factor-1. J. Biol. Chem. 286, 14291–14303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolli N., Payne E. M., Grabher C., Lee J. S., Johnston A. B., Falini B., Kanki J. P., Look A. T. (2010) Expression of the cytoplasmic NPM1 mutant (NPMc+) causes the expansion of hematopoietic cells in zebrafish. Blood 115, 3329–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng K., Sportoletti P., Ito K., Clohessy J. G., Teruya-Feldstein J., Kutok J. L., Pandolfi P. P. (2010) The cytoplasmic NPM mutant induces myeloproliferation in a transgenic mouse model. Blood 115, 3341–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vassiliou G. S., Cooper J. L., Rad R., Li J., Rice S., Uren A., Rad L., Ellis P., Andrews R., Banerjee R., Grove C., Wang W., Liu P., Wright P., Arends M., Bradley A. (2011) Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat. Genet. 43, 470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. den Besten W., Kuo M. L., Williams R. T., Sherr C. J. (2005) Myeloid leukemia-associated nucleophosmin mutants perturb p53-dependent and independent activities of the Arf tumor suppressor protein. Cell Cycle 4, 1593–1598 [DOI] [PubMed] [Google Scholar]
- 36. Bhat U. G., Jagadeeswaran R., Halasi M., Gartel A. L. (2011) Nucleophosmin interacts with FOXM1 and modulates the level and localization of FOXM1 in human cancer cells. J. Biol. Chem. 286, 41425–41433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taura M., Suico M. A., Fukuda R., Koga T., Shuto T., Sato T., Morino-Koga S., Okada S., Kai H. (2011) MEF/ELF4 transactivation by E2F1 is inhibited by p53. Nucleic Acids Res. 39, 76–88 [DOI] [PMC free article] [PubMed] [Google Scholar]